Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder and the most common form of dementia, affecting more than 5.4 million people in the USA. Although the cause of AD is not well understood, the cholinergic, amyloid and tau hypotheses were proposed to explain its development. Drug discovery for AD based on the cholinergic and amyloid theories have not been effective. In this article we summarize tau-based natural products as AD therapeutics from a variety of biological sources, including the anti-amyloid agent curcumin, isolated from turmeric, the microtubule stabilizer paclitaxel, from the Pacific Yew Taxus brevifolia, and the Streptomyces-derived Hsp90 inhibitor, geldanamycin. The overlooked approach of clearing tau aggregation will most likely be the next objective for AD drug discovery.
Alzheimer’s disease (AD) was first described in 1906 by Aloysius ‘Alois’ Alzheimer, a German psychiatrist and neuropathologist who identified and described amyloid plaques (Aβ) and neurofibrillary tangles (NFTs) from a 51-year old patient named Auguste Deter who showed strangely impaired behavior [1]. The observed symptoms for AD are related to cognitive decline, memory loss, confusion, problems with reading, writing and speaking, along with changes in mood and personality. As the disease progresses, AD patients withdraw more and more from work and social activities to depend on total care from caregivers [2]. Since Alois Alzheimer described the disease, its cause remains unknown except for less than 5% of the cases that are genetic, with mutations observed in amyloid precursor protein [3], presenilin-1 and -2 [4], displaying autosomal-dominant familial AD, and in the autosomal-recessive AD apolipoprotein E-e4 (ApoE4) [5]. Several hypotheses have been proposed to explain the onset and development of AD. The cholinergic hypothesis states that low production of acetylcholine (ACh) initiates AD, while the observation of Aβ and NFTs lead to the amyloid and tau hypotheses as causes for AD development.
The acetylcholine strategy
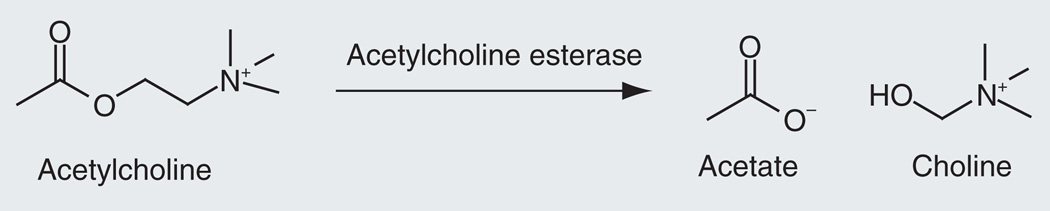
The cholinergic hypothesis of AD arose after deficits in the neurotransmitter ACh were noted in AD patients. ACh activity is regulated by the serine protease AChE, which breaks down ACh in the CNS by catalyzing the hydrolysis of ACh to acetate and choline (Figure 1) [6]. Therapy is based on drugs inhibiting AChE, which inactivates ACh at the synapse. The inhibition of AChE prevents the normal breakdown of ACh to compensate for the low concentrations of ACh that are characteristic of AD.
Figure 1.
Breaking down of acetylcholine by acetylcholine esterase.
From the amyloid hypothesis to tau
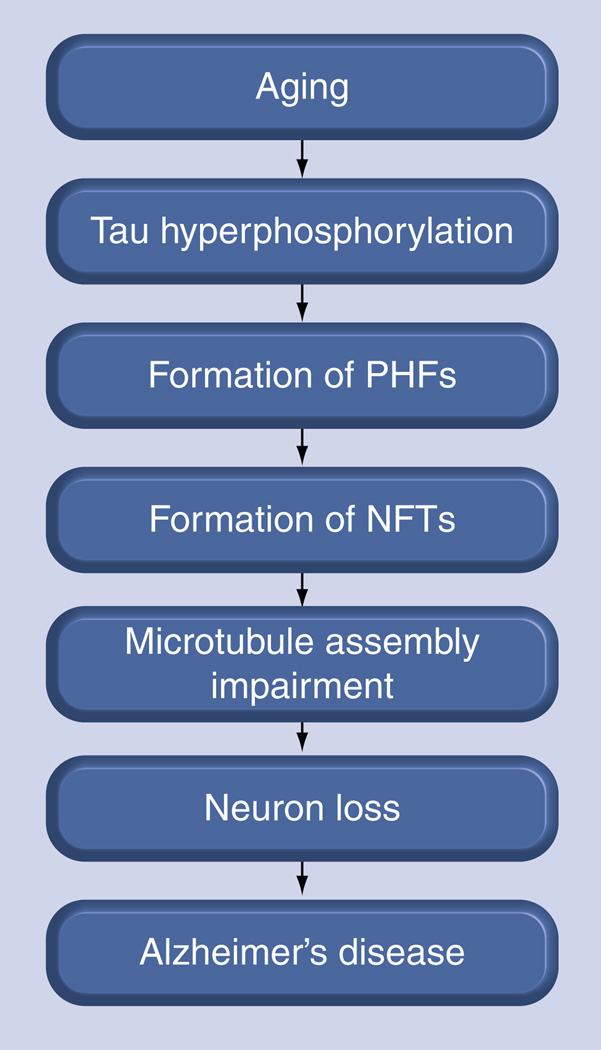
The hallmarks of AD are largely twofold: insoluble deposits of Aβ located between neurons and clumps of NFTs composed of tau aggregates found within nerve cells. Initially, the Aβ theory prevailed against the tau theory, especially as mutations were identified in the amyloid precursor protein located on chromosome 21 that corresponds to amyloid build-up and AD phenotypes. More recently, however, the tau hypothesis has gained traction. The tau protein stabilizes microtubules in neurons, but abnormal hyperphosphorylation of tau leads to aggregate formation. Diseases known to present with these aggregates are termed tauopathies, of which the most well-known is AD [7–12]. Although these insoluble visible tangles correlate strongly with AD severity post-mortem [13], they may merely be the cell’s response to the advent of AD pathology. Soluble tau intermediates are more neurotoxic than higher order aggregates and are responsible for the cognitive dysfunction in AD and other tauopathies (Figure 2) [14–17]. Mutations in the coding and noncoding portions of the tau gene have also been directly associated with the development of the condition referred to as frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17) [18,19]. Thus, despite a few efforts aimed at preventing tau aggregation [15,20], a more beneficial approach towards AD therapeutics may be to enhance tau clearance [21]. Targeting the microtubule-associated protein tau (MAPT) that pathologically accumulates in AD and the paired helical filaments (PHFs) that are indicative of AD NFTs may perhaps be the most effective strategy for treating post-symptomatic AD [14–16,22–26]. Furthermore, the recent failures of Aβ-targeted therapeutics in Phase III clinical trials suggest that it is both timely and prudent to consider alternative drug-discovery strategies for AD.
Figure 2. The tau hypothesis of Alzheimer’s disease progression.
NFT: Neurofibrillary tangles; PHF: Paired helical filaments.
Pharmaceutical AD treatments
Currently, there are no cures for AD, and only five available drugs for treating symptoms: four AChE inhibitors, drugs developed based solely on the cholinergic hypothesis and one N-methyl-d-aspartate (i.e., NMDA)-receptor antagonist (Table 1) [27,201]. The first AD drugs, starting with Cognex® (tacrine) in 1993, were developed based on the cholinergic hypothesis of AD. Cognex®, Aricept® (donepezil), Exelon® (rivastigmine) and Razadyne® (galantamine) are all AChE inhibitors, and the hope was that inhibiting AChE activity would maintain ACh levels in AD patients (Table I). The more recently approved drug memantine differs slightly in its approach by acting on the glutamatergic system instead, but its efficacy seems restricted to those with moderate to severe AD, and it displays limited effects [28,29].
Table I.
Alzheimer’s disease drugs and selected compounds in various stages of clinical trials.
| Compound | Trade name | Source | Pathway | Status | Year of approval (US FDA) |
|---|---|---|---|---|---|
| Tacrine | Cognex® | Synthetic | AChE inhibitors | Market | 1993 |
| Donepezil | Aricept® | Synthetic | AChE inhibitors | Market | 1996 |
| Rivastigmine | Exelon® | Synthetic (natural product-inspired) | AChE inhibitors | Market | 2000 |
| Galantamine | Razadyne® | Natural product (plant) | AChE inhibitors | Market | 2001 |
| Memantine | Axura®, Namenda®, Abixa® and Memox | Synthetic | N-methyl-d-aspartate receptor antagonist | Market | 2004 |
| Curcumin | Natural product (plant) | Aβ plaques | Phase II | ||
| Homotaurine | Alzhamed™ | Natural product (algae) | Aβ plaques | Phase III (failed) | |
| R-flurbiprofen | Flurizan™ | Synthetic | Aβ plaques | Phase III (failed) | |
| Minocycline | Synthetic (natural product-inspired) | Aβ/tau/microglia | Phase II | ||
| Methylene blue | Rember™ | Synthetic | Tau aggregation | Phase II | |
| Tideglusib | Zentylor™ | Synthetic | Tau aggregation | Phase II |
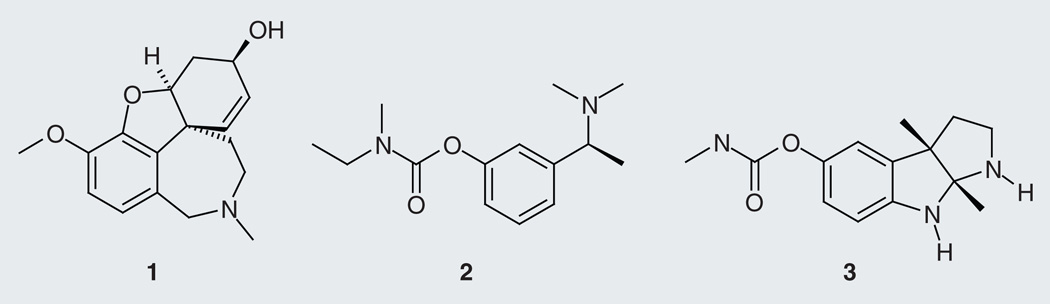
Among the available drugs, one is a natural product. Galantamine (1; Figure 3) is an alkaloid isolated from the bulbous herbaceous plant Galanthus woronowii (Caucasian snow-drop) and bulbs of different species in the Amaryllidaceae family (Amaryllis, Hippeastrum, Lycoris, Ungernia, Leucojum, Narcissus, Zephyranthes, Hymenocallis and Haemanthus genera)[30]. It is important to note that rivastigmine (2) is a synthetic analog of the cholinesterase inhibitor alkaloid physostigmine (3) from the poisonous seeds of Physostigma venosum (calabar bean) [31]. The cholinergic-based drugs are considered to only ease the symptoms of AD and not to prevent the progression of the disease; however, AChE inhibitors may have disease-modifying effects [32].
Figure 3.
AChE inhibitors galantamine (1), rivastigmine (2) and physostigmine (3).
Recent focus on future AD therapeutics has been on reducing Aβ levels, and NFTs production resulting from the hyperphosphorylation of the tau protein has received little attention, despite clinical trials suggesting that tau-based therapies may be more relevant than anti-Aβ compounds in patients already presenting with AD symptoms [33]. Therefore, there is a significant need for efficient drugs against AD with tau-reducing properties. These drugs can be synthesized or harvested from nature, the advantage of the latter being the potential for chemical diversity, biological selectivity and favorable properties. The majority of current drugs on the market are natural product-derived compounds [34].
Current approaches to reduce the effects of tau dysfunction in AD
A number of strategies have been used to search for the best way to decrease tau levels in neurons. They vary from inhibiting formation of tau aggregates, regulating tau using kinases, controlling tau degradation via chaperones and stabilizing tau microtubules. Current biochemical assays focus on inhibiting tau fibrillization [15,35]. While this approach may yield novel compounds, recent work suggests tau aggregation may actually be a protective mechanism employed by neurons and the most toxic entities are tau intermediates [15,36,37]. A number of proline-directed kinases (ERK2, GSK-3 and CDK5), nonproline-directed enzymes (CK1 and PKA) and microtubule affinity-regulating kinases (MARKs) are known to be involved in the process of tau phosphorylation [38–40]. Manipulations of kinases by drugs have been shown to be an effective way to reduce tau levels; for example, a small-molecule inhibitor of GSK-3β kinase was effective in reducing phosphorylated tau [41,42]. Alternatively, affecting molecular chaperone protein functions may have deleterious effects on tau as well, since inhibiting the molecular chaperone Hsp90 showed positive effects in reducing phosphorylated and misfolded tau [21]. Hyperphosphorylated tau is also known to destabilize microtubules and cause impairment in microtubule function and axonal transport, leading to the idea that microtubule-stabilizing agents may help compensate for these losses [43,44].
Natural products as tau targeting agents
Several natural products already evaluated for their efficacy in treating AD have been previously been summarized in literature [45,46]. Since recent clinical trials suggested tau-based therapies may be more effective than anti-Aβ treatments for patients already presenting AD symptoms, the relative paucity of tau-reducing agents needs to be addressed. Examples of anti-tau diets (diets aiding in reducing tau) indicate the potential of utilizing natural products as future treatments for AD. Summarized below are natural products reported to date, from terrestrial and marine plants, invertebrates and algae, as well as microorganisms, which have been found active in tau-related screens.
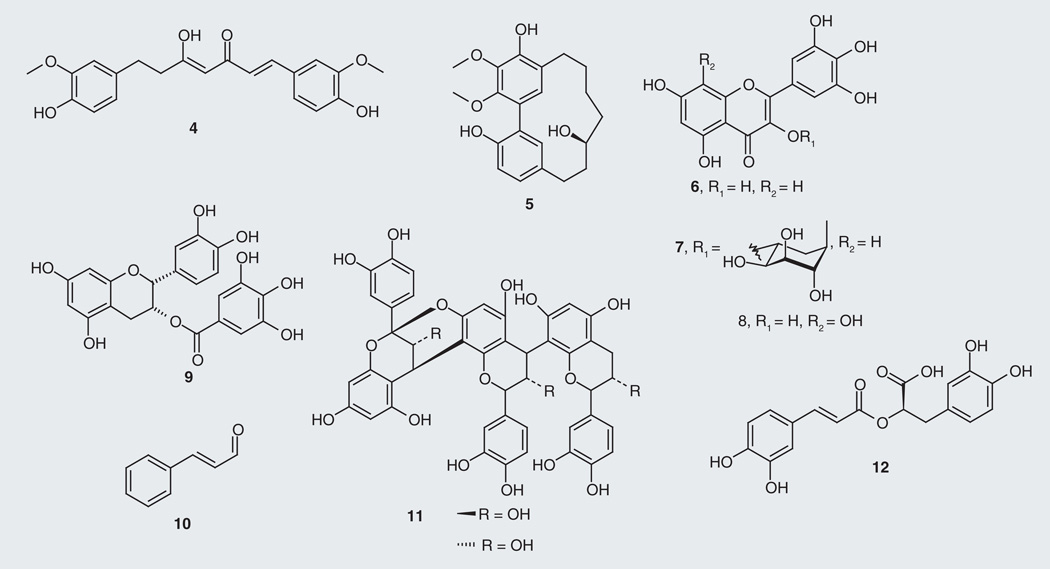
Scientists have looked to dietary sources, including extracts and preparations of ethnobotanical plants, for relief of neurodegenerative disorders [47,48]; recent efforts to uncover the chemical basis of these materials have identified a number of bioactive metabolites, some with drug-development potential. Many anti-tau natural products made by plants are polyphenols such as curcumin (4; Figure 4), a linear diarylheptanoid present at 66.8% of an optimized turmeric (Curcuma longa) extract [49]. This extract, in addition to acting as an antioxidant, was observed to significantly increase production of the anti-inflammatory cytokine IL-4 and to reduce Aβ and tau levels in Aβ-overexpressing mice [50]. Furthermore, our group has identified a potent macrocyclic diarylheptanoid from bayberry root bark (Myrica cerifera) extract, (+)-aR,11S-myricanol (5) that reduces tau levels [51]. Compound 5 reduced tau levels ex vivo in a cell culture model of tauopathy (in HeLa-C3 cells) with an EC50 value of 35 µM and is a suitable scaffold for AD drug discovery [101]. The isolation of (+)-aR,11S-myricanol (5) was accompanied by additional bayberry flavonoids, including myricetin (6) and its rhamoside glycoside myricitrin (7). Low micromolar tau filament formation inhibition of myricetin was previously reported in vitro as well as for two other members of the flavonoid family the roselle (Hibiscus sabdariffa)-derived gossypetin (8) [52] and the green tea (Camellia sinensis)-derived (−)-epicatechin-3-gallate (9) [53] showing IC50 values at 1.2, 2.0 and 1.8 µM, respectively [54]. A cinnamon (Cinnamonium zeylanicum) extract inhibiting aggregation of human tau in vitro leads to an inhibitory activity attributable to both compounds cinnamaldehyde (10) and A-type doubly linked procyanidin oligomers of the catechins/epicatechin structural classes (11) [55]. Similar procyanidins identified from grape seed (Vitis vinifera)-derived polyphenolic extracts were found to prevent tau fibrillization into neurotoxic aggregates [56]. Investigation of sage (Salvia offinalis) as a culinary source for improving cognition and memory showed that the active ingredient was the polyphenol rosmarinic acid (12), which reduced tau hyperphosphorylation in addition to attenuating several AD pathways, such as reactive oxygen species formation, lipid peroxidation, DNA fragmentation, caspase-3 activation and Aβ accumulation [57].
Figure 4.
Plant-derived tau-targeting natural products.
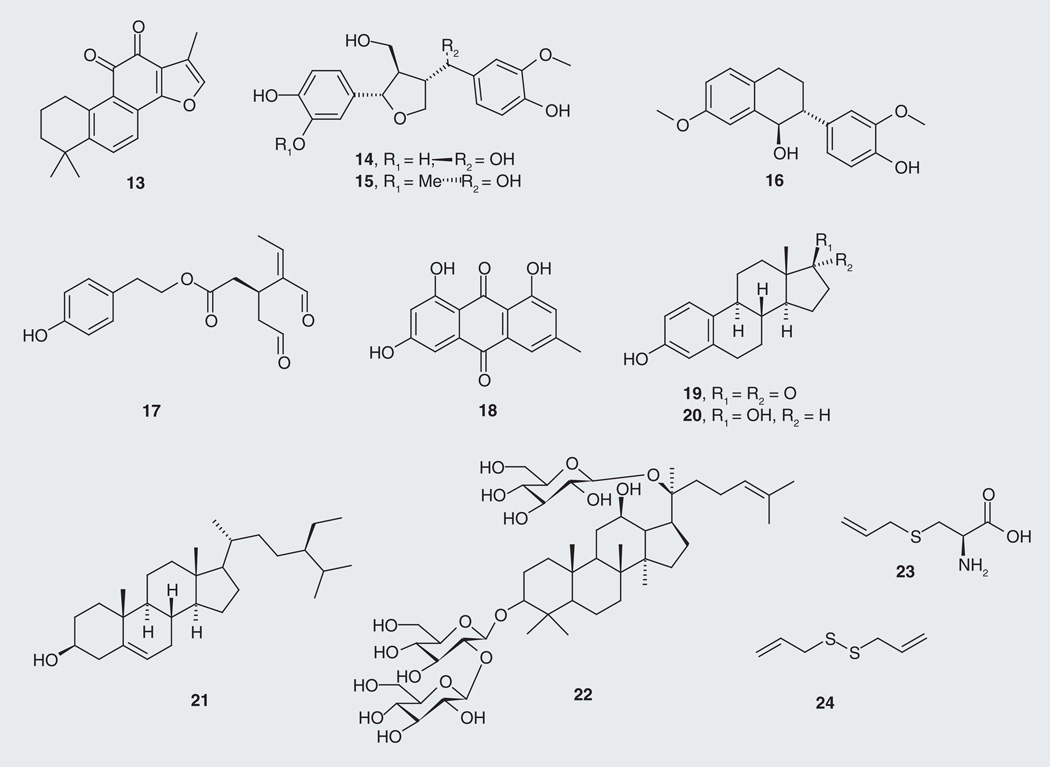
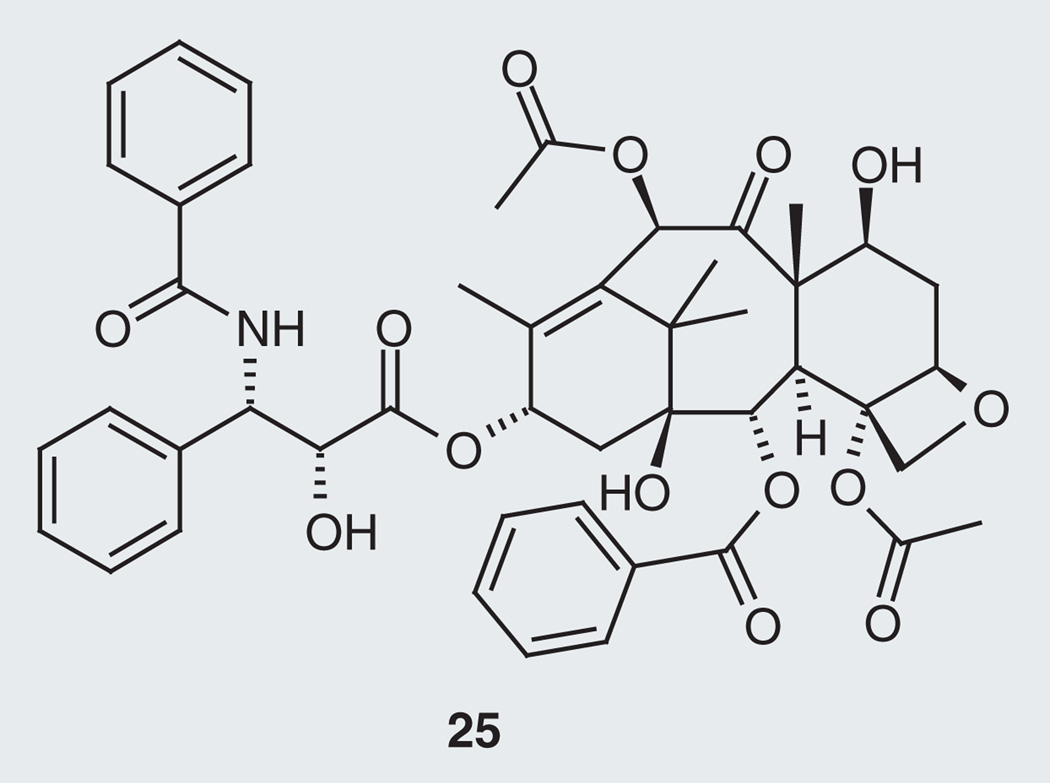
Other plant and dietary sources with tau-modifying effects produce compounds that are not polyphenols. Tanshinone IIA (13; Figure 5), a norditerpene from red sage (Salvia miltiorrhiza), possesses protective effects against neurotoxicity and tau hyperphosphorylation induced by β-amyloid at 10 µM [58], whereas the lignans 14 and 15, and the isoflavane 16, from the water soluble extract of Chinese Yew, Taxus yunnanensis, revealed stimulatory effects on GSK-3β at 10 µM, preferentially phosphorylating serine residues rather than threonine residues on recombinant human tau protein (rhTP) [59]. The known microtubule stabilizer drug (MSD) paclitaxel (25; Figure 6) from the Pacific Yew, Taxus brevifolia, showed positive results in neurodegenerative tauopathy by counteracting ‘loss-of-function’ effects of tau pathology in a transgenic mouse model [44]. Oleocanthal (17), from olive oil (Oleaeuropaea), was tested for inhibition of filament formation of the longest tau isoform T40 and the corresponding microtubule-binding region K18, displaying IC50 values of 20 and 2.9 µM, respectively [60]. The anthroquinone emodin (18), isolated from the root and rhizome of rhubarb, Rheum palmatum, displays inhibition of PHFs [61]. Levels of tau-1 were found to decrease in ovariectomized rats (which hold similar pathology to menopausal women with AD) after 4 weeks of drinking the juice of young coconut (Cocos nucifera). Young coconut juice contains estrogen-like substances such as estrone (19), 17-β-estradiol (20) and β-sitosterol (21), which are implicated in the observed effects on tau [62]. The steroid glycoside ginsenoside Rd (22) from Asian ginseng, Panax ginseng, shows in vivo and in vitro reduction of neurotoxicity and tau hyperphosphorylation by enhancing the activities of PP-2A [63]. Aged garlic (Allium sativum) extract has sulfur-containing constituents S-allyl-cysteine (23) (water-soluble component of garlic) and diallyl-disulfide (24) (lipid-soluble component of garlic), which exhibited anti-amyloidogenic, anti-inf lammatory and anti-tangle effects [64].
Figure 5.
Plant-derived tau-targeting natural products.
Figure 6. Paclitaxel.
Plant-derived microtubule stabilizer drug as tau-targeting natural product.
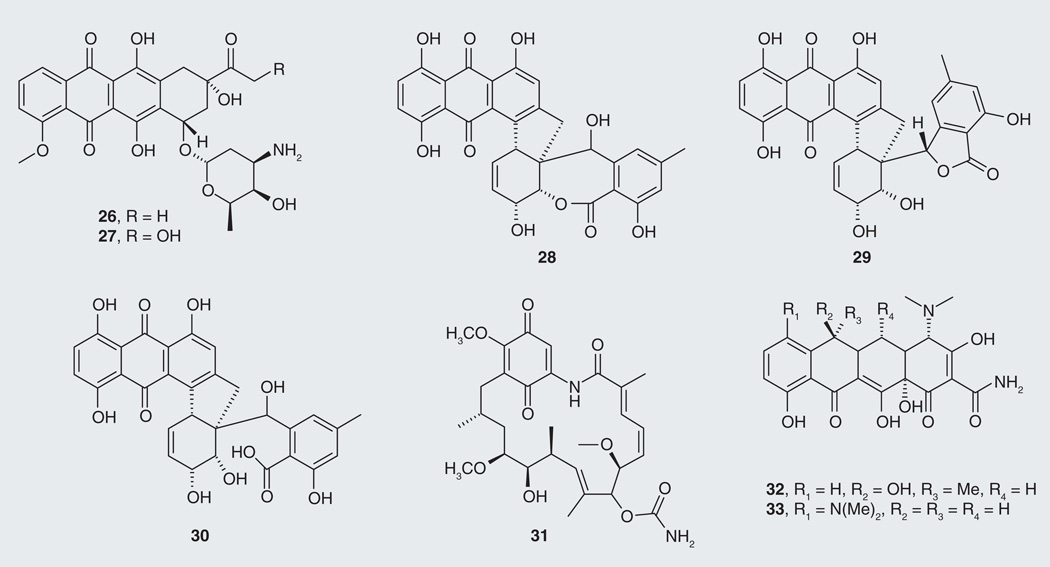
Tau-reducing compounds are not limited to plant sources; however, in addition to those listed above, bacterial- and fungal-derived compounds with anti-tau activity have been reported. Most are quinone derivatives such as the anthraquinones, daunorubicin (26; Figure 7) and adriamycin (27) isolated from the bacterium Streptomyces peucetius, along with the previously discussed compound emodin (18). Both compounds 26 and 27 displayed tau aggregation inhibition activity, dissolving PHFs in vitro and in cells [61]. From the fungal kingdom, the anthraquinone rubellins were isolated from the phytopatogenic fungus Ramularia collo-cygni [65]. After broad bioactivity profiling, rubellins B, D and E (28–30) were found to inhibit the formation of tau aggregates with IC50 values of 1.2, 0.9 and 0.9 µM, respectively, and to promote the disassembly of tau aggregates with DC50’s of 1.6, 2.2 and 0.5 µM, respectively. Benzoquinone macrocyclic polyketide geldanamycin (31), isolated from the related microbe Streptomyces hygroscopicus [66], was identified as an inhibitor of Hsp90 and reported to reduce levels of hyperphosphorylated tau [21]. While tetracycline (32), a compound isolated from Streptomyces spp., inhibited and disassembled Aβ fibrils [67], its synthetic derivative minocycline (33) showed additional capacities such as neuroprotection [68], microglial activation [69], caspase-3 activation inhibition, and reduction of tau hyperphosphorylation [70]. Minocycline is currently being tested in a Phase II clinical trial in AD (Table 1).
Figure 7.
Microbe-derived tau-targeting natural products.
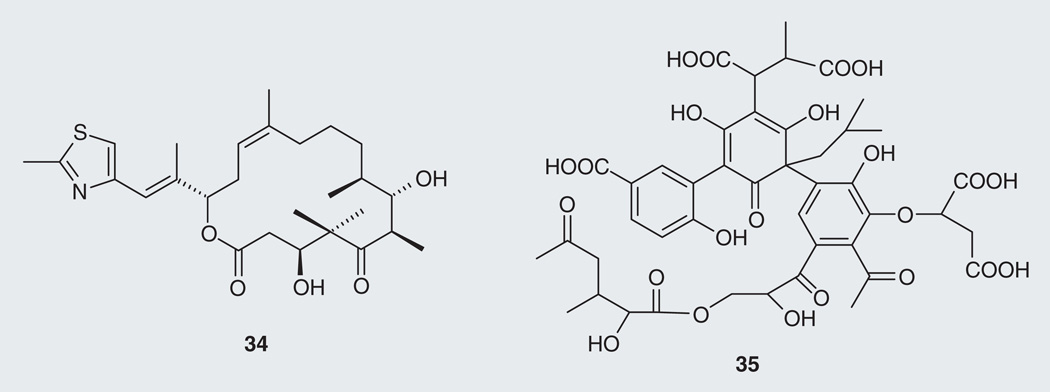
Transgenic mice with existing tau pathology treated with the myxobacteria (Sorangium cellulosum)-derived MSD macrocyclic polyketide epothilone D (34; Figure 8) had less forebrain tau pathology, with a reduction of axonal dystrophy and an increase of axonal microtubule density [71]. Tau tangles are still present in the optic nerve, which is expected. Improving MT function and axonal integrity with a MSD may not alter tau phosphorylation and aggregation, but may compensate for the loss-of-function of tau [44].
Figure 8.
Microbe-derived tau-targeting natural products (34 & 35).
Microbial action on plant litter polyphenolics produces complex mixtures of higher order polyphenolics known as fulvic and humic acids. Fulvic acid standard I (35) (Suwannee River I 1S101F), was found to inhibit aggregation of tau fibrils in vitro with an IC50 value of 37 µM and promote the disassembly of tau fibrils with a DC50 value of 95 µM [72]. Fulvic acid also represents a major component of the Indian Ayurvedic traditional medicine shilajit, a tar-like substance reported to control aging-related cognitive disorders [73].
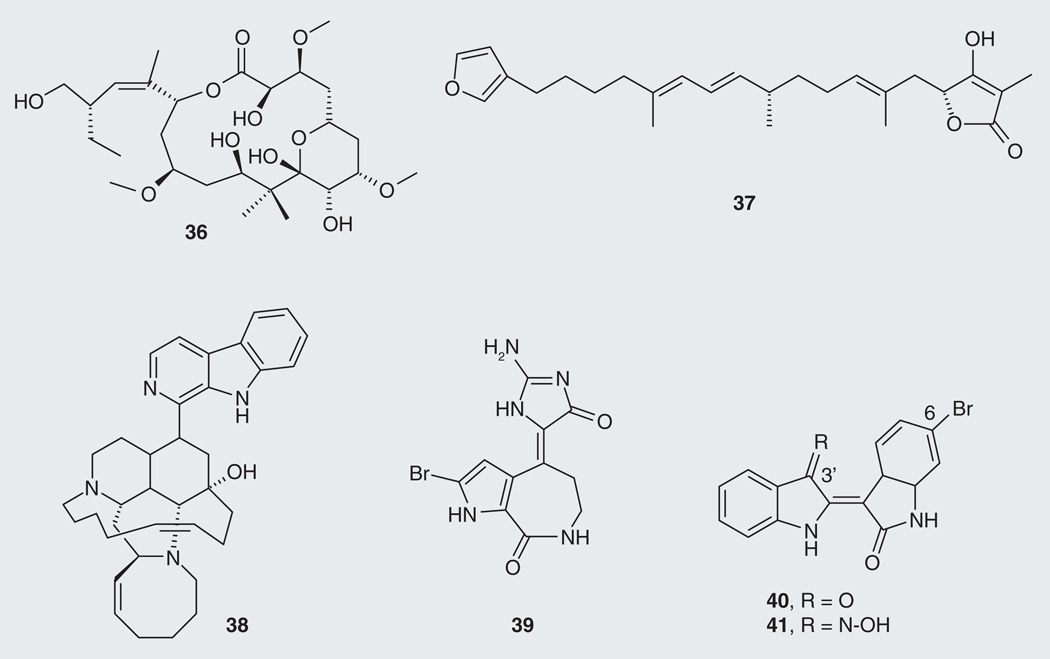
Further tau-reducing compounds are found in marine organisms such as sponges and sea snails. Peloruside A (36; Figure 9) is a MSD isolated from the marine sponge Mycale hentscheli with the same mode of action as paclitaxel [74]. Palinurin (37), a linear furanosesterterpene from sponges of the genus Ircinia (I. variabilis, I. dendroides and I. oros), emerged as a non-ATP competitive inhibitor of GSK-3β with an IC50 value of 4.5 µM [75,102], resulting in reduction of tau hyperphosphorylation. Marine alkaloids have also been evaluated against GSK-3β. Manzamine A (38), isolated from the sponge Haliclona sp. [76], and hymenaldisine (39), first isolated from two sponges Axinella verrucosa and Acanthella aurantiaca [77], inhibited cyclin-dependent kinases, displaying IC50 values toward GSK-3β of 10 µM [78] and 35 nM, respectively [79]. Finally, the bis-indole alkaloids indirubins are constituents of a dye extracted from gasteropod mollusks of the Muricidae and Thaididae families. 6-bromoinduribin (40) from the Mediterranean mollusk Hexaplex trunculus and its synthetic derivative 6-bromoinduribins-3´-oxime (41) display an impressive selectivity toward, and inhibition of, GSK-3β with IC50 values of 45 and 5 nM, respectively [80]. These outstanding results show that bromominated bis-indoles may prove to be good candidates to develop for their ability to reduce tau.
Figure 9.
Marine macro-organism-derived tau-targeting natural products.
Development of natural products as tau-modifying compounds in AD
Little is currently known about structure–activity or structure–property relationships of these natural products. In the case of flavonoid polyphenols, it appears ortho-substituted phenol groups are required for tau reduction, based on comparison of (−)-epicatechin-3-gallate (9) to (−)-epicatechin, the latter of which is not active [54]. The same observation is noted with polyphenols for α-synolein and prion protein aggregation [81,82]. A protein/small-molecule interaction mechanism study revealed that the tau fibrillogenic fragment K18 is likely to be covalently modified by oleocanthal through Schiff base formation between the ε-amino group of the lysine residues and oleocanthalaldehyde carbonyls, which inhibits tau fibrillation [83]. However, further studies are needed to clarify the interaction between tau-reducing small molecules and the protein. In addition, the exact mechanism of tau oligomer formation remains to be elucidated. Furthermore the blood–brain barrier (BBB), the semi-permeable protective shield surrounding the brain to restrict substances in the blood from entering the CNS, can present developmental challenges for drugs in AD. Crossing the BBB requires a drug with low molecular weight and lipid solubility [84]. Few permeability studies have been conducted on the natural products discussed above. However, curcumin (4) was found effective at crossing the BBB [85]. Other tau-reducing polyphenols of the proanthocyanidin and flavonoid classes may be good candidates for AD leads as a result of having similar chemical character to curcumin [86,87]. On the other hand, the anticancer MSD paclitaxel (25) and related taxanes showed poor BBB permeability, suggesting smaller or more lipophilic natural products may be more successful [88]. Indeed, epothilone D (34), another MSD, is a brain penetrant and safe at low doses [71]. The indirubins displayed in vivo efficacy, highlighting their facility in crossing the BBB and suggesting these compounds may warrant development as therapeutic agents in AD and other neurodegenerative disorders [79].
Future perspective
Overall, we have focused this review on tau-based therapeutics for AD. We have summarized current literature describing anti-tau compounds from nature as potential agents to treat AD. Natural products from different biological sources, such as plants, fungi, bacteria, marine sponges and mollusks have been evaluated for their tau protein modulation. Discovery and development of compounds along this line will require stronger knowledge of the mode of action of the disease. Furthermore, any newly discovered drug leads must have favorable BBB permeability and suitable pharmacodynamic properties.
Executive summary.
Understanding Alzheimer’s disease progression is crucial to finding a cure for a rising aging population
-
▪
Current commercially available drugs for Alzheimer’s disease (AD) are AChE inhibitors. Tacrine, donepezil, and the natural product-derived galantamine and rivastigmine can alleviate symptoms of AD and may have modifying effect on the disease.
-
▪
Mechanisms of AD development and progression are not well known. However, two major theories are the amyloid and tau hypotheses.
A switch in drug-discovery strategy from the Aβ to the tau hypothesis
-
▪
Due to the limited success of Aβ-based drugs in clinical trials, tau-based therapeutics have emerged as a potential alternative.
-
▪
Two drugs based on the tau hypothesis are already in Phase II clinical trials. The outcomes of these trials will provide more information about the viability of the tau strategy.
Natural products are a rich source of diverse scaffolds for AD drug discovery
-
▪
Many drugs are derived from natural products including two of the four available AD drugs based on AChE.
-
▪
Based on current successes, natural products may provide new resources for the discovery of novel and more effective drugs for AD.
Acknowledgments
The authors of this review were supported by Grants from NIH/NINDS (R01NS073899), the Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation and CurePSP.
Key Terms
- Alzheimer’s disease
Most common form of neurodegenerative disorder affecting memory, thinking and behavior. It is characterized by the gradual loss of neurons and synapses in the brain and leads to death.
- Tau
Microtubule-stabilizing protein abundant in neurons of the CNS. The abnormal function of tau leads to neurodegenerative disorders such as Alzheimer’s disease.
- Galantamine
AChE inhibitor that is the first and, currently, only natural product used as a drug to treat symptoms of Alzheimer’s disease.
- Alkaloid
Organic substance containing basic nitrogen atoms.
- Polyphenol
Organic substance characterized by the presence of multiple phenol structural units.
- Curcumin
Principal constituent of the indian spice turmeric (Curcuma longa). A broad range of therapeutic properties toward cancer, autoimmune diseases and Alzheimer’s disease are attributed to curcurmin.
- Paclitaxel
Yew tree-derived antimitotic drug used in cancer chemotherapy that has been studied as a treatment for Alzheimer’s disease.
- Quinone
Aromatic organic substance characterized by fully conjugated diiones.
- Minocycline
Tetracycline antibiotic primarily used in treating acne. Minocycline shows a broad spectrum of activities, including Alzheimer’s disease-treatment properties.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Prince M, Bryce R, Ferri C. World Alzheimer Report. London, UK: Alzheimer’s Disease International; 2011. pp. 1–36. [Google Scholar]
- 2.Freeman WJ. Alzheimer: the life of a physician and the career of a disease. J. Am. Med. Assoc. 2005;293:745–746. [Google Scholar]
- 3.Lott IT, Head E. Alzheimer disease and down syndrome: factors in pathogenesis. Neurobiol. Aging. 2005;26:383–389. doi: 10.1016/j.neurobiolaging.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Waring SC, Rosenberg RN. Genome-wide association studies in Alzheimer disease. Arch. Neurol. 2008;65:329–334. doi: 10.1001/archneur.65.3.329. [DOI] [PubMed] [Google Scholar]
- 5.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology including Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartus RT, Dean RL, Pontecorvo MJ, Flicker C. The cholinergic hypothesis: a historical overview, current perspective, and future directions. Ann. NY Acad. Sci. 1985;444:332–358. doi: 10.1111/j.1749-6632.1985.tb37600.x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia ML, Cleveland DW. Going new places using an old MAP: tau, microtubules and human neurodegenerative disease. Curr. Opin. Cell Biol. 2001;13:41–48. doi: 10.1016/s0955-0674(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 8.Cassimeris L, Spittle C. Regulation of microtubule associated protein. Int. Rev. Cytol. 2001;210:163–226. doi: 10.1016/s0074-7696(01)10006-9. [DOI] [PubMed] [Google Scholar]
- 9.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathywith presenile dementia. Proc. Natl Acad. Sci. USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5´-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 11.Hardy J, Orr H. The genetics of neurodegenerative diseases. J. Neurochem. 2006;97:1690–1699. doi: 10.1111/j.1471-4159.2006.03979.x. [DOI] [PubMed] [Google Scholar]
- 12.Goedert M, Spillantini MG, Crowther RA, et al. Tau gene mutation in familial progressive subcortical gliosis. Nat. Med. 1999;5:454–457. doi: 10.1038/7454. [DOI] [PubMed] [Google Scholar]
- 13.Van Marum RJ. Current and future therapy in Alzheimer’s disease. Fundam. Clin. Pharmacol. 2008;22:265–274. doi: 10.1111/j.1472-8206.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- 14.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–448. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spires-Jones TL, de Calignon A, Matsui T, et al. In vivo imaging reveals dissociation between caspase activation and acute neuronal death in tangle-bearing neurons. Neuroscience. 2008;28:862–867. doi: 10.1523/JNEUROSCI.3072-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Congdon EE, Necula M, Blackstone RD, Kuret J. Potency of a tau fibrillization inhibitor is influenced by its aggregation state. Arch. Biochem. Biophys. 2007;465:127–135. doi: 10.1016/j.abb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oddo S, Vasilevko V, Caccamo A, Kitazawa M, Cribbs DH, La Ferla FM. Reduction of soluble Aβ and tau, but not Soluble Aβ alone, ameliorates cognitive decline in transgenic mice with plaques and tangles. J. Biol. Chem. 2006;281:39413–39423. doi: 10.1074/jbc.M608485200. [DOI] [PubMed] [Google Scholar]
- 18.Goedert M. Tau gene mutations and their effects. Mov. Disord. 2005;20:45–52. doi: 10.1002/mds.20539. [DOI] [PubMed] [Google Scholar]
- 19. Goedert M, Jakes R. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2005;1739:240–250. doi: 10.1016/j.bbadis.2004.08.007. ▪ The clear influence of the tau protein in neurodegenerative diseases, such as Alzheimer’s disease and the inherited frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17).
- 20.Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregationby phenothiazines. Proc. Natl Acad. Sci. USA. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickey CA, Kamal A, Lundgren K, et al. The high-affinity HSP90–CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green RC, Schneider LS, Amato DA, et al. Effect of Tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease. J. Am. Med. Assoc. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauthier S, Aisen PS, Ferris SH, et al. Effect of Tramiprosate in Patients with Mild-tomoderate Alzheimer’s disease: exploratory analyses of the MRI sub-group of the Alphase study. J. Nutr. Health Aging. 2009;13:550–557. doi: 10.1007/s12603-009-0106-x. [DOI] [PubMed] [Google Scholar]
- 24.Lyketsos CG, Breitner JC, Green RC, et al. Naproxen and celecoxib do notprevent AD in early results from a randomized controlled trial. Neurology. 2007;68:1800–1808. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 25.Crystal H, Dickson D, Fuld P, et al. Clinicopathological studies in dementia: nondemented subjects with pathological confirmed Alzheimer’s disease. Neurology. 1988;38:1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Ber.) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 27.Kramp VP. List of drugs in development for neurodegenerative diseases: update October 2011. Neurodegen. Dis. 2012;9:210–283. doi: 10.1159/000335520. [DOI] [PubMed] [Google Scholar]
- 28.Mount C, Downton C. Alzheimer disease: progress or profit? Nat. Med. 2006;12:780–784. doi: 10.1038/nm0706-780. [DOI] [PubMed] [Google Scholar]
- 29.Areosa SA, Sherriff F, McShane R. Menantine for dementia. Coch. Data. Syst. Rev. 2006;2:1–77. [Google Scholar]
- 30.Brekov S, Georgieva L, Kondakova V, et al. Plant sources of Galathamine: phytochemical and biotechnological aspects. Biotechnol. Biotechnol. Eq. 2009;23:1170–1176. [Google Scholar]
- 31.Nordberg A, Svensson AL. Cholinesterase inhibitors in the treatment of Alzheimer disease: a comparison of tolerability and pharmacology. Drug Saf. 1998;19:465–480. doi: 10.2165/00002018-199819060-00004. [DOI] [PubMed] [Google Scholar]
- 32.Al-Rashid ZF, Hsung RP. (+)-arisugacin A – computational evidence of a dual binding site covalent inhibitor of acetylcholinesterase. Bioorg. Med. Chem. Lett. 2011;21:2687–2691. doi: 10.1016/j.bmcl.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polydoro M, Acker CM, Duff K, Castillo PE, Davies PJ. Age-dependent impairment of cognitive and synaptic function in the tau mouse model of tau pathology. Neurosci. 2009;29:10741–10749. doi: 10.1523/JNEUROSCI.1065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. ▪ This coverage of 30 years of natural products shows that natural products are an inestimable source of drug discovery.
- 35.Necula M, Chirita CN, Kuret J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: implications for the treatment of tauopathic neurodegenerative diseases. Biochemistry. 2005;44:10227–10237. doi: 10.1021/bi050387o. [DOI] [PubMed] [Google Scholar]
- 36.de Calignon A, Fox LM, Pitstick R, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–1204. doi: 10.1038/nature08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenberg D, Nelson R, Sawaya MR, et al. The structural biology of protein aggregation diseases: fundamental questions and some answers. Acc. Chem. Res. 2006;39:568–575. doi: 10.1021/ar0500618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanger DP, Anderton BH, Noble W. Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat. Rev. Drug Discov. 2007;6:464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- 40.Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr. Med. Chem. 2008;15:2321–2328. doi: 10.2174/092986708785909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina M, Castro A. Glycogen synthase kinase-3 (GSK-3) inhibitors reach the clinic. Curr. Opin. Drug Discov. Dev. 2008;11:533–543. [PubMed] [Google Scholar]
- 42.Selenica ML, Jensen HS, Larsen AK, et al. Efficacy of small-molecule glycogen synthase kinase-3 inhibitors in the post natal rat model of tau hyperphosphorylation. Br. J. Pharmacol. 2007;152:959–979. doi: 10.1038/sj.bjp.0707471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishihara T, Hong M, Zhang B, et al. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, Maiti A, Shively S, et al. Microtubule-binding drugs offset tau sequestration by stabilizing microtubules and reversing fast axonal transport deficits in a tauopathy model. Proc. Natl Acad. Sci. USA. 2005;102:227–223. doi: 10.1073/pnas.0406361102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams P, Sorribas A, Howes MJR. Natural products as a source of Alzheimer’s drug leads . Nat. Prod. Rep. 2011;28:48–77. doi: 10.1039/c0np00027b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Da Rocha MD, Dias Viegas FP, Campos HC, et al. The role of natural products in the discovery of new drug candidates for the treatment of neurodegenerative disorders II: Alzheimer’s disease. CNS Neurol. Disord. Drug Targets. 2011;10:251–270. doi: 10.2174/187152711794480429. [DOI] [PubMed] [Google Scholar]
- 47.Gu Y, Nieves JW, Stern Y, Luchsinger JA, Scarmeas N. Food combination and Alzheimer disease risk: a protective diet. Arch. Neurol. 2010;67:699–706. doi: 10.1001/archneurol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howes MJ, Houghton PJ. Ethnobotanical treatment strategies against Alzheimer’s disease. Curr. Alzheimer Res. 2012;9:67–85. doi: 10.2174/156720512799015046. [DOI] [PubMed] [Google Scholar]
- 49.Shytle RD, Bickford PC, Rezai-zadeh K, et al. Optimized turmeric extracts have potent anti-amyloidogenic effects. Curr. Alzheimer Res. 2009;6:564–571. doi: 10.2174/156720509790147115. [DOI] [PubMed] [Google Scholar]
- 50.Shytle RD, Tan J, Bickford PC, et al. Optimized turmeric extract reduces β-amyloid and phosphorylated tau protein burden in Alzheimer’s transgenic mice. Curr. Alzheimer Res. 2012;9:500–506. doi: 10.2174/156720512800492459. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51. Jones J, Lebar MD, Jinwal UK, et al. The diarylheptanoid (+)-aR,11S-myricanol and two flavones from bayberry (Myricacerifera) destabilize the microtubule-associated protein tau. J. Nat. Prod. 2011;74:38–44. doi: 10.1021/np100572z. ▪▪ (+)-aR,11S-myricanol represents a new scaffold for tau-targeting natural products and synthetic derivatives development.
- 52.Mounnissamy VM, Kavimani S, Gunasegaran R. Antibacterial activity of gossypetin isolated from hibiscus sabdariffa. Antiseptic. 2009;99:81–82. [Google Scholar]
- 53.Shiota S, Shimizu M, Mizushima T. Marked reduction in the minimum inhibitory concentration (MIC) of beta-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechingallate, an ingredient of green tea (Camellia sinensis) Biol. Pharm. Bull. 1999;22:1388–1390. doi: 10.1248/bpb.22.1388. [DOI] [PubMed] [Google Scholar]
- 54.Taniguchi S, Suzuki N, Masuda M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005;280:7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 55.Peterson DW, George RC, Scaramozzino F, Lapointe NE, Anderson RA, Graves DJ, et al. Cinnamon extract inhibits tau aggregation assiocated with Alzheimer’s disease in vitro. J. Alzheimers Dis. 2009;17:585–597. doi: 10.3233/JAD-2009-1083. [DOI] [PubMed] [Google Scholar]
- 56.Pasinetti GM, Ksiezak-Reding H, Santa- Maria I, Wang J, Ho L. Development of a grape seed polyphenolic extract with antioligomeric activity as a novel treatment in progressive supranuclear palsy and other tauopathies. J. Neurochem. 2010;114:1557–1568. doi: 10.1111/j.1471-4159.2010.06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luvone T, De Filippis D, Esposito G, D’Amico A, Izzo AA. The spice sage and its active ingredient rosmarinicacid protect PC12 cells from amyloid-β peptide-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2006;317:1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- 58.Shi LL, Yang WN, Chen XL, et al. The protective effects of tanshinone IIA on neurotoxicity induced by β-amyloid protein through calpain and the p35/Cdk5 pathway in primary cortical neurons. Neurochem. Int. 2012;61(2):227–235. doi: 10.1016/j.neuint.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 59.Ohtsuki K, Miyai S, Yamaguchi A, Morikawa K, Okano T. Biochemical characterization of novel lignansisolated from the wood of Taxus yunnanensis as effective stimulators for glycogen synthase kinase-3β and the phosphorylation of basic brain proteins by the kinase in vitro. Biol. Pharm. Bull. 2012;35:385–393. doi: 10.1248/bpb.35.385. [DOI] [PubMed] [Google Scholar]
- 60.Li W, Sperry JB, Crowe A, et al. Inhibition of tau fibrillation by oleocanthal via the reaction of the amino group of tau. J. Neurochem. 2009;110:1339–1351. doi: 10.1111/j.1471-4159.2009.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pickhardt M, Gazova Z, von Bergen M, et al. Anthraquinones inhibit tau aggregation and dissolve Alzheimer’s paired helical filaments in vitro and in cells. J. Biol. Chem. 2005;280:3628–3635. doi: 10.1074/jbc.M410984200. [DOI] [PubMed] [Google Scholar]
- 62.Radenahmad N, Saleh S, Sawangjaroen K, et al. Young coconut juice, a potential therapeutic agent that could significantly reduce some pathologies associated with Alzheimer’s disease: novel findings. Br. J. Nutr. 2011;105:738–746. doi: 10.1017/S0007114510004241. [DOI] [PubMed] [Google Scholar]
- 63.Li L, Liu J, Yan X, et al. Protective effects of ginsenoside Rd against okadaic acid-induced neurotoxicity in vivo and in vitro. J. Ethnopharmacol. 2011;138:135–141. doi: 10.1016/j.jep.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 64.Chaudan NB. Effect of aged garlic extract on APP processing and tau phosphorylationin Alzheimer’s transgenic model Tg2576. J. Ethnopharm. 2006;108:385–394. doi: 10.1016/j.jep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 65.Miethbauer S, Gaube F, Möllmann U, et al. Antimicrobial, antiproliferative, cytotoxic, and tau inhibitory activity of rubellins and caeruleoramularin produced by the phytopathogenic fungusrcollo-cygni. Planta Med. 2009;75:1523–1525. doi: 10.1055/s-0029-1185835. [DOI] [PubMed] [Google Scholar]
- 66.Bedin M, Gaben AM, Saucier C, Mester J. Geldanamycin, an inhibitor of the chaperone activity of HSP90, induces MAPKindependent cell cycle arrest. Int.J. Cancer. 2004;109:643–652. doi: 10.1002/ijc.20010. [DOI] [PubMed] [Google Scholar]
- 67.Forloni G, Colombo L, Girola, Tagliavini F, Salmona M. Anti-amyloidogenic activity of tetracyclines: studies in vitro. FEBS Lett. 2007;487:404–407. doi: 10.1016/s0014-5793(00)02380-2. [DOI] [PubMed] [Google Scholar]
- 68.Choi Y, Kim H, Shin KY, et al. Minocycline attenuates neuronal cell death and improves cognitive impairment in Alzheimer’s disease models. Neuropsychopharmacology. 2007;32:2393–2404. doi: 10.1038/sj.npp.1301377. [DOI] [PubMed] [Google Scholar]
- 69.Familian A, Boshuizen RS, Eikelenboom P, Veerhuis R. Inhibitory effect of minocycline on amyloid beta fibril formation and human microglial activation. Glia. 2006;53:233–240. doi: 10.1002/glia.20268. [DOI] [PubMed] [Google Scholar]
- 70.Noble W, Garwood C, Stephenson J, Kinsey AM, Hanger DP, Anderton BH. Minocycline reduces the development of abnormal tau species in models of Alzheimer’s disease. FASEB J. 2009;23:739–750. doi: 10.1096/fj.08-113795. [DOI] [PubMed] [Google Scholar]
- 71.Zhang B, Carroll J, Trojanowski JQ, et al. The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J. Neurosci. 2012;32:3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cornejo A, Jimenez JM, Caballero L, Melo F, Maccioni RB. Fulvic acid inhibits of tau fibrils associated with Alzheimer’s disease. J. Alzheimer Dis. 2011;27:143–153. doi: 10.3233/JAD-2011-110623. [DOI] [PubMed] [Google Scholar]
- 73.Carrasco-Gallardo C, Guzman L, Maccioni RB. Shilajit: a natural phytocomplex with potential precognitive activity. Int. J. Alzheimer Dis. 2012:1–4. doi: 10.1155/2012/674142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das V, Miller JH. Microtubule stabilization by peloruside A and paclitaxel rescues degenerating neurons from okadaic acid-induced tau phosphorylation. Eur. J. Neurosci. 2012;35:1705–1717. doi: 10.1111/j.1460-9568.2012.08084.x. [DOI] [PubMed] [Google Scholar]
- 75.Alfano G, Cimino G, De Stefano S. Palinurin, a new linear sesterterpene from a marine sponge. Experientia. 1979;35:1136–1137. [Google Scholar]
- 76.Sakai R, Higa T, Jefford CW, Bernardinelli G. Manzamine A, a novel antitumor alkaloid from a sponge. J. Am. Chem. Soc. 1986;108:6404–6405. [Google Scholar]
- 77.Cimino G, de Rosa S, de Stefano S, Mazzarella L, Puliti R, Sodano G. Isolation and x-ray crystal structure of a novel bromo-compound from two marine sponges. Tetra. Lett. 1982;23:767–768. [Google Scholar]
- 78.Hamann M, Alonso A, Martin-Aparicio E, et al. Glycogen synthasekinase-3 (GSK-3) inhibitory activity and structure-activity relationship (SAR) studies of themanzamine alkaloids. Potential for Alzheimer’s disease. J. Nat. Prod. 2007;70:1397–1405. doi: 10.1021/np060092r. [DOI] [PubMed] [Google Scholar]
- 79.Meijer L, Thunnissen A, White AW, et al. Inhibition of cyclin-dependent kinases, GSK-3β and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 2000;7:51–63. doi: 10.1016/s1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 80. Meijer L, Skaltsounis AL, Magiatis P, et al. GSK-3-selective inhibitors derived from tyrianpurple indirubins. Chem. Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. ▪ The mollusk-derived bromoindirubins provide a promising scaffold for the development of selective and strong inhibitors of GSK-3β, which can be triggered to reduce phosphorylated tau.
- 81.Zhu M, Rajamani S, Kaylor J, Han S, Zhou F, Fink AL. The flavonoid baicalein inhibits fibrillation of alpha-synuclein and disaggregates existing fibrils. J. Biol. Chem. 2004;279:26846–26857. doi: 10.1074/jbc.M403129200. [DOI] [PubMed] [Google Scholar]
- 82.Kocisko DA, Baron GS, Rubenstein R, Chen J, Kuizon S, Caughey B. Drugs and natural products protein formation in a library of 2,000 new inhibitors of scrapie-associated prion. J. Virol. 2003;77:10288–10294. doi: 10.1128/JVI.77.19.10288-10294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Monti MC, Margarucci L, Tosco A, Riccio R, Casapullo A. New insights on the interaction mechanism between tau protein and oleocanthal, an extra-virgin olive-oil bioactive component. Food Funct. 2011;2:423–428. doi: 10.1039/c1fo10064e. [DOI] [PubMed] [Google Scholar]
- 84. Alam MI, Beg S, Samad A, et al. Strategy for effective brain drug delivery. Eur. J. Pharm. Sci. 2010;40:385–403. doi: 10.1016/j.ejps.2010.05.003. ▪ Highlights the importance of drug delivery to make the CNS-related disease treatment effective.
- 85.Yang F, Lim GP, Begum AN. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 86.Janle EM, Lila MA, Wood L, et al. Kinetics and tissue distribution on 14C-labeled grape polyphenol fractions. FASEB J. 2007;21:837. [Google Scholar]
- 87.Jager AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16:1471–1485. doi: 10.3390/molecules16021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin J, Lee WS, Joo KM, et al. Preparation of blood–brain barrier-permeable paclitaxelcarrier conjugate and its chemotherapeutic activity in the mouse glioblastoma model . Med. Chem. Commun. 2011;2:270–273. [Google Scholar]
Patents
- 101.Dickey C, Lebar MD, Baker BJ, Jones J. WO2012012798. 2012
- 102.Gordillo DA, Diaz ID, Gil AM, et al. WO054221. 2004
Website
- 201. ClinicalTrials.gov. http://clinicaltrials.gov.