Abstract
Many species in the animal kingdom are characterized by alternative mating tactics (AMTs) within a sex. In males, such tactics include mate guarding versus sneaking behaviours, or territorial versus female mimicry. Although AMTs can occur in either sex, they have been most commonly described in males. This sex bias may, in part, reflect the increased opportunity for sexual selection that typically exists in males, which can result in a higher probability that AMTs evolve in that sex. Consequently, females and polyandry can play a pivotal role in governing the reproductive success associated with male AMTs and in the evolutionary dynamics of the tactics. In this review, we discuss polyandry and the evolution of AMTs. First, we define AMTs and review game theoretical and quantitative genetic approaches used to model their evolution. Second, we review several examples of AMTs, highlighting the roles that genes and environment play in phenotype expression and development of the tactics, as well as empirical approaches to differentiating among the mechanisms. Third, ecological and genetic constraints to the evolution of AMTs are discussed. Fourth, we speculate on why female AMTs are less reported on in the literature than male tactics. Fifth, we examine the effects of AMTs on breeding outcomes and female fitness, and as a source, and possibly also a consequence, of sexual conflict. We conclude by suggesting a new model for the evolution of AMTs that incorporates both environmental and genetic effects, and discuss some future avenues of research.
Keywords: tactic, strategy, mate choice, polymorphism, sexual selection, disruptive selection
1. Introduction
Many species in the animal kingdom are characterized by alternative mating tactics (AMTs) within a sex [1–3]. For example, males within a species may use different behaviours to attract or otherwise mate with females. In the mating system of the ruff (Philomachus pugnax), as one well-studied example, some males set up and defend small mating territories on a lek and court females [4]. Other males instead occupy areas on the boundaries of territorial-holding males and attempt to sneak copulations with females that enter the territory. AMTs have been reported in many taxa and can include striking differences in behaviour, morphology, physiology and life history [2,5]. Although AMTs can occur in either sex, they have most commonly been described in males.
In the early days of behavioural ecology, during the 1970s and 1980s, there was growing interest in using evolutionary game theory to understand the evolution of AMTs and the concept of evolutionarily stable strategies (ESS) was introduced [6,7]. ESS theory detailed the concepts of pure and mixed strategies, where ‘strategies’ were viewed as simple rules that specified what phenotype (‘tactic’) an organism expressed. A pure strategy is one where the rule gives rise to a single phenotype such as ‘fight’. A one-to-one relationship between the strategy and the phenotype was assumed. A mixed strategy, in its original formulation, is one where the rule gives rise to two or more phenotypes based on probability. For example, a mixed strategy might specify ‘fight’ with probability of 0.9 and ‘sneak’ with probability of 0.1 (see Nash [8]). The probability presumably involved something analogous to a coin flip (with a weighted coin) and might be performed once with the phenotype then adopted for life, or be performed each time a mating event or contest occurred. In either case, however, the coin flip was assumed to be strictly probabilistic and was not influenced by the environment, including aspects of the mating event or competitor.
The probabilistic underpinnings of the mixed strategy met with criticism in their application to living organisms, which were thought to be incapable of performing the required randomization process [9,10]. Gross [1] consequently argued that the mixed strategy had not been realized, albeit as discussed below, it is difficult to provide the necessary empirical evidence to demonstrate a mixed strategy. Gross also suggested that no two individuals would have equal ‘status’ (relative condition or competitive ability) because, for example, of the multitude of factors that could affect status (e.g. environmental influences such as disease and trauma, genetic variance or ontogeny). This assertion invalidates the evolutionary stability of the mixed strategy [7,11]. Flaxman [12], on the other hand, drawing on a framework developed by Crowley [13], argued that situations could exist when individuals are incapable of assessing their status or at least incapable of assessing a difference in their status relative to a competitor (i.e. two competitors would be deemed equal in status) and that it was therefore plausible that mixed strategies could exist. Those situations include when assessments fall into a finite number of categories, perhaps when body size is assessed as small, medium or large, as opposed to on a continuous scale (see Flaxman [12]).
When AMTs instead represent two pure strategies, two conditions are required for the tactics to be evolutionarily stable (referred to as an ESS or a mixed ESS): (i) negative frequency-dependent selection; and (ii) equal fitness of the tactics when the population is at the equilibrium frequency. Negative frequency-dependent selection simply requires that, when a tactic is rare, it has higher fitness than the alternative tactic. For example, if the two tactics are fight and sneak, when fighters are rare in the population relative to sneakers, they must have higher fitness than sneakers. Conversely, at the other end of the frequency distribution, when fighters are common in the population, sneakers must have higher fitness. Given these conditions, the two frequency-dependent fitness functions associated with fight and sneak tactics must intersect, and it is at the frequency defined by that point of intersection that determines the equilibrium frequency and ESS (see box 2 in Gross [1]).
A common early research approach was to try to find examples of alternative strategies (mating strategies or otherwise) in natural systems and show, by direct fitness measurements or indirect inferences, that these strategies had more or less equal fitness. For instance, Jane Brockmann and Richard Dawkins studied digger wasps' (Sphex ichneumoneus) digging behaviour and argued that two observed nesting strategies exemplified the so-called mixed ESS where the two alternative reproductive modes were assumed to be genetic and to have more or less equal fitness [14]. A few years later, Gross [15] presented empirical evidence for disruptive selection towards two different male reproductive strategies (‘jacks’ and ‘hooknoses’) in salmon, and estimated their relative fitness, based on spawning observations, to be equal [15].
Gross later, however, argued that a mixture of pure strategies was unlikely to explain most examples of AMTs [1]. Instead, he argued that most AMTs represent a conditional strategy whereby individuals express the tactic associated with the highest fitness (pay-off) given their relative condition (Gross termed this ‘status’). The conditional strategy differs from the original mixed strategy insomuch as the phenotype that is expressed is based on an assessment of oneself—such as one's competitive ability—relative to others in the population as opposed to probabilistically. For example, a conditional strategy might provide the decision rule to sneak when small and fight when large. Importantly, the conditional strategy is a single decision rule used by all individuals in the population, and therefore, the model makes no predication of equal fitness of the alternative tactics. Indeed, Gross argued that alternative tactics in a conditional strategy would have unequal fitness with low-condition (or low-status) individuals assumed to be ‘making the best of a bad job’ in adopting a certain tactic [1]. This claim has met with resistance from researchers that posit that alternative tactics that have unequal fitness must be evolutionarily unstable [16]. The problem with the concept of ‘best of a bad job’ strategy is that natural selection does not favour those genotypes who do a bad job, however well they might be doing it, but rather those who do a better job than all other genotypes across strategies (W. R. Rice 1998, personal communication). Thus, from an evolutionary viewpoint, what matters is not that a genotype is ‘best’ within its strategy set, but its fitness with respect to all other genotypes in the population. Gross counters this argument by implying that the decision rule underlying a conditional strategy is coded by a single gene that is fixed in the population (he refers to it as a genetic monomorphism) and, therefore, there is no evolutionary game; at least not until a mutation gives rise to a second, alternative gene coding for a different decision rule [1]. Additionally, condition should have a strong environmental component that can introduce a stochastic element and maintain phenotypic variation within a population. The idea that AMTs could be coded by a single gene fixed in the population has itself been criticized as being too simplistic [17]. In terms of quantitative genetic theory and terminology, Gross's view of a population having a single, condition-dependent ‘decision rule’ is equivalent to stating that there is no genetic variation in plasticity and reaction norms within the population, i.e. no genotype-by-environment interaction (G × E). Such a statement is a strong claim, particularly in the light of a body of evidence that G × Es are common in natural populations [18–20]. Certainly, seeking a fuller understanding of the genetic architecture of AMTs is critical to the debate.
Today, there are several examples of AMTs in both males and females that have been shown to have a firm genetic basis (‘polymorphisms’), and in others that appear to mainly be influenced by environmental factors (‘polyphenisms’; figure 1). A non-exhaustive list of animal species, including both vertebrates and invertebrates, is presented in table 1. Overall, alternative male mating tactics linked to colour or discrete morphs dominate our list. For example, male dung beetles (Onthophagus taurus) are dimorphic, with some males developing horns and other males remaining hornless [45]. Research has shown that larval food quantity predictably determines the development of either horned or hornless males irrespective of paternal phenotype, suggesting a conditional strategy [45,46]. Furthermore, determination of fitness functions for both male types has shown that individual males maximize their fitness by adopting the appropriate life history (i.e. horned or hornless) given their size [47]. Whether there are G × E effects on the tactic adopted, or the size-dependent switch point associated with the transition from the hornless to horned tactic is genetically fixed in a population, is not yet known.
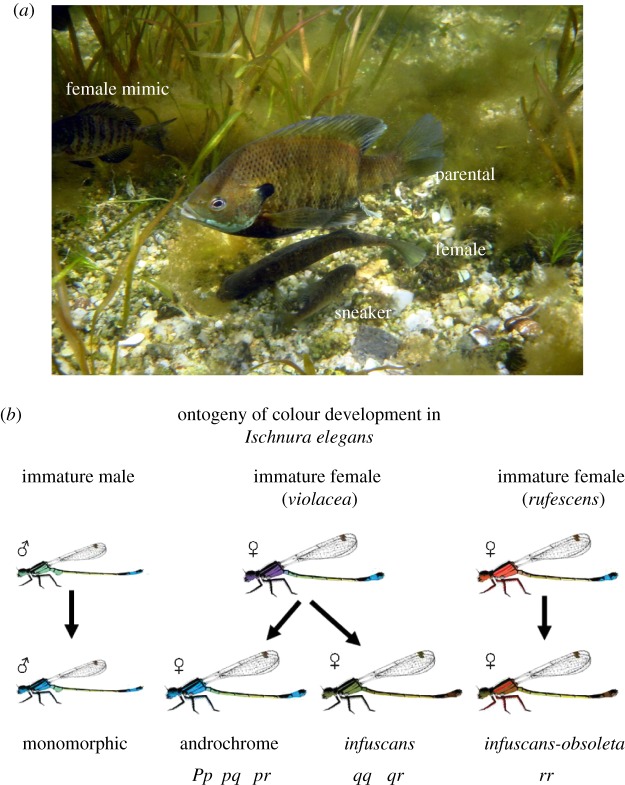
Figure 1.
Two examples of alternative mating tactics. (a) Males of the bluegill sunfish (Lepomis macrochirus) are characterized by two life histories termed parental and cuckolder. Parentals defend nesting territories, court females, and provide sole parental care for the developing eggs and offspring. Cuckolders adopt a sneaking tactic when small and a female mimicry tactic when larger. The parental and cuckolder life histories likely represent a conditional strategy. (b) Females of the blue-tailed damselfly (Ischnura elegans) are characterized by three hertiable morphs, one of which mimics male coloration (androchrome). Breeding experiments implicate a single gene with three alleles and sex-limited hierarchical expression with three alleles in a dominance hierarchy: p (androchrome) which is dominant to q (infuscans) which in turn is dominant to r (infuscans–obsoleta). These heritable morphs thus represent alternative strategies. Note that males are phenotypically monomorphic and that there are also age-related colour changes, in addition to the genetic variation among the three adult female morphs.
Table 1.
Sample of species with alternative mating tactics (AMTs). Listed are the species, the AMTs and a brief description of the associated behaviours and trait differences, the proposed evolutionary mechanism and genetic basis underlying phenotypic expression of the tactics, and the reference.
| species | AMTs | mechanism | reference |
|---|---|---|---|
| male AMTs | |||
| bluegill sunfish (Lepomis macrochirus) | (i) parental: nest building, courting, and sole parental care | possible conditional strategy with cuckolder males having higher fitness than parental males | [5,21] |
| (ii) cuckolder: sneak or female mimicry | |||
| plainfin midshipman (Porichthys notatus) | (i) type 1: guard nest, court females, and sole parental care | unknown | [22] |
| (ii) type 2: sneak or female mimicry | |||
| ruff (Philomachus pugnax) | (i) independent: guard lek territory, court females | alternative strategies with one gene, two alleles implicated | [23] |
| (ii) satellite: sneak | |||
| swordtail (Xiphophorus sp.) | (i) court | alternative strategies determined by sex-linked gene that regulates size at maturation; males with small size at maturation sneak | [24,25] |
| (ii) sneak | |||
| scorpionflies (Panorpa sp.) | (i) court and provide nuptial gift of insect carcass | conditional strategy based on dominance or foraging ability | [26] |
| (ii) court and provide nuptial gift of nutrient-rich saliva | |||
| (iii) force copulate (no nuptial gift provided) | |||
| dung beetle (Onthophagus sp.) | (i) horned-males: guard territories and females | conditional strategy based on body size with larger males developing horns | [27,28] |
| (ii) hornless males: sneak | |||
| isopod (Paracerceis sculpta) | (i) alpha male: guard territory and females | alternative strategies with one gene, three alleles implicated | [29,30] |
| (ii) beta male: mimic female | |||
| (iii) gamma male: sneak | |||
| white-throated sparrow (Zonotrichia albicollis) | males (and females) differing in colour (tan- or white-striped morphs) and colour morph is associated with aggression levels, territoriality and promiscuity | morph determination genetic and expressed in both sexes due to a chromosomal inversion | [31–34] |
| female AMTs | |||
| damselflies (several species of Ischnura) | male mimicry, differential resistance to male mating harassment, differential fitness tolerance to mating harassment | alternative strategies with one gene, three alleles determined from Mendelian segregation and breeding experiments | [35–37] |
| diving beetles (family Dytiscidae) | female resistance to male mating attempts, elythral structure influencing male clasping ability of females | alternative mating strategies with one and two alleles determined from Mendelian segregation and breeding experiments | [38] |
| side-blotched lizard (Uta stansburiana) | unknown, but female morphs differ in life-history traits, immune function and hormone profiles | quantitative genetic and pedigree-based heritability estimates based on comparisons between relatives in an individually marked free-roaming population | [39,40] |
| gouldian finch (Erythrura gouldiae) | female differ in immunity and hormonal profiles and modify patterns of sex allocation, investment in clutch and egg size, and amount of parental care in relation to mate properties | male and female colour morph influenced by a Z-linked gene and two alternative alleles (‘red’ and ‘black’) with allelic dominance | [41–44] |
Conclusive evidence for alternative strategies is less common in the literature. In the ruff, independent and satellite mating tactics appear to be controlled by a single gene with two alternative alleles—one allele giving rise to the independent tactic and the other allele giving rise to the satellite tactic [23]. It remains unclear, however, whether the two strategies have equal fitness [48]. Furthermore, a third tactic involving female mimicry has recently been described in some populations [49]. A slightly more complex genetic model has been proposed for the marine isopod Paracerceis sculpta, which is characterized by three male mating tactics [50]. In this case, the three tactics may have equal fitness as expected for evolutionary stability ([29]; for other examples, see references [24,51]). In most cases, the genetic basis has been demonstrated through breeding experiments or parent–offspring regressions, but the actual molecular-genetic or genomic basis of morph differentiation is unknown. A notable exception is the white-throated sparrow (Zonotrichia albicollis), which has a striking plumage polymorphism in both males and females that is associated with variation in mating behaviour, aggression and territoriality [31] and is caused by a chromosomal pericentric inversion [32–34].
There are considerably fewer examples of AMTs within females, with a notable exception in some insect families such as coenagrionid damselflies [35,36] and diving beetles (Dytiscidae) [38]. In both these insect groups, the AMTs have been shown to have a genetic basis, and have been linked to striking behavioural phenotypes that differ in terms of coloration or elythral structures [38,52].
There is also a small, but growing number of cases where heritable male colour polymorphisms that are linked to mating tactics also have female counterparts within the same populations. Such female colour morphs have been shown to differ with respect to behaviours, life-history traits, hormones and immunological traits [31,39,41,42,53–55].
2. Genetic and plastic alternative mating tactics
There are several major conceptual and methodological problems in the assumptions behind both the traditional game theory perspective as well as the proposed alternative by Gross in the form of ‘status-dependent tactics’ [17,56]. First, it is of course correct that one cannot a priori assume that an observed tactic is ‘genetic’ without proper experiments (e.g. breeding experiments). However, assuming the opposite, that of complete environmental determination and lack of additive genetic variance for the trait, is an equally extreme position. Most phenotypic traits are likely to show some additive genetic variance, the question is then only how much, which becomes an empirical question rather than a theoretical one. Additive genetic variance can lead to heritability of the tactic [57], albeit it does not necessarily preclude the evolutionary stability of a single decision gene [58]. Second, an unfortunate dichotomy was, prematurely, established between ‘genetic’ versus ‘environmental’ causes of phenotypic variation in mating tactics, when in practice the existence of one factor does not exclude the other (figure 2). Indeed, as noted above, genes and environments typically interact via G × E interactions [20]. Third, and partly related, phenotypic plasticity can and does often have a genetic basis and hence can evolve, just like ‘condition’ does not imply only environmental effects but is often, at least partly, genetic [57,59]. By logical extension, a condition-dependent strategy can therefore evolve through genetic variation in ‘threshold reaction norms’ [60,61] or ‘switch points’ [56]. Evolutionary theory thus does not necessarily see phenotypic plasticity as nuisance and as an alternative to genetic evolution by natural or sexual selection, but rather an interesting process in its own right, which is able to influence both short- and long-term evolutionary change [62].
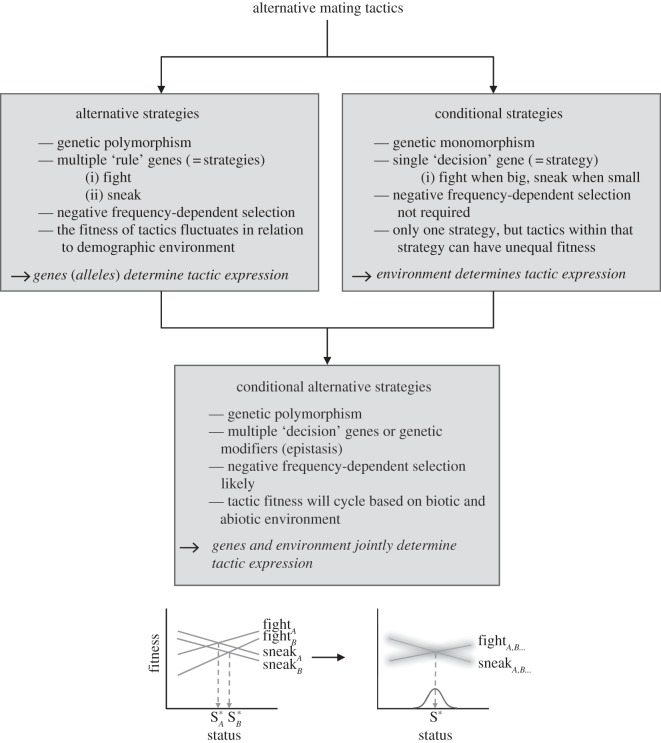
Figure 2.
A unified theory for the evolution and phenotypic expression of alternative mating tactics (AMTs). Past theory has focused on two approaches comprising alternative strategies and the conditional strategy. Alternative strategies imply a genetic polymorphism in a population that give rise to genetically determined AMTs such as fight versus sneak. Negative frequency-dependent selection is required for evolutionary stability and the strategies/tactics are expected to have equal fitness. A conditional strategy implies a genetically monomorphic decision gene that is condition-dependent such as fight when large versus sneak when small. Negative frequency-dependent selection is not required for evolutionary stability, and the tactics are expected to have unequal fitness. Conditional alternative strategies incorporate quantitative genetic theory, and combine genetic and environmental effects on tactic phenotypic expression. Regardless of whether the condition-dependent decision gene shows genetic variation within a population, it is expected to be influenced by polymorphic, modifier loci via epistasis (fightA/sneakA versus fightB/sneakB, where the A and B denote different genotypes) as well as by biotic and abiotic environmental factors, leading to genotype-by-environment interactions (G × E). G × Es reflect variation in reaction norms and tactic switch points within populations. Temporal variation in the environment will lead to a shifting fitness landscape, which will change the relative fitness of the tactics. Equal fitness is not expected at any given time period.
There are three additional methodological and conceptual problems with the application of the traditional game theory approach to the study of AMTs. First, the assumption of equal fitness is statistically impossible to address empirically—it is impossible to prove, beyond doubt, that two strategies do not differ in fitness, whereas the null hypothesis of no difference can be rejected. This asymmetry creates an unfortunate bias against finding a mixed ESS in nature. Second, the assumption of equal fitness assumes that evolutionary equilibrium has been reached, but in many natural populations, sexual and natural selection can fluctuate, implicating a dynamic adaptive landscape where populations have not yet reached their adaptive peaks, and might often be far from these peaks, or where peaks are perpetually shifting through time [63–65]. Third, the basic assumption in a phenotypic modelling approach such as game theory and related methods (e.g. ‘adaptive dynamics’) is that strategies are modelled assuming asexual inheritance and lack of recombination [52]. The assumption of equal fitness of genotypes at equilibrium is clearly wrong in many cases, for instance, when overdominant selection (‘heterozygote advantage’) maintains a genetic polymorphism at a locus [66]. In combination with the equally questionable assumption that populations are at evolutionary equilibrium, these limitations challenge the generality, utility and validity of game theoretical approaches in the study of AMTs [52]. Rather, population and quantitative genetic approaches might be more appropriate tools in this research field, in combination with modern genomic techniques, rigorous breeding experiments, and detailed behavioural and experimental studies.
3. Ecological factors and genetic constraints influencing alternative mating tactics
At this stage, it is difficult to make any generalizations regarding the ecological factors that favour the evolution of genetic versus phenotypically plastic AMTs. However, early population genetic theory dealing with the maintenance of polymorphisms has emphasized spatial [67] or temporal variation in selection [68]. When the environment varies in a coarse-grained fashion and selection is ‘soft’ (i.e. selection is influenced by density- and frequency-dependent competition), rather than ‘hard’ (i.e. independent of the demographic environment), a genetic polymorphism can be maintained at a single locus, when more than one ecological niche is available [69]. Conversely, when the environment is more fine grained, such as when habitat patches are small relative to average dispersal distances between generations, phenotypic plasticity rather than genetic polymorphisms is favoured by selection [70]. In some types of environments, more canalized genetic habitat specialists can even coexist with more plastic habitat generalists [71].
These models apply, with some basic adjustments, to the maintenance of AMTs, when the tactics do have a genetic basis. When applying these classical models to the analysis of alternative tactics, the term ‘habitat’ or ‘niche’ has to be replaced by ‘social environment’ or ‘environment of competitors’, but otherwise the formalism can be retained. However, this connection to early population genetic models has been made only in a few cases [53]. This paucity is unfortunate, as there are many interesting new avenues of research that open up using this classical population genetic perspective, as opposed to relying only on game theoretical approaches. For instance, the possibility that coexisting morphs differ in their degree of phenotypic plasticity versus canalization [71] has been investigated in only a few cases [41,53], but should be a fruitful line of investigation.
A relatively unexplored area of research is if certain genetic architectures (i.e. the number and effect size of loci influencing morph determination) are more conducive than others to the evolutionary emergence of AMTs. For instance, are genes of major effect or single locus systems more favourable to evolution of genetically based alternative strategies than when traits are governed by multiple loci? Many of the best examples of genetically based mating strategies come from systems with discrete colour polymorphisms, and where morphs differ in life-history traits, physiology and mating behaviours [36,39,50,51]. As colour is often governed by one or a few genes [52], one could speculate whether such colour polymorphic systems are ‘pre-adapted’ to evolve into systems with AMTs. And if so, do the different colour morphs subsequently diverge in suites of other traits, apart from colour?
Be that as it might, it is quite far-fetched to explain the multi-factorial trait differences in morphology, physiology and life-history traits between morphs in such polymorphic systems [72] as the result of only a single locus with many pleiotropic effects. Rather, these multi-trait differences are likely to have arisen owing to correlational selection for different optimal trait combinations in the different morphs [72]. Correlational selection results in the formation of linkage disequilibrium (LD) and the build-up of adaptive genetic correlations between traits [40,72]. Such LD will of course be opposed by recombination, and the realized level of LD will reflect a balance between the strength of correlational selection versus the eroding effects of recombination [72]. The recombination load can potentially be mitigated or reduced by the evolution of various genomic modifications and arrangements, including chromosomal inversions [34], suppressed recombination, translocations or the evolution of tight linkage or even ‘supergenes’ [72].
An interesting question is also about cause–effect relationships in terms of the genetic basis of AMTs. Did the AMTs arise because of a simple genetic architecture facilitating the evolution of discrete genetic morphs, or was it rather the other way around, the existence of alternative strategies affected the underlying genetic architecture? Some theoretical models suggest that frequency-dependent disruptive selection in itself can favour the transition from a polygenic architecture with many loci of small effect to an oligogenic system of a few loci of large effect [73]. Disruptive selection can, depending on ecological circumstances and assumptions in different models, either lead to the splitting of populations and speciation [74], the evolution of the genetic architecture such as dominant–recessive and protected polymorphisms [75,76] or the evolution of sexual dimorphism and ecological niche segregation between males and females [77]. Finally, systems with genetic polymorphisms can potentially evolve into systems with plastic morph determination, and systems with plastic morphs could in turn evolve into genetic morph determination [78]. These questions can be addressed using comparative phylogenetic methods [78], an exciting area where very little research has been undertaken to date.
Two aspects of genetic constraints are worth considering in the context of AMTs. First, morphs, and hence genetic variation, can be lost, particularly in small populations where negative frequency-dependent selection is opposed by genetic drift [79]. Second, genetic correlations between the sexes and between morphs will constrain their independent evolutionary divergence that can delay the approach to the two sexes' different adaptive peaks [40,80], which is the basic source driving intralocus sexual conflict [81,82]. Just as males and females can be viewed as occupying different adaptive peaks or trying to climb these peaks [83,84], different morphs within sexes can be viewed as occupying different adaptive peaks [72]. The evolution of sexual dimorphism and the evolution of discrete morphs within a species have in common that intersexual and intermorph genetic correlations, and the sharing of a common underlying gene pool, constrain the divergence of the different phenotypes (males, females and morphs within sexes) [80].
4. Alternative mating tactics: more common in males or just overlooked in females?
Currently, most examples of AMTs, and particularly those with a known genetic bias, come from studies of males (table 1). Although systems of female polymorphisms are gradually starting to accumulate in the literature, fewer examples of female alternative reproductive strategies are known to date [52]. Why is this so? Is it because such examples of alternative female mating strategies are rarer than in males, or is it because they have been overlooked? Or has there been a bias in the research community to mainly focus on male reproductive strategies while ignoring female variation and processes such as male mate choice [85]?
Current data do not allow us firmly to evaluate these explanations, but we suspect that AMTs in females might have been largely overlooked, and might be more common than has been recognized. Part of the problem is that while it is relatively easy to identify the ecological factors generating male variance in fitness such as the number of mates, the factors generating fitness variance among female morphs or genotypes are less well understood. Yet, we do now know that there is additive genetic variance in female fitness within populations, as revealed by hemi clonal analysis of fruitfly Drosophila melanogaster [86,87]. How does such additive genetic variance arise? Two possibilities are genetic variation in female condition (which in turn affects fitness) and genetic variation in female resistance and tolerance towards male mating harassment [37,52,88]. For instance, if females are subject to unwanted male mating harassment, and such harassment is costly to fitness, then females could cope with such harassment by either increasing their resistance or their fitness tolerance to male harassment [88,89].
Other possible factors that can generate variation in female fitness are direct or indirect fitness costs and benefits of mating. For instance, in free-roaming crickets (Gryllus campestris), female fitness is positively correlated with the number of matings the females obtain [90]. This correlation indicates the potential existence of indirect fitness benefits of mating with multiple partners in females in this species, in contrast to the traditional view that only males benefit from mating multiply [91,92]. Indirect benefits are, however, thought to be a relatively weak evolutionary force, particularly when compared with direct fitness benefits affecting female survival or fecundity ([93–95], but see [96]). Certainly, AMTs are more likely to evolve in females in mating systems where there is high variance in fitness among females (sensu [3]). Finally, it should be kept in mind that genetic variation in female mating behaviours need not be adaptive at all, and could even be maladaptive, if traits such as mating rates simply reflect a correlated response to selection for promiscuity in males and a strong intersexual genetic correlation for mating behaviour across the sexes [97].
Importantly, when AMTs exist within both males and females of a species, the alternative phenotypes in one of the sexes might simply reflect a correlated response to selection on individuals of the other sex. In this case, the AMTs may be non-adaptive or even maladaptive in one of the sexes owing to a shared gene pool of the males and females (i.e. intralocus sexual conflict; [40,81]). For instance, selection for male promiscuity could simply result in increased female mating rates owing to a shared underlying intersexual genetic correlation between the sexes. Such underlying intersexual genetic correlations will prevent the evolution of independent mating rates in the males and females. This pattern was recently demonstrated in an elegant quantitative genetic study of zebra finches, where female promiscuity appears to arise as a correlated response to selection for male promiscuity [97]. Moreover, in white-throated sparrows, the same chromosomal inversion apparently determines both male and female plumage colour: white males and females are both aggressive and promiscuous, consistent with an intersexual genetic correlation influencing mating behaviour in both sexes [32,34]. By contrast, in the context of mating preferences, male and female behaviours in the Australian fruitfly (Drosophila serrata) are not genetically correlated across the sexes, suggesting that the genes for female mating preferences are not the same genes as those influencing male mate preferences (T. P. Gosden & S. F. Chenoweth 1998, personal communication). When not genetically correlated with males, variation in female mating rates, behaviour, morphology and life-history traits is likely under selection and can be adaptive (also see [36,37]).
5. Alternative mating tactics, polyandry and sexual selection
Sexual selection can both drive the evolution and expression of AMTs, as well as vice versa: once AMTs have evolved, they will affect sexual selection. This bidirectional relationship between sexual selection and AMTs means that AMTs will be a source as well as a target of sexual selection, creating scope for dynamic feedback loops between the two. Female choice, for instance, can promote or suppress the phenotypic expression (and presumably evolution) of male AMTs. There are several examples of variation in female mating preferences for males based on body coloration [98], which can maintain phenotypic diversity within males if females prefer rare male colour phenotypes. Negatively frequency-dependent selection, where females prefer to mate with novel or otherwise rare males, could therefore promote the evolution of male AMTs [99]. On the other hand, female choice can also suppress the coexistence of AMTs. Even if conditions exist that favour the expression of AMTs, such as competition among males for mating access to females, strong female choice for one of the two tactics can result in a significant reduction or even absence of the less preferred male type [100]. Certainly, any kind of differential (sexual) selection between male types can affect the fitness benefit associated with each tactic, which will alter the evolutionarily stable frequencies of the tactics [1]. For example, in swordtail fish (Xiphophorus sp.), males use either a courting or sneaking tactic to mate with females. Females prefer to mate with courting males, but there is variation in the strength of the preference both within and among populations [101,102]. Populations that contain females characterized by strong preferences for the courting male type also have a higher proportion of those males at the expense of fewer sneaker males [101].
When male AMTs have differential effects on female fitness, sexual conflict can arise. Female fitness may be affected directly via her survival or condition, or that of her offspring, or indirectly via the genetic quality of the offspring [103]. For example, in the scorpionflies (Panorpa spp.), males use one of three condition-based mating tactics: (i) defend a food resource such as an insect carcass; (ii) secrete nutrient-rich saliva on a leaf as a nuptial gift; or (iii) offer females nothing but instead try to copulate with them by force [26]. Males are able to switch among the tactics, and generally the smallest and least competitive males will adopt the force copulation behaviour. In this mating system, females benefit directly by mating with males that defend carcasses or provide the nuptial saliva gift as it increases their condition and presumably their survivorship. Conversely, females are in conflict with males that adopt the force copulation tactic, which provides no direct benefit to the female.
In bluegill sunfish (Lepomis macrochirus), males adopt one of two life histories termed parental and cuckolder (figure 1; [5]). Parental males defend nesting territories and provide sole parental care of the young. Cuckolder males instead use a sneaking or female mimicry tactic to steal fertilizations from parental males. Cuckolder males may have higher genetic quality (‘good genes’) as their offspring grow faster and have higher survivorship than those of parental males even when the offspring have the same mother (i.e. when comparing maternal half-siblings; [21,104]). Conversely, parental males cue into rates of cuckoldry and adjust their level of parental care, providing less care to broods that have higher levels of cuckoldry [105,106]. Consequently, females are faced with a trade-off between obtaining good genes from cuckolder males and good care from parental males. This trade-off also leads to sexual conflict, with females preferring a mixture of offspring sired by parental and cuckolder males, but parental males preferring no cuckoldry in their nest, and individual cuckolder males preferring high rates of cuckoldry. Neff [107] modelled this trade-off and used empirical data from a bluegill population to argue that high-quality females were negotiating the trade-off by mating with parental males that experienced lower cuckoldry overall but also what cuckoldry occurred was done disproportionately by cuckolder males that mimic females (as opposed to cuckolder males that sneak). The lower cuckolder rates translated into higher levels of paternal care ([107]; also see [106]). Additionally, cuckoldry by female mimics should have a lower impact on offspring care, because parental males cannot detect the cuckoldry until after the eggs hatch [106,108]. Thus, by mating in nests where the cuckoldry occurs disproportionately by female mimics, high-quality females may be obtaining good genes in some of their offspring but also minimizing the negative impact that cuckoldry has on the direct benefits of paternal care.
Sexual conflict can also itself serve as an agent of selection that promotes the evolution of AMTs. In the blue-tailed damselfly (Ischnura elegans), for example, males often harass females over mating. Mating can be costly to females insomuch as copulation can last for several hours, taking time away from foraging and potentially exposing the pair to increased predation [109]. As a potential counter-strategy, female blue-tailed damselflies have evolved three colour morphs, one of which mimics male coloration (referred to as androchrome females; [35,36]). Androchrome females mate less often with males and presumably have evolved to reduce the cost associated with male mate harassment [109]. An analogous polymorphism exists in diving beetles (Dytiscidae), where females are either male-like in morphology or have evolved modified backs with various armaments to reduce male mating success during copulation and presumably costly, excessive male mating attempts [38].
6. Conclusion and future directions
Past research on AMTs has focused on two alternative frameworks: the game theoretical ‘alternative strategies’ and the ‘conditional strategy’. This approach has led to an unfortunate dichotomy between genetic versus environmental causes of phenotypic variation. Here, we propose that these two frameworks instead represent the extremes on a continuum and that most AMTs fall somewhere in between the two, with both genes and environment contributing to phenotypic expression of the tactics (figure 2). G × E interactions play predominately into our framework, which will lead to variation among individuals in the status-dependent switch points. We envision that, regardless of whether there is a single decision gene in the population that shows no variation in DNA sequence (‘monomorphism’), other loci are likely always to be important in modifying the expression or effect of the decision gene through epistasis. This epistasis will ultimately impact the expression of phenotype (tactic). The modifier loci are unlikely to be genetically monomorphic in a population, and their effects are also likely to be influenced by the environment, resulting in the G × E effects on tactic phenotypic expression. Because environments are rarely homogenous and static through time, the fitness landscape and adaptive peaks associated with the different tactics will continually shift, resulting in changes to the relative fitness of the AMTs. Consequently, at any particular point in time, equal fitness of the tactics is not expected. This framework leads to two important assertions. First, expecting equal relative fitness of AMTs is not likely to be fruitful insomuch as the tactics may or may not have equal fitness based on the particular environmentally and genetically determined fitness landscape at the time point that the calculation is made. Second, while controlled breeding experiments can provide insight into the genetic architecture of tactic phenotypic expression, they cannot fully capture G × E effects and the complexities introduced by potential modifier loci (i.e. epistasis). To better understand G × E effects, breeding experiments could be conducted in multiple, natural environments. Such experiments might reveal environmentally induced variation in tactic switch points and hence norms of reaction, a key prediction of our model. Indeed, G × E effects no doubt play a major role in governing phenotypic variation in general and across traits that show either discrete variation, such as AMTs, as well as traits that are more continuous in distribution.
Another major challenge for future research will be to investigate the nature of selection on alternative female phenotypes, when they exist. One important goal must be to understand whether these alternative phenotypes simply reflect a correlated response to selection on males and are hence non-adaptive or even maladaptive owing to a shared gene pool of males and females (i.e. intralocus sexual conflict). We suspect that in most cases, AMTs in females will not be the result of correlated selection on males (or vice versa). Exceptions might be in species where the tactics have recently evolved and there has been insufficient time for mutation and natural selection to modify expression of the tactics in the sex where they are maladaptive.
Sexual conflict can arise when male AMTs have differential effects on female fitness. The intensity of this conflict and its impact on the ensuring male–female evolutionary arms race warrants further investigation. Additionally, female choice can either promote or inhibit the evolution (and phenotypic expression) of male AMTs. Polymorphisms in female preferences for alternative male mating types are straightforward to assess using, for example, two-choice experiments. However, it is less clear whether female choice can actually generate the conditions required to lead to the evolution of male AMTs in the first place. Ascertaining the role of female choice and female resistance to male mating attempts, and how they might promote or inhibit the evolution or phenotypic expression of alternative male tactics, is more difficult. Mate choice experiments or experiments assessing interspecific variation in female resistance towards mating harassment in an explicit phylogenetic context might provide insight with respect to this latter regard. Moreover, the relative importance of female choice, female resistance towards mating attempts and mating harassment, and male–male competition in the evolution of AMTs, is poorly understood and might benefit from a thorough phylogenetic analysis.
Acknowledgements
Funding for B.D.N. was provided by the Natural Sciences and Engineering Research Council of Canada and for E.I.S. by a grant from the Swedish Research Council (VR). We thank the anonymous reviewers for their valuable comments. Karrianne DeBaeremaeker and Shawn Garner provided comments on the manuscript and assisted with figure preparation.
References
- 1.Gross MR. 1996. Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol. Evol. 11, 92–98 10.1016/0169-5347(96)81050-0 (doi:10.1016/0169-5347(96)81050-0) [DOI] [PubMed] [Google Scholar]
- 2.Oliveira RF, Taborsky M, Brockmann HJ. 2008. Alternative reproductive tactics: an integrative approach. Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Taborsky M. 1994. Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv. Study Behav. 23, 1–100 10.1016/S0065-3454(08)60351-4 (doi:10.1016/S0065-3454(08)60351-4) [DOI] [Google Scholar]
- 4.Van Rhijn JG. 1991. The ruff. London, UK: T and AD Poyser [Google Scholar]
- 5.Gross MR. 1982. Sneakers, satellites and parentals: polymorphic mating strategies in North American sunfishes. Z. Tierpsychol. 60, 1–26 [Google Scholar]
- 6.Dawkins R. 1976. The selfish gene. Oxford, UK: Oxford University Press [Google Scholar]
- 7.Maynard Smith J. 1982. Evolution and the theory of games. Cambridge, UK: Cambridge University Press [Google Scholar]
- 8.Nash JF. 1950. Equilibrium points in n-person games. Proc. Natl Acad. Sci. USA 36, 48–49 10.1073/pnas.36.1.48 (doi:10.1073/pnas.36.1.48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aumann R. 1985. What is game theory trying to accomplish? In Frontiers of economics (eds Arrow K, Honkapohja S.), pp. 909–924 Oxford, UK: Blackwell [Google Scholar]
- 10.Rubinstein A. 1991. Comments on the interpretation of game theory. Econometrica 59, 909–924 10.2307/2938166 (doi:10.2307/2938166) [DOI] [Google Scholar]
- 11.Selten R. 1980. A note on evolutionarily stable strategies in asymmetric animal conflicts. J. Theor. Biol. 84, 93–101 10.1016/S0022-5193(80)81038-1 (doi:10.1016/S0022-5193(80)81038-1) [DOI] [PubMed] [Google Scholar]
- 12.Flaxman SM. 2000. The evolutionary stability of mixed strategies. Trends Ecol. Evol. 15, 482–484 10.1016/S0169-5347(00)01966-2 (doi:10.1016/S0169-5347(00)01966-2) [DOI] [PubMed] [Google Scholar]
- 13.Crowley PH. 2000. Hawks, doves, and mixed-symmetry games. J. Theor. Biol. 204, 543–563 10.1006/jtbi.2000.2037 (doi:10.1006/jtbi.2000.2037) [DOI] [PubMed] [Google Scholar]
- 14.Brockmann HJ, Grafen A, Dawkins R. 1979. Evolutionarily stable nesting strategy in a digger wasp. J. Theor. Biol. 77, 473–96 10.1016/0022-5193(79)90021-3 (doi:10.1016/0022-5193(79)90021-3) [DOI] [PubMed] [Google Scholar]
- 15.Gross MR. 1985. Disruptive selection for alternative life histories in salmon. Nature 313, 47–48 10.1038/313047a0 (doi:10.1038/313047a0) [DOI] [Google Scholar]
- 16.Shuster SM, Wade MJ. 2003. Mating systems and strategies. Princeton, NJ: Princeton University Press [Google Scholar]
- 17.Tomkins JL, Hazel W. 2007. The status of the conditional evolutionarily stable strategy. Trends Ecol. Evol. 22, 522–528 10.1016/j.tree.2007.09.002 (doi:10.1016/j.tree.2007.09.002) [DOI] [PubMed] [Google Scholar]
- 18.Hutchings JA. 2011. Old wine in new bottles: reaction norms in salmonid fishes. Heredity 106, 421–437 10.1038/hdy.2010.166 (doi:10.1038/hdy.2010.166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchings JA, Swain DP, Rowe S, Eddington JD, Puvanendran V, Brown JA. 2007. Genetic variation in life-history reaction norms in a marine fish. Proc. R. Soc. B 274, 1693–1699 10.1098/rspb.2007.0263 (doi:10.1098/rspb.2007.0263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch M, Walsh JB. 1998. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates [Google Scholar]
- 21.Neff BD, Lister JS. 2007. Genetic life history effects on juvenile survival in bluegill. J. Evol. Biol. 20, 517–525 10.1111/j.1420-9101.2006.01265.x (doi:10.1111/j.1420-9101.2006.01265.x) [DOI] [PubMed] [Google Scholar]
- 22.Bass A. 1992. Dimorphic male brains and alternative reproductive tactics in a vocalizing fish. Trends Neurosci. 15, 139–145 10.1016/0166-2236(92)90356-D (doi:10.1016/0166-2236(92)90356-D) [DOI] [PubMed] [Google Scholar]
- 23.Lank DB, Smith CM, Hanotte O, Burke T, Cooke F. 1995. Genetic polymorphism for alternative mating behavior in lekking male ruff Philomachus pugnax. Nature 378, 59–62 10.1038/378059a0 (doi:10.1038/378059a0) [DOI] [Google Scholar]
- 24.Ryan MJ, Causey BA. 1989. ‘Alternative’ mating behavior in the swordtails Xiphophorus nigrensis and Xiphophorus pygmaeus (Pisces: Poeciliidae). Behav. Ecol. Sociobiol. 24, 341–348 10.1007/BF00293262 (doi:10.1007/BF00293262) [DOI] [Google Scholar]
- 25.Kallman KD. 1989. Genetic control of size at maturity in Xiphophorus. In Ecology and evolution of livebearing fishes (eds Meffe GK, Snelson FF.), pp. 163–184 Englewood Cliffs, NJ: Prentice Hall [Google Scholar]
- 26.Thornhill R. 1981. Panorpa (Mecoptera: Panorpidae) scorpionflies: systems for understanding resource-defense polygyny and alternative male reproductive efforts. Annu. Rev. Ecol. Syst. 12, 355–386 10.1146/annurev.es.12.110181.002035 (doi:10.1146/annurev.es.12.110181.002035) [DOI] [Google Scholar]
- 27.Eberhard WG. 1982. Beetle horn dimorphism: making the best of a bad lot. Am. Nat. 119, 420–426 10.1086/283920 (doi:10.1086/283920) [DOI] [Google Scholar]
- 28.Emlen DJ. 1994. Environmental control of horn length dimorphism in the beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Proc. R. Soc. Lond. B 256, 131–136 10.1098/rspb.1994.0060 (doi:10.1098/rspb.1994.0060) [DOI] [Google Scholar]
- 29.Shuster SM, Wade MJ. 1991. Equal mating success among male reproductive strategies in a marine isopod. Nature 350, 608–610 10.1038/350608a0 (doi:10.1038/350608a0) [DOI] [Google Scholar]
- 30.Shuster SM. 1989. Male alternative reproductive strategies in a marine isopod crustacean (Paracerceis sculpta): the use of genetic markers to measure differences in fertilization success among α-, β-, and γ-males. Evolution 43, 1683–1698 10.2307/2409384 (doi:10.2307/2409384) [DOI] [PubMed] [Google Scholar]
- 31.Formica VA, Tuttle EM. 2009. Examining the social landscapes of alternative reproductive strategies. J. Evol. Biol. 22, 2395–2408 10.1111/j.1420-9101.2009.01855.x (doi:10.1111/j.1420-9101.2009.01855.x) [DOI] [PubMed] [Google Scholar]
- 32.Davis JK, Mittel B, Lowman JJ, Thomas PJ, Maney DL, Martin CL, Thomas JW. 2011. Haplotype-based genomic sequencing of a chromosomal polymorphism in the white-throated sparrow (Zonotrichia albicollis). J. Hered. 102, 380–390 10.1093/jhered/esr043 (doi:10.1093/jhered/esr043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huynh LY, Maney DL, Thomas JW. 2011. Chromosome-wide linkage disequilibrium caused by an inversion polymorphism in the white-throated sparrow (Zonotrichia albicollis). Heredity 106, 537–546 10.1038/hdy.2010.85 (doi:10.1038/hdy.2010.85) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas JW, Cáceres M, Lowman JJ, Morehouse CB, Short ME, Baldwin EL, Maney DL, Martin CL. 2008. The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement and suppressor of recombination. Genetics 179, 1455–1468 10.1534/genetics.108.088229 (doi:10.1534/genetics.108.088229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cordero A. 1990. The inheritance of female polymorphism in the damselfly Ischnura graellsii (Rambur) (Odonata: Coenagrionidae). Heredity 64, 341–346 10.1038/hdy.1990.42 (doi:10.1038/hdy.1990.42) [DOI] [Google Scholar]
- 36.Svensson EI, Abbott J, Hardling R. 2005. Female polymorphism, frequency dependence, and rapid evolutionary dynamics in natural populations. Am. Nat. 165, 567–576 10.1086/429278 (doi:10.1086/429278) [DOI] [PubMed] [Google Scholar]
- 37.Gosden TP, Svensson EI. 2009. Density-dependent male mating harassment, female resistance, and male mimicry. Am. Nat. 173, 709–721 10.1086/598491 (doi:10.1086/598491) [DOI] [PubMed] [Google Scholar]
- 38.Hardling R, Bergsten J. 2006. Nonrandom mating preserves intrasexual polymorphism and stops population differentiation in sexual conflict. Am. Nat. 167, 401–409 10.1086/498946 (doi:10.1086/498946) [DOI] [PubMed] [Google Scholar]
- 39.Sinervo B, Svensson E, Comendant T. 2000. Density cycles and an offspring quantity and quality game driven by natural selection. Nature 406, 985–988 10.1038/35023149 (doi:10.1038/35023149) [DOI] [PubMed] [Google Scholar]
- 40.Svensson EI, McAdam AG, Sinervo B. 2009. Intralocus sexual conflict over immune defense, gender load, and sex-specific signaling in a natural lizard population. Evolution 63, 3124–3135 10.1111/j.1558-5646.2009.00782.x (doi:10.1111/j.1558-5646.2009.00782.x) [DOI] [PubMed] [Google Scholar]
- 41.Pryke SR, Astheimer LB, Buttemer WA, Griffith SC. 2007. Frequency-dependent physiological trade-offs between competing colour morphs. Biol. Lett. 3, 494–497 10.1098/rsbl.2007.0213 (doi:10.1098/rsbl.2007.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pryke SR, Astheimer LB, Griffith SC, Buttemer WA. 2012. Covariation in life-history traits: differential effects of diet on condition, hormones, behavior, and reproduction in genetic finch morphs. Am. Nat. 179, 375–390 10.1086/664078 (doi:10.1086/664078) [DOI] [PubMed] [Google Scholar]
- 43.Pryke SR, Griffith SC. 2006. Red dominates black: agonistic signalling among head morphs in the colour polymorphic Gouldian finch. Proc. R. Soc. B 273, 949–957 10.1098/rspb.2005.3362 (doi:10.1098/rspb.2005.3362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pryke SR, Griffith SC. 2009. Genetic incompatibility drives sex allocation and maternal investment in a polymorphic finch. Science 323, 1605–1607 10.1126/science.1168928 (doi:10.1126/science.1168928) [DOI] [PubMed] [Google Scholar]
- 45.Hunt J, Simmons LW. 1997. Patterns of fluctuating asymmetry in beetle horns: an experimental examination of the honest signalling hypothesis. Behav. Ecol. Sociobiol. 41, 109–114 10.1007/s002650050370 (doi:10.1007/s002650050370) [DOI] [Google Scholar]
- 46.Moczek AP, Emlen DJ. 2000. Male horn dimorphism in the scarab beetle, Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? Anim. Behav. 59, 459–466 10.1006/anbe.1999.1342 (doi:10.1006/anbe.1999.1342) [DOI] [PubMed] [Google Scholar]
- 47.Hunt J, Simmons LW. 2001. Status-dependent selection in the dimorphic beetle Onthophagus taurus. Proc. R. Soc. Lond. B 268, 2409–2414 10.1098/rspb.2001.1758 (doi:10.1098/rspb.2001.1758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Widemo F. 1998. Alternative reproductive strategies in the ruff, Philomachus pugnax: a mixed ESS? Anim. Behav. 56, 329–336 10.1006/anbe.1998.0792 (doi:10.1006/anbe.1998.0792) [DOI] [PubMed] [Google Scholar]
- 49.Jukema J, Piersma T. 2006. Permanent female mimics in a lekking shorebird. Biol. Lett. 2, 161–164 10.1098/rsbl.2005.0416 (doi:10.1098/rsbl.2005.0416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shuster SM, Sassaman C. 1997. Genetic interaction between male mating strategy and sex ratio in a marine isopod. Nature 388, 373–377 10.1038/41089 (doi:10.1038/41089) [DOI] [Google Scholar]
- 51.Sinervo B, Lively CM. 1996. The rock-paper-scissors game and the evolution of alternative male strategies. Nature 380, 240–243 10.1038/380240a0 (doi:10.1038/380240a0) [DOI] [Google Scholar]
- 52.Svensson EI, Abbott JK, Gosden TP, Coreau A. 2009. Female polymorphisms, sexual conflict and limits to speciation processes in animals. Evol. Ecol. 23, 93–108 10.1007/s10682-007-9208-2 (doi:10.1007/s10682-007-9208-2) [DOI] [Google Scholar]
- 53.Svensson E, Sinervo B, Comendant T. 2001. Condition, genotype-by-environment interaction, and correlational selection in lizard life-history morphs. Evolution 55, 2053–2069 10.1554/0014-3820(2001)055[2053:CGBEIA]2.0.CO;2 (doi:10.1554/0014-3820(2001)055[2053:CGBEIA]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 54.Svensson E, Sinervo B, Comendant T. 2001. Density-dependent competition and selection on immune function in genetic lizard morphs. Proc. Natl Acad. Sci. USA 98, 12 561–12 565 10.1073/pnas.211071298 (doi:10.1073/pnas.211071298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Svensson EI, Sinervo B, Comendant T. 2002. Mechanistic and experimental analysis of condition and reproduction in a polymorphic lizard. J. Evol. Biol. 15, 1034–1047 10.1046/j.1420-9101.2002.00452.x (doi:10.1046/j.1420-9101.2002.00452.x) [DOI] [Google Scholar]
- 56.Roff DA. 2011. Alternative strategies: the evolution of switch points. Curr. Biol. 21, R285–R287 10.1016/j.cub.2011.03.016 (doi:10.1016/j.cub.2011.03.016) [DOI] [PubMed] [Google Scholar]
- 57.Lister JS, Neff BD. 2006. Paternal genetic effects on foraging decision-making under the risk of predation. Ethology 112, 963–970 10.1111/j.1439-0310.2006.01248.x (doi:10.1111/j.1439-0310.2006.01248.x) [DOI] [Google Scholar]
- 58.Gross MR, Repka J. 1998. Stability with inheritance in the conditional strategy. J. Theor. Biol. 192, 445–453 10.1006/jtbi.1998.0665 (doi:10.1006/jtbi.1998.0665) [DOI] [PubMed] [Google Scholar]
- 59.Rowe L, Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421 10.1098/rspb.1996.0207 (doi:10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- 60.Piche J, Hutchings JA, Blanchard W. 2008. Genetic variation in threshold reaction norms for alternative reproductive tactics in male Atlantic salmon, Salmo salar. Proc. R. Soc. B 275, 1571–1575 10.1098/rspb.2008.0251 (doi:10.1098/rspb.2008.0251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomkins JL, Moczek AP. 2009. Patterns of threshold evolution in polyphenic insects under different developmental models. Evolution 63, 459–468 10.1111/j.1558-5646.2008.00563.x (doi:10.1111/j.1558-5646.2008.00563.x) [DOI] [PubMed] [Google Scholar]
- 62.West-Eberhard MJ. 2003. Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 63.Calsbeek R, Gosden TP, Kuchta SR, Svensson EI. 2012. Fluctuating selection and dynamic adaptive landscapes. In The adaptive landscape in evolutionary biology (eds Svensson EI, Calsbeek R.), pp. 89–103 Oxford, UK: Oxford University Press [Google Scholar]
- 64.Gosden TP, Svensson EI. 2008. Spatial and temporal dynamics in a sexual selection mosaic. Evolution 62, 845–856 10.1111/j.1558-5646.2008.00323.x (doi:10.1111/j.1558-5646.2008.00323.x) [DOI] [PubMed] [Google Scholar]
- 65.Siepielski AM, DiBattista JD, Carlson SM. 2009. It's about time: the temporal dynamics of phenotypic selection in the wild. Ecol. Lett. 12, 1261–1276 10.1111/j.1461-0248.2009.01381.x (doi:10.1111/j.1461-0248.2009.01381.x) [DOI] [PubMed] [Google Scholar]
- 66.Hartl DL, Clark AG. 1997. Principles of population genetics, 3rd edn Sunderland, MA: Sinauer Associates [Google Scholar]
- 67.Gillespie J. 1973. Polymorphism in random environments. Theory Popul. Biol. 4, 193–195 10.1016/0040-5809(73)90028-2 (doi:10.1016/0040-5809(73)90028-2) [DOI] [Google Scholar]
- 68.Haldane J, Jayakar S. 1963. Polymorphism due to selection of varying direction. J. Genet. 58, 237–242 10.1007/BF02986143 (doi:10.1007/BF02986143) [DOI] [Google Scholar]
- 69.Levene H. 1953. Genetic equilibrium when more than one ecological niche is available. Am. Nat. 87, 331–333 10.1086/281792 (doi:10.1086/281792) [DOI] [Google Scholar]
- 70.Kirkpatrick M. 1996. Genes and adaptation: a pocket guide to the theory. In Adaptation (eds Rose MR, Lauder GV.), pp. 125–146 San Diego, CA: Academic Press [Google Scholar]
- 71.Lively CM. 1986. Canalization versus developmental conversion in a spatially variable environment. Am. Nat. 128, 561–572 10.1086/284588 (doi:10.1086/284588) [DOI] [Google Scholar]
- 72.Sinervo B, Svensson E. 2002. Correlational selection and the evolution of genomic architecture. Heredity 89, 329–338 10.1038/sj.hdy.6800148 (doi:10.1038/sj.hdy.6800148) [DOI] [PubMed] [Google Scholar]
- 73.Kopp M, Hermisson J. 2006. The evolution of genetic architecture under frequency-dependent disruptive selection. Evolution 60, 1537–1550 10.1554/06-220.1 (doi:10.1554/06-220.1) [DOI] [PubMed] [Google Scholar]
- 74.Dieckmann U, Doebeli M. 1999. On the origin of species by sympatric speciation. Nature 400, 354–357 10.1038/22521 (doi:10.1038/22521) [DOI] [PubMed] [Google Scholar]
- 75.Rueffler C, Van Dooren TJM, Leimar O, Abrams PA. 2006. Disruptive selection and then what? Trends Ecol. Evol. 21, 238–245 10.1016/j.tree.2006.03.003 (doi:10.1016/j.tree.2006.03.003) [DOI] [PubMed] [Google Scholar]
- 76.Van Dooren TJM. 1999. The evolutionary ecology of dominance-recessivity. J. Theor. Biol. 198, 519–532 10.1006/jtbi.1999.0929 (doi:10.1006/jtbi.1999.0929) [DOI] [PubMed] [Google Scholar]
- 77.Bolnick DI, Doebeli M. 2003. Sexual dimorphism and adaptive speciation: two sides of the same ecological coin. Evolution 57, 2433–2449 10.1554/02-595 (doi:10.1554/02-595) [DOI] [PubMed] [Google Scholar]
- 78.Schwander T, Leimar O. 2011. Genes as leaders and followers in evolution. Trends Ecol. Evol. 26, 143–151 10.1016/j.tree.2010.12.010 (doi:10.1016/j.tree.2010.12.010) [DOI] [PubMed] [Google Scholar]
- 79.Corl A, Davis AR, Kuchta SR, Sinervo B. 2010. Selective loss of polymorphic mating types is associated with rapid phenotypic evolution during morphic speciation. Proc. Natl Acad. Sci. USA 107, 4254–4259 10.1073/pnas.0909480107 (doi:10.1073/pnas.0909480107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abbott JK, Svensson EI. 2010. Morph-specific variation in intersexual genetic correlations in an intra-specific mimicry system. Evol. Ecol. Res. 12, 105–118 [Google Scholar]
- 81.Bonduriansky R, Chenoweth SF. 2009. Intralocus sexual conflict. Trends Ecol. Evol. 24, 280–288 10.1016/j.tree.2008.12.005 (doi:10.1016/j.tree.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 82.Cox RM, Calsbeek R. 2009. Sexually antagonistic selection, sexual dimorphism, and the resolution of intralocus sexual conflict. Am. Nat. 173, 176–187 10.1086/595841 (doi:10.1086/595841) [DOI] [PubMed] [Google Scholar]
- 83.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 10.2307/2407393 (doi:10.2307/2407393) [DOI] [PubMed] [Google Scholar]
- 84.Rice WR, Chippindale AK. 2001. Intersexual ontogenetic conflict. J. Evol. Biol. 14, 685–693 10.1046/j.1420-9101.2001.00319.x (doi:10.1046/j.1420-9101.2001.00319.x) [DOI] [Google Scholar]
- 85.Bonduriansky R. 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. 76, 305–339 10.1017/S1464793101005693 (doi:10.1017/S1464793101005693) [DOI] [PubMed] [Google Scholar]
- 86.Lew TA, Morrow EH, Rice WR. 2006. Standing genetic variance for female resistance to harm from males and its relationship to intralocus sexual conflict. Evolution 60, 97–105 10.1111/j.0014-3820.2006.tb01085.x (doi:10.1111/j.0014-3820.2006.tb01085.x) [DOI] [PubMed] [Google Scholar]
- 87.Linder JE, Rice WR. 2005. Natural selection and genetic variation for female resistance to harm from males. J. Evol. Biol. 18, 568–575 10.1111/j.1420-9101.2004.00872.x (doi:10.1111/j.1420-9101.2004.00872.x) [DOI] [PubMed] [Google Scholar]
- 88.Svensson EI, Råberg L. 2010. Resistance and tolerance in animal enemy-victim coevolution. Trends Ecol. Evol. 25, 267–274 10.1016/j.tree.2009.12.005 (doi:10.1016/j.tree.2009.12.005) [DOI] [PubMed] [Google Scholar]
- 89.Gosden TP, Svensson EI. 2007. Female sexual polymorphism and fecundity consequences of male mating harassment in the wild. PLoS ONE 2, e580 10.1371/journal.pone.0000580 (doi:10.1371/journal.pone.0000580) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rodriguez-Munoz R, Bretman A, Slate J, Walling CA, Tregenza T. 2010. Natural and sexual selection in a wild insect population. Science 328, 1269–1272 10.1126/science.1188102 (doi:10.1126/science.1188102) [DOI] [PubMed] [Google Scholar]
- 91.Bateman AJ. 1948. Intra-sexual selection in Drosophila. Heredity 2, 349–368 10.1038/hdy.1948.21 (doi:10.1038/hdy.1948.21) [DOI] [PubMed] [Google Scholar]
- 92.Trivers RL. 1972. Parental investment and sexual selection. In Sexual selection and the descent of man (ed. Campbell B.), pp. 136–179 Chicago, IL: Aldine [Google Scholar]
- 93.Arnqvist G, Kirkpatrick M. 2005. The evolution of infidelity in socially monogamous passerines: the strength of direct and indirect selection on extrapair copulation behavior in females. Am. Nat. 165, S26–S37 10.1086/429350 (doi:10.1086/429350) [DOI] [PubMed] [Google Scholar]
- 94.Kirkpatrick M, Barton NH. 1997. The strength of indirect selection on female mating preferences. Proc. Natl Acad. Sci. USA 94, 1282–1286 10.1073/pnas.94.4.1282 (doi:10.1073/pnas.94.4.1282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Møller AP, Alatalo RV. 1999. Good-genes effects in sexual selection. Proc. R. Soc. Lond. B 266, 85–91 10.1098/rspb.1999.0607 (doi:10.1098/rspb.1999.0607) [DOI] [Google Scholar]
- 96.Neff BD, Pitcher TE. 2005. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38 10.1111/j.1365-294X.2004.02395.x (doi:10.1111/j.1365-294X.2004.02395.x) [DOI] [PubMed] [Google Scholar]
- 97.Forstmeier W, Martin K, Bolund E, Schielzeth H, Kempenaers B. 2011. Female extrapair mating behavior can evolve via indirect selection on males. Proc. Natl Acad. Sci. USA 108, 10 608–10 613 10.1073/pnas.1103195108 (doi:10.1073/pnas.1103195108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maan ME, Seehausen O, Söderberg L, Johnson L, Ripmeester EAP, Mrosso HDJ, Taylor MI, van Dooren TJM, van Alphen JJM. 2004. Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proc. R. Soc. Lond. B 271, 2445–2452 10.1098/rspb.2004.2911 (doi:10.1098/rspb.2004.2911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alonzo SH, Warner RR. 2000. Female choice, conflict between the sexes and the evolution of male alternative reproductive behaviours. Evol. Ecol. Res. 2, 149–170 [Google Scholar]
- 100.Henson SA, Warner RR. 1997. Male and female alternative reproductive behaviors in fishes: a new approach using intersexual dynamics. Annu. Rev. Ecol. Syst. 28, 571–592 10.1146/annurev.ecolsys.28.1.571 (doi:10.1146/annurev.ecolsys.28.1.571) [DOI] [Google Scholar]
- 101.Rios-Cardenas O, Tudor MS, Morris MR. 2007. Female preference variation has implications for the maintenance of an alternative mating strategy in a swordtail fish. Anim. Behav. 74, 633–640 10.1016/j.anbehav.2007.01.002 (doi:10.1016/j.anbehav.2007.01.002) [DOI] [Google Scholar]
- 102.Tudor MS, Morris MR. 2011. Frequencies of alternative mating strategies influence female mate preference in the swordtail Xiphophorus multilineatus. Anim. Behav. 82, 1313–1318 10.1016/j.anbehav.2011.09.014 (doi:10.1016/j.anbehav.2011.09.014) [DOI] [Google Scholar]
- 103.Alonzo SH. 2008. Male and female alternative reproductive behaviors and conflict within and between the sexes. In Alternative reproductive tactics (eds Oliveira RF, Taborsky M, Brockmann J.), pp. 435–450 Cambridge, UK: Cambridge University Press [Google Scholar]
- 104.Neff BD. 2004. Increased performance of offspring sired by parasitic males in bluegill sunfish. Behav. Ecol. 15, 327–331 10.1093/beheco/arh016 (doi:10.1093/beheco/arh016) [DOI] [Google Scholar]
- 105.Neff BD, Gross MR. 2001. Dynamic adjustment of parental care in response to perceived paternity. Proc. R. Soc. Lond. B 268, 1559–1565 10.1098/rspb.2001.1678 (doi:10.1098/rspb.2001.1678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neff BD. 2003. Decisions about parental care in response to perceived paternity. Nature 422, 716–719 10.1038/nature01528 (doi:10.1038/nature01528) [DOI] [PubMed] [Google Scholar]
- 107.Neff BD. 2008. Alternative mating tactics and mate choice for good genes or good care. In Alternative reproductive tactics: an integrative approach (eds Oliveira RF, Taborsky M, Brockmann HJ.), pp. 421–434 Cambridge, UK: Cambridge University Press [Google Scholar]
- 108.Neff BD, Sherman PW. 2003. Nestling recognition via direct cues by parental male bluegill sunfish (Lepomis macrochirus). Anim. Cogn. 6, 87–92 10.1007/s10071-003-0166-y (doi:10.1007/s10071-003-0166-y) [DOI] [PubMed] [Google Scholar]
- 109.Cordero A, Carbone SS, Utzeri C. 1998. Mating opportunities and mating costs are reduced in androchrome female damselflies, Ischnura elegans (Odonata). Anim. Behav. 55, 185–197 10.1006/anbe.1997.0603 (doi:10.1006/anbe.1997.0603) [DOI] [PubMed] [Google Scholar]