Abstract
Purpose
The incidence of adolescent idiopathic scoliosis (AIS) has rapidly increased, and with it, physician consultations and expenditures (about one and a half times) in the last 5 years. Recent etiological studies reveal that AIS is a complex genetic disorder that results from the interaction of multiple gene loci and the environment. For personalized treatment of AIS, a tool that can accurately measure the progression of Cobb's angle would be of great use. Gene analysis utilizing single nucleotide polymorphism (SNP) has been developed as a diagnostic tool for use in Caucasians but not Koreans. Therefore, we attempted to reveal AIS-related genes and their relevance in Koreans, exploring the potential use of gene analysis as a diagnostic tool for personalized treatment of AIS therein.
Materials and Methods
A total of 68 Korean AIS and 35 age- and sex-matched, healthy adolescents were enrolled in this study and were examined for 10 candidate scoliosis gene SNPs.
Results
This study revealed that the SNPs of rs2449539 in lysosomal-associated transmembrane protein 4 beta (LAPTM4B) and rs5742612 in upstream and insulin-like growth factor 1 (IGF1) were associated with both susceptibility to and curve severity in AIS. The results suggested that both LAPTM4B and IGF1 genes were important in AIS predisposition and progression.
Conclusion
Thus, on the basis of this study, if more SNPs or candidate genes are studied in a larger population in Korea, personalized treatment of Korean AIS patients might become a possibility.
Keywords: Adolescent idiopathic scoliosis, gene, single nucleotide polymorphism
INTRODUCTION
Adolescent idiopathic scoliosis (AIS) is characterized by a structural lateral curvature of the spine with a rotary component deviation in otherwise healthy individuals. When defined as a Cobb's angle of at least 10°, AIS is the most common pediatric spinal deformity, with reports of incidences ranging from 0.5% to 10% worldwide.1 A report published in Korea revealed a scoliosis prevalence of 3.26% among 1134890 school children, higher than those of other countries.2 According to a notification released by the Korea Health Insurance Review and Assessment Service, the number of scoliosis patients and expenditures related to its treatment increased by 12.2% and 40.3% over the past five years, respectively.3
AIS is usually noticed while bathing or during physical activity by a child's parents. Sometimes, early detection of AIS occurs during periodic school screening upon discovery of a waist or rib hump.4 The Adams forward bending test is helpful in detecting a thoracic rib hump or lateralized lifting of the buttock, but exact diagnosis is difficult. A hump and/or the Adams forward bending test are sensitive but not specific. Upon suspicion of AIS, radiographs can be used to confirm the diagnosis.
Clinical features and radiographic findings suggestive of minimal or mild idiopathic scoliosis do not adequately predict the future progression of the deformity. Even mild idiopathic scoliosis has been found to progress, so that regular medical assessment over a period of years has become necessary to detect and assess progression of the deformity.1,5,6 The current standards of care suggest that children and teens with AIS should be monitored until they cease growing, usually with serial radiographs, for curve progression. If serial radiographs confirm progression of Cobb's angle (20<Cobb's angle<45), a brace should be administered as the next step in the primary treatment thereof. Approximately 8% to 10% of all patients with adolescent idiopathic scoliosis are prescribed a brace at some point during their treatment. If the spinal curve continues to progress (Cobb >45), spinal fusion is often recommended.
Brace treatment comprising wearing of a brace for a minimum of 15 hours per day is considered effective. However, practically, it is difficult to ensure that adolescents wear a brace 15 hours a day.7 In order to overcome non compliance, electronic monitoring of these individuals is now followed in certain places.8 Due to difficulties faced during brace treatment, a prognostic method is direly needed to assess whether the deformity will progress or not. Such methods could help clinicians plan the treatment schedule of AIS on an individual and more effective manner.
Factors known to predict curve progression include maturity, curve size, and position of the curve apex. Progression equations have also been developed to quantify the risk of progression. Peterson and Nachemson9 used a method combining Risser sign, level of apex, presence of trunk imbalance, and chronological age to the aforementioned list of factors to predict curve progression. Although this method might be of some help to clinicians and patients, some patients do not follow this equation, and a short period brace or exercise only often improve Cobb's angle. In some cases, progression of deformity occurs rapidly, and physicians and their patients may miss the proper timing of surgery (Fig. 1). To solve this problem, a more accurate diagnostic tool is needed to determine whether or not deterioration will occur.
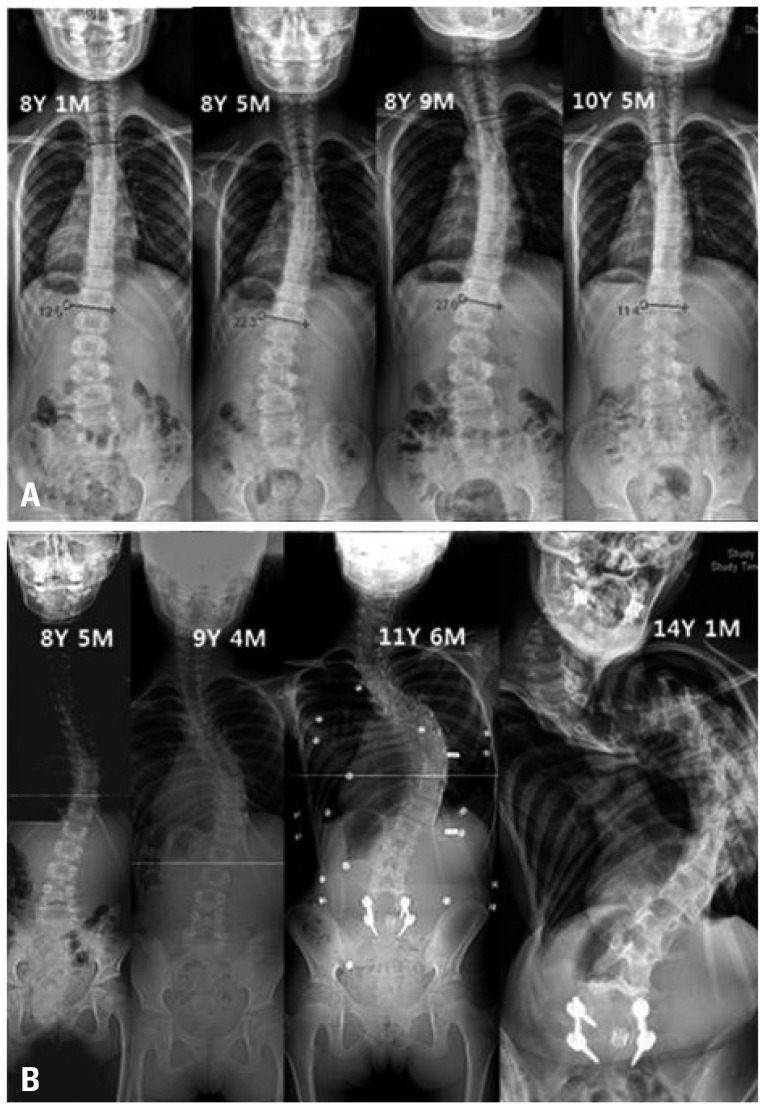
Fig. 1.
(A) A female patient (8 years 1 month) when first discovered in had a Cobb's of 12.5 degrees. She was kept under observation but the Cobb's angle deteriorated to 27 degrees. Therefore, she was treated with brace and exercise. After 2 years and 4 months of non-surgical treatment, Cobb's angle improved to 11.4 degrees. (B) Female patient (8 years 5 month) when first diagnosed, had 27.5 degrees Cobb's and was treated with brace and exercise but the Cobb's angle deteriorated to 140 degrees. Finally, her scoliosis and pulmonary functions deteriorated enough to cause serious danger to her life.
A prognostic test with a sufficient negative predictive value (NPV) can alter the monitoring schedule of a patient. Moreover, such test can reveal patients whose Cobb's angle will not undergo serious progression. Accordingly, low risk patients with a definite NPV might be spared the long-term negative effects of repeated exposure to radiation, unnecessary treatments, emotional stress, cost of direct care (physician's fee, hospital charge) and indirect cost of care, such as time away from a patient's studies and hospital visit expenses.10,11 In those who might undergo serious progression, however, it is important that AIS does not get worse. An effective prognostic test would allow physicians to develop new clinical protocols for managing high risk AIS patients.
Genetic associations in AIS have been described for decades.11 Recent etiologic studies revealed that AIS is a complex genetic disorder that results from the interaction of multiple gene loci and the environment.12,13 Linkage studies of these families revealed multiple potential genetic loci, chromosomes 6, 9, 16 and 17, that may predispose individuals to the condition.14 Several genes such as bone morphogenetic protein 4, interleukin-6, leptin, matrix metalloproteinase-3, melatonin 1B receptor (MTNR1B), etc., have been shown to be associated with a combinatorial effect.15 Recently, Ward insisted that by using 53 gene marker SNPs known to be associated with AIS, a NPV greater than 99% could be obtained.5
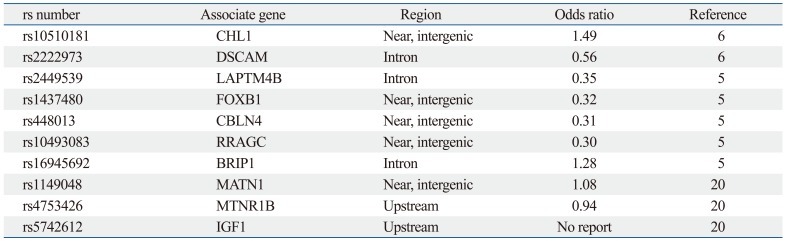
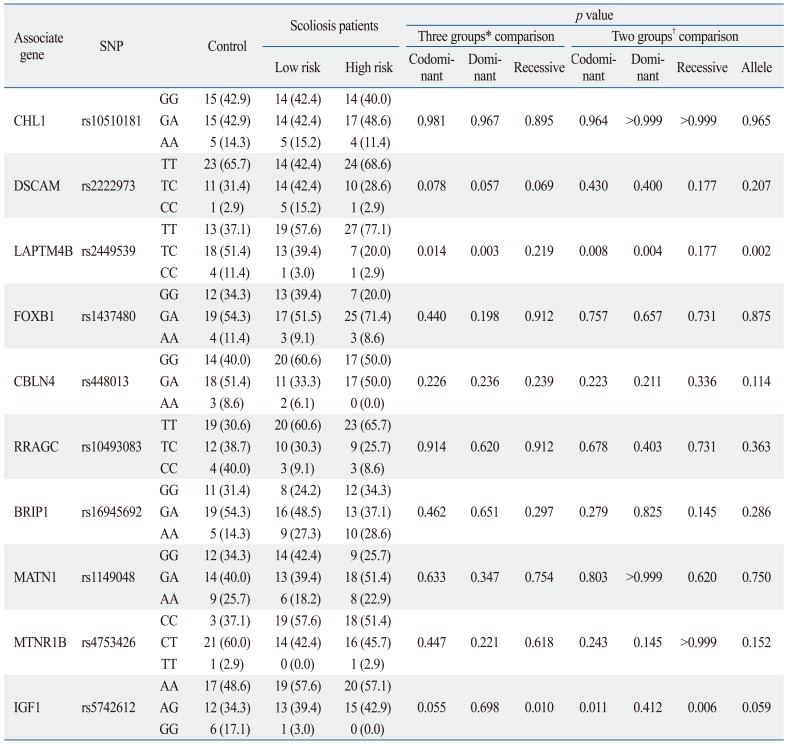
Ward, et al.5 also reported that these 53 SNPs were closely associated with AIS severity. The odds ratios for 5 of these SNPs (rs2449539, rs1437480, rs448013, rs10493083, rs16945692) with severe scoliosis ranged from 0.35 to 1.28, as shown in Table 1. Based on their study, a diagnostic kit was developed that could predict the progression of Cobb's angle and was sold commercially. Subsequently, several studies were carried out in Oriental countries concerning AIS candidate genes and SNP markers thereof.16 Sharma, et al.6 genotyped the SNPs of an additional chromosome, 3p26.3, and tested replication in two follow-up case-control cohorts, obtaining stronger results when all three cohorts were combined (rs10510181 odds ratio of 1.49). In addition, other significant associations in their genome-wide association study (GWAS) were discovered for SNPs (rs2222973) in the Down syndrome cell adhesion molecule (DSCAM) gene, which encodes for an axon guidance protein in the same structural class.
Table 1.
Selected SNP Markers in This Study
CHL1, close homolog of L1; DSCAM, Down syndrome cell adhesion molecule; LAPTM4B, lysosomal-associated transmembrane protein 4B; FOXB1, forkhead box protein B1; CBLN4, cerebellin 4 precursor; RRAGC, ras-related GTP binding C; BRIP1, fanconi anemia group J protein; MATN1, matrilin 1, cartilage matrix protein, MTNR1B, melatonin receptor 1B; IGF1, insulin-like growth factor 1; SNP, single nucleotide polymorphism.
Ethnically, Koreans are much more similar to the Japanese than Caucasians. Therefore, from a database of Japanese SNPs, we selected SNPs for study with a minor allele frequency of more than 5%. Other controversial SNPs in Asian populations were also included for confirmation. Common SNPs in the genes of matrilin 1 (MATN1) and insulin-like growth factor 1 (IGF1) are reported to be associated with AIS in Chinese, but studies to uncover candidate genes or marker SNPs for AIS in Koreans have yet to be performed. Furthermore, only one paper has reported on a relationship between AIS and osteoporosis in Korea,17 and the lack of data has limited the ability of physicians in Korea to personalize treatment of AIS, unlike Caucasian populations. The objective of this study was to reveal AIS-related genes and their relevance in Koreans, exploring the potential use of gene analysis as a diagnostic tool for personalized treatment of AIS therein.
MATERIALS AND METHODS
Prior to conducting our study, we received Institutional Review Board and Clinical Research Ethics Committee approval of our study protocols. A total of 68 Korean adolescents (8-18 years of age), newly diagnosed with AIS at the authors' institution, in addition to 35 age- and sex-matched, healthy adolescents were enrolled in our study after applying the exclusion criteria detailed below. Some of the healthy controls comprised students from the school where the authors conducted the study. The following candidates were excluded: those with a history of congenital anomalies, neuromuscular diseases, endocrine disorders, skeletal dysplasia or connective tissue disorders, or mental retardation or mental illness, as well as psychiatric patients on medication affecting bone metabolism. From the 68 patients in the scoliosis group, 33 patients with a Cobb's angle between 10-40 degrees allowing for non-surgical treatment were subdivided into a lower risk group, while 35 patients whose Cobb's angle was more than 40 degrees were divided into the high risk group as they required surgery.
Evaluation of scoliosis angle
Normal standing postero-anterior radiographs were taken for each AIS patient upon their first visit. Standard techniques for measuring Cobb's angle were used, and if more than one curve was discovered, the most severe curve was selected for measurement. A Cobb's angle of less than 10 degrees was considered normal.
Anthropometric measurements
Anthropometric measurements were done about body height and weight. Calculation of height for patients was corrected by use of Bjure's formula [Log y=0.011x-0.177, where y is the loss of trunk height (cm) due to a deformed spine and x is the greatest Cobb's angle of the primary curve].17,18 Calculation of body mass indices (BMIs) was done by dividing weight (kg) by uncorrected height squared (m2).17
Dual-energy X-ray absorptiometry
Lumbar spinal bone mineral density (LSBMD) and femoral neck BMD (FNBMD) of the non-dominant proximal femur were measured by dual-energy X-ray absorptiometry (XR-36; Norland Corp., Fort Atkinson, WI, USA). LSBMDs were measured at L1-L4 in the anterior-posterior view.17 There was no difficulty in measuring BMD in the control group and lower risk group, but severely rotated vertebrae made the measurements difficult in the high risk group. To minimize this problem, each spine of the high risk group was pre-scanned once, after which a reference line was drawn to join the highest points of the iliac crests, which usually passed between the third and fourth lumbar spinous processes. On that reference line, a rectangle was erected to include L2-L4, and this was defined as the scan area.17 As the standardized T score for adolescents has not yet been exactly defined in Korea, total bone mass was used for bone mineral density.
Biochemical markers of bone turnover
Blood samples were collected between 8:00 and 10:00 a.m. after an overnight fast. Plasma and serum samples were analyzed in a routine laboratory using standard procedures. Osteocalcin and serum carboxy-terminal collagen crosslinks (CTX) levels in heparinized plasma were measured using a solid-phase, two-site chemiluminescent enzyme-labeled immunometric assay (Immulite Osteocalcin, Diagnostic Product Corporation, Los Angeles, CA, USA). Additionally, serum alkaline phosphatase (ALP) by radio immune essay (RIA) (Tandem-R Ostase, Beckman Coulter, Fullerton, CA, USA) and serum 25(OH)D3 levels by RIA using insulin-depleted saline (Immunodiagnostic Systems Limited, Boldon, UK) were measured.17 The intra-assay and inter-assay variability for 25(OH)D3 were below 10%.17
SNP marker selection
Reviewing many papers, we selected for study the most significant SNPs documented in the most recent genome-wide association studies, as well as other important studies. Two SNP markers (rs10510181, rs2222973) selected from Sharma, et al.'s paper, 5 SNP markers (rs2449539, rs1437480, rs448013, rs10493083, rs16945692) from Ward, et al.'s paper, and 3 SNP markers (rs1149048, rs4753426, rs5742612) from the aforementioned Chinese paper were selected.
Genotyping method
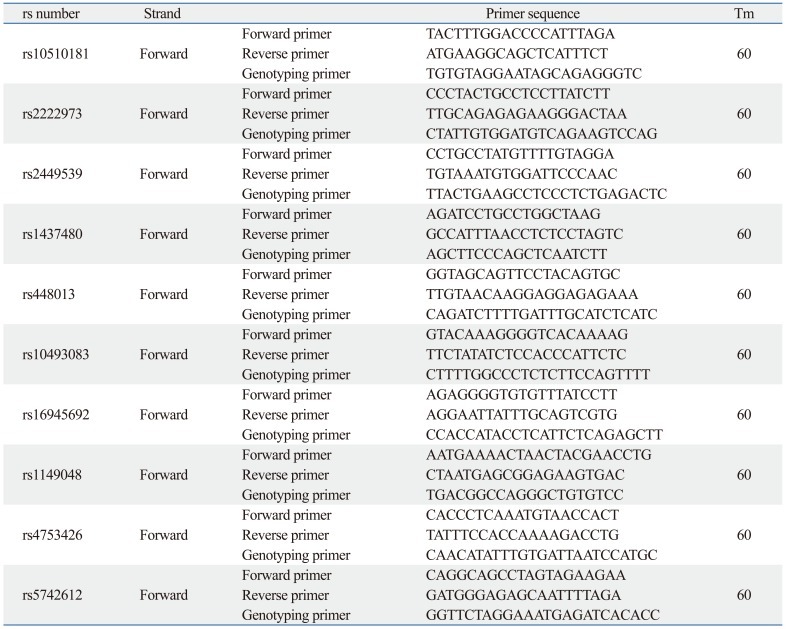
Genotypes were screened via single base primer extension assay using an ABI PRISM SNaPShot Multiplex kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's recommendations. Briefly, genomic DNA flanking the SNPs of interest was amplified via polymerase chain reaction (PCR) with forward and reverse primer pairs (Table 2) and standard PCR reagents in a 10 microliter reaction volume, containing 10 ng of genomic DNA, 0.5 pM of each oligonucleotide primer, 1 microliter of 10X PCR buffer, 250 M dNTP (2.5 mM each) and 0.25 units of i-StarTaq DNA Polymerase (5 unit/µL) (iNtRON Biotechnology, Seongnam, Korea). The PCR conditions were as follows: 10 min at 95℃ for 1 cycle, and 35 cycles at 95℃ for 30s, 60℃ for 1 min, 72℃ for 1 min, followed by 1 cycle of 72℃ for 10 min. After amplification, the PCR products were treated with 1 unit each of shrimp alkaline phosphatase (SAP) (USB Corporation, Cleveland, OH, USA) and exonuclease I (USB Corporation, Cleveland, OH, USA) at 37℃ for 75 minutes and 72℃ for 15 minutes to purify the amplified products. One micro liter of the purified amplification products were added to a SNaPshot Multiplex Ready reaction mixture containing 0.15 pmols of genotyping primer for primer extension reaction. The primer extension reaction was carried out for 25 cycles at 96℃ for 10 seconds, 50℃ for 5 seconds, and 60℃ for 30 seconds. The reaction products were then treated with 1 unit of SAP at 37℃ for 1 hour and 72℃ 15 minutes to remove excess fluorescent dye terminators. One microliter of the final reaction samples containing the extension products was then added to 9 microliters of Hi-Di formamide (ABI, Foster City, CA, USA). The mixture was incubated at 95℃ for 5 min, followed by 5 min on ice, and then analyzed by electrophoresis in an ABI Prism 3730xl DNA analyzer. Analysis was carried out using Gene Mapper software (version 4.0; Applied Biosystems, Foster City, CA, USA). Table 3 shows the primer sets and Tm used for the SNaPshot assay.
Table 2.
Primer Sets and Tm for the SNaPshot Assay
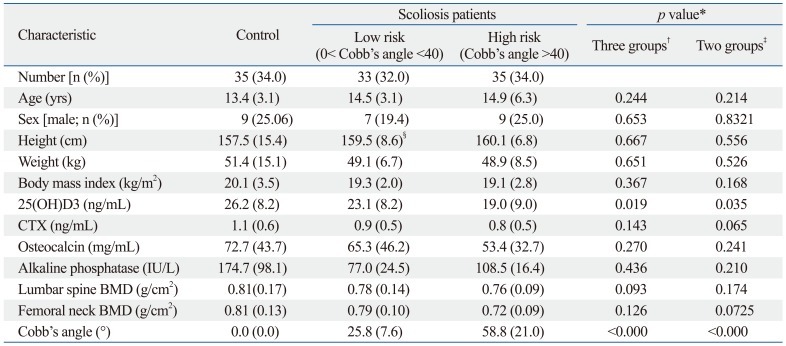
Table 3.
Baseline Characteristics of the Subjects
CTX, carboxy-terminal collagen crosslinks; BMD, bone mineral density.
*χ2 test or t-test were used where appropriate.
†Three groups: control group vs. high risk group vs. lower risk group.
‡Two groups: control group vs. scoliosis group.
§Corrected height using Bjure's formula.
Statistical analysis
Statistical analysis was performed using statistical package for social science (SPSS) software version 11.5 for Windows (SPSS, Chicago, IL, USA). Data are expressed as the mean±standard deviation. Groups were compared using t-test and analysis of variance (ANOVA), whenever appropriate. p-values <0.05 were considered significant for clinical parameters such as BMI.
The Hardy-Weinberg equilibrium was tested for each SNP in the patient and control groups using the chi-square test. The Hardy-Weinberg equilibrium principle states that genetic variation in a population will remain constant from one generation to the next in the absence of disturbing factors. Frequency distributions of genotypes in patients and controls were compared using the chi-square test for each SNP studied. One-way ANOVA was used to compare Cobb's angles among different genotypes. Inter-group comparisons were made using t-test, a p-value of <0.01 was considered significant in genotype or allele comparisons.
RESULTS
In this study, the following three groups were compared: 35 subjects in the control group, 33 patients in the lower risk group and 35 patients in the high risk group (Table 3). The mean Cobb's angle for lower risk group patients was 25.8° (SD=7.6°) and 58.8° (SD=21.0°) for high risk group patients. Age, sex, height and weight had no statistical significant difference among the three groups. BMI was 20.1 (SD=3.5) in the control group, 19.3 (SD=2.0) in the low risk group and 19.1 (SD=2.8) in the high risk group, and this mathematical variation was not statistically significant bone formation marker alkaline phosphatase (ALP) levels were 174.7 IU (SD=98.1) in the control group, 77.0 IU (SD=24.5) in the low risk group and 108.5 (SD=16.4) the in high risk group. Again, despite variation, its statistical value was questionable. Also, differences in CTX, representative of the degree of bone destruction, and osteocalcin, a bone formation marker, were found to be statistically inconclusive. Mean lumbar spine and femur neck bone mineral density (FNBMD) in AIS patients tended to be lower than that in controls, yet the difference failed to reach statistical significance. Vitamin 25(OH)D3 levels were 19.0 ng/mL (SD=9.0) in the high risk group, 23.1 ng/mL (SD=8.2) in the lower risk group and 26.2 ng/mL (SD=8.2) in the control group, and vitamin D levels were shown to be significantly lower in the high risk group than those in the lower risk and control groups (p=0.019).
Genotypes were shown to be in Hardy-Weinberg equilibrium. The distribution of the genotypes and alleles of the cases and controls for the ten promoter SNPs are presented in Table 4. No variations of statistical importance were observed in genotype frequencies and allele frequencies of the eight promoter SNPs among the lower risk group, high risk group and controls in the Korean population, when the SNPs were studied independently (Table 4).
Table 4.
Frequency of Genetic Variations in Control and Scoliosis Patients
SNP, single nucleotide polymorphism; CHL1, close homolog of L1; DSCAM, Down syndrome cell adhesion molecule; LAPTM4B, lysosomal-associated transmembrane protein 4 beta; FOXB1, forkhead box protein B1; CBLN4, cerebellin 4 precursor; RRAGC, ras-related GTP binding C; BRIP1, fanconi anemia group J protein; MATN1, matrilin 1, cartilage matrix protein, MTNR1B, melatonin receptor 1B; IGF1, insulin-like growth factor 1.
*Three groups: control group vs. high risk group vs. lower risk group.
†Two groups: control group vs. scoliosis group.
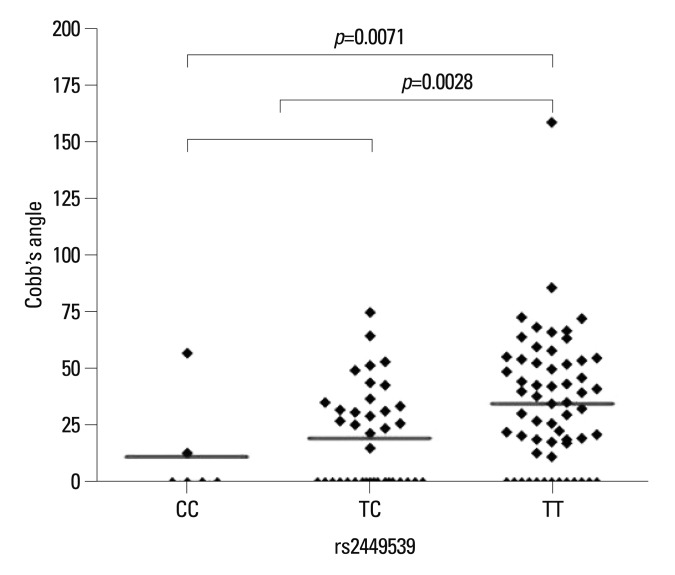
The lysosomal-associated transmembrane protein 4 beta (LAPTM4B) polymorphism (rs2449539) was found to be significantly different among the controls and lower risk and high risk groups. In comparison of the three groups individually, the frequency of TT genotype was most common in the high risk group, followed by the low risk group and control group, respectively (p=0.014). In contrast, the frequency of the TC genotype was most common in the control group, followed by the low risk group and high risk group, in that order (p=0.003). In comparison between two groups (controls versus AIS patient group), the frequency of TT genotype was most common in the AIS patients group (p=0.008). Similarly, the frequency of TC genotype was most common in the control group as compared to the AIS patients group (p=0.004). These results are summarized in Fig. 2.
Fig. 2.
Reviewing the relation between rs2449539 SNP and the Cobb's angle, genotype TT was compared with the CC, TC type and a significant difference (p=0.0028) was found. Comparison between CC type and TT type also showed a significant difference (p=0.0071). SNP, single nucleotide polymorphism.
Additionally, IGF1 polymorphism (rs5742612) was also found to be significantly different among the controls and the lower risk and high risk groups. In comparison of the three groups individually, the frequency of GG genotype was most common in the control group, followed by the low risk group and high risk group, in that order (p=0.010). Between the two groups (controls versus AIS patient group), the frequency of GG genotype was most common in the control group (p=0.006). However, there was no significant difference in allele frequencies for one SNP (rs5742612) (p=0.059).
As shown in Table 4, two SNPs at rs2449539 (p=0.003) and rs5742612 (p=0.006) were found to exhibit significant associations with AIS in Koreans (p<0.01). The other 8 SNPs demonstrated no such significant associations. There was a significant difference in allele frequencies for one SNP (rs2449539) (p=0.002).
DISCUSSION
In Korea, AIS patients make about 100000 visits to physicians annually, thereby incurring an expenditure of millions of dollars or even more, and this expenditure has increased steeply over the past five years (Fig. 1). Clinicians and patients need to be aware of the risk of curve progression as they make treatment decisions. Peterson and Nachemson's9 progression equation might be of some help to clinicians and patients who want to base their treatment on the risk of progression. But some patients do not follow this equation, and to solve this problem, a more accurate diagnosis and diagnostic tool that can predict whether or not deterioration will occur is needed.
Though AIS is thought to be multifactorial disorder, differing degrees of interaction between multiple factors depend on linear and summation causality. Although AIS has been linked to multifactorial causes, genetic factors are considered the most important cause.
There are many reports about the supportive role of genetic inheritance in the development of AIS. A meta-analysis of different twin studies has been performed and found a concordance rate for AIS of 73% in monozygous twins versus 36% in dizygous twins.20 With recent advances of genetic analysis techniques, many different gene loci linked to AIS is reported by family-linkage studies. Miller, et al.21 reported that gene loci in the primary regions on chromosomes 6, 9, 16, and 17 and in the secondary regions on chromosomes 1, 3, 5, 7, 8, 11, 12, and 19, after scanning 202 families and 1198 individuals, were linked to AIS in 2005. Yet, other studies failed to achieve the same results, and instead, found other candidate genes. Therefore, it is hard to establish whether the etiology of AIS is due to single gene or not.22,23 Recently, many genetic association studies with the candidate gene approach have been performed with the help of information gathered from the international HapMap project. In previous studies, the selection of candidate gene selection followed to prior knowledge of the expression of observed phenotypes in AIS and different SNPs studied with the case-only and case-control analysis. More recently, a more comprehensive explanation on the possible genes involved in the etiopathogenesis of AIS has been presented by GWASs of all SNPs in the genome. In the recent report of Ward, et al.,5 the comparison of a GWAS of 1.8 million genetic markers was performed between 1200 AIS patients and 1500 controls. Their study found 202 markers associated to curve progression, and after further refinement, 30 markers were reported as the most useful prognostic markers for curve progression. After verifying the results of this study, they launched the development of commercially available kit with a NPV greater than 99% for AIS-PT. However, there was an important limitation to their work; genotype frequencies for several of the SNP markers used in the AIS-PT kit vary by race. Accordingly, their AIS-PT kit has been validated for whites only. Thus, Korean specific SNP tests should be developed to personalize the treatment of AIS therein.
In this study, the authors selected a control group that was age- and sex-matched, so that age and sex did not differ among the three groups compared. BMI was 20.1 (SD=3.5) in the control group, 19.3 (SD=2.0) in the lower risk group, 19.1 (SD=2.8) in the high risk group and arithmetically low in the AIS patient group (lower+high risk group), but this difference was not statistically significant. Several authors reported that scoliotic girls had lower mean weight and a lower bone mineral status compared to those in the control population.24,25 Leptin along with soluble leptin receptor was shown to play an important role in the regulation of bone and energy metabolism in children. Liu, et al.25 insisted that leptin and soluble leptin receptor were abnormal and associated with deranged growth and anthropometric phenotypes in AIS girls. According to Liu's study, abnormality of leptin receptor leads to lower BMI, and osteoporosis is accompanied by the possibility of scoliosis. Although our study did not show any variation of significance, increasing the sample size of the study population may help in establishing any correlation that may exist between decreased BMI and AIS.
In the present study, vitamin 25(OH)D3 level was 26.2 (SD=8.2) in the control group, 23.1 (SD=8.2) in the lower risk group and 19.0 (SD=9.0) in the high risk group, the difference of which was statistically significant in the surgically treated, high risk group (p=0.019). The BMDs of the spine were 0.81 g/cm2 (SD=0.17) in the control group, 0.78 (SD=0.14) in the lower risk group and 0.76 (SD=0.09) in the high risk group, a difference which tended to be lower in the high risk group, but was not statistically significant (p=0.093). Generalized low bone mass and osteopenia in the axial and peripheral skeleton have been described in patients with AIS.26,27 The precise mechanism of bone loss in these patients is unclear. Recently, many studies have reported that gene polymorphism is related to osteoporosis, but such results vary and are difficult to interpret.27,28 Once again by increasing the size of the study population, the relationship between osteoporosis and AIS may be established.
In a previous GWAS, Sharma, et al.6 insisted that chromosome 3p26.3 SNPs in the proximity of the cell adhesion molecule with homology to the L1CAM (CHL1) gene (rs10510181) as well as SNPs in the DSCAM gene (rs222973) that encodes for an axon guidance protein were strongly associated with AIS. The prevalence of the rs10510181 genotype was nearly the same as that in the control, lower risk and high risk groups of the present study. The prevalences of the rs2222973 genotype were 42.4% (TT), 42.4% (TC) and 15.22% (CC). In particular, lower risk AIS patients more frequently exhibited the TC genotype, but this was not significant (p=0.057, 0.078). By studying SNPs, in particular rs2222973, in a larger population in Korea, we may be able to develop SNPs as race-specific markers of AIS in Koreans.
Among the 53 SNPs reported by Ward, et al. we studied the following five SNPs: rs2449539, rs1437480, rs448013, rs10493083 and rs16945692. Except for the SNP rs2449539 that encodes the LAPTM4B gene, genotype and allele frequency between the three groups did not differ (p-values from 0.198 to 0.914). LAPTM4B is a protein that in humans is encoded by the LAPTM4B gene encoded on chromosome 8q22, which has been extensively studied in cancer research.29 We found that allele T of rs2449539 involved a significant predisposition to AIS (p=0.002), and individuals with the TT genotype were at a higher risk for AIS, in comparison to TC and CC (p=0.08, 0.04). In this study, we identified that rs2449539 polymorphism in the LAPTM4B gene is associated with both susceptibility and disease progression in AIS. Accordingly, we concluded that the LAPTM4B gene may be important to disease predisposition and progression. Additionally, we also reconfirmed previous reports of ethnic differences in the association between AIS and rs2449539 polymorphism of the LAPTM4B gene. No statistically significant differences were found in the genotype frequencies of the remaining 4 SNPs between AIS patients and controls in the Korean population when the SNPs were studied independently. Similarly, the allele frequencies were comparable between cases and controls (Table 4). Thus, rs2449539 polymorphism of the LAPTM4B gene may be important to the complex genetic etiopathogenesis of AIS.
Comparing Ward's study and our study, there are lot of genetic differences between Caucasians and Asians.5 Two Chinese papers reported the associations of rs1149048 near MATN1, rs10488682 near MTNR1B and rs4753426 near insulin-IGF1 with AIS in Chinese.30,31 However, these associations were not shown to exist in a Japanese population.19 With exception of rs5742612 SNP upstream IGF1, genotype and allele frequency of two SNPs between the three groups did not differ (p from 0.145 to 0.999). We found that allele A of rs5742612 was not significantly related with a predisposition to AIS (p=0.059), but individuals with genotype GG were shown to be at a lower risk of AIS in comparison to AA and AG (p=0.006). In our study, the SNPs of rs1149048 and rs4753426 exhibited no association with AIS predisposition in Koreans, nor did we find any association for these two SNPs with curve severity. However, the rs5742612 SNP in the upstream IGF1 gene was shown to be closely related with AIS in Koreans. Our result fell somewhere between those for Chinese and Japanese populations.
There were some limitations to this study. The number of samples tested was relatively small, which diminishes the statistical power of the study and the possibility of detecting correlations. Further studies with a larger patient population are recommended. The association with other factors, such as the markers of bone metabolism and other candidate genes, should be tested.
In conclusion, this study provides evidence that the SNP rs2449539 in LAPTM4B and rs5742612 in upstream IGF1 genes were associated with both susceptibility to and curve severity in AIS. Our results suggest that LAPTM4B and IGF1 may both be important to AIS predisposition and progression. Nevertheless, expanding our database by increasing the study size so that more SNPs or candidate genes can be studied in the Korean population would prove immensely beneficial in developing a SNP marker for AIS specifically designed for the Korean population.
ACKNOWLEDGEMENTS
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication.
Funding from the College of Medicine in Yonsei University (topic number: 6-2010-0039) was received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA. Adolescent idiopathic scoliosis. Lancet. 2008;371:1527–1537. doi: 10.1016/S0140-6736(08)60658-3. [DOI] [PubMed] [Google Scholar]
- 2.Suh SW, Modi HN, Yang JH, Hong JY. Idiopathic scoliosis in Korean schoolchildren: a prospective screening study of over 1 million children. Eur Spine J. 2011;20:1087–1094. doi: 10.1007/s00586-011-1695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scoliosis is increasing in teenager from 2006 to 2010 in Republic of Korea. Press report in Korea health insurance review and assessment service. [accessed on 2011 November 27]. Available at: http://www.hira.or.kr/cms/rc/rce_news/1208414_10816.html.
- 4.Renshaw TS. Screening school children for scoliosis. Clin Orthop Relat Res. 1988;(229):26–33. [PubMed] [Google Scholar]
- 5.Ward K, Ogilvie JW, Singleton MV, Chettier R, Engler G, Nelson LM. Validation of DNA-based prognostic testing to predict spinal curve progression in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2010;35:E1455–E1464. doi: 10.1097/BRS.0b013e3181ed2de1. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Gao X, Londono D, Devroy SE, Mauldin KN, Frankel JT, et al. Genome-wide association studies of adolescent idiopathic scoliosis suggest candidate susceptibility genes. Hum Mol Genet. 2011;20:1456–1466. doi: 10.1093/hmg/ddq571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz DE, Herring JA, Browne RH, Kelly DM, Birch JG. Brace wear control of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2010;92:1343–1352. doi: 10.2106/JBJS.I.01142. [DOI] [PubMed] [Google Scholar]
- 8.Rahman T, Borkhuu B, Littleton AG, Sample W, Moran E, Campbell S, et al. Electronic monitoring of scoliosis brace wear compliance. J Child Orthop. 2010;4:343–347. doi: 10.1007/s11832-010-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson LE, Nachemson AL. Prediction of progression of the curve in girls who have adolescent idiopathic scoliosis of moderate severity. Logistic regression analysis based on data from The Brace Study of the Scoliosis Research Society. J Bone Joint Surg Am. 1995;77:823–827. doi: 10.2106/00004623-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Kleinerman RA. Cancer risks following diagnostic and therapeutic radiation exposure in children. Pediatr Radiol. 2006;36(Suppl 2):121–125. doi: 10.1007/s00247-006-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yawn BP, Yawn RA. The estimated cost of school scoliosis screening. Spine (Phila Pa 1976) 2000;25:2387–2391. doi: 10.1097/00007632-200009150-00019. [DOI] [PubMed] [Google Scholar]
- 12.Wang WJ, Yeung HY, Chu WC, Tang NL, Lee KM, Qiu Y, et al. Top theories for the etiopathogenesis of adolescent idiopathic scoliosis. J Pediatr Orthop. 2011;31(1 Suppl):S14–S27. doi: 10.1097/BPO.0b013e3181f73c12. [DOI] [PubMed] [Google Scholar]
- 13.Ogilvie J. Adolescent idiopathic scoliosis and genetic testing. Curr Opin Pediatr. 2010;22:67–70. doi: 10.1097/MOP.0b013e32833419ac. [DOI] [PubMed] [Google Scholar]
- 14.Cheng JC, Tang NL, Yeung HY, Miller N. Genetic association of complex traits: using idiopathic scoliosis as an example. Clin Orthop Relat Res. 2007;462:38–44. doi: 10.1097/BLO.0b013e3180d09dcc. [DOI] [PubMed] [Google Scholar]
- 15.Mórocz M, Czibula A, Grózer ZB, Szécsényi A, Almos PZ, Raskó I, et al. Association study of BMP4, IL6, Leptin, MMP3, and MTNR1B gene promoter polymorphisms and adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2011;36:E123–E130. doi: 10.1097/BRS.0b013e318a511b0e. [DOI] [PubMed] [Google Scholar]
- 16.Fei Q, Wu Z, Wang H, Zhou X, Wang N, Ding Y, et al. The association analysis of TBX6 polymorphism with susceptibility to congenital scoliosis in a Chinese Han population. Spine (Phila Pa 1976) 2010;35:983–988. doi: 10.1097/BRS.0b013e3181bc963c. [DOI] [PubMed] [Google Scholar]
- 17.Suh KT, Eun IS, Lee JS. Polymorphism in vitamin D receptor is associated with bone mineral density in patients with adolescent idiopathic scoliosis. Eur Spine J. 2010;19:1545–1550. doi: 10.1007/s00586-010-1385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siu King Cheung C, Tak Keung Lee W, Kit Tse Y, Ping Tang S, Man Lee K, Guo X, et al. Abnormal peri-pubertal anthropometric measurements and growth pattern in adolescent idiopathic scoliosis: a study of 598 patients. Spine (Phila Pa 1976) 2003;28:2152–2157. doi: 10.1097/01.BRS.0000084265.15201.D5. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, Matsumoto M, Karasugi T, Watanabe K, Chiba K, Kawakami N, et al. Lack of association between adolescent idiopathic scoliosis and previously reported single nucleotide polymorphisms in MATN1, MTNR1B, TPH1, and IGF1 in a Japanese population. J Orthop Res. 2011;29:1055–1058. doi: 10.1002/jor.21347. [DOI] [PubMed] [Google Scholar]
- 20.Andersen MO, Thomsen K, Kyvik KO. Adolescent idiopathic scoliosis in twins: a population-based survey. Spine (Phila Pa 1976) 2007;32:927–930. doi: 10.1097/01.brs.0000259865.08984.00. [DOI] [PubMed] [Google Scholar]
- 21.Miller NH, Justice CM, Marosy B, Doheny KF, Pugh E, Zhang J, et al. Identification of candidate regions for familial idiopathic scoliosis. Spine (Phila Pa 1976) 2005;30:1181–1187. doi: 10.1097/01.brs.0000162282.46160.0a. [DOI] [PubMed] [Google Scholar]
- 22.Gurnett CA, Alaee F, Bowcock A, Kruse L, Lenke LG, Bridwell KH, et al. Genetic linkage localizes an adolescent idiopathic scoliosis and pectus excavatum gene to chromosome 18 q. Spine (Phila Pa 1976) 2009;34:E94–E100. doi: 10.1097/BRS.0b013e31818b88a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward K, Ogilvie J, Argyle V, Nelson L, Meade M, Braun J, et al. Polygenic inheritance of adolescent idiopathic scoliosis: a study of extended families in Utah. Am J Med Genet A. 2010;152A:1178–1188. doi: 10.1002/ajmg.a.33145. [DOI] [PubMed] [Google Scholar]
- 24.Barrios C, Cortés S, Pérez-Encinas C, Escrivá MD, Benet I, Burgos J, et al. Anthropometry and body composition profile of girls with nonsurgically treated adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2011;36:1470–1477. doi: 10.1097/BRS.0b013e3181f55083. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Tam EM, Sun GQ, Lam TP, Zhu ZZ, Sun X, et al. Abnormal leptin bioavailability in adolescent idiopathic scoliosis: an important new finding. Spine (Phila Pa 1976) 2012;37:599–604. doi: 10.1097/BRS.0b013e318227dd0c. [DOI] [PubMed] [Google Scholar]
- 26.Cheng JC, Qin L, Cheung CS, Sher AH, Lee KM, Ng SW, et al. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res. 2000;15:1587–1595. doi: 10.1359/jbmr.2000.15.8.1587. [DOI] [PubMed] [Google Scholar]
- 27.Lee WT, Cheung CS, Tse YK, Guo X, Qin L, Lam TP, et al. Association of osteopenia with curve severity in adolescent idiopathic scoliosis: a study of 919 girls. Osteoporos Int. 2005;16:1924–1932. doi: 10.1007/s00198-005-1964-7. [DOI] [PubMed] [Google Scholar]
- 28.Chen WJ, Qiu Y, Zhu F, Zhu ZZ, Sun X, Liu Z, et al. [Vitamin D receptor gene polymorphisms: no association with low bone mineral density in adolescent idiopathic scoliosis girls] Zhonghua Wai Ke Za Zhi. 2008;46:1183–1186. [PubMed] [Google Scholar]
- 29.Zhou L, He XD, Yu JC, Zhou RL, Shan Y, Rui JA. Overexpression of LAPTM4B-35 attenuates epirubucin-induced apoptosis of gallbladder carcinoma GBC-SD cells. Surgery. 2011;150:25–31. doi: 10.1016/j.surg.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Tang NL, Cao X, Qiao D, Yi L, Cheng JC, et al. Promoter polymorphism of matrilin-1 gene predisposes to adolescent idiopathic scoliosis in a Chinese population. Eur J Hum Genet. 2009;17:525–532. doi: 10.1038/ejhg.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu XS, Tang NL, Yeung HY, Lee KM, Hung VW, Ng BK, et al. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2007;32:1748–1753. doi: 10.1097/BRS.0b013e3180b9f0ff. [DOI] [PubMed] [Google Scholar]