Abstract
Sarcopenia is the age-related loss of skeletal muscle mass and function. It is now recognised as a major clinical problem for older people and research in the area is expanding exponentially. One of the most important recent developments has been convergence in the operational definition of sarcopenia combining measures of muscle mass and strength or physical performance. This has been accompanied by considerable progress in understanding of pathogenesis from animal models of sarcopenia. Well-described risk factors include age, gender and levels of physical activity and this knowledge is now being translated into effective management strategies including resistance exercise with recent interest in the additional role of nutritional intervention. Sarcopenia is currently a major focus for drug discovery and development although there remains debate about the best primary outcome measure for trials, and various promising avenues to date have proved unsatisfactory. The concept of ‘new tricks for old drugs’ is, however, promising, for example, there is some evidence that the angiotensin-converting enzyme inhibitors may improve physical performance. Future directions will include a deeper understanding of the molecular and cellular mechanisms of sarcopenia and the application of a lifecourse approach to understanding aetiology as well as to informing the optimal timing of interventions.
Keywords: sarcopenia, skeletal muscle, ageing, pathogenesis, diagnosis, exercise, nutrition, drug treatment, life course, epidemiology, older people
Introduction
Sarcopenia, the age-related loss of skeletal muscle mass and function, is coming of age. It is now recognised as a major clinical problem for older people and research in the area is expanding exponentially [1]. This interest stems from the fact that sarcopenia is both common and associated with serious health consequences in terms of frailty, disability, morbidity and mortality. The estimated direct healthcare cost attributable to sarcopenia in the USA in 2000 was £18.5 bn [2]. Furthermore, sarcopenia is associated with major co-morbidity such as obesity, osteoporosis and type 2 diabetes [3]. But perhaps the most powerful indication that the loss of skeletal muscle, in particular strength, is important comes from the evidence that it predicts future mortality in middle-aged as well as older adults [4].
One of the most important recent developments has been convergence in the operational definition of sarcopenia. The European Working Group on Sarcopenia in Older People published guidelines in 2010 where specific parameters to identify sarcopenia have been identified [5]. Similar (although not identical) guidelines have subsequently emerged from the USA [6]. This step forward in defining sarcopenia is leading to opportunities in two major areas: first in understanding aetiology and secondly in developing treatments. This progress is underpinned by studies in animal (especially rodent) models of sarcopenia [7]. These provide the opportunity for intensive experimentation to define the molecular and cellular changes that lead to, and are the hallmarks of, this age-related condition.
Well-described risk factors for sarcopenia include age, gender and level of physical activity, and resistance exercise is particularly effective for slowing the age-related loss of skeletal muscle. Nevertheless many gaps in our knowledge remain and there are a number of promising new horizons. This review will focus on current understanding as well as new developments in the pathogenesis, diagnosis and management of sarcopenia.
Pathogenesis
Skeletal muscle comprises primarily two main types of muscle fibre (myofibre): type 1 myofibres have a slow contraction time, utilise oxidative pathways and resist fatigue. In contrast, type 2 myofibres have a quick contraction time, rely on glycolytic pathways and fatigue more easily. The age-related loss of human skeletal muscle mass is due to a decrease in myofibre size and number with the loss of both fast and slow type myofibres, although the loss of fast myofibres tends to start earlier, at ∼70 years [8]. Many factors influence the decrease in muscle mass. A significant contributor is an anabolic resistance of older skeletal muscle to protein nutrition [9] as seen during immobilisation [10] which can be ameliorated at least in part by resistance exercise and dietary supplementation [11]. Other intensive areas of research are related to the loss of innervation [12] and oxidative damage [13]. Loss of myofibre innervation is a characteristic of ageing muscles with changes occurring at many levels, from the central and peripheral nervous system to cells in skeletal muscle tissue. These include the loss of motoneurons in the central nervous system (CNS), diminished function of the remaining motoneurons, demyelination of axons and withdrawal of nerve terminals from the neuromuscular junctions (NMJs) [12, 14, 15].
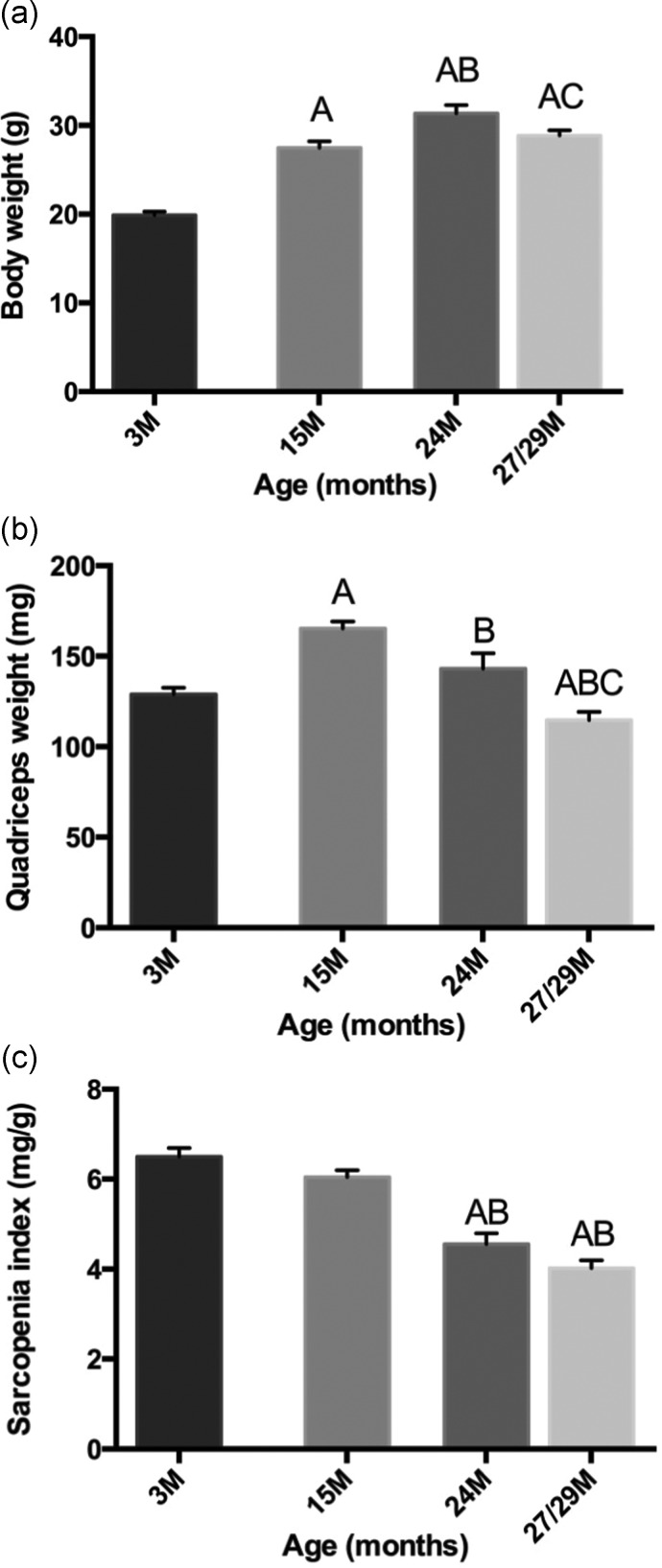
Many studies that describe the pathogenesis of ageing skeletal muscle have been carried out in rodent models. In contrast with human muscles that are composed predominantly of slow myofibres, mouse muscles are mainly fast: such differences between species need to be considered when extending observation from animal models to humans. Furthermore, there is more time for more secondary consequences to become pronounced in humans where sarcopenia becomes progressively manifest over 20–30 years, whereas the duration is far shorter in mice; >1 year (from ∼18 to 30 months), with the normal lifespan of mice being a mere 3 years or less (Figure 1). Innervation of myofibres is clearly required for skeletal muscle contraction in mice and humans, but different conclusions may be reached from initial studies. Examination of aged mice (up to 29 months old) revealed marked denervation of NMJs of hind limb muscles without any change in number or size of motorneuron cell bodies in the lumbar spinal cord, suggesting a primary problem at the level of the muscles per se [16]. In contrast, many changes in motorneurone function are noted from electrophysiological studies in ageing humans supporting changes in the CNS [15], although it is difficult to determine whether these changes are secondary to earlier NMJ changes, since invasive examination of the NMJ status is rare in human studies. Further experiments in animal models can help to define the precise timing of these key events.
Figure 1.
Mice as a model for sarcopenia: changes in (a) body mass and (b) weight of quadriceps muscles over the life-span of female C57Bl/6J mice expressed as (c) a sarcopenia index [16]. The loss of quadriceps muscle mass starts sometime after 15 months of age, is evident by 24 months and pronounced by 29 months. Statistically significant differences between ages (P < 0.05) are shown as A: different from 3 months, B: different from 15 months and C: different from 24 months. The extent of sarcopenia is influenced by gender (being more pronounced in females than males) and may vary between different muscles.
In older age, an accumulation of reactive oxygen species (ROS) may lead to oxidative damage of biomolecules, and contribute to the loss of muscle mass and strength. It is well documented that elevated oxidative stress is associated with many clinical situations of muscle wasting, but the precise nature of the oxidative stress in different situations and their complex in vivo interactions remain unclear [17]. Irreversible oxidation of macromolecules such as proteins and lipids results in the accumulation of a pigment called lipofuscin that is a classic marker for ageing tissues [18]. Other forms of ROS cause reversible oxidation of protein thiols to modulate the function of many proteins (e.g. involved in signalling regulating protein synthesis and degradation, muscle contraction), although the contribution of such thiol oxidation to sarcopenia has barely been evaluated [17]. Since different anti-oxidants target specific types of ROS, it is essential to know exactly what forms of ROS are elevated in sarcopenia in order to select the appropriate therapeutic drug or supplement.
Diagnosis
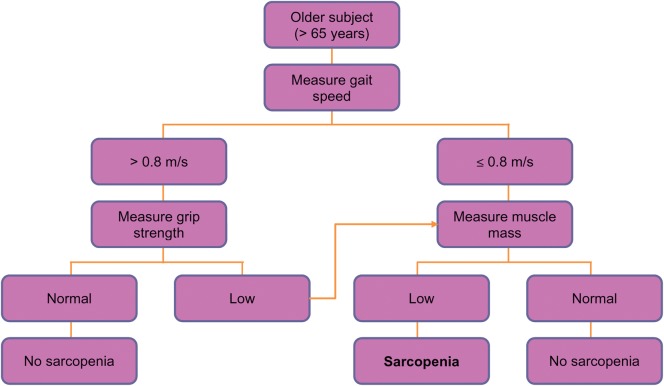
The European Working Group on Sarcopenia in Older People (EWGSOP) have recently developed a practical clinical definition as well as consensus criteria for sarcopenia allowing an important step forward in the field in terms of standardising diagnosis [5]. The recommendation is to use the presence of both low muscle mass and low muscle function (strength or performance) as summarised in an algorithm (Figure 2). The approach applies these characteristics to further define conceptual stages as presarcopenia, sarcopenia and severe sarcopenia.
Figure 2.
EWGSOP-suggested algorithm for sarcopenia case finding in older individuals [5].
Encouragingly there seems to be international convergence in the approach to defining sarcopenia. Fielding and colleagues published US guidelines last year on behalf of the International Working Group on Sarcopenia suggesting that a diagnosis of sarcopenia could be made on the basis of low gait speed and an objectively measured low muscle mass [6]. This area is important because an operational definition is needed to allow development and evaluation of interventions for prevention or treatment and with the emergence of a number of candidate therapies, this has become more pressing [19].
Management
There is considerable interest in the role of lifestyle and diet in the aetiology of sarcopenia, and the extent to which interventions to change behaviour could make useful contributions to its management [20].
Exercise
The established link between inactivity and losses of muscle mass and strength suggests that physical activity should be protective for sarcopenia. A range of exercise interventions, that include resistance, power and functional training, as well as endurance and aerobic training regimes, have been used. In particular, progressive resistance exercise training (PRT), in which participants exercise against an increasing external load, has been shown to have positive effects on strength and physical function. A 2009 Cochrane review of 121 randomised controlled trials showed PRT to be an effective intervention for improving physical functioning in older people [21]; observed benefits included large positive effects on muscle strength as well as improved performance in some assessed activities. Most commonly the exercise interventions were carried out two to three times per week. Strength training appears to evoke not only muscle hypertrophy, but also beneficial changes in neuromuscular function [15] and other types of exercise interventions, involving gait, balance, co-ordination and functional exercises, may also be effective in reducing the risk and rate of falls [22] as well as improving balance in older people [23].
Diet and nutrition
In comparison with the clear benefits of exercise, we know much less about the influence of diet in older age on muscle mass and strength, and much of the research in this area is relatively new. Food intake falls by ∼25% between 40 and 70 years of age, putting older people at risk of having inadequate nutrient intakes. There is a growing literature that suggests that diet could be an important modifiable influence on sarcopenia [24]—with the most consistent evidence pointing to roles for protein, vitamin D and antioxidant nutrients.
Dietary protein provides amino acids needed for the synthesis of muscle protein, and absorbed amino acids have a stimulatory effect on protein synthesis after feeding. Branched chain amino acids, such as leucine, have been shown to boost signalling pathways that lead to increased protein translation in both humans and rodents [25, 26]. However, there is concern that these anabolic responses may be blunted in older people [27], raising the possibility that recommendations for protein intake should be increased [28]. There is good observational evidence that links low-protein intake to declining muscle mass [29], and supplementation with protein and/or amino acids should therefore have the potential to slow sarcopenic muscle loss. However, the results from trials have been inconsistent. A Cochrane review found that the use of protein and energy supplements in older people at risk of malnutrition produced a small but consistent weight gain and mortality appeared to be reduced in those who were undernourished [30]. However, there was no evidence of functional benefit and further work is needed to establish protein and specific amino acid requirements to support optimal physical function in older people.
The current widespread interest in the contribution of low vitamin D status to poor health also extends to effects on muscle strength and physical function in older adults. The vitamin D receptor (VDR) has been isolated from skeletal muscle, and polymorphisms of the VDR have been linked to differences in muscle strength [31]. Much of the observational literature is consistent with direct effects of vitamin D on muscle function. For example, a fourfold increase in the risk of frailty has been described in older men and women with low vitamin D status [32] and a meta-analysis indicated that vitamin D supplementation (700–1000 IU/day) reduces risk of falls in older people [33]. However, supplementation is not consistently linked to measurable improvements in physical function, and its benefits remain controversial [34]. Nevertheless as vitamin D insufficiency is common among older adults [32], further trial data are needed.
The implicated role of oxidative stress in the aetiology of sarcopenia has focused interest on the effects of dietary antioxidants [35]. Observational studies have shown better physical function among older adults with a higher antioxidant status and, importantly, low status is predictive of a decline in measures of function such as walking speed over time [36]. To date there have been few studies to determine how antioxidant supplementation of older adults affects muscle strength, and the benefits are uncertain. Interventions based on simple suppression of activity of ROS, through the use of non-specific antioxidants, may be unlikely to improve age-related declines in muscle mass and function [37], but this remains an important question to be addressed.
Much of the existing evidence that links diet to muscle mass and strength in older people is observational. Because dietary components are often highly correlated with each other, identifying the effects of individual nutrients is problematic. For example, high fruit and vegetable consumption may be indicators of other dietary differences which could be important for muscle function, such as greater intakes of vitamin D and antioxidant nutrients [38]. There is some evidence that ‘healthy’ diets, characterised by greater fruit and vegetable consumption and wholemeal cereals, are associated with greater muscle strength in older adults [24]. Interventions to change dietary patterns would be expected to change intakes of a range of nutrients, and therefore could be more effective than single-nutrient supplements in preventing age-related losses in muscle mass and strength. Despite the challenges of changing dietary behaviour, nutrition interventions in older community-dwelling adults have been shown to be effective [39]. The potential of whole-diet interventions for the management of sarcopenia is significant, and needs to be explored.
Diet and exercise
A final consideration for maintenance of healthy muscle is the possibility of interactions between diet and exercise in their influence on muscle mass and strength, and the extent to which interventions that combine supplementation and exercise training may be more effective than either alone. Interactive effects of diet and exercise on physical function have been studied most extensively in relation to protein/amino acid supplementation. Although synergistic effects of protein feeding and exercise have been described [40], it is not clear whether there are additional benefits of protein/amino acid supplementation on the skeletal muscle response to prolonged resistance exercise training [41]. A recent report showed that initial benefits in older subjects were blunted over time [9]. At present the implications for long-term effects of combined exercise training and high-protein intakes for management of sarcopenia are uncertain. Further research is needed into the interactions between nutrition, exercise and skeletal muscle adaptations in order to define effective strategies to prevent and treat sarcopenia in the future [41].
Drug treatment
Sarcopenia is now a major focus for drug discovery and development [42]. However, debate about the best primary outcome measure for trials is ongoing and various promising therapeutic avenues to date have proved unsatisfactory. Growth hormone has been used for some years to improve muscle mass, but the data supporting an associated increase in function have not been convincing [43]. The therapeutic use of testosterone had a setback recently when a major trial had to be stopped early because of side effects [44]. Perhaps a more promising area at present is the idea of ‘new tricks for old drugs’. For example, there is some evidence for the benefit of angiotensin-converting enzyme inhibitors in improving physical function which may be mediated through a direct effect on muscle, and a number of studies are currently underway in this area [45]. An important consideration that has previously often been overlooked is the potential for commonly used drugs to also have adverse effects on skeletal muscle function. For example, we have shown that many prescribed medications are associated with lower grip strength and this appears to be partly independent of co-morbidities [46].
Future directions
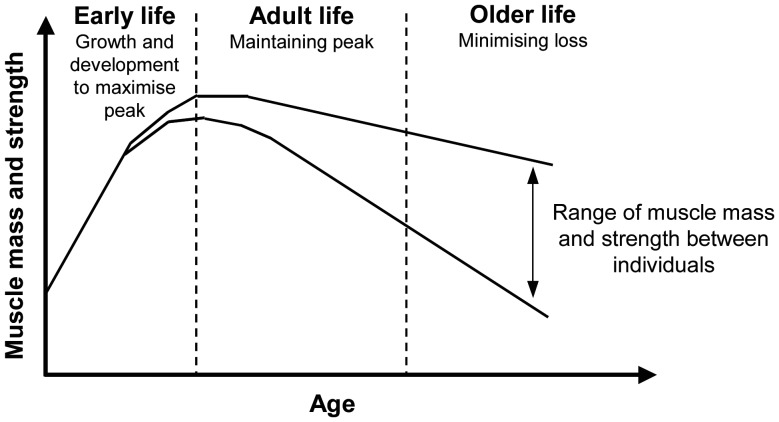
A deeper understanding of the molecular and cellular mechanisms of sarcopenia, derived from both human and animal studies, has great potential to identify novel targets for drug and other treatment strategies as well as to develop better biomarkers to monitor the efficacy of various interventions. Another exciting new area is the application of a lifecourse approach to the understanding and management of sarcopenia. This approach recognises that muscle mass and function in later life reflect not only the rate of muscle loss, but also the peak attained earlier in life (Figure 3) [47]. Therefore, in addition to identifying the determinants of skeletal muscle loss, there needs to be focus on the factors associated with peak muscle mass and strength such as birth weight [48] and early nutrition [49] as well as the mechanisms underlying these associations [50]. Importantly, the lifecourse approach suggests that there is potential for prevention and intervention at earlier stages of life rather than just when sarcopenia has become established although to date the evidence is scanty. This is a fertile area for future research.
Figure 3.
A lifecourse approach to sarcopenia [47].
Conflicts of interest
None declared.
References
- 1.Sayer AA. Sarcopenia. BMJ. 2010;341:c4097. doi: 10.1136/bmj.c4097. [DOI] [PubMed] [Google Scholar]
- 2.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 3.Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care. 2005;28:2541–2. doi: 10.2337/diacare.28.10.2541. [DOI] [PubMed] [Google Scholar]
- 4.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–56. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shavlakadze T, Grounds M. Of bears, frogs, meat, mice and men: complexity of factors affecting skeletal muscle mass and fat. Bioessays. 2006;28:994–1009. doi: 10.1002/bies.20479. [DOI] [PubMed] [Google Scholar]
- 8.Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6:588–95. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 9.Farnfield MM, Breen L, Carey KA, Garnham A, Cameron-Smith D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl Physiol Nutr Metab. 2012;37:21–30. doi: 10.1139/h11-132. [DOI] [PubMed] [Google Scholar]
- 10.Murton AJ, Greenhaff PL. Muscle atrophy in immobilization and senescence in humans. Curr Opin Neurol. 2009;22:500–505. doi: 10.1097/WCO.0b013e32832f15e1. [DOI] [PubMed] [Google Scholar]
- 11.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–59. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- 12.Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One. 2011;6:e28090. doi: 10.1371/journal.pone.0028090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Neill ED, Wilding JP, Kahn CR, et al. Absence of insulin signalling in skeletal muscle is associated with reduced muscle mass and function: evidence for decreased protein synthesis and not increased degradation. Age (Dordr) 2010;32:209–22. doi: 10.1007/s11357-009-9125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edstrom E, Altun M, Bergman E, et al. Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol Behav. 2007;92:129–35. doi: 10.1016/j.physbeh.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 15.Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20:49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- 16.Shavlakadze T, McGeachie J, Grounds MD. Delayed but excellent myogenic stem cell response of regenerating geriatric skeletal muscles in mice. Biogerontology. 2010;11:363–76. doi: 10.1007/s10522-009-9260-0. [DOI] [PubMed] [Google Scholar]
- 17.Arthur PG, Grounds MD, Shavlakadze T. Oxidative stress as a therapeutic target during muscle wasting: considering the complex interactions. Curr Opin Clin Nutr Metab Care. 2008;11:408–416. doi: 10.1097/MCO.0b013e328302f3fe. [DOI] [PubMed] [Google Scholar]
- 18.Tohma H, Hepworth AR, Shavlakadze T, Grounds MD, Arthur PG. Quantification of ceroid and lipofuscin in skeletal muscle. J Histochem Cytochem. 2011;59:769–79. doi: 10.1369/0022155411412185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper C, Dere W, Evans W, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int. 2012;23:1839–48. doi: 10.1007/s00198-012-1913-1. [DOI] [PubMed] [Google Scholar]
- 20.Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clin Interv Aging. 2010;5:259–70. doi: 10.2147/cia.s6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;3:CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howe TE, Rochester L, Neil F, Skelton DA, Ballinger C. Exercise for improving balance in older people. Cochrane Database Syst Rev. 2011;11:CD004963. doi: 10.1002/14651858.CD004963.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson S, Cooper C, Aihie SA. Nutrition and sarcopenia: a review of the evidence and implications for preventive strategies. J Aging Res. 2012;2012:510801. doi: 10.1155/2012/510801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson JM, Fry CS, Drummond MJ, et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–62. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anthony JC, Reiter AK, Anthony TG, et al. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E-BP1 or S6K1 phosphorylation. Diabetes. 2002;51:928–36. doi: 10.2337/diabetes.51.4.928. [DOI] [PubMed] [Google Scholar]
- 27.Rattan SI. Synthesis, modification and turnover of proteins during aging. Adv Exp Med Biol. 2010;694:1–13. doi: 10.1007/978-1-4419-7002-2_1. [DOI] [PubMed] [Google Scholar]
- 28.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–55. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 30.Milne AC, Potter J, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2005;1:CD003288. doi: 10.1002/14651858.CD003288.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports. 2010;20:182–90. doi: 10.1111/j.1600-0838.2009.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilhelm-Leen ER, Hall YN, Deboer IH, Chertow GM. Vitamin D deficiency and frailty in older Americans. J Intern Med. 2010;268:171–80. doi: 10.1111/j.1365-2796.2010.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bischoff-Ferrari HA, wson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annweiler C, Schott AM, Berrut G, Fantino B, Beauchet O. Vitamin D-related changes in physical performance: a systematic review. J Nutr Health Aging. 2009;13:893–8. doi: 10.1007/s12603-009-0248-x. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Wilson JM, Lee SR. Dietary implications on mechanisms of sarcopenia: roles of protein, amino acids and antioxidants. J Nutr Biochem. 2010;21:1–13. doi: 10.1016/j.jnutbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser M, Bandinelli S, Lunenfeld B. Frailty and the role of nutrition in older people. A review of the current literature. Acta Biomed. 2010;81(Suppl. 1):37–45. [PubMed] [Google Scholar]
- 37.Jackson MJ. Strategies for reducing oxidative damage in ageing skeletal muscle. Adv Drug Deliv Rev. 2009;61:1363–8. doi: 10.1016/j.addr.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Robinson S, Syddall H, Jameson K, et al. Current patterns of diet in community-dwelling older men and women: results from the Hertfordshire Cohort Study. Age Ageing. 2009;38:594–9. doi: 10.1093/ageing/afp121. [DOI] [PubMed] [Google Scholar]
- 39.Bandayrel K, Wong S. Systematic literature review of randomized control trials assessing the effectiveness of nutrition interventions in community-dwelling older adults. J Nutr Educ Behav. 2011;43:251–62. doi: 10.1016/j.jneb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Symons TB, Sheffield-Moore M, Mamerow MM, Wolfe RR, Paddon-Jones D. The anabolic response to resistance exercise and a protein-rich meal is not diminished by age. J Nutr Health Aging. 2011;15:376–81. doi: 10.1007/s12603-010-0319-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koopman R. Dietary protein and exercise training in ageing. Proc Nutr Soc. 2011;70:104–113. doi: 10.1017/S0029665110003927. [DOI] [PubMed] [Google Scholar]
- 42.Brass EP, Sietsema KE. Considerations in the development of drugs to treat sarcopenia. J Am Geriatr Soc. 2011;59:530–35. doi: 10.1111/j.1532-5415.2010.03285.x. [DOI] [PubMed] [Google Scholar]
- 43.Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr Rev. 2012;33:314–77. doi: 10.1210/er.2012-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witham MD, Sumukadas D, McMurdo ME. ACE inhibitors for sarcopenia—as good as exercise training? Age Ageing. 2008;37:363–5. doi: 10.1093/ageing/afn124. [DOI] [PubMed] [Google Scholar]
- 46.Ashfield TA, Syddall HE, Martin HJ, Dennison EM, Cooper C, Aihie SA. Grip strength and cardiovascular drug use in older people: findings from the Hertfordshire Cohort Study. Age Ageing. 2010;39:185–91. doi: 10.1093/ageing/afp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging. 2008;12:427–32. doi: 10.1007/BF02982703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayer AA, Syddall HE, Gilbody HJ, Dennison EM, Cooper C. Does sarcopenia originate in early life? Findings from the Hertfordshire Cohort Study. J Gerontol. 2004;59A:930–34. doi: 10.1093/gerona/59.9.m930. [DOI] [PubMed] [Google Scholar]
- 49.Robinson SM, Simmonds SJ, Jameson KA, et al. Muscle strength in older community-dwelling men is related to type of milk feeding in infancy. J Gerontol A Biol Sci Med Sci. 2012;67:990–6. doi: 10.1093/gerona/gls061. [DOI] [PubMed] [Google Scholar]
- 50.Patel H, Jameson K, Syddall H, et al. Developmental Influences, Muscle Morphology, and Sarcopenia in Community-Dwelling Older Men. J Gerontol A Biol Sci Med Sci. 2012;67:82–7. doi: 10.1093/gerona/glr020. [DOI] [PubMed] [Google Scholar]