Abstract
A facile one-step strategy is explored for the achievement of uniform luminescent AuNPs in two-dimensional ultrathin silica matrix based on simultaneous reaction and assembly at the liquid-liquid interface. Meanwhile, the as-prepared AuNPs/silica nanocomposites can be further employed to fabricate micrometer-thick film with bifunctional luminescent and superhydrophobic properties. Such versatile concept is an ideal candidate for the development of multifunctional devices.
Keywords: fluorescence, gold NPs, ultrathin film, interfacial assembly, superhydrophobic
The assembly of zero dimensional nanoparticles (NPs) into two-dimensional (2D) or 3D architectural materials is a critical step in the development of novel optoelectronic, magnetic and biological devices with NPs as functional components.[1] To date, various types of NPs such as quantum dots (QDs), plasmonic metal NPs, and magnetic NPs have served as building blocks for this purpose.[2] In addition, a variety of elegant techniques have been developed for assembling NPs into a targeted 2D membrane, including Langmuir-Blodgett technique,[3] layer-by-layer process,[4] molecular-directed templates,[5] field-induced interaction[6], etc. In particular, liquid-liquid interface, as a versatile platform, plays an important role in the synthesis of NPs with uniform size distribution and the control of their assembly behaviors.[7] While successful creation of diverse functional 2D nanomaterials has been made by minimizing interfacial energy,[8] most of the as-prepared 2D membranes composed of densely packed NPs are vulnerable due to the weak interactions among NPs. In order to fabricate mechanically robust and elastic membranes with NPs, chemical crosslinking through functional ligands on the particle surface are needed.[9] Russell’s group designed a series of cross-linking routes to prepare stable membranes of QDs and gold NPs (AuNPs) at fluid interfaces.[10] As an alternative, a cross-linking approach based on coordination chemistry has also been employed to create robust membrane of FePt NPs at the liquid-liquid interface.[11] Moreover, a nonchemical strategy for constructing durable magnetic colloidosomes at the toluene/water interface was also reported.[12] Despite these great progresses, it is still a great challenge to simultaneously realize the interfacial synthesis of NPs and the assembly of them into robust 2D membrane at the liquid-liquid interface.
Herein, we demonstrate a one-step procedure to fabricate stable, luminescent and ultrathin (average thickness of ~3.3 nm) AuNPs membrane in silica matrix (AuNPs/silica membrane) at the toluene/water interface. Luminescent AuNPs with triplet-state emission are a type of intermediate gold nanostructures that bridge a link between the few-atom gold nanoclusters and traditional surface plasmonic AuNPs.[13] These AuNPs exhibited bright emissions with lifetimes on the microsecond time scale due to the ligand-metal charge transfer between gold(I) and the surface ligands.[14] The tunable emission with large stokes shift and easily modified surface chemistry enable these luminescent AuNPs to serve as a new platform for chemical sensing.[15] For example, luminescent AuNPs have demonstrated great promise as optical indicators for sensing metal ions (e.g., Cu2+, Hg2+),[16] small biomolecules,[17] and protein.[18] While these luminescent AuNPs are particularly interesting, very limited efforts have been devoted to integrating individual luminescent AuNPs into 2D membranes, where they can serve as key components in the potential portable sensing devices in the future. In this work, we report a facile interfacial assembly method for the fabrication of ultrathin luminescent AuNPs/silica membrane. Different from most of the classical procedures, where NPs were prepared first and then followed by an additional assembly step, in this work, the fabrication and organization processes of NPs were accomplished in one-step, which could offer a new route for the assembly techniques.
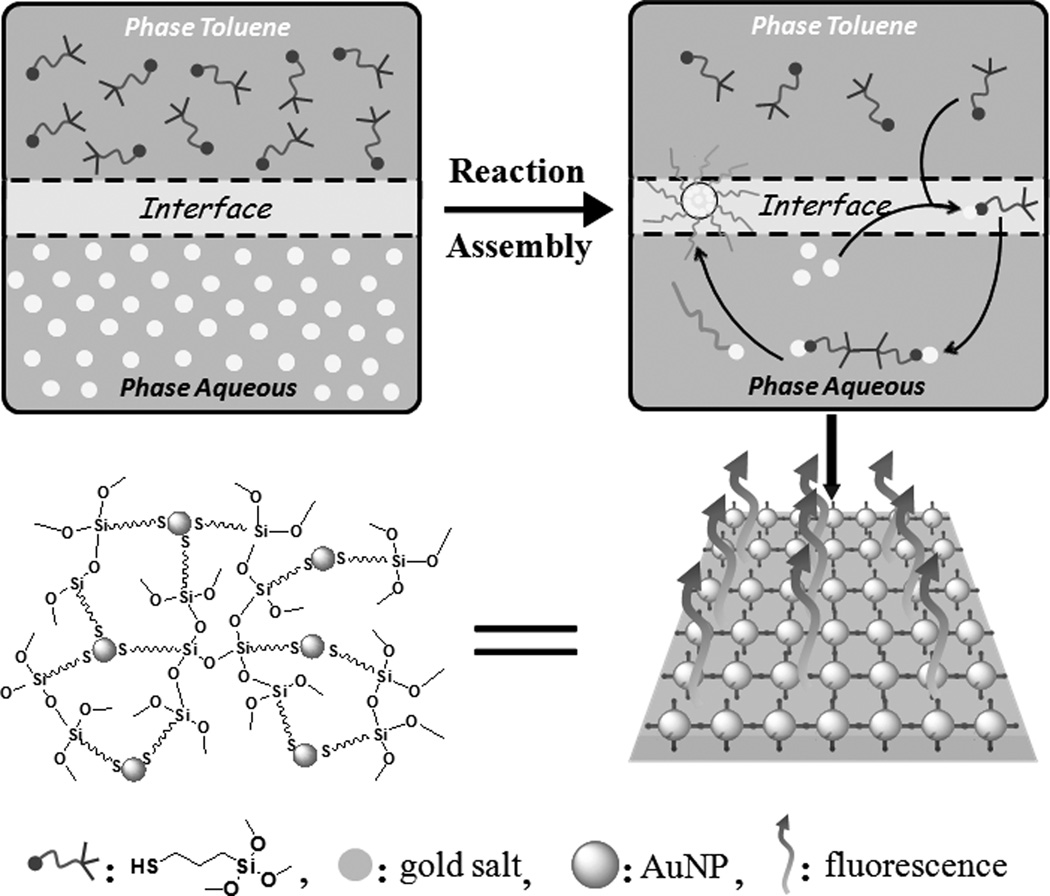
In the present study, ultrathin luminescent AuNPs/silica composite membrane was achieved in one-step at the toluene/water interface using 3-mercaptopropyltrimethoxysilane (MPTS) as a bifunctional ligand (Figure 1). Initially, MPTS immediately reacted with gold(III) ions to form Au(I)-MPTS polymers at the toluene/water interface due to the reduction of thiol group by forming the strong Au(I)-S bond. At the same time, similar to the Stöber method of hydrolyzing tetraethylorthosilicate (TEOS),[19] the rapid acid-catalyzed hydrolysis of MPTS also took place immediately to form silica matrix because of the presence of H+ from HAuCl4. Subsequently, the Au(I)-MPTS polymers gradually dissociated into luminescent AuNPs,[13a, 20] which were naturally and homogeneously in-situ embedded in the silica matrix. The FT-IR spectrum indicated the characteristic asymmetrical stretching peaks of the siloxane bond (Si-O-Si) at 1100 and 1025 cm−1 (Figure S2), which confirmed the formation of cross-linked silica matrix. It should be noted that a typical weak peak of the free thiol group at ~2560 cm−1 still existed because of the excess MPTS in the reaction system. The luminescence properties of the AuNPs/silica composites were examined in the powder form using solid-state fluorescence spectroscopy (Figure S3). The maximum excitation and emission of AuNPs/silica composites were located at 360 nm and 635 nm, respectively (Figure 2A). The large Stokes shift (275 nm) and full-width at half-maximum (fwhm, ~150 nm) indicated that the photoluminescence mainly originated from donor-acceptor (MPTS→Au(I)) charge transfer.[14] Figure 2B shows the time-resolved photoluminescence of AuNPs/silica. The corresponding fitting curve was calculated with an exponential decay based on nonlinear least-square (equal S1), and the average lifetime τ̄ was calculated to be ~16.64 µs, this long excited-state lifetime suggested that the red emission of AuNPs/silica at ~635 nm could be assigned to triplet excited states,[13, 14, 22] which is consistent with the observed charge transfer behavior between MPTS and Au(I).
Figure 1.
Schematic illustration of the synthesis and assembly of ultrathin luminescent AuNPs/silica membrane at the toluene/water interface. See Figure S1 in the Supporting Information for the same figure with color.
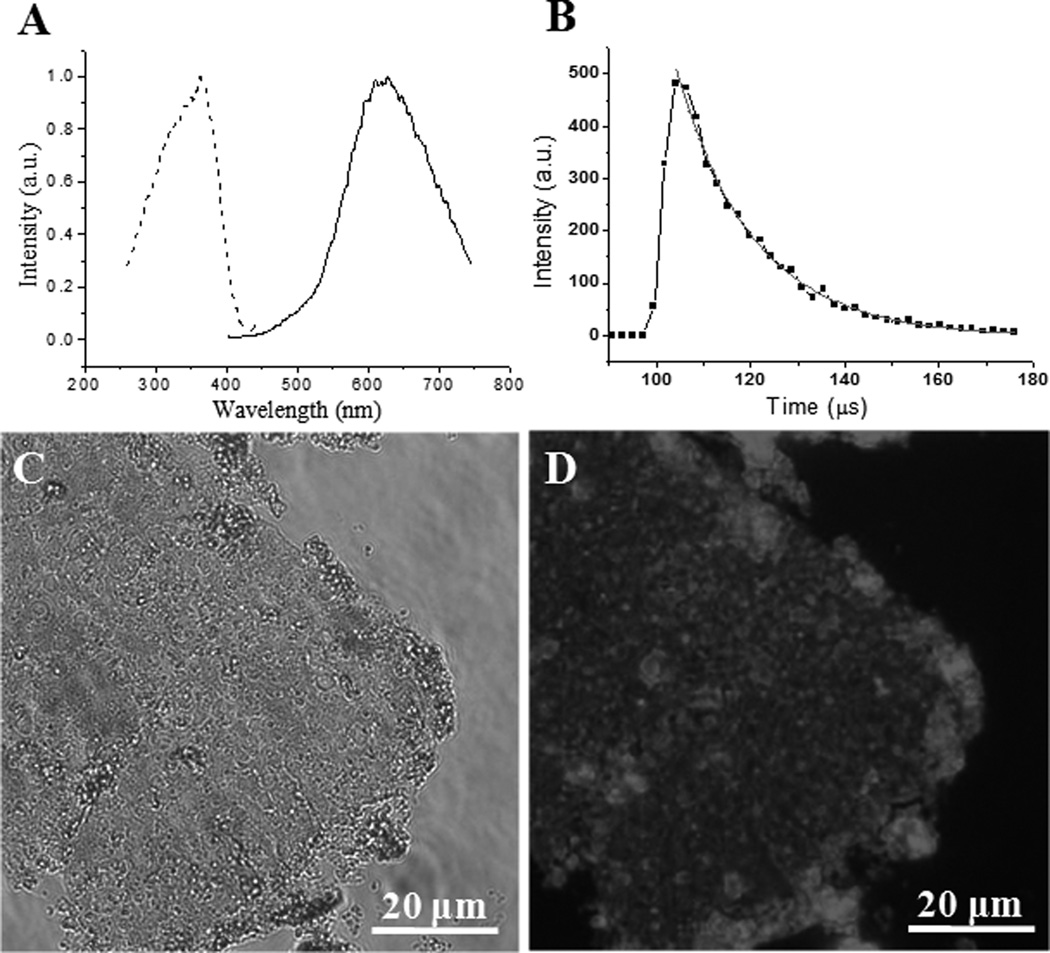
Figure 2.
Luminescence properties and microscopy images of the luminescent AuNPs/silica membrane. (A) Solid state excitation (λemmax = 640 nm, dash line) and emission (λexmax = 360 nm, solid line) spectra. (B) Time-resolved luminescence decay curve measured at emission peak maxima of 640 nm. The sample was excited at 360 nm. Exponential decay function was used for satisfactory fitting (χ2 < 1.15). (C, D) Brightfield (C) and luminescence (D) microscopy images.
The high interfacial tension at the liquid-liquid interface allows both molecular reactions and particle assembly to be rapidly accomplished. Hence, unlike previous reactions carried out in homogenous system,[21] where the hydrolysis of MPTS produced spherical structures, the AuNPs/silica composites showed an ultrathin 2D structure, in virtue of the confinement effect of the liquid-liquid interface. The membrane of luminescent AuNPs/silica obtained at the toluene/water interface can be transferred onto a microscope cover glass by drop casting membrane on water surface, which can be directly imaged using fluorescence microscopy. As shown in the bright-field image (Figure 2C), the AuNPs/silica film displayed a uniform 2D membrane structure in a relatively large scale (tens of micrometer). The corresponding luminescence image also revealed an evenly distributed luminescent signal (Figure 2D), suggesting the luminescent AuNPs were homogenously embedded in the continuous amorphous silica matrix.
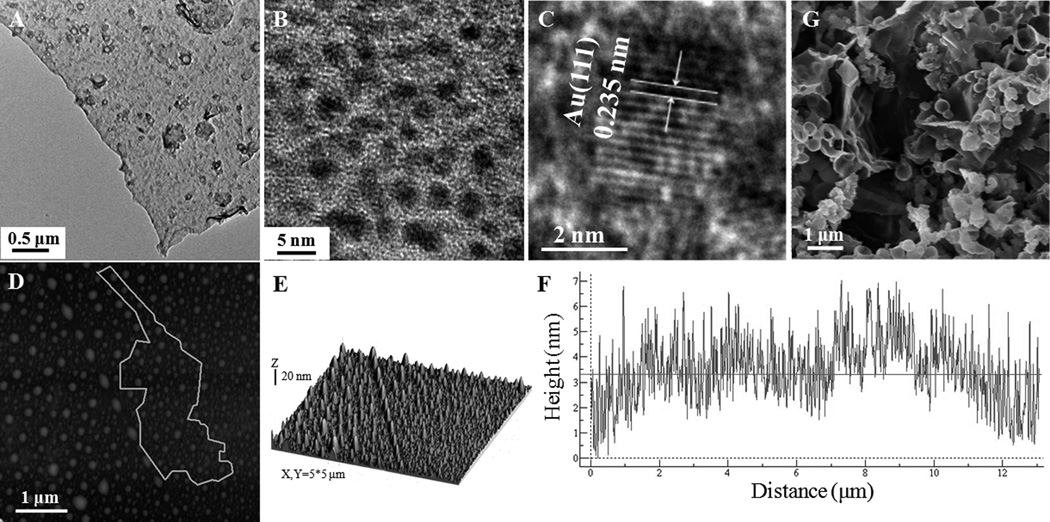
To further validate the formation of AuNPs and map their distribution in the silica matrix, transmission electron microscopy (TEM) was used to directly observe the morphologies of the AuNPs/silica membrane. As shown in the TEM image (Figure 3A), the AuNPs/silica membrane appeared to be flat and foldable. It should be noted that some agglomerates were adsorbed on the AuNPs/silica membrane. This is due to the formation of small AuNPs/silica capsules during the sample preparation, and the reasons for the appearance of capsules will be explained in the following section. The high-resolution TEM (HRTEM) image revealed that monodisperse AuNPs with the diameter of 2.34±0.32 nm were uniformly incorporated in the silica matrix (Figure 3B and S4). The lattice space of the AuNPs measured from the HRTEM images (Figure 3C) is 0.235 nm, corresponding to the d spacing of the (111) crystal plane of face centered cubic (FCC) Au.[24] The structural features of AuNPs/silica membrane were further analyzed using atom force microscopy (AFM). The tapping mode AFM data demonstrated protuberances on the film after AuNPs/silica assemblies were transferred from toluene/water interface to the silicon wafer (Figures 3D and E). The formation of protuberances can be partially ascribed to the flexibility of AuNPs/silica composite membrane, but primarily because of its hydrophobic trait, which facilitated the formation of water droplets encapsulated in the AuNPs/silica membrane. Similar phenomena was found in the formation of ultrathin capsules of quantum dots at oil/water interface.[7c, 10b] To further verify whether the AuNPs/silica membrane has a trend to engender 3D capsule structure, the membrane at the toluene/water interface was vigorously shaken in a vial and then transferred into a glass dish for microscope observation. The AuNPs/silica membrane coated water droplets with a diameter of ~10 µm were clearly seen in the bright-field and luminescence images (Figure S5A, B). In fact, large capsules (even macroscopic capsules at millimeter scale, data are not shown here) can be obtained by soft shaking, and these large capsules could be divided into many smaller capsules via strong shaking, although it is hard to control the size and polydispersity of the capsules at the current stage.[7c]
Figure 3.
(A-C) Low magnification (A) and high resolution TEM images of luminescent AuNPs/silica membrane formed at toluene/water interface, showing well-dispersed individual AuNPs in silica matrix. (D, E) AFM images of luminescent AuNPs/silica membrane in taping mode. (F) Line profile along the line in (D). (G) SEM image of luminescent AuNPs/silica capsules achieved via vigorously shaking of the corresponding membrane.
Subsequently, to acquire scanning electron microscope (SEM) image, a capsule sample obtained by intensively shaking was transferred onto a solid substrate. As shown in Figure 3G, the hollow spherical feature was maintained in the capsules with a diameter less than ~2 µm. However, the large capsules collapsed because the mechanical strength of AuNPs/silica composites is not strong enough to maintain their capsular structures during the dry process. Based on these results, we can conclude that the formation of protuberances on the AuNPs/silica membrane is ascribed to its flexibility and hydrophobicity, and inevitable fluctuation in the transfer process. Consequently, in order to detect the real thickness of the membrane, the protuberances were avoided in the statistic analysis. The step height analysis illustrated that the average thickness of the AuNPs/silica membrane is ~3.3 nm (Figure 3F), which is a little larger than the diameter of the AuNPs (~2.3 nm), suggesting that the AuNPs were nearly incorporated into the silica matrix as monolayer. The average height of the entire AuNPs/silica membrane is ~6 nm, which is slightly higher than the thickness of the membrane due to the existence of protuberances (Figure S6). Furthermore, the fluorescence of this assembled 2D AuNPs/silica membrane was remarkably stable at the toluene/water interface as well as in the dried powder form for at least six months under R.T., allowing for potential sensor application. Different from most of the previous reports,[2b, 7b, 23] where the pre-fabricated AuNPs were closely packed in the thin films via weak interaction, the assembly strategy of AuNPs by covalent cross-linking of functional ligands is undoubtedly efficient to achieve robust membrane. On one hand, the silica matrix in this system via cross-linking of MPTS has flexibility similar to polymers, implying a straightforward approach to prepare stable, large scale and multilayered membranes. On the other hand, the silica matrix also offers other great advantages over polymers, such as high thermal stability and good phase compatibility. Therefore, this facile interfacial strategy provides a promising route to fabricate diverse new functional composite membranes for practical application. Meanwhile, we believe that the procedure described here is not only limited to the AuNPs, but also can be explored as a general strategy for the accomplishment of diverse 2D nanomaterials.
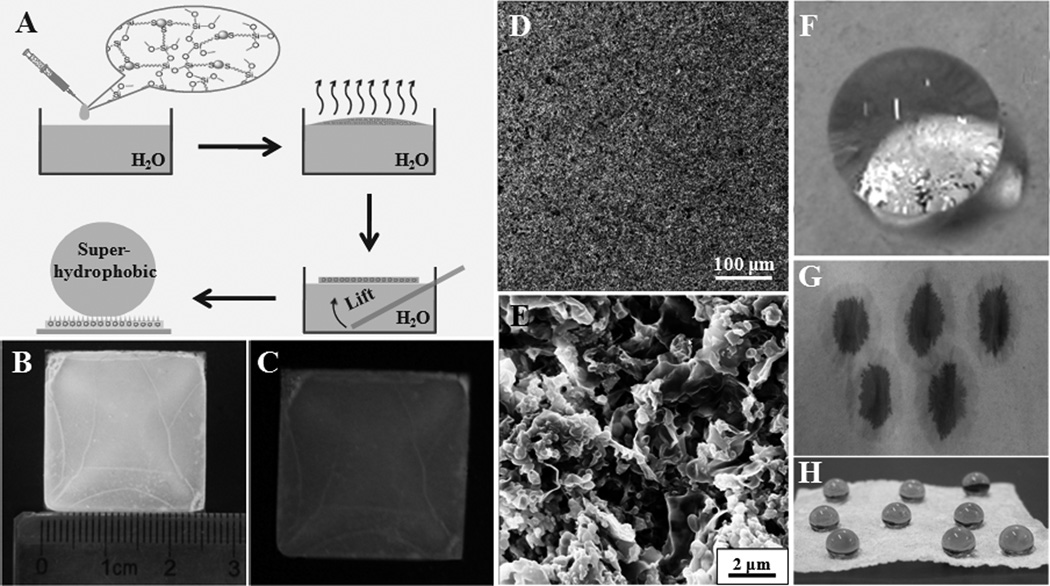
The as-prepared ultrathin luminescent AuNPs/silica membrane exhibited hydrophobic property due to its ability to float at the toluene/water interface. To investigate whether these AuNPs/silica nanocomposites could construct superhydrophobic film, we performed further assembly process using the interface effect. In general, a surface with a water contact angle (CA) higher than 150° is termed as superhydrophobic surface, which has attracted great interests owing to its importance in fundamental research, inspired mimetic attempts and industrial applications.[25] Initially, the water in the sample was removed and the AuNPs/silica nanocomposites was dispersed in toluene by sonication, and then the toluene solution containing AuNPs/silica nanocomposites was added onto the water surface in a glass dish. The thickness and scale of the formed multilayered AuNPs/silica film can be easily tuned by varying the volume of toluene solution containing AuNPs nanocomposites (Figure 4A). Figure 4B, C showed typical images of as-prepared superhydrophobic AuNPs/silica film under daylight and ultraviolet light (365 nm), respectively. As indicated in these images, the film represented smooth trait and stable red fluorescence in macroscale. Upon dropping water (8 µL) on the luminescent AuNPs/silica film (Figure 4F), the water displayed a spherical droplet on the surface. The CA calculated by Young-Laplace equation was about 153° as demonstrated in Figure S8. In this case, the superhydrophobic properties of the film of luminescent AuNPs/silica nanocomposites could be assigned to the micro/nanoscales binary microsturctures (Figure 4D, E), allowing air to be trapped and expressing unique superhydrophobicity.[26] The thickness of AuNPs/silica film in this typical procedure is ~35 µm according to the SEM image of the cross-section of the film (Figure S9). The effects of the film thickness on the hydrophobicity were also investigated. Typically, the AuNPs/silica nanocomposite film with the thickness of ~30 µm had superhydrophobicity (Figure S10). As the film thickness increased, the water repellency slowly decreased (Figure S10). Such phenomenon can be attributed to the reduced surface roughness of the thicker film. During the film formation process, toluene was gradually volatilized to leave AuNPs/silica nanocomposites to form the film and thus the thicker film required relatively long time, which allowed the AuNPs/silica nanocomposites to build a compact structure with low roughness surface. This process was confirmed by the SEM images of the ~165 µm-thick AuNPs/silica nanocomposite film, displaying lower roughness microstructures compared to the ~35 µm-thick film (Figure S11). Moreover, the luminescent AuNPs/silica nanocomposites could be employed to tune the wettability of other materials. To be specific, a piece of hygroscopic paper was immersed in the toluene solution of AuNPs/silica nanocomposites (~1 wt%) and dried in room atmosphere. The photographs of the hygroscopic paper without and with the modification of AuNPs/silica nanocomposites, respectively, implied obvious switch of the superhydrophilicity to superhydrophobicity of the paper surface (Figure 4G, H). SEM images showed the microstructure difference of the paper with and without the treatment using AuNPs/silica nanocomposites (Figure S12). Without the modification of AuNPs/silica, the hygroscopic paper had the knitted fibrous microstructure (Figure S12A, B). After the modification with AuNPs/silica nanocomposites, many flow-like AuNPs/silica microclusters were deposited on the paper, as shown in Figure S12C, D. These microclusters not only enhanced the surface roughness but also reduced the surface energy, leading to the formation of superhydrophobic surface on the paper. In comparison with the previously reported works,[27] the bifunctional film with luminescence and superhydrophibicity fabricated at the interface offers many advantages, such as simplified process, good stability, and easy scale-up. These results suggested that the interfacial assembly strategy has profound prospects in the achievement of multifunctional nanomaterials.
Figure 4.
(A) Schematic representation of the procedure for fabricating the luminescent and superhydrophobic film composed of AuNPs/silicananocomposites. (B, C) Photographs of the assembled superhydrophobic AuNPs/silica film taken under daylight (B) and UV light (365 nm, C), respectively. (D, E) Low and high magnification SEM images of superhydrophobic AuNPs/silica film. (F) Profile of a water droplet (8 µL) on the AuNPs/silica film. (G, H) Photographs of water drops (dyed by trypan blue) on the hygroscopic paper before (G) and after (H) the modification with AuNPs/silica nanocomposites. See Figure S7 in the Supporting Information for the same figure with color.
In summary, we have developed a one-step interfacial strategy for the synthesis and assembly of luminescent AuNPs into silica matrix. To the best of our knowledge, this work is the first example on the MPTS-directed synthesis of luminescent AuNPs at interface. The integration of fabrication and assembly process in one-step may provide a pervasive concept in the achievement of 2D nanoparticle arrays. Moreover, the as-prepared AuNPs/silica nanocomposites can be further employed to fabricate luminescent and superhydrophobic films with multifunctional applications. Further efforts paid on the development of various composite membranes at interface will hold promise for the integration of desirable properties in versatile devices.
Experimental Section
Experimental materials and methods as well as additional results can be found in the Supporting Information.
Supplementary Material
Acknowledgements
This work was supported in part by the NIH (R21EB009853 to J.Z.), CPRIT (RP120588 to J.Z) and the start-up fund from the University of Texas at Dallas (J.Z). Authors also thanks Sreekar Marpu, Qi Wang, Mohammad A. Omary in Department of Chemistry at University of North Texas for helping on the measurements of luminescence lifetime and solid-state luminescence spectra of luminescent AuNPs/silica membrane.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
References
- 1.a) Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. Nature. 1996;382:607. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]; b) Shenhar R, Norsten TB, Rotello VM. Adv. Mater. 2005;17:657. [Google Scholar]; c) Kiely CJ, Fink J, Brust M, Bethell D, Schiffrin DJ. Nature. 1998;396:444. [Google Scholar]; d) Whitesides GM, Grzybowski B. Science. 2002;295:2418. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]; e) Park S, Lim J-H, Chung S-W, Mirkin CA. Science. 2004;303:348. doi: 10.1126/science.1093276. [DOI] [PubMed] [Google Scholar]; f) Nie Z, Petukhova A, Kumacheva E. Nat. Nanotechnol. 2010;5:15. doi: 10.1038/nnano.2009.453. [DOI] [PubMed] [Google Scholar]; g) Dong A, Chen J, Vora PM, Kikkawa JM, Murray CB. Nature. 2010;466:474. doi: 10.1038/nature09188. [DOI] [PubMed] [Google Scholar]; h) Lu W, Lieber CM. Nat. Mater. 2007;6:841. doi: 10.1038/nmat2028. [DOI] [PubMed] [Google Scholar]
- 2.a) Tang Z, Zhang Z, Wang Y, Glotzer SC, Kotov NA. Science. 2006;314:274. doi: 10.1126/science.1128045. [DOI] [PubMed] [Google Scholar]; b) Alivisatos AP, Johnsson KP, Peng X, Wilson T, Loweth CJ, Bruchez MP., Jr Nature. 1996;382:609. doi: 10.1038/382609a0. [DOI] [PubMed] [Google Scholar]; c) Zheng R, Gu H, Xu B, Fung KK, Zhang X, Ringer SP. Adv. Mater. 2006;18:2418. [Google Scholar]
- 3.a) Aleksandrovic V, Greshnykh D, Randjelovic I, Frömsdorf A, Kornowski A, Roth SV, Klinke C, Weller H. ACS Nano. 2008;2:1123. doi: 10.1021/nn800147a. [DOI] [PubMed] [Google Scholar]; b) Acharya S, Hill JP, Ariga K. Adv. Mater. 2009;21:2959. [Google Scholar]; c) Chen X, Lenhert S, Hirtz M, Lu N, Fuchs H, Chi L. Acc. Chem. Res. 2007;40:393. doi: 10.1021/ar600019r. [DOI] [PubMed] [Google Scholar]
- 4.a) Jiang C, Tsukruk VV. Adv. Mater. 2006;18:829. [Google Scholar]; b) Markutsya S, Jiang C, Pikus Y, Tsukruk VV. Adv. Funct. Mater. 2005;15:771. [Google Scholar]
- 5.a) Mao Z, Xu H, Wang D. Adv. Funct. Mater. 2010;20:1053. [Google Scholar]; b) Zhang J, Li Y, Zhang X, Yang B. Adv. Mater. 2010;22:4249. doi: 10.1002/adma.201000755. [DOI] [PubMed] [Google Scholar]
- 6.a) Min Y, Akbulut M, Kristiansen K, Golan Y, Israelachvili J. Nat. Mater. 2008;7:527. doi: 10.1038/nmat2206. [DOI] [PubMed] [Google Scholar]; b) Ahniyaz A, Sakamoto Y, Bergström L. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17570. doi: 10.1073/pnas.0704210104. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Ryan KM, Mastroianni A, Stancil KA, Liu H, Alivisatos AP. Nano Lett. 2006;6:1479. doi: 10.1021/nl060866o. [DOI] [PubMed] [Google Scholar]; d) Bahns JT, Sankaranarayanan SKRS, Gray SK, Chen L. Phys. Rev. Lett. 2011;106:095501. doi: 10.1103/PhysRevLett.106.095501. [DOI] [PubMed] [Google Scholar]
- 7.a) Binder WH. Angew. Chem. Int. Ed. 2005;44:5172. doi: 10.1002/anie.200501220. [DOI] [PubMed] [Google Scholar]; b) Duan H, Wang D, Kurth DG, Möhwald H. Angew. Chem. Int. Ed. 2004;43:5639. doi: 10.1002/anie.200460920. [DOI] [PubMed] [Google Scholar]; c) Lin Y, Skaff H, Emrick T, Dinsmore AD, Russell TP. Science. 2003;299:226. doi: 10.1126/science.1078616. [DOI] [PubMed] [Google Scholar]; d) Wang X, Zhuang J, Peng Q, Li Y. Nature. 2005;437:121. doi: 10.1038/nature03968. [DOI] [PubMed] [Google Scholar]; e) Rao TUB, Pradeep T. Angew. Chem. Int. Ed. 2010;49:3925. doi: 10.1002/anie.200907120. [DOI] [PubMed] [Google Scholar]
- 8.a) Capito RM, Azevedo HS, Velichko YS, Mata A, Stupp SI. Science. 2008;319:1812. doi: 10.1126/science.1154586. [DOI] [PubMed] [Google Scholar]; b) Yang S, Wang C-F, Chen S. J. Am. Chem. Soc. 2011;133:8412. doi: 10.1021/ja201194d. [DOI] [PubMed] [Google Scholar]; c) Tang SKY, Derda R, Mazzeo AD, Whitesides GM. Adv. Mater. 2011;23:2413. doi: 10.1002/adma.201100067. [DOI] [PubMed] [Google Scholar]
- 9.Böker A, He J, Emrick T, Russell TP. Soft Matter. 2007;3:1231. doi: 10.1039/b706609k. [DOI] [PubMed] [Google Scholar]
- 10.a) Lin Y, Skaff H, Böker A, Dinsmore AD, Emrick T, Russell TP. J. Am. Chem. Soc. 2003;125:12690. doi: 10.1021/ja036919a. [DOI] [PubMed] [Google Scholar]; b) Skaff H, Lin Y, Tangirala R, Breitenkamp K, Böker A, Russell TP, Emrick T. Adv. Mater. 2005;17:2082. [Google Scholar]; c) Glogowski E, Tangirala R, He J, Russell TP, Emrick T. Nano Lett. 2007;7:389. doi: 10.1021/nl062581h. [DOI] [PubMed] [Google Scholar]
- 11.Arumugam P, Patra D, Samanta B, Agasti SS, Subramani C, Rotello VM. J. Am. Chem. Soc. 2008;130:10046. doi: 10.1021/ja802178s. [DOI] [PubMed] [Google Scholar]
- 12.Duan H, Wang D, Sobal NS, Giersig M, Kurth DG, Möhwald H. Nano Lett. 2005;5:949. doi: 10.1021/nl0505391. [DOI] [PubMed] [Google Scholar]
- 13.a) Zhou C, Sun C, Yu M, Qin Y, Wang J, Kim M, Zheng J. J. Phys. Chem. C. 2010;114:7727. doi: 10.1021/jp9122584. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zheng J, Zhang C, Dickson RM. Phys. Rev. Lett. 2004;93:077402. doi: 10.1103/PhysRevLett.93.077402. [DOI] [PubMed] [Google Scholar]
- 14.a) Link S, Beeby A, FitzGerald S, El-Sayed MA, Schaaff TG, Whetten RLJ. Phys. Chem. B. 2002;106:3410. [Google Scholar]; b) Lin CAJ, Yang TY, Lee CH, Huang SH, Sperling RA, Zanella M, Li JK, Shen JL, Wang HH, Yeh HI, Parak WJ, Chang WH. ACS Nano. 2009;3:395. doi: 10.1021/nn800632j. [DOI] [PubMed] [Google Scholar]; c) Zhou R, Shi M, Chen X, Wang M, Chen H. Chem. Eur. J. 2009;15:4944. doi: 10.1002/chem.200802743. [DOI] [PubMed] [Google Scholar]; d) Zheng J, Nicovich PR, Dickson RM. Annu. Rev. Phys. Chem. 2007;58:409. doi: 10.1146/annurev.physchem.58.032806.104546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang L, Dong S, Nienhaus GU. Nano Today. 2011;6:401. [Google Scholar]
- 16.a) Chen W, Tu X, Guo X. Chem. Commun. 2009:1736. doi: 10.1039/b820145e. [DOI] [PubMed] [Google Scholar]; b) Huang C-C, Yang Z, Lee K-H, Chang H-T. Angew. Chem. Int. Ed. 2007;46:6824. doi: 10.1002/anie.200700803. [DOI] [PubMed] [Google Scholar]
- 17.a) Wen F, Dong Y, Feng L, Wang S, Zhang S, Zhang X. Anal. Chem. 2011;83:1193. doi: 10.1021/ac1031447. [DOI] [PubMed] [Google Scholar]; b) Shiang Y-C, Huang C-C, Chang H-T. Chem. Commun. 2009:3437. doi: 10.1039/b901916b. [DOI] [PubMed] [Google Scholar]
- 18.Shiang Y-C, Lin C-A, Huang C-C, Chang H-T. Analyst. 2011;136:1177. doi: 10.1039/c0an00889c. [DOI] [PubMed] [Google Scholar]
- 19.Stöber W, Fink A, Bohn E. J. Colloid Interface Sci. 1968;26:62. [Google Scholar]
- 20.Yu M, Zhou C, Liu J, Hankins JD, Zheng J. J. Am. Chem. Soc. 2011;133:11014. doi: 10.1021/ja201930p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.a) Beaudet L, Pitre R, Robillard L, Mercier L. Chem. Mater. 2009;21:5349. [Google Scholar]; b) Lu Z, Sun L, Nguyen K, Gao C, Yin Y. Langmuir. 2011;27:3372. doi: 10.1021/la1048216. [DOI] [PubMed] [Google Scholar]
- 22.Yam VW-W, Cheng EC-C, Zhou Z-Y. Angew. Chem., Int. Ed. 2000;39:1683. doi: 10.1002/(sici)1521-3773(20000502)39:9<1683::aid-anie1683>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.a) Yu A, Liang Z, Cho J, Caruso F. Nano Lett. 2003;3:1203. [Google Scholar]; b) Fan H, Yang K, Boye DM, Sigmon T, Malloy KJ, Xu H, LóPez GP, Brinker CJ. Science. 2004;304:567. doi: 10.1126/science.1095140. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu T, Teranishi T, Hasegawa S, Miyake M. J. Phys. Chem. B. 2003;107:2719. [Google Scholar]
- 25.a) Barthlott W, Neinhuis C. Planta. 1997;202:1. [Google Scholar]; b) Blossey R. Nat. Mater. 2003;2:301. doi: 10.1038/nmat856. [DOI] [PubMed] [Google Scholar]; c) Gao X, Jiang L. Nature. 2004;432:36. doi: 10.1038/432036a. [DOI] [PubMed] [Google Scholar]
- 26.a) Ma ML, Hill RM. Curr. Opin. Colloid Interface Sci. 2006;11:193. [Google Scholar]; b) Yao X, Song Y, Jiang L. Adv. Mater. 2011;23:719. doi: 10.1002/adma.201002689. [DOI] [PubMed] [Google Scholar]
- 27.a) Hou L, Wang C, Chen L, Chen S. J. Mater. Chem. 2010;20:3863. [Google Scholar]; b) Hong J, Bae WK, Lee H, Oh S, Char K, Caruso F, Cho J. Adv. Mater. 2007;19:4364. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.