Abstract
Purpose
We investigated the effects of desiccating stress on murine corneal apical epithelial cell area and desquamation by using 4 defined parameters and evaluated the effects of the metalloproteinase inhibitor doxycycline on this process.
Methods
C57BL/6 mice were subjected to experimental dry eye (EDE) for 5 days without or with topical therapy with doxycycline 0.025% or 0.0025% or vehicle 4 times a day. C57BL/6 mice that were not exposed to desiccating stress served as controls. Whole mount corneas from each group were immunostained for occludin and visualized by laser scanning confocal microscopy. The images were analyzed in a masked fashion, and mean individual cell area, apical cell density, average cell number loss, and average percent loss were recorded.
Results
EDE caused a significant decrease in apical corneal cell area (1073 ± 135.9 μm2), an increase in apical cell density (895.8 ± 115.4 cells per mm2) and a greater percent of epithelial loss (21.29% ± 13.40%) than controls (1341 ± 95.28 μm2, 714.4 ± 55.60 cells per mm2, 2.897% ± 3.452%, P < 0.001 for all, respectively). Treatment with 0.025% doxycycline preserved cell area (1337 ± 144.6 μm2) and the apical cell density (721.0 ± 91.62 cells per mm2) and decreased percentage loss (5.117% ± 6.757%) compared with the vehicle control group (1154 ± 88.10 μm2, 830.2 ± 49.76 cells per mm2, 22.14 ± 9.616%, P < 0.001 for all, respectively).
Conclusions
Desiccating stress decreases apical corneal epithelial cell area, increases apical cell density, and promotes epithelial cell loss. Treatment with the metalloproteinase inhibitor doxycycline during desiccating stress preserves cell area and apical cell density and prevents EDE-induced corneal epithelial cell loss. These findings suggest that metalloproteinases mediate apical corneal epithelial loss during desiccating stress.
Keywords: confocal microscopy, corneal epithelium, cell density, metalloproteinase, doxycycline
Dry eye is one of the most common ophthalmologic diseases. Although the prevalence of dry eye may vary depending on the criteria used to diagnose the condition, the 2007 International Dry Eye Workshop estimated that 5%–30% of the population older than 50 years suffers from symptoms of dry eye.1 The Salisbury Eye Study showed that 14.6% of the population aged 65 or above will suffer from one of the 6 common dry eye symptoms at some point, whereas a study of patients older than 65 years in Taiwan showed an astonishing 33.7% prevalence of one of the 6 symptoms.2,3 Because of advancing demographic trends in the United States and per the midrange prevalence estimate by the Dry Eye Workshop, by the year 2020, almost 9 million adults older than 65 years will have at least 1 symptom of dry eye (based on 2000 US Census Bureau data).
It is well recognized that dry eye causes disease of the superficial corneal epithelium.4 It has been demonstrated previously that matrix metalloproteinase-9 (MMP-9) levels are increased in the tears of mice with experimental dry eye (EDE) and in patients with dry eye with corneal epithelial disease or sterile corneal ulcers.5–8 MMP-9 has been demonstrated to increase both retinal endothelial and corneal epithelial permeability and to degrade the tight junction protein occludin.5,9 The expression of MMP-9 is upregulated by the proinflammatory cytokines interleukin-1 and tumor necrosis factor-α, both of which are increased in the ocular surface epithelia in murine EDE and in patients with dry eye disease.6,10,11 Also, MMP-9 production in the corneal epithelium may be stimulated by mitogen activated protein kinase pathways.11 Cor-neal epithelial disease is less severe in mice with an MMP-9 gene deletion than in wild-type mice.5 Also, when topical MMP-9 was administered to that knockout strain, the corneal permeability increased, indicating that the metalloproteinase disrupted corneal barrier function.5
Doxycycline was discovered in the early 1960s as a semisynthetic long-acting tetracycline derivative useful as a bacterial ribosome inhibitor in a wide variety of microbes. In subantimicrobial doses, it is also an effective primary treatment for rosacea and sterile corneal ulcerations and an effective adjunctive treatment for adult periodontitis.12–14 It also may play a role in prophylaxis for abdominal aortic aneurysms because they are thought to arise from inflammation and proteolysis in the vessel wall.15,16 Doxycycline has been shown to effectively inhibit MMP-9 in a wide variety of mouse and human cells, including prostate epithelium, epidermal keratinocytes, and the aortic endothelium.17–19 One mechanism by which doxycycline decreases the production of MMP-9 is its ability to inhibit the intracellular p38 and C-Jun-N-terminal kinase mitogen-activated protein kinase signaling cascade.20,21 We have previously shown that doxycycline preserved the apical corneal epithelium barrier function and prevented the development of corneal surface irregularities associated with EDE.22 It has also been shown that doxycycline can inhibit the production of the inflammatory cytokine interleukin-1β.23
The purpose of this study was to determine if doxycycline preserves apical corneal epithelial integrity during desiccating stress by measuring apical cell area and desquamation.
MATERIALS AND METHODS
Mouse Model of Dry Eye
The research protocol was approved by the Baylor College of Medicine Center for Comparative Medicine, and it conformed to Association for Research in vision and Ophthalmology standards. All applicable institutional and governmental regulations concerning the ethical use of animals were followed during this research.
EDE was induced in 20 C57BL/6 mice aged 6–8 weeks of mixed sex by placing them in a dehumidified room (30%–40% ambient humidity) and exposing them to an air draft for 18 hours per 24-hour period for 5 days. Four times daily (8 am, 11 am, 2 pm, and 5 pm), they were given sterile subcutaneous injections of 0.5 mg/0.1 mL scopolamine hydrobromide (Sigma–Aldrich, St. Louis, MO) into alternating flanks for five 24-hour periods as reported previously.5,22
Five groups were evaluated: untreated control mice not exposed to EDE or treated topically (UT), EDE control mice that received no topical medication (5D), EDE + drug vehicle that was exposed to EDE and given 1 μL/eye of drug vehicle (hydroxypropyl methylcellulose, glycerin, and excipients, pH 5.5) topically 4 times a day (5D + vehicle), EDE + 0.025% doxycycline that was exposed to EDE and given 1 μL per eye of 0.025% doxycycline (Alacrity Biosciences Inc., Laguna Hills, CA) topically 4 times a day (5D + doxycycline 0.025%), and EDE + 0.0025% doxycycline that was exposed to EDE and given 1 μL per eye of 0.0025% doxycycline topically 4 times a day (5D + doxycycline 0.0025%).
Immunofluorescent Staining and Laser Scanning Confocal Microscopy
After 5 days of EDE, all groups of mice were euthanized and 4 corneas per experimental group from 4 different animals were excised and fixed in cold methanol (4°C) for 10 minutes. After fixation, they were permeabilized with phosphate-buffered saline (PBS) containing 0.1% Triton X-100 for 10 minutes. After blocking with 20% goat serum in PBS for 60 minutes, we applied primary polyclonal rabbit antisera against occludin (1:50 dilution, 5 μg/mL; Zymed, San Francisco, CA) and incubated for 1 hour at room temperature. Secondary antibody, Alexa Fluor 488–conjugated goat anti-rabbit immunoglobulin G (1:300), was applied and allowed to incubate in a dark chamber for 1 hour, followed by counterstaining with propidium iodide (2 μg/mL in PBS) for 30 minutes. The whole corneas were flattened on microscope slides and covered with antifade mounting medium (Gel/-Mount; Fisher, Atlanta, GA), and coverslips were applied. Digital images (512 × 512 pixels) were captured using a laser scanning confocal microscope (LSM 510; Zeiss with krypton–argon and He–Ne laser; Carl Zeiss Meditec Ltd., Thornwood, NY) with 488-nm excitation and 543-nm emission filters, LP505 and LP560, respectively. They were acquired with a 40/1.3× oil immersion objective. From each whole mount cornea, images of 5 or 6 different areas were taken. Each of the 5 experimental groups yielded at least 25 digital pictures for analysis. The digital images were analyzed on Metavue 6.24r software (Molecular Devices, Sunnyvale, CA). Images were labeled with a letter (A–E) designating their experimental group. All the images were analyzed at one time by the same masked observer. The cells were circumscribed, and both the cell and image field areas were calculated by the software, and these values were entered into an Excel spreadsheet (Microsoft, Redmond, WA). Also, the number of superficial nuclei was counted, and areas of obvious cell desquamation were outlined and entered into the spreadsheet. Four parameters were compiled: mean epithelial cell area, apical epithelial density, mean epithelial area percent loss, and mean cell number loss.
Mean corneal epithelial cell area was measured by outlining 12–15 cells per field per digital image, and the mean peripheral circumferential cell measurement was calculated. Apical cell density was calculated using the previously calculated mean cell area and dividing into the known area (50,000 μm2) of each photographic image field. The percent loss was calculated by outlining the areas of obvious cell loss, either individual cells or cells in a cluster, and cells that were actively being desquamated, and dividing into the known photographic field area. The mean cell number loss was calculated by dividing the total area desquamated in a given image by the average cell area for that given field.
Statistical Analysis
Statistical analysis was performed with 1-way analysis of variance with Tukey post hoc testing using GraphPad Prism 3.0 software (GraphPad Software, San Diego, CA). A P ≥ 0.05 was considered statistically significant.
RESULTS
Effects of EDE and Doxycycline on the Mouse Corneal Surface
To evaluate the effects of EDE and doxycycline on the corneal surface, we immunostained for the tight junction protein occludin because it was previously found in the apical corneal epithelial cells.24 As shown in Figure 1, occludin was observed to demarcate the cell membranes of the apical cells in UT corneas. The observation that EDE corneas had smaller and more cells than UT corneas prompted further investigation.
FIGURE 1.
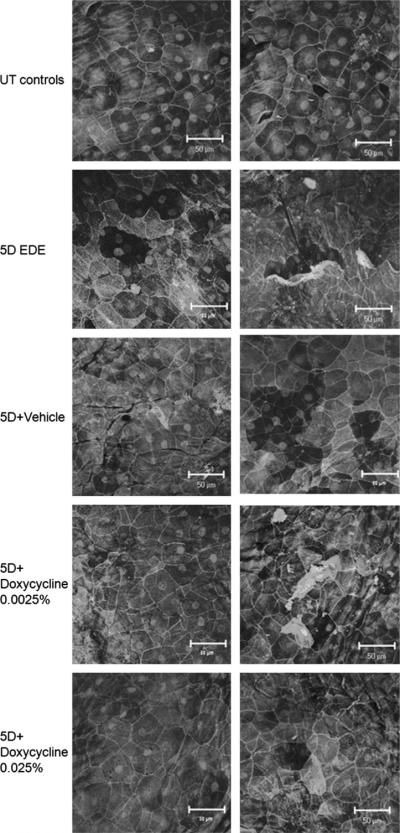
Laser scanning confocal microscopy of whole mount murine corneas stained for occludin with propidium iodide nuclear counterstaining, organized into UT, 5D (EDE), and the 3 treatment groups. The untreated controls (UT) show typical cell area (column on left) and a low level of desquamation (column on right). The EDE corneas, in contrast, have a much smaller cell area and a high level of apical cell loss. The corneas treated with 5D + doxycycline 0.025% had a cell area similar to that of the UT corneas and little of the desquamative loss seen in either the 5D-treated or 5D + vehicle-treated groups.
Apical Corneal Epithelial Cell Area
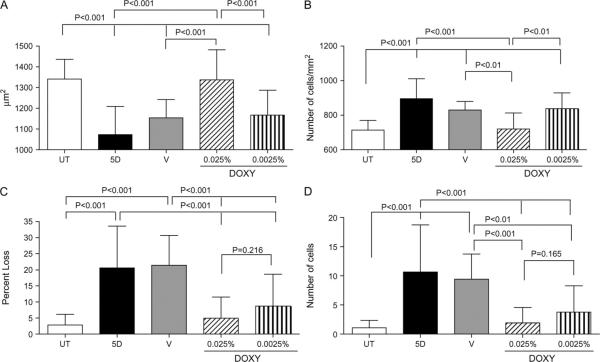
Apical cell area was calculated by outlining 12–15 individual cells in digital images (n = 25) and computing the average for each experimental group. EDE significantly decreased the mean apical corneal epithelial cell area compared with the UT control group (mean ± standard deviation of 1073 ± 135.9 μm2 v.s 1341 ± 95.28 μm2, P < 0.001, respectively). Apical corneal epithelial cell area of corneas treated with 0.025% doxycycline was significantly greater than that of corneas treated with the vehicle control (1337 ± 144.6 μm2 vs 1154 ± 88.10 μm2, P < 0.001, respectively; Figs. 1, 2A) Eyes treated with lower dose 0.0025% doxycycline were not statistically different from the 5D + vehicle group (1167 ± 119.6 μm2, P > 0.05; Fig. 2A).
FIGURE 2.
Mean ± standard deviation of (A) epithelial cell area, (B) apical epithelial cell density, (C) epithelial percent loss, and (D) cells lost per field. In comparison with the UT group, the 5D group has a smaller cell area, higher apical density, higher percent loss, and more cells lost per field. The 5D + doxycycline 0.025% group showed a rescue for each of these parameters from the 5D values to the UT values, whereas the vehicle alone had no significant effect. The low-dose doxycycline had less of an effect.
Apical Cell Density
After the mean cell area was calculated, the apical cell density was determined by dividing by the known photographic field area (50,000 μm2) and multiplying by 106 to standardize the results to cells per square millimeter for each image. This calculation was repeated for each digital image (n = 25), and the results were averaged within each group. EDE significantly increased the number of cells observed per field compared with the UT controls (895.8 ± 115.4 vs 714.4 ± 55.60 cells per mm2, P < 0.001, respectively). Treatment with high-dose doxycycline preserved apical cell density compared with the 5D + vehicle control (721.0 ± 91.62 vs 830.2 ± 49.76 cells per mm2, P < 0.01, respectively). The low-dose doxycycline treatment group was not statistically different from the vehicle (837.9 ± 90.84 cells per mm2, P > 0.05; Figs. 1, 2B).
Epithelial Area Percent Loss
The mean epithelial area percent loss was calculated by outlining areas of apical epithelial desquamation in digital images (n = 25) dividing by the known field area (50,000 μm2) and averaging the results for each group. The EDE corneas had 21.29% ± 13.40% of the epithelium lost to desquamation, which was significantly higher than that in the UT control mice, with just 2.90% ± 3.45% lost (P < 0.001). Both concentrations of doxycycline (0.025% and 0.0025%) significantly decreased the percentage of apical epithelial loss to EDE when compared with the 5D + vehicle corneas (5.12% ± 6.76% and 8.96% ± 10.27% vs 22.14% ± 9.62% P < 0.001, respectively). Doxycycline 0.025% had the greatest effect on reducing cell loss; however, the effect was not significantly greater than that of low-dose doxycycline (P = 0.216; Figs. 1, 2C).
Cell Number Loss
Cell number loss represents the area of cells lost per field divided by the mean cell area for that field. This calculation was repeated for each digital image (n = 25), and the results were averaged by treatment group. The EDE corneas had 10.67 ± 8.07 cells lost per field, whereas the UT controls lost 1.08 ± 1.26 cells (P < 0.001). The group treated with 5D + vehicle lost 9.42 ± 4.34 cells, which was significantly higher than the loss in both the groups treated with 0.025% doxycycline (1.93 ± 2.64, P < 0.001) and 0.0025% doxycycline (3.77 ± 4.52, P < 0.01) (Fig. 2D). Although corneas treated with both concentrations of the doxycycline were significantly different from the 5D + vehicle group, the greatest effect was observed in the doxycycline 0.025% group (Figs. 1, 2D).
DISCUSSION
The ocular surface epithelium is covered by noncornified stratified epithelia whose apical cells produce mucins that maintain hydration and lubrication of the ocular surface, in addition to providing protection from micro-organisms, toxins, and foreign objects.25 We have previously observed that desiccating stress increased desquamation of the apical corneal epithelial cells.22 The present study used quantitative digital image analysis parameters to evaluate the effects of EDE on the apical corneal epithelial cell size and area. We also evaluated the effects of the metalloproteinase inhibitor doxycycline on these parameters. We observed that at baseline, the apical cell layer of corneal epithelium harbors large cells with minimal desquamation being present. Desiccating stress promoted loss of the larger apical cells with exposure of smaller, less well-differentiated subapical cells. This process was significantly inhibited by topical administration of the metalloproteinase inhibitor doxycycline.
We initially noted a 20% reduction in apical corneal epithelial cell area in EDE (from 1341 μm2 in UT to 1073 μm2 after EDE). This decrease in mean cell area was considered to most likely represent exposure of smaller, more basal epithelial cells after loss of larger apical epithelia. To confirm, we evaluated the number of cells per field. If smaller cells were being exposed in the apical corneal epithelium, an increase in superficial cell density should occur. We observed that the EDE group had many more cells per square millimeter than the UT group (895.8 ± 115.4 vs 714.4 ± 55.60 cells per mm2), which corroborates our hypothesis. The percent loss reflects normal desquamation of murine corneal epithelium and the accelerated loss in response to EDE. UT controls had an intact apical cell layer with minimal corneal epithelial cell loss (2.90%). The cell loss was increased almost 10-fold after EDE (21.29%), indicating that desiccating stress promotes desquamation. Administration of topical 0.025% doxycycline preserved cell area and apical density at UT levels and minimized the loss of the epithelium (5.12%). In contrast, the vehicle treatment had no effect on epithelial loss, and the percent loss in the vehicle-treated group (22.14%) was nearly equal to that in the EDE group. However, the lower concentration of doxycycline, 0.0025%, did not show the same level of rescue as the more concentrated formulation, and it was not shown to be significantly different from the vehicle with regard to cell area and apical cell density. The lower concentration doxycycline showed 8.96% loss, which, although not as close to normal as the higher concentration, was a significant improvement over the EDE and vehicle-treated groups. Our results also demonstrate that the vehicle has some restorative effect on cell area (1154 ± 88.10 μm2), perhaps secondary to a rehydration effect, but it was not effective in preventing apical epithelial cell loss.
Our results are significant because we show a quantifiable effect on corneal epithelial cell parameters. It has been shown that the expression of MMP-9 is increased in both dry eye disease in humans and EDE in mice.5–8 It has been previously reported that doxycycline given topically in EDE will increase corneal smoothness and preserve the interepithelial cell tight junctions in the murine cornea.22 We have previously reported that mice lacking a functional MMP-9 gene have a decreased dry eye phenotype in response to EDE compared with their wild-type counterparts.5 However, administration of topical MMP-9 to the MMP-9–knockout mice disrupted corneal barrier function to the extent seen in the wild-type controls. We show here that topical doxycycline treatment has a similar effect in MMP-9–knockout mice in preventing apical corneal epithelial desquamation induced by EDE. It is likely that the mechanism of action of doxycycline in preventing apical epithelial cell loss is through its ability to inhibit MMP-9.
We were unable to find any previously reported ex vivo confocal microscopic studies of murine corneas evaluated for epithelial cell area or apical density. However, multiple in vivo photographic studies of human and murine corneas have been done. A study26 of relatively young, healthy human eyes (mean age of 45 years) showed that the mean area of superficial corneal epithelial cells was 913 μm2 and the superficial cell density was 1213 cells per mm2. This study also showed an increase in epithelial cell density as the scan moved more basally with a decrease in cell area (5699 cells per mm2, 177 μm2). Additional in vivo studies in humans have shown an increase in epithelial cell density and decrease in cell area in the basal corneal epithelium.27,28 In vivo studies of corneas of laboratory animals reveal a similar trend in rabbits, rats, and mice, with an increase in epithelial cell density and a decrease in cell area basally, but the cell changes were not quantified.29 These results help support our theory that as the apical layers of the epithelium are lost to desquamation in EDE, the smaller, more numerous basal cells are exposed, thus increasing the superficial cell density and decreasing the overall cell area. These findings also suggest that these objective confocal image analysis methods couldbeusedasefficacy parameters in humanclinicaltrials of dry eye therapies.
Our study shows that EDE can induce a quantifiable decrease in mean cell area and an increase in both apical cell density and apical desquamation. Doxycycline can rescue the corneal epithelium from apical cell loss in response to desiccating stress, and this effect is noted to be stronger with a higher concentration. It remains to be determined if doxycycline will induce a similar rescue in cell area and desquamation in human dry eye disease.
ACKNOWLEDGMENTS
The study was supported by grant NIH EY11915 (S.C.P.) and EY016928-01 (C.S.D.P.) and by unrestricted research grant from Alacrity Biosciences. D.F.P. is an employee of Alacrity Biosciences but played no part in the collection or interpretation of the data.
REFERENCES
- 1.Smith JA, Albeitz J, Begley C, et al. The epidemiology of dry eye disease: Report of the Epidemiology Subcommittee of the International Dry Eye Workshop (2007) Ocul Surf. 2007;5:93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 2.Schein OD, Munoz B, Tielsch JM, et al. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124:723–728. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 3.Lin PY, Cheng CY, Hsu WM, et al. Association between symptoms and signs of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Invest Ophthalmol Vis Sci. 2005;46:1593–1598. doi: 10.1167/iovs.04-0864. [DOI] [PubMed] [Google Scholar]
- 4.Pflugfelder SC. Anti-inflammatory therapy of dry eye. Am J Ophthalmol. 2004;137:337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 5.Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon A, Dursun D, Liu Z, et al. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- 7.Smith VA, Rishmawi H, Hussein H, et al. Tear film MMP accumulation and corneal disease. Br J Ophthalmol. 2001;85:147–153. doi: 10.1136/bjo.85.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrales RM, Stern ME, De Paiva CS, et al. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47:3293–3302. doi: 10.1167/iovs.05-1382. [DOI] [PubMed] [Google Scholar]
- 9.Behzadian MA, Wang XL, Windsor LJ, et al. TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest Ophthalmol Vis Sci. 2001;42:853–859. [PubMed] [Google Scholar]
- 10.Barton K, Monroy DC, Nava A, et al. Inflammatory cytokines in the tears of patients with ocular rosacea. Ophthalmology. 1997;104:1868–1874. doi: 10.1016/s0161-6420(97)30014-1. [DOI] [PubMed] [Google Scholar]
- 11.Luo L, Li DQ, Doshi A, et al. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 12.Akpek EK, Merchant A, Pinar V, et al. Ocular rosacea: patient characteristics and follow-up. Ophthalmology. 1997;104:1863–1867. [PubMed] [Google Scholar]
- 13.Seedor JA, Perry HD, McNamara TF, et al. Systemic tetracycline treatment of alkali-induced corneal ulceration in rabbits. Arch Ophthalmol. 1987;105:268–271. doi: 10.1001/archopht.1987.01060020122043. [DOI] [PubMed] [Google Scholar]
- 14.Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521–532. doi: 10.1902/jop.2000.71.4.521. [DOI] [PubMed] [Google Scholar]
- 15.Mosorin M, Juvonen J, Biancari F, et al. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34:606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 16.Wilson WR, Anderton M, Schwalbe EC, et al. Matrix metalloproteinase-8 and -9 are increased at the site of abdominal aortic aneurysm rupture. Circulation. 2006;113:438–445. doi: 10.1161/CIRCULATIONAHA.105.551572. [DOI] [PubMed] [Google Scholar]
- 17.Lokeshwar BL. MMP inhibition in prostate cancer. Ann N Y Acad Sci. 1999;878:271–289. doi: 10.1111/j.1749-6632.1999.tb07690.x. [DOI] [PubMed] [Google Scholar]
- 18.Hanemaaijer R, Visser H, Koolwijk P, et al. Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv Dent Res. 1998;12:114–118. doi: 10.1177/08959374980120010301. [DOI] [PubMed] [Google Scholar]
- 19.Qin X, Corriere MA, Matrisian LM, et al. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler Thromb Vasc Biol. 2006;26:1510–1516. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 20.Kim HS, Luo L, Pflugfelder SC, et al. Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2005;46:840–848. doi: 10.1167/iovs.04-0929. [DOI] [PubMed] [Google Scholar]
- 21.De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 22.De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006;47:2847–2856. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- 23.Solomon A, Rosenblatt M, Li DQ, et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Invest Ophthalmol Vis Sci. 2000;41:2544–2557. [PubMed] [Google Scholar]
- 24.Ban Y, Dota A, Cooper LJ, et al. Tight junction-related protein expression and distribution in human corneal epithelium. Exp Eye Res. 2003;76:663–669. doi: 10.1016/s0014-4835(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 25.Farjo AA, Soong HK. Corneal epithelium. In: Yanoff M, editor. Ophthalmology. 2nd ed Chapter 56. Mosby Inc; St. Louis, MO: 2004. pp. 413–420. [Google Scholar]
- 26.Mustonen RK, McDonald MB, Srivannaboon S, et al. Normal human corneal cell populations evaluated by in vivo scanning slit confocal microscopy. Cornea. 1998;17:485–492. doi: 10.1097/00003226-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Quadrado MJ, Popper M, Morgado AM, et al. Diabetes and corneal cell densities in humans by in vivo confocal microscopy. Cornea. 2006;25:761–768. doi: 10.1097/01.ico.0000224635.49439.d1. [DOI] [PubMed] [Google Scholar]
- 28.Harrison DA, Joos C, Ambrosio R., Jr Morphology of corneal basal epithelial cells by in vivo slit-scanning confocal microscopy. Cornea. 2003;22:246–248. doi: 10.1097/00003226-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Labbe A, Liang H, Martin C, et al. Comparative anatomy of laboratory animal corneas with a new-generation high-resolution in vivo confocal microscope. Curr Eye Res. 2006;31:501–509. doi: 10.1080/02713680600701513. [DOI] [PubMed] [Google Scholar]