Summary
The interplay of the immune system with other aspects of physiology is continually being revealed and in some cases studied in considerable mechanistic detail. A prime example is the influence of metabolic cues on immune responses. It is well appreciated that upon activation, T cells take on a metabolic profile profoundly distinct from that of their quiescent and anergic counterparts; however, a number of recent breakthroughs have greatly expanded our knowledge of how aspects of cellular metabolism can shape a T-cell response. Particularly important are findings that certain environmental cues can tilt the delicate balance between inflammation and immune tolerance by skewing T-cell fate decisions toward either the T-helper 17 (Th17) or T-regulatory (Treg) cell lineage. Recognizing the unappreciated immune modifying potential of metabolic factors and particularly those involved in the generation of these functionally opposing T-cell subsets will likely add new and potent therapies to our repertoire for treating immune mediated pathologies. In this review, we summarize and discuss recent findings linking certain metabolic pathways, enzymes, and byproducts to shifts in the balance between Th17 and Treg cell populations. These advances highlight numerous opportunities for immune modulation.
Keywords: Th17, Treg, metabolism, T-cell differentiation
Introduction
An effective adaptive immune system must possess the ability to respond to a wide variety of infectious and malignant insults. This versatility is achieved in large part by the functions of highly specialized effector T-helper (Th) subsets generated by the activation of uncommitted precursors. These T-effector lineages and their characteristic cytokines can mean the difference between host resistance and susceptibility to a range of pathogens and diseases. For instance, naive CD4+ T cells can differentiate into Th1 cells that produce interferon-γ (IFNγ) and offer protection against intracellular viral, bacterial, and parasitic infection. The Th1 response is also beneficial for anti-tumor immunity. Alternatively, commitment to the Th2 lineage yields producers of interleukin-4 (IL-4) that are critical for controlling extracellular parasites such as helminthes. A more recently appreciated subset, the Th17 cells are responsible for combating extracellular bacteria and fungi through secretion of interleukin-17A (IL-17A), IL-17F and IL-22 but are far better known for their role in autoimmune responses (1).
The activity of all these effector T cells is attenuated by the anti-inflammatory action of regulatory T cells (Tregs) that inhibit T-cell proliferation and T-effector function. Tregs are often classified as one of two subpopulations. Induced regulatory T cells (iTregs) arise in the periphery from naive T cells upon exposure to transforming growth factor-β (TGF-β) and are propagated by IL-2. Like their thymus-derived counterparts the naturally occurring regulatory T cells (nTregs), iTregs are an important mechanism for the regulation of all types of effector responses. Both iTregs and nTregs express forkhead box protein 3 (Foxp3) as a core subset-specific transcription factor that is key for maintaining both their characteristic function (2) and gene expression profile typified by repression of effector genes and upregulation of those needed for immune suppression. These cells are critical for the prevention of excessive immune activation and autoimmune responses perpetrated by self-reactive T cells (3-6).
Generation of effector T cells or iTregs from naive precursors is referred to as T-cell differentiation – a process heavily influenced by the prevailing environmental inputs present during T-cell activation. This series of events consists of signal transduction initiated at the T cell’s receptors for antigen, cytokine, and costimulatory molecules. The predominance of certain key cytokines over others in the microenvironment can drive the differentiation of naive CD4+ T cells into certain effector lineages, while antagonizing the acquisition of other phenotypes. These cytokine signals are relayed by well-characterized signal transducer and activator of transcription (STAT) pathways also linked to particular lineages. These signals in turn skew the gene expression machinery at work in the cell in a like-wise lineage specific manner dramatically remodeling of the gene expression landscape. The result of this cascade is a committed, functionally specialized T cell.
Recent developments have uncovered that in addition to cytokines and costimulatory molecules, an expansive number of environmental cues linked to various metabolic states and processes can impact T-cell differentiation. These metabolic factors can indeed mean the difference between the generation of suppressive anergic T cells or Tregs and activated T-effector cells (7-10). Furthermore, a considerable body of literature identifies specific metabolic sensors, enzymes, and byproducts as regulators of the highly medically important balance between the developmentally linked yet functionally divergent Th17 and iTreg lineages. The relative dominance of these populations can mean the difference between autoimmune pathology and tolerance. Therefore a clear understanding of the role played by metabolism in shifting this balance and the identification of novel targets for modulation is directly relevant to a number of human diseases. In this review, we discuss a number of breakthroughs that have greatly enhanced our understanding of the interplay between metabolism and the processes governing the relative Th17 and Treg cell balance and the exciting implications they hold for potential immune modulating therapies.
The reciprocal nature of Th17/Treg differentiation
Th17 and Treg cells represent two CD4+ T-cell subsets that share important developmental elements but ultimately bifurcate into distinct phenotypes with remarkably opposite activities, Th17 cells being pro-inflammatory and Tregs being anti-inflammatory. The initial events of Th17 differentiation require functional IL-6 signaling in addition to TGF-β. Subsequently, IL-23 and IL-21 play a key role in the maintenance of the Th17 lineage by enhancing the transcription of IL-17 and other Th17 signature cytokines. Interestingly, the signaling pathways initiated by IL-6, IL-21, and IL-23 all activate STAT3, which is critical for the effects of these cytokines on Th17 cell differentiation (11-13). It is now known that STAT3 is required for Th17 differentiation. Demonstrating this, CD4+ T cells from STAT3 deficient mice fail to upregulate IL-17 and fail to induce severe disease in the experimental autoimmune encephalitis (EAE) model of multiple sclerosis (MS). These observations reflect the importance of STAT3 signaling for induction of retinoid orphan receptor γt (RORγt) gene expression, the key transcription factor required for Th17 development (14). Interestingly, the defect in Th17 differentiation seen in the absence of STAT3 can be only partially reversed by ectopic RORγt expression (15), suggesting additional factors contribute to the generation of Th17 cells.
Th17 cells are important for host defense at mucosal sites and mediating immunity to extracellular bacterial and fungal pathogens. However, dysregulated Th17 responses, characterized by over-production of IL-17A and IL-17F, are particularly important causative or compounding factors in autoimmune diseases, such as MS (EAE in mice) and inflammatory bowel disease (IBD). It was also demonstrated recently that Th17 responses play a critical role in promoting inflammation-associated carcinogenesis. Conversely, the activity of Treg cells restrains the pathological activity of Th17 cells in these and numerous autoimmune disease models (4). In addition to the transcription factor Foxp3, the cytokine TGF-β is also important to the development and function of Treg cells. TGF-β has been shown to maintain peripheral nTreg cells that develop in the thymus, and its deficiency leads to the development of early lethal autoimmunity. Moreover, TGF-β induces Foxp3 expression in peripheral naive T cells, leading to the differentiation of iTregs, which exhibit a suppressive phenotype similar to that seen in nTreg cells (3). Interestingly, this cytokine is also important in Th17 differentiation.
Despite their opposing functions, Th17 and Treg cells share elements in their development including a common requirement for the cytokine TGF-β (4, 16-19). In response to TGF-β in vitro as well as in vivo, many T cells co-express RORγt and Foxp3 (18, 20). Also supporting the notion of an uncommitted precursor stage, studies tracing the fate of IL-17+ cells in the gut revealed that many, at some point, had expressed Foxp3 (21) and transient expression of Foxp3 by non-Tregs has also been reported (22). Eventually, however, depending on the interplay between additional environmental cues, such as the relative amounts of IL-6 and TGF-β and certain metabolic factors (to be discussed here), one or the other phenotype emerges as dominant. TGF-β in the absence of IL-6 induces Foxp3 and represses IL-23R transcription. Foxp3, in turn, can bind to RORγt protein and antagonize its ability to bind DNA, thus pushing T-cell differentiation away from the Th17 transcriptional program and decidedly toward the Treg lineage. In contrast, pro-inflammatory cytokines, such as IL-6 or IL-21 in the presence of low TGF-β concentrations, activate STAT3, which overcomes Foxp3 inhibition of RORγt transcriptional activity. This leads to the upregulation of the IL-23R, thus pushing T-cell differentiation toward a Th17 fate (11). Ultimate Th17 differentiation is associated with Foxp3 downregulation and sustained, unopposed RORγt and STAT3 transcriptional activity. However, the mechanisms underlying Foxp3 loss during Th17 lineage commitment are just beginning to be understood.
Understanding the molecular events that drive uncommitted CD4+ T cells towards one of these highly divergent cell fates is currently the aim of considerable research effort. Surprisingly, a number of findings implicate that the pathways and cues that determine or indicate a cell’s metabolic state can ‘tip the balance’ during T-cell differentiation in favor of Th17 or iTreg lineage commitment. The list of metabolic-immune system intersections regulating the balance between Th17 and Treg cells is just beginning to be compiled.
Meeting the metabolic requirements of T effectors and Treg cells
Quiescent, naive T cells have relatively modest biosynthetic and energetic demands that are typically met by glucose oxidation via the tricarboxylic acid (TCA) cycle and the oxidation of lipids with low levels of glycolysis. The yields of these processes are mostly used to maintain cellular homeostasis (9). T-cell activation drastically increases the cellular metabolic demands. An increase in cell size, division rate (proliferation) coupled with a need for energy to fuel the synthesis of macromolecules, intracellular mediators and effector gene products (cytokines) all require a metabolic reprogramming of T cells upon activation (7, 8). Interestingly, memory T cells and Treg cells also are known to have lighter metabolic demands similar to resting T cells, and the role played by the dominant metabolic processes in these cells are distinct from those of effector T cells.
To meet the demands of their activated lifestyle, T cells downregulate the pathways characteristic of resting cells in favor of aerobic glycolysis and glutamine catabolism (8). This is mediated by the signaling cascades initiated by engagement of the T-cell receptor (TCR), costimulatory molecules, and cytokines and involve mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK), phosphoinositol-3 kinase (PI3K)/Akt, mammalian target of rapamycin (mTOR), and nuclear factor-κB (NFκB) pathways (7, 8). These signals bring about the induction of the transcription factors Myc and HIF-1α that are known to induce a number of genes important for glycolysis and glutaminolysis. To differentiate from uncommitted naive CD4+ precursors into specialized effectors, proper upregulation of glucose metabolism is an absolute requisite. An inability to do so inhibits T-cell differentiation both in vitro and in vivo (23) and instead results in anergy (24). This crossroads of T-cell fate was largely uncovered by studies of mTOR, an important metabolic sensor.
mTOR
It is impossible to discuss the interplay of metabolism and T-cell differentiation without continuous reference to mTOR. While the fate of newly activated T cells is influenced by a variety of factors including strength of TCR signal, the presence of costimulatory or co-inhibitory molecules and cytokines, a variety of other environmental cues are also integrated into this decision. These signals, which include nutrient, oxygen, energy, and stress levels, are all integrated by mTOR (25) and regulate cellular size, growth, proliferation, survival, and metabolism. The numerous signaling pathways governed by this serine/threonine kinase, their impact on the T-cell response, as well as their intersection with other metabolic pathways have been intensely studied (reviewed in 10, 25, 26).
mTOR itself contains twin N-terminal HEAT domains important for protein-protein interactions, an FAT domain, an FRB region (the site of rapamycin/FKBP12 binding), a kinase domain, and a structurally supportive C-terminal FATC domain (10). It is activated by amino acids, oxidative stress, and nutrients in the microenvironment. It is also activated by CD28-initiated PI3K/Akt signals and cytokines such as IL-1, IL-2, and IL-4. Due to its importance as a metabolic sensor, mTOR is at the crux of the figurative decision faced by T cells to either differentiate into effectors or become anergic, a hypoactive state often accompanied by immune suppression and Foxp3 induction. Stimulation of naive CD4+ T cells under conditions inducing suboptimal mTOR activity, such as nutrient starvation, weak or abbreviated TCR stimulation, or inadequate costimulation fail to generate effector T cells and lead instead to the development of Foxp3+ Treg cells. Chemical inhibition of mTOR also yields similar results, and furthering the negative relationship between mTOR activity and the Treg lineage is the observation that Tregs (unlike T effectors) only display transiently upregulation of mTOR activity during the early stages of their activation that is typically not sustained (10).
Optimal mTOR activation, on the other hand, results in the upregulation of glycolysis and STAT signaling needed to support commitment to the Th1, Th2, and Th17 effector lineages. mTOR signaling arises from its participation in either of two distinct kinase complexes, determined by the assemblage of GTPases, scaffolding proteins, and adapter molecules. These complexes are known as mTORC1 and mTORC2 (10, 25). The activity of these mTOR complexes is crucial in the differentiation processes leading naive precursors towards effector T-cell fates, a point made dramatically clear by genetic mTOR deficiency. Naive CD4+ T cells that lack both mTORC1 and mTORC2 signaling fail to differentiate into any T-effector lineage (Th1, Th2, or Th17) and instead, readily take on a regulatory T-cell phenotype. Mechanistically, the inability to become effector cells in mTOR null T cells is associated with a failure to upregulate appropriate Th subset-specific transcription factors (such as Tbet for Th1 cells). These mice also display decreased STAT activation in response to various skewing cytokines(27). Also, treatment of naive CD4+ T cells with the notorious mTOR inhibitor rapamycin results in potent suppression of mTOR signaling and recapitulates the phenotype seen with genetic knockouts causing a surge in Treg generation marked by an increase in Foxp3 expression (10).
While wholesale mTOR deficiency or inhibition suppresses T-effector differentiation in general, specific targeting or deleting components of its individual signaling complexes interestingly yields a more directed modulation of the immune response. This stems from the specific effects of mTORC1 and mTORC2 on T effector subsets. Ras homolog enriched in brain (Rheb)-deficient T cells, which lack mTORC1 activity, fail to become Th1 and Th17 but can still differentiate into Th2. Interestingly, knocking out Rictor and thereby generating mTORC2-deficient T cells results in cells able to differentiate into Th1 and Th17 but not Th2 cells (10, 25). The importance of mTORC1 signaling in the pro-inflammatory Th1 and Th17 cells is demonstrated using mice lacking Rheb (and mTORC activity) in the EAE model. Compared to their wildtype counterparts, these mice mount deficient Th1 and Th17 responses (in vitro and in vivo) and are less susceptible to the traditional symptoms of ascending paralysis seen in EAE (28). The above study highlights mTORC1’s importance to both the Th1 and Th17 responses. However, mTOR activity has also been shown to have specific roles during Th17 development. For instance, the cytokine IL-1 promotes Th17 cell differentiation and proliferation specifically through mTOR activation (29). Additionally, mTOR inactivation can undermine Th17 differentiation through heightened T-cell sensitivity to TGF-β (10), potentially overcoming the relative influence of STAT3 utilizing, pro-Th17 cytokines (such as IL-6 or IL-21). Another major contribution of mTOR and specifically mTORC1 to the generation of Th17 cells appears to be the metabolic shift needed for activated, proliferative, and functional immune effectors. One mechanism responsible for establishing the requisite metabolic landscape is the induction of hypoxia inducible factor 1α (HIF-1). This molecule mediates the upregulation of numerous glycolysis genes and, as discussed in the following section, is a key point of intersection between several metabolic cues and the developing T-cell response.
HIF-1
HIF-1, like mTOR, is a well-known integrator of metabolic cues responsible for initiating an adaptive cellular response to environmental conditions. Particularly, HIF-1 is crucial sensor of oxygen levels responsible for initiating the cellular response to hypoxia. Among the many genes regulated by HIF-1 are those encoding glycolysis participants (30). Disrupting HIF-1 in T cells results in stunted expression of several glycolysis genes including Glut1 (a glucose transporter), hexokinase 2, glucose-6-phosphate isomerase, enolase 1, pyruvate kinase muscle, and lactate dehydrogenase (31). Another HIF-1 target gene, pyruvate dehydrogenase kinase (PDK), promotes glycolysis by preventing the shuttling of pyruvate into the TCA by inhibiting its conversion to acetyl-CoA by pyruvate dehydrogenase (25) making HIF-1’s contribution to glycolysis both direct and indirect. HIF-1 is expressed by nearly all mammalian cells. It is a helix-loop-helix type transcription factor composed of a constitutively expressed β subunit (HIF1β, also called ARNT) and an α subunit (either HIF1α or HIF2α) that is tightly regulated by oxygen availability at the protein level. Under normoxic conditions, HIF-1 protein is hydroxylated at prolines 402 and 564 by members of a family of prolyl hydroxylases domain proteins (PHD1, PHD2, and PHD3), which require oxygen for their function, marking HIF-1 for degradation through the ubiquitin-proteasome pathway. Polyubiquitylation of PHD-modified HIF-1 is achieved by interaction with the von Hippel-Lindau (VHL) protein that recruits an E3 ubiquitin ligase allowing for its removal from the cell via the proteasome (32, 33). With the absence or scarcity of oxygen, inactive PHDs do not initiate HIF-1 modification and protein expression is relatively stable. Upon translocation to the nucleus, HIF-1 subunit dimerization occurs followed by interaction with the cofactor p300 and binding to the promoters of target genes at defined five-nucleotide sequences termed hypoxia response elements (HREs, 5′-[A/G]CGTG-3′) (32). In tumors, genes regulated by HIF-1 include those controlling various cellular processes including metabolism, angiogenesis, and apoptosis to name a few (30, 34, 35).
Transcription of HIF1α seems to be constitutively ’on’ at basal levels; it is at the protein level that oxygen dependent mechanisms result in the stringent regulation of the molecule. However, numerous non-hypoxic stimuli including TCR activation (36), nitric oxide, lipopolysaccharides, and infectious microbes can result in HIF-1 upregulation even under normoxic conditions (37). Inflamed tissue sites are very often hypoxic due to tissue damage resulting in compromised vasculature or the rapid proliferation and infiltration of leukocytes or pathogens (or both). As a result, HIF-1 expression is readily detected in a variety of inflamed and damaged tissues (37). This and the numerous stimuli capable of inducing HIF-1 transcription suggest that this oxygen sensor is likely to play an important role in the biology of leukocytes engaged in an immune response. In fact, the interplay between the cellular responses to hypoxia and inflammation responsible for shaping the innate immune response has been intensely studied (and reviewed in detail in references (37, 38).
Examples of HIF-1’s importance in the innate immune system are many. In general, HIF-1 stabilization and induction in phagocytes and neutrophils appears to enhance their microbicidal and pro-inflammatory capacities. Conversely, HIF-1 deficiencies go hand-in-hand with compromised resistance to a number of pathogens (37). Since TCR signaling causes increased HIF-1 protein in peripheral human T cells and TCR activation coupled with hypoxia is required for induction of HIF-1 in peripheral human T cells (36), it stands to reason that T cells are also impacted by HIF-1. Initial studies of HIF-1 in T-cell function suggested that the molecule acts as a suppressor of T-cell activation, proliferation and effector cytokine production (39). Interestingly, however, several recent studies have revealed a role for HIF-1 in T-cell differentiation that suggests a decidedly pro-inflammatory contribution.
HIF-1-mediated metabolic changes are needed for Th17 generation
Differentiation of naive T cells into the Th17 lineage, or any effector phenotype for that matter, requires the upregulation of glycolysis. Shi et al. found that HIF-1 plays a critical role in setting the metabolic stage for Th17 development (31). Comparing glycolytic activity across the major T-cell subsets revealed that Th17 cells relied much more heavily on this metabolic pathway than Treg cells. Suspecting that this metabolic pathway preferred by the Th17 lineage was initiated by HIF-1, a known inducer of glycolytic enzymes, these investigators measured HIF-1 protein and mRNA levels in Th1, Th2, Treg, and Th17 cells. In agreement with findings from our own group (40), HIF-1 was highly expressed by the latter subset. Activation of naive T cells from conditional HIF-1 knockout mice revealed that the process of glycolysis and expression of numerous genes important for glycolysis were stunted relative to wildtype cells (31). Reflecting the link between glycolysis, HIF-1, and Th17 cells, in vitro induction of IL-17 as well as the Th17-associated factors IL-17F, IL-21, IL-22, and IL-23R are defective in T cells from conditional HIF-1 knockout mice. Interestingly, Foxp3 levels were oppositely affected by T-cell-specific HIF-1 deficiency, reflecting a role for HIF-1 a reciprocal regulation of Treg and Th17 cells (31).
To investigate the in vivo relevance of their observations, these investigators turned to a Th17-dependent, adoptive transfer model of MOG-immunization model of EAE. Mice having HIF-1-deficient CD4+ T cells not only generated reduced frequencies of IL-17+ cells, but impressively, even after further ex vivo expansion under Th17 skewing conditions, these cells proved to be poor mediators of EAE compared to wildtype cells when injected into healthy mice. Again reflecting an inverse relationship between the Treg and Th17 lineages, knockout animals were found to harbor elevated proportions of Foxp3+ cells during EAE, another observation supported by our findings (31, 40). Interestingly, administration of the glycolysis inhibitor (2-DG) at concentrations below that known to suppress T-cell proliferation significantly interfered with glycolysis and Th17 skewing while elevating Foxp3 expression in this study. Obtaining similar results using the well-known mTOR inhibitor rapamycin and observing that HIF-1 expression was also suppressed along with the machinery for glycolysis, the authors propose a model in which under Th17-inducing conditions, mTOR spurs on glycolysis via its upregulation of HIF-1, which is responsible for expression of glycolysis genes (31). It is unclear from this study how much the other highly glycolytic effector lineages, the Th1 and Th2 cells, are impacted by HIF-1 deficiency. It is possible that the remarkable inhibition seen in the experiments described above due to the highly Th17 dominated contexts studied in the paper’s in vitro and in vivo experiments. Subjecting HIF-1-deficient T cells to Th1 and Th2 dependent model systems may reveal how specific HIF-1’s role is to the Th17 differentiation process.
One wonders if HIF-1’s contribution to Th17 differentiation at the expense of iTreg generation is due to effects specific to lineage choice or the more general events of T-cell activation. There is evidence to suggest that although the process of glycolysis supports T-effector generation in general, HIF-1 may be more important for Th17 development than the initial, less lineage specific events of T-cell activation. These finding include that Myc alone can provide the necessary metabolic shift to support general T-cell activation. Interestingly, Wang et al. (41) found that despite the fact that both HIF-1 and Myc are both induced following T-cell activation, acute inducible HIF-1 deficiency resulted in T cells with glycolytic capacities similar to wildtype controls. This finding suggests that HIF-1’s alteration of the metabolic strategy may be dispensable for activation while being required for optimal Th17 differentiation. These authors did allude to a potential role for HIF-1 in the maintenance of glycolytic metabolism post-activation since HIF-1-deficient T cells did have some glycolytic impairment at later time points. Alternatively, it could also be that HIF-1 has pro-Th17 effects beyond augmentation of glycolysis in T cells.
HIF-1 as a multi-level promoter of Th17 differentiation
Work from our group demonstrates that besides participating in the pro-Th17 glycolytic pathway, HIF-1 contributes at several points in the genetic program leading to commitment of naive CD4+ T cells to the Th17 lineage. Previous works showing that HIF-1 is a target gene for activated STAT3 in tumor cells (42) and that STAT3 activation is critical for Th17 development (12) both allude to a potential role for HIF-1 in Th17 development. Testing this notion, we, in agreement with Shi et al., found that Th17 cells express high levels of HIF-1 suggesting a key role for this molecule in Th17 differentiation. Furthermore, naive CD4+ T cells from a CD4-restricted HIF-1 knockout mouse (HIF-1αFlox/Flox × CD4cre+) fail to upregulate IL-17 upon activation in vitro under Th17-inducing conditions. Additionally, these CD4 HIF-1-deficient mice were largely spared the severe EAE symptoms elicited in their wildtype counterparts following MOG immunization. These results suggest a significant role for HIF-1 in Th17 differentiation. In support of this, intermittent in vitro hypoxia, which stabilizes HIF-1 protein levels, augmented Th17 development compared to normoxic culture conditions in a HIF-1 dependent manner (40).
Upon further investigation, we found that the defect in Th17 differentiation seen in in the absence of HIF-1 is tied to reduced expression of the key Th17 transcription factor RORγt. Reporter assays revealed that HIF-1 readily associates with and activates transcription at the RORγt promoter and ChIP analysis of in vitro differentiated Th17 cells confirmed that HIF-1 indeed transactivates RORγt gene expression (40). Interestingly, ectopic RORγt expression does not rescue IL-17 production in HIF-1−/− T cells, even though IL-17 levels are successfully elevated by forced expression of the Th17 transcription factor in wildtype T cells, even in the absence of Th17-inducing cytokine. These results suggest that HIF-1 not only directly mediates RORγt gene expression but also is required for the optimal function of RORγt in IL-17A gene transcription. Reporter assays and co-immunoprecipitate (co-IP) assays both supported a combined HIF-1 and RORγt co-regulation of IL-17A transcription, as the two transcription factors were found to physically interact in T cells cultured under Th17-skewing conditions and as optimal activity at the IL-17 promoter required their collaboration (40). Further experimentation showed that the HIF-1/RORγt complex activates Th17-associated genes by recruiting yet another element of the gene expression machinery.
Realizing that the histone acetyltransferase (HAT) known as p300 is known to be critical for HIF-1-mediated gene activation under hypoxia, we hypothesized that HIF-1 might function in a complex with RORγt by recruiting this transcriptional co-regulator to target genes such as IL-17A. Through ChIP analysis, we detected the binding of RORγt, HIF-1α, and p300 at the IL-17A promoter as well as other Th17 signature genes such as IL-17F and IL-23R. Also supporting this notion, histones H3 and H4 around the IL-17A promoter region were highly acetylated (indicative of an ‘open’ chromatin structure) in wildtype compared to HIF-1−/− T cells under Th17-inducing conditions. Furthermore, the role of this tripartite RORγt/HIF-1α/p300 complex in the activation of the IL-17A promoter was also confirmed by reporter assays showing that synergistic activation of the il17 promoter requires functional versions of all three proteins (40). These results demonstrate HIF-1’s dual role in regulating IL-17 by both directly activating RORγt transcription and subsequently joining with RORγt at the IL-17A promoter to recruit p300, generating a permissive chromatin structure.
While these findings demonstrate HIF-1’s direct pro-Th17 influence during T-cell differentiation, they reflect only half of this molecule’s contribution to the relative balance between Th17 and Treg cells. In addition to their defect in Th17 differentiation, HIF-1-deficient CD4+ T cells were also prone to accumulation of Foxp3 upon in vitro skewing and in vivo during EAE. Curiously however, Foxp3 mRNA levels in wildtype and HIF-1−/− T cells stimulated under Th17 skewing conditions were essentially identical, implying that HIF-1 might be involved in Foxp3 protein modulation in addition to its role in the transcriptional activation of the Th17 pathway. Support for this hypothesis comes from the observation that HIF-1−/− T cells cultured under Treg conditions also expressed substantially more Foxp3 than did wildtype cells. Adding increasing amounts of IL-6 to these cultures (which leads to higher levels of HIF-1 induction) results in progressive loss of Foxp3 protein in wildtype but not HIF-1−/− T cells (40). Based on these findings, we hypothesized that HIF-1 downregulates Foxp3 protein levels through a mechanism completely distinct from its classic role as a transcriptional regulator.
It has been well established that degradation of HIF-1 itself occurs via proline hydroxylation at amino acid positions 402 and 564 by prolyl hydroxylases (PHDs). Hydroxylated HIF-1 is subsequently marked for degradation by the 26S proteasome (32). Interestingly, HIF-1’s ability to induce Foxp3 protein degradation is not only dependent upon the physical interaction of the two molecules, but it also requires the process of HIF-1 degradation. A HIF-1 mutant resistant to hydroxylation by PHDs and subsequent degradation failed to degrade Foxp3 (40), suggesting that HIF-1-mediated Foxp3 loss may occur as a co-elimination process. Taken together, these findings suggest that under both Treg and Th17-skewing conditions, HIF-1 downregulates Foxp3 protein levels. Supporting this notion was our observation that skewing of naive T cells with TGF-β and IL-2 under periodic hypoxia significantly reduced Foxp3 levels relative to continuous normoxic culture. Collectively, these in vitro and in vivo results suggest a model in which HIF-1 is induced via STAT3 signaling in differentiating T cells, resulting in the enhancement of the Th17 genetic program via tripartite, HIF-1/RORγt/p300-dependent activation of transcription of Th17 loci, and the repression of the Treg transcriptional program through induced degradation of Foxp3 protein. The findings that HIF-1 is upregulated by STAT3, and is needed for both the optimal induction of RORγt and IL-17 as well as the removal of Foxp3 in the presence of pro-Th17 cytokines is consistent with those of Yang et al. (20). They found that while RORγt/RORα were needed for optimal IL-17 induction, a STAT3-dependent, RORγt-independent mechanism was response for loss of Foxp3 upon Th17 skewing of Treg cells (20). This novel, multifaceted role for HIF-1 in shaping the T-cell response suggests that targeting this molecule should shift a decidedly pro-inflammatory T-cell-generating process to one that is immune suppressive.
HIF-1 as a sensor of hypoxia/hypoxia and Th17 responses
Low oxygen levels can be the result of enhanced T-cell metabolic activity, and it may serve as the catalyst for drastic metabolic reprogramming in these cells. Hypoxia often accompanies inflammation. In fact, the overlap between the cellular response to oxygen scarcity and inflammation is extensive (37). Interestingly, HIF-1 appears to have important functions in both. In keeping with the well-established stabilization of HIF-1 by hypoxia and the apparent role of HIF-1 in promoting glycolysis and T-effector cell generation, intermittent exposure of developing Th17 cells to hypoxia augmented their production of IL-17(40). These results were in agreement with those of an earlier study that found exposure to human PBMCs to hypoxia increased their production of IL-17 (43). Another study by Ikejiri et al. (44) also found that exposure to hypoxia enhanced IL-17 levels in T cells. Interestingly however, this study found that optimal Th17 differentiation occurs not upon oxygen deprivation alone but only when cells are primed with a period of hypoxia (inducing HIF-1 accumulation) followed by re-oxygenation (44). Furthermore this hypoxic priming was found to be dependent on mTORC1 induced expression of HIF-1, and the effect on IL-17 induction was enhanced upon ablation of the HIF-1 degradation machinery (44).
The importance of the re-oxygenation period in this study may be due to the dynamic and reciprocal nature of HIF-1 protein level fluctuations and those of Foxp3. Should a period of hypoxic ‘priming’ temporarily stabilize HIF-1 protein stores as expected when the degradation machinery stalls, a re-exposure to oxygen would bring about a ‘perfect storm’ of Th17 permissive elements. In such a scenario, these re-oxygenated cells should have their Foxp3 protein rapidly removed by an expanded HIF-1 pool simultaneously driving IL-17 induction via enhanced RORγt expression and activity – effectively jump-starting the genetic program for the development of IL-17 producing cells. Interestingly, however, this group did not observe changes in Foxp3 upon re-oxygenation post-hypoxia (44). While this seems to argue against the scenario described above, it is also possible that Foxp3 protein levels are also highly dynamic, in which case the timing of measurement could be problematic.
This study’s authors speculate that a physiologically relevant instance of hypoxic priming followed by re-oxygenation could occur as developing T-effector cells are activated in lymphoid tissue (that can be relatively hypoxic) and then exit into the peripheral, oxygen-rich circulation. The transition through such dynamic environments may provide a ‘built in’ mechanism to ensure potent priming of these inflammatory cells prior to their entry into peripheral tissues that does not result in the up-regulation of effector cytokine (IL-17) until these cells are closer to foci of inflammation (44).
This notion of an inherent HIF-1-mediated mode of immune activation is reminiscent of one postulated for the contribution of this molecule to innate immunity (37, 38). It was reasoned that since oxygen availability in the circulation can exceed 10%, HIF-1 signaling in blood dwelling neutrophils should be inoperative or at least suppressed. However, as these cells exit the blood stream and traverse the tissues toward sites of inflammation, they encounter a gradient of decreasing oxygen levels. Additionally, HIF-1 expression is stabilized transcriptionally as they ‘home in’ on the epicenter of inflammation containing pro-inflammatory cytokines such as IL-1β and TNFα and microbial products (such as LPS) allowing optimal immune activation to occur restricted to sites of inflammation. A major unanswered question concerning oxygen abundance and HIF-1’s effect on IL-17 and Foxp3 levels concerns how the balance is altered under chronic or prolonged hypoxia. In the studies referenced above, HIF-1’s role in the regulation of IL-17 relied on intermittent, or short term hypoxia followed, or punctuated by exposure to normoxia (40, 44). Therefore, they do not necessarily speak to the relationship between IL-17 and Foxp3 in T cells under chronic hypoxia. Interestingly, since short-term hypoxic priming followed by normoxia was optimal for IL-17 upregulation while continual oxygen deprivation proved ineffectual for induction of the cytokine (44), it is tempting to speculate that during prolonged oxygen deprivation additional mechanisms may be acting on the Treg/Th17 balance.
The issue of chronic hypoxia is also relevant to the tumor-lymphocyte interface. If Foxp3 degradation is wholly dependent upon the co-degradation of HIF-1, then with prolonged or uninterrupted periods of hypoxia, it would be expected that Foxp3 levels alongside those of HIF should stabilize. Certainly the reliable observation that Foxp3+ T cells seem to accumulate in solid tumors, even with IL-17+ cells, seems to support this notion. However, the revelation that hypoxic tumor masses readily recruit Foxp3-expressing Tregs (45, 46) that are likely induced elsewhere (47) offer a highly plausible alternative scenario in which HIF-1 may be degrading Foxp3 within the tumor in conjunction with a replenished Foxp3+ population thanks to newly infiltrating Tregs. The relative importance of HIF-1 and HIF-2 in these conditions must be investigated further. There is reason to suspect these molecules may be relied upon differently, perhaps depending on the extent or duration of hypoxia. While HIF-1 was susceptible to polyubiquitin tagging by the E3ligase CHIP/Stub1 and subsequent degradation under prolonged hypoxia, HIF-2 was not (48), suggesting the possibility that HIF-2 may still be active. Of course, HIF-2’s importance, if any, in the Th17/Treg balance is lacking is currently not established.
It is possible that a brief period of modest upregulation of HIF-1 has a greater impact as a Th17 promoter. Germaine to this discussion is the observation that even in the absence of oxygen, some elements of the HIF-1 degradation machinery can be active. Despite a temporary respite from the tight regulation of HIF-1 in low oxygen, degradation of HIF-1 protein is eventually resumed through multiple mechanisms (48, 49). How this added regulatory mechanism affects HIF-1 in differentiating T cells is unknown. Perhaps some rather counter-intuitive findings from a model of punctuated hypoxia (modeling sleep disorder apneas) showed that HIF-1 is actually induced more strongly by intermittent than continuous hypoxia (50) and moreover, it induced mTOR activation responsible for robust HIF-1 accumulation that persisted for an extended period even after re-oxygenation (51). These results from non-T cells at the very least support a model were the effects of hypoxic priming may be operative outside a narrow window of time. Ischemia/reperfusion (I/R) injury is another physiologically relevant setting in which hypoxic charging followed by re-oxygenation could bring about HIF-1-dependent inflammation. Indeed, studies in I/R models suggest that HIF-1 contributes to tissue damage upon re-oxygenation (52, 53). It will be interesting to determine if this HIF-1-associated damage was Th17/IL-17 dependent.
HIF-1 drives Th17 cell function and longevity
The studies mentioned above clearly demonstrate a role for HIF-1 in the initial events of a Th17 response, specifically in the differentiation of Th17 cells from naive precursors. Another role for HIF-1 in the Th17 response was reported by Kryczek et al. (54). They found that human Th17 cells, which represent a profoundly long-lived and highly plastic population, express heightened HIF-1 message. Suspecting that HIF-1 was responsible for the longevity of Th17 cells, the authors tested the consequences of targeting the molecule. In support of their hypothesis, they found that treatment of Th17 cells in vitro with the HIF-1 inhibitor echinomycin reduced IL-17 production and their ability to persist in vivo after injection into mice. Additionally HIF-1 was found to be important for preventing apoptosis in Th17 cells by virtue of its activation of the Notch signaling pathway and anti-apoptotic genes (54), resulting in a highly persistent pro-inflammatory T cell.
All these findings suggest that the importance of HIF-1 in the Th17 response is not limited to the developmental stage of IL-17-producing T cells but that it can continue to promote the persistence/survival of these cells during post-differentiation as well. The possibility that HIF-1 mediated sensing of metabolic inputs can contribute to the other major characteristic of human Th17 cells, namely their ability to upregulate cytokines of other lineages (such as IFNγ), was not explored in this report. Perhaps further study will shed light on this interesting aspect of Th17 cells.
Targeting HIF-1 to modify the Th17/Treg balance
The role of HIF-1 as a positive regulator of the Th17 response has been established by the work of several groups including our own (Fig. 1). Studies using mice having T-cell-restricted HIF-1 deficiency have been important for these discoveries, however they do not speak to the suitability of HIF-1 expression/function modulation as a therapy to counter undesirable Th17 responses. Thankfully, the intense interest in HIF-1 within the cancer field should aid efforts to evaluate the feasibility and efficacy of HIF-1 targeting. HIF-1’s importance in tumor-promoting processes is well established. These include the promotion of angiogenesis, the upregulation of glycolytic metabolism, and the processes important for tumor cell metastasis. Because of this multifaceted role, HIF-1 has been viewed as a tempting target for new anti-cancer therapies and a robust assortment of compounds with HIF-1 inhibiting activities have been discovered (55-59). These agents include an impressively diverse group of drugs with a variety of HIF-1 antagonizing mechanisms. For instance, the well-characterized HIF-1 inhibiting compounds digoxin, acriflavine, and echinomycin suppress HIF-1 activity by reducing its expression (55), preventing HIF-1α/HIF-1β dimer formation (56), and interfering with HIF-1 DNA binding (58, 59), respectively. Among this cache may be several agents well suited for modulating the Th17/Treg balance to treat immune pathologies. Addition enthusiasm should be taken from the fact that several of these compounds have already seen use in human patients and as such have already been vetted for safety. Also, studies have already demonstrated the utility of HIF-1 inhibitors to suppress Th17 responses. For instance, Kryczek et al. (60) successfully used echinomycin to suppress Th17 function and persistence in vivo.
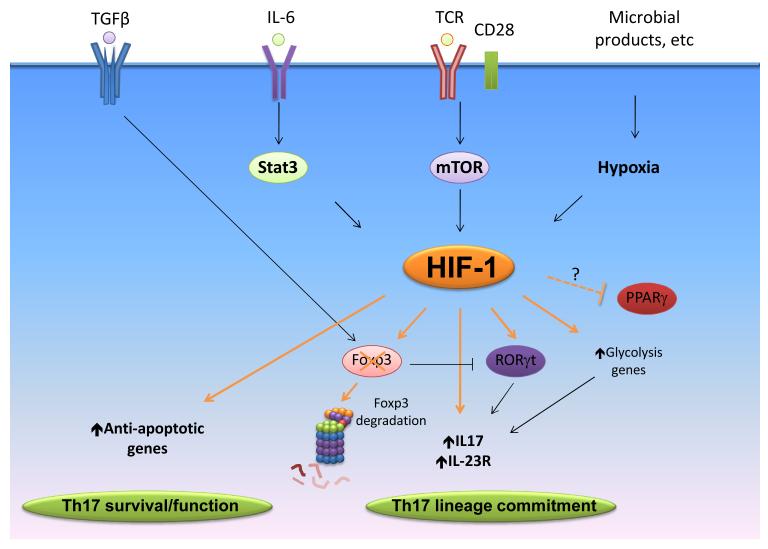
Fig. 1. Reported and potential mechanisms for the regulation of the Th17/Treg balance by HIF-1.
HIF-1 is upregulated in naïve Cd4+ T cells by a range of stimuli including TCR stimulation, LPS, mTORC1 activation, hypoxia, and pro-inflammatory cytokines. HIF-1 induces glycolysis genes that support the differentiation of Th17 cells. HIF-1 has also been reported to activate expression of the Th17 master regulator RORγt. HIF-1 then interacts with RORγt and p300 in order to induce optimal transcription at the il17 gene and other Th17 associated loci. HIF-1 also binds Foxp3 and mediates its proteosomal degradation. HIF-1 also drives expression of survival genes that aid in Th17 persistence. HIF-1 may also suppress expression of the anti-Th17 factor PPAR γ.
Another well-known inhibitor of HIF-1, digoxin, has been demonstrated to be a potent downmodulator of the Th17 response. When screening for agents capable of antagonizing the activity of the Th17 master regulator RORγt, Huh et al. (61) came across the cardiac glycoside digoxin. They found that digoxin treatment of naive T cells in vitro prevented induction of IL-17 even during Th17 skewing conditions and in vivo digoxin treatment during EAE delayed the onset and reduced the severity of disease compared to vehicle controls. Importantly, less toxic derivatives of digoxin were also potent Th17 inhibitors. These drugs also increased the tendency of cells to upregulate Foxp3 compared to controls. Similar results were obtained in another study from Fujita-Sato et al. (62). Unfortunately, it is unclear if HIF-1 was effectively inhibited by the digoxin/digoxin derivative doses used in these studies. Nevertheless, it is tempting to speculate on the possibility that the Th17 suppressing activity of digoxin could be linked to its negative effects on HIF-1 (55). It is possible that in the face of digoxin-suppressed levels of HIF-1, RORγt transcriptional activity would be suboptimal as predicted by our findings (40). Alternatively, the interaction of these inhibitors with RORγt’s ligand-binding domain could be impeding the HIF-1-RORγt association and the subsequent enhanced transcriptional activity. It would be enlightening to determine whether or not the binding of digoxin or other promising Th17 antagonizing ligands (63, 64) to RORγt obscures the region important for the interaction between it and HIF-1. Supporting the ability of HIF-1 targeting to undermine the Th17 response, the HIF-1 inhibitor acriflavine, a very specific HIF-1 inhibitor (65) with a distinct mechanism of action (56) is capable of recapitulating the in vivo effects of digoxin treatment in the EAE model (our unpublished data).
HIF-1 and autoimmune disease
Common themes across autoimmune pathologies include dysregulated Th17 responses and/or Treg deficits. As HIF-1 has been shown by several groups to play a significant role in determining the relative dominance of these opposing T-cell populations, targeting this molecule should prove a highly beneficial intervention for a variety of autoimmune diseases. A convincing case has been made for targeting HIF-1’s contribution to Th17 development to ameliorate MS by studies using T-cell-specific HIF-1 knockout mice and known HIF-1 inhibitors in the EAE model (31, 40, 61, 63, 64). Chemical inhibition of mTOR, thought to be upstream of HIF-1 in the upregulation of glycolysis, also protected mice from developing severe EAE symptoms (31). Collectively, these animal model findings are most encouraging and beg advanced vetting of HIF pathway-targeting strategies in the setting of MS.
HIF-1 has been implicated as a key player in inflammatory bowel disease (IBD). In the diseased gut, the heightened metabolic activity of the inflamed tissue further drives hypoxia (66), which stabilizes HIF-1 expression. Reflecting this, HIF-1 was found to be expressed much more strongly in the afflicted tissues of IBD patients (67) compared to healthy tissue. Additionally, since Th17 cells are known to contribute to IBD pathology in mice and humans, targeting HIF-1 should be an effective strategy for undermining a colitogenic T-cell response. Despite this, the overall role played by HIF-1 in colitis models is not completely clear. HIF-1 in epithelial cells has been proposed as a step to resolve inflammation in mouse models of colitis (68). However, others report that HIF-1 can play a deleterious role in colitis (68-71). Judging from the patents filed intending HIF-1 modulation as a treatment, it appears that equal enthusiasm exists for both enhancing and inhibiting HIF-1 during IBD (72). It is possible that this discrepancy arises from HIF-1’s role in various cell types pertinent to IBD and well as the intricacies of the experimental models for colitis that involve their individual methods of induction, T-cell dependence, T-helper subsets invoked, intensity, and chronic vs. acute nature. These issues will need to be clarified in order for HIF-1 targeting to be adapted into a viable treatment for IBD.
HIF-1 and cancer
Chemically targeting HIF-1 appears to be a highly viable anti-cancer strategy with multiple potential benefits arising from suppressing tumor-promoting processes. An additional result of HIF-1 inhibition during cancer may be the suppressed production of IL-17, a cytokine that contributes to tumor progression (73, 74). Undermining the Th17 differentiation pathway along with multiple pro-tumor processes by chemically targeting general HIF-1 function is a tempting therapeutic strategy. However, studies using mice with HIF-1 deficient T cells sound a note of caution when considering HIF-1 inhibition as monotherapy cancer treatment.
While HIF-1 inhibitors can interfere with the tumor-promoting processes of angiogenesis and the favoring of glycolytic metabolism, they may, as suggested by the previous work of our group and others, also elevate the frequency of immune suppressive Treg cells. These cells are known to stymie anti-tumor immune response by promoting immune tolerance, a state permissive to cancer persistence and progression. Nevertheless, it is still likely that HIF-1 inhibition may yet prove particularly advantageous in the treatment of cancer. It may be prudent to evaluate the efficacy of HIF-1 inhibition as a cancer therapy in combination with other agents aimed at counteracting the potential increases in suppressor cell generation such as the drugs used to deplete Treg cells. Such a combinational approach should simultaneously neutralize two tumor-promoting T-cell populations. Additionally, since some studies suggest that in the latter stages of tumor development, Th17 cells have anti-tumor effects, restricting the therapeutic window of HIF-1 inhibition to early developing tumors may prove more effective as a treatment strategy. Future studies should include the possible role of HIF-1 in other IL-17-expressing cells. Recently identified ’natural Th17 cells’ seem to develop in the thymus without peripheral antigen encounters and have requirements for their generation that distinguish them from their ‘traditional Th17’ (75). Whether the requisites for optimal IL-17 production in these or other cell types such as γδ T cells or innate IL-17-producing cells (76) include HIF-1 remains to be determined.
Related to this question is the possible role for HIF-1 in bringing about the Foxp3+IL-17+ cells found in cancers (77, 78). At least one study suggests hypoxia (and therefore potentially HIF-1) is involved in the acquisition of this double producer phenotype. Whether these cells reflect a transient intermediate cell state as former Tregs lose Foxp3 while simultaneously gaining effector function or if the tumor microenvironment stabilizes HIF-1 (through hypoxia) as well as Foxp3 (through tolerogenic mechanisms or reduced HIF-1 degradation) is unknown. These additional sources of IL-17 may or may not depend upon the HIF pathway of IL-17/IL-17-associated cytokine induction, and further experimentation should be taken on to investigate this possibility.
Lipid oxidation driving Treg over Th17 differentiation
While an upsurge in glycolysis is strongly linked to mTOR activation, HIF-1 expression and T-effector differentiation, and particularly commitment to the Th17 lineage, the oxidative metabolism of lipids on the other hand is associated with Treg dominance. As discussed in the following section, this process and the genes encoding its key participants for the most part favor the generation and functions of Tregs directly or do so by antagonizing the development of Th17 and other T-effector cells.
Resting T cells, memory T cells, and Treg cells are not faced with the same heavy demands for energy and biosynthesis characteristic of CD4+ T-effector cell populations. mTOR activity is highly important for this metabolic shift crucial towards the glucose metabolism needed to support Th1, Th2, and Th17 generation and function. Inhibition of mTOR in naive T cells by rapamycin treatment prevents T-effector cell differentiation but generates large proportions of Foxp3+ Treg cells (10) demonstrating that these cells can be sustained and even flourish as a subset by alternative metabolic pathways.
Indeed the process of Treg induction has been shown to be largely independent of the metabolic reprogramming seen during T-cell activation that is required for T-effector generation. Measuring mitochondrial potential and palmitate oxidation levels upon activation of different CD4+ T-cell subsets reveals that Tregs are the least glycolytic and the most committed to lipid oxidation (79). In keeping with this observation, Tregs were found to upregulate the glucose transporter protein Glut1 (which is important for glucose uptake and support of glycolysis) to a lesser extent than other the subsets (79). Additionally Tregs do not sustain considerable levels of mTOR activation upon TCR activation (10).
Confirming that Tregs are preferentially reliant upon lipid oxidation to support their persistence in the absence of glycolysis, Michalek et al. (79) found that treatment of naive T cells with Etomoxir (Etx), an inhibitor of fatty acid β-oxidation, prevented rapamycin induced accumulation of Tregs. Furthermore, exposing differentiating T cells to exogenous fatty acids effectively suppressed T-effector differentiation while enhancing the generation as well as the suppressive capabilities of Tregs (79). In keeping with the often-observed reciprocal Treg/Th17 regulation, lipid oxidation has a decidedly negative effect on Th17 differentiation. By supplying exogenous fatty acids in the culture media during the in vitro Th17 differentiation of CD4+ T cells inhibited Th17 differentiation (79).
Disrupting fatty acid metabolism does not solely impinge upon Treg cells. Since the CPT-1 inhibitor Etx is capable of disrupting the fatty acid metabolism that Treg cells rely upon and in vitro it prevents their generation in the absence of glucose metabolism (79), one would expect that such an agent might unleash a robust T-effector response. Interestingly, in vivo administration of Etx actually tempered EAE severity and reduced the Th1 response in these mice (80). Even though the effect of CPT-1 inhibition on the balance between Foxp3+ and IL-17+ cells is not completely clear, this study does suggest that fatty acid oxidation may still have a role in the T-effector response as well as in Tregs. Nevertheless, a general association exists between Treg generation and the metabolic factors involved in fatty acid metabolism as well as between promoters of glycolysis and Th17 differentiation. Examining the enzyme AMP-activated protein kinase (or AMPK), an important inhibitor of mTOR and driver of lipid metabolism, and its impact of the Th17/Treg axis further supports this generalization.
AMPK
AMP-activated protein kinase (AMPK) is a heterotrimeric kinase consisting of a catalytic α subunit, a regulatory β subunit, and an AMP-binding γ subunit. α and β subunits occur as either of two isoforms (e.g. α1 or α2), while three different γ subunits are possible. AMPK acts as a sensor for intracellular energy and is activated by low energy levels indicated by fluctuations in AMP:ATP ratios. Binding of AMP to the γ subunit results in allosteric activation of this enzyme (81). AMPK can also be activated in an AMP-independent manner. This is mediated by phosphorylation of threonine-172 of the α subunit by upstream kinases. Once activated, in order to restore ATP homeostasis, AMPK upregulates a suite of genes involved in energy yielding processes such as glucose oxidation and, importantly, lipid oxidation. Among these is carnitine palmitoyl transferase (CPT-1), which mediates the influx of fatty acids into the mitochondria, a process crucial for oxidative lipid metabolism (82). AMPK also preserves CPT-1 activity by inhibiting acetyl-CoA carboxylase which is itself a suppressor of CPT-1, thus further promoting fatty acid oxidation (81).
As previously described as a contrast to the metabolic requirements of effector T cells, Tregs are associated with the process of fatty acid oxidation. Inhibition of CPT-1 with Ext curtails the ability of Tregs to thrive in the absence of glucose metabolism and recall that fatty acids positively affect Treg function and generation in vitro. Furthermore Michalek et al. (79) showed that both nTregs and iTregs displayed a high degree of AMPK activity, and pharmacological activation of AMPK by the drug metformin increased lipid oxidation in T cells in vitro. Moreover, in vivo administration of metformin was sufficient to decrease Glut1 expression and increase Treg numbers in an asthma model (79). In addition to upregulating the genes for lipid metabolism, AMPK has also been reported to promote the uptake of glucose and the process of glycolysis (83). Interestingly, despite the fact that both mTOR and AMPK are induced by TCR stimulation and are important for cellular metabolism, the latter kinase is a potent negative regulator of the former. This inhibition occurs when nutrients are limiting, and the process is mediated by AMPK’s phosphorylation of the suppressive tuberous sclerosis 2 (TSC2) protein of the TSC1/2 complex. Phosphorylation of TSC2 by AMPK activates the complex that prevents the interaction of Rheb with mTORC1, suppressing its activation (25). Reflecting this inverse relationship AMPK deficiency in multiple cell types results in enhanced mTORC1 signaling accompanied by higher glycolytic rates and elevated production of effector cytokine (84, 85). TSC1 knockout yields a similar phenotype (86), reinforcing the importance of the molecule in AMPK-mediated suppression of mTORC1 activity.
AMPK-deficient mice and AMPK-activating compounds in animal models for various autoimmune diseases have considerably illuminated the role of this kinase in the immune response. In mice afflicted with EAE, AMPK protein levels appear extremely low in T cells and other immune cells as well suggesting a link between low AMPK activity and immune pathology. Interestingly, this downregulation of AMPK was accomplished post-transcriptionally, as mRNA levels were no different between diseased and healthy samples. Genetic deletion of AMPK in mice rendered them susceptible to more intense EAE symptoms. In this study, AMPK−/− mice had higher clinical scores, showed heightened central nervous system (CNS) cellular infiltration, and higher ex vivo levels of IFNγ production than their wildtype counterparts (87). A similar phenotype was seen upon knocking out one of the upstream activators of AMPK, the serine/threonine kinase liver kinase β1 (LKB1). This kinase is important for AMPK activation, and in its absence, AMPK activity is reduced. MacIver et al. (84) found that T cells lacking LKB1, like those of AMPK−/− mice, display more robust mTORC1 activity and pro-inflammatory cytokine production than wildtype controls. Also naive LKB1−/− CD4+ T cells were more capable of differentiating into Th17 and Th1 cells in vitro. Interesting, these authors found that while both CD4+ and CD8+ T cells from LBK1−/− mice produced more IFNγ and IL-17 than wildtype cells, AMPK deficiency resulted in higher than normal pro-inflammatory cytokine production in the CD8+ compartment only. In contrast to the case of LKB1 deficiency, AMPK−/− CD4+ T cells were no more inclined towards the Th17 lineage than wildtype controls (84).
Since LKB1 is known to activate 13 other kinases in the AMPK family (88), it is possible that LKB1’s effects on Th17 differentiation are mediated through other intermediates related to AMPK. This may explain the curious finding that in the EAE model, splenocytes of the more severely afflicted AMPK−/− mice did not produce more IL-17 than wildtype controls (87). It remains unclear, however, whether or not Foxp3 levels were altered by AMPK deficiency. Nevertheless, agonists of AMPK do show promise as potential anti-inflammatory agents.
Activators of AMPK as immune modifiers
The prospect of therapeutically activating AMPK in the hopes of ameliorating autoimmune disease has been explored and encouraging results have been reported. In one study, a compound called 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR), an agonist of AMPK, showed significant promise in a mouse model of colitis. In that study, daily AICAR treatment beginning either after induction of acute colitis by TNBS or in a chronic/relapsing model significantly improved disease outcome as evidenced by body weight loss and colon histological inflammation. Moreover, this treatment inhibited macrophage activation and reduced Th1 and Th17 cell populations and production of their characteristic cytokines amongst gut and lymph node dwelling leukocytes. The authors concluded that AICAR-initiated AMPK activation may serve to downregulate ongoing innate and adaptive immune responses such as those involved in colitis (89, 90).
Additionally, AICAR showed promise as a modulator of the T-cell response in the EAE model. Administering this AMPK agonist either before or during active disease lessened symptom severity compared to that seen in untreated mice. AICAR was also found to have reduced leukocyte infiltration of CNS tissues, suppressed T-cell responses, reduced Th1 cytokines, and elevated production of the anti-inflammatory cytokine IL-10 compared to controls (91). This same group also found that the widely used diabetes drug metformin, which activates AMPK, could also reduce the severity of disease in the EAE model for MS. In addition to reductions in disease score, metformin-treated mice showed reduced mononuclear cell infiltration as well as lower pro-inflammatory cytokine message including IFNγ and IL-17 in the CNS. Transcripts for the Th1 and Th17 master regulators Tbet and RORγt were also reduced as was the capacity for ex vivo pro-inflammatory cytokine production by splenocytes in the treatment group (87).
While Foxp3+ cell frequencies were not measured these studies, the large-scale reduction in immune pathology and increased IL-10 production is consistent with elevated Treg presence and/or function. In another study however, Michalek et al. (79) showed that metformin treatment could in fact result in elevated Foxp3+ cell numbers in an asthma model, suggesting that AMPK activation can serve as a two-pronged immune modulator, inhibiting harmful effectors directly and through the enrichment of suppressor cells. These studies provide a prime example of a metabolic factor with significant impact on the immune system. AMPK appears to favor Treg generation over that of effector cells including Th17 cells, either through directly by promoting fatty acid oxidation, or indirectly by inhibiting mTOR (and presumably HIF-1 and glycolysis). The link between lipid metabolism and the prevalence of the Treg lineage over that of Th17 is not limited to this kinase, however.
The PPARs
Peroxisome proliferator-activated receptors (PPARs) are nuclear hormone receptors that bind to and are activated by fatty acids. PPARs occur as three different isoforms (α, β/δ, and γ), each having their own preferential ligands (92). The PPARs are transcription factors and as such contain a DNA-binding domain, a ligand-binding domain, and a hinge region (81). They are known to form heterodimers with retinoid X receptors (RXRs) to regulate the transcription of a number of genes relevant to lipid metabolism including (92). The general mechanisms of transcriptional activation by the PPARs can involve the binding a conserved DNA sequence (a PPRE) and the recruitment of co-activator complexes to the promoter. Repression of gene expression can be mediated by direct binding of a PPAR to a transcription factor, either obstructing its ability to bind DNA or bringing it to complex with co-repressor molecules (92). Additionally, the binding PPARs modified by SUMOylation to co-repressor complexes can prevent their removal from the promoters of target genes to be silenced (93).
PPARα is an intercellular sensor of endogenous fatty acids, and like other PPAR isoforms, it is known to bind and be activated by a variety of fatty acids (81, 92). PPARα controls a number of metabolic processes, including mitochondrial fatty acid β-oxidation, fatty acid uptake, and glucose homeostasis. The γ isoform (PPARγ) is regarded as a master regulator of adipocyte metabolism and development (25, 94). Despite their importance in the above processes, PPARs do not only serve to regulate lipid-metabolizing genes. PPARs are expressed by macrophages, T and B cells, as well as dendritic cells, and they have been reported to have anti-inflammatory activity in these cells of the immune system (reviewed in 92). Among the genes repressed by the PPARs include several important for immune activation and inflammation (including the transcription factors AP1 and NF-kB and IL-2 (95-97). Like its homolog PPARα, PPARγ has been shown to act as an immune suppressor. In EAE models, mice partially deficient in PPARγ (PPARγ+/−) fair worse than their wildtype counterparts, and PPARγ antagonists similarly enhanced EAE disease severity (98). In contrast, ligation of PPARγ with any of several PPARγ agonists results in lower clinical scores as well as other improved disease metrics including reduced leukocyte infiltration of the CNS, pro-inflammatory cytokine production, and overall duration of disease (92).
PPARγ’s protective action in EAE was initially attributed to its negative effect on the Th1 response. With the surge in interest in the relatively newly discovered Th17 cell subset, the possibility that PPARγ may also impact this pro-inflammatory T-cell population has been investigated. Since EAE has a significant Th17 component, it would be consistent with the results if PPARγ ligation undermines Th17 differentiation. A study by Klotz et al. (99) found that the γ isoform was a robust inhibitor of Th17 differentiation. In addition to lower IL-17 production, PPARγ activation resulted in stunted RORγt induction. These anti-Th17 effects of PPARγ were found to be due to a negative impact on STAT3 signaling (99). While not effecting STAT3 levels or phosphorylation of STAT3 in response to IL-6 treatment, PPARγ disrupted STAT3 transcriptional activity by interfering with its DNA binding capacity, in keeping with previous observations (100). This suppression of STAT3 function was mediated by the physical interaction of PPAR with the transcription factor and the ability of ligated PPARγ to facilitate the association of phospho-STAT3 with a co-repressor complex rendering it transcriptionally inactive. Additionally, this study showed that chemically activating PPARγ in T cells from MS patients could also result in successfully suppressed production of Th17 cytokines (99).
In this study, PPARγ agonism did not affect differentiation of other T-helper subsets (Th1, Th2), while other studies have found PPAR activity results in suppression of Th1 responses as well (101, 102). This observation may suggest that certain ligands (such as pioglitazone, in this case) may have subset specific effects, while others have a more generalized anti-inflammatory impact. It is also possible that different ligands may affect different cells through distinct mechanisms during EAE. Understanding the basis for such observations would permit the development of therapeutics more tailored to modify specific immune pathologies. As with PPARγ, ligation of PPARβ/δ also suppresses disease severity in the EAE model. The action of this form of PPAR effectively reduced both Th17 and Th1 cells. IL-10 levels on the other hand were increased by a PPARβ/δ agonist while PPARβ/δ knockout mice experience enhanced Th17/Th1 responses and prolonged disease in the EAE model (103). Additionally, it was reported that ligation of PPARα effectively protected mice from developing severe EAE by inducing a shift in the prevailing Th cytokine response from a typically strong Th1 response to one displaying increased Th2 cytokine (IL-4). Also, similar to PPARγ, activation of PPARα also suppresses the production of pro-inflammatory cytokines by CNS resident cells (microglia and astrocytes) (92).
While the studies referenced above establish a clear anti-inflammatory role for the PPARs, their effect on the Treg population and the balance between these cells and the Th17 lineage is less clear. Even though they did not report a role for PPAR in Treg generation coincident with the suppression of Th17 and Th1 cell generation, they did include observations of enhanced production of IL-10 as reported for PPARδ ligation, possibly indicating enhanced suppressor cell presence or function. Other studies, however, clearly demonstrate a link between PPAR activation and Treg-mediated immune control. Hontecillas et al. (104) found that T-cell-specific PPARγ deficiency enhanced pro-inflammatory cytokine production and rendered mice susceptible to chemically induced colitis. Additionally, knockout Tregs could not prevent colitis in lymphopenic recipients when co-injected with naive T cells (104). Another study reported the PPARγ agonist ciglitazone mediated tolerogenic effects of retinoic acid (RA) on DCs, causing them to become inducers of Tregs (105). This same ligand also proved to be a potent inducer of Tregs and protective in a model of graft versus host disease (106). Another study found that PPARα and PPARγ ligation in conjunction with TGF-β treatment enhanced Treg generation of human Treg cells (107). These findings suggest that PPARγ agonists do not only suppress generation of T effectors, including Th17 cells, but they can also mediate Treg induction and suppressive function.
A recent study also found a role for PPARγ in a unique subset of Treg cells. Visceral adipose tissues (VATs) house a unique subset of Treg cells that are associated with reduced inflammation in adipose tissue. Suppressing this low level fat-associated inflammation is linked to a reversal of insulin resistance. VAT Tregs were found to uniquely express PPARγ, and they display a gene expression profile significantly distinct from their spleen and lymph node dwelling counter parts. Recently, it was shown that mice specifically lacking PPARγ in Treg cells (Foxp3-cre/Ppargf/f) have a reduced VAT Treg population and as a consequence display increased insulin resistance and potentially heightened susceptibility to diabetic pathology (108). It is possible that the bevy of genes for lipid metabolism and synthesis impart VAT Tregs with a survival advantage optimal for this fatty niche. This study found that in order to reap the full benefits of the anti-diabetes drug and PPARγ agonist pioglitazone required PPARγ expression by VAT Tregs (108), demonstrating the important of these cells in diabetes.
A number of questions remain concerning the immunomodulation attributed to PPAR activation. For instance, there exists some uncertainty whether the anti-inflammatory effects of the PPAR agonists are actually dependent on these receptors. This notion arises from the observations that antagonists (to PPARγ) and agonists (to PPARγ and PPARα) seem to be equally effective at altering the course of EAE in both wildtype mice and those deficient in the corresponding PPAR (92). Whether this is an indicator of promiscuity between PPARs and their ligands or a high degree of redundancy in their immune-modifying function remains to be seen. Compounding the situation is the observation that PPAR agonists can directly activate AMPK (81), which can similarly suppress inflammation.
Despite an apparent anti-inflammatory role for the molecule, PPARγ knockout CD4+ T cells do not induce widespread autoimmune pathology (109). Also, despite its suppressive effect on Th1 and Th17 cells, PPARγ appears to be activated by mTOR, while PPARα, having similar anti-inflammatory properties, is suppressed by mTOR (26). Whether this counterintuitive relationship is operative in T cells remains to be seen, and if so, one speculates if this could be indicative of a negative feedback mechanism in the mTOR pathway known to be important for induction of Th17 cells. Additionally, it is unknown if the same mechanisms underlying the anti-Th17 effects of PPARγ are employed during activation of other PPARs. Work in adipocytes suggests that the β and δ isoforms of PPAR also inhibit STAT3, although this study found that it was achieved by ERK1/2 inhibition and the prevention of a STAT3-Hsp90 complex (110).
There appears to be a sex-linked difference in PPAR expression patterns and their effects on the Th1 and Th17 pro-inflammatory cytokines. Male mice express higher levels of PPARα and IL-17 in their T cells than female mice, which preferentially express PPARγ. This profile is apparently male sex hormone dependent and can be reversed by over-expression or siRNA knockdown of the gender-preferred PPAR (111). It has been suggested that this sex hormone-dependent PPAR/pro-inflammatory cytokine expression profile may explain the heightened susceptibility of females to certain autoimmune diseases.
PPAR activation as therapy
The PPARs boast a rather large ligand-binding pocket capable of interacting with a variety of synthetic ligands that constitute an impressive list of efficacious PPAR activators (92). One family of compounds in particular, the thiazolidinediones (TZDs), include commonly prescribed diabetes drugs that have been shown in numerous studies to be potent PPAR activators with immune-modifying activities (112). This body of work should form the basis for developing new immune-modulating therapies relying on PPAR agonism. Thus far, there is cause for optimism as experimental ligation of PPARγ has shown promise in several animal models of autoimmune disease (92, 113-115). The PPARγ ligands rosiglitazone and pioglitazone were found to reduce IL-17 as well as Th2 cytokines and afford protection from asthma. Protection was negated by administering exogenous IL-17 (113), suggesting that these agents can be used to combat Th17-driven pathologies.
Prompted by these encouraging studies, PPARγ agonists are currently being tested in clinical trials for autoimmune diseases. In one MS trial, despite having no significant improvement in disease score (as determined by expanded disability status scale or EDSS), MRI data from recipients of the PPARγ agonist pioglitazone (in combination with IFNβ-1α) showed less gray matter atrophy and promising trends toward decreased lesions after one year. Rosiglitazone has been tested as an intervention for ulcerative colitis in a randomized placebo-controlled study and was found to be an effective strategy (116). These findings offer encouragement to further explore the immunosuppressive capabilities of PPAR agonists. It does not appear to be true that all factors associated with fatty acid metabolism oppose Th17 differentiation. Li et al. (119) found that epidermal fatty acid-binding protein (E-FABP), a lipid chaperone with similar ligand binding specificity as the PPAR molecules, had the opposite effect on the Th17/Treg balance.
Fatty acid-binding proteins
FABPs are cytosolic molecules capable of binding hydrophobic ligands such as lipids and directing them to intracellular compartments (117). These FABPs are highly tissue specific. One such variety epidermal fatty acid-binding protein, E-FABP, is a lipid chaperone with known regulatory effects on inflammation particularly mediated through antigen-presenting cells (118). Findings from Li et al. (119) suggest that the FABPs may also be metabolic players with potent influence on the Th17/Treg balance. These authors found that E-FABP is upregulated in vitro under Th17-skewing conditions, and deficiency of this FABP in naive CD4+ T cells results in reduced Th17 skewing capability. This phenotype could also be achieved by treatment of cells with an FABP inhibitor (119).
CD4+ T cells from these knockout mice also showed a slightly greater inclination towards Foxp3 upregulation under Th17-inducing conditions that was markedly enhanced upon inclusion of RA in the Th17 skewing cocktail. Reflecting this shift in the balance of Th17/Treg generation favoring enhanced tolerance, E-FABP-deficient mice developed much less severe EAE than their wildtype counterparts after immunization with MOG peptide. Analysis of lymph node, spleen, and CNS-infiltrating cells confirmed that E-FABP acts as a pro-Th17 factor. The apparent pro-Th17 effect of E-FABP was attributed to the molecule’s negative regulation of the Th17 suppressor PPARγ. Knockout of E-FABP resulted in a specific upregulation of mRNA for PPARγ in activated T cells, and elevated PPARγ activity was also seen in the absence of E-FABP. Treatment of E-FABP knockout CD4+ T cells with the PPARγ antagonist GW9662 during Th17 skewing returned the frequency of IL-17-producing cells to near wildtype levels (119). Despite inducing lower RORγt and RORα levels, E-FABP knockout T cells activated in the presence of IL-6 possess undisturbed levels of STAT3, and phosphorylation of this critical signaling component of the pro-Th17 cytokine was also intact. However, in agreement with previous reports of PPARγ’s interference with STAT3 activity (99, 100) and in keeping with elevated PPARγ presence, E-FABP-deficient T cells displayed compromised STAT3 DNA binding (119). These results suggest that the PPARγ’s suppression of the Th17 axis may be counteracted by the action of FABPs.
Prior work implicated that at least one additional FABP found in adipose tissue, A-FABP, may possess an important role in the immune system and particularly the Th17 response. The involvement of this protein family in promoting T-effector responses, including those of Th17 cells, is most curious since they share considerable ligand (lipid) specificity with the PPARs that undermine Th1 and Th17 responses. The fact that PPARs and FABPs seem to fall on opposing metabolic sides of the Th17/Treg balance is even more curious given the fact that they are known to cooperate in the regulation of lipid relevant gene expression (120). Further investigation may reveal the reason for this odd relationship between the PPARs and the FABPs and their respective immune roles.
Sterol Sensing and Cholesterol Metabolism
Another instance of metabolic influence in T-cell fate decisions can be found in the machinery for cholesterol metabolism. Liver X receptor (LXR) is an orphan nuclear receptor occurring as one of two different isoforms: LXRα or LXRβ, both of which can be expressed in CD4+ T cells regulating gene products that control intracellular cholesterol homeostasis (121). It binds DNA as a heterodimer when joined to the retinoid X receptor (RXR) and regulates cholesterol and fatty acid metabolism genes, such as ABCA1, ABCG1, SREBP1C, and fatty acid synthase. Given this apparent association between LXR and lipid metabolism as well as its partnership with the RXR, it seems that a negative impact of Th17 generation would be likely. Indeed, synthetic LXR agonists were found to decrease the severity of EAE when administered prior to myelin peptide immunization (122) as well as after initiation of disease (123). In the latter study, LXR ligation effectively suppressed IL-17 and IFNγ expression by T cells as well. While in another study, retroviral over-expression of LXR in T cells inhibited Th17 differentiation. Conversely, knocking it out resulted in enhanced IL-17 production. Treatment of differentiating cells with LXR agonists also suppressed expression of IL-17, RORγt, and other Th17-associate genes (124).
The mechanism behind LXR-mediated Th17 suppression appears to depend upon downstream target genes. One LXR-regulated gene product, sterol regulatory element binding protein (SREBP-1), is a known regulator of lipogenic enzymes (125) and was found to associate with the il17 promoter, where it interacts with and inhibits the activity of the aryl hydrocarbon receptor (Ahr)(124). This transcription factor is known to be important for expression of Th17-associated genes (15). In this way, LXR disrupts transcription of the IL-17 gene. Intriguingly, the authors of this study point out that in past clinical trials, MS patients were found to have lower cholesterol levels than healthy individuals, and they propose that such a low cholesterol status may be linked to autoimmunity(124). While establishing such a relation will require considerable work, this study’s findings suggest it may exist.
ERRα
Another recent and notable discovery linking a metabolic factor to the generation of T-effector cells including Th17 cells involved estrogen-related receptor (ERRα). This hormone nuclear receptor is known to regulate energy metabolism pathways and is particularly important in the biogenesis of mitochondria (126).
CD4+ naive T cells express very low levels of ERRα, but upon activation in the presence of adequate costimulation, it is upregulated. Inhibition of ERRα by activating CD4+ T cells in the presence of XCT790, a compound that causes ERRα degradation, reduced T-cell activation and glucose metabolism. A component of the pyruvate dehydrogenase (PDH) complex was downregulated while an inhibitor of PDH called pyruvate dehydrogenase kinase 1 (PDK1) was upregulated by ERRα antagonism. This altered pattern of gene expression shunts pyruvate away from conversion into acetyl-CoA and entry into the TCA cycle (an effect also reported to be downstream of HIF-1 activity). CPT-1, a promoter of lipid oxidation, was simultaneously upregulated in these treated cells, suggesting ERRα inhibition was preventing glucose metabolism as well as mitochondrial metabolism.
Using T cells from ERRα-deficient mice confirmed these results and also revealed that despite their altered metabolism, these cells were comparable to wildtype in regards to TCR signaling and initial T-cell viability. Even though the initial events in T-cell activation seemed intact without functional ERRα, such inhibition did markedly reduce T-cell proliferative capacity. Supplying exogenous fatty acids could rescue proliferation even in the presence of XCT790, due to elevated levels of CPT1 levels, suggesting that ERRα inhibited cells could rely on fatty acid oxidation to support cell division (127).
Fatty acid supplementation, however, could not rescue T-effector cell phenotypes in the XCT790-treated cells. In accord with other reports of fatty acid oxidation in the cross-regulation of T-effector and Treg cell fate, in the absence of ERRα, lipid metabolism could only sustain a Treg cell phenotype. From this observation the authors conclude that ERRα is critical for T-effector cell generation. Supporting this idea, mice deficient in ERRα as well as mice treated with ERRα inhibitor experienced delayed onset and reduced severity of disease compared to untreated, wildtype mice after immunization with MOG peptide in the EAE model. ERRα targeting also dramatically reduced the frequencies of IL-17-producing cells in the lymph nodes of these mice. Curiously but consistent with the in vitro effects of ERRα inhibition without exogenous fatty acids, the lymph node Foxp3+ populations were not significantly different between the three groups (127). This could possibly suggest that while ERRα is critical for in vivo T-effector generation, other factors determine the representation of Tregs.
Amino acid abundance
The relative abundance or scarcity of amino acids is a T-cell relevant metabolic metric. mTOR is activated by the presence of free amino acids, especially branch chain amino acids (BCAA).This can be mediated through Rag GTPases and their interaction with mTORC1 (128, 129). In fact even when other factors are plentiful, mTORC1 activity depends upon the presence of amino acids (10), suggesting that amino acid abundance should favor T-effector differentiation and activation over that of Treg induction and anergy. While the presence of high levels of such mTOR-activating amino acids should permit a robust Th17 response, one would expect that general amino acid scarcity should conversely impede such a response. It has been proposed that one mechanism by which Tregs promote immune anergy/tolerance is by consuming BCAA, which results in mTOR inactivation (130). Furthermore, a recent report demonstrated direct link between the regulation of Th17 differentiation and the machinery for sensing amino acid available in the T cell’s microenvironment.
Amino acid scarcity elicits expression of several genes involved in amino acid transport, biogenesis and their assemblage into higher proteins. Elements of such an ‘amino acid starvation response’ (AAR) were found to have a negative effect on Th17 responses. Importantly, Sundrud et al. (131) found that artificial activation of the AAR response by the compound halofuginone inhibited Th17 differentiation in vitro by interfering with the maintenance of STAT3 signaling. Halofuginone treatment also resulted in a reciprocal increase in Treg generation in vitro, suppressed the Th17 response, and provided significant protection to mice in the EAE model (131). These results identify a compound with unappreciated potential as an immune modulating therapeutic. Shortages of particular amino acids can also sway the balance between T-effector and Treg generation. The process of glutaminolysis generates fodder for the TCA cycle from the breakdown of the amino acid glutamine (26). Efficient glutamine uptake and catabolism in conjunction with glycolysis was shown by Wang et al. (8) to be important during T-cell activation and is likely requisite for proper effector T-cell differentiation. However, the amino acid tryptophan has garnered much attention for the role its metabolism plays in T-cell differentiation.
The enzyme indoleamine 2,3-dioxygenase (IDO) mediates the initial reaction in the breakdown of tryptophan. By depleting this already rare amino acid, a localized amino acid scarcity of sorts is induced causing an increase in uncharged tRNA results in proximal T cells activating the stress responsive general control nonrepressed 2 (GCN2) kinase. This molecule can suppress Th17 differentiation when activated as part of the AAR. Downstream products of tryptophan catabolism also have been implicated in the suppressive effects of IDO on T cells with cumulative suppression of Th17 cells arising from both direct inhibition by tryptophan catabolites and indirect suppression resulting from a heightened iTreg population (132, 133). Reflecting the potency of IDO as an element of immune control, its expression by DCs could suppress EAE (132). Additionally, Sharma et al. (134) and Baban et al. (135) both reported that the action of IDO can also prevent the conversion of Foxp3+ cells to IL-17 producers. Suggesting that newly generated Tregs can perpetuate a tolerogenic state initiated by IDO is the observation that cytotoxic T-lymphocyte antigen-4 signaling is capable of inducing expression of the enzyme (136). This tendency of amino acid starvation to expand Tregs and promote tolerance at the expense of T-effector responses is apparently not limited to the amino acid tryptophan. Besides IDO, the activities of asparaginase and arginase I can also result in deprivation of their respective amino acids and activation of GCN2 kinase (8). In at least the latter case, enzymatic activity has been shown to mediate tolerance in human T cells (137). These studies clearly show that the actual or perceived availability of amino acids can have a significant impact on the Th17 and Treg populations.
PI3K/Akt
Activation of T cells without costimulatory signals results in anergy rather than activation (138). Interestingly, a similar fate awaits T cells activated while incapable of extensive glucose metabolism. It has been shown that co-stimulation through CD28 mediates glucose metabolism in T cells (139, 140), particularly through Glut1 surface expression and trafficking (141, 142). It is also known that the status of mTOR activation at the time of TCR stimulation is a crucial factor in deciding the difference between T-cell anergy and T-cell activation much in the same manner (24, 143). mTOR itself is be activated by PI3K/Akt signaling initiated by TCR activation and costimulation, and it has been reported that HIF-1 induction upon TCR activation is dependent on both PI3K/Akt as well as mTOR (36). These molecules are known to cooperate in the establishment of a glycolytic metabolism (144). Therefore the PI3K pathway is highly relevant to the metabolic input affecting the generation of Tregs and T-effector cells in general. Reflecting its place in the Th17 promoting chain of events, involving mTOR and HIF-1, PI3K/Akt signaling appears to have a hand in skewing the Treg/Th17 balance. A report by Kurebayashi et al. (145) found that not only was the PI3K/Akt mediated activation of mTOR’s TORC1 pathway important forTh17 differentiation, but they also attribute its effects to a concerted nuclear trafficking of RORγt and suppression of Gfi-1, an inhibitor of Th17 differentiation.
Factors interfering with PI3K/Akt pathway are associated with the Treg generation and T-cell anergy. Recently, programmed death-1 (PD-1) was implicated as suppressing the progression of T cells through the cell cycle (146). PD-1 was previously shown to suppress glucose uptake and the rate of glycolysis in T cells by inhibiting CD28-mediated activation of PI3K and subsequent phosphorylation of Akt (147). This in turn would mean reduced mTOR activation and the loss of several downstream pro-Th17 molecular events. A link between PD1/PDL1 signaling and Treg generation and function has been shown by Francisco et al. (148). Demonstrating at least a negative association between PD1 signaling and Th17 cells D’Addio et al. (149) found that blockade of PDL1 resulted in reduced proportions of Treg cells and a coinciding increase in Th17 differentiation in an alloantigen-specific model of fetomaternal tolerance. In recent years, the development of numerous isoform-specific inhibitors and knockout mice have permitted a ‘fine focus’ for observing the individual roles played by the different species of PI3K. Past studies have found that specific inhibition or genetic knockout of the delta isoform of PI3K (PI3Kδ) suppressed a variety of T-effector subsets (150, 151) including Th17 cells. Curiously, it has been reported that PI3Kγ is not important for Th17 despite PI3Kγ-deficient mice being relatively protected from EAE (152, 153). However more recently, Bergamini et al. reported the discovery of a novel PI3Kγ inhibitor capable of suppressing not only IL-17 but also RORγt making the compound termed ‘CZC24832’ a potent Th17 antagonist (154).
A relationship between the Treg population and inhibition of PI3K signaling is also evident. It would seem that some degree of PI3K activity is needed to maintain Tregs, while high or constitutive levels of PI3K/Akt activation are detrimental for Treg function and generation (155). Perhaps supporting this notion is the observation that Treg cells display lower levels of mTOR activations upon stimulation by IL-2 due to higher levels of the inhibitor of the PI3K/Akt pathway PTEN (156), and Tregs display very low levels of Akt activity (157). During Treg differentiation, PD-1 has been reported to suppress both mTOR and Akt signaling (157). Other negative regulators of PI3K/Akt signaling such as PTEN (158, 159) are important for Foxp3+ Treg generation (155). In the absence of PTEN, T cells failed to upregulate Foxp3 and become Tregs upon exposure to TGF-β. This deficit could be corrected by simultaneously inhibiting PI3K–demonstrating a strong negative association of the PI3Kinase/Akt pathway with the Treg side of the Th17/Treg balance. Furthermore, Patterson et al. (160) showed that the PH-domain leucine-rich-repeat protein phosphatase (PHLPP1), which deactivates Akt, was also highly expressed by Tregs compared to other T cells and plays an important role in Treg function and differentiation. In their study, Tregs from PHLPP1−/− mice lost suppressive function and the generation of iTregs from PHLPP1 deficient precursors was reduced (160). The negative effects of these phosphatases on PI3Kinase/Akt signaling clearly impact the function and frequency of the Treg populations, even though their importance in Th17 differentiation remains in question. In contrast, the PI3Kinase/Akt antagonizing phosphatase SHIP-1 appears to have little impact on the function of Tregs in vitro and in vivo. Even more surprising was the observation that deletion of SHIP-1 in mice results in reduced Th17 differentiation and an increased tendency to become Treg cells under Th17-inducing in vitro conditions (161).
Targeting specific isoforms of PI3K may translate into effective therapies capable of altering the Th17/Treg balance. Supporting this, the PI3Kγ specific inhibitor AS605240, at certain doses, induce Tregs in vivo and reduces the severity of colitis in mice (162), although any potential effect on IL-17 and Th17 cells was not investigated in these studies. Additionally, there are a number of questions involving the isoforms of PI3K and their role in determining the Th17/Treg balance. For example, there are data to support the notion that certain PI3K isoforms may be more important than others in suppressing Treg generation. Specifically, inhibiting PI3Kα proved to be most effective at inducing Foxp3 expression, while other isoform-specific inhibitors failed to do so or did so at doses associated with a loss of specificity (155). Therefore PI3K’s impact on the Th17/Treg balance may be multifaceted with it being highly important to encourage mTOR activity and glucose uptake and these and possibly other pro-Th17 and anti-Treg effects may be mediated by distinct PI3K isoenzymes. Further investigation may yet advance the use of highly specific PI3K inhibitors to use as therapeutic immune-modifying drugs.
Potential metabolic cross-talk and feedback loops
Antagonistic crosstalk plays a major role in regulating T-helper cell differentiation. Cytokines characteristic of one T-effector subset often drive the generation and function of that subset while working to downplay commitment to alternative cell fates. In fact, this antagonism extends from the level of cytokine signaling to the STATs and the lineage master regulators as well. For instance, in addition to promoting Th2 cell differentiation, IL-4 suppresses commitment to other lineages. Similarly, GATA3 suppresses expression of the typical Th1 cytokine IFNγ (163). GATA3 can also interfere with STAT4 and IL-12Receptor expression, while Tbet negatively regulates Th2 development by reducing induction of GATA3 (164). This theme of antagonistic cross-talk is not limited the Th1 and Th2 lineages. IL-2/STAT5 signaling is necessary for the generation of certain T-effectors as well as iTreg cells yet it strongly inhibits Th17 differentiation (165). Also Th17 cells may be reprogrammed into Th1-like cells upon stimulation with IL-12 (166). These observations clearly illustrate the highly degree of cross-talk at work during T-effector differentiation. Examining the literature concerning the metabolic pathways now known to favor Th17 or Treg cell generation reveals that considerable cross-talk exists between them as well. Just as is the case for competing sets of cytokines/transcription factors, it is likely that the effects of metabolism on T-cell differentiation are the result of complex networks of cues. The reports mentioned below provide no small amount of encouragement for future research into the interplay of the metabolic pathways in question during T-effector differentiation. Investigating whether or not these instances of metabolic cross-talk or counter-regulation are operative in T cells may reveal further layers to the regulation of the Th17/Treg balance and could represent additional opportunities to modulate the T-helper cell response.
AMPK, which drives fatty acid oxidation, inhibits mTOR signaling that governs glycolytic metabolism. Work with AMPK agonists demonstrate that the T-effector differentiation made possible by mTOR can be ablated by AMPK activation (87, 91). Examples of counter-regulatory cross-talk do not stop with these molecules by any means. We have already discussed the impact of HIF-1 and Foxp3 during Th17 differentiation as well as the antagonism of E-FABP and PPARγ. Many more potential instances of metabolic cross-talk may exist with potent effects on the balance between inflammation and Th17 generation and T-cell anergy and Treg cells. Reports from non-T cells reveal possible crosstalk between HIF-1 and PPAR may impact the Th17/Treg balance. HIF-1 negatively regulates PPARγ expression. Specifically, expression of the PPARγ2 isoform is repressed through the action of the HIF-1 target gene Dec1/Stra13 (167). While it has yet to be demonstrated experimentally, it is an intriguing possibility that an additional mechanism for driving Th17 differentiation exists in the suppression of a suppressor. Supporting this notion is the observation that under conditions of hypoxia, which stabilize HIF-1 expression, PPARγ activity is reduced (168).
Another potential example of cross-regulation may be found in the functions of HIF-1 and a molecule known as Nr4a1 or Nur77. Additionally, metabolic factors may be altering the balance between Tregs and other cells in the thymus. Immature T cells in the thymus with autoreactive TCRs face either deletion or being shunted from a conventional T-cell fate to become Tregs (169). Nur77 is an immediate-early response gene induced by TCR and can serve as an indicator for strength of TCR signals. This nuclear receptor superfamily member is important for glycolysis (170). Nr4a1/Nur77 knockout mice have elevated thymic Tregs, and it has been proposed that this gene may negatively regulate these Tregs by promoting expression of glycolysis genes (169) in a manner similar to that seen for Tregs in the periphery (79). Interestingly, the promoter of Nur77 can be bound by HIF-1, suggesting a role for HIF-1 in promoting Nur77 expression (171). Additionally, Kim et al. (172) have reported that Nur77 is capable of inhibiting pVHL, thereby stabilizing HIF-1 expression. These findings could indicate that yet another mechanism for the promotion of glycolysis, suppression of Treg generation, and reciprocal commitment to the Th17 lineage could be mediated by HIF-1. Moreover, the mutually positive effect these molecules exert on each other in non-T cells could indicate the existence of a positive feedback loop.
Negative feedback loops may also involve HIF-1 and its role in determining T-cell fate. Hypoxia is a potent stabilizer of HIF-1 expression, and mTORC1 activates HIF-1 expression. However, hypoxia and HIF-1 can suppress mTORC1 activation through the REDD1 protein in an AMPK-independent, TSC1/2 complex-dependent manner (173). Could this be evidence of a negative feedback loop? Could these observations explain the interesting finding that intermittent hypoxia is a more potent stimulus for mTORC1 activity and HIF-1 expression? Findings that REDD1 also suppresses HIF-1 expression seem to support the notion of a negative feedback loop in the mTORC1/HIF-1 pathway. However, the observation that enhancement of HIF-1 expression in the face of REDD1 deficiency is mostly mTORC1 independent complicates the matter (174).
In another thought-provoking study, the activity of serine/threonine kinase known as glycogen synthase kinase 3 (GSK3) was found to be a promoter of the Th17 response. This prevents the insulin-initiated conversion of glucose to glycogen by glycogen synthase (175). Interestingly, it also appears to enhance STAT3 activation in T cells in response to IL-6, IL-21, and IL-23 as well as IL-6 production by antigen-presenting cells (176), a process that may be mediated by GSK3’s interaction with STAT3 and recruitment to cytokine receptors (175). Reflecting this, GSK3 inhibition effectively reduced IL-17 expression in several tissues including the gut, the infected lung, and the EAE-afflicted CNS (176). GSK3 activity may be thought of a promoter of glucose availability and by extension the metabolic states conducive to Th17 differentiation and as an enhancer of STAT3 signaling, one would expect it to work in concert with HIF-1. Surprisingly, GSK3 has been reported to actually drive the degradation of HIF-1 (177). This could be another indication that negative feedback is an important means of controlling HIF-1 function. As mentioned previously, studies of unexplored stimuli such as chronic hypoxia could reveal if more complex layers of regulation such as these are relevant to the HIF-1’s role in the T cell.
Further work in this area will no doubt yield insights into the impact of these metabolic players on the shaping of the immune response. Since these studies were mostly done in non-T cells of course it remains to be seen if these concepts hold true in T cells. There is some cause for optimism that such may be indeed prove to be the case. Several of the studies above were done in cancer cells, which have energetic and biosynthetic needs similar to activated T cells and appear to invoke similar metabolic shifts to meet these demands.
Concluding remarks
The immune system, particularly the T-cell response, is heavily influenced by metabolic factors. The extent of this influence can be seen in T cells activated without the means or ability to fuel their rapid proliferation and effector functions. Such cells, like those lacking the machinery for glycolysis or under mTOR deficiency or inhibition, are directed towards a Treg-like fate typified by anergy and suppressive functions. In this way the prevailing metabolic state of a T cell can mean the difference between general T-effector commitment and Treg generation. It is also clear that certain cellular metabolic elements can execute even more focused modification of T-cell fate – directing cells to either the Th17 or iTreg lineage during their initially overlapping differentiation events (Fig. 2). While we have concentrated on the influence of metabolic cues during the generation of Th17 and iTreg cells from naive precursors, the possibility that these metabolic cues can even skew committed T cells types from one phenotype to another also deserves consideration. Certainly the findings of Kryczek et al. (54, 60) support the possibility that metabolic factors can influence T-cell subsets beyond the initial differentiation process. In that study, Th17 cells relied on HIF-1 both for optimal IL-17 production, and to stave off apoptosis as well. These authors also reported that human Th17 cells are highly capable of producing the cytokines of other Th lineages such as IFNγ as well as the transcription factor Foxp3 in certain pathological settings (54, 60). Others have also reported an ability of Th17 cells to produce a range of other cytokines beyond those typical of the lineage apparently depending on the pathophysiological setting (178, 179). Could metabolic factors mean the difference between a strict adherence to the canonical Th17 phenotype (IL-17 production) or the shift to a more versatile one? Likewise, can metabolic cues affect the stability of the Treg phenotype? Since the concept of fully committed Tregs acquiring T-effector functions or outright conversion to other lineages remains controversial (21, 22, 180, 181), uncovering a role for metabolic stability or instability of Foxp3+ cells will be highly interesting and significant to the field. Exposure to hypoxia has been reported to result in IL-17 expression by Foxp3+ Treg cells (77), suggesting that metabolic cues such as those indicating or related to hypoxia may impact Th17 and Treg cell populations well beyond the window of T-cell differentiation by acting on committed lineage members. Further investigation into the role of metabolic cues in T-cell function will no doubt shed light on this and other queries. Particularly interesting is the possibility that this phenomenon of metabolic meddling in the functions of T cells could explain the convoluted/complicated role of IL-17 and Th17 cells in cancer biology (182).
Fig. 2. The effect of metabolic factors on Th17 and iTreg differentiation.
The process of glycolysis and factors promoting it favor Th17 differentiation as do environmental cues such as low oxygen levels, amino acid, and glucose abundance. Lipid oxidation on the other hand promotes Tregs generation. Fatty acids and metabolic cues linked to AMPK activation and poor mTOR activation (amino acid scarcity, lack of glucose metabolism) also promote Treg induction.
In this review, we have mentioned numerous examples of metabolic influences on the predominance of either Th17 or iTreg cells as well as their potential as targets immune modifying therapies. As these instances of metabolic immune control have rapidly accumulated, for the most part, over the past half-decade, it is most likely that the near future will witness additional revelations of this immunometabolic intersection. Additional metabolic factors likely to impact the Treg and Th17 populations include the vitamin D receptor. While important for the regulation of calcium, ligation of this transcription factor has been shown to inhibit Th17 cell differentiation and increase Treg generation and ameliorate autoimmune disease in animal models. This may explain reports that a poor vitamin D status in humans may be linked to autoimmunity (183-186). There also may be an intersection between factors regulating the cellular calcium flux and HIF-1. The phosphatase calcineurin has been shown to dephosphorylate RACK1, preventing it from binding to HIF-1 and recruiting the ubiquitin tagging enzyme. The ubiquitination and subsequent degradation of HIF-1 can thereby be prevented by calcineurin (187), potentially altering the Th17/Treg balance through this molecule. This may explain why calcineurin inhibition has a negative effect on Th17 development (188). However, it is curious that calcineurin inhibition therapies in kidney transplant patients are sometimes associated with elevated frequencies of Th17 cells. This could reflect calcinuerin’s importance for production of IL-2, a cytokine needed to support Tregs and some T-effector cells but negatively linked to Th17 differentiation (189). Regardless, further exploration of calcineurin’s impact on the Treg/Th17 balance will no doubt aid the improvement of transplant-associated treatments. While in this review we have chosen to focus on the factors actively participating in metabolic pathways, the byproducts of metabolism may also hold considerable sway over the Th17/Treg axis. For instance, it has been proposed that lactic acid produced by tumor cells increases IL-23 and Th17 generation (190). Additionally, accumulation of mitochondrial reactive oxygen species (ROS), a consequence of oxidative respiration and paradoxically, moderate hypoxia also can impact Th17 development. Zhi et al. (191) found that deletion of the immediate early response gene X-1 (IEX-1), a negative regulator of mitochondrial ROS build up, enhanced Th17 differentiation. Furthermore, the heightened Th17 responses of IEX-1 knockout mice led to more severe disease in an arthritis model. This study found that levels of the pro-Th17 transcription factor Batf to be elevated in along with ROS levels in these knockout mice (191). Interestingly, increases in ROS have also been linked to upregulation of another pro-Th17 metabolic factor, HIF-1. These results suggest that the end products of metabolic processes also may significantly influence the T-cell response.
These and other yet-to-be discovered intersections of the immune system and metabolic pathways will add to our understanding of the nuances of immune regulation and form the basis for new and potent immunomodulatory therapies, as several of those discussed in this review already have. Indeed, speculations based on work in non-T cells concerning potential cross-talk among immunologically relevant metabolic factors may also lead to additional findings and opportunities for immune modulation. Many of the recent studies describing metabolic influence over T-cell fate have proposed or actually included the initial evaluation of new molecular agonists and antagonists in animal models of autoimmune disease. With the advancement of some to the proving ground of clinical trials, already underway in some cases, we are likely to see this surge in interest in metabolic immune modification yield novel and effective new therapies and hope for those afflicted with pathologies stemming from immune dysregulation.
Acknowledgments
We thank the members of Dr. Pan and Dr. Pardoll’s laboratories, particularly Dr. Huang-Yu Yang for figure preparation. These investigators are supported by grants from the NIH, Melanoma Research Alliance, The Cancer Research Institute and The Crohns and Colitis Foundation of America. Dr. Pan is the recipient of the Stewart Trust Scholar Award.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Barnes MJ, Powrie F. Regulatory T cells reinforce intestinal homeostasis. Immunity. 2009;31:401–411. doi: 10.1016/j.immuni.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Green DR. The immune diet: meeting the metabolic demands of lymphocyte activation. F1000 Biol Rep. 2012;4:9. doi: 10.3410/B4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang R, Green DR. Metabolic reprogramming and metabolic dependency in T cells. Immunol Rev. 2012;249:14–26. doi: 10.1111/j.1600-065X.2012.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris TJ, et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J Immunol. 2007;179:4313–4317. doi: 10.4049/jimmunol.179.7.4313. [DOI] [PubMed] [Google Scholar]
- 13.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Zhou L, Littman DR. Transcriptional regulatory networks in Th17 cell differentiation. Curr Opin Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 17.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 18.Veldhoen M, Stockinger B, TGFbeta1 a. "Jack of all trades": the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 2006;27:358–361. doi: 10.1016/j.it.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 19.O’Quinn DB, Palmer MT, Lee YK, Weaver CT. Emergence of the Th17 pathway and its role in host defense. Adv Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- 20.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends Immunol. 2012;33:168–173. doi: 10.1016/j.it.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. Anergic T cells are metabolically anergic. J Immunol. 2009;183:6095–6101. doi: 10.4049/jimmunol.0803510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol Rev. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waickman AT, Powell JD. Mammalian target of rapamycin integrates diverse inputs to guide the outcome of antigen recognition in T cells. J Immunol. 2012;188:4721–4729. doi: 10.4049/jimmunol.1103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulen MF, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dang CV. The interplay between MYC and HIF in the Warburg effect. Ernst Schering Found Symp Proc. 2007:35–53. doi: 10.1007/2789_2008_088. [DOI] [PubMed] [Google Scholar]
- 31.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007:cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 33.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18:534–543. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura H, et al. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol. 2005;174:7592–7599. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]
- 37.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]
- 39.Lukashev D, et al. Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol. 2006;177:4962–4965. doi: 10.4049/jimmunol.177.8.4962. [DOI] [PubMed] [Google Scholar]
- 40.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Q, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 43.Yin Y, Li G. Hypoxia induces T Helper 17 cell upregulation in cultured peripheral blood mononuclear cells from chronic stage patients of severe cerebral infarction. Microbiol Immunol. 2011;55:130–134. doi: 10.1111/j.1348-0421.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- 44.Ikejiri A, et al. Dynamic regulation of Th17 differentiation by oxygen concentrations. Int Immunol. 2012;24:137–146. doi: 10.1093/intimm/dxr111. [DOI] [PubMed] [Google Scholar]
- 45.Yan M, et al. Recruitment of regulatory T cells is correlated with hypoxia-induced CXCR4 expression, and is associated with poor prognosis in basal-like breast cancers. Breast Cancer Res. 2011;13:R47. doi: 10.1186/bcr2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Facciabene A, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Lee JH, Kim CH. Optimal population of FoxP3+ T cells in tumors requires an antigen priming-dependent trafficking receptor switch. PLoS One. 2012;7:e30793. doi: 10.1371/journal.pone.0030793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo W, Zhong J, Chang R, Hu H, Pandey A, Semenza GL. Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1alpha but Not HIF-2alpha. J Biol Chem. 2010;285:3651–3663. doi: 10.1074/jbc.M109.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uchida T, et al. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression in lung epithelial cells: implication of natural antisense HIF-1alpha. J Biol Chem. 2004;279:14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- 50.Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kannan KB, et al. Hypoxia-inducible factor plays a gut-injurious role in intestinal ischemia reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2011;300:G853–861. doi: 10.1152/ajpgi.00459.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feinman R, et al. HIF-1 mediates pathogenic inflammatory responses to intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2010;299:G833–843. doi: 10.1152/ajpgi.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kryczek I, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105:19579–19586. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee K, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc Natl Acad Sci USA. 2009;106:17910–17915. doi: 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Beppu K, Nakamura K, Linehan WM, Rapisarda A, Thiele CJ. Topotecan blocks hypoxia-inducible factor-1alpha and vascular endothelial growth factor expression induced by insulin-like growth factor-I in neuroblastoma cells. Cancer Res. 2005;65:4775–4781. doi: 10.1158/0008-5472.CAN-04-3332. [DOI] [PubMed] [Google Scholar]
- 58.Rapisarda A, et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62:4316–4324. [PubMed] [Google Scholar]
- 59.Kong D, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65:9047–9055. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- 60.Kryczek I, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 61.Huh JR, et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujita-Sato S, et al. Structural basis of digoxin that antagonizes RORgamma t receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J Biol Chem. 2011;286:31409–31417. doi: 10.1074/jbc.M111.254003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Solt LA, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J Biol Chem. 2011;286:22707–22710. doi: 10.1074/jbc.C111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia Y, Choi HK, Lee K. Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem. 2012;49:24–40. doi: 10.1016/j.ejmech.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 66.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pierdomenico M, et al. New insights into the pathogenesis of inflammatory bowel disease: transcription factors analysis in bioptic tissues from pediatric patients. J Pediatr Gastroenterol Nutr. 2011;52:271–279. doi: 10.1097/MPG.0b013e3182034d08. [DOI] [PubMed] [Google Scholar]
- 68.Shah YM, et al. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. Gastroenterology. 2008;134:2036–2048. doi: 10.1053/j.gastro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karhausen J, Furuta GT, Tomaszewski JE, Johnson RS, Colgan SP, Haase VH. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirota SA, Beck PL, MacDonald JA. Targeting hypoxia-inducible factor-1 (HIF-1) signaling in therapeutics: implications for the treatment of inflammatory bowel disease. Recent Pat Inflamm Allergy Drug Discov. 2009;3:1–16. doi: 10.2174/187221309787158434. [DOI] [PubMed] [Google Scholar]
- 73.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70:10112–10120. doi: 10.1158/0008-5472.CAN-10-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim JS, Smith-Garvin JE, Koretzky GA, Jordan MS. The requirements for natural Th17 cell development are distinct from those of conventional Th17 cells. J Exp Med. 2011;208:2201–2207. doi: 10.1084/jem.20110680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 77.Yang S, et al. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. 2011;89:85–91. doi: 10.1189/jlb.0910506. [DOI] [PubMed] [Google Scholar]
- 78.Huang C, Fu ZX. Localization of IL-17+Foxp3+ T cells in esophageal cancer. Immunol Invest. 2011;40:400–412. doi: 10.3109/08820139.2011.555489. [DOI] [PubMed] [Google Scholar]
- 79.Michalek RD, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shriver LP, Manchester M. Inhibition of fatty acid metabolism ameliorates disease activity in an animal model of multiple sclerosis. Sci Rep. 2011;1:79. doi: 10.1038/srep00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee WH, Kim SG. AMPK-dependent metabolic regulation by PPAR agonists. PPAR Res. 2010;2010:549101. doi: 10.1155/2010/549101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rinaldo P, Matern D, Bennett MJ. Fatty acid oxidation disorders. Annu Rev Physiol. 2002;64:477–502. doi: 10.1146/annurev.physiol.64.082201.154705. [DOI] [PubMed] [Google Scholar]
- 83.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase--development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.MacIver NJ, et al. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J Immunol. 2011;187:4187–4198. doi: 10.4049/jimmunol.1100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carretero J, et al. Dysfunctional AMPK activity, signalling through mTOR and survival in response to energetic stress in LKB1-deficient lung cancer. Oncogene. 2007;26:1616–1625. doi: 10.1038/sj.onc.1209951. [DOI] [PubMed] [Google Scholar]
- 86.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nath N, et al. Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity. Biochem Biophys Res Commun. 2009;386:16–20. doi: 10.1016/j.bbrc.2009.05.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lizcano JM, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bai A, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80:1708–1717. doi: 10.1016/j.bcp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 90.Bai A, et al. Novel anti-inflammatory action of 5-aminoimidazole-4-carboxamide ribonucleoside with protective effect in dextran sulfate sodium-induced acute and chronic colitis. J Pharmacol Exp Ther. 2010;333:717–725. doi: 10.1124/jpet.109.164954. [DOI] [PubMed] [Google Scholar]
- 91.Nath N, Giri S, Prasad R, Salem ML, Singh AK, Singh I. 5-aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J Immunol. 2005;175:566–574. doi: 10.4049/jimmunol.175.1.566. [DOI] [PubMed] [Google Scholar]
- 92.Yang Y, Lovett-Racke AE, Racke MK. Regulation of Immune Responses and Autoimmune Encephalomyelitis by PPARs. PPAR Res. 2010;2010:104705. doi: 10.1155/2010/104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pascual G, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–367. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- 95.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 96.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 97.Yang XY, et al. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor gamma (PPARgamma) agonists. PPARgamma co-association with transcription factor NFAT. J Biol Chem. 2000;275:4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 98.Raikwar HP, Muthian G, Rajasingh J, Johnson C, Bright JJ. PPARgamma antagonists exacerbate neural antigen-specific Th1 response and experimental allergic encephalomyelitis. J Neuroimmunol. 2005;167:99–107. doi: 10.1016/j.jneuroim.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 99.Klotz L, et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J Exp Med. 2009;206:2079–2089. doi: 10.1084/jem.20082771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang LH, et al. Transcriptional inactivation of STAT3 by PPARgamma suppresses IL-6-responsive multiple myeloma cells. Immunity. 2004;20:205–218. doi: 10.1016/s1074-7613(04)00030-5. [DOI] [PubMed] [Google Scholar]
- 101.Drew PD, Storer PD, Xu J, Chavis JA. Hormone regulation of microglial cell activation: relevance to multiple sclerosis. Brain Res Brain Res Rev. 2005;48:322–327. doi: 10.1016/j.brainresrev.2004.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Storer PD, Xu J, Chavis J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists inhibit the activation of microglia and astrocytes: implications for multiple sclerosis. J Neuroimmunol. 2005;161:113–122. doi: 10.1016/j.jneuroim.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 103.Kanakasabai S, Chearwae W, Walline CC, Iams W, Adams SM, Bright JJ. Peroxisome proliferator-activated receptor delta agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis. Immunology. 2010;130:572–588. doi: 10.1111/j.1365-2567.2010.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hontecillas R, Bassaganya-Riera J. Peroxisome proliferator-activated receptor gamma is required for regulatory CD4+ T cell-mediated protection against colitis. J Immunol. 2007;178:2940–2949. doi: 10.4049/jimmunol.178.5.2940. [DOI] [PubMed] [Google Scholar]
- 105.Housley WJ, et al. PPARgamma regulates retinoic acid-mediated DC induction of Tregs. J Leukoc Biol. 2009;86:293–301. doi: 10.1189/jlb.1208733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wohlfert EA, Nichols FC, Nevius E, Clark RB. Peroxisome proliferator-activated receptor gamma (PPARgamma) and immunoregulation: enhancement of regulatory T cells through PPARgamma-dependent and -independent mechanisms. J Immunol. 2007;178:4129–4135. doi: 10.4049/jimmunol.178.7.4129. [DOI] [PubMed] [Google Scholar]
- 107.Lei J, Hasegawa H, Matsumoto T, Yasukawa M. Peroxisome proliferator-activated receptor alpha and gamma agonists together with TGF-beta convert human CD4+CD25-T cells into functional Foxp3+ regulatory T cells. J Immunol. 2010;185:7186–7198. doi: 10.4049/jimmunol.1001437. [DOI] [PubMed] [Google Scholar]
- 108.Cipolletta D, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Housley WJ, et al. Peroxisome proliferator-activated receptor gamma is required for CD4+ T cell-mediated lymphopenia-associated autoimmunity. J Immunol. 2011;187:4161–4169. doi: 10.4049/jimmunol.1101731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Serrano-Marco L, et al. Activation of peroxisome proliferator-activated receptor-beta/-delta (PPAR-beta/-delta) ameliorates insulin signaling and reduces SOCS3 levels by inhibiting STAT3 in interleukin-6-stimulated adipocytes. Diabetes. 2011;60:1990–1999. doi: 10.2337/db10-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang MA, et al. Peroxisome proliferator-activated receptor (PPAR)alpha and - gamma regulate IFNgamma and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci USA. 2012;109:9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhatia V, Viswanathan P. Insulin resistance and PPAR insulin sensitizers. Curr Opin Investig Drugs. 2006;7:891–897. [PubMed] [Google Scholar]
- 113.Park SJ, et al. Peroxisome proliferator-activated receptor gamma agonist down-regulates IL-17 expression in a murine model of allergic airway inflammation. J Immunol. 2009;183:3259–3267. doi: 10.4049/jimmunol.0900231. [DOI] [PubMed] [Google Scholar]
- 114.Rhee KJ, et al. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009;77:1708–1718. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adachi M, et al. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55:1104–1113. doi: 10.1136/gut.2005.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewis JD, et al. Rosiglitazone for active ulcerative colitis: a randomized placebo-controlled trial. Gastroenterology. 2008;134:688–695. doi: 10.1053/j.gastro.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hertzel AV, Bernlohr DA. The mammalian fatty acid-binding protein multigene family: molecular and genetic insights into function. Trends Endocrinol Metab. 2000;11:175–180. doi: 10.1016/s1043-2760(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 118.Reynolds JM, et al. Deficiency of fatty acid-binding proteins in mice confers protection from development of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:313–321. doi: 10.4049/jimmunol.179.1.313. [DOI] [PubMed] [Google Scholar]
- 119.Li B, Reynolds JM, Stout RD, Bernlohr DA, Suttles J. Regulation of Th17 differentiation by epidermal fatty acid-binding protein. J Immunol. 2009;182:7625–7633. doi: 10.4049/jimmunol.0804192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tan NS, et al. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22:5114–5127. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Repa JJ, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hindinger C, et al. Liver X receptor activation decreases the severity of experimental autoimmune encephalomyelitis. J Neurosci Res. 2006;84:1225–1234. doi: 10.1002/jnr.21038. [DOI] [PubMed] [Google Scholar]
- 123.Xu J, Wagoner G, Douglas JC, Drew PD. Liver X receptor agonist regulation of Th17 lymphocyte function in autoimmunity. J Leukoc Biol. 2009;86:401–409. doi: 10.1189/jlb.1008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cui G, et al. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest. 2011;121:658–670. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bengoechea-Alonso MT, Ericsson J. SREBP in signal transduction: cholesterol metabolism and beyond. Curr Opin Cell Biol. 2007;19:215–222. doi: 10.1016/j.ceb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 126.Mirebeau-Prunier D, et al. Estrogen-related receptor alpha and PGC-1-related coactivator constitute a novel complex mediating the biogenesis of functional mitochondria. FEBS J. 2010;277:713–725. doi: 10.1111/j.1742-4658.2009.07516.x. [DOI] [PubMed] [Google Scholar]
- 127.Michalek RD, et al. Estrogen-related receptor-alpha is a metabolic regulator of effector T-cell activation and differentiation. Proc Natl Acad Sci USA. 2011;108:18348–18353. doi: 10.1073/pnas.1108856108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10:935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sancak Y, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cobbold SP, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sundrud MS, et al. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yan Y, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Terness P, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma MD, et al. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102–6111. doi: 10.1182/blood-2008-12-195354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baban B, et al. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–2483. doi: 10.4049/jimmunol.0900986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boasso A, Herbeuval JP, Hardy AW, Winkler C, Shearer GM. Regulation of indoleamine 2,3-dioxygenase and tryptophanyl-tRNA-synthetase by CTLA-4-Fc in human CD4+ T cells. Blood. 2005;105:1574–1581. doi: 10.1182/blood-2004-06-2089. [DOI] [PubMed] [Google Scholar]
- 137.Kropf P, et al. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol. 2007;37:935–945. doi: 10.1002/eji.200636542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baxter AG, Hodgkin PD. Activation rules: the two-signal theories of immune activation. Nat Rev Immunol. 2002;2:439–446. doi: 10.1038/nri823. [DOI] [PubMed] [Google Scholar]
- 139.Frauwirth KA, et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 140.Frauwirth KA, Thompson CB. Activation and inhibition of lymphocytes by costimulation. J Clin Invest. 2002;109:295–299. doi: 10.1172/JCI14941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jacobs SR, et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology. 2009;127:459–465. doi: 10.1111/j.1365-2567.2009.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kurebayashi Y, et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep. 2012;1:360–373. doi: 10.1016/j.celrep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 146.Patsoukis N, Brown J, Petkova V, Liu F, Li L, Boussiotis VA. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46. doi: 10.1126/scisignal.2002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.D’Addio F, et al. The link between the PDL1 costimulatory pathway and Th17 in fetomaternal tolerance. J Immunol. 2011;187:4530–4541. doi: 10.4049/jimmunol.1002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Okkenhaug K, Patton DT, Bilancio A, Garcon F, Rowan WC, Vanhaesebroeck B. The p110delta isoform of phosphoinositide 3-kinase controls clonal expansion and differentiation of Th cells. J Immunol. 2006;177:5122–5128. doi: 10.4049/jimmunol.177.8.5122. [DOI] [PubMed] [Google Scholar]
- 151.Patton DT, et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2006;177:6598–6602. doi: 10.4049/jimmunol.177.10.6598. [DOI] [PubMed] [Google Scholar]
- 152.Berod L, et al. PI3Kgamma deficiency delays the onset of experimental autoimmune encephalomyelitis and ameliorates its clinical outcome. Eur J Immunol. 2011;41:833–844. doi: 10.1002/eji.201040504. [DOI] [PubMed] [Google Scholar]
- 153.Rodrigues DH, Vilela MC, Barcelos LS, Pinho V, Teixeira MM, Teixeira AL. Absence of PI3Kgamma leads to increased leukocyte apoptosis and diminished severity of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;222:90–94. doi: 10.1016/j.jneuroim.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 154.Bergamini G, et al. A selective inhibitor reveals PI3Kgamma dependence of T(H)17 cell differentiation. Nat Chem Biol. 2012;8:576–582. doi: 10.1038/nchembio.957. [DOI] [PubMed] [Google Scholar]
- 155.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zeiser R, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Han JM, Patterson SJ, Levings MK. The Role of the PI3K Signaling Pathway in CD4(+) T Cell Differentiation and Function. Front Immunol. 2012;3:245. doi: 10.3389/fimmu.2012.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hagenbeek TJ, et al. The loss of PTEN allows TCR alphabeta lineage thymocytes to bypass IL-7 and Pre-TCR-mediated signaling. J Exp Med. 2004;200:883–894. doi: 10.1084/jem.20040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Patterson SJ, et al. Cutting edge: PHLPP regulates the development, function, and molecular signaling pathways of regulatory T cells. J Immunol. 2011;186:5533–5537. doi: 10.4049/jimmunol.1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Locke NR, Patterson SJ, Hamilton MJ, Sly LM, Krystal G, Levings MK. SHIP regulates the reciprocal development of T regulatory and Th17 cells. J Immunol. 2009;183:975–983. doi: 10.4049/jimmunol.0803749. [DOI] [PubMed] [Google Scholar]
- 162.Dutra RC, et al. Inhibitor of PI3Kgamma ameliorates TNBS-induced colitis in mice by affecting the functional activity of CD4+CD25+FoxP3+ regulatory T cells. Br J Pharmacol. 2011;163:358–374. doi: 10.1111/j.1476-5381.2011.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yagi R, et al. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity. 2010;32:507–517. doi: 10.1016/j.immuni.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Usui T, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 166.Mukasa R, et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–341. doi: 10.1016/s1534-5807(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 168.Narravula S, Colgan SP. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. J Immunol. 2001;166:7543–7548. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- 169.Fassett MS, Jiang W, D’Alise AM, Mathis D, Benoist C. Nuclear receptor Nr4a1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proc Natl Acad Sci USA. 2012;109:3891–3896. doi: 10.1073/pnas.1200090109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chao LC, et al. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes. 2009;58:2788–2796. doi: 10.2337/db09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Choi JW, Park SC, Kang GH, Liu JO, Youn HD. Nur77 activated by hypoxia-inducible factor-1alpha overproduces proopiomelanocortin in von Hippel-Lindau-mutated renal cell carcinoma. Cancer Res. 2004;64:35–39. doi: 10.1158/0008-5472.can-03-0145. [DOI] [PubMed] [Google Scholar]
- 172.Kim BY, Kim H, Cho EJ, Youn HD. Nur77 upregulates HIF-alpha by inhibiting pVHL-mediated degradation. Exp Mol Med. 2008;40:71–83. doi: 10.3858/emm.2008.40.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brugarolas J, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Horak P, et al. Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci USA. 2010;107:4675–4680. doi: 10.1073/pnas.0907705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. J Biol Chem. 2008;283:21934–21944. doi: 10.1074/jbc.M802481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Beurel E, Yeh WI, Michalek SM, Harrington LE, Jope RS. Glycogen synthase kinase-3 is an early determinant in the differentiation of pathogenic Th17 cells. J Immunol. 2011;186:1391–1398. doi: 10.4049/jimmunol.1003511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Flugel D, Gorlach A, Michiels C, Kietzmann T. Glycogen synthase kinase 3 phosphorylates hypoxia-inducible factor 1alpha and mediates its destabilization in a VHL-independent manner. Mol Cell Biol. 2007;27:3253–3265. doi: 10.1128/MCB.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hirota K, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhou X, Bailey-Bucktrout S, Jeker LT, Bluestone JA. Plasticity of CD4(+) FoxP3(+) T cells. Curr Opin Immunol. 2009;21:281–285. doi: 10.1016/j.coi.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wilke CM, et al. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Joshi S, et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31:3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bruce D, Yu S, Ooi JH, Cantorna MT. Converging pathways lead to overproduction of IL-17 in the absence of vitamin D signaling. Int Immunol. 2011;23:519–528. doi: 10.1093/intimm/dxr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Spanier JA, Nashold FE, Olson JK, Hayes CE. The ifng gene is essential for vdr gene expression and vitamin d3-mediated reduction of the pathogenic T cell burden in the central nervous system in experimental autoimmune encephalomyelitis, a multiple sclerosis model. J Immunol. 2012;189:3188–3197. doi: 10.4049/jimmunol.1102925. [DOI] [PubMed] [Google Scholar]
- 186.Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285:38751–38755. doi: 10.1074/jbc.C110.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu YV, et al. Calcineurin promotes hypoxia-inducible factor 1alpha expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J Biol Chem. 2007;282:37064–37073. doi: 10.1074/jbc.M705015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bacchetta R, Gambineri E, Roncarolo MG. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007;120:227–235. doi: 10.1016/j.jaci.2007.06.023. quiz 236-227. [DOI] [PubMed] [Google Scholar]
- 189.Li Y, et al. CNI induced Th17/Treg imbalance and susceptibility to renal dysfunction in renal transplantation. Int Immunopharmacol. 2011;11:2033–2038. doi: 10.1016/j.intimp.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 190.Shime H, et al. Tumor-secreted lactic acid promotes IL-23/IL-17 proinflammatory pathway. J Immunol. 2008;180:7175–7183. doi: 10.4049/jimmunol.180.11.7175. [DOI] [PubMed] [Google Scholar]
- 191.Zhi L, Ustyugova IV, Chen X, Zhang Q, Wu MX. Enhanced Th17 Differentiation and Aggravated Arthritis in IEX-1-Deficient Mice by Mitochondrial Reactive Oxygen Species-Mediated Signaling. J Immunol. 2012;189:1639–1647. doi: 10.4049/jimmunol.1200528. [DOI] [PMC free article] [PubMed] [Google Scholar]