Abstract
A systematic series of previously inaccessible key C20' urea and thiourea derivatives of vinblastine were prepared from 20'-aminovinblastine that was made accessible through a unique Fe(III)/NaBH4-mediated alkene functionalization reaction of anhydrovinblastine. Their examination defined key structural features of the urea-based analogues that contribute to their properties and provided derivatives that match or exceed the potency of vinblastine by as much as 10-fold in cell-based functional assays, which is directly related to their relative tubulin binding affinity. In contrast to expectations based on apparent steric constraints of the tubulin binding site surrounding the vinblastine C20' center depicted in an x-ray co-crystal structure, remarkably large C20' urea derivatives are accommodated.
INTRODUCTION
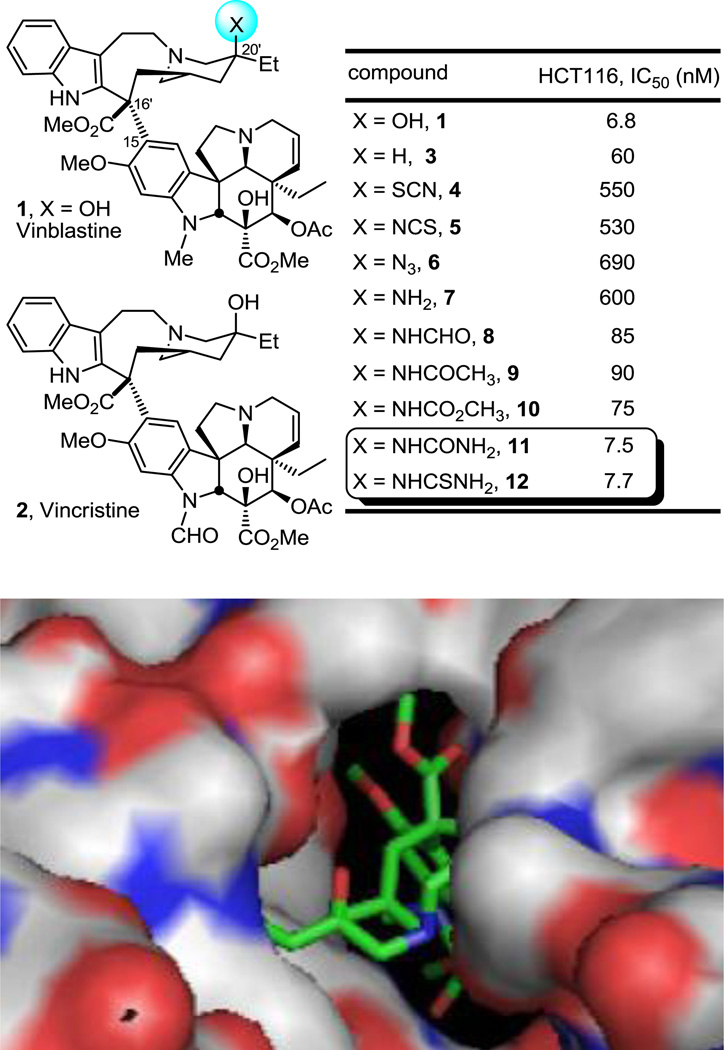
Vinblastine (1) and vincristine (2) are the most widely recognized members of the Vinca alkaloids as a result of their clinical use as antitumor drugs, and their discovery represent one of the earliest important contributions that plant-derived natural products have made to cancer chemotherapy (Figure 1).1 Originally isolated from the leaves of Catharanthus roseus (L) G. Don in trace quantities,2,3 vinblastine and vincristine were among the first small molecules shown to bind tubulin and to inhibit microtubule formation and mitosis.1,4 Due to their clinical importance, low natural abundance, and structural complexity, they have been the subject of extensive and continuing biological and synthetic investigations.4,5,6
Figure 1.
Top: Natural products and initial C20' vinblastine analogues. Bottom: x-ray structure of vinblastine bound to tubulin highlighting the region surrounding vinblastine C20' site (PDB ID: 1Z2B)14.
We previously reported the total synthesis of vinblastine6 and its extension to the total synthesis of related natural products including vincristine and key analogues that utilizes a one-pot, two-step, biomimetic Fe(III)-promoted single electron oxidative coupling of catharanthine and vindoline and a subsequent Fe(III)/NaBH4-mediated in situ alkene oxidation to generate vinblastine directly.7,8,9 Recently, we detailed the results of investigation of the Fe(III)/NaBH4-mediated free radical oxidation of the anhydrovinblastine trisubstituted alkene used to introduce the vinblastine C20' tertiary alcohol,6b extending the reaction to provide a simple method for direct functionalization of unactivated alkenes.10,11 In these studies, the broad alkene substrate scope was defined, the exclusive Markovnikov addition regioselectivity was established, the excellent functional group tolerance was revealed, alternative free radical traps were introduced, the Fe(III) salt and initiating hydride source were examined, and remarkably mild reaction conditions (0–25 °C, 5–30 min) were introduced that are relatively insensitive to the reaction parameters. The interest in this Fe(III)/NaBH4-mediated reaction emerged not only from its use in accessing vinblastine, but the opportunity it presented for the late-stage, divergent12 preparation of otherwise inaccessible vinblastine analogues incorporating alternative C20' functionality. Although this site is known to be critical to the properties of vinblastine13 and is found deeply embedded in the tubulin bound complex (Figure 1),14 the prior exploration of C20' substituent effects has been limited to semi-synthetic O-acylation of the C20' alcohol or its elimination and subsequent alkene reduction or superacid-catalyzed additions.15 These invariably led to substantial reductions in biological potency of the resulting derivative, albeit with examination of only a limited number of key analogues. Consequently, in the course of the development of the Fe(III)/NaBH4-mediated alkene functionalization reaction, its use was extended to the preparation of a series of key vinblastine analogues bearing alternative C20'-functionality. Those of initial interest included the C20' azide 6 and amine 7, both of which proved to be approximately 100-fold less potent than vinblastine (1) and 10-fold less potent than 20'-deoxyvinblastine (3). However, acylation of the C20' amine improved activity 10-fold and installation of the unsubstituted C20' urea 11 or thiourea 12 provided compounds that nearly matched the potency of vinblastine itself (Figure 1). Herein and based on these observations, we report a systematic exploration of C20' amine, urea, and thiourea derivatives of vinblastine that have not only provided C20' urea-based analogues that substantially exceed the potency of vinblastine, but that also exhibit good activity against a Pgp-overexpressing, vinblastine-resistant tumor cell line. Just as remarkably and in contrast to expectations based on the steric constraints of the tubulin binding site surrounding the vinblastine C20' center as depicted in the x-ray co-crystal structure of a tubulin bound complex,14 large C20' urea derivatives are accommodated, exhibiting potent functional activity in cell-based proliferation assays and effectively binding tubulin.
RESULTS AND DISCUSSION
Chemistry
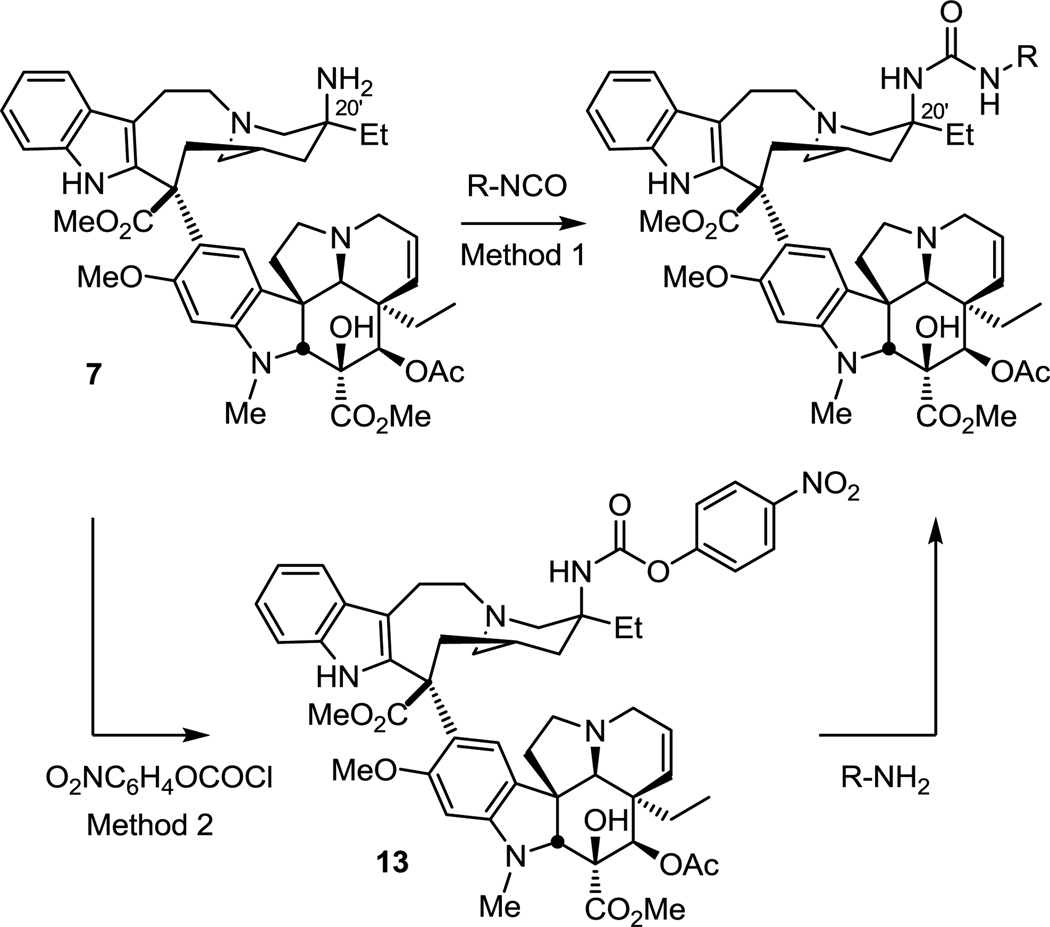
The targeted vinblastine C20' urea analogues were prepared by two methods (Scheme 1). The first method (Method 1) involved treating the recently accessible 20'-aminovinblastine (7)10 with available isocyanates to provide the corresponding ureas. In the instances when the isocyanates were not readily available, Method 2 was used. This entailed treating 20'-aminovinblastine (7) with p-nitrophenylchloroformate to provide the activated carbamate 13, which was then treated with a series of amines to provide the additional C20' urea analogues.
Scheme 1.
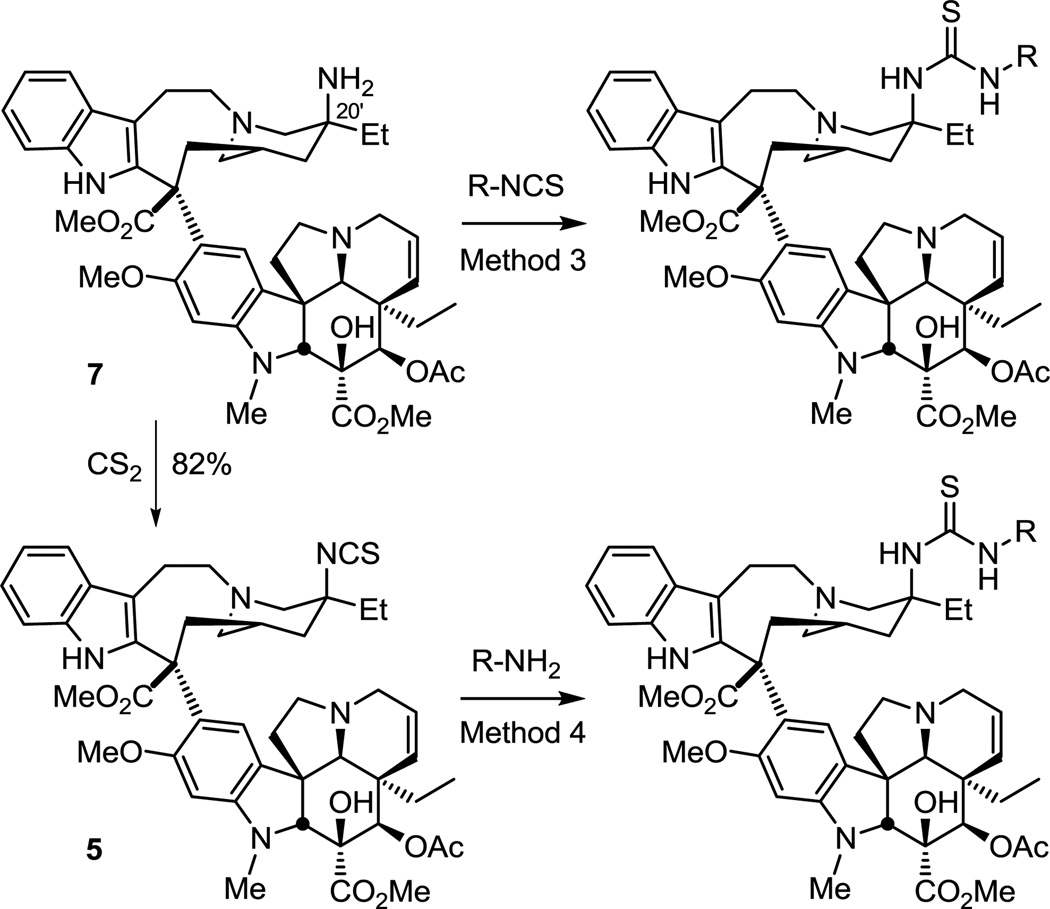
The vinblastine C20' thiourea analogues were prepared also using two complementary methods (Scheme 2). Thus, treatment of 20'-aminovinblastine (7) with a small series of commercially available isothiocyanates (Method 3) versus isocyanates provided the corresponding C20' thiourea analogues. Alternatively, treatment of 20'-isothiocyanovinblastine (5),10 made from the reaction of 20'-aminovinblastine (7) and carbon disulfide (82%) or that is available upon direct Fe(III)/NaBH4-mediated functionalization of anhydrovinblastine (KSCN),10 with available amines (Method 4) also provided a set of C20' thiourea vinblastine analogues.
Scheme 2.
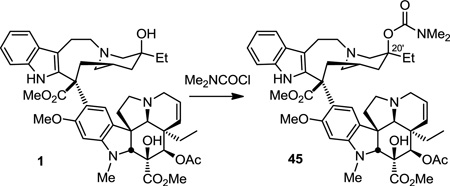
A series of N,N-disubstituted C20' urea and thiourea vinblastine analogues were also prepared using Method 2 and 4 and commercially available N,N-disubstituted amines. Additionally, the carbamate analogue 45 was synthesized for direct comparison with what proved to be the potent C20' urea and thiourea derivatives. Thus, vinblastine was treated with the N,N-dimethylcarbamyl chloride to provide the C20' carbamate 45 (eq 1).
 |
(1) |
Biological Activity
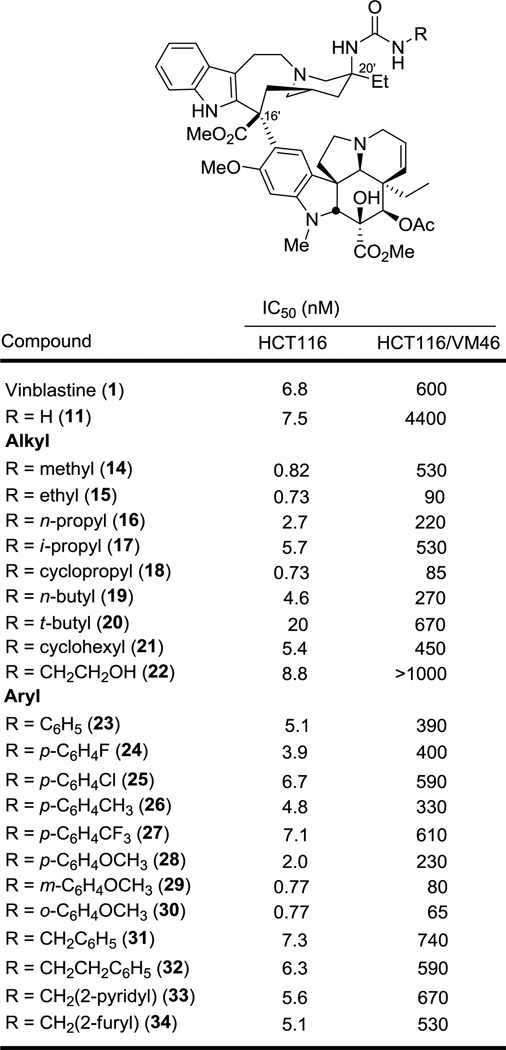
The C20' substituted vinblastine analogues were examined for cell growth inhibitory activity against the HCT116 (human colon cancer), and HCT116/VM46 (resistant human colon cancer) tumor cell lines, the latter of which exhibits resistance (100-fold) to vinblastine through overexpression of Pgp.16,17 As we reported, the unsubstituted urea 11 on which the studies are based approached but did not match the potency of vinblastine. The results of the examination of the systematically varied monosubstituted C20' urea derivatives are summarized in Figure 2 alongside those of 1 and 11. Terminal N-alkyl substituents were not only well tolerated, but provided significant enhancements in activity, improving on the potency of 11 and providing derivatives that substantially surpass that of vinblastine itself. Most notable of these are the urea derivatives 14–18, bearing small N-alkyl substituents, some of which exhibited IC50s of 700–800 pM against HCT116, improving activity against HCT116 10-fold relative to 11 and substantially surpassing the potency of vinblastine itself (ca. 10-fold). Even the larger N-alkyl derivatives 19 and 21 matched or slightly surpassed the activity of vinblastine and only 20, bearing the large t-butyl substituent, experienced a small, but surprisingly modest loss in activity given expectations. Introduction of a polar group that can serve as either a H-bond donor or acceptor on the alkyl substituent in 22 maintained the activity observed with other small alkyl substituents in the HCT116 cell line; however, it had a deleterious effect in the potency against the resistant cell line (IC50 >1000 nM, HCT116/VM46), similar to that seen in the parent urea 11. The cell growth inhibition activity against the L1210 (mouse leukemia) tumor cell line was also measured and the results were qualitatively and quantitatively (IC50) nearly identical to those observe with the HCT116 cell line providing IC50 values for the urea derivatives that matched or exceeded the activity of vinblastine itself (see Supporting Information for L1210 cytotoxic activity data).
Figure 2.
Activity of vinblastine C20' urea analogues
The examination of the monosubstituted C20' urea derivatives bearing N-aryl substituents (23–30) proved even more unexpected. All exhibited cell growth inhibitory activity at levels exceeding the parent urea 11, matching or surpassing the potency of vinblastine itself. Electron-withdrawing or electron-donating substituents on the parent N-phenyl urea 23 are well tolerated and although no strongly polar substituents were examined, it is notable that the p-methoxy substituent proved to be among the best of the p-substituents. As a result, the impact of a m- and o-methoxy phenyl substituent was also examined. Significantly, 29 and 30 bearing N-(m-methoxyphenyl) and N-(o-methoxyphenyl) urea substituents respectively, exhibited exceptional activity, substantially exceeding the potency of vinblastine nearly 10-fold (IC50 770 pM vs 6.8 nM, HCT116) and displaying uniquely potent activity against the vinblastine-resistant HCT116 cell line (IC50 = 80 and 65 nM, HCT116/VM46). This activity along with that of 15 and 18 represents a 10-fold improvement over vinblastine and may represent activity at a level of clinical significance.
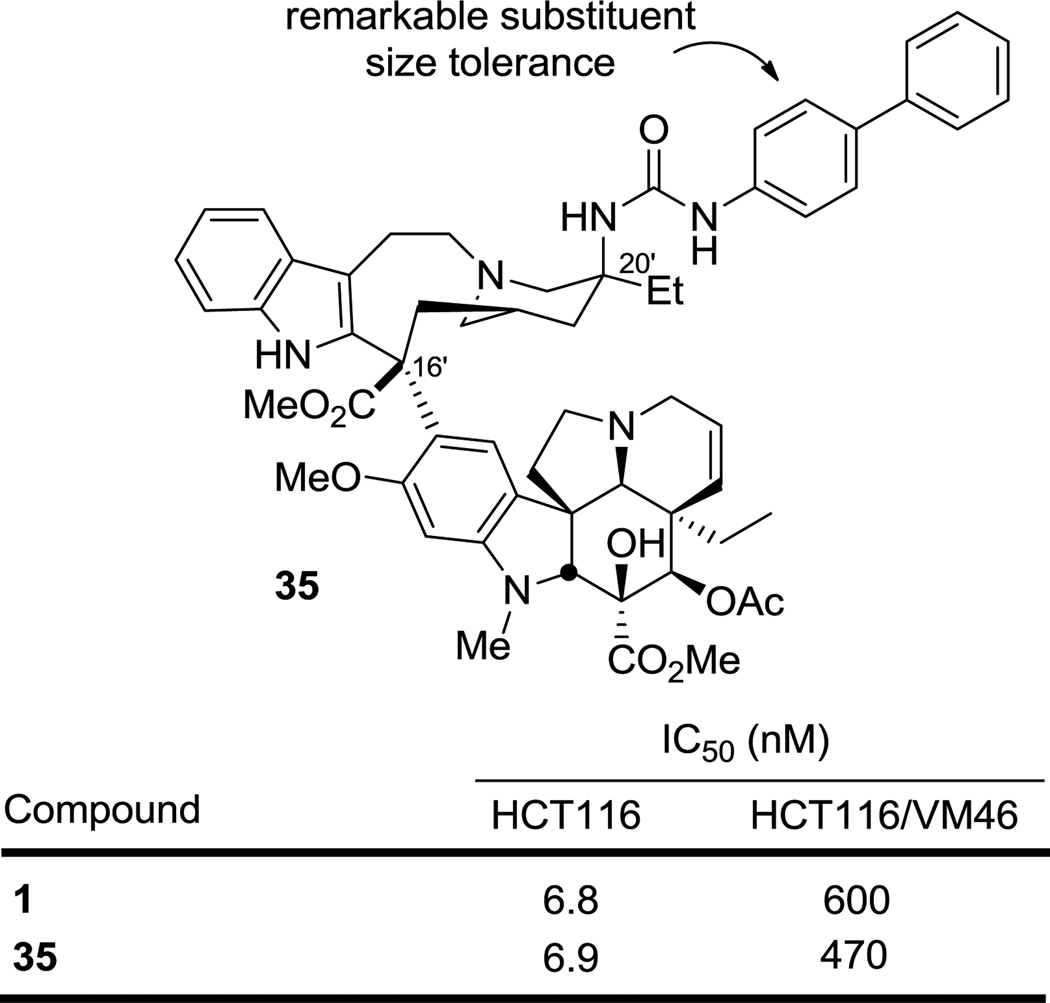
Placement of a one or two methylene spacer between the phenyl ring and urea nitrogen (31 and 32) maintained activity with both derivatives matching the activity of both 23 and vinblastine itself. Replacement of the phenyl ring in 31 with a heteroaromatic ring (furan or pyridine) provided 33 and 34, which displayed comparable activity to the parent 31, matching or slightly exceeding the potency of vinblastine. All these observations are unexpected given the apparent steric constraints of the tubulin binding site surrounding the vinblastine C20' center observed in the x-ray crystal structure of a tubulin bound complex.14 To further probe just how much space may be available to a C20' derivative, the rigid N-biphenyl urea 35 was prepared and examined. Remarkably, it displayed cell growth inhibitory activity at a level indistinguishable from vinblastine (Figure 3). This result indicates that sterically demanding modifications to such C20' urea derivatives are possible even beyond what we have probed herein. As a result and in addition to improvements in potency, this may be a superb site for modulating the physical and chemical properties of the drug that impact additional features including Pgp efflux,18 in vivo drug distribution, selective cellular uptake, and metabolism.
Figure 3.
Unprecedented C20' steric tolerance
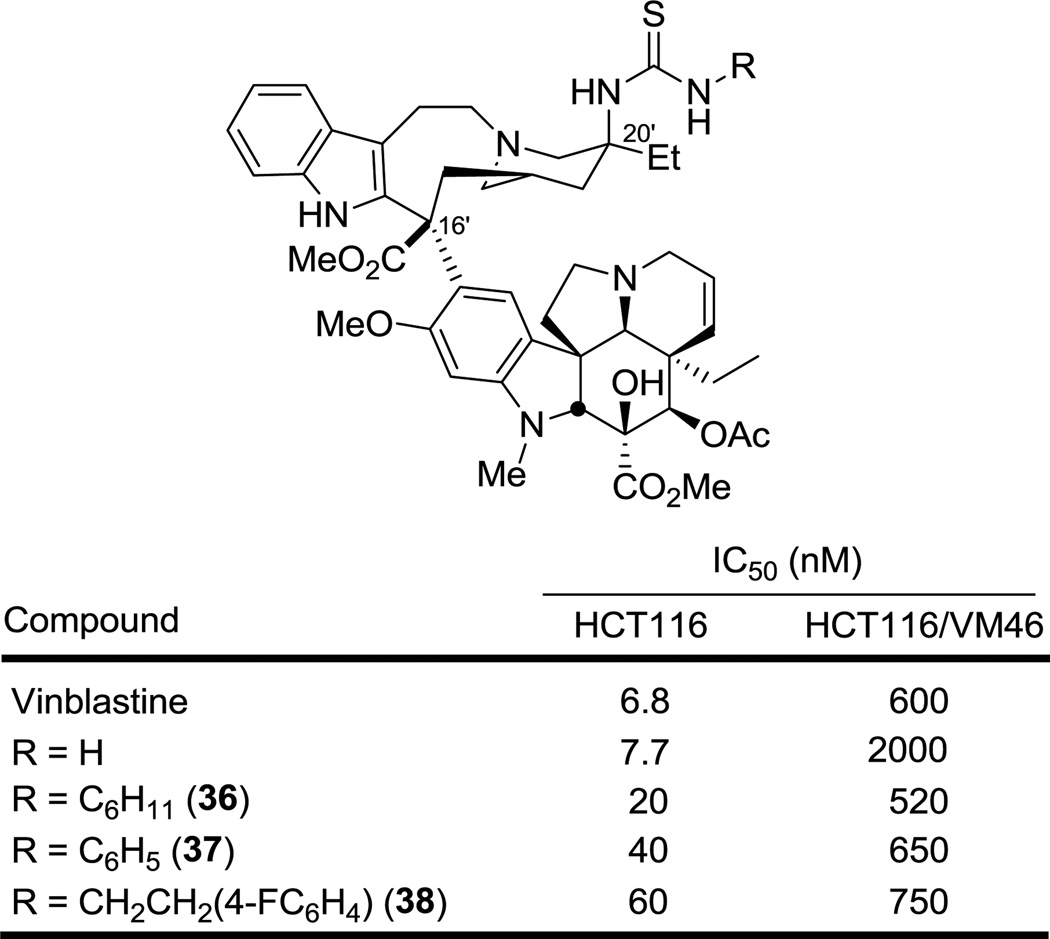
A series of thiourea derivatives were also examined (Figure 4). Although the unsubstituted thiourea derivative 12 approached the activity of the corresponding urea 11 in our original studies (IC50 = 7.7 vs 7.5 nM, HCT116), the monosubstituted N-alkyl or N-aryl derivatives 36 and 37 proved to be 3- to 4-fold less active than the corresponding ureas 21 and 23 (HCT116). However, it is notable that the activity difference between sensitive and vinblastine-resistant cell lines diminishes in this thiourea series (15- to 25-fold vs 100-fold), suggesting they may be transported by Pgp somewhat less effectively.18
Figure 4.
Activity of vinblastine C20' thiourea analogues
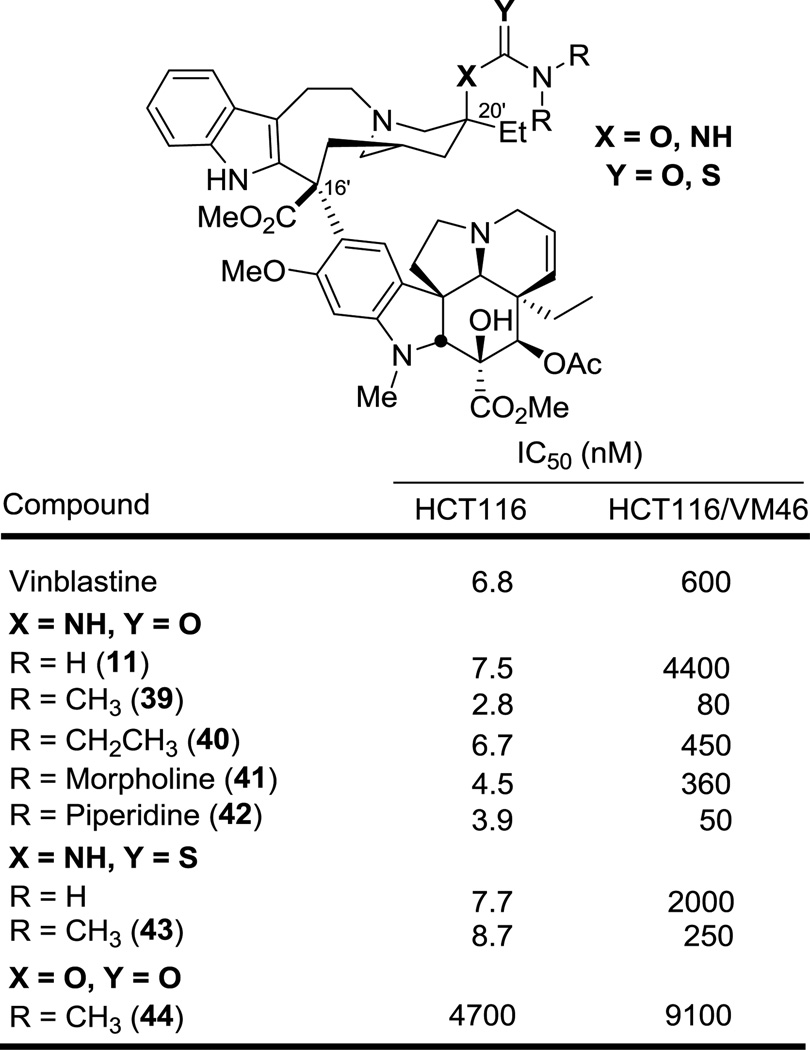
A small, but important series of N,N-disubstituted ureas and thioureas were also examined in order to establish whether the terminal urea nitrogen could be fully substituted or whether the derivatives require or benefit from the presence of an N–H H-bond donor (Figure 5). Remarkably, all the N,N-disubstituted ureas exhibited potent cell growth inhibitory activity matching or surpassing the activity of vinblastine and indicating that a terminal H-bond donor site is not important to their functional activity. However, both 39 and 40 were less active than the corresponding monosubstituted urea derivatives 14 and 15. Ureas with cyclic substituents on the terminal nitrogen, 41 and 42, exhibited a similar potency to the acyclic derivatives 39 and 40. An analogous observation was made with the N,N-dimethyl thiourea derivative 43, which approached the potency of vinblastine but exhibited activity slightly lower than the corresponding N,N-dimethyl urea 38. Interestingly, and like the thiourea derivatives 36 and 37, 43 and especially the urea derivatives 39 and 42 exhibited a diminished activity difference between sensitive and vinblastine-resistant HCT116 cell lines (10-fold vs 100-fold). Finally, comparison of the N,N-dimethyl urea or thiourea 39 and 43 with the N,N-dimethylcarbamate 44 (>1000-fold less active) clearly illustrates the distinction and importance of the C20' amine versus C20' alcohol functionalization, suggesting the H-donor capabilities of the former may be important.
Figure 5.
Activity of vinblastine C20' disubstituted urea and thiourea analogues
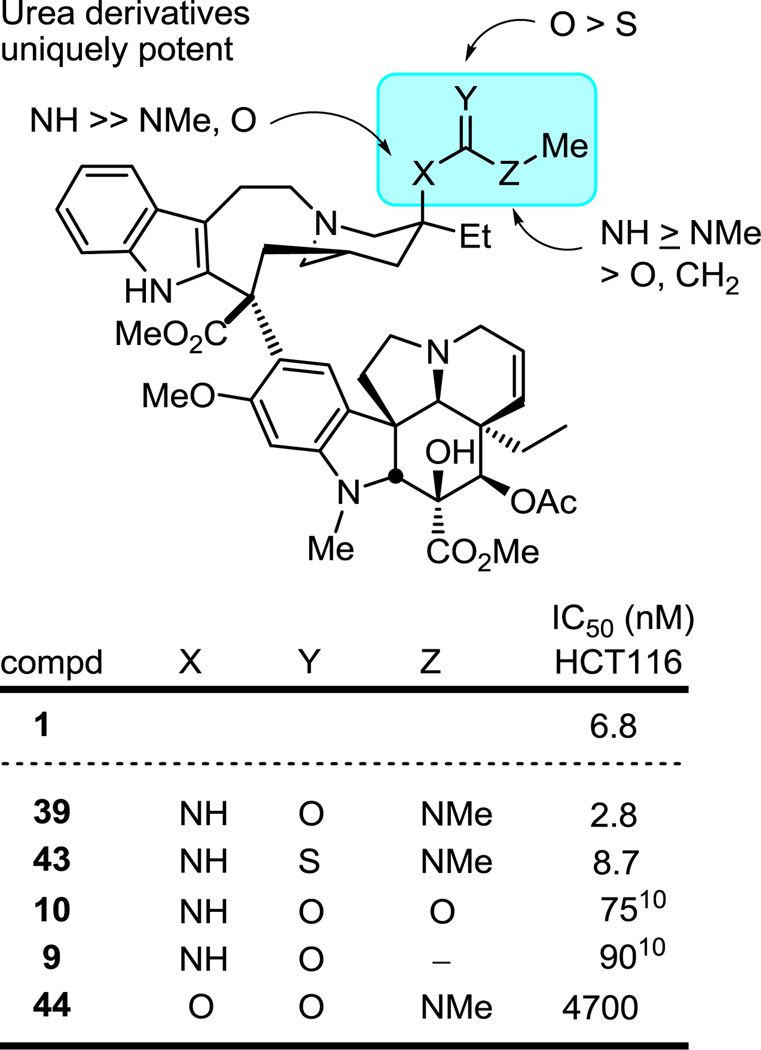
Although less pronounced, but as detailed in our initial work,10 the amide 9 and methyl carbamate 10 were found to be more than 10-fold less active than the urea 11, further highlighting a unique role the urea terminal nitrogen plays in potentiating the activity (Figure 6). As a result and given the size of substituents tolerated, even the intermediate p-nitrophenylcarbamate 13 used to prepare the ureas herein was tested and proved to be a surprisingly effective agent (IC50 = 55 nM, HCT116), matching the activity of the methyl carbamate 10.
Figure 6.
Importance of the C20' amine versus alcohol functionalization and distinctions between urea/thiourea versus carbamate/amide derivatives
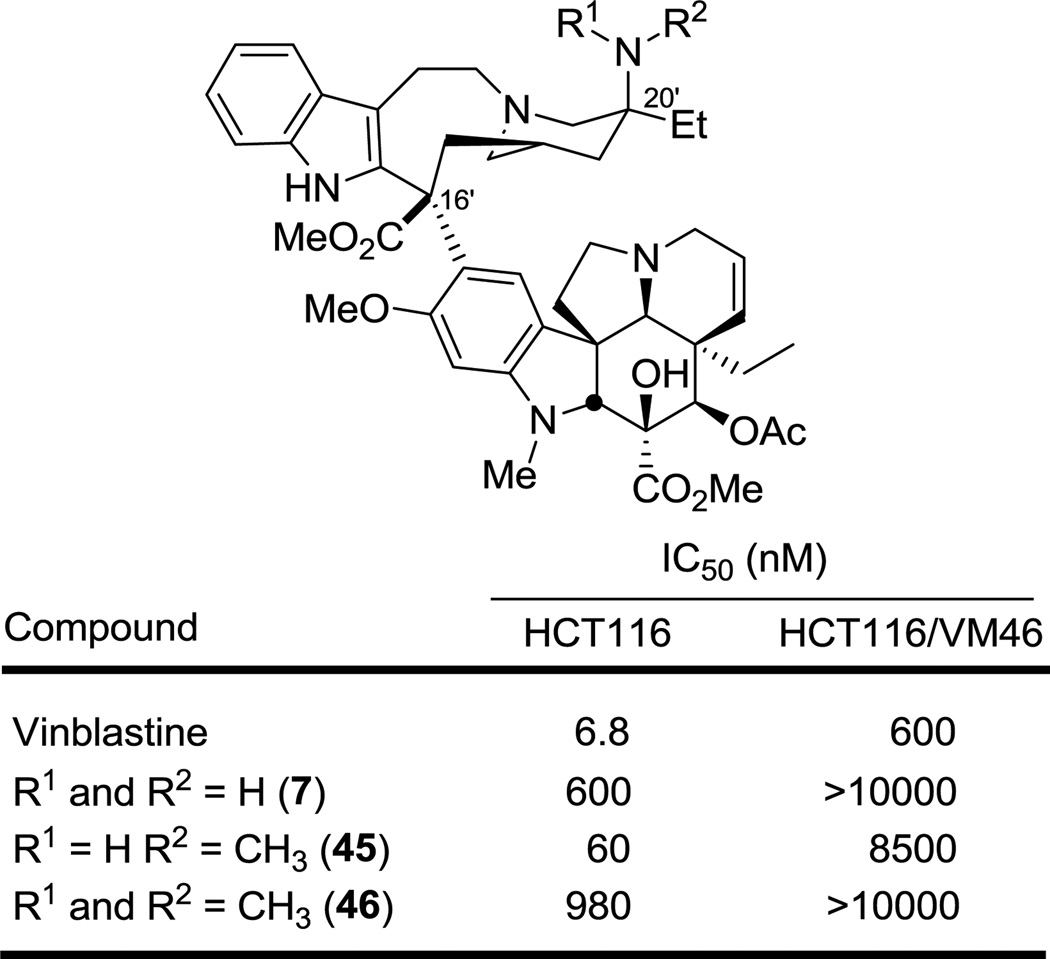
Finally, two additional C20' amines (45 and 46) were prepared by reductive amination (H2CO (5 equiv), NaBH3CN (20 equiv), THF, 4 h, 32% (45) NHMe, 33% (46) NMe2) of 20'-aminovinblastine (7) in order to establish the generality of the observations made with the unsubstituted primary amine 7 itself (Figure 7). Although the C20' methylamine proved more potent than 7, it was still 10-fold less active than vinblastine and the C20' dimethylamine derivative 46 was the least active of the C20' amines, suggesting the importance of a H-bond donor with regard to biological activity.
Figure 7.
Activity of C20' amines
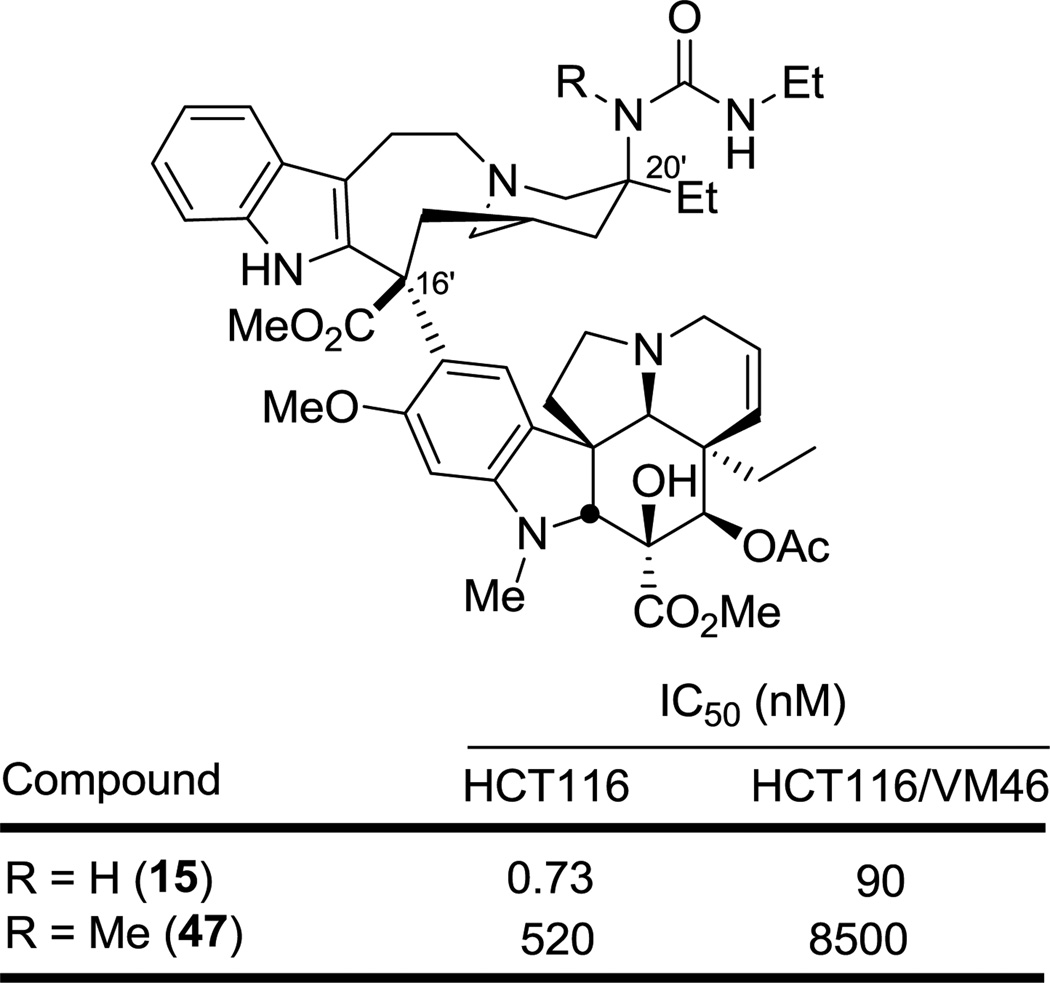
Finally, the 20'-(methylamino)vinblastine (45) was enlisted to establish whether the active urea derivatives require or benefit from the H-bond donor site of the derivatized C20' amine. Thus, treatment of 45 with ethyl isocyanate provided the N-methyl urea 47 for comparison with 15 (Figure 8). There was a 700-fold decrease in activity between 15 and 47 (HCT116), clearly illustrating the importance of a H-bond donor site on the C20' position. This observation clearly suggests that the incorporation of a urea functionality maintains a key H-bond site directly attached to the C20' position and that it best approximates the acidity of the vinblastine C20' alcohol, while allowing for further functionalization on the urea terminal nitrogen that maintains or in many cases improves the activity of the compound.
Figure 8.
Impact of C20' nitrogen H-bond donor
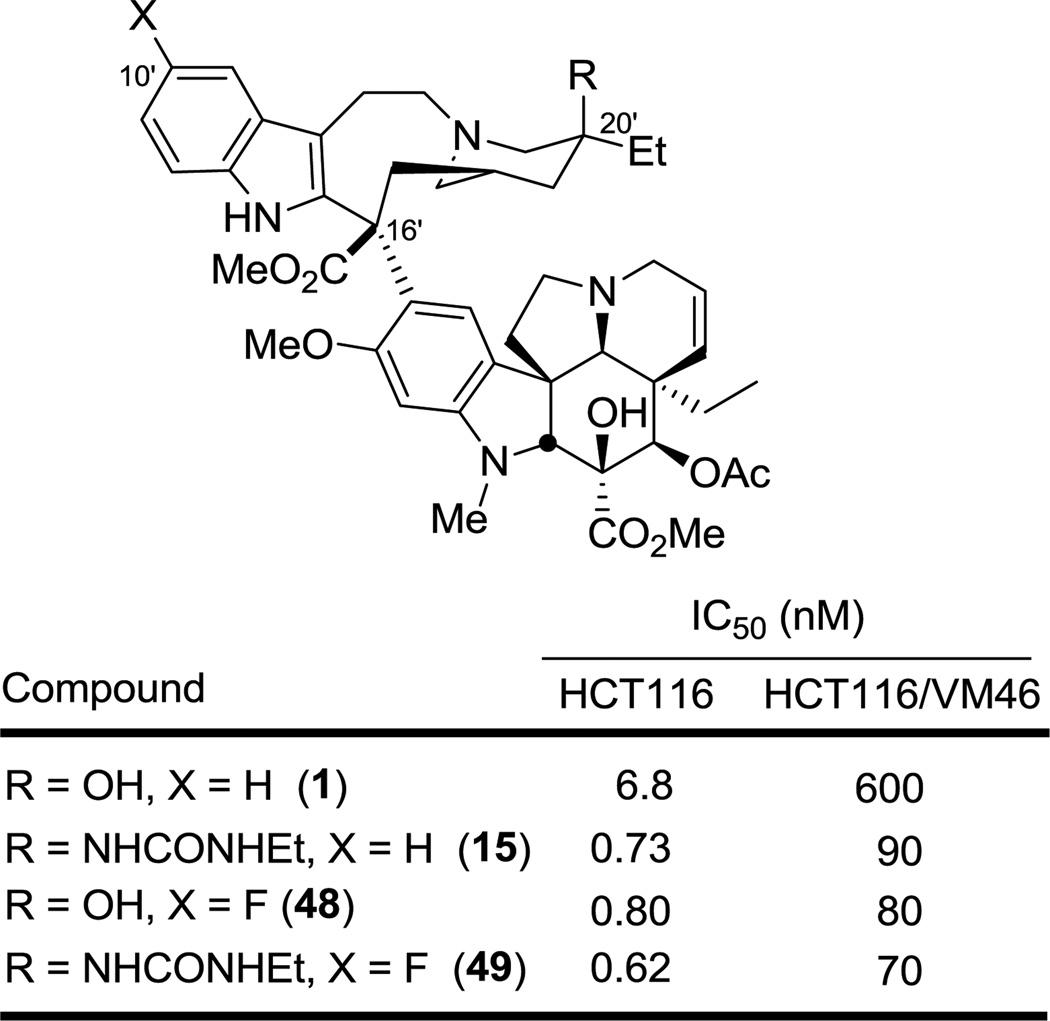
In recent work, the incorporation of a fluorine atom at the 10' position provided a potent molecule (48) with an 8-fold improvement in activity over vinblastine itself.9a We were interested in determining whether the incorporation of both the 10'-F substituent and a C20' urea would have an additive effect in enhancing the potency of vinblastine. An analogue 49 with both functionalities was prepared and evaluated (Figure 9). Only a modest improvement in potency was observed in 49 relative to 15 and 48, suggesting that these modifications are not fully additive. Nonetheless, the modified vinblastine 49 is at least 10-fold more potent than vinblastine exhibiting a sub nanomolar IC50 for cell growth inhibition (620 pM, HCT116) and a nearly 10-fold improved activity against a vinblastine-resistant cell line (IC50 = 70 nM, HCT116/VM46).
Figure 9.
Urea derivative of 10'-fluorovinblastine
Binding to Tubulin
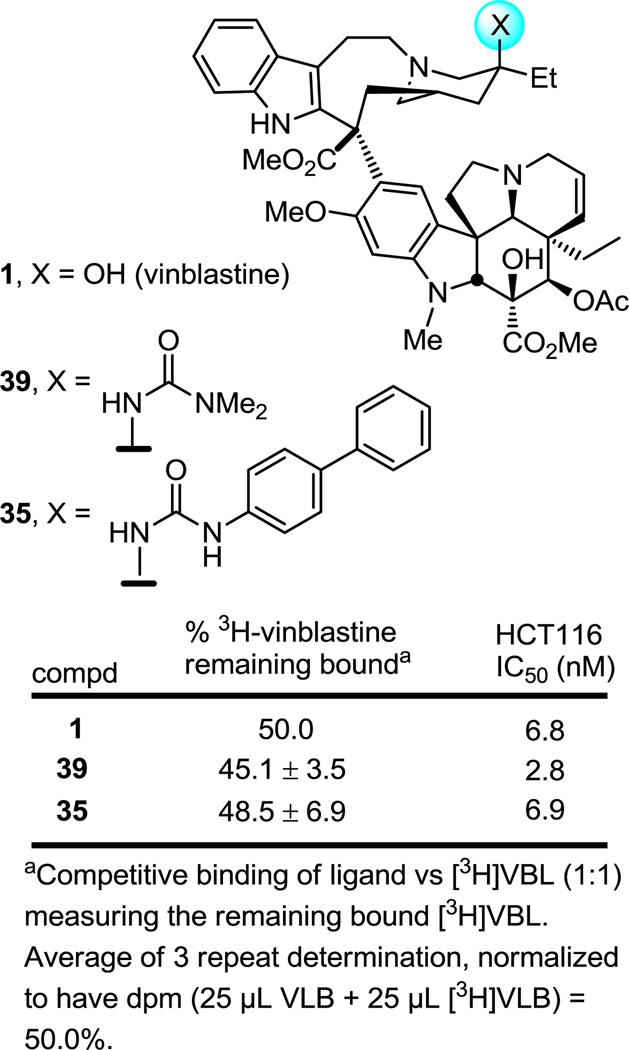
Given the apparent steric constraints of the tubulin binding site surrounding the vinblastine C20' center (Figure 1) and the size of the C20' urea substituents that support and improve on the functional potency of vinblastine itself in the cytotoxic cell growth assays, it was not clear whether these effects could be related to their target (tubulin) binding affinity or derived from their impact on other properties of the molecules (e.g., cell permeability, metabolism, solubility). As a result, two representative C20' urea derivatives 35 and 39 were examined in a well-established tubulin binding assay conducted by measuring the competitive displacement of 3H-vinblastine from porcine tubulin (Figure 10).19 Notably, 35 contains the large biphenyl urea substituent yet matches the functional activity of vinblastine, whereas 39 bears the much smaller N,N-dimethylurea whose functional activity slightly exceeds that of vinblastine (≤ 3-fold). Importantly, these binding studies confirmed that 39 binds tubulin with a slightly better affinity than vinblastine and further established that even 35, bearing the large biphenyl substituent, remarkably binds with an affinity matching or even slightly exceeding that of vinblastine. Thus, the effects of the urea 39 as well as 35 observed in the functional assays correlate directly with their target tubulin binding affinities. These unanticipated observations with 35 highlight that the vinblastine interaction with tubulin surrounding the C20' center is flexible and capable of reorganization to accommodate even a very large substituent. It is notable that this site is adjacent to the nucleotide binding site involving the T5 loop in the N-terminal β1 tubulin nucleotide binding domain and adjacent to the H6 helix and H6–H7 loop that links the nucleotide binding domain to the intermediate domain. It is likely this region is capable of significant reorganization to accommodate the binding of 35 or that the urea substituent may extend into the nucleotide binding site and displace a bound nucleotide.
Figure 10.
Tubulin binding assay
CONCLUSIONS
A remarkable series of previously inaccessible C20' urea derivatives of vinblastine were prepared and found to match or substantially exceed the potency of vinblastine in functional cell-based growth inhibition assays. In addition to defining structural features of the urea required for or potentiating their activity that are directly related to their relative tubulin binding affinity, the studies established an unprecedented steric tolerance for the size of a C20' substituent. A H-bond donor on the C20' position was unequivocally shown to be an important feature of the potent vinblastine analogues. Although this site is known to be critical to the properties of vinblastine and is located deeply embedded in the tubulin bound complex where such substituents would be apparently sterically constrained, the studies revealed that sterically demanding ureas are not only tolerated but that functionalization of this site offers a superb opportunity for enhancing potency as much as 10-fold. In addition to improvements in potency, this may also be a superb site for modulating the physical and chemical properties of the drug that impact additional features including Pgp efflux, in vivo drug distribution, selective cellular uptake, and metabolism.
EXPERIMENTAL SECTION
General Procedures
All commercial reagents were used without further purification unless otherwise noted. THF was distilled prior to use. All reactions were performed in oven-dried (200 °C) glassware and under an inert atmosphere of anhydrous Ar unless otherwise noted. Column chromatography was performed with silica gel 60. TLC was performed on Whatman silica gel (250 µm) F254 glass plates and spots visualized by UV. PTLC was performed on Whatman silica gel (250 and 500 µm) F254 glass plates. Optical rotations were determined on a Rudolph Research Analytical Autopol III automatic polarimeter using the sodium D line (λ = 589 nm) at room temperature (23 °C) and are reported as follows: [α]D23, concentration (c = g/100 mL), and solvent. FT-IR spectroscopy was recorded on a Nicolet 380 FT-IR instrument. 1H NMR was recorded on a Bruker 600 MHz spectrometer. Chemical shifts are reported in ppm from an internal standard of residual CHCl3 (δ 7.26 for 1H). Proton chemical data are reported as follows: chemical shift (δ), multiplicity (ovlp = overlapping, br = broad, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), coupling constant, and integration. High resolution mass spectra were obtained on an Agilent ESI-TOF/MS using Agilent ESI-L low concentration tuning mix as internal high resolution calibration standards. The purity of each tested compound (>95%) was determined on an Agilent 1100 LC/MS instrument using a ZORBAX SB-C18 column (3.5 mm, 4.6 mm × 50 mm, with a flow rate of 0.75 mL/min and detection at 220 and 254 nm) with a 10–98% acetonitrile/water/0.1% formic acid gradient (two different gradients).
General methods for the synthesis of ureas
Method 1
A solution of 20'-aminovinblastine (7, 3.5 mg, 0.004 mmol) in THF (3 mL) was treated with an isocyanate (0.008 mmol). The reaction mixture was stirred for 2 h at 25 °C and then was quenched with the addition of distilled H2O (3 mL). The mixture was extracted with 10% MeOH in CH2Cl2 and the combined organic extracts were washed with saturated aqueous NaCl (3 mL). The organic layer was dried over Na2SO4, and concentrated under reduced pressure. PTLC (SiO2, EtOAc:MeOH:Et3N = 97:3:3) provided the urea (15, 19–21, 23–32, and 34–35); yields (35–98%). Isocyanates used include: ethyl isocyanate, n-butyl isocyanate, t-butyl isocyanate, cyclohexyl isocyanate, phenyl isocyanate, benzyl isocyanate, phenethyl isocyanate, o-methoxyphenyl isocyanate, m-methoxyphenyl isocyanate, p-methoxyphenyl isocyanate, p-fluorophenyl isocyanate, p-chlorophenyl isocyanate, p-tolyl isocyanate, p-trifluoromethylphenyl isocyanate, furfuryl isocyanate and p-biphenyl isocyanate.
Method 2
A solution of 20'-aminovinblastine (7, 5.7 mg, 0.007 mmol) in THF (3 mL) was treated with 4-nitrophenyl chloroformate (2.1 mg, 0.011 mmol, 1.5 equiv). The reaction mixture was stirred for 4 h at 25 °C and then was concentrated under reduced pressure. PTLC (SiO2, EtOAc:MeOH = 95:5) provided 13 (4.6 mg, 67%, off white solid). A solution of 13 (4.0 mg, 0.004 mmol) in THF (3 mL) was treated with an amine (0.008 mmol). The reaction mixture was stirred for 1 h at 25 °C and then was quenched with the addition of distilled H2O (3 mL). The mixture was extracted with 10% MeOH in CH2Cl2, and the combined organic extracts were washed with saturated aqueous NaCl (3 mL). The organic layer was dried over Na2SO4, and concentrated under reduced pressure. PTLC (SiO2, EtOAc:MeOH:Et3N = 97:3:3) provided the urea (14, 16–18, 22, 33, and 39–42); yields (40–99%). The amines used include: methylamine, propylamine, isopropylamine, cyclopropylamine, dimethylamine, diethylamine, 2-(aminomethyl)pyridine, morpholine, piperidine, and ethanolamine.
General methods for the synthesis of thioureas
Method 3
A solution of 20'-aminovinblastine (7, 8.0 mg, 0.010 mmol) in THF (4 mL) was treated with an isothiocyanate (0.031 mmol). The reaction mixture was stirred for 2 h at 25 °C and then was quenched with the addition of distilled H2O (3 mL), the mixture was extracted with 10% MeOH in CH2Cl2, and the combined organic extracts were washed with saturated aqueous NaCl (3 mL). The organic layer was dried over Na2SO4, and concentrated under reduced pressure. PTLC (SiO2, EtOAc:MeOH:Et3N = 97:3:3) provided the thiourea (37); yields (70%). The isothiocyanate used: phenylisothiocyanate.
Method 4
A solution of 20'-aminovinblastine (7, 8.2 mg, 0.010 mmol) in THF (3 mL) was treated with carbon disulfide (915 µL, 15 mmol). The reaction mixture was stirred for 13 h and then was quenched with the addition of distilled H2O (5 mL), the mixture was extracted with 10% MeOH in CH2Cl2, and washed with saturated aqueous NaCl (2 mL). The organic layer was dried over Na2SO4, and concentrated under reduced pressure. PTLC (SiO2, EtOAc:MeOH:Et3N = 97:3:3) provided 5 (7.0 mg, 82%, white solid).10 A solution of 20'-isothiocyanovinblastine (5, 6.0 mg, 0.007 mmol) in THF (3 mL) was treated with an amine (0.011 mmol). The reaction mixture was stirred for 1 h at 25 °C and then was quenched with the addition of distilled H2O (3 mL). The mixture was extracted with 10% MeOH in CH2Cl2, and the combined extracts were washed with saturated aqueous NaCl (3 mL). The organic layer was dried over Na2SO4, and concentrated under reduced pressure. PTLC (SiO2, EtOAc:MeOH:Et3N = 97:3:3) provided the thiourea (36, 38, and 43); yields (26–92%). The amines used include: cyclohexylamine, 4-fluorophenethylamine and dimethylamine.
(R = 20'-NHCO2C6H4NO2) 13
Yield: 67%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.87 (br s, 1H), 8.11 (d, J = 9.2 Hz, 2H), 8.04 (br s, 1H), 7.53 (d, J = 7.8 Hz, 1H), 7.18–7.14 (m, 1H), 7.14–7.08 (m, 2H), 6.76 (d, J = 9.1 Hz, 2H), 6.63 (s, 1H), 6.11 (s, 1H), 5.85 (dd, J = 10.0, 3.9 Hz, 1H), 5.48 (s, 1H), 5.30 (d, J = 9.8 Hz, 1H), 4.00 (t, J = 13.2 Hz 1H), 3.81 (s, 3H), 3.79 (s, 3H), 3.72 (s, 1H), 3.63 (s, 3H), 3.60–3.55 (m, 4H), 3.43 (d, J = 13.6 Hz, 1H), 3.37 (dd, J = 15.9, 4.5 Hz, 1H), 3.35–3.26 (m, 2H), 3.20–3.13 (m, 2H), 2.98 (d, J = 13.8 Hz, 1H), 2.85–2.77 (m, 2H), 2.70 (s, 3H), 2.46–2.42 (m, 1H), 2.35 (d, J = 11.9 Hz, 1H), 2.24 (d, J = 13.0 Hz, 1H), 2.20–2.13 (m, 1H), 2.11 (s, 3H), 1.85–1.76 (m, 2H), 1.64 (d, J = 14.8 Hz, 1H), 1.47–1.40 (m, 2H), 1.37–1.29 (m, 2H), 0.96 (t, J = 7.4 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H); IR (film) νmax 3467, 2924, 1736, 1218 cm−1; HRESI-TOF m/z 975.4492 (C53H62N6O12 + H+, required 975.4498); [α]D23 −13 (c 0.1, CHCl3).
(R = 20'-NHCONHCH3) 14
Yield: 85%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.83 (br s, 1H), 7.97 (br s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.19–7.13 (m, 1H), 7.13–7.07 (m, 2H), 6.64 (s, 1H), 6.09 (s, 1H), 5.86 (dd, J = 10.0, 4.4 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 10.7 Hz, 2H), 4.54 (br s, 1H), 4.33 (br s, 1H), 3.84 (t, J = 13.2 Hz 1H), 3.80 (s, 6H), 3.74 (s, 1H), 3.60 (s, 3H), 3.39–3.36 (m, 2H), 3.32–3.21 (m, 3H), 3.20–3.10 (m, 2H), 2.86 (d, J = 4.8 Hz, 3H), 2.83 (d, J = 16.6 Hz, 1H), 2.71 (s, 3H), 2.68 (s, 1H), 2.58 (d, J = 13.8 Hz, 1H), 2.45 (dd, J = 16.9, 10.5 Hz, 1H), 2.38 (d, J = 12.8 Hz, 1H), 2.20–2.15 (m, 2H), 2.11 (s, 3H), 1.85–1.74 (m, 4H), 1.69 (d, J = 13.7 Hz, 2H), 1.44–1.30 (m, 2H), 0.82 (t, J = 7.4 Hz, 3H), 0.77 (t, J = 7.4 Hz, 3H); IR (film) νmax 3539, 2870, 1721, 1554, 1461, 1223, 1039, 712 cm−1; HRESI-TOF m/z 867.4631 (C53H62N6O12 + H+, required 867.4651); [α]D23 −1.5 (c 0.05, CHCl3).
(R = 20'-NHCONHCH2CH3) 15
Yield: 95%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.84 (br s, 1H), 7.98 (br s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.19–7.14 (m, 1H), 7.14–7.07 (m, 2H), 6.64 (s, 1H), 6.09 (s, 1H), 5.86 (dd, J = 9.9, 4.0 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 9.8 Hz, 1H), 4.59 (br s, 1H), 4.29 (br s, 1H), 3.83 (t, J = 12.0 Hz, 1H), 3.80 (s, 6H), 3.74 (s, 1H), 3.60 (s, 3H), 3.37 (d, J = 15.0 Hz, 2H), 3.34–3.19 (m, 4H), 3.18–3.13 (m, 2H), 2.83 (d, J = 16.0 Hz, 1H), 2.71 (s, 3H), 2.68 (s, 1H), 2.57 (d, J = 13.1 Hz, 1H), 2.47–2.43 (m, 1H), 2.38 (d, J = 13.4 Hz, 1H), 2.20–2.15 (m, 2H), 2.11 (s, 3H), 1.86–1.73 (m, 4H), 1.68 (d, J = 14.3 Hz, 2H), 1.41–1.38 (m, 4H), 1.19 (t, J = 7.2 Hz, 3H), 0.82 (t, J = 7.4 Hz, 3H), 0.77 (t, J = 7.4 Hz, 3H); IR (film) νmax 3463, 2921, 1739, 1500, 1459, 1228, 1039, 739 cm−1; HRESI-TOF m/z 881.4797 (C49H64N6O9 + H+, required 881.4808); [α]D23 +5.4 (c 0.08, CHCl3).
(R = 20'-NHCONHCH2CH2CH3) 16
Yield: 40%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.84 (br s, 1H), 7.98 (br s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.18–7.14 (m, 1H), 7.14–7.08 (m, 2H), 6.64 (s, 1H), 6.09 (s, 1H), 5.86 (dd, J = 10.1, 4.3 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 9.8 Hz, 1H), 4.63 (br s, 1H), 4.28 (br s, 1H), 3.83 (t, J = 12.0 Hz, 1H), 3.80 (s, 6H), 3.74 (s, 1H), 3.60 (s, 3H), 3.37 (dd, J = 15.9, 4.7 Hz, 2H), 3.34–3.27 (m, 2H), 3.27–3.11 (m, 4H), 2.83 (d, J = 16.2 Hz, 1H), 2.71 (s, 3H), 2.68 (s, 1H), 2.57 (d, J = 13.8 Hz, 1H), 2.47–2.42 (m, 1H), 2.38 (d, J = 13.1 Hz, 1H), 2.24–2.15 (m, 2H), 2.09 (s, 3H), 1.87–1.75 (m, 4H), 1.60–1.52 (m, 6H), 1.40–1.34 (m, 2H), 0.95 (t, J = 7.4 Hz, 3H), 0.82 (t, J = 7.4 Hz, 3H), 0.77 (t, J = 7.4 Hz, 3H); IR (film) νmax 3436, 2952, 1739, 1547, 1459, 1243, 1032, 755 cm−1; HRESI-TOF m/z 895.4953 (C50H66N6O9 + H+, required 895.4964); [α]D23 +4.0 (c 0.05, CHCl3).
(R = 20'-NHCONHCH(CH3)2) 17
Yield: 59%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.84 (br s, 1H), 8.00 (br s, 1H), 7.52 (d, J = 7.9 Hz, 1H), 7.17–7.08 (m, 3H), 6.65 (s, 1H), 6.09 (s, 1H), 5.87 (dd, J = 10.1, 3.7 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 9.3 Hz, 1H), 4.29 (br s, 1H), 4.14 (br s, 1H), 3.96–3.91 (m, 1H), 3.81–3.77 (m, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.74 (s, 1H), 3.73–3.71 (m, 1H), 3.60 (s, 3H), 3.37 (d, J = 14.8 Hz, 2H), 3.34–3.26 (m, 2H), 3.23 (t, J = 11.9 Hz, 1H), 3.18–3.11 (m, 2H), 2.83 (d, J = 16.0 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 1H), 2.56 (d, J = 13.6 Hz, 1H), 2.47–2.43 (m, 1H), 2.36 (d, J = 14.4 Hz, 1H), 2.20–2.15 (m, 2H), 2.11 (s, 3H), 1.86–1.73 (m, 4H), 1.47–1.42 (m, 1H), 1.37–1.29 (m, 3H), 1.20 (dd, J = 6.4, 2.5 Hz, 6H), 0.82 (t, J = 7.4 Hz, 3H), 0.77 (t, J = 7.4 Hz, 3H); IR (film) νmax 3471, 3323, 2923, 1741, 1459, 1239, 1041 cm−1; HRESI-TOF m/z 917.4761 (C50H66N6O9 + Na+, required 917.4783); [α]D23 +6.1 (c 0.1, CHCl3).
(R = 20'-NHCONHCH(CH2)2) 18
Yield: 70%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.86 (br s, 1H), 8.02 (br s, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.18–7.13 (m, 1H), 7.13–7.07 (m, 2H), 6.62 (s, 1H), 6.10 (s, 1H), 5.85 (dd, J = 10.2, 4.4 Hz, 1H), 5.47 (s, 1H), 5.30 (d, J = 6.6 Hz, 1H), 5.17 (s, 1H), 4.66 (s, 1H), 3.91 (t, J = 13.8 Hz, 1H), 3.80 (s, 6H), 3.74 (s, 1H), 3.56 (s, 3H), 3.41–3.13 (m, 6H), 3.08–3.00 (m, 2H), 2.83 (d, J = 16.2 Hz, 1H), 2.77 (br s, 1H), 2.71 (s, 3H), 2.70–2.68 (m, 1H), 2.67 (s, 1H), 2.63 (d, J = 13.5 Hz, 1H), 2.47–2.43 (m, 1H), 2.38 (d, J = 12.1 Hz, 1H), 2.24 (d, J = 14.2 Hz, 1H), 2.19 (dd, J = 12.9, 7.7 Hz, 1H), 2.15 (s, 1H), 2.11 (s, 3H), 1.86–1.70 (m, 4H), 1.52–1.49 (m, 2H), 0.81 (t, J = 7.3 Hz, 3H), 0.76 (t, J = 7.3 Hz, 3H), 0.70–0.69 (m, 2H), 0.50–0.48 (m, 2H); IR (film) νmax 3629, 2952, 1740, 1506, 1458, 1245, 998, 748 cm−1; HRESI-TOF m/z 893.4792 (C50H64N6O9 + H+, required 893.4808); [α]D23 +19 (c 0.09, CHCl3).
(R = 20'-NHCONHCH2CH2CH2CH3) 19
Yield: 45%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.85 (br s, 1H), 7.98 (br s, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.18–7.13 (m, 1H), 7.13–7.07 (m, 2H), 6.64 (s, 1H), 6.09 (s, 1H), 5.86 (dd, J = 10.2, 4.4 Hz, 1H), 5.46 (s, 1H), 5.31 (d, J = 11.1 Hz, 1H), 4.56 (br s, 1H), 4.27 (br s, 1H), 3.80 (s, J = 1.6 Hz, 6H), 3.74 (s, 1H), 3.60 (s, 3H), 3.37 (dd, J = 15.9, 4.7 Hz, 2H), 3.31–3.20 (m, 4H), 3.15 (br s, 2H), 3.09 (dd, J = 14.5, 7.2 Hz, 4H), 2.83 (d, J = 16.2 Hz, 1H), 2.71 (s, 3H), 2.68 (s, 1H), 2.55 (d, J = 13.8 Hz, 1H), 2.47–2.43 (m, 1H), 2.38 (d, J = 12.1 Hz, 1H), 2.20–2.15 (m, 2H), 2.11 (s, 3H), 2.07 (s, 1H), 1.86–1.72 (m, 4H), 1.56–1.49 (m, 2H), 1.38–1.33 (m, 4H), 0.93 (t, J = 7.3 Hz, 3H), 0.82 (t, J = 7.3 Hz, 3H), 0.77 (t, J = 7.3 Hz, 3H); IR (film) νmax 3544, 2952, 1736, 1501, 1458, 1240, 1030, 761 cm−1; HRESI-TOF m/z 909.5106 (C51H68N6O9 + H+, required 909.5121); [α]D23 +14 (c 0.06, CHCl3).
(R = 20'-NHCONHC(CH3)3) 20
Yield: 45%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.83 (br s, 1H), 7.99 (br s, 1H), 7.53 (d, J = 8.0 Hz, 1H), 7.19–7.14 (m, 1H), 7.13–7.07 (m, 2H), 6.65 (s, 1H), 6.09 (s, 1H), 5.86 (dd, J = 10.1, 3.9 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 10.2 Hz, 1H), 4.54 (br s, 1H), 4.10 (br s, 1H), 3.83 (t, J = 14.1 Hz, 1H), 3.80 (s, 3H), 3.78 (s, 3H), 3.74 (s, 1H), 3.60 (s, 3H), 3.40–3.22 (m, 5H), 3.17–3.12 (m, 2H), 2.71 (s, 3H), 2.68 (s, 1H), 2.54 (d, J = 13.6 Hz, 1H), 2.47–2.42 (m, 1H), 2.35 (d, J = 13.4 Hz, 1H), 2.21–2.14 (m, 2H), 2.11 (s, 3H), 1.87–1.75 (m, 2H), 1.73–1.62 (m, 4H), 1.49–1.41 (m, 2H), 1.38 (s, 9H), 1.37–1.31 (m, 2H), 0.82 (t, J = 7.4 Hz, 3H), 0.77 (t, J = 7.4 Hz, 3H); IR (film) νmax 3457, 2941, 1741, 1556, 1458, 1244, 1036, 741 cm−1; HRESI-TOF m/z 909.5128 (C51H68N6O9 + H+, required 909.5121); [α]D23 +3.0 (c 0.1, CHCl3).
(R = 20'-NHCONHC6H11) 21
Yield: 51%, Method 1.1H NMR (600 MHz, CDCl3) δ 9.84 (br s, 1H), 7.99 (br s, 1H), 7.52 (d, J = 7.9 Hz, 1H), 7.20–7.14 (m, 1H), 7.14–7.07 (m, 2H), 6.64 (s, 1H), 6.09 (s, 1H), 5.86 (dd, J = 10.3, 4.1 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 9.3 Hz, 1H), 4.53 (br s, 1H), 4.22 (br s, 1H), 4.02 (d, J = 9.0 Hz, 1H), 3.83 (t, J = 12.9 Hz, 1H), 3.79 (s, 3H), 3.79 (s, 3H), 3.74 (s, 1H), 3.60 (s, 3H), 3.50–3.45 (m, 1H), 3.39–3.21 (m, 3H), 3.16–3.12 (m, 2H), 2.83 (d, J = 16.1 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 1H), 2.55 (d, J = 13.5 Hz, 1H), 2.47–2.42 (m, 1H), 2.37 (d, J = 12.7 Hz, 1H), 2.21–2.14 (m, 2H), 2.11 (s, 3H), 2.04–1.90 (m, 2H), 1.86–1.74 (m, 4H), 1.64–1.59 (m, 2H), 1.37–1.31 (m, 6H), 1.20–1.02 (m, 6H), 0.82 (t, J = 7.4 Hz, 3H), 0.77 (t, J = 7.4 Hz, 3H); IR (film) νmax 3460, 2919, 1738, 1556, 1459, 1242, 1033, 752 cm−1; HRESI-TOF m/z 935.5276 (C53H70N6O9 + H+, required 935.5277); [α]D23 +3.8 (c 0.09, CHCl3).
(R = 20'-NHCONHCH2CH2OH) 22
Yield: 60%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.78 (br s, 1H), 7.99 (br s, 1H), 7.52 (d, J = 7.8 Hz, 1H), 7.19–7.09 (m, 3H), 6.69 (s, 1H), 6.08 (s, 1H), 5.89–5.85 (m, 1H), 5.48 (s, 1H), 5.31 (d, J = 11.8 Hz, 1H), 4.59 (br s, 1H), 3.83–3.72 (m, 4H), 3.80 (s, 3H), 3.79 (s, 3H), 3.76 (s, 1H), 3.57 (s, 3H), 3.57–3.54 (m, 1H), 3.42–3.37 (m, 3H), 3.34–3.29 (m, 1H), 3.26–3.17 (m, 4H), 3.08–3.03 (m, 1H), 2.87 (br s, 1H), 2.82 (d, J = 16.0 Hz, 1H), 2.72 (s, 3H), 2.68 (s, 1H), 2.60–2.42 (m, 1H), 2.33–2.17 (m, 1H), 2.14–2.09 (m, 1H), 2.11 (s, 3H), 1.97–1.77 (m, 2H), 1.75–1.64 (m, 3H), 1.50–1.45 (m, 1H), 1.38–1.34 (m, 2H), 1.16 (dd, J = 14.3, 4.6 Hz, 2H), 0.83–0.81 (m, 3H), 0.77 (t, J = 7.1 Hz, 3H); IR (film) νmax 3399, 2927, 1738, 1502, 1458, 1232, 1040 cm−1; HRESI-TOF m/z 897.4753 (C49H64N6O10 + H+, required 897.4756); [α]D23 −14 (c 0.2, CHCl3).
(R = 20'-NHCONHC6H5) 23
Yield: 87%, Method 1. 1H NMR (600 MHz, CDCl3) δ 1H NMR (600 MHz, CDCl3) δ 9.87 (br s, 1H), 7.98 (br s, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.31 (t, J = 7.8 Hz, 2H), 7.16–7.14 (m, 1H), 7.13–7.05 (m, 3H), 6.62 (s, 1H), 6.09 (s, 1H), 5.85 (dd, J = 10.3, 4.6 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 9.4 Hz, 1H), 4.80 (s, 1H), 3.80 (s, 6H), 3.74 (s, 1H), 3.60 (s, 3H), 3.39–3.35 (m, 2H), 3.32–3.28 (m, 1H), 3.22–3.18 (m, 1H), 3.10 (s, 3H), 2.82 (d, J = 16.3 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 1H), 2.59 (d, J = 13.8 Hz, 1H), 2.47–2.42 (m, 1H), 2.36–2.28 (m, 2H), 2.25–2.13 (m, 2H), 2.11 (s, 3H), 2.04 (s, 1H), 1.85–1.76 (m, 3H), 1.71–1.65 (m, 2H), 1.64–1.54 (m, 2H), 1.24–1.20 (m, 2H), 0.82 (t, J = 7.4 Hz, 3H), 0.80 (t, J = 7.4 Hz, 3H); IR (film) νmax 3468, 2940, 1742, 1501, 1460, 1237, 1031, 760 cm−1; HRESI-TOF m/z 929.4807 (C53H64N6O9 + H+, required 929.4807); [α]D23 +16 (c 0.9, CHCl3).
(R = 20'-NHCONH(4-fluorophenyl)) 24
Yield: 47%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.81 (br s, 1H), 7.97 (br s, 1H), 7.49 (d, J = 8.1 Hz, 1H), 7.42 (dd, J = 8.8, 4.8 Hz, 2H), 7.18–7.14 (m, 1H), 7.12–7.08 (m, 2H), 7.01 (t, J = 8.6 Hz, 2H), 6.63 (s, 1H), 6.09 (s, 1H), 5.86 (dd, J = 10.2, 4.5 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 12.1 Hz, 1H), 4.71 (br s, 1H), 3.80 (s, 6H), 3.75 (s, 1H), 3.61 (s, 3H), 3.40–3.36 (m, 2H), 3.32–3.28 (m, 1H), 3.21–3.06 (m, 4H), 2.82 (d, J = 16.5 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 1H), 2.59 (d, J = 13.7 Hz, 1H), 2.47–2.42 (m, 1H), 2.34 (d, J = 12.7 Hz, 1H), 2.20–2.15 (m, 2H), 2.11 (s, 3H), 1.85–1.75 (m, 4H), 1.59–1.50 (m, 4H), 1.23–1.21 (m, 2H), 0.82 (t, J = 7.4 Hz, 3H), 0.79 (t, J = 7.4 Hz, 3H); IR (film) νmax 3490, 2921, 1745, 1509, 1455, 1228, 1043, 736 cm−1; HRESI-TOF m/z 947.4726 (C53H63FN6O9 + H+, required 947.4713); [α]D23 −4.4 (c 0.08, CHCl3).
(R = 20'-NHCONH(4-chlorophenyl)) 25
Yield: 63%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.80 (br s, 1H), 7.95 (br s, 1H), 7.40 (d, J = 7.9 Hz, 2H), 7.38 (d, J = 8.1 Hz, 1H), 7.19–7.07 (m, 5H), 6.65 (s, 1H), 6.08 (s, 1H), 5.86 (dd, J = 10.2, 4.5 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 12.1 Hz, 1H), 4.81 (br s, 1H), 3.79 (s, 6H), 3.75 (s, 1H), 3.60 (s, 3H), 3.36 (d, J = 15.7 Hz, 2H), 3.31–3.26 (m, 2H), 3.22–3.15 (m, 2H), 3.01–2.91 (m, 1H), 2.82 (d, J = 15.7 Hz, 1H), 2.72 (s, 3H), 2.67 (s, 1H), 2.61–2.55 (m, 2H), 2.49–2.43 (m, 2H), 2.21–2.14 (m, 2H), 2.10 (s, 3H), 1.83–1.74 (s, 4H) 1.36–1.27 (m, 4H), 1.16–1.04 (m, 2H), 0.82 (t, J = 7.4 Hz, 3H), 0.79 (t, J = 7.4 Hz, 3H); IR (film) νmax 3376, 2923, 1736, 1493, 1455, 1240, 1090, 765 cm−1; HRESI-TOF m/z 963.4407 (C53H63ClN6O9 + H+, required 963.4418); [α]D23 +21 (c 0.07, CHCl3).
(R = 20'-NHCONH(4-methylphenyl)) 26
Yield: 40%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.86 (br s, 1H), 7.99 (br s, 1H), 7.47 (d, J = 7.9 Hz, 1H), 7.33 (d, J = 8.1 Hz, 2H), 7.18–7.07 (m, 3H), 6.61 (s, 1H), 6.08 (s, 1H), 5.85 (d, J = 5.9 Hz, 1H), 5.47 (s, 1H), 5.30 (d, J = 10.3 Hz, 1H), 4.74 (br s, 1H), 4.30 (br s, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.74 (s, 1H), 3.67–3.62 (m, 3H), 3.61 (s, 3H), 3.40–3.34 (m, 2H), 3.31–3.27 (m, 1H), 3.20–3.16 (m, 1H), 2.81 (d, J = 15.7 Hz, 1H), 2.71 (s, 3H), 2.66 (s, 1H), 2.58 (d, J = 13.3 Hz, 1H), 2.46–2.41 (m, 1H), 2.32 (s, 3H), 2.24–2.14 (m, 2H), 2.11 (s, 3H), 2.03 (s, 1H), 1.87 (d, J = 13.8 Hz, 2H), 1.84–1.76 (m, 2H), 1.63–1.53 (m, 4H), 1.38–1.34 (m, 2H), 1.21 (dd, J = 14.5, 5.7 Hz, 2H), 0.81 (t, J = 7.6 Hz, 3H), 0.78 (t, J = 7.6 Hz, 3H); IR (film) νmax 3369, 2912, 1722, 1507, 1445, 1230, 1017, 729 cm−1; HRESI-TOF m/z 943.4949 (C54H66N6O9 + H+, required 943.4964); [α]D23 +36 (c 0.1, CHCl3).
(R = 20'-NHCONH(4-trifluoromethylphenyl)) 27
Yield: 60%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.82 (br s, 1H), 7.94 (br s, 1H), 7.58 (d, J = 8.6 Hz, 2H), 7.51 (d, J = 8.6 Hz, 2H), 7.48 (d, J = 7.9 Hz, 1H), 7.18–7.14 (m, 1H), 7.13–7.07 (m, 2H), 6.65 (s, 1H), 6.09 (s, 1H), 5.87 (dd, J = 10.0, 4.2 Hz, 1H), 5.46 (s, 1H), 5.31 (d, J = 10.3 Hz, 1H), 4.99 (br s, 1H), 3.90–3.84 (m, 2H), 3.80 (s, 6H), 3.75 (s, 1H), 3.67–3.63 (m, 1H), 3.61 (s, 3H), 3.40–3.36 (m, 2H), 3.33–3.29 (m, 1H), 3.27–3.20 (m, 1H), 3.13–3.10 (m, 1H), 2.83 (d, J = 15.8 Hz, 1H), 2.72 (s, 3H), 2.68 (d, J = 11.9 Hz, 1H), 2.60 (d, J = 13.8 Hz, 1H), 2.48–2.43 (m, 1H), 2.40 (d, J = 13.3 Hz, 1H), 2.33–2.24 (m, 2H), 2.20–2.15 (m, 1H), 2.10 (s, 3H), 2.01 (s, 1H), 1.90–1.87 (m, 2H), 1.84–1.75 (m, 2H), 1.66 (d, J = 14.9 Hz, 2H), 1.40–1.32 (m, 2H), 0.82 (t, J = 7.2 Hz, 6H); IR (film) νmax 3334, 2931, 1716, 1537, 1472, 1232, 1023, 745 cm−1; HRESI-TOF m/z 997.4685 (C54H63F3N6O9 + H+, required 997.4681); [α]D23 −18 (c 0.04, CHCl3).
(R = 20'-NHCONH(4-methoxyphenyl)) 28
Yield: 36%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.80 (br s, 1H), 8.01 (br s, 1H), 7.38 (d, J = 7.9 Hz, 1H), 7.24–7.20 (m, 1H), 7.18–7.09 (m, 2H), 6.93 (d, J = 8.7 Hz, 2H), 6.88 (d, J = 8.9 Hz, 2H), 6.39 (s, 1H), 6.34 (s, 1H), 6.08 (s, 1H), 5.88 (dd, J = 10.0, 4.7 Hz, 1H), 5.41 (s, 1H), 5.30 (d, J = 10.1 Hz, 1H), 4.60 (d, J = 12.0 Hz, 1H), 4.04 (t, J = 12.0 Hz, 1H), 3.82 (s, 3H), 3.80 (s, 3H), 3.79 (s, 3H), 3.77 (s, 1H), 3.66 (s, 3H), 3.65–3.58 (m, 2H), 3.52–3.48 (m, 1H), 3.38–3.33 (m, 2H), 3.31–3.27 (m, 1H), 3.02–2.93 (m, 2H), 2.86–2.79 (m, 2H), 2.73 (s, 3H), 2.66 (s, 1H), 2.48–2.41 (m, 1H), 2.18–2.14 (m, 2H), 2.11 (s, 3H), 2.04–1.97 (m, 1H), 1.83–1.74 (m, 2H), 1.35–1.28 (m, 2H), 0.87 (t, J = 7.3 Hz, 3H), 0.79 (t, J = 7.3 Hz, 3H); IR (film) νmax 3447, 2899, 1742, 1507, 1459, 1234, 1036, 736 cm−1; HRESI-TOF m/z 959.4917 (C54H66N6O10 + H+, required 959.4913); [α]D23 −9.4 (c 0.04, CHCl3).
(R = 20'-NHCONH(3-methoxyphenyl)) 29
Yield: 50%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.82 (br s, 1H), 7.96 (br s, 1H), 7.47 (d, J = 7.9 Hz, 1H), 7.19 (t, J = 8.1 Hz, 1H), 7.16–7.13 (m, 1H), 7.12–7.06 (m, 3H), 6.99 (d, J = 8.6 Hz, 1H), 6.62 (s, 1H), 6.61 (d, J = 8.1 Hz, 1H), 6.08 (s, 1H), 5.85 (dd, J = 10.2, 4.6 Hz, 1H), 5.46 (s, 1H), 5.30 (d, J = 9.9 Hz, 1H), 4.82 (s, 1H), 3.79 (s, 6H), 3.78 (s, 3H), 3.74 (s, 1H), 3.66–3.62 (m, 3H), 3.59 (s, 3H), 3.38–3.35 (m, 2H), 3.31–3.27 (m, 1H), 3.22–3.18 (m, 1H), 3.15–3.10 (m, 1H), 3.03 (d, J = 13.3 Hz, 2H), 2.82 (d, J = 16.1 Hz, 1H), 2.71 (s, 3H), 2.66 (s, 1H), 2.58 (d, J = 13.8 Hz, 1H), 2.47–2.40 (m, 1H), 2.34 (d, J = 13.0 Hz, 1H), 2.22–2.14 (m, 2H), 2.10 (s, 3H), 1.84–1.76 (m, 3H), 1.75–1.69 (m, 2H), 1.38–1.31 (m, 2H), 1.23–1.21 (m, 1H), 0.81 (ovlp t, J = 7.4 Hz, 3H), 0.80 (ovlp t, J = 7.4 Hz, 3H); IR (film) νmax 3483, 2985, 1745, 1501, 1454, 1228, 1033, 760 cm−1; HRESI-TOF m/z 959.4905 (C54H66N6O10 + H+, required 959.4913); [α]D23 −19 (c 0.05, CHCl3).
(R = 20'-NHCONH(2-methoxyphenyl)) 30
Yield: 35%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.85 (br s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01 (br s, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.17–7.12 (m, 1H), 7.13–7.07 (m, 2H), 7.05–6.95 (m, 2H), 6.88–6.86 (m, 1H), 6.64 (s, 1H), 6.10 (s, 1H), 5.85 (dd, J = 10.0, 4.7 Hz, 1H), 5.47 (s, 1H), 5.30 (d, J = 10.1 Hz, 1H), 5.13–5.10 (m, 1H), 4.76 (br s, 1H), 4.57 (br s, 1H), 3.85 (s, 3H), 3.80 (s, 3H), 3.80 (s, 3H), 3.74 (s, 1H), 3.56 (s, 3H), 3.40–3.36 (m, 2H), 3.34–3.20 (m, 2H), 3.14–3.07 (m, 2H), 2.71 (s, 3H), 2.67 (s, 1H), 2.60 (d, J = 13.7 Hz, 1H), 2.50–2.42 (m, 1H), 2.39 (d, J = 12.9 Hz, 1H), 2.27 (d, J = 13.7 Hz, 1H), 2.20–2.15 (m, 2H), 2.11 (s, 3H), 2.09–1.99 (m, 2H), 1.84–1.76 (m, 4H), 1.50–1.39 (m, 2H), 1.38–1.28 (m, 2H), 0.81 (ovlp t, J = 7.4 Hz, 3H), 0.80 (ovlp t, J = 7.4 Hz, 3H); IR (film) νmax 3451, 2919, 1730, 1531, 1461, 1257, 952, 721 cm−1; HRESI-TOF m/z 959.4909 (C54H66N6O10 + H+, required 959.4913); [α]D23 +24 (c 0.03, CHCl3).
(R = 20'-NHCONHCH2C6H5) 31
Yield: 96%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.84 (br s, 1H), 7.98 (br s, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 8.0 Hz, 2H), 7.31 (t, J = 7.8 Hz, 2H), 7.17–7.08 (m, 4H), 6.62 (s, 1H), 6.09 (s, 1H), 5.85 (dd, J = 10.3, 4.6 Hz, 1H), 5.47 (s, 1H), 5.30 (d, J = 9.4 Hz, 1H), 4.68 (s, 1H), 4.48–4.31 (m, 2H), 3.80 (s, 3H), 3.79 (s, 3H), 3.74 (s, 1H), 3.60 (s, 3H), 3.38–3.33 (m, 2H), 3.31–3.26 (m, 1H), 3.22–3.18 (m, 1H), 3.10 (s, 3H), 2.83 (d, J = 16.3 Hz, 1H), 2.70 (s, 3H), 2.67 (s, 1H), 2.59 (d, J = 13.8 Hz, 1H), 2.47–2.42 (m, 1H), 2.36–2.28 (m, 2H), 2.25–2.13 (m, 2H), 2.11 (s, 3H), 2.04 (s, 1H), 1.83–1.71 (m, 3H), 1.71–1.65 (m, 2H), 1.64–1.54 (m, 2H), 1.24–1.20 (m, 2H), 0.83 (t, J = 7.4 Hz, 3H), 0.80 (t, J = 7.4 Hz, 3H); IR (film) νmax 3454, 2992, 1737, 1501, 1459, 1231, 1033, 729 cm−1; HRESI-TOF m/z 943.4950 (C54H66N6O9 + H+, required 943.4964); [α]D23 +29 (c 0.3, CHCl3).
(R = 20'-NHCONHCH2CH2C6H5) 32
Yield: 98%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.83 (br s, 1H), 7.97 (br s, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.31 (t, J = 7.8 Hz, 2H), 7.17–7.08 (m, 6H), 6.39 (s, 1H), 6.07 (s, 1H), 5.89 (dd, J = 10.3, 4.6 Hz, 1H), 5.39 (s, 1H), 5.32 (d, J = 9.4 Hz, 1H), 4.52 (s, 1H), 3.80 (s, 3H), 3.78 (s, 3H), 3.74 (s, 1H), 3.65 (s, 3H), 3.64–3.63 (m, 2H), 3.49–3.43 (m, 1H), 3.37–3.32 (m, 1H), 3.31–3.19 (m, 1H), 3.10 (s, 3H), 2.99 (d, J = 15 Hz, 1H), 2.95–2.89 (m, 2H), 2.72 (s, 3H), 2.67 (s, 1H), 2.58–2.50 (m, 1H), 2.45–2.37 (m, 1H), 2.40–2.30 (m, 2H), 2.25–2.13 (m, 2H), 2.11 (s, 3H), 2.00–1.94 (m, 1H), 1.84–1.70 (m, 4H), 1.71–1.65 (m, 2H), 1.64–1.54 (m, 2H), 1.34–1.28 (m, 2H), 0.87 (t, J = 7.4 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H); IR (film) νmax 3448, 2927, 1742, 1555, 1461, 1236, 1038, 749 cm−1; HRESI-TOF m/z 957.5106 (C55H68N6O9 + H+, required 957.5121); [α]D23 −17 (c 0.06, CHCl3).
(R = 20'-NHCONHCH2(2-pyridyl)) 33
Yield: 79%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.84 (br s, 1H), 8.52 (s, 1H), 8.04 (br s, 1H), 7.66 (t, J = 8.1 Hz, 1H), 7.48 (d, J = 7.9 Hz, 2H), 7.39 (d, J = 7.7 Hz, 1H), 7.18–7.08 (m, 3H), 6.67 (s, 1H), 6.09 (s, 1H), 5.87–5.83 (m, 1H), 5.54 (br s, 1H), 5.47 (s, 1H), 5.30 (d, J = 10.4 Hz, 1H), 4.62–4.52 (m, 2H), 3.87–3.83 (m, 1H), 3.80 (s, 6H), 3.74 (s, 1H), 3.57 (s, 3H), 3.39–3.28 (m, 3H), 3.23–3.17 (m, 1H), 3.10–3.02 (m, 1H), 2.83 (d, J = 15.0 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 1H), 2.57 (d, J = 13.8 Hz, 1H), 2.48 (q, J = 9.5 Hz, 1H), 2.35 (d, J = 14.6 Hz, 1H), 2.28 (d, J = 9.8 Hz, 1H), 2.21–2.16 (m, 1H), 2.11 (s, 3H), 1.99 (s, 1H), 1.45–1.32 (m, 1H), 1.86–1.70 (m, 8H), 1.27–1.22 (m, 2H), 0.80 (t, J = 6.8 Hz, 3H), 0.73 (t, J = 7.1 Hz, 3H); IR (film) νmax 3375, 2925, 1737, 1503, 1459, 1230, 1039 cm−1; HRESI-TOF m/z 944.4913 (C53H65N7O9 + H+, required 944.4916); [α]D23 +2.7 (c 0.2, CHCl3).
(R = 20'-NHCONHCH2(2-furyl)) 34
Yield: 96%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.83 (br s, 1H), 7.98 (br s, 1H), 7.50 (d, J = 8.2 Hz, 1H), 7.35 (s, 1H), 7.17–7.08 (m, 3H), 6.66 (s, 1H), 6.31 (d, J = 9.0 Hz, 2H), 6.21 (br s, 1H) 6.09 (s, 1H), 5.86 (dd, J = 9.8, 4.7 Hz, 1H), 5.47 (s, 1H), 5.30 (d, J = 10.0 Hz, 1H), 4.40–4.37 (m, 2H), 3.80 (s, 6H), 3.74 (s, 1H), 3.59 (s, 3H), 3.40–3.28 (m, 2H), 3.26–3.20 (m, 1H), 3.22–3.18 (m, 1H), 3.10–3.00 (m, 3H), 2.83 (d, J = 17.3 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 1H), 2.55 (d, J = 14.2 Hz, 1H), 2.47–2.42 (m, 1H), 2.35 (d, J = 12.8 Hz, 1H), 2.25–2.15 (m, 1H), 2.11 (s, 3H), 2.02 (s, 1H), 1.85–1.76 (m, 3H), 1.71–1.65 (m, 4H), 1.37–1.20 (m, 4H), 0.81 (t, J = 7.4 Hz, 3H), 0.72 (t, J = 7.5 Hz, 3H); IR (film) νmax 3388, 2925, 1739, 1504, 1459, 1230, 1039 cm−1; HRESI-TOF m/z 933.4736 (C52H64N6O10 + H+, required 933.4756); [α]D23 +11 (c 0.1, CHCl3).
(R = 20'-NHCONH(4-biphenyl)) 35
Yield: 44%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.82 (br s, 1H), 7.98 (br s, 1H), 7.58–7.51 (m, 6H), 7.45 (d, J = 8.0 Hz, 1H), 7.41 (t, J = 7.6 Hz, 2H), 7.31 (t, J = 7.4 Hz, 1H), 7.17–7.13 (m, 1H), 7.13–7.06 (m, 2H), 6.64 (s, 1H), 6.09 (s, 1H), 5.86 (dd, J = 10.4, 4.8 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 10.2 Hz, 1H), 4.82 (br s, 1H), 3.82 (t, J = 13.8 Hz, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.75 (s, 1H), 3.61 (s, 3H), 3.39–3.36 (m, 2H), 3.32–3.28 (m, 1H), 3.19 (t, J = 11.4 Hz, 1H), 3.14–3.06 (m, 1H), 3.02 (d, J = 12.5 Hz, 2H), 2.82 (d, J = 15.7 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 1H), 2.60 (d, J = 13.8 Hz, 2H), 2.47–2.42 (m, 1H), 2.35 (d, J = 13.0 Hz, 1H), 2.23 (d, J = 14.2 Hz, 1H), 2.21–2.14 (m, 1H), 2.11 (s, 3H), 1.82–1.77 (m, 4H), 1.73–1.65 (m, 4H), 1.37–1.34 (m, 1H), 0.82 (t, J = 7.4 Hz, 6H); IR (film) νmax 3444, 2967, 1735, 1523, 1459, 1240, 1039, 744 cm−1; HRESI-TOF m/z 1005.5122 (C59H68N6O9 + H+, required 1005.5121); [α]D23 −8.8 (c 0.1, CHCl3).
(R = 20'-NHCSNHC6H11) 36
Yield: 26%, Method 4. 1H NMR (600 MHz, CDCl3) δ 9.85 (br s, 1H), 8.03 (br s, 1H), 7.51 (d, J = 7.7 Hz, 1H), 7.16–7.14 (m, J = 7.0 Hz, 1H), 7.12–7.06 (m, 2H), 6.62 (s, 1H), 6.10 (s, 1H), 5.84 (dd, J = 10.1, 4.1 Hz, 1H), 5.47 (s, 2H), 5.35–5.33 (m, 1H), 5.29 (d, J = 9.1 Hz, 1H), 3.79 (s, 3H), 3.78 (s, 3H), 3.73 (s, 1H), 3.67–3.63 (m, 2H), 3.60 (s, 3H), 3.44 (s, 1H), 3.39–3.33 (m, 2H), 3.32–3.26 (m, 2H), 3.24–3.14 (s, 2H), 3.09 (q, J = 7.7 Hz, 1H), 2.81 (d, J = 14.7 Hz, 1H), 2.70 (s, 3H), 2.65 (s, 1H), 2.62–2.60 (m, 1H), 2.47–2.42 (m, 1H), 2.38–2.26 (m, 3H), 2.24–2.14 (m, 3H), 2.10 (s, 3H), 2.08–1.97 (m, 5H), 1.83–1.66 (m, 4H), 1.44–1.36 (m, 4H), 1.20–1.06 (m, 2H), 0.87 (t, J = 6.9 Hz, 3H), 0.81 (t, J = 6.9 Hz, 3H); IR (film) νmax 3467, 2912, 1727, 1506, 1456, 1230, 1087, 774 cm−1; HRESI-TOF m/z 951.5051 (C53H70N6O8S + H+, required 951.5048); [α]D23 +9.2 (c 0.2, CHCl3).
(R = 20'-NHCSNHC6H5) 37
Yield: 70%, Method 3. 1H NMR (500 MHz, CDCl3) δ 9.82 (br s, 1H), 8.09 (br s, 1H), 7.54 (m, 4H), 7.49 (d, J = 8.0 Hz, 1H), 7.40 (t, J = 7.2 Hz, 1H), 7.24–7.19 (m, 1H), 7.19–7.14 (m, 2H), 6.60 (s, 1H), 6.25 (s, 1H), 6.18 (s, 1H), 5.92 (dd, J = 10.0, 4.3 Hz, 1H), 5.56 (s, 1H), 5.37 (d, J = 10.0 Hz, 1H), 3.89 (s, 3H), 3.88 (s, 3H), 3.80 (s, 1H), 3.70 (s, 3H), 3.49–3.32 (m, 4H), 3.17–3.07 (m, 2H), 2.94–2.80 (m, 3H), 2.78 (s, 3H), 2.65–3.62 (s, 1H), 2.52–2.43 (m, 1H), 2.33–2.22 (m, 4H), 2.19 (s, 3H), 1.94–1.80 (m, 2H), 1.44–1.37 (m, 3H), 1.14 (d, J = 9.2 Hz, 1H), 0.88 (t, J = 6.3 Hz, 6H); IR (film) νmax 3449, 2930, 1737, 1498, 1458, 1231, 1039, 751 cm−1; HRESI-TOF m/z 945.4573 (C53H64N6O8S + H+, required 945.4579); [α]D23 +8.1 (c 0.2, CHCl3).
(R = 20'-NHCSNHCH2CH2(4-fluorophenyl)) 38
Yield: 91%, Method 4. 1H NMR (600 MHz, CDCl3) δ 9.83 (br s, 1H), 8.01 (br s, 1H), 7.51 (d, J = 8.8 Hz, 1H), 7.26–7.24 (m, 2H), 7.17–7.08 (m, 3H), 6.97–6.94 (m, 2H), 6.64 (s, 1H), 6.12 (s, 1H), 5.85 (dd, J = 10.1, 3.4 Hz, 1H), 5.75 (br s, 1H), 5.48 (s, 1H), 5.30 (d, J = 10.2 Hz, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.77–3.74 (m, 3H), 3.61 (s, 3H), 3.60 (s, 1H), 3.42–3.35 (m, 2H), 3.32–3.28 (m, 1H), 3.25–3.10 (m, 3H), 3.02–2.97 (m, 1H), 2.96–2.91 (m, 1H), 2.83–2.80 (m, 1H), 2.72 (s, 3H), 2.66 (s, 1H), 2.55 (d, J = 13.7 Hz, 1H), 2.47–2.42 (m, 1H), 2.32 (d, J = 13.8 Hz, 1H), 2.22–2.17 (m, 2H), 2.11 (s, 3H), 1.84–1.65 (m, 4H), 1.54–1.44 (m, 2H), 1.37–1.22 (m, 5H), 0.82 (t, J = 7.2 Hz, 3H), 0.74 (t, J = 7.4 Hz, 3H); IR (film) νmax 3468, 2961, 1737, 1507, 1461, 1226, 1038 cm−1; HRESI-TOF m/z 991.4790 (C55H67N6O8S + H+, required 991.4798); [α]D23 +6.6 (c 0.2, CHCl3).
(R = 20'-NHCON(CH3)2) 39
Yield: 99%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.84 (br s, 1H), 7.98 (br s, 1H), 7.48 (d, J = 8.2 Hz, 1H), 7.17–7.13 (m, 1H), 7.12–7.07 (m, 2H), 6.62 (s, 1H), 6.09 (s, 1H), 5.86 (dd, J = 10.0, 5.1 Hz, 1H), 5.46 (s, 1H), 5.30 (d, J = 10.3 Hz, 1H), 4.68 (s, 1H), 3.90 (t, J = 14.7 Hz, 1H), 3.79 (s, 3H), 3.79 (s, 3H), 3.73 (s, 1H), 3.58 (s, 3H), 3.38–3.35 (m, 2H), 3.31–3.27 (m, 2H), 3.23–3.19 (m, 2H), 3.17–3.12 (m, 2H), 3.05 (s, 6H), 2.82 (d, J = 16.0 Hz, 1H), 2.70 (s, 3H), 2.67 (s, 1H), 2.58 (d, J = 13.9 Hz, 1H), 2.47–2.42 (m, 1H), 2.38 (d, J = 13.1 Hz, 1H), 2.23–2.14 (m, 2H), 2.10 (s, 1H), 1.87–1.74 (m, 4H), 1.69 (d, J = 13.9 Hz, 2H), 1.40–1.33 (m, 4H), 0.81 (t, J = 7.4 Hz, 3H), 0.75 (t, J = 7.4 Hz, 3H); IR (film) νmax 3472, 2955, 1723, 1500, 1459, 1258, 1038, 777 cm−1; HRESI-TOF m/z 881.4790 (C49H64N6O9 + H+, required 881.4808); [α]D23 −50 (c 0.04, CHCl3).
(R = 20'-NHCON(CH2CH3)2) 40
Yield: 88%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.87 (br s, 1H), 8.02 (br s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.18–7.13 (m, 1H), 7.13–7.07 (m, 2H), 6.62 (s, 1H), 6.10 (s, 1H), 5.85 (dd, J = 10.2, 4.1 Hz, 1H), 5.47 (s, 1H), 5.30 (d, J = 10.2 Hz, 1H), 4.35 (br s, 1H), 3.85 (t, J = 12.7 Hz, 2H), 3.79 (s, 6H), 3.74 (s, 1H), 3.59 (s, 3H), 3.51–3.45 (m, 1H), 3.40–3.20 (m, 3H), 3.17–3.12 (m, 1H), 2.83 (d, J = 16.2 Hz, 1H), 2.71 (s, 7H), 2.58 (d, J = 13.7 Hz, 1H), 2.47–2.42 (m, 1H), 2.39 (d, J = 12.7 Hz, 1H), 2.23 (d, J = 14.4 Hz, 1H), 2.20–2.14 (m, 1H), 2.11 (s, 3H), 2.08 (s, 1H), 1.88–1.67 (m, 4H), 1.37–1.32 (m, 1H), 1.29–1.25 (m, 4H), 1.23 (t, J = 7.1 Hz, 6H), 0.81 (t, J = 7.3 Hz, 3H), 0.75 (t, J = 7.4 Hz, 3H); IR (film) νmax 3486, 2940, 1741, 1520, 1458, 1233, 1042, 709 cm−1; HRESI-TOF m/z 909.5130 (C51H68N6O9 + H+, required 909.5121); [α]D23 −38 (c 0.08, CHCl3).
(R = 20'-NHCO-(morpholine)) 41
Yield: 99%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.84 (br s, 1H), 8.06 (s, 1H), 7.99 (br s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.19–7.14 (m, 1H), 7.13–7.07 (m, 2H), 6.62 (s, 1H), 6.10 (s, 1H), 5.85 (dd, J = 10.1, 4.1 Hz, 1H), 5.47 (s, 1H), 5.30 (d, J = 10.1 Hz, 1H), 4.48 (s, 1H), 3.85 (d, J = 10.9 Hz, 3H), 3.80 (s, 3H), 3.80 (s, 3H), 3.79–3.77 (m, 1H), 3.77–3.64 (m, 11H), 3.62–3.56 (m, 4H), 3.48–3.44 (m, 1H), 3.38–3.36 (m, 1H), 3.33–3.05 (m, 2H), 2.83 (d, J = 16.1 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 1H), 2.60 (d, J = 13.6 Hz, 1H), 2.47–2.42 (m, 1H), 2.40 (d, J = 12.5 Hz, 1H), 2.29–2.22 (m, 2H), 2.20–2.15 (m, 2H), 2.11 (s, 3H), 1.90–1.74 (m, 2H), 1.70–1.67 (m, 2H), 1.37–1.32 (m, 2H), 0.81 (t, J = 7.3 Hz, 3H), 0.76 (t, J = 7.5 Hz, 3H); IR (film) νmax 3448, 2919, 1740, 1538, 1449, 1247, 1032, 711 cm−1; HRESI-TOF m/z 923.4897 (C51H66N6O10 + H+, required 923.4913); [α]D23 −8.0 (c 0.1, CHCl3).
(R = 20'-NHCO-(piperidine)) 42
Yield: 34%, Method 2. 1H NMR (600 MHz, CDCl3) δ 9.85 (br s, 1H), 8.02 (br s, 1H), 7.50 (d, J = 7.4 Hz, 1H), 7.17–7.14 (m, 1H), 7.11–7.09 (m, 2H), 6.59 (s, 1H), 6.09 (s, 1H), 5.84 (dd, J = 10.1, 4.1 Hz, 1H), 5.45 (s, 1H), 5.29 (d, J = 10.4 Hz, 1H), 4.45 (s, 1H), 3.82 (t, J = 14.7 Hz, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.73 (s, 1H), 3.59 (s, 3H), 3.52–3.48 (m, 2H), 3.43–3.40 (m, 2H), 3.38–3.34 (m, 2H), 3.31–3.26 (m, 4H), 3.18–3.12 (m, 2H), 2.82 (d, J = 16.6 Hz, 1H), 2.70 (s, 3H), 2.66 (s, 1H), 2.47–2.38 (m, 4H), 2.28–2.20 (m, 2H), 2.18–2.14 (m, 2H), 2.10 (s, 3H), 2.07 (s, 1H), 1.90–1.76 (m, 4H), 1.69 (s, 1H), 1.35–1.27 (m, 5H), 0.80 (t, J = 7.3 Hz, 3H), 0.75 (t, J = 7.3 Hz, 3H); IR (film) νmax 3458, 2958, 1791, 1509, 1466, 1251, 1061, 737 cm−1; HRESI-TOF m/z 921.5103 (C52H68N6O9 + H+, required 921.5121); [α]D23 −9.2 (c 0.02, CHCl3).
(R = 20'-NHCSN(CH3)2) 43
Yield: 71%, Method 4. 1H NMR (600 MHz, CDCl3) δ 9.83 (br s, 1H), 7.98 (br s, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.18–7.13 (m, 1H), 7.12–7.08 (m, 2H), 6.64 (d, J = 3.9 Hz, 1H), 6.10 (s, 1H), 5.86 (dd, J = 10.0, 5.1 Hz, 1H), 5.54 (s, 1H), 5.47 (s, 1H), 5.31 (d, J = 10.0 Hz, 1H), 4.70–4.67 (m, 1H), 3.81 (s, 3H), 3.80 (s, 3H), 3.75 (s, 1H), 3.59 (s, 3H), 3.44 (s, 6H), 3.38 (d, J = 12.2 Hz, 2H), 3.31 (d, J = 4.7 Hz, 1H), 3.27–3.15 (m, 3H), 3.14–3.03 (m, 2H), 2.83 (d, J = 15.2 Hz, 1H), 2.72 (s, 3H), 2.70 (s, 1H), 2.67 (s, 1H), 2.54 (d, J = 13.7 Hz, 1H), 2.48–2.43 (m, 1H), 2.38 (d, J = 14.1 Hz, 1H), 2.23–2.16 (m, 2H), 2.11 (s, 3H), 1.96 (d, J = 15.2 Hz, 1H), 1.85–1.74 (m, 2H), 1.38–1.29 (m, 4H), 0.81 (t, J = 7.4 Hz, 3H), 0.75 (t, J = 7.4 Hz, 3H); IR (film) νmax 3458, 2963, 1728, 1536, 1474, 1235, 1061, 737 cm−1; HRESI-TOF m/z 897.4559 (C49H64N6O8S + H+, required 897.4579); [α]D23 −56 (c 0.07, CHCl3).
(R = 20'-OCON(CH3)2) 44
Yield: 88%. 1H NMR (600 MHz, CDCl3) δ 9.86 (br s, 1H), 8.02 (br s, 1H), 7.50 (d, J = 7.8 Hz, 1H), 7.17–7.12 (m, 1H), 7.12–7.07 (m, 2H), 6.61 (s, 1H), 6.09 (s, 1H), 5.84 (dd, J = 10.2, 4.1 Hz, 1H), 5.46 (s, 1H), 5.29 (d, J = 10.0 Hz, 1H), 4.34 (s, 1H), 3.84 (t, J = 12.7 Hz, 1H), 3.79 (s, 6H), 3.74 (s, 1H), 3.58 (s, 3H), 3.47 (dd, J = 14.6, 7.2 Hz, 2H), 3.40–3.19 (m, 4H), 3.16–3.12 (m, 2H), 2.82 (d, J = 16.2 Hz, 1H), 2.70 (s, 9H), 2.66 (s, 1H), 2.57 (d, J = 13.7 Hz, 1H), 2.46–2.41 (m, 1H), 2.38 (d, J = 12.7 Hz, 1H), 2.22 (d, J = 14.4 Hz, 1H), 2.20–2.13 (m, 1H), 2.10 (s, 3H), 2.07 (s, 1H), 1.86–1.76 (m, 2H), 1.72 (d, J = 14.5 Hz, 2H), 1.38–1.30 (m, 2H), 0.80 (t, J = 7.3 Hz, 3H), 0.75 (t, J = 7.4 Hz, 3H); IR (film) νmax 3472, 2969, 1731, 1539, 1459, 1224, 1048, 740 cm−1; HRESI-TOF m/z 882.4642 (C49H63N5O10 + H+, required 882.4648); [α]D23 −64 (c 0.03, CHCl3).
(R = 20'-NHCH3) 45
A solution 20'-aminovinblastine (8.8 mg, 0.011 mmol) in THF (3 mL) was treated with a 37% formaldehyde in water solution (4 µL, 0.05 mmol). The reaction mixture was stirred for 4 h at 25 °C and then was treated with sodium cyanoborohydride (12 mg, 0.20 mmol). The reaction mixture was stirred for 1 h at 25 °C and then was quenched with distilled H2O (3 mL). The mixture was extracted with 10% MeOH in CH2Cl2, and the combined organic extracts were washed with saturated aqueous NaCl (3 mL). The organic layer was dried over Na2SO4, and concentrated under reduced pressure. PTLC (SiO2, EtOAc:MeOH:Et3N = 97:3:3) provided 45 (2.8 mg, 32%, off white solid) and 46 (2.9 mg, 33%, off white solid). For 45: 1H NMR (600 MHz, CDCl3) δ 9.87 (br s, 1H), 8.01 (br s, 1H), 7.51 (d, J = 7.5 Hz, 1H), 7.18–7.14 (m, 1H), 7.11–7.08 (m, 2H), 6.58 (br s, 1H), 6.09 (s, 1H), 5.85 (dd, J = 9.9, 4.0 Hz, 1H), 5.46 (s, 1H), 5.30 (d, J = 9.8 Hz, 1H), 4.07–3.91 (m, 2H), 3.80 (s, 3H), 3.79 (s, 3H), 3.73 (s, 1H), 3.61 (s, 3H), 3.36 (dd, J = 16.3, 4.6 Hz, 1H), 3.31–3.26 (m, 1H), 3.12–3.04 (m, 2H), 2.83 (d, J = 16.1 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 3H), 2.46–2.41 (m, 1H), 2.37–2.31 (m, 1H), 2.31–2.20 (m, 1H), 2.17–2.13 (m, 1H), 2.11 (s, 3H), 1.87–1.73 (m, 2H), 1.18–1.08 (m, 2H), 0.81 (t, J = 7.4 Hz, 3H), 0.76 (t, J = 7.4 Hz, 3H); IR (film) νmax 3487, 2953, 1729, 1505, 1461, 1239, 1037, 735 cm−1; HRESI-TOF m/z 824.4576 (C47H61N5O8 + H+, required 824.4593); [α]D23 −114 (c 0.03, CHCl3).
(R = 20'-N(CH3)2) 46
1H NMR (600 MHz, CDCl3) δ 9.89 (br s, 1H), 7.98 (br s, 1H), 7.52 (d, J = 7.7 Hz, 1H), 7.17–7.11 (m, 3H), 6.57 (br s, 1H), 6.10 (s, 1H), 5.85 (dd, J = 9.5, 4.3 Hz, 1H), 5.46 (s, 1H), 5.30 (d, J = 10.1 Hz, 1H), 3.97–3.92 (m, 1H), 3.80 (s, 3H), 3.79 (s, 3H), 3.73 (s, 1H), 3.61 (s, 3H), 3.38–3.27 (m, 4H), 3.20 (d, J = 13.8 Hz, 1H), 3.17–3.12 (m, 1H), 2.84 (d, J = 16.2 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 3H), 2.46–2.41 (m, 1H), 2.38 (s, 6H), 2.31–2.29 (m, 1H), 2.18–2.14 (m, 1H), 2.11 (s, 3H), 1.87–1.73 (m, 6H), 1.49–1.42 (m, 2H), 1.36–1.31 (m, 2H), 0.83–0.78 (m, 6H); IR (film) νmax 3404, 2951, 1734, 1501, 1459, 1227, 1037, 731 cm−1; HRESI-TOF m/z 838.4752 (C48H63N5O8 + H+, required 838.4749); [α]D23 +16 (c 0.01, CHCl3).
(R = 20'-NMeCONHCH2CH3) 47
Yield: 95%, Method 1. 1H NMR (600 MHz, CDCl3) δ 9.83 (br s, 1H), 7.95 (br s, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.16–7.09 (m, 3H), 6.66 (s, 1H), 6.08 (s, 1H), 5.86 (dd, J = 9.9, 3.9 Hz, 1H), 5.48 (s, 1H), 5.31 (d, J = 10.6 Hz, 1H), 4.54 (br s, 1H), 4.29 (br s, 1H), 3.80 (s, 3H), 3.77 (s, 3H), 3.74 (s, 1H), 3.70–3.65 (m, 1H), 3.57 (s, 3H), 3.43–3.37 (m, 2H), 3.33–3.18 (m, 5H), ), 3.05–3.02 (m, 2H), 2.98 (br s, 3H), 2.82 (d, J = 16.1 Hz, 1H), 2.71 (s, 3H), 2.67 (s, 1H), 2.56 (d, J = 14.2 Hz, 1H), 2.47–2.43 (m, 1H), 2.36 (d, J = 11.6 Hz, 1H), 2.24–2.16 (m, 2H), 2.11 (s, 3H), 1.87–1.75 (m, 3H), 1.38–1.32 (m, 5H), 1.19 (t, J = 7.2 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H), 0.79 (t, J = 7.3 Hz, 3H); IR (film) νmax 3462, 3400, 2926, 1735, 1504, 1460, 1243, 1039 cm−1; HRESI-TOF m/z 895.4956 (C50H66N6O9 + H+, required 895.4964); [α]D23 +17 (c 0.1, CHCl3).
Compound 49
Iron(III) chloride hexahydrate (55 mg, 0.21 mmol) was added to a solution of (−)-vindoline (19 mg, 0.041 mmol) and 10'-fluorocatharanthine9a (15 mg, 0.041 mmol) in CF3CH2OH (0.1 mL), aqueous 0.1 N HCl (1.0 mL) and H2O (1.0 mL) at 23 °C under Ar. The reaction mixture was stirred for 2 h at 23 °C. Meanwhile, in a separate flask, a mixture of iron(III) oxalate hexahydrate (198 mg, 0.41 mmol) in degassed H2O (40 mL) was cooled to 0 °C and placed under Ar. CsN3 (215 mg, 1.23 mmol) was added to the mixture at 0 °C, followed by the vindoline coupling solution and NaBH4 (31 mg, 0.81 mmol) in H2O (1 mL). The resulting mixture was stirred for 30 min before being quenched by addition of 28–30% aqueous NH4OH (4 mL). The mixture was extracted with 10% MeOH in CH2Cl2, the organic layer was dried over Na2SO4, and concentrated under reduced pressure. PTLC (SiO2, EtOAc:MeOH:Et3N = 97:3:3) provided 10'-fluoro-20'-azidovinblastine (50, 2.4 mg, 7%, white solid), and 10'-fluoro-20'-azidoleurosidine (6.0 mg, 17%, white solid). A solution of compound 50 (2.4 mg, 0.0028 mmol) in THF/H2O (1/1 mL) was treated with CoCl2• 6H2O (35 mg, 0.15 mmol) followed by NaBH4 (17 mg, 0.45 mmol). The reaction mixture was stirred for 2 h before being quenched with the addition of saturated NaHCO3 (1 mL). The mixture was extracted with 10% MeOH in CH2Cl2, and washed with saturated aqueous NaCl (1 mL). The organic layer was dried over Na2SO4, and concentrated under reduced pressure. PTLC (SiO2, EtOAc:MeOH = 90:10) provided 10'-fluoro-20'-aminovinblastine (51, 1.6 mg, 70%, white solid). A solution 51 (1.6 mg, 0.0019 mmol) in THF (3 mL) was treated with ethyl isocyanate (2 µL 0.025 mmol). The reaction mixture was stirred for 4 h at 25 °C and then was concentrated under reduced pressure. PTLC (SiO2, CH2Cl2:MeOH = 92:8) provided 49 (1.0 mg, 59%, white solid). 50: 1H NMR (600 MHz, CDCl3) δ 9.76 (br s, 1H), 8.00 (br s, 1H), 7.39 (dd, J = 8.8, 5.1 Hz, 1H), 6.86–6.82 (m, 1H), 6.76 (dd, J = 9.6, 2.4 Hz, 1H), 6.56 (s, 1H), 6.10 (s, 1H), 5.86 (dd, J = 10.3, 4.6 Hz, 1H), 5.48 (s, 1H), 5.29 (d, J = 10.2 Hz, 1H), 3.93 (t, J = 14.1 Hz, 1H), 3.82–3.76 (m, 1H), 3.80 (s, 6H), 3.72 (s, 1H), 3.63 (s, 3H), 3.40–3.33 (m, 2H), 3.30–3.25 (m, 2H), 3.13–3.07 (m, 1H), 2.95 (d, J = 14.4 Hz, 1H), 2.83–2.73 (m, 3H), 2.69 (s, 3H), 2.66 (s, 1H), 2.62 (s, 1H), 2.43–2.40 (m, 2H), 2.25 (d, J = 13.9 Hz, 1H), 2.18–2.14 (m, 1H), 2.10 (s, 3H), 2.07 (s, 1H), 1.85–1.77 (m, 2H), 1.63–1.52 (m, 2H), 1.47–1.41 (m, 2H), 0.93 (t, J = 7.5 Hz, 3H), 0.78 (t, J = 7.4 Hz, 3H); IR (film) νmax 2925, 2109, 1734, 1614, 1460, 1228, 1038 cm−1; HRESI-TOF m/z 854.4218 (C46H56FN7O8 + H+, required 854.4247); [α]D23 −6.0 (c 0.08, CHCl3). 51: 1H NMR (600 MHz, CDCl3) δ 9.81 (br s, 1H), 8.00 (br s, 1H), 7.40–7.37 (m, 1H), 6.89–6.85 (m, 1H), 6.78 (d, J = 9.2 Hz, 1H), 6.51 (s, 1H), 6.10 (s, 1H), 5.89 (dd, J = 10.1, 4.0 Hz, 1H), 5.45 (s, 1H), 5.31 (d, J = 10.1 Hz, 1H), 3.92 (t, J = 14 Hz, 1H), 3.82–3.77 (m, 1H), 3.81 (s, 6H), 3.74 (s, 1H), 3.63 (s, 3H), 3.38 (d, J = 16.0, 4.7 Hz, 2H), 3.32–3.27 (m, 1H), 3.10–3.05 (m, 2H), 2.84 (d, J = 15.7 Hz, 1H), 2.75–2.68 (m, 3H), 2.71 (s, 3H), 2.64 (s, 1H), 2.50–2.45 (m, 1H), 2.17 (d, J = 3.4 Hz, 1H), 2.11 (s, 3H), 2.08 (s, 1H), 2.05 (s, 1H), 1.87–1.75 (s, 4H), 1.42–1.15 (m, 6H), 0.88 (t, J = 7.4 Hz, 3H), 0.78 (t, J = 7.3 Hz, 3H); HRESI-TOF m/z 828.4311 (C46H58FN5O8 + H+, required 828.4311); [α]D23 −23 (c 0.10, CHCl3). 49: 1H NMR (600 MHz, CDCl3) δ 9.77 (br s, 1H), 7.95 (br s, 1H), 7.39 (dd, J = 8.8, 5.2 Hz, 1H), 6.86 (t, J = 9.4 Hz, 1H), 6.77 (d, J = 9.3 Hz, 1H), 6.58 (s, 1H), 6.09 (s, 1H), 5.88 (dd, J = 9.8, 4.6 Hz, 1H), 5.47 (s, 1H), 5.31 (d, J = 11.4 Hz, 1H), 4.41 (br s, 1H), 4.21 (br s, 1H), 3.84–3.77 (m, 2H), 3.80 (s, 6H), 3.75 (s, 1H), 3.61 (s, 3H), 3.40 (dd, J = 17.0, 5.2 Hz, 2H), 3.35–3.19 (m, 4H), 3.16–3.08 (m, 2H), 2.82 (d, J = 14.9 Hz, 1H), 2.71 (s, 3H), 2.65 (s, 1H), 2.57 (d, J = 14.1 Hz, 1H), 2.47–2.43 (m, 1H), 2.39 (d, J = 12.8 Hz, 1H), 2.22–2.17 (m, 2H), 2.11 (s, 3H), 1.87–1.75 (m, 4H), 1.69 (d, J = 14.6 Hz, 2H), 1.43–1.38 (m, 3H), 1.19 (t, J = 7.1 Hz, 3H), 0.79 (t, J = 7.4 Hz, 3H), 0.77 (t, J = 7.4 Hz, 3H); HRESI-TOF m/z 899.4713 (C49H63FN6O9 + H+, required 899.4713).
Cell growth inhibition assay
Compounds were tested for their cell growth inhibition of L1210 (ATCC #CCL-219, mouse lymphocytic leukemia, see Supporting Information) cells, HCT116 (ATCC #CCL-247, human colorectal carcinoma) cells, and HCT116/VM46 (a vinblastine-resistant strain of HCT116) cells in culture. A population of cells (> 1 × 106 cells/mL as determined with a hemocytometer) was diluted with an appropriate amount of Dulbecco-modified Eagle Medium (DMEM, Gibco) containing 10% fetal bovine serum (FBS, Gibco) to a final concentration of 30,000 cells/mL. To each well of a 96-well plate (Corning Costar), 100 µL of the cell-media solution was added with a multichannel pipette. The cultures were incubated at 37 °C in an atmosphere of 5% CO2 and 95% humidified air for 24 h. The test compounds were added to the plate as follows: test substances were diluted in DMSO to a concentration of 1 mM and 10-fold serial dilutions were performed on a separate 96-well plate. Fresh culture medium (100 µL) was added to each well of cells to constitute 200 µL of medium per well followed by 2 µL of each test agent. Compounds were tested in duplicate (n = 2–8 times) at six concentrations between 0–1,000 nM or 0–10,000 nM. Following addition, cultures were incubated for an additional 72 h.
A phosphatase assay was used to establish the IC50 values as follows: the media in each cell was removed and 100 µL of phosphatase solution (100 mg phosphatase substrate in 30 mL 0.1 M NaOAc, pH 5.5, 0.1% Triton X-100 buffer) was added to each well. The plates were incubated at 37 °C for either 5 min (L1210) or 15 min (HCT116 and HCT116/VM46). After the given incubation time, 50 µL of 0.1 N NaOH was added to each well and the absorption at 405 nm was determined using a 96 well plate reader. As the absorption is directly proportional to the number of living cells, the IC50 values were calculated and the reported values represent of the average of 4–16 determinations (SD ±10%).
Tubulin binding competition assay
The competitive displacement of 3H-vinblastine (obtained from Moravek Biochemicals, Inc.) from purified porcine tubulin (tubulin and general tubulin buffer obtained from Cytoskeleton, Inc.) was measured using a procedure previously described.19 As described, a 100 µL sample of tubulin solution diluted with 850 µL buffer was incubated with 25 μL of 7.2 × 10−5 M 3H-vinblastine for 15 min at 37 °C, after which 25 µL of 7.2 × 10−5 M unlabeled alkaloid was added and incubation continued for 60 min. Tubulin-bound 3H-vinblastine was adsorbed onto DEAE filter paper and counted directly. Millipore Steriflip filter units were used to wash the filter paper with buffer under mild suction.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the financial support of the National Institute of Health (CA042056, CA115526). K.K.D. is a Skaggs Fellow and T.J.B. is a NIH postdoctoral fellow (CA165303).
ABBREVIATIONS USED
- Pgp
P-glycoprotein
Footnotes
Supporting Information
Cytotoxic activity data for the L1210 cell line for all compounds reported. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) Review: Neuss N, Neuss MN. Therapeutic use of bisindole alkaloids from catharanthus. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. San Diego: Academic; 1990. pp. 229–240. (b) Review: Pearce HL. Medicinal chemistry of bisindole alkaloids from catharanthus. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. San Diego: Academic; 1990. pp. 145–204. (c) Review: Kuehne ME, Marko I. Syntheses of vinblastine-type alkaloids. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. San Diego: Academic; 1990. pp. 77–132.
- 2.Noble RL, Beer CT, Cutts JH. Role of chance observations in chemotherapy: Vinca rosea. Ann. N. Y. Acad. Sci. 1958;76:882–894. doi: 10.1111/j.1749-6632.1958.tb54906.x. [DOI] [PubMed] [Google Scholar]; (a) Noble RL. Catharanthus roseus (vinca rosea) - importance and value of a chance observation. Lloydia. 1964;27:280–281. [Google Scholar]; (b) Svoboda GH, Nuess N, Gorman M. Alkaloids of Vinca rosea Linn. (Catharanthus roseus G. Don.). V. Preparation and characterization of alkaloids. J. Am. Pharm. Assoc. Sci. Ed. 1959;48:659–666. doi: 10.1002/jps.3030481115. [DOI] [PubMed] [Google Scholar]
- 3.(a) Noble RL. Catharanthus roseus (vinca rosea) - importance and value of a chance observation. Lloydia. 1964;27:280–281. [Google Scholar]; (b) Svoboda GH, Nuess N, Gorman M. Alkaloids of Vinca rosea Linn. (Catharanthus roseus G. Don.). V. Preparation and characterization of alkaloids. J. Am. Pharm. Assoc. Sci. Ed. 1959;48:659–666. doi: 10.1002/jps.3030481115. [DOI] [PubMed] [Google Scholar]
- 4.(a) Fahy J. Modifications in the “upper” velbenamine part of the Vinca alkaloids have major implications for tubulin interacting activities. Curr. Pharm. Design. 2001;7:1181–1197. doi: 10.2174/1381612013397483. [DOI] [PubMed] [Google Scholar]; (b) Potier P. Synthesis of the antitumor dimeric indole alkaloids from catharanthus species (vinblastine group) J. Nat. Prod. 1980;43:72–86. [Google Scholar]; (c) Kutney JP. Plant cell culture combined with chemistry: a powerful route to complex natural products. Acc. Chem. Res. 1993;26:559–566. [Google Scholar]; (d) Miyazaki T, Yokoshima S, Simizu S, Osada H, Tokuyama H, Fukuyama T. Synthesis of (+)-vinblastine and its analogues. Org. Lett. 2007;9:4737–4740. doi: 10.1021/ol702040y. [DOI] [PubMed] [Google Scholar]
- 5.(a) Langlois N, Gueritte F, Langlois Y, Potier P. Application of a modification of the Polonovski reaction to the synthesis of vinblastine-type alkaloids. J. Am. Chem. Soc. 1976;98:7017–7024. doi: 10.1021/ja00438a046. [DOI] [PubMed] [Google Scholar]; (b) Kuehne ME, Matson PA, Bornmann WG. Enantioselective syntheses of vinblastine, leurosidine, vincovaline and 20’-epi-vincovaline. J. Org. Chem. 1991;56:513–528. [Google Scholar]; (c) Bornmann WG, Kuehne ME. A common intermediate providing syntheses of Ψ-tabersonine, coronaridine, iboxyphylline, ibophyllidine, vinamidine, and vinblastine. J. Org. Chem. 1992;57:1752–1760. [Google Scholar]; (d) Yokoshima S, Ueda T, Kobayashi S, Sato A, Kuboyama T, Tokuyama H, Fukuyama T. Stereocontrolled total synthesis of (+)-vinblastine. J. Am. Chem. Soc. 2002;124:2137–2139. doi: 10.1021/ja0177049. [DOI] [PubMed] [Google Scholar]; (e) Kuboyama T, Yokoshima S, Tokuyama H, Fukuyama T. Stereocontrolled total synthesis of (+)-vincristine. Proc. Natl. Acad. Sci. USA. 2004;101:11966–11970. doi: 10.1073/pnas.0401323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Ishikawa H, Colby DA, Boger DL. Direct coupling of catharanthine and vindoline to provide vinblastine: total synthesis of (+)- and ent-(−)-vinblastine. J. Am. Chem. Soc. 2008;130:420–421. doi: 10.1021/ja078192m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. J. Am. Chem. Soc. 2009;131:4904–4916. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Ishikawa H, Elliott GI, Velcicky J, Choi Y, Boger DL. Total synthesis of (−)- and ent-(+)-vindoline and related alkaloids. J. Am. Chem. Soc. 2006;128:10596–10612. doi: 10.1021/ja061256t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Elliott GI, Fuchs JR, Blagg BSJ, Ishikawa H, Tao H, Yuan Z, Boger DL. Intramolecular Diels–Alder/1,3-dipolar cycloaddition cascade of 1,3,4-oxadiazoles. J. Am. Chem. Soc. 2006;128:10589–10595. doi: 10.1021/ja0612549. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Choi Y, Ishikawa H, Velcicky J, Elliott GI, Miller MM, Boger DL. Total synthesis of (−)- and ent-(+)-vindoline. Org. Lett. 2005;7:4539–4542. doi: 10.1021/ol051975x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yuan Z, Ishikawa H, Boger DL. Total synthesis of natural (−)- and ent-(+)-4-desacetoxy-6,7-dihydrovindorosine and natural and ent-minovine: oxadiazole tandem intramolecular Diels–Alder/1,3-dipolar cycloaddition reaction. Org. Lett. 2005;7:741–744. doi: 10.1021/ol050017s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wilkie GD, Elliott GI, Blagg BSJ, Wolkenberg SE, Soenen DB, Miller MM, Pollack S, Boger DL. Intramolecular Diels–Alder and tandem intramolecular Diels–Alder/1,3-dipolar cycloaddition reactions of 1,3,4-oxadiazoles. J. Am. Chem. Soc. 2002;124:11292–11294. doi: 10.1021/ja027533n. [DOI] [PubMed] [Google Scholar]
- 8.(a) Va P, Campbell EL, Robertson WM, Boger DL. Total synthesis and evaluation of a key series of C5-substituted vinblastine derivatives. J. Am. Chem. Soc. 2010;132:8489–8495. doi: 10.1021/ja1027748. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sasaki Y, Kato D, Boger DL. Asymmetric total synthesis of vindorosine, vindoline, and key vinblastine analogues. J. Am. Chem. Soc. 2010;132:13533–13544. doi: 10.1021/ja106284s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kato D, Sasaki Y, Boger DL. Asymmetric total synthesis of vindoline. J. Am. Chem. Soc. 2010;132:3685–3687. doi: 10.1021/ja910695e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tam A, Gotoh H, Robertson WM, Boger DL. Catharanthine C16 substituent effects on the biomimetic coupling with vindoline: preparation and evaluation of a key series of vinblastine analogues. Bioorg. Med. Chem. Lett. 2010;20:6408–6410. doi: 10.1016/j.bmcl.2010.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Gotoh H, Duncan KK, Robertson WM, Boger DL. 10'-Fluorovinblastine and 10'-fluorovincristine: synthesis of a key series of modified Vinca alkaloids. ACS Med. Chem. Lett. 2011;2:948–952. doi: 10.1021/ml200236a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gotoh H, Sears JE, Eschenmoser A, Boger DL. New insights into the mechanism and an expanded scope of the Fe(III)-mediated vinblastine coupling reaction. J. Am. Chem. Soc. 2012;134:13240–13243. doi: 10.1021/ja306229x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leggans EK, Barker TJ, Duncan KK, Boger DL. Iron(III)/NaBH4-mediated additions to unactivated alkenes: synthesis of novel 20'-vinblastine analogues. Org. Lett. 2012;14:1428–1431. doi: 10.1021/ol300173v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker TJ, Boger DL. Fe(III)/NaBH4-mediated free radical hydrofluorination of unactivated alkenes. J. Am. Chem. Soc. 2012;134:13588–13591. doi: 10.1021/ja3063716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boger DL, Brotherton CE. Total synthesis of azafluoranthine alkaloids: rufescine and imelutine. J. Org. Chem. 1984;49:4050–4055. [Google Scholar]
- 13.Borman LS, Kuehne ME. Functional hot spot at the C-20’ position of vinblastine. In: Brossi A, Suffness M, editors. The Alkaloids. Vol. 37. San Diego: Academic; 1990. pp. 133–144. [Google Scholar]
- 14.Gigant B, Wang C, Ravelli RBG, Roussi F, Steinmetz MO, Curmi PA, Sobel A, Knossow M. Structural basis for the regulation of tublin by vinblastine. Nature. 2005;435:519–522. doi: 10.1038/nature03566. [DOI] [PubMed] [Google Scholar]
- 15. Miller JC, Gutowski GE, Poore GA, Boder GB. Alkaloids of Vinca rosea L. (Catharanthus roseus G. Don). 38. 4'-dehydrated derivatives. J. Med. Chem. 1977;20:409–413. doi: 10.1021/jm00213a019. Miller JC, Gutowski G. Vinca alkaloid derivatives. 2753791. E. Ger Patent. (Chem. Abstr.1978, 89, 129778). Gerzon K, Miller JC. Oxazolidinedione sulfide compounds. 55602. Eur. Patent. (Chem. Abstr.1982, 97, 163310). (d) Review of superacid functionalization: Duflos A, Kruczynski A, Baret J-M. Novel aspects of natural and modified Vinca alkaloids. Curr. Med. Chem. Anti-Cancer Agents. 2002;2:55–75. doi: 10.2174/1568011023354452.
- 16.Lampidis TJ, Kolonias D, Podona T, Israel M, Safa AR, Lothstein L, Savaraj N, Tapiero H, Priebe W. Circumvention of P-gp MDR as a function of anthracycline lipophilicity and charge. Biochemistry. 1997;36:2679–2685. doi: 10.1021/bi9614489. [DOI] [PubMed] [Google Scholar]
- 17.Perego P, De Cesare M, De Isabella P, Carenini N, Beggiolin G, Pezzoni G, Palumbo M, Tartaglia L, Prtesi G, Pisano C, Carminati P, Scheffer GL, Zunino F. A novel 7-modified camptothecin analog overcomes breast cancer resistance protein-associated resistance in a mitoxantrone-selected colon carcinoma cell line. Cancer Res. 2001;61:6034–6037. [PubMed] [Google Scholar]
- 18.Hitchcock SA. Structural modifications that alter the P-glycoprotein efflux properties of compounds. J. Med. Chem. 2012;55:4877–4895. doi: 10.1021/jm201136z. [DOI] [PubMed] [Google Scholar]
- 19.Owellen RJ, Donigian DW, Hartke CA, Hains FO. Correlation of biologic data with physicochemical properties among the Vinca alkaloids and their congeners. Biochem. Pharmacol. 1977;26:1213–1219. doi: 10.1016/0006-2952(77)90108-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.