Abstract
Sperm competition represents an important component of post-copulatory sexual selection. It has been argued that the level of sperm competition declines in birds towards the equator. However, to date, sperm competition estimates have been available mainly for avian species inhabiting the northern temperate zone. Here we apply a novel approach, using the coefficient of between-male variation (CVbm) in sperm size as an index for sperm competition risk, in a comparative analysis of 31 Afrotropical and 99 northern temperate zone passerine species. We found no difference in sperm competition risk between the two groups, nor any relationship with migration distance. However, a multivariate model indicated that sperm competition risk was highest in species with a combination of low body mass and few eggs per clutch. The effect of clutch size was most pronounced in tropical species, which indicates that sperm competition risk in tropical and temperate species is differently associated with particular life-history traits. Although tropical species had lower sperm competition risk than temperate zone species for overlapping clutch sizes, the idea of a generally reduced risk of sperm competition in tropical birds was not supported by our analysis.
Keywords: clutch size, extra-pair paternity, life history, post-copulatory sexual selection, sperm phenotype
1. Introduction
Female promiscuity is a prerequisite for sperm competition [1] and a possible mechanism promoting sexual selection in socially monogamous taxa [2–4]. In birds, sperm competition shows high interspecific variation, as indicated by the wide range in extra-pair paternity (EPP) rates (proxy measure of sperm competition) in pair-bonding species [5–7]. Unlike with other life-history and ecological traits [8,9], patterns of variation of avian EPP rates along the latitudinal gradient of environmental conditions remain poorly understood, particularly because adequate information is only available for temperate zone species of the Northern Hemisphere [10]. However, there is a prevailing assumption of low levels of sperm competition in tropical species [10,11] that stems from known differences in life-history traits between tropical and temperate zone species [9]. For example, the ‘life history’ hypothesis of EPP predicts an inverse relationship between promiscuity and lifespan since males of species with ‘fast’ life histories (short lifespans) are expected to tolerate higher EPP rates than males of species with ‘slow’ life histories (long lifespans) [12,13]. Across birds, EPP rates indeed tend to be low in slowly reproducing long-living species [13,14], and most tropical birds are characterized by low adult mortality and reduced annual reproductive rates [9,15], associated with small clutch sizes [9] and a generally slow pace of life [16]. Furthermore, tropical birds do not migrate for long distances, whereas EPP rates have been suggested to increase with migration distance and associated effects of short breeding seasons, high breeding synchrony and rapid pair formation [17,18]. On the other hand, low demands for parental care seem to be associated with increased sperm competition in birds [14,19,20], and many tropical birds are characterized by reduced parental investments into a single breeding attempt [21–23].
Even after more than 20 years of extensive study on avian EPP [6,7], the supposition of low levels of sperm competition in tropical birds still continues to be a matter of theoretical reasoning rather than robust empirical evidence. In fact, rates of EPP have been published for fewer than 0.5 per cent of all bird species living in the tropical regions (approx. 5000 species) compared with more than 10 per cent of the approximately 1250 temperate zone species (reviewed in [24]). The strong ‘temperate zone bias’ (sensu [10]) in data available for comparative studies on EPP in birds precludes any rigorous comparison of sperm competition in the tropical and temperate zone birds. It may also lead to biased interpretations of the causes and correlates of sperm competition in birds [10]. The only study that contrasted temperate and tropical songbirds used relative testes mass as a proxy for sperm competition levels, and found some evidence for higher levels of sperm competition in North American than in Neotropical passerines [11].
Recently, another proxy for the risk of sperm competition has been suggested, based on the fact that sperm competition should have a profound effect on sperm morphology [25–27]. Intense sperm competition may lead to stabilizing selection on sperm phenotypes, and thus reduced variation in sperm size among males [25,28]. Theoretical models also predict lower variation in sperm dimensions within males under strong sperm competition levels [29,30]. In line with these predictions, comparative studies have shown a strong inverse relationship between sperm competition indices and coefficients of both between-male (CVbm) and within-male (CVwm) variation in sperm length in insects [31] and birds [32,33]. In contrast to sperm CVwm, the relationship between sperm CVbm and sperm competition risk does not show any phylogenetic bias (cf. [31,33]), which makes sperm CVbm a suitable index to predict levels of sperm competition in species for which such information is missing [27].
Here we test whether sperm competition is lower in tropical than temperate zone passerines using sperm CVbm as an index for sperm competition risk in a comparative analysis with phylogenetic control. We compared 31 species from tropical Africa (Cameroon and Nigeria) and 99 from the northern temperate zone (Europe and Canada). However, as tropical and temperate species may differ in various ecology and life-history traits previously reported to be associated with sperm competition in birds (see above; also [34,35]), we included four such traits (i.e. clutch size, body mass, migration distance and social mating system) as covariates in our analysis. We used data on clutch size and body mass as proxies for current reproductive investments [9,22] and the longevity/lifespan [36], respectively. From the ‘life history’ theory of EPP [12], we would expect high sperm competition risk to be associated with large clutches and small body size (i.e. ‘fast’ life histories). We furthermore predicted increased sperm competition risk in long-distance migrants [18], and in species with more polygynous social mating systems [14,34].
2. Material and methods
(a). Data collection
Sperm samples were collected in Cameroon (23 species) and Nigeria (8 species) in tropical west Africa, and in Canada (38 species), Czech Republic (10 species) and southern Norway (51 species) in the northern temperate zone (see the electronic supplementary material, table S1 for detailed list of species and sampling sites details). Sperm sampling took place during the breeding season in the respective areas (i.e. during April to July in 2006–2010 in the northern temperate zone, and during October to December 2008 and 2010 in Cameroon and during March to September 2010 in Nigeria). Sperm samples (approx. 0.5–3 µl) were obtained by gently massaging the cloacal protuberance, using a similar technique as described in [37], collected with a microcapillary, and diluted in 200 µl of approximately 5 per cent formalin solution for fixation. All birds were released immediately after sampling. Slides were prepared by spreading a drop (approx. 15 µl) of the fixed sperm sample on a clean microscope slide and air-dried. Using a digital camera (DFC420, Leica Microsystems, Heerbrugg, Switzerland) mounted onto a digital light microscope (DM6000 B, Leica Microsystems), we captured high-resolution digital images of individual spermatozoa at microscope magnifications of 200×, 320× or 400× (depending on the actual sperm length). Leica Application Suite (v. 2.6.0 R1) was used to measure total sperm length (±0.1 µm), from the anterior tip of the acrosome to the posterior tip of the flagellum, of 10 intact spermatozoa from each male. Measuring 10 spermatozoa provides a representative estimate of a male's mean sperm total length [38]. The repeatability (sensu [39]) of measurements of the total length of individual spermatozoa was high (r = 0.98) [38]. All measurements were performed by T.L. As a measure of sperm length variability, we calculated the coefficient of between-male variation in sperm length, and as coefficient of variation is likely to be underestimated for small sample sizes, we adjusted for this using the following equation: adjusted CV = s.d./mean × 100 × (1 + 1/4n−1) [40] (see also [38]). Numbers of males used to calculate sperm CVbm for each species are provided in the electronic supplementary material, table S1.
Information on body mass, mean clutch size and social mating system were obtained from the compendia by Cramp et al. [41], Fry et al. [42] and Ridgely & Tudor [43], as well as Birds of North America Online (BNA, http://bna.birds.cornell.edu/bna), and further cross-checked with published databases [34] and results of on-going field studies by U.O. for Psalidoprocne obscura. Given the scarcity of information about social mating system for tropical birds we only distinguished between predominantly ‘monogamous’ species with bi-parental care (though this group included species with occasional polygyny and variable forms of parental care) and species with little or no male parental care and strong (harem) polygyny (Agelaius, Euplectes, all Ploceus except P. bannermanni). The latter were considered ‘polygynous’ species in analyses. Even when applying these relaxed criteria, there were five tropical species with uncertain social mating status (treated as monogamous in the main analysis; see the electronic supplementary material, table S1). To evaluate the effect of mating system on sperm CVbm, we also ran the analysis with these five species excluded from the dataset.
Migration distance was estimated in Google Earth (http://earth.google.com) using the linear distances (rounded to intervals of 103 km) between the sperm sampling locality and the centre of winter distribution presented in compendia cited above ([41–43] and BNA). Moreover, the distance for each species in areas where sperm samples were obtained was checked using local migration atlases (e.g. Norway [44], Czech Republic [45]). Several species were partial migrants (i.e. part of the population migrates for short distances whereas some individuals are sedentary). In the analyses, migration distance for sedentary and/or short-distance/partial migratory species (migration distance < 500 km) was set to 0 km. Most African species are sedentary or seasonal short-distance migratory [42] (U.O., unpublished data), but data on migration habits are merely missing for most tropical species included in our analyses. However, it is unlikely that these tropical species would migrate for a distance of more than 500 km [42,46] (U.O., unpublished data). Hence, migration distance was set to the lowest category (0–500 km) for all tropical species.
(b). Statistical analyses
All analyses were performed in the statistical software R v. 2.13.1 [47] using the package APE [48]. To account for non-independence in the data due to shared ancestry among species, we applied a generalized least-squares (GLS) method in a phylogenetic framework (hereafter PGLS [49,50]; see the electronic supplementary material, figure S1 for phylogeny used) when testing for effects of predictor variables on sperm competition (using sperm CVbm as the response variable). Branch lengths were set to one, because the species tree was based on a consensus phylogeny. Through a maximum-likelihood framework, an index of phylogenetic association, λ, was estimated, with values ranging from 0 (indicating phylogenetic independence) to 1 (indicating complete phylogenetic dependence). Symbols following the λ estimate throughout the manuscript refer to likelihood-ratio tests against models with λ = 0 or λ = 1 (∗p < 0.05 and †p > 0.05, respectively). Prior to analysis, all continuous variables were checked for normality, and body mass and sperm CVbm were log10-transformed to achieve normal distribution. In multiple regression PGLS, the variables best associated with the dependent variable (sperm CVbm) were chosen by backward elimination of the full model, which also included interaction terms, until the minimal adequate model [51] was obtained. Changes in deviance, degrees of freedom and accompanied t-test after removing the term of interest from models were used to estimate the significance of that term during the process of model simplification. The variation in data explained by the minimal adequate model was compared with the variation associated with the null model using F-statistics. In cases when results suggested significant zone × covariate interaction, the difference in sperm CVbm between zones is reported (i) as a zone difference in intercepts of regressions between sperm CVbm and the covariate (i.e. at zero value of the covariate), and (ii) for overall covariates' mean (the input variable centred around its overall mean value; i.e. by setting the mean as 0, and expressing other values as the positive or negative difference from that mean value; see [52] for further details).
3. Results
(a). Sperm competition in tropical and temperate species
Controlling for common ancestry, we found no evidence for a difference in sperm competition risk between tropical and temperate passerine species (PGLS: F1,129 = 0.06, p = 0.81, r2 = 0.00, λ = 0.40*,*, figure 1). Moreover, the observed sperm CVbm values showed largely the same range of variation in both regions (figure 1). The full list of sperm CVbm values (along with associated estimates of EPP rate for the species) is listed in the electronic supplementary material, table S1. It is notable that in some tropical families sperm CVbm values were either generally high (e.g. Estrildidae) or generally low (e.g. Nectariniidae), indicating low and high risk of sperm competition, respectively, among related species. In other families, such as Ploceidae, our data indicated relatively large contrasts in sperm competition risk among related species.
Figure 1.
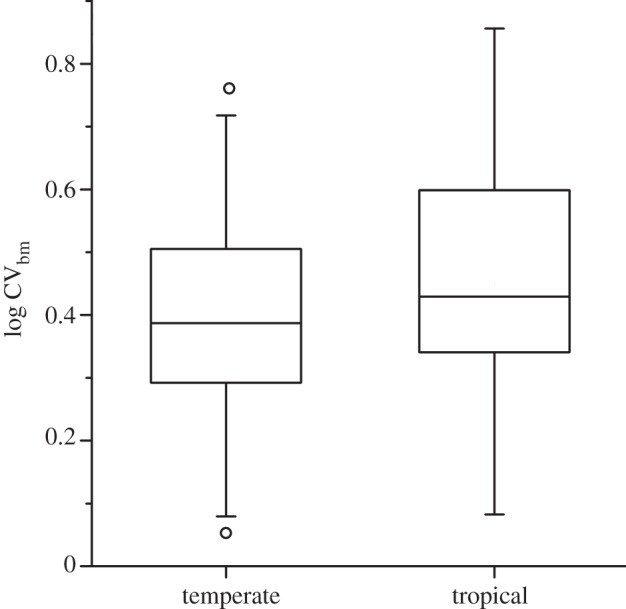
Sperm length variation in tropical and temperate zone passerines. The figure shows coefficients of between-male variation in sperm total length (log CVbm) estimates (median, 25 and 75% quartiles) for 31 Afrotropical (tropical) and 99 temperate zone (temperate) passerine species (see electronic supplementary material, figure S1 and table S1 for further details).
(b). Sperm competition and life-history variables
For the combined dataset of 130 species, univariate models identified only one variable that explained a significant amount of variation in sperm competition risk across species, namely the social mating system. Polygynous species (n = 6) had significantly higher sperm competition risk than monogamous species (PGLS: F1,129 = 4.84, p = 0.03, r2 = 0.04, λ = 0.43*,*). Excluding five tropical species with uncertain information about the mating system gave the same conclusion (PGLS: F1,124 = 4.17, p = 0.043, r2 = 0.033, λ = 0.39*,*). The non-significant univariate test results for migration distance, body mass and clutch size are given in electronic supplementary material, table S2. Nevertheless, as there is potential for interaction effects among several of these variables, including different life-history patterns between temperate and tropical species, and various life-history traits that might be inter-correlated, we also ran a multivariate PGLS analysis of all variables. The minimal adequate model revealed several significant partial effects on sperm competition risk (table 1; λ = 0.17†,*), explained 14 per cent of the variation in sperm competition risk across all species and did not include migration distance (addition of migration distance to the minimal adequate model did not improve the fit: t = −0.02, Δd.f. = 1, p = 0.98, Δr2 < 0.01). The effect of social mating system was upheld, and in addition there was a significant effect of body mass, and a significant interaction term between clutch size and climatic zone (table 1). Temperate species had significantly larger clutches than tropical species (5.09 versus 2.74 eggs; F1,129 = 107.51, p < 0.001, r2 = 0.46, λ = 0.99*,†), and the significant interaction term implies that the two climatic zones have different slopes for the relationship between clutch size and sperm competition risk. The difference between zones in sperm CVbm increased with increasing clutch size: sperm competition risk was predicted the same for temperate and tropical species based on intercepts (for clutch size = 0 the difference in intercepts is −0.22 ± 0.21 (s.e.); table 1), but the difference was 0.55 ± 0.16 when the clutch size was centred around the overall mean (4.52 eggs). The patterns are illustrated by scatter plots of species data points in figure 2. A separate PGLS on tropical species revealed that only clutch size was a significant predictor of sperm CVbm (F1,30 = 9.04, p = 0.005, r2 = 0.24, λ = 0.0†,*, slope: 0.20 ± 0.067; figure 2), suggesting reduced sperm competition risk in tropical species that lay relatively large clutches. No such clear trend was evident among temperate species (F1,98 = 0.50, p = 0.48, r2 = 0.005, λ = 0.31*,*, slope: 0.02 ± 0.022), and this analysis also failed to find any effect of migration and body mass on sperm competition risk (the significance of full model involving body mass, clutch size and migration distance: F3,96 = 1.21, p = 0.31, r2 = 0.04, λ = 0.24*,*; partial effects of explanatory variables all p > 0.10).
Table 1.
The overview and parameter estimates for the minimal adequate model resulting from backward selection of model evaluating the effects of life-history correlates (clutch size and body mass), migration distance and climate zone (tropical and temperate) on the coefficient of between-male variation in sperm length (log CVbm) in 130 passerine species. Higher values of CVbm indicate lover levels of sperm competition. For details see §2.
| term | estimate | s.e. | t-value | p-value |
|---|---|---|---|---|
| (intercept) | 0.263 | 0.244 | 1.08 | 0.283 |
| zonea | −0.223 | 0.212 | −1.05 | 0.294 |
| log(body mass) | 0.157 | 0.059 | 2.66 | 0.009 |
| mating systemb | −0.346 | 0.169 | −2.05 | 0.042 |
| clutch size | 0.038 | 0.023 | 1.67 | 0.097 |
| clutch size : zone | 0.171 | 0.066 | 2.59 | 0.011 |
a1, temperate; 2, tropical.
b1, monogamous; 2, polygynous.
Figure 2.
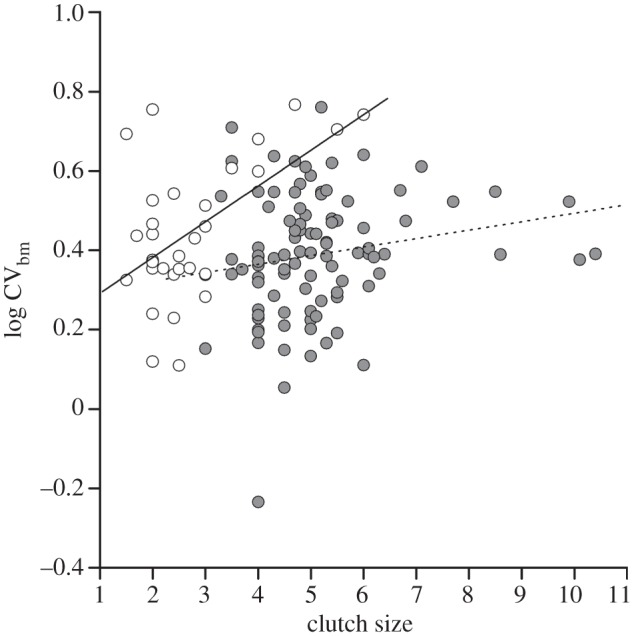
Relationship between clutch size and coefficient of between-male variation in sperm total length (CVbm) for 31 Afrotropical (open circles) and 99 temperate zone (filled circles) passerine species. The association between clutch size and CVbm is significant in tropical species only (see §3 for further details) and is indicated by a solid line. Plotted points are species averages, and relationships are not controlled for phylogeny. Higher values of CVbm indicate lower values of EPP, and hence sperm competition (see the main text for further details).
4. Discussion
We found no evidence supporting the view of generally lower sperm competition risk in tropical birds than in temperate zone birds. Our results indicate that Afrotropical species exhibit the same wide range in sperm competition risk as documented for temperate species. However, we found some evidence that the covariation between sperm competition and certain life-history traits, in particular clutch size, may differ between zones, although the combined effects of various predictor variables explained only 14 per cent of the variation in sperm competition risk across all species. This means that most of the variation in sperm competition risk among passerine bird species is still not accounted for, and the enigma also exists for tropical species.
Our findings cast doubt on a long-standing view of the association between climatic zone (latitude) and sexual promiscuity in birds [10,18,23], and contrast with results of previous studies that found smaller relative testis size in tropical birds [11] or a general decrease in EPP (another proxy measure of sperm competition) with decreasing latitude [18]. However, the general supposition of low intensity of sperm competition in the tropics seems to have been drawn from rather weak and indirect evidence. First, some authors [11] only used samples of testes from museum collections, which may have provided biased estimates of real testis size if inferred from preserved old material (see [53] for discussion), and used in several cases just one individual per species. Similarly, another study [18] used a rather heterogeneous sample of passerine and non-passerine species in which most tropical passerines were represented by island populations, characterized by low sperm competition levels [54]. Our analysis is based on a relatively high number of tropical species and a proxy for sperm competition risk that is closely associated with EPP frequencies in passerine species [25,27,33]. Our results support the view that tropical avian species exhibit variable levels of sperm competition, similar to that observed among temperate species. Recently, this view has been supported by data from 13 tropical species with published EPP rates [24], although the confidence intervals of the estimates of EPP are large due to generally low sample sizes (sensu [6]).
The lack of an overall difference in the sperm competition risk between tropical and temperate birds is surprising given that tropical birds differ from temperate birds in many aspects, some of which traditionally provide proximate (levels of testosterone [55]; breeding density and synchrony [10]) or ultimate (longevity [9,15]) explanations for variation in sperm competition levels in avian populations (see also [6,7,12]). However, multivariate PGLS modelling provided a more complex picture. First, the results confirmed a previous finding of comparatively high levels of sperm competition in polygynous species with reduced parental care [14,34]. The model has also indicated a decrease in sperm competition levels in large-bodied species, a pattern already demonstrated in passerines [56]. This finding seems to be in congruence with Mauck et al.'s [12] idea of low EPP rates in species with long lifespans, assuming that body mass is in general a good proxy for lifespan in passerines [36]. It should be noted, however, that many tropical passerines have been found to live longer than their temperate zone counterparts of the same body mass [9,15], and body mass itself was unlikely to explain variation in sperm competition risk between zones. Unfortunately, exact data on lifespans are unavailable for most species included in our analysis. On the other hand, our finding of an inverse relationship between clutch size and sperm competition risk in tropical birds was contrary to expectations of the ‘life history’ hypothesis of EPP (see also [12–14]). It could be compatible with a general pattern of higher sperm competition risk in avian species with reduced male parental care [14], provided that tropical species with small clutches exhibit reduced male parental care. Reduced reproductive value of a single breeding attempt [9,21] may also imply reduced potential for direct selection against female promiscuity (sensu [57,58]) in tropical species. However, this may not apply to species with large clutch sizes in a climatic zone characterized by constant low abundance of food resources [59] and high predation rates [8,9]. We were unable to infer this possibility with our dataset, but the results indicate that tropical species with relatively large clutch sizes exhibit lower risk of sperm competition than temperate zone species of the same clutch size (figure 2). However, as we pointed out above, no overall difference in sperm competition risk between the tropical and temperate species was detectable.
It has been argued that migration is the most important predictor of the latitudinal trend of promiscuity [18], responsible for high sperm competition risk in temperate zone birds. Migratory behaviour, which characterizes many species at higher latitudes [16,60], may affect promiscuous behaviour through several pathways. First, migration could be associated with short breeding seasons, leading to hasty and inaccurate choice of social mate [17] or with condition-dependent arrival to the breeding grounds that allows for better assessment of social and potential extra-pair mates [18]. In addition, breeding synchrony [11] or individual genetic diversity [61] may increase with migration, thus promoting promiscuity [56,62] (but see [63]). Sedentary species may also keep the same territory and partners over multiple breeding seasons, which reduces potential for seeking extra-pair mates during the search and selection of new social mates [64]. However, we found no support for a relationship between migration distance and sperm competition in our sample of 99 temperate passerine species, nor in the total dataset involving tropical species. Altogether, our data indicate that migration does not contribute significantly to the difference in sperm competition risk between the climatic zones.
5. Conclusion
Sperm competition risk is likely to be a function of several evolutionary processes and complex ecological constraints [6,7,57]. On the one hand, variables such as low breeding densities, low breeding synchrony and low annual mortality rates that are characteristic for birds breeding in the tropical zone [9–11] may reduce the potential for sperm competition [6]. On the other hand, our data suggest that the covariation of sperm competition risk with clutch size differs between the zones (although the proximate mechanism for this difference remains unclear) and sperm competition risk (as inferred from sperm CVbm) of tropical passerines with clutch sizes typical for that climate zone is the same as that of temperate zone species. Exploring the variation in sperm competition risk in the entire variety of avian phylogenetic lineages in tropical regions may help to examine the generality of conclusions about the ecology and life-history correlates of the intensity of post-copulatory selection in birds. Assessing these effects on broader spatial scales in future comparative studies is encouraged, but requires more detailed information about the breeding biology and ecology of tropical birds [9,65].
Acknowledgements
We are grateful to all who assisted us in the fieldwork in Cameroon, Canada, the Czech Republic and Norway. Ernest Vunan, Lars Erik Johannessen, Silje Hogner and Martin Mikes deserve special thanks. The research was conducted in adherence with the Norwegian and Czech regulations for the use of animals in research, and approved by the Canadian Wildlife Service (permit no. CA 0155), Queen's University Animal Care Committee (protocol no. Robertson-2005-014-R1) and Cameroonian Ministry of Science and Innovation (permit no. 124/MINRESI/B00/C00/C10/C13). The study was funded by the Czech Science Foundation (project nos. P505/11/1617 and P506/12/2472), the Research Council of Norway (project nos. 170853 and 196554), and the Natural Sciences and Engineering Research Council of Canada. T.A. was partially supported by the Academy of Sciences of the Czech Republic (project no. M200930971). This is contribution no. 57 from A. P. Leventis Ornithological Research Institute.
References
- 1.Birkhead TR, Møller AP. 1998. Sperm competition and sexual selection. London, UK: Academic Press [Google Scholar]
- 2.Albrecht T, Vinkler M, Schnitzer J, Polakova R, Munclinger P, Bryja J. 2009. Extra-pair fertilizations contribute to selection on secondary male ornamentation in a socially monogamous passerine. J. Evol. Biol. 22, 2020–2030 10.1111/j.1420-9101.2009.01815.x (doi:10.1111/j.1420-9101.2009.01815.x) [DOI] [PubMed] [Google Scholar]
- 3.Webster M, Pruett-Jones S, Westneat D, Arnold S. 1995. Measuring the effects of pairing success, extra-pair copulations and mate quality on the opportunity for sexual selection. Evolution 49, 1147–1157 10.2307/2410439 (doi:10.2307/2410439) [DOI] [PubMed] [Google Scholar]
- 4.Pizzari T, Parker GA. 2006. Sperm competition and sperm phenotype. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, Pitnick S.), pp. 207–245 Oxford, UK: Academic Press [Google Scholar]
- 5.Birkhead TR, Møller AP. 1992. Sperm competition in birds: evolutionary causes and consequences. London, UK: Academic Press [Google Scholar]
- 6.Griffith S, Owens IPF, Thuman K. 2002. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 11, 2195–2212 10.1046/j.1365-294X.2002.01613.x (doi:10.1046/j.1365-294X.2002.01613.x) [DOI] [PubMed] [Google Scholar]
- 7.Westneat D, Stewart I. 2003. Extra-pair paternity in birds: causes, correlates, and conflict. Annu. Rev. Ecol. Evol. Syst. 34, 365–396 10.1146/annurev.ecolsys.34.011802.132439 (doi:10.1146/annurev.ecolsys.34.011802.132439) [DOI] [Google Scholar]
- 8.Skutch A. 1949. Do tropical birds rear as many young as they can nourish? Ibis 91, 430–458 10.1111/j.1474-919X.1949.tb02293.x (doi:10.1111/j.1474-919X.1949.tb02293.x) [DOI] [Google Scholar]
- 9.Ricklefs R, Wikelski M. 2002. The physiology/life-history nexus. Trends Ecol. Evol. 17, 462–468 10.1016/S0169-5347(02)02578-8 (doi:10.1016/S0169-5347(02)02578-8) [DOI] [Google Scholar]
- 10.Stutchbury BJM, Morton ES. 2001. Behavioral ecology of tropical songbirds. London, UK: Academic Press [Google Scholar]
- 11.Stutchbury B, Morton E. 1995. The effect of breeding synchrony on extra-pair mating systems in songbirds. Behaviour 132, 675–690 10.1163/156853995X00081 (doi:10.1163/156853995X00081) [DOI] [Google Scholar]
- 12.Mauck R, Marschall E, Parker P. 1999. Adult survival and imperfect assessment of parentage: effects on male parenting decisions. Am. Nat. 154, 99–109 10.1086/303216 (doi:10.1086/303216) [DOI] [PubMed] [Google Scholar]
- 13.Arnold K, Owens IPF. 2002. Extra-pair paternity and egg dumping in birds: life history, parental care and the risk of retaliation. Proc. R. Soc. Lond. B 269, 1263–1269 10.1098/rspb.2002.2013 (doi:10.1098/rspb.2002.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett PM, Owens IPF. 2002. Evolutionary ecology of birds. Oxford, UK: Oxford University Press [Google Scholar]
- 15.Peach W, Hanmer D, Oatley T. 2001. Do southern African songbirds live longer than their European counterparts? Oikos 93, 235–249 10.1034/j.1600-0706.2001.930207.x (doi:10.1034/j.1600-0706.2001.930207.x) [DOI] [Google Scholar]
- 16.Wiersma P, Munoz-Garcia A, Walker A, Williams JB. 2007. Tropical birds have a slow pace of life. Proc. Natl Acad. Sci. USA 104, 9340–9345 10.1073/pnas.0702212104 (doi:10.1073/pnas.0702212104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stutchbury B. 1998. Female mate choice of extra-pair males: breeding synchrony is important. Behav. Ecol. Sociobiol. 43, 213–215 10.1007/s002650050483 (doi:10.1007/s002650050483) [DOI] [Google Scholar]
- 18.Spottiswoode C, Møller A. 2004. Extrapair paternity, migration, and breeding synchrony in birds. Behav. Ecol. 15, 41–57 10.1093/beheco/arg100 (doi:10.1093/beheco/arg100) [DOI] [Google Scholar]
- 19.Govaty PA. 1996. Battle of the sexes and origins of monogamy. In Partnerships in birds: the study of monogamy (ed. Black JM.), pp. 21–52 Oxford, UK: Oxford University Press [Google Scholar]
- 20.Mulder RA, Dunn PO, Cockburn A, Lazenby-Cohen KA, Howell MJ. 1994. Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proc. R. Soc. Lond. B 255, 223–229 10.1098/rspb.1994.0032 (doi:10.1098/rspb.1994.0032) [DOI] [Google Scholar]
- 21.Ghalambor CK, Martin TE. 2001. Fecundity–survival trade-offs and parental risk-taking in birds. Science 292, 494–497 10.1126/science.1059379 (doi:10.1126/science.1059379) [DOI] [PubMed] [Google Scholar]
- 22.Jetz W, Sekercioglu CH, Boehning-Gaese K. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biol. 6, 2650–2657 10.1371/journal.pbio.0060303 (doi:10.1371/journal.pbio.0060303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNamara JM, Barta Z, Wikelski M, Houston AI. 2008. A theoretical investigation of the effect of latitude on avian life histories. Am. Nat. 172, 331–345 10.1086/589886 (doi:10.1086/589886) [DOI] [PubMed] [Google Scholar]
- 24.Macedo RH, Karubian J, Webster MS. 2008. Extrapair paternity and sexual selection in socially monogamous birds: are tropical birds different? Auk 125, 769–777 10.1525/auk.2008.11008 (doi:10.1525/auk.2008.11008) [DOI] [Google Scholar]
- 25.Calhim S, Immler S, Birkhead TR. 2007. Postcopulatory sexual selection is associated with reduced variation in sperm morphology. PLoS ONE 2, e413. 10.1371/journal.pone.0000413 (doi:10.1371/journal.pone.0000413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitnick S, Hosken DJ, Birkhead TR. 2006. Sperm morphological diversity. In Sperm biology: an evolutionary perspective (eds Birkhead TR, Hosken DJ, Pitnick S.), pp. 69–149 Oxford, UK: Academic Press [Google Scholar]
- 27.Lifjeld JT, Laskemoen T, Kleven O, Albrecht T, Robertson RJ. 2010. Sperm length variation as a predictor of extrapair paternity in passerine birds. PLoS ONE 5, e13456. 10.1371/journal.pone.0013456 (doi:10.1371/journal.pone.0013456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Birkhead T, Pellatt E, Brekke P, Yeates R, Castillo-Juarez H. 2005. Genetic effects on sperm design in the zebra finch. Nature 434, 383–387 10.1038/nature03374 (doi:10.1038/nature03374) [DOI] [PubMed] [Google Scholar]
- 29.Parker G, Begon M. 1993. Sperm competition and sperm games: sperm size and number under gametic control. Proc. R. Soc. Lond. B 253, 255–262 10.1098/rspb.1993.0111 (doi:10.1098/rspb.1993.0111) [DOI] [PubMed] [Google Scholar]
- 30.Hunter F, Birkhead T. 2002. Sperm viability and sperm competition in insects. Curr. Biol. 12, 121–123 10.1016/S0960-9822(01)00647-9 (doi:10.1016/S0960-9822(01)00647-9) [DOI] [PubMed] [Google Scholar]
- 31.Fitzpatrick JL, Baer B. 2011. Polyandry reduces sperm length variation in social insects. Evolution 65, 3006–3012 10.1111/j.1558-5646.2011.01343.x (doi:10.1111/j.1558-5646.2011.01343.x) [DOI] [PubMed] [Google Scholar]
- 32.Immler S, Calhim S, Birkhead TR. 2008. Increased postcopulatory sexual selection reduces the intramale variation in sperm design. Evolution 62, 1538–1543 10.1111/j.1558-5646.2008.00393.x (doi:10.1111/j.1558-5646.2008.00393.x) [DOI] [PubMed] [Google Scholar]
- 33.Kleven O, Laskemoen T, Fossøy F, Robertson RJ, Lifjeld JT. 2008. Intraspecific variation in sperm length is negatively related to sperm competition in passerine birds. Evolution 62, 494–499 10.1111/j.1558-5646.2007.00287.x (doi:10.1111/j.1558-5646.2007.00287.x) [DOI] [PubMed] [Google Scholar]
- 34.Pitcher T, Dunn P, Whittingham L. 2005. Sperm competition and the evolution of testes size in birds. J. Evol. Biol. 18, 557–567 10.1111/j.1420-9101.2004.00874.x (doi:10.1111/j.1420-9101.2004.00874.x) [DOI] [PubMed] [Google Scholar]
- 35.Dunn P, Whittingham L, Pitcher T. 2001. Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution 55, 161–175 10.1554/0014-3820 (doi:10.1554/0014-3820) [DOI] [PubMed] [Google Scholar]
- 36.Lindstedt SL, Calder WA. 1976. Body size and longevity in birds. Condor 78, 91–145 10.2307/1366920 (doi:10.2307/1366920) [DOI] [Google Scholar]
- 37.Wolfson A. 1952. The cloacal protuberance: a means for determining breeding condition in live male passerines. Bird Banding 23, 159–165 10.2307/4510381 (doi:10.2307/4510381) [DOI] [Google Scholar]
- 38.Laskemoen T, Kleven O, Fossøy F, Lifjeld JT. 2007. Intraspecific variation in sperm length in two passerine species, the Bluethroat Luscinia svecica and the Willow Warbler Phylloscopus trochilus. Ornis Fenn. 84, 131–139 [Google Scholar]
- 39.Lessells CM, Boag PT. 1987. Unrepeatable repeatabilities: a common mistake. Auk 104, 116–121 10.2307/4087240 (doi:10.2307/4087240) [DOI] [Google Scholar]
- 40.Sokal RR, Rohlf FJ. 1981. Biometry: the principles and practice of statistics in biological research, 2nd edn New York, NY: W. H. Freeman and Co [Google Scholar]
- 41.Cramp S, Simmons KEL, Perrins CM. 1978–1994. The birds of the Western Palearctic. Oxford, UK: Oxford University Press [Google Scholar]
- 42.Fry CH, Keith S, Newman K, Urban EK. 1982–2004. The birds of Africa. Princeton, NJ: Princeton University Press [Google Scholar]
- 43.Ridgely SR, Tudor G. 2009. Birds of South America: passerines. London, UK: Christopher Helm [Google Scholar]
- 44.Bakken V, Runde O, Tjørve E. 2006. Norsk ringmerkingsatlas, vol. 2 Stavanger, Norway: Stavanger Museum [Google Scholar]
- 45.Cepák J, Klvaňa P, Škopek J, Schröpfer L, Jelínek M, Hořák D, Formánek J, Zárybnický J. 2008. Czech and Slovak bird migration atlas [in Czech]. Prague, Czech Republic: Aventinum [Google Scholar]
- 46.Fraser KC, Kyser TK, Ratcliffe LM. 2008. Detecting altitudinal migration events in neotropical birds using stable isotopes. Biotropica 40, 269–272 10.1111/j.1744-7429.2008.00408.x (doi:10.1111/j.1744-7429.2008.00408.x) [DOI] [Google Scholar]
- 47.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing
- 48.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 10.1093/bioinformatics/btg412 (doi:10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 49.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877–884 10.1038/44766 (doi:10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 50.Freckleton R, Harvey P, Pagel M. 2002. Phylogenetic analysis and comparative data: a test and review of evidence. Am. Nat. 160, 712–726 10.1086/343873 (doi:10.1086/343873) [DOI] [PubMed] [Google Scholar]
- 51.Crawley MJ. 2007. The R book. Chichester, UK: John Willey & Sons Ltd [Google Scholar]
- 52.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113 10.1111/j.2041-210X.2010.00012.x (doi:10.1111/j.2041-210X.2010.00012.x) [DOI] [Google Scholar]
- 53.Calhim S, Birkhead TR. 2007. Testes size in birds: quality versus quantity—assumptions, errors, and estimates. Behav. Ecol. 18, 271–275 10.1093/beheco/arl076 (doi:10.1093/beheco/arl076) [DOI] [Google Scholar]
- 54.Griffith S, Stewart I, Dawson D, Owens I, Burke T. 1999. Contrasting levels of extra-pair paternity in mainland and island populations of the house sparrow (Passer domesticus): is there an ‘island effect’? Biol. J. Linnean Soc. 68, 303–316 10.1111/j.1095-8312.1999.tb01171.x (doi:10.1111/j.1095-8312.1999.tb01171.x) [DOI] [Google Scholar]
- 55.Hau M, Ricklefs RE, Wikelski M, Lee KA, Brawn JD. 2010. Corticosterone, testosterone and life-history strategies of birds. Proc. R. Soc. B 277, 3203–3212 10.1098/rspb.2010.0673 (doi:10.1098/rspb.2010.0673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrie M, Doums C, Møller A. 1998. The degree of extra-pair paternity increases with genetic variability. Proc. Natl Acad. Sci. USA 95, 9390–9395 10.1073/pnas.95.16.9390 (doi:10.1073/pnas.95.16.9390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnqvist G, Kirkpatrick M. 2005. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 165, S26–S37 10.1086/429350 (doi:10.1086/429350) [DOI] [PubMed] [Google Scholar]
- 58.Albrecht T, Kreisinger J, Pialek J. 2006. The strength of direct selection against female promiscuity is associated with rates of extrapair fertilizations in socially monogamous songbirds. Am. Nat. 167, 739–744 10.1086/502633 (doi:10.1086/502633) [DOI] [PubMed] [Google Scholar]
- 59.Ashmole NP. 1963. The regulation of numbers of tropical oceanic birds. Ibis 103, 458–473 [Google Scholar]
- 60.Garamszegi LZ, Hirschenhauser K, Bokony V, Eens M, Hurtrez-Bousses S, Møller AP, Oliveira RF, Wingfield JC. 2008. Latitudinal distribution, migration, and testosterone levels in birds. Am. Nat. 172, 533–546 10.1086/590955 (doi:10.1086/590955) [DOI] [PubMed] [Google Scholar]
- 61.Fitzpatrick S. 1994. Colourful migratory birds: evidence for a mechanism other than parasite resistance for maintenance of good genes sexual selection. Proc. R. Soc. Lond. B 257, 155–160 10.1098/rspb.1994.0109 (doi:10.1098/rspb.1994.0109) [DOI] [Google Scholar]
- 62.Stutchbury B. 1998. Breeding synchrony best explains variation in extra-pair mating system among avian species. Behav. Ecol. Sociobiol. 43, 221–222 10.1007/s002650050485 (doi:10.1007/s002650050485) [DOI] [Google Scholar]
- 63.Weatherhead P, Yezerinac S. 1998. Breeding synchrony and extra-pair mating in birds. Behav. Ecol. Sociobiol. 43, 217–219 10.1007/s002650050484 (doi:10.1007/s002650050484) [DOI] [Google Scholar]
- 64.Slagsvold T, Lifjeld JT. 1997. Incomplete knowledge of male quality may explain variation in extra-pair paternity in birds. Behaviour 134, 353–371 10.1163/156853997X00584 (doi:10.1163/156853997X00584) [DOI] [Google Scholar]
- 65.Stutchbury BJM, Morton ES. 2008. Recent advances in the behavioral ecology of tropical birds: the 2005 Margaret Morse Nice Lecture. Wilson J. Ornithol. 120, 26–37 10.1676/07-018.1 (doi:10.1676/07-018.1) [DOI] [Google Scholar]


