Abstract
Bar-headed geese are renowned for migratory flights at extremely high altitudes over the world's tallest mountains, the Himalayas, where partial pressure of oxygen is dramatically reduced while flight costs, in terms of rate of oxygen consumption, are greatly increased. Such a mismatch is paradoxical, and it is not clear why geese might fly higher than is absolutely necessary. In addition, direct empirical measurements of high-altitude flight are lacking. We test whether migrating bar-headed geese actually minimize flight altitude and make use of favourable winds to reduce flight costs. By tracking 91 geese, we show that these birds typically travel through the valleys of the Himalayas and not over the summits. We report maximum flight altitudes of 7290 m and 6540 m for southbound and northbound geese, respectively, but with 95 per cent of locations received from less than 5489 m. Geese travelled along a route that was 112 km longer than the great circle (shortest distance) route, with transit ground speeds suggesting that they rarely profited from tailwinds. Bar-headed geese from these eastern populations generally travel only as high as the terrain beneath them dictates and rarely in profitable winds. Nevertheless, their migration represents an enormous challenge in conditions where humans and other mammals are only able to operate at levels well below their sea-level maxima.
Keywords: high altitude, avian migration, exercise performance, physiology
1. Introduction
A significant proportion of the world's bar-headed geese (Anser indicus) make biannual migrations between breeding areas in Mongolia, northern China and the Tibetan Plateau (latitude between 29°N and 37°N, mean elevation of 4800 m) and wintering areas in India, crossing the Himalayan mountains (along the southern edge of the Tibetan plateau) en route [1–8]. This species has become renowned for a paradigm of extreme high-altitude migration, being frequently cited as flying regularly above 8000 m [4,7,9–32] following the original report of observation of birds over the summits of the Himalayas [30]. Up to now, however, the published reports that indicated that birds may be flying at very high altitudes have been only qualitative, either because the recording devices did not directly measure altitude [4,7] or because they were based on incidental auditory and visual observations [30,32]. Such observations may also be subject to unknown errors by observers, particularly given that neurological ability is known to be affected by high altitude [33], and they do not indicate whether such flights are common and whether they represent sustainable, aerobically powered flights. Thus, personal accounts of this type lack scientific accuracy with respect to prevailing atmospheric conditions. For example, Swan [30] wrote of hearing bar-headed geese flying over the summit of Mount Makalu (at 8487 m elevation, the fifth highest mountain on Earth) while standing at 4800 m on the Barun Glacier on a still night. Given that he would have been around 6000 m away from the summit of Makalu (see the electronic supplementary material, figure S1) and that sound attenuates as the inverse square of the distance from the source (and more quickly in hypodense air, i.e. at high altitude), this interpretation is highly speculative compared with the idea that the geese were simply flying within and along the enormous valley below Mount Makalu. Thus, there is a requirement to obtain objective and quantitative data on how often these birds fly at extreme altitudes, under what environmental circumstances and whether they represent true powered flight.
Swan [30] speculated that extreme high-altitude flight would both facilitate navigation and reduce the risk of being blown into mountainsides. This hypothesis does not seem sufficient to explain why this energetically demanding migration might primarily take place high above the land surface, and thus in conditions of very low partial pressure of oxygen and air density (and consequently poor lift), when lower-altitude routes through the Himalayas exist. Given that flight is a particularly costly form of transport, in terms of the rate of oxygen consumption, and that oxygen becomes progressively more rarefied at higher altitudes, powered flight should become progressively more challenging with altitude. For example, at 8000 m, the minimum mechanical power required for flight is 50 per cent greater than that at sea level [34], whereas the partial pressure of oxygen is 40 per cent lower than that at sea level [35]. The postulated high-altitude migration of bar-headed geese is thus a paradox of avian migration ecology and physiology [17]. However, no direct measurements of extreme high-altitude flight by bar-headed geese have ever been collected, and the migratory flights of bar-headed geese have yet to be placed into any quantitative context, either with regard to their overall migratory strategy, their associated environmental conditions or their physiological nature.
Specific weather conditions may be exploited by migrating birds to provide significant energetic savings [36]. For example, because winds reach speeds that are similar to the most efficient transit speeds for birds [37], migratory costs could be halved by flying in tailwind conditions, if the magnitude of turbulence remains the same. General patterns of wind are globally quite predictable (e.g. winds are typically stronger over seas and at high altitude) and consequently many species of birds have been shown to seek tailwinds during migration [37,38]. In addition, vertical components of wind (e.g. orographic updrafts and ‘thermals’) can be used by both small and large bird species to generate lift and reduce the costs of climbing flight [9,39,40], and could be used to negotiate demanding vertical features such as mountain ranges. However, this may not always be the case [41–43] and we have previously shown that for a group of bar-headed geese migrating northwards over the Himalayas, many birds began their flights in what was probably the still of the night and made use of low-altitude passes (up to 5800 m) [1,6].
In this study, we describe the movements and timing of a larger group of bar-headed geese, including those in the study of Hawkes et al. [1]. On the basis of our previous findings, we posit the hypotheses that, because migratory wild animals should tend to minimize energy expenditure, bar-headed geese will: (i) migrate at the lowest altitudes possible; and (ii) make use of favourable winds (i.e. tailwinds).
Global Positioning System (GPS) data are collected objectively over wide spatial and temporal scales, and are thus well suited to test these hypotheses. In this study, bar-headed geese (n = 91) were fitted with GPS satellite transmitters (Microwave Telemetry solar Argos-GPS PTT-100; http://microwavetelemetry.com/Bird_PTTs/30g_gps.php) at two sites in India in December 2008 (Chilika Lake, 19.694°N, 85.307°E, n = 15; Koonthankulum Bird Sanctuary, 8.472°N, 77.705°E, n = 10), at Qinghai Lake, China in March 2007 and April 2008 (36.907°N, 100.139°E, n = 29) and at Terkhiin Tsagaan Lake, Mongolia in July 2008 and July 2009 (48.147°N, 99.576°E, n = 37). The transmitters had a mass of 30 g, which represents approximately 1 per cent of the 2.26 kg mean body mass of the study individuals, and incorporated estimates of altitude to ±22 m accuracy, up to 20 480 m. They were attached using Teflon and elastic harnesses; see §4 for discussion of the potential effects of transmitter tags. Data were analysed using R (www.r-project.org) and integrated with environmental variables using custom script in Matlab (www.mathworks.com). See §4 for more details.
2. Results
(a). Migratory patterns
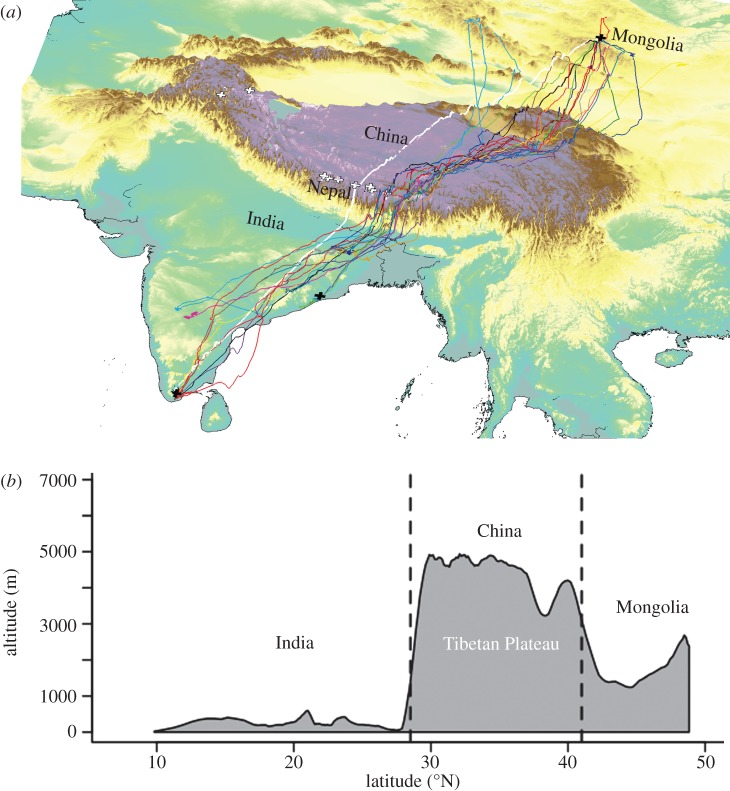
Global Positioning System data described a migratory corridor extending from northern Mongolia to southern India, with many of the birds deployed from Mongolia passing over the eastern Himalayas near the border between Nepal and Bhutan, where Himalayan valleys are lowest and where the width of the Himalayan mountain range tends to its narrowest [44] (figure 1). Tags from 53 geese failed to provide sufficient data to describe their migration in detail and were therefore excluded from further analyses. Tags from the remaining 38 geese provided data for a median 453 days (range 135–1216 days), sending a median nine GPS locations per day (range 0–24). Geese demonstrated a migratory dichotomy [6]: (i) some geese wintered in India near sea level (‘crossers’; n = 19); and (ii) other geese remained north of the Himalayas, wintering in eastern Tibet at altitudes between 3190 and 4440 m (range of medians per goose; ‘non-crossers’; n = 19).
Figure 1.
Satellite tracks of migrating bar-headed geese: (a) three-dimensional map showing release locations (black crosses) of bar-headed geese in India (n = 2 sites) and Mongolia (n = 1 site). Coloured lines represent 16 individual geese, and coloured background shading indicates elevation. Solid thick white line shows the great circle route. White crosses show locations of the 14 ‘eight-thousanders’ (the world's highest mountains, each over 8000 m in elevation). Five peaks are largely obscured owing to their proximity to other peaks. (b) Cross-section of land elevation under the arithmetic mean bar-headed goose northwards migration from Indian wintering (left side of plot) to Chinese and Mongolian breeding grounds (right side of plot).
(b). Do bar-headed geese migrate at the lowest altitudes possible?
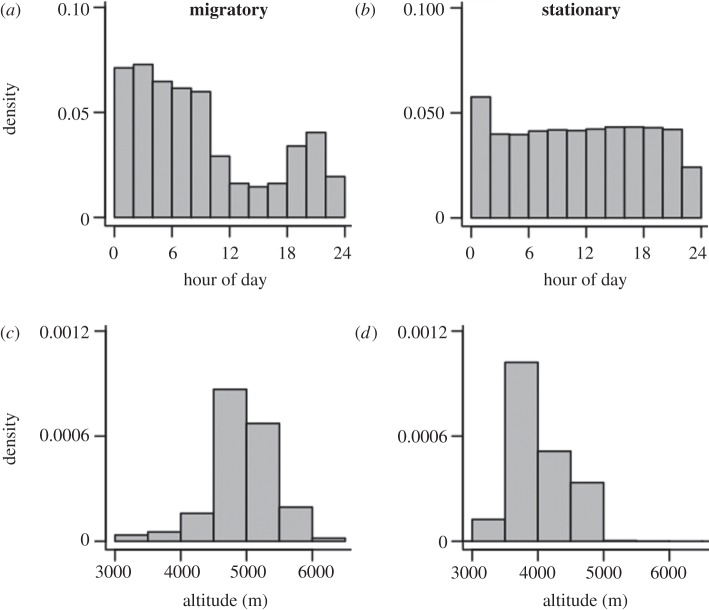
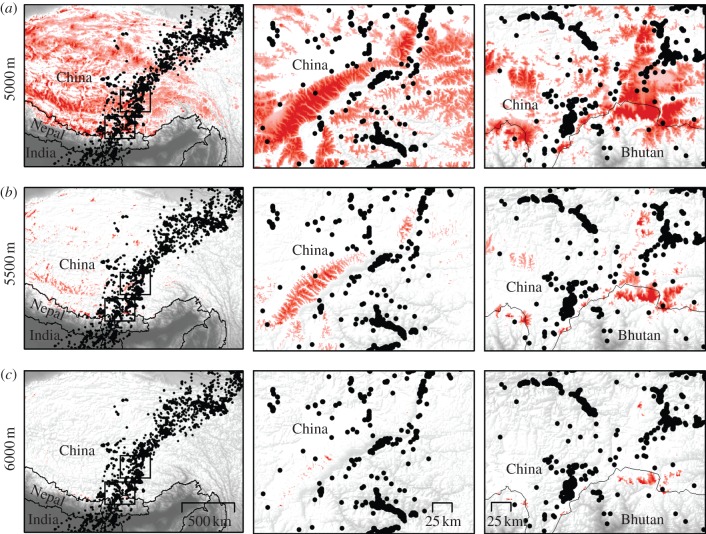
Geese migrating southwards in the autumn flew up to 7290 m altitude (maximum altitude recorded for one goose over northern Bhutan), with crossers migrating a median 3152 km southwards from Mongolia and China to India (maximum 4542 km) in a median 81 days (maximum 149 days). By extracting migratory phases from stationary phases, it was evident that there was a diel pattern in the timing of locations received during migration, but not during stationary phases, suggesting that geese mainly migrated at night and during the early morning (figure 2a,b). Geese migrating northwards in spring sustained high climb rates for hours while crossing the Himalayas (see also [1]) and one was tracked as high as 6540 m. The birds travelled a median 3000 km distance (maximum 5140 km) in a median 47 days (maximum 92 days). In total, 95 per cent of filtered locations gathered while birds were in flight over the Tibetan Plateau (where the highest ground elevations they would have experienced, including the Himalayas, were located) were received from lower than 5784 m (n = 207 locations). Although our data cannot preclude that geese could have occasionally flown higher than this during inter-location intervals (which were 1–2 h long), it seems unlikely that significant flights were missed. Only 10 geese were recorded higher than 5784 m (and only one goose higher than 6500 m), contributing just 11 locations. What is more, all but one of the 11 locations were received during the night and early morning, between 21.00 and 11.00, when the density altitude (the corresponding virtual altitude in the International Standard Atmosphere for the actual conditions experienced by the geese) may have been some hundreds of metres lower than they would have experienced during the day, owing to the colder air temperatures (see also [1]). The majority of goose locations occurred less than 591 m from the ground (upper quartile; median 188 m) and geese flew closest to the ground on the Tibetan Plateau (median 62 m). We also compared the locations received from geese over terrain higher than 5000 m with a null model of randomly dispersed locations in the same area (see §4; electronic supplementary material, figure S2). Terrain over which geese travelled was in the lowest 1 per cent of elevation values in the null model, suggesting that the majority of geese choose to minimize altitude by flying along valleys where possible and do not usually cross mountain summits (figure 3).
Figure 2.
Timing and altitude of locations: histograms showing relative frequencies (densities) of (a,b) timing of locations and (c,d) altitudes for (a,c) migrating and (b,d) stationary bar-headed geese (e.g. during stop-over events, breeding and wintering).
Figure 3.
Bar-headed goose migration and altitude: map showing GPS locations received from bar-headed geese (black dots) on the Tibetan Plateau (the Himalayan mountains are in northern Nepal near the southern Chinese border). Background greyscale shading shows elevation from SRTM, and red shading shows elevations (a) above 5000 m, (b) above 5500 m and (c) above 6000 m. Left column shows migration across Tibetan Plateau. Middle and right columns show zoomed extents of commonly used valleys. Extents shown as black boxes.
(c). Do bar-headed geese make use of favourable winds?
At the resolution of our modelled data describing general wind patterns, there was no evidence that southbound geese selected specific weather conditions in which to migrate (gross wind speeds and directions were not significantly different from those experienced by stationary geese; electronic supplementary material, figures S3 and S4; see also §4), but northbound geese migrated in wind speeds that were significantly slower than those that they experienced when stationary (Mann–Whitney–Wilcoxon test, W = 109, p < 0.01). During migration, geese flew at 17.1 m s−1 (15.5–18.9 inter-quartile range; n = 38 geese), with a maximum recorded GPS ground speed of 35.3 m s−1. However, more than half of the ground speeds were marginally slower than the altitude-corrected predicted ‘minimum power air speed’ (the air speed required to have travelled with the minimum power between two locations) from flight biomechanical theory [34], and were correlated with and almost equal to minimum transit speeds (the minimum ground speed required to have travelled between two successive locations and their corresponding time stamps; figure 4a,b). Although detailed wind data with which to estimate airspeed were not available (airspeed = groundspeed–wind vector; which would have permitted the estimation of the tailwind ratio), our data therefore suggest that geese may have made headway regardless of wind conditions [43]. However, we do not know what the local conditions were in the immediate vicinity of the geese. As expected from flight biomechanical theory, geese flew significantly faster at higher altitudes, travelling 3.7 m s−1 faster above 5000 m than at sea level (Spearman's correlation coefficient S = 177 458, p < 0.01, n = 38 geese; figure 4c).
Figure 4.
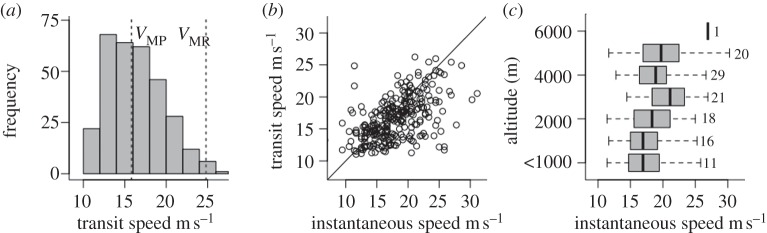
Bar-headed goose flight ground speeds. (a) Frequency histogram of minimum transit speeds received during migration. Vertical dashed lines show VMP (minimum power speed) and VMR (maximum range speed, the most efficient speed per unit of distance) at sea level. (b) Scatter plot of instantaneous GPS ground speed and minimum transit speeds. Black line shows line of equality, where geese could not have stopped between transmitted locations. (c) Box plot showing median instantaneous ground speeds in 1000 m altitudinal bins (comprising median of median values per goose). Black line shows median value, box shows inter-quartile range, dashed line shows total range. Number of locations for each altitudinal band indicated.
Bar-headed goose migration described a route that was 112 km longer than the great-circle route (the minimum distance between wintering and breeding sites; figure 1a, white line), with a deviation up to 450 km eastwards on the Tibetan Plateau. At latitudinally and temporally coincident locations on the great-circle route, modelled wind speeds were significantly stronger than those experienced by migrating bar-headed geese (median 11.1 versus 2.6 m s−1; Wilcoxon–Mann–Whitney two-sample U-test, U = 1119, p < 0.01), and were also likely to be highly turbulent and frequently against the direction of travel (figure 5). We have previously recorded apparent selection for calmer, less windy conditions at night for bar-headed geese making the 8 h northern migration over the Himalayan mountain range [1].
Figure 5.
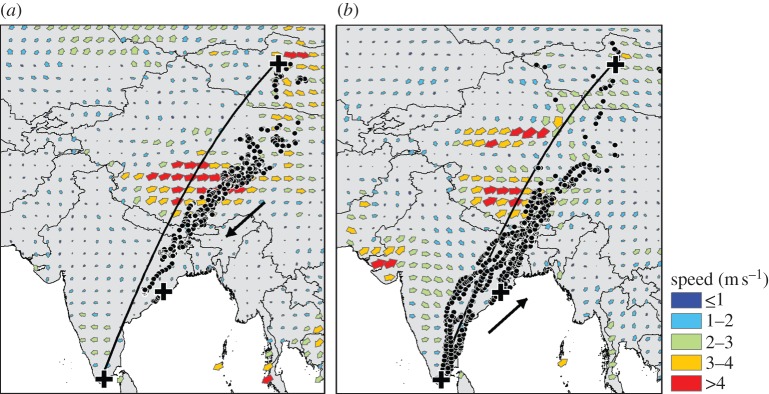
Winds experienced by migrating bar-headed geese: wind patterns (mean wind speed and direction) during (a) southward migration (1 October–31 December) and (b) northward migration (1 March–31 May), where arrows indicate wind speed and direction (largest arrow size and red colour denotes strongest wind speeds; see legend). Black arrow shows schematic of travel. Black dots show GPS locations received from bar-headed geese at coincident temporal scale to winds.
3. Discussion
The reputed extreme high-altitude migration of bar-headed geese above the peaks of the Himalayas [30] has puzzled ecologists and physiologists alike for over 30 years; indeed, Black & Tenney ([17], p. 236) suggested that ‘there must be a good explanation for why the birds fly to the extreme altitudes … particularly since there are passes through the Himalaya at lower altitudes, and which are used by other migrating bird species’. In this study, we find no evidence to support a general paradigm of extreme high-altitude migration (>8000 m), although there were a couple of flights over 6000 m altitude for short periods of time. Geese in this study comprised two major wintering aggregations and two breeding populations, of which one, Qinghai province in China, hosts almost one-third of the world's nesting population for this species [2,3,5,6]. This suggests that a major portion of the global population of bar-headed geese (>17 000 individuals) might be expected to be found along this flyway [45].
The strategy adopted by the majority of bar-headed geese passing through the eastern Tibetan plateau was potentially to reduce overall flight costs by flying over the lowest part of the mountain range and not much higher than the underlying terrain dictated. There was no evidence that geese made regular use of tailwinds, although we note the resolution of the meteorological data we used precludes detailed analysis of this hypothesis. The rare higher flights that were recorded in this study may represent brief negotiation of particularly high terrain where no lower-altitude routes exist and/or may have been associated with favourable winds or night-time reductions in density altitude. Bar-headed geese do also cross the mountains further west [4,7], where there is a greater percentage of the Himalayas' highest peaks and ridges. Thus, future studies may address whether extreme high-altitude flight is more prevalent for other populations and whether they may make use of favourable wind conditions (e.g. tailwinds and lift from vertical up-currents). Our dataset, however, suggest that higher-altitude flights in the eastern Himalayas and Tibetan plateau are a relatively uncommon event, and supports our hypothesis that geese migrate via the lowest routes available to them. It does not, however, support our second hypothesis that they make use of tailwind conditions to reduce energy expenditure.
We have shown that the majority of bar-headed geese typically fly up to 6000 m above sea level. This is still an extraordinary feat. Even at these altitudes, the barometric pressure in the standard atmosphere is more than halved, yielding not only less aerodynamic lift, but also half the partial pressure of oxygen with which to fuel flight. We are unable, at present, to determine whether the geese migrate at these altitudes solely under their own power. If they do, it would be particularly impressive as they are relatively large birds with a potentially negative allometric scaling ratio of power available to power required, compared with smaller birds [34,46,47], and are not known to exhibit exceptional external morphological adaptations to facilitate flight at high altitude [15]. By contrast, humans can only exercise sub-maximally at such altitudes [48]. Occasional rare flights to even higher altitudes do occur, and it is not clear whether these flights are assisted by favourable wind and temperature conditions [49] or whether they can be sustained entirely by the various physiological adaptations for flight at high altitude, which have been described in some detail for bar-headed geese [10,27,49].
4. Material and methods
(a). Satellite tracking
Global Positioning System tags obtained locations hourly (n = 25 tags) or 2-hourly (n = 66) and transmitted the accumulated, compressed data to the Argos satellite location system every 2 days. The units also transmitted the goose's instantaneous flight ground speed (to±0.3 m s−1 accuracy at speeds > 11.1 m s−1) and heading (to±1° accuracy). Transmitters were attached, using Teflon and elastic harnesses. Externally mounted telemetry tags are known to have negative effects on a range of avian species [50–54] (e.g. causing an increase in energy expenditure during flight owing to the additional drag induced by the tag). Such tag attachment effects may have existed for bar-headed geese in this study. Although it is unclear whether it may have affected their altitudinal maxima to some degree, it seems unlikely that geese would have flown higher than necessary without the burden of tags given the relatively small size of the tags and the increasing costs of flight with altitude.
(b). Flight biomechanics
The ground speed data transmitted by the tags were compared with an estimate of the most efficient air speed per unit of time (the minimum power air speed; VMP) and distance (the maximum range air speed; VMR) [34]. These estimates were computed in Flight v. 1.22 [34] using the following input parameters: (i) 125 per cent of the mean capture mass of bar-headed geese in the present study (125% = 2.82 kg; geese were captured during their wing moult and many weeks prior to fattening and migration [55]); (ii) a mean measured wing span for bar-headed geese of 1.46 m; and (iii) a mean wing area of 0.25 m2 (from Lee et al. [15]), yielding estimates of VMP and VMR for an average goose at sea level in still air. Estimated VMP and VMR were then recalculated over 500 m altitudinal increments to 7500 m above sea level in Flight for comparison with the tracking data at altitude. During migration however, as fat stores are used up (and replenished at stop-over sites), body mass will change, meaning that actual VMP and VMR are dynamic while our estimates are not. They will therefore obviously not be accurate for all geese at all times of the year, but can be parsimoniously used as a broad indicator of optimum flight speeds.
(c). Data management
Global Positioning System data were managed using the Satellite Tracking and Analysis Tool [56] and hosted on Movebank (http://www.movebank.org) [57]. GPS location data (n = 149 320 locations) were used in analyses and we did not use Argos-derived locations. The time zone in which each location was transmitted was determined and used to convert each GPS time (in coordinated universal time) to local time, and compared with local sunrise and sunset times to assign each location to day or night. Migratory periods were extracted from the data using the minimum straight-line displacement distance from original release location to each subsequent GPS location. Periods of migration (persistent movement, shown as steep increases in displacement) can then be identified as distinct from stationary phases such that migratory timing can be extracted (e.g. stop-overs, breeding and wintering shown as flat sections where displacement distance does not change, retaining 5% of locations). Data were further filtered to retain locations corresponding only to transit ground speeds greater than 11 m s−1 (25% slower than the estimated VMP [34], retaining 5.4 per cent of the filtered data). All retained data had corresponding estimates of instantaneous GPS-derived ground speed and 97 per cent had altitudinal estimates.
(d). Environmental data layers
Land elevation data coincident to goose locations were obtained by overlaying tracks on the NASA/NGA 90 m Shuttle Radar Topography Mission (SRTM; http://www2.jpl.nasa.gov/srtm/) topographic data product. Spatio-temporally congruent weather data for the central Asian region were obtained from the European Centre for Medium-Range Weather Forecasts ERA interim dataset on a fixed grid of 1.5° and at 6-hourly temporal resolution (http://www.ecmwf.int [58]). Coincident ground elevation from SRTM was subtracted from each transmitted flight altitude to derive flight height above ground, and used to select the relevant pressure level from the ERA dataset for each location. The spatio-temporally congruent U and V components, the vectoral components of wind speed and direction, were then extracted from the dataset and used to calculate wind direction and speed for each goose location. Wind data were plotted using the R package ‘oce’ and circular statistics carried out using ‘CircStats’. We calculated the great circle route (shortest route) between the deployment and terminus locations, using the R package ‘geosphere’.
(e). Statistical analysis of route elevation
We tested whether geese selected routes through the Himalayas and across the Qinghai–Tibetan Plateau that encompassed statistically lower altitudes than might be expected at random by testing the observed goose data against a null model [59]. The null model was constructed by randomly scattering 5000 points across a rectangular extent that encompassed the generalized flyway across the Qinghai–Tibetan Plateau (see the electronic supplementary material, figure S2). The elevation of the ground under each modelled point was then sampled from the SRTM data layer and compared with the ground elevations under bar-headed goose locations that lay within the same extent. We then enumerated the proportion in each that lay above and below 5000 m elevation.
(f). Statistical analyses
Global Positioning System and meteorological data were not normally distributed (Shapiro–Wilk test, p < 0.05), and measures of central tendency are therefore represented as medians with inter-quartile ranges. Wilcoxon tests were used to compare between two groups [60].
Acknowledgements
This work was carried out through funding from the British Biotechnology and Biological Sciences Research Council award to C.M.B. and P.J.B. (grant no. BB/F015615/1), from the NSERC of Canada to W.K.M., and from the Max Planck Institute for Ornithology. ECMWF ERA-Interim data used in this study have been obtained from the ECMWF data server. Support was also provided by the US Geological Survey, Western Ecological and Patuxent Wildlife Research Centers and Avian Influenza Programme, as well as the United Nations Food and Agriculture Organization (J. Lubroth, J. Domenech), and field and analysis assistance from the Qinghai Lake National Nature Reserve staff (Z. Xing, Y. Hou, D. Zhang), Qinghai Forestry Bureau (S. Li), Chinese Academy of Sciences (F. Lei, X. Hu, L. Hu, N. Kong, Y. Li, Z. Luo, J. Liu), US Geological Survey (S. Schwarzbach, W. Perry, S. Heath, E. Palm, S. Muzaffar, A. Schultz, K. Spragens), Ministry of Environment and Forests, Chief Wildlife Wardens of Tamil Nadu and Orissa, Mongolia—the Mongolian Academy of Sciences, Wildlife Science and Conservation Center. We also thank the Global Bathymetric Chart of the Oceans, the Shuttle Radar Topography Mission from NASA and the British Atmospheric Data Centre for access to meteorological station data. The project was supported by field teams in both India and Mongolia, to whom we are very grateful. Work was carried out under permit in both India and Mongolia. The use of trade names in this document is for descriptive purposes only and does not imply endorsement by the US Government. Designed research: L.A.H., C.M.B., P.J.B., J.Y.T., M.W.; performed research: L.A.H., S.B., N.B., P.J.B., P.B.F., W.K.M., S.H.N., D.J.P., Y.H., B.Y., G.R.S., P.S., J.Y.T., C.M.B.; analysed data: L.A.H., M.J.W., C.M.B.; contributed analytical tools: D.C.D., J.Y.T.; wrote the paper: L.A.H, C.M.B., P.J.B., with additional contributions from all authors.
References
- 1.Hawkes LA, et al. 2011. The trans-Himalayan flights of bar-headed geese (Anser indicus). Proc. Natl Acad. Sci. USA 108, 9516–9519 10.1073/pnas.1017295108 (doi:10.1073/pnas.1017295108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prosser DJ, et al. 2011. Wild bird migration across the Qinghai–Tibetan Plateau: a transmission route for highly pathogenic H5N1. PLoS ONE 6, e17622. 10.1371/journal.pone.0017622 (doi:10.1371/journal.pone.0017622) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo-Gang Z, Dong-Ping L, Yun-Qiu H, Hong-Xing J, Ming D, Fa-Wen Q, Jun L, Zhi X, Feng-Shan L. 2011. Migration routes and stop-over sites determined with satellite tracking of bar-headed geese Anser indicus breeding at Qinghai Lake, China. Waterbirds 34, 112–116 10.1675/063.034.0115 (doi:10.1675/063.034.0115) [DOI] [Google Scholar]
- 4.Köppen U, Yakovlev A, Barth R, Kaatz M, Berthold P. 2010. Seasonal migrations of four individual bar-headed geese Anser indicus from Kyrgyzstan followed by satellite telemetry. J. Ornithol. 151, 703–712 10.1007/s10336-010-0492-1 (doi:10.1007/s10336-010-0492-1) [DOI] [Google Scholar]
- 5.Cui P, et al. 2011. Movement patterns of bar-headed geese Anser indicus during breeding and post-breeding periods at Qinghai Lake, China. J. Ornithol. 152, 83–92 10.1007/s10336-010-0552-6 (doi:10.1007/s10336-010-0552-6) [DOI] [Google Scholar]
- 6.Takekawa JY, et al. 2009. Geographic variation in bar-headed geese Anser Indicus: connectivity of wintering areas and breeding grounds across a broad front. Wildfowl 59, 100–123 [Google Scholar]
- 7.Javed S, Takekawa J, Douglas D, Rahmani A, Kanai Y, Nagendran M, Choudhury B. 2000. Tracking the spring migration of a bar-headed goose (Anser indicus) across the Himalaya with satellite telemetry. Glob. Environ. Res. 4, 195–205 [Google Scholar]
- 8.Bishop M, Yanling S, Zhouma C, Binyuan G. 1997. Bar-headed Geese Anser indicus wintering in south-central Tibet. Wildfowl 48, 118–126 [Google Scholar]
- 9.Ward S, Bishop CM, Woakes AJ, Butler PJ. 2002. Heart rate and the rate of oxygen consumption of flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus). J. Exp. Biol. 205, 3347–3356 [DOI] [PubMed] [Google Scholar]
- 10.Faraci FM. 1991. Adaptations to hypoxia in birds: how to fly high. Annu. Rev. Physiol. 53, 59–70 10.1146/annurev.ph.53.030191.000423 (doi:10.1146/annurev.ph.53.030191.000423) [DOI] [PubMed] [Google Scholar]
- 11.Faraci FM, Fedde MR. 1986. Regional circulatory responses to hypocapnia and hypercapnia in bar-headed geese. Am. J. Physiol. Regul. Integr. Comp. Physiol. 250, R499–R504 [DOI] [PubMed] [Google Scholar]
- 12.Faraci FM, Kilgore DL, Fedde MR. 1984. Attenuated pulmonary pressor response to hypoxia in bar-headed geese. Am. J. Physiol. Regul. Integr. Comp. Physiol. 247, R402–R403 [DOI] [PubMed] [Google Scholar]
- 13.Faraci FM, Kilgore DL, Fedde MR. 1984. Oxygen delivery to the heart and brain during hypoxia: Pekin duck versus bar-headed goose . Am. J. Physiol. Regul. Integr. Comp. Physiol. 247, R69–R75 [DOI] [PubMed] [Google Scholar]
- 14.Fedde MR, Orr JA, Shams H, Scheid P. 1989. Cardiopulmonary function in exercising bar-headed geese during normoxia and hypoxia. Respir. Physiol. 77, 239–252 10.1016/0034-5687(89)90010-8 (doi:10.1016/0034-5687(89)90010-8) [DOI] [PubMed] [Google Scholar]
- 15.Lee SY, Scott GR, Milsom WK. 2008. Have wing morphology or flight kinematics evolved for extreme high altitude migration in the bar-headed goose? Comp. Biochem. Physiol. C, Toxicol. Pharmacol. 148, 324–331 10.1016/j.cbpc.2008.05.009 (doi:10.1016/j.cbpc.2008.05.009) [DOI] [PubMed] [Google Scholar]
- 16.Liang Y, Hua Z, Liang X, Xu Q, Lu G. 2001. The crystal structure of bar-headed goose hemoglobin in deoxy form: the allosteric mechanism of a hemoglobin species with high oxygen affinity. J. Mol. Biol. 313, 123–137 10.1006/jmbi.2001.5028 (doi:10.1006/jmbi.2001.5028) [DOI] [PubMed] [Google Scholar]
- 17.Black CP, Tenney SM. 1980. Oxygen transport during progressive hypoxia in high-altitude and sea-level waterfowl. Respir. Physiol. 39, 217–239 10.1016/0034-5687(80)90046-8 (doi:10.1016/0034-5687(80)90046-8) [DOI] [PubMed] [Google Scholar]
- 18.Saunders DK, Fedde MR. 1991. Physical conditioning: effect on the myoglobin concentration in skeletal and cardiac muscle of bar-headed geese. Comp. Biochem. Physiol. A, Physiol. 100, 349–352 10.1016/0300-9629(91)90481-Q (doi:10.1016/0300-9629(91)90481-Q) [DOI] [Google Scholar]
- 19.Weber RE. 2007. High-altitude adaptations in vertebrate hemoglobins. Respir. Physiol. Neurobiol. 158, 132–142 10.1016/j.resp.2007.05.001 (doi:10.1016/j.resp.2007.05.001) [DOI] [PubMed] [Google Scholar]
- 20.Scott GR, Schulte PM, Egginton S, Scott ALM, Richards JG, Milsom WK. 2011. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol. 28, 351–363 10.1093/molbev/msq205 (doi:10.1093/molbev/msq205) [DOI] [PubMed] [Google Scholar]
- 21.Scott GR, Egginton S, Richards JG, Milsom WK. 2009. Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc. R. Soc. B 276, 3645–3653 10.1098/rspb.2009.0947 (doi:10.1098/rspb.2009.0947) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott GR, Richards JG, Milsom WK. 2009. Control of respiration in flight muscle from the high-altitude bar-headed goose and low-altitude birds. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1066–R1074 10.1152/ajpregu.00241.2009 (doi:10.1152/ajpregu.00241.2009) [DOI] [PubMed] [Google Scholar]
- 23.Scott GR, Milsom WK. 2007. Control of breathing and adaptation to high altitude in the bar-headed goose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R379–R391 10.1152/ajpregu.00161.2007 (doi:10.1152/ajpregu.00161.2007) [DOI] [PubMed] [Google Scholar]
- 24.Scott GR. 2011. Elevated performance: the unique physiology of birds that fly at high altitudes. J. Exp. Biol. 214, 2455–2462 10.1242/jeb.052548 (doi:10.1242/jeb.052548) [DOI] [PubMed] [Google Scholar]
- 25.Storz JF, Scott GR, Cheviron ZA. 2010. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 213, 4125–4136 10.1242/jeb.048181 (doi:10.1242/jeb.048181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott GR, Cadena V, Tattersall GJ, Milsom WK. 2008. Body temperature depression and peripheral heat loss accompany the metabolic and ventilatory responses to hypoxia in low and high altitude birds. J. Exp. Biol. 211, 1326–1335 10.1242/jeb.015958 (doi:10.1242/jeb.015958) [DOI] [PubMed] [Google Scholar]
- 27.Scott GR, Milsom WK. 2006. Flying high: a theoretical analysis of the factors limiting exercise performance in birds at altitude. Respir. Physiol. Neurobiol. 154, 284–301 10.1016/j.resp.2006.02.012 (doi:10.1016/j.resp.2006.02.012) [DOI] [PubMed] [Google Scholar]
- 28.Scott GR, Meir JU, Hawkes LA, Frappell PB, Milsom WK. 2011. Point: high altitude is for the birds!. J. Appl. Physiol. 111, 1514–1515 10.1152/japplphysiol.00821.2011 (doi:10.1152/japplphysiol.00821.2011) [DOI] [PubMed] [Google Scholar]
- 29.Petschow D, Wurdinger I, Baumann R, Duhm J, Braunitzer G, Bauer C. 1977. Causes of high blood O2 affinity of animals living at high altitude. J. Appl. Physiol. 42, 139–143 [DOI] [PubMed] [Google Scholar]
- 30.Swan LW. 1961. Ecology of the high Himalayas. Sci. Am. 205, 68–78 10.1038/scientificamerican1061-68 (doi:10.1038/scientificamerican1061-68) [DOI] [Google Scholar]
- 31.Faraci FM. 1986. Circulation during hypoxia in birds. Comp. Biochem. Physiol. A, Physiol. 85, 613–620 10.1016/0300-9629(86)90270-7 (doi:10.1016/0300-9629(86)90270-7) [DOI] [PubMed] [Google Scholar]
- 32.Blum A. 1998. Annapurna: a woman‘s place, p. 272 San Francisco, CA: Sierra Book Clubs [Google Scholar]
- 33.West JB. 2006. Human responses to extreme altitudes. Integr. Comp. Biol. 46, 25–34 10.1093/icb/icj005 (doi:10.1093/icb/icj005) [DOI] [PubMed] [Google Scholar]
- 34.Pennycuick CJ. 2008. Modelling the flying bird. London, UK: Academic Press [Google Scholar]
- 35.West JB, Lahiri S, Maret KH, Peters RM, Pizzo CJ. 1983. Barometric pressures at extreme altitudes on Mt. Everest: physiological significance. J. Appl. Physiol. 54, 1188–1194 [DOI] [PubMed] [Google Scholar]
- 36.Klaassen M, Beekman J, Kontiokorpi J, Mulder RW, Nolet B. 2004. Migrating swans profit from favourable changes in wind conditions at low altitude. J. Ornithol. 145, 142–151 10.1007/s10336-004-0025-x (doi:10.1007/s10336-004-0025-x) [DOI] [Google Scholar]
- 37.Liechti F. 2006. Birds: blowin’ by the wind? J. Ornithol. 147, 202–211 10.1007/s10336-006-0061-9 (doi:10.1007/s10336-006-0061-9) [DOI] [Google Scholar]
- 38.Hedenström A, Alerstam T, Green M, Gudmundsson G. 2002. Adaptive variation of airspeed in relation to wind, altitude and climb rate by migrating birds in the Arctic. Behav. Ecol. Sociobiol. 52, 308–317 10.1007/s00265-002-0504-0 (doi:10.1007/s00265-002-0504-0) [DOI] [Google Scholar]
- 39.Bohrer G, Brandes D, Mandel JT, Bildstein KL, Miller TA, Lanzone M, Katzner T, Maisonneuve C, Tremblay JA. 2012. Estimating updraft velocity components over large spatial scales: contrasting migration strategies of golden eagles and turkey vultures. Ecol. Lett. 15, 96–103 10.1111/j.1461-0248.2011.01713.x (doi:10.1111/j.1461-0248.2011.01713.x) [DOI] [PubMed] [Google Scholar]
- 40.Sapir N, Wikelski M, McCue MD, Pinshow B, Nathan R. 2010. Flight modes in migrating European bee-eaters: heart rate may indicate low metabolic rate during soaring and gliding. PLoS ONE 5, e13956. 10.1371/journal.pone.0013956 (doi:10.1371/journal.pone.0013956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finn J, Carlsson J, Kelly T, Davenport J. 2012. Avoidance of headwinds or exploitation of ground effect—why do birds fly low? J. Field Ornithol. 83, 192–202 10.1111/j.1557-9263.2012.00369.x (doi:10.1111/j.1557-9263.2012.00369.x) [DOI] [Google Scholar]
- 42.Grönroos J, Green M, Alerstam T. 2012. To fly or not to fly depending on winds: shorebird migration in different seasonal wind regimes. Anim. Behav. 83, 1449–1457 10.1016/j.anbehav.2012.03.017 (doi:10.1016/j.anbehav.2012.03.017) [DOI] [Google Scholar]
- 43.Pennycuick C, Griffin L, Colhoun K, Angwin R. 2011. A trial of a non-statistical computer program for monitoring fuel reserves, response to wind and other details from GPS tracks of migrating geese. J. Ornithol. 152, 87–99 10.1007/s10336-010-0633-6 (doi:10.1007/s10336-010-0633-6) [DOI] [Google Scholar]
- 44.Fort M. 2000. Glaciers and mass wasting processes: their influence on the shaping of the Kali Gandaki valley (higher Himalaya of Nepal). Quat. Int. 65/66, 101–119 10.1016/S1040-6182(99)00039-7 (doi:10.1016/S1040-6182(99)00039-7) [DOI] [Google Scholar]
- 45.Miyabayashi Y, Mundkur T. 1999. Atlas of key sites for Anatidae in the east Asian flyway. Tokyo, Japan: Wetlands International [Google Scholar]
- 46.Pennycuick CJ. 1975. Mechanics of flight. In Avian biology (eds Farner DS, King JR, Parkes KC.), pp. 1–75 New York, NY: Academic Press [Google Scholar]
- 47.Bishop CM. 2005. Circulatory variables and the flight performance of birds. J. Exp. Biol. 208, 1695–1708 10.1242/jeb.01576 (doi:10.1242/jeb.01576) [DOI] [PubMed] [Google Scholar]
- 48.West JB, et al. 1983. Maximal exercise at extreme altitudes on Mount Everest. J. Appl. Physiol. 55, 688–698 [DOI] [PubMed] [Google Scholar]
- 49.Butler PJ. 2010. High fliers: the physiology of bar-headed geese. Comp. Biochem. Physiol. A, Mol. Integr. Physiol. 156, 325–329 10.1016/j.cbpa.2010.01.016 (doi:10.1016/j.cbpa.2010.01.016) [DOI] [PubMed] [Google Scholar]
- 50.Phillips RA, Xavier JC, Croxall JP, Burger AE. 2003. Effects of satellite transmitters on albatross and petrels. Auk 120, 1082–1090 [Google Scholar]
- 51.Wilson RP, McMahon CR. 2006. Measuring devices on wild animals: what constitutes acceptable practice? Front. Ecol. Environ. 4, 147–154 10.1890/1540-9295(2006)004[0147:MDOWAW]2.0.CO;2 (doi:10.1890/1540-9295(2006)004[0147:MDOWAW]2.0.CO;2) [DOI] [Google Scholar]
- 52.Barron DG, Brawn JD, Weatherhead PJ. 2010. Meta-analysis of transmitter effects on avian behaviour and ecology. Methods Ecol. Evol. 1, 180–187 10.1111/j.2041-210X.2010.00013.x (doi:10.1111/j.2041-210X.2010.00013.x) [DOI] [Google Scholar]
- 53.Vandenabeele S, Shepard E, Grogan A, Wilson RP. 2012. When three per cent may not be three per cent: device-equipped seabirds experience variable flight constraints. Mar. Biol. 159, 1–14 10.1007/s00227-011-1784-6 (doi:10.1007/s00227-011-1784-6) [DOI] [Google Scholar]
- 54.Pennycuick C, Fast P, Ballerstädt N, Rattenborg N. 2012. The effect of an external transmitter on the drag coefficient of a bird's body, and hence on migration range, and energy reserves after migration. J. Ornithol. 153, 633–644 10.1007/s10336-011-0781-3 (doi:10.1007/s10336-011-0781-3) [DOI] [Google Scholar]
- 55.Portugal SJ, Green JA, Butler PJ. 2007. Annual changes in body mass and resting metabolism in captive barnacle geese (Branta leucopsis): the importance of wing moult. J. Exp. Biol. 210, 1391–1397 10.1242/jeb.004598 (doi:10.1242/jeb.004598) [DOI] [PubMed] [Google Scholar]
- 56.Coyne MS, Godley BJ. 2005. Satellite Tracking and Analysis Tool (STAT): an integrated system for archiving, analyzing and mapping animal tracking data. Mar. Ecol. Prog. Ser 301, 1–7 10.3354/meps301001 (doi:10.3354/meps301001) [DOI] [Google Scholar]
- 57.Kranstauber B, Cameron A, Weinzerl R, Fountain T, Tilak S, Wikelski M, Kays R. 2011. The Movebank data model for animal tracking. Environ. Model. Softw. 26, 834–835 10.1016/j.envsoft.2010.12.005 (doi:10.1016/j.envsoft.2010.12.005) [DOI] [Google Scholar]
- 58.Dee DP, et al. 2011. The ERA-Interim reanalysis: configuration and performance of the data assimilation system. Q. J. R. Meteorol. Soc. 137, 553–597 10.1002/qj.828 (doi:10.1002/qj.828) [DOI] [Google Scholar]
- 59.Lipp HP, Vyssotski AL, Wolfer DP, Renaudineau S, Savini M, Troster G, Dell'Omo G. 2004. Pigeon homing along highways and exits. Curr. Biol. 14, 1239–1249 10.1016/j.cub.2004.07.024 (doi:10.1016/j.cub.2004.07.024) [DOI] [PubMed] [Google Scholar]
- 60.Zar JH. 1999. Biostatistical analysis, 4th edn, p. 663 Upper Saddle River, NJ: Pearson Education [Google Scholar]