Abstract
The sites that receive ligament and tendon insertions (entheses) on the cortical surfaces of long bones are poorly understood, particularly as regards modeling and regulation. Entheses are classified as either fibrocartilagenous or fibrous based on their structures. Fibrous entheses typically insert into the metaphysis or diaphysis of a long bone, bear a periosteal component, and are modeled during long bone growth. This modeling forms a root system by which the insertions attach to the cortical surface. In the case of the medial collateral ligament, modeling drives actual migration of the ligament along the cortical surface in order to accommodate linear growth, whereas in other sites modeling may excavate a deep cortical root system (e.g., the teres major insertion) or a shallow root system with a large footprint (e.g., the latissimus dorsi insertion). We report here that conditionally deleting parathyroid hormone-related protein (PTHrP) in fibrous entheses via Scleraxis-Cre targeting causes modeling to fail in these three iterations of osteoclast-driven enthesis excavation or migration. These iterations appear to represent formes frusts of a common modeling strategy, presumably differing from each other as a consequence of differences in biomechanical control. In sites in which PTHrP is not induced, either physiologically or because of conditional deletion, modeling does not take place and fibrocartilage is induced. These findings represent the initial genetic evidence that PTHrP regulates periosteal/intramembranous bone cell activity on cortical bone surfaces and indicate that PTHrP serves as a load-induced modeling tool in fibrous insertion sites during linear growth.
Keywords: cortical bone, modeling, enthesis, insertion sites, PTHrP
Introduction
The surface of long bones bears a continuous connective tissue covering. The simplest such structure is the periosteum, which is composed of an outer fibrous layer and an inner cambial layer, the source of the periosteal osteoblasts and osteoclasts that model and sculpt the bone surface (1). This cortical surface also receives the insertions of tendons, ligaments, and capsules, known collectively as entheses.(2-4) These connective tissue structures join muscle to muscle, bone to bone, and muscle to bone and arise via mesenchymal condensations that form coincident with those that will give rise to the bones themselves.(2-4, 5,6) Scleraxis (Scx) is a key transcription factor that is associated with these connective tissue structures throughout life. (5-8)
Entheses are classified into two major subgroups. Fibrocartilagenous insertions are the most complex of the two, comprising four layers (tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone) that provide a graded distribution of load from major muscles to bone. These tend to insert into the epiphyseal end of long bones as well as into ridges that form on the bone surface in association with major muscles.(2-4,8,9) Fibrocartilagenous entheses lack a periosteal component, and their associated ridges and tuberosities form via the same endochondral pathway that forms the long bones themselves.(2-4) The second group are referred to as fibrous insertions. These typically insert into the metaphysis or diaphysis of a long bone, do have a periosteal component, and form not via the endochondral pathway but rather by the intramembraneous (periosteal) pathway.(4) These insertions are sometimes likened to the root systems of trees, some entering at acute angles and burrowing deeply and others spreading over shallow but large surfaces.(2,3) So-called periosteal-muscle insertions are a simplified version of a fibrous enthesis in which a muscle attaches over a broad surface area of a bone via little more than the two-layer periosteum.(2-4)
The entheses are important structures biologically and clinically,(2-4,9) yet they are poorly understood, especially as regards regulation. A recent study has provided considerable insight into the formation and growth of the fibrocartilagenous insertions that are associated with bone ridges such as the deltoid tuberosity.(8) Scx-Cre was used to conditionally delete BMP4 at the sites of these ridge-associated entheses, which caused a failure of their induction during development.(8) A number of such sites were affected, but none of these corresponded to the fibrous sites studied in the work reported here.
Parathyroid hormone-related protein (PTHrP) is an autocrine/paracrine regulatory factor with a number of functions during development. These include formation of the mammary epithelium, regulation of the chondrocyte differentiation program in endochondral bone, and driving the eruption of teeth.(10-14) PTHrP was recently indentified in the fibrous layer of the PO and in fibrous entheses sites.(13,14) In the insertion sites, PTHrP seems to be mechanically-induced, and its expression level is highest during peak linear long-bone growth.(13-15) We report here the effects of conditionally deleting PTHrP in selective fibrous entheses sites via Scx-Cre. We find that PTHrP normally regulates the postnatal modeling of these sites by osteoclasts and that deleting PTHrP is associated with a complete failure of modeling.
Materials and Methods
Mice
The Scx-Cre mouse(8) was outbred onto a CD-1 background for three generations and crossed sequentially to PTHrP-lacZ and PTHrPlox/lox mice to create Scx-Cre/PTHrPlacZ/lox cKO and littermate control PTHrPlacZ/lox mice; gender- and age-matched wild-type CD-1 mice were used as controls for all experiments. The TM-unloading experiment was performed at 3.5 weeks via a simple subcutaneous procedure in which the TM origin at the inferior angle of the scapula was exposed and sectioned bloodlessly at its connective-tissue origin from the scapular cortex, and the mice were sacrificed at 9 weeks of age (see supplementary data and Fig. S6). All mice were handled according to the United State Department of Agriculture guidelines and with the approval of the Yale University Animal Care and Use Committee.
Anatomical and histological analyses
Specimens were analyzed by micro-CT X-ray tomography using a Scanco microCT-35 at a maximum 10μ voxel size, with an integration time of 500 ms and an energy of 55 kVp. We generated 2D views and 3D volume renderings of the micro-CT data with AltaViewer software, version 1.1.2 (Numira Biosciences, Salt Lake City, UT). The chemical(14) and beetle larvae(12) clearing protocols were as described. The statistics applied to the length measurements involved one-way Anova using Tukey’s Multiple Comparison Test. Plastic embedding, sectioning, staining (toluidine blue, von Kossa) and dynamic histomorphometry (Osteomeasure software) were carried out in the Physiology Core of the Yale Core Center for Muscular Skeletal Disorders.(13,14,16) Fixing, decalcification, paraffin embedding, sectioning and staining using X-gal, TRAP, and AP techniques were as described.(13,14,16) Immunohistochemistry for Col-2 (Abcam) and RANKL (Santa Cruz) employed protocols from the supplier. All data shown were replicated in triplicate unless otherwise indicated.
Results
We used Scx-Cre to target the PTHrP gene in fibrous insertion sites in the mouse. A PTHrP-lacZ replacement construct served as the PTHrP-null allele in this system, providing a convenient lacZ marker of PTHrP-expression sites in the conditionally-deleted PTHrP mouse (Scx-Cre/PTHrPlacZ/lox), referred to here as “PTHrP cKO”. Cross-comparison of β-gal-expressing patterns in the PTHrP-lacZ and Scx-Cre/R26R mice allowed us to identify those sites in which PTHrP and Scx gene expression were concordant and therefore candidate sites of interest in the cKO mouse (see Fig. S1). In all experiments, gender-and-age-matched wild-type CD-1 mice served as the controls, and in the humerus experiments CD-1 as well as PTHrPlacZ/lox (cKO littermates) served as controls. We focused primarily on the period of linear growth between 3 and 12 weeks in the mouse, particularly at 6-7 weeks (during peak growth, when the cellular changes in the insertions should be most apparent) and 12-14 weeks (when skeletal maturity is reached and postnatal development of the insertions is essentially complete).(15)
The medial collateral ligament (MCL)
The MCL tethers the distal femur to the proximal tibia and provides medial stability to the knee (Fig. S1F). The tibial MCL insertion is a fibrous site that lies on the surface of the medial proximal tibial metaphysis, just beneath the growth plate. Since this site lies distal to the growth plate, it must migrate during linear growth in order to maintain biomechanical integrity as a collateral ligament. The time course of this process has been well-described in the rat,(17) and the tibial site has been found to express the PTHrP gene at a high level during growth.(14) This site expresses Scx-Cre in high-abundance as well (Fig. S1 B-E).
In wild-type CD-1-mice, anterior-medial 3D views revealed a highly reproducible cortical depression corresponding to the osteoclast-induced surface canal by which the MCL migrates during growth (Fig. 1A, Fig. S2 A-F). This cortical depression provides a highly reproducible and very sensitive index of osteoclast-driven MCL migration in the CD-1 mouse (Fig. S2 and see below). The final resting place of the insertion in wild-type mice is identifiable in most mice by a small mineralized crest on the medial cortical surface referred to as the proximal tibial crest(18) (Fig. 1A and B and Figs. S2 and S3). In contrast, the cortical depression is abortive to entirely absent in the cKO mice; migration fails, and the proximal tibial crest is replaced by a large tuberosity that distorts the proximal medial surface of the tibia (Fig. 1D-E). These abnormalities are highly reproducible and were seen in 11/11 cKO mice of both genders studied at 11-16 weeks, although the degree of distortion and size of the tuberosities varied somewhat (Figs. S2 and S3). The bottom panels of Fig. 1 contain low-magnification plastic sections of 16-week-old tibia that further illustrate the normal MCL and proximal tibial crest (panel G) as well as the tuberosity and distortion of the medial tibia in the cKO mouse (H,I). In addition, the MCL in the cKO mouse is both markedly thickened (compare G and I and Fig. S4 E and F) and abnormally mineralized (I).
Fig. 1.
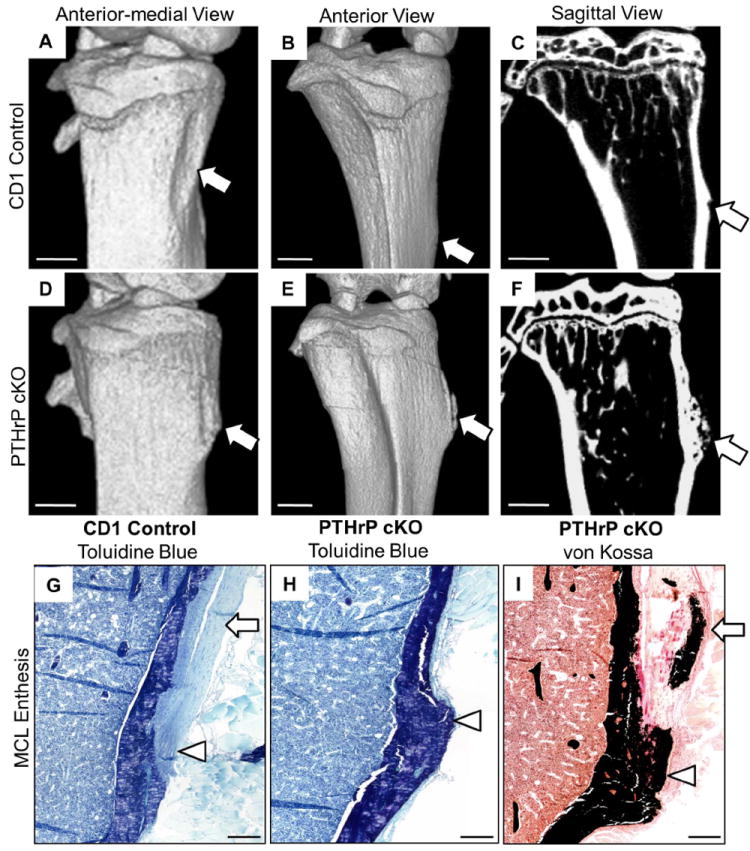
Conditional deletion of PTHrP impairs modeling and migration of the MCL insertion. (A-F) Images of right-sided tibia from 14-week-old CD-1 (A-C) and cKO (D-F) mice, as anterior-medial 3D (A,D), anterior (B,E), and sagittal (C,F) views. In the CD-1 images (A-C), the scalloped cortical surface represents the cortical depression by which the MCL insertion migrates (arrow in panel A), and the proximal tibial crest is shown in panels B and C (arrows). In the cKO images (D-F), the cortical depression is abortive (D); migration has failed, and a large tuberosity has formed at the site of a proximal tibial crest (arrows in E,F). The distances from the top of tibia to the proximal tibial crest in the CD-1 control or mid-tuberosity in the cKO mice was measured in the six examples of each genotype included in Fig. S3 and were 2.02 ± 0.21 and 1.32 ± 0.11 (± SD) mm, respectively (p<0.003 by t test). The tuberosity has a moth-eaten mineralization pattern in cross-section (F). Panels G-I contain 4X dissecting scope images from plastic sections of 16-week-old CD-1 (G) and cKO (H,I) female mice stained with toluidine blue (G,H) or von Kossa (I) reagents. Note the tuberosity and distortion in H and I, and mineralization within the tuberosity and tendon itself in I. The MCL tendon is identified by arrows in G and I and the insertion site by arrowheads in G-I. The thickening of the MCL tendon in I is also well seen in Fig. S 4F. Scale bars in A-F are 1 mm and in G-I 200 μm.
In a similar group of PTHrP-lacZ mice at 12 weeks, we found a more modest but clearcut hapoloinsufficient phenotype at the MCL site that might well have been missed were it not for the sensitive readout provided by the cortical depression (Figs. S2 and S3).
We next examined the modeling of the tibial MCL enthesis histologically, first to characterize its development during growth in control mice and subsequently its failure in cKO mice. In control mice, the MCL is free-standing along the medial surface until it reaches its insertion site (Fig. 2A-C). The cortical surface flanking the free-standing MCL contains the cortical depression, a region that is excavated by numerous osteoclasts that lie subjacent to the mesenchymal cell population that expresses PTHrP (Fig. 2A and C). As expected, the mesenchymal cells that lie between the PTHrP-expressing population and the osteoclasts on the cortical surface are strongly positive by immunohistochemistry for RANK-ligand (RANKL) (Fig. 2G). The insertion site itself and its more distal elements normally contain alkaline phosphatase (AP)-expressing periosteal osteoblasts that will cement the tendon to the cortical surface (Fig. 2B and C). The insertion itself comprises two components, one derived from the fused tendon elements and the other from the subjacent mesenchymal layers on the cortical surface (the so-called enthesis component). Of these images, the montage in Fig. 2C provides the most useful depiction of the histological and functional anatomy of the normal MCL insertion site.
Fig. 2.
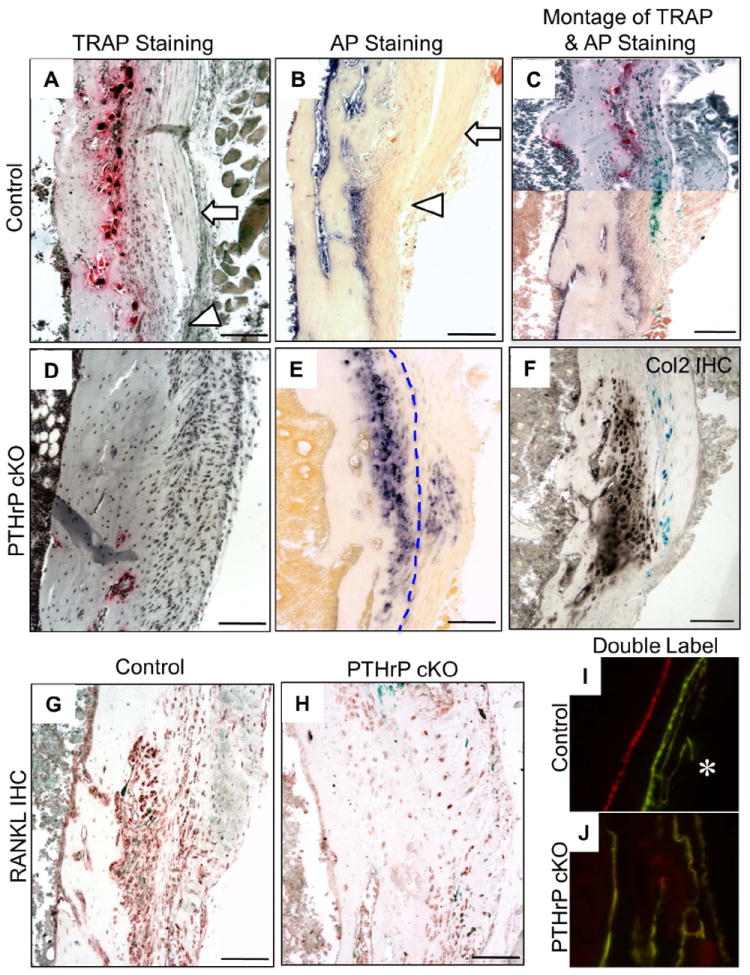
Histological findings associated with normal and failed MCL modeling at 6-8 weeks of age. (A and B) TRAP– and alkaline phosphatase (AP)-stained sections from CD-1 mice at 6 weeks. (A) Cortical depression flanked by the free-standing MCL (arrow) above its actual insertion site (arrowhead). (B) Free-standing MCL (arrow) above the insertion site (arrowhead), which contains AP-positive periosteal osteoblasts/mesenchymal cells that cement the MCL into the cortical surface. These “excavating” and “cementing” components of the MCL site are also presented as a montage in panel C using sections from PTHrP-lacZ mice to illustrate the location of PTHrP gene expression in the site. This montage was prepared from contiguous sections from a 6-week-old PTHrP lacZ mouse mounted so as to reconstruct the upper (TRAP- and X-gal-stained; free-standing MCL) and lower (AP- and X-gal-stained; MCL insertion site) portions of the MCL insertion (see also Fig. S4). (D and E) TRAP- and AP-stained sections in 6-to 8-week-old cKO sections. Panel D lacks any evidence of a cortical depression. Panel E reveals AP-expressing fibrochondrocytes that have formed in both the enthesis component and tendinous component of the insertion site (these populations are separated by a dotted line and are referred to as enthesis and sesamoid fibrochondrocytes, respectively (2,3)). (F) Collagen-2 immunohistochemistry in a 7-week cKO section; there is no such staining in control sections (Fig. S6 I). (G) RANKL-positive mesenchymal cells in CD-1 sections lie between the PTHrP-expressing cells and the osteoclasts on the cortical surface (see panel C). (H) RANKL immunohistochemistry in a section contiguous to E contains only background-level staining. (I and J) Calcein (green) and alizarin (red) double florescent labels in plastic sections from a CD-1 mouse in the region of the MCL (I, showing endocortical double label), and the MCL tuberosity in a cKO mouse (J, showing a chaotic labeling pattern). Scale bars = 100 μm.
The process described above fails entirely in the cKO mice at 6-8 weeks (Fig. 2D and E). The cortical depression does not form and is replaced by what appears to be essentially a connective tissue scar (Fig. 2D). On AP-staining, many of these cells turn out to be fibrochondrocytes, confirmed as such by collagen-2 immunohistochemistry (Fig. 2F). As expected from the absent osteoclasts, immunohistochemistry reveals no RANKL staining in the mesenchymal cells of the cKO MCL (Fig. 2H). The fibrochondrocytes seem to be derived from both the enthesis and tendinous components of the insertion (Fig. 2E), and both have mineralized (see Fig. 1I) by what appears to be a disorganized process (Fig. 2J). The loading of the site that has driven the fibrochondrocyte formation is reflected also by a much thickened MCL in the cKO mice (Fig. S4 C and D). The extent of the destruction of the MCL site in the cKO mouse exhibited by 6 weeks suggested that this process might be evident earlier, and this was confirmed by TRAP staining in 3-week-old CD-1 and cKO mice, which revealed a paucity of osteoclasts in the cKO site (Fig. S4 C and D).
Teres Major and Latissimus Dorsi Insertions
The cKO mouse displays a number of abnormal entheses other than at the MCL site. These include the origins and/or insertions of the pronator teres and brachioradialis in the humerus and radius, several regions on the scapula, and the medial surface of the humerus (below and Fig. S5). Of these, we chose to focus on the medial humerus because this region is profoundly abnormal by micro-CT in the cKO mouse (Fig. 3) and is an area that is rich in insertions of muscles that adduct the shoulder. In cleared and histological specimens, this region co-expresses both the Scx and PTHrP genes in high abundance (Fig. S1 G-J).
Fig. 3.
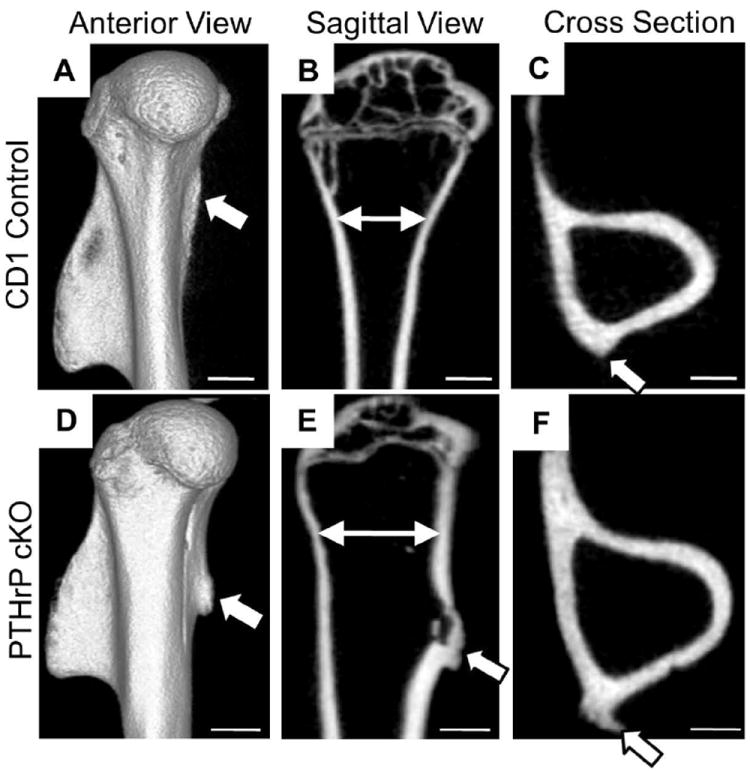
Conditional deletion of PTHrP impairs modeling of adductor insertion sites in the proximal humerus. Micro-CT images of the humerus in 15-week-old CD-1 and cKO mice. (A-C) 3D, sagittal, and cross-sections from CD-1 mice; (D-F) corresponding images in a cKO mouse. The arrowheads identify the ridge and tuberosity in the CD-1 and cKO mice, respectively. Note the distortion of the proximal humerus in panel E as compared to B (high-lighted by the two-headed arrows), apparent also in the cross-sections (panels C versus F). Scale bars = 1 mm.
The sites in question turn out to be the insertions of the teres major (TM) and the latissimus dorsi (LD), both of which attach to the humerus in a groove at the angle of its medial and lateral surfaces (see Fig. 4A and supplementary text). The TM is a thick, short, and compact muscle that originates from the inferior angle of the scapula and inserts into the humerus at an acute (near-90°) angle, and the LD originates from the spine in the lower back and is a broad and thin muscle that converges to insert at a shallow (about 10°) angle over a relatively long portion of the medial surface of the humerus. Neither site is thought to be migratory.
Fig. 4.
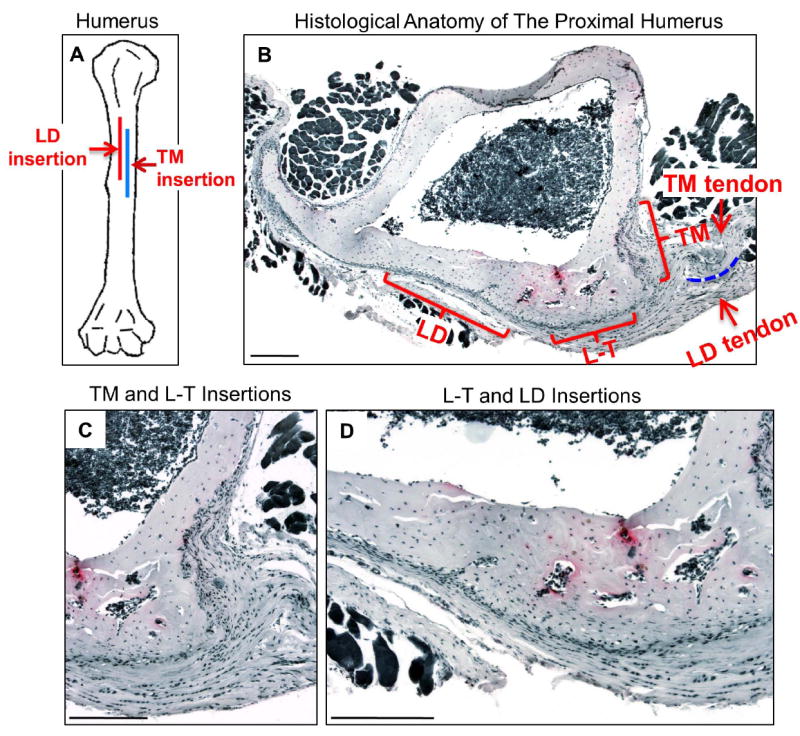
Anatomy of adductor insertions in the proximal humerus. (A) Cartoon depicting the insertion sites of the teres major and latissimus dorsi in the proximal humerus. (B-D) Cross-sections of the proximal humerus at 9 weeks in a CD-1 mouse (TRAP-stained sections chosen as an anatomical visual aid rather than for the few osteoclasts apparent at this stage). The insertion sites of the teres major (TM) and latissimus dorsi (LD) are indicated by brackets, as are their tendons (arrows) and the bursa that separates them proximally (dotted line). More distally, the tendons merge, and the region subjacent to this area is referred to as the T-L insertion site. Panel C is a higher magnification of the TM and T-L sites and D of the LD and T-L sites. Scale bars = 200 μm.
Anatomically, these adductor insertion sites correspond to a ridge at the posterior-medial angle of the humerus in the adult CD-1 mouse (Fig. 3A and C). However, in the cKO mouse these sites comprise a large tuberosity in all three micro-CT views of the humerus (Fig. 3D-F). Note that the cKO humerus appears shorter and fatter on the 3D image (Fig. 3A and D) and that its proximal portion is badly distorted on the sagittal view, presumably by traction (compare Fig. 3 B and E). There is no evidence of haploinsufficiency in the PTHrP-lacZ mouse in the proximal humerus histologically or via micro-CT (Fig. S5 G and H). Correspondingly, we used both CD-1 and PTHrP-lacZ mice interchangeably as controls in this analysis because of the usefulness of the β-gal marker for PTHrP expression in histological sections.
Fig. 4B-D introduces the histological anatomy of a 9-week-old CD-1 humerus as seen in cross section. In essence, the TM site (Fig. 4B and C) corresponds to a near-90° insertion associated with a deep excavation on the posterior surface and the LD site (panels B and D) to a gradual as well as shallow excavation over much of the medial surface. It is known that the connective tissues associated with muscles of similar function (e.g., adductors) sometimes comingle and/or fuse near their insertion sites (2,3), and this is the case for the TM and LD connective tissues shown in Fig. 4. We refer to this fused region as the “teres-latissimus” (T-L) site, and it corresponds to a relatively gradual insertion covering the medial surface near the posteromedial angle in the humerus. Here there is no excavation whatsoever (panels B and C).
We will initially describe the normal developmental histology in these humeral sites before summarizing the findings in the cKO mice. TRAP-stained sections from PTHrP-lacZ mice at 6 weeks show the excavations taking place at a time of peak linear growth (Fig. 5A-C). Note the correspondence of the major and more modest β-gal and TRAP readouts at the TM (panel B) and LD (panel C) insertions, respectively, as well as the absence of both β-gal and TRAP staining at the T-L site (panel A). Immunohistochemistry for RANKL revealed a correspondence of RANKL-positivity and TRAP as well as β-gal activities in the TM and LD versus the T-L sites (D-F). At 9 weeks, long bone growth is approaching skeletal maturity in the mouse(15) and with it the maturation of the sites in terms of anchoring them to the cortical surface. In the TM (panel G) and LD sites (panel H) this process is driven by deep-zone periosteal osteoblasts, while by 9 weeks the TL site has declared itself as a fibrocartilaginous site (panel I), with a cortical surface comprised entirely of enthesis fibrochondrocytes.
Fig. 5.
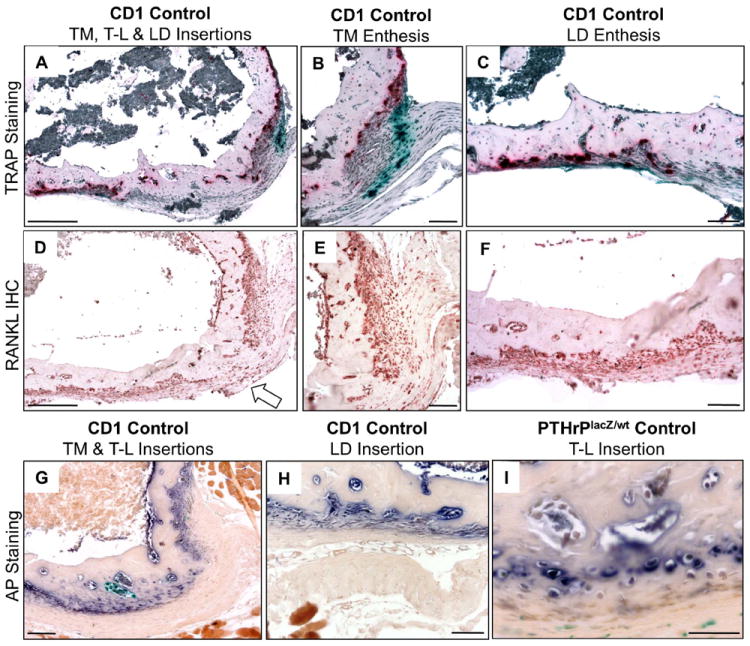
Histology of postnatal modeling of humeral adductor insertion sites. (A-C) TRAP-stained TM, LD, and T-L insertion sites at 6 weeks from a PTHrP-lacZ mouse. (B and C) higher-magnification images of the TM (B) and LD (C) insertions, respectively. Note that the T-L site contains neither β-gal activity nor osteoclasts. (D-F) RANKL immunohistochemistry in 6-week-old CD-1 sections at low magnification (D) and at higher magnification at TM (E) and LD (F) sites; note that there is no staining on the cortical surface at the T-L site (arrow in D). (G-I) AP staining in 9-week-old CD-1 (G and H) and PTHrP-lacZ (I) mice. Panel G shows the TM and T-L sites, H the LD site, and I a higher magnification of the T-L site. Note that at 9 weeks the T-L site is populated by enthesis fibrochondrocytes. Scale bars in A-H are 100 μm and in I 50 μm.
In brief summary, the normal adductor insertions in the proximal humerus in the mouse are three in number. Two of these are PTHrP-expressing sites at which osteoclasts excavate a cortical root system via apparent RANKL mediation, and in these sites there seems to be a clear correlation between the β-gal, RANKL, and osteoclast levels and activities. The third site lacks these three features, is not excavated, and proves to be a sessile fibrocartilagenous site at about 9 weeks and beyond.
In the cKO sections, TRAP-staining revealed no TRAP-positive cells or excavations on the cortical surface of the cKO specimen and what appeared to be enlarging and distorted TM and T-L sites at 6 weeks (Fig. 6A). These features were even more evident in AP-stained sections (Fig. 6B and C), in which it was clear that both the TM and T-L insertions had become distorted fibrocartilagenous tuberosities. The T-L site had formed both sesamoid and enthesis populations of fibrochondrocytes, and the destroyed and now fibrocartilagenous tuberosity at the TM site was easily identified as such by the residual β-gal surface staining pattern that defined its TM heritage (Fig. 6B and C). There was no RANKL staining at either the TM or LD sites (D and E). Given the degree of destruction of the insertion site seen at 6 weeks, we looked more carefully at 3 weeks. Here, we found robust TRAP-positive cells in CD-1 (panel G) sections at the TM site as compared to a totally quiescent TM site in the cKO sections (panel H). AP-staining the cKO TM site displayed a burgeoning population of enthesis fibrochondrocytes even at this age (panel I).
Fig. 6.
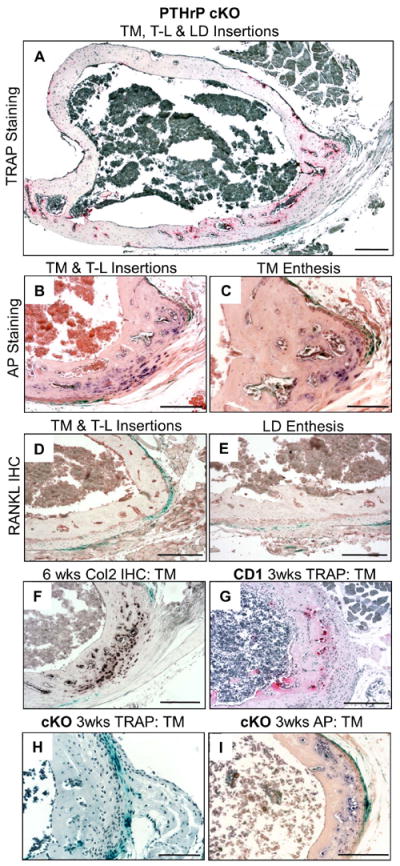
Failed modeling and fibrocartilagenous tuberosity formation of adductor insertion site in PTHrP cKO mice. (A-F) sections at 6 weeks. (A) TRAP-stained cKO cross-section showing no osteoclasts on the cortical surface. (B and C) AP-stained cKO sections of the TM and T-L sites, which are populated entirely by fibrochondrocytes (note that in the T-L site both enthesis and sesamoid populations of fibrochondrocytes have formed). (D and E) RANKL immunohistochemistry in TM (D) and LD (E) sites; note that neither site has been excavated and that RANKL staining is at background as compared to Fig. 5E-F. (F) Collagen-2 immunohistochemistry in cKO section at 6 weeks (no fibrochondrocytes or col-2 staining were found in the control mice at this age (see Fig. S6I)). (G-I) Sections from 3-week-old CD-1 (G) and cKO (H and I) mice stained for TRAP (G and H) or AP (I). Note that no cortical depression or osteoclasts whatsoever were found in the cKO section (H) and that enthesis fibrochondrocytes have already begun to form in the cKO specimen (I). Scale bars in C and H are 100 μm and in remaining panels 200 μm.
The dominant abnormality at the adductor insertion sites in the cKO mouse corresponds to a fibrocartilagenous tuberosity with apparent contributions from both the TM and T-L sites. We became curious as to the loading that might be primarily driving this abnormality and carried out a TM unloading experiment in the cKO mouse that indicated that the latissimus dorsi was largely responsible for loading the T-L site (see supplementary text and Fig. S6 A-F). One implication of this finding is that a single tendon may insert via two quite different types of entheses (one fibrous and the other fibrocartilagenous), the principal regulatory difference between them being the induction of PTHrP associated with the fibrous site.
Discussion
Summary and Model (Fig. 7)
Fig. 7.
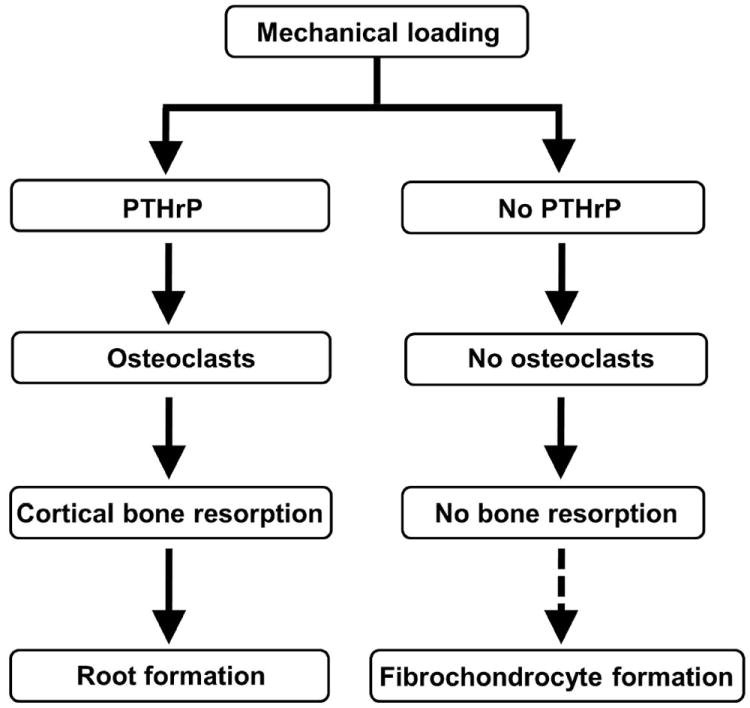
Flow diagram of the proposed responses to mechanical loading on the cortical surfaces of long bones when PTHrP is induced by the loading and when it is not. The steps regulating fibrochondrocyte formation in response to loading are unknown.
We conclude that PTHrP is deployed in fibrous insertion sites as a load-induced modeling tool that directs osteoclasts to excavate the root system by which these sites attach to the cortical surface (Fig. 7). We describe three formes frustes of this pathway, one migratory (MCL), one associated with an acute insertion and a deep root system (TM), and a third associated with a long and gradual insertion and a shallow saw-tooth-like root system (LD). We also describe a contiguous insertion in the humerus that is clearly heavily loaded but does not express PTHrP, induce RANKL, or drive osteoclastic surface resorption; this site becomes fibrocartilagenous as it approaches adult form. This is a convenient comparison site, as it indicates that the biological decision to sculpt an insertion site is made at the level of PTHrP induction. When PTHrP is deleted by Scx-Cre, fibrocartilagenous tuberosities and deformities develop at these sites. Thus, in the absence of PTHrP, it appears that induction of fibrochondrocytes may be more or less a default pathway in response to both normal and abnormal loading (Fig. 7). Fibrochondrocytes are well known to be load-induced, not only in classical locations such as the Achilles and quadriceps insertions but also in response to abnormal loading such as the MCL insertion on the cKO mice summarized above.(2-4) Fibrochondrocytes can be derived from a variety of cell types and locations, including the tenocytes in tendon (so-called sesamoid fibrochondrocytes) and the mesenchymal cells in entheses (so-called enthesis fibrochondrocytes). The fibrocartilage in question may or may not mineralize.
PTHrP was initially identified as a tumor-derived factor that induces hypercalcemia in patients with a malignancy.(10) It is a member of a small gene family that includes parathyroid hormone (PTH) itself, a heritage that is reflected in highly homologous PTH and PTHrP N-terminal sequences that share a common receptor, referred to as the type 1 PTH-PTHrP receptor or PTHR1.(10) The biological specificity of the two proteins is the result of their different domains of influence: PTH is a classical systemic peptide hormone, whereas as PTHrP acts primarily as an autocrine/paracrine regulatory factor. PTHrP has been shown to be mechanically induced in a number of tissues,(10,12-14) and its known functions include regulation of the chondrocyte differentiation program during long bone growth, regulating smooth muscle tone, supplying bone mineral to the mammary gland during lactation, and creating the osteoclastic tunnel by which teeth erupt.(10-14, 19)
As is the case for many local regulatory molecules, PTHrP is a low-abundance product, and it was identified on the surface of long bones only with the sensitivity provided by a PTHrP-lacZ marker system.(13,14) Here, PTHrP was found to be expressed in the fibrous layer of the periosteum and the periosteal component of fibrous insertion sites, with peak levels occurring during linear growth.(13,14) In several such sites, PTHrP was shown to be mechanically-induced.(14) PTHrP was not found to be expressed in any bone or stromal cell population within bone (i.e., in trabecular or endochondral bone). Given these findings and its known regulatory factors summarized above, PTHrP was clearly a good candidate for the functions described in this manuscript.
The MCL insertion into the proximal tibial metaphysis is a fibrous site which migrates during linear growth. This process has been well studied at a descriptive level.(2,3,17,20,21) The ligament itself grows at an appositional rate (i.e., at the same rate as the organism as a whole), but the rate of linear bone growth driven by the growth plate is some 7 times greater than the appositional rate, necessitating the migration.(22) This process is entirely a postnatal event, with the osteoclasts that drive it beginning to be induced in the rat at day 8, forming the cortical depression by day 20, and between days 20-60 displaying the characteristic pattern of proximal osteoclastic bone resorption and distal periosteal bone formation that models and anchors the MCL insertion.(17) The persistence of the migratory canal visualized my micro-CT as well as the intensity of the cortical resorption seen histologically suggest that the migration of the MCL is driven by an osteoclastic resorption process that is completely uncoupled from osteoblastic bone formation. Between days 60 and 120 the insertion site itself matures to ultimately become lamellar cortical bone, and all cellular activity by this time is quiescent.(17) For biomechanical reasons the final resting site of the proximal tibial crest/insertion reflects a net proximal-distal migration;(17-19,21) if the tibia insertion were to remain in its initial embryonic site, it would be far too short to provide meaningful biomechanical support. Becoming stuck in situ appears to be more-or-less what happens in the PTHrP cKO mouse, in which case it takes the form of what we refer to as a traction-driven tuberosity together with distortion of the entire medial tibial metaphysis. The thickening of the MCL in this setting also presumably reflects loading.(2,3,23)
The proximal humerus is a very instructive location in several ways. First, the TM and LD sites here extend the spectrum as to how the PTHrP gene is deployed to create root systems of different character. The differences here would appear to be driven by different biomechanical inputs, as suggested by the relative numbers and/or sizes of the PTHrP- and RANKL-expressing cells, the numbers of osteoclasts, and the sizes of the cortical excavations. Second, the so-called T-L site does not deploy this program and thus provides a convenient comparison site in this regard. We do not fully understand the biomechanical events at the adductor sites in either the normal or control or cKO mice. It does appear in the cKO mouse there is a substantial transfer of LD loading from its destroyed insertion site to the so-called T-L site, which drives the major portion of the so-called traction tuberosity that forms there. It is of interest that the normal T-L site does not declare itself by fibrochondrocyte formation until some 4-5 weeks after osteoclasts form in the TM and LD sites, so that forming the fibrocartilagenous T-L enthesis occurs later in postnatal development than sculpting the fibrous insertion sites. This relatively delayed postnatal developmental pattern is also seen at the Achilles insertion.(24) Clearly, the key biological difference that distinguishes the T-L from the TM and LD sites is the deployment of PTHrP and the associated downstream modeling program at the TM and LD sites.
The cKO long bones are foreshortened, which is likely the result of spillover of the high-level Scx-Cre expression in the surface connective tissues into the peripheral layer of PTHrP-expressing growth chondrocytes (see Fig. S1A and supporting text). The 10% or so reduction in long bone length would appear to have little relevance to the findings described in this manuscript, and the borderline shortening in the PTHrP-lacZ limbs is of unclear biological significance (see supporting data). The haploinsufficiency at the MCL site in the PTHrP-lacZ mice, however, is highly reproducible (Figs. S2 and S3) and presumably reflects a requirement for the full complement of PTHrP expression and function at this site in order to drive migration. We do not see micro-CT or histological evidence of a haploinsufficient phenotype elsewhere in the cKO mouse, and apart from the functional aspect at the MCL just noted the relevance of this observation is unclear. There is a single example in the radiological literature of what is described as a “traction exostosis” at the MCL site in a patient with Albright’s hereditary osteodystrophy.(25) There has also been a family with brachydactyly type E described with PTHrP haploinsufficiency, but there was no mention of bone surface abnormalities in these individuals.(26)
As noted in the Introduction, the fibrous sites described here differ in structure, location, timing, and the regulatory mechanisms involved from the findings reported by Blitz et al in bone ridges.(8) That manuscript described the role of Scx-regulated BMP4 expression in the induction of bone ridge formation during embryogenesis, followed by their later growth in response to muscle loading. These ridges do not include any fibrous entheses, and the various phenotypic findings in the PTHrP cKO mouse do not involve the bony ridges.(8) In addition, PTHrP does not appear to exert its regulatory effects until the postnatal period.
It also seems clear that PTHrP is not involved in the regulatory pathway that leads to fibrochondrocytes; the regulation of these cells at the cortical surface is yet another issue that is poorly understood. Sesamoid and enthesis as well as unmineralized and mineralized populations of fibrochondrocytes form at the PTHrP cKO-associated traction tuberosities, but these are clearly pathological processes. As noted in the text, the biological decision to sculpt a fibrous enthesis seems to be made at the level of PTHrP gene induction in the mesenchymal cells at these sites, and the neighboring LD and T-L sites in the humerus might provide a powerful “plus - minus“ system for pursuing the upstream regulation of PTHrP in this regard.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants R01-DK62515 (to AEB) and P30-AR-46032 to the Yale Center for Musculoskeletal Disorders.
Authors’ roles: Study design: AB, JH, RJ, and MW. Study conduct: JH, AN and MW. Data collection and analysis: AB, JH, AN, and MW. Data interpretation: AB, JH, and MW. Drafting and revising manuscript content: AB and MW. Approving final version of manuscript: AB, JH, RJ, AN, and MW. AB takes responsibility for the integrity of the data analyses.
We thank C. Macica for assistance with immunohistochemistry, G. Liang and N. Troiano for technical assistance, and A. DeCosta for preparing the manuscript.
Supported by NIH grants (to RLJ and AEB).
Footnotes
Supplemental data are included.
Disclosure
All authors state that they have no conflicts of interest.
References
- 1.Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35(5):1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 2.Shaw HM, Benjamin M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand J Med Sports. 2007;17(4):303–315. doi: 10.1111/j.1600-0838.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (“Entheses”) in relation to exercise and/or mechanical load. J Anat. 2006;208(4):471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doschak MR, Zernicke RF. Structure, function and adaptation of bone-tendon and bone-ligament complexes. J Musculoskeletal Neuronal Interact. 2005;5(1):35–40. [PubMed] [Google Scholar]
- 5.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121(4):1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 6.Brent AE, Tabin CJ. Somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- 7.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007;134(14):2697–2708. doi: 10.1242/dev.001933. [DOI] [PubMed] [Google Scholar]
- 8.Blitz E, Viukov S, Sharir A, Shwartz Y, Galloway JL, Pryce BA, Johnson RL, Tabin CJ, Schweitzer R, Zelzer E. Bone ridge patterning during musculoskeletal assembly is mediated through Scx regulation of BMP4 at the tendon-skeleton junction. Developmental Cell. 2009;17(6):861–873. doi: 10.1016/j.devcel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228–237. doi: 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wysolmerski JJ. Chapter 26. Parathyroid Hormone–Related Protein. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7 Washington, DC: American Society for Bone and Mineral Research; 2008. pp. 127–133. [Google Scholar]
- 11.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 12.Philbrick WM, Dreyer BE, Nakchbandi IA, Karaplis AC. Parathyroid hormone-related protein is required for tooth eruption. Proc Natl Acad Sci USA. 1998;95(20):11846–11851. doi: 10.1073/pnas.95.20.11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Macica CM, Dreyer BE, Hammond VE, Hens JR, Philbrick WM, Broadus AE. Initial characterization of PTH-related protein gene-driven lacZ expression in the mouse. J Bone Miner Res. 2006;21(1):113–23. doi: 10.1359/JBMR.051005. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Macica CM, Nasiri A, Judex S, Broadus AE. Mechanical regulation of PTHrP expression in entheses. Bone. 2007;41(5):752–759. doi: 10.1016/j.bone.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roach HI, Mehta G, Oreffo ROC, Clarke NMP, Cooper C. Temporal analysis of rat growth plates: cessation of growth with age despite presence of physis. J Histochem Cytochem. 2003;51(3):373–383. doi: 10.1177/002215540305100312. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Macica CM, Nasiri A, Broadus AE. Regulation of articular chondrocyte proliferation and differentiation by Indian hedgehog and parathyroid hormone-related protein in mice. Arthritis Rheum. 2008;58:3788–3797. doi: 10.1002/art.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei X, Messner K. The postnatal development of the insertion of the medial collateral ligament in the rat knee. Anat Embryol. 1996;193(1):53–59. doi: 10.1007/BF00186833. [DOI] [PubMed] [Google Scholar]
- 18.Bab I, Hajbi-Yonissi C, Gabet Y, Müller R. Micro-Tomographic Atlas of the Mouse Skeleton. New York: Springer; 2007. p. 173. [Google Scholar]
- 19.Qing H, Ardeshirpour L, Pajevic PD, Dusevich V, Jähn K, Kato S, Wysolmerski J, Bonewald LF. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J Bone Miner Res. 2012;27(5):1018–1029. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matyas JR, Bodie D, Anderson M, Frank CB. The developmental morphology of a “periosteal” ligament insertion: growth and maturation of the tibial insertion of the rabbit medial collateral ligament. J Orthop Res. 1990;8(3):412–24. doi: 10.1002/jor.1100080313. [DOI] [PubMed] [Google Scholar]
- 21.Dörfl J. Migration of tendinous insertions. I. Cause and mechanism. J Anat. 1980;131(Pt 1):179–195. [PMC free article] [PubMed] [Google Scholar]
- 22.Woo SL-Y, Buckwalter JA. Am Orthop Surg. Park Ridge, IL: 1998. Injury and Repair of the Musculoskeletal Soft Tissues; pp. 45–101. [DOI] [PubMed] [Google Scholar]
- 23.Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, Ilic D, Baskaran H, Sakai T. Conversion of mechanical force into TGF-β-mediated biochemical signals. Current Biology. 2011;21(11):933–41. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang G, Katz LD, Insogna KL, Carpenter TO, Macica CM. Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in hyp mice. Calcif Tissue Int. 2009;85(3):235–246. doi: 10.1007/s00223-009-9270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson LC, Hall CM. Albright’s hereditary osteodystrophy and pseudohypoparathroidism. Semin Musculoskelet Radiol. 2002;6(4):273–83. doi: 10.1055/s-2002-36726. [DOI] [PubMed] [Google Scholar]
- 26.Klopocki E, Hennig BP, Dathe K, Koll R, de Ravel T, Baten E, Blom E, Gillerot Y, Weigel JF, Krüger G, Hiort O, Seemann P, Mundlos S. Deletion and point mutations of PTHLH cause brachydactyly type E. Am J Human Genetics. 2010;86(3):434–439. doi: 10.1016/j.ajhg.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


