Abstract
Successful treatment of pneumonic infection with Francisella tularensis, the causative agent of tularemia, requires rapid initiation of antibiotic therapy, yet even then treatment failures may occur. Consequently, new treatments are needed to enhance the effectiveness of antimicrobial therapy for acute pneumonic tularemia. In a prior study, immunization with F. tularensis membrane protein fraction (MPF) antigens 3 days prior to challenge was reported to induce significant protection from inhalational challenge. We therefore hypothesized that MPF immunization might also be effective in enhancing infection control if combined with antibiotic therapy and administered after infection as post-exposure immunotherapy. To address this question, a 24 h post-exposure treatment model of acute pulmonary Schu S4 strain of F. tularensis infection in BALB/c mice was used. Following exposure, mice were immunized with MPF and treated with low-dose gentamicin, alone or in combination and the effects on survival, bacterial burden and dissemination were assessed. We found that immunization with MPF significantly increased the effectiveness of subtherapeutic gentamicin therapy for post-exposure treatment of pneumonic tularemia, with 100% of combination-treated mice surviving long-term. Bacterial burdens in the liver and spleen were significantly reduced in combination MPF-gentamicin treated mice at 7 days after challenge. Passively transferred antibodies against MPF antigens also increased the effectiveness of gentamicin therapy. Thus, we concluded that post-exposure immunization with MPF antigens was an effective means of enhancing conventional antimicrobial therapy for pneumonic tularemia.
Keywords: Francisella tularensis, membrane proteins, antibodies
Introduction
Francisella tularensis (Ft), a Gram negative, intracellular bacterium, is the causative agent of tularemia. Ft has an extremely low infectious dose [1, 2], and can be transmitted to humans via several routes including inhalation which is associated with higher mortality in humans [3, 4]. The Ft subspecies of clinical significance are tularensis (type A), which is associated with the most severe form of tularemia and includes the Schu S4 strain and holarctica (type B), which is less virulent and includes the live vaccine strain (LVS). Ft infections are treatable with a wide variety of antibiotics, including aminoglycosides, such as gentamicin [5-9], provided they are administered shortly following exposure. Improper diagnosis of tularemia can delay antibiotic treatment and result in increased mortality and disease relapse [3, 10]. In some cases, antibiotics fail to completely clear the infection or have toxic side effects such as hearing loss or kidney damage [11-16]. Therefore, increased dose or prolonged administration is not ideal.
The combination of immunotherapy with antibiotic therapy has been demonstrated for other bacterial infections and could reduce or eliminate treatment failures. Using the mouse pathogen, Ft novicida, Pammit et al. administered intranasal interleukin-12 (IL-12) to mice in conjunction with gentamicin and observed a 50% increase in survival [17]. Likewise, gentamicin augmented by interferon gamma (IFN-γ) increased survival by 41% in Swiss mice infected with drug-resistant Enterococcus faecalis [18]. We reported that IFN-γ used in combination with ceftazidime exerted synergistic activity against Burkholderia pseudomallei [19]. Thus, immune augmentation of antibiotic therapy is a promising approach to developing therapeutics against bacterial infections.
Humoral immunity must be considered when developing an immunotherapy strategy for controlling Ft infections. Several studies show that anti-Ft live vaccine strain (LVS) antibodies provide protection against virulent murine tularemia [20-23] and adoptive transfer of Ft LVS immune serum fully protected mice when administered 24 h prior to a lethal, respiratory Ft LVS infection [22]. The administration of immune serum not only augmented bacterial clearance, but also limited dissemination [22]. The membrane and surface antigens of Ft are likely targets for an immunotherapeutic strategy encompassing adaptive immunity. Huntley et al. identified 15 outer membrane proteins of Ft LVS and Schu S4 that, when given to mice, protected against intranasal Ft Schu S4 challenge [24, 25] and induced both Th1 and Th2 antibody responses in mice [24]. Ireland et al. administered the membrane protein fraction (MPF) from Ft LVS along with cationic liposomal DNA complex (CLDC) adjuvant three days prior to a virulent Ft Schu S4 challenge in mice, and achieved 100% protection [26]. Thus, the rapid immune response generated against CLDC-MPF along with adaptive immunity to the membrane proteins led us to hypothesize that MPF of Ft LVS can be applied as a post-exposure combination therapeutic with a conventional antibiotic against tularemia.
In the present study, we established a subtherapeutic gentamicin treatment model of tularemia in mice and applied this model to demonstrate that post-exposure vaccination with MPF allowed for survival of animals infected with virulent Ft. We further demonstrated that anti-MPF antibodies elicited by post-exposure vaccination were protective when passively transferred to mice infected with Ft Schu S4.
Materials and Methods
Mice
Specific pathogen-free 8 week old female BALB/cJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were used between 8 and 12 weeks of age. All studies were conducted in ABSL-3 facilities and were approved by the Institutional Animal Care and Use Committee at Colorado State University. The pre-determined end points of infection were 20% weight loss, lack of spontaneous movement for more than 10 seconds, respiratory distress and lack of response to tactile stimulus.
Bacteria and culture conditions
Ft strains Schu S4 and LVS were grown as previously described [27]. Frozen stocks of Ft for murine infections were prepared by adding 15% glycerol to the 50 ml culture and dividing the sample into 1 ml aliquots. Aliquots were stored at −80°C and titered prior to use. Ft LVS used to generate MPF was cultured in Modified Mueller Hinton (MMH) and harvested in late-log phase.
Preparation of MPF, CLDC, and antibiotics
MPF was prepared as previously described [26]. Lyophilized CLDC was provided by Juvaris BioTherapeutics (Burlingame, CA) and reconstituted with 5% dextrose water (D5W) to a concentration of 205 μg/ml of non-coding DNA. Gentamicin (Sigma, St. Louis, MO) was diluted in Dulbecco's phosphate buffered saline (dPBS) for injections.
Intranasal challenge
Mice were anesthetized by intraperitoneal (i.p.) injection of 100 mg/kg ketamine plus 10 mg/kg zylazine diluted in sterile water. Mice were infected with 50 colony forming units (CFU) of Ft in a total volume of 20 μl of dPBS, administered in sequential droplets on alternating nares.
Bacterial Burden in Organs
Lungs, liver, and spleen were harvested and placed in 5 ml of sterile dPBS (pH 7.4). Organs were homogenized using a Seward Stomacher 80 Biomaster (Seward, Bohemia, NY). Homogenates were diluted 1:10 and 100 μl was inoculated on 9% chocolatized sheep blood agar (CHAB) plates and placed at 35°C for 48 h after which, CFUs were enumerated.
MPF, CLDC, and gentamicin treatment
MPF was administered to mice at a concentration of 10 μg in 200 μl of D5W i.p. on days 1 and 4 post-infection. CLDC (102.5 μg/ml) was administered i.p. in 200 μl of D5W on days 1 and 4 post-infection. For experiments where MPF was mixed with CLDC, the MPF and CLDC were diluted to a 2× concentration in D5W and mixed 1:1 resulting in a final concentration of 10 μg of MPF and 102.5 μg/ml of CLDC. Gentamicin was diluted to 40, 20, 10, or 5 mg/kg in 200 μl and injected i.p., daily, for 10 days, post-infection.
Passive Immunization
To obtain immune serum, naïve mice received two MPF treatments (10 μg in 200 μl D5W i.p.) four days apart. Seven days after the first MPF injection, the mice were anesthetized as described above and immune serum was collected via terminal cardiac puncture. IgM and IgG titers in the immune serum were quantified via ELISA prior to transfer. Passive immunization was performed in mice infected with Ft Schu S4 by administering immune serum (300 μl i.p.) at one and four days post-infection in lieu of MPF treatment. Mice were also given daily gentamicin (10 mg/kg) for 10 days post-infection and survival was monitored for 25 days.
Serum Isotyping
Antibody responses were assessed as previously described [28, 29]. Briefly, ELISA plates were coated overnight with 0.75 μg/ml of MPF, blocked in PBST containing 5% non-fat dry milk and incubated with immune sera serially diluted (1:2) in blocking buffer for 1 h at room temperature. All secondary antibodies (diluted 1:2000 in blocking buffer) were conjugated to horseradish peroxidase including rat anti-mouse IgM (BD Biosciences), goat anti-mouse IgG3 (Abcam, Cambridge, MA), goat anti-mouse IgG2a (Abcam), rat anti-mouse IgG1 (BD biosciences) and rat anti-mouse IgG2b (Abcam). 3,3′,5,5′-Tetramethylbenzidine (TMB) (Sigma) substrate was used to develop the plates. The reaction was stopped by the addition of 100 μl of 1N HCl. The absorbance at 405 nm was determined using a Thermo multiskan EX spectrophotometer.
PAGE and Western blot
MPF was separated by SDS-PAGE and electroblotted as previously described [27]. The membranes were incubated overnight at room temperature with either anti-Ft LPS monoclonal antibodies (1:250) (Immuno-Precise Antibodies Ltd., Victoria, BC, Canada), or MPF immune sera (1:400) from either day 7 uninfected mice or day 7 Ft Schu S4 infected mice that received gentamicin and MPF immunotherapy. Membranes were incubated with alkaline phosphatase conjugated anti-mouse IgG or IgM (1:5000 in TBS) (Sigma) for 1 h at room temperature and developed using SigmaFast BCIP/NBT alkaline phosphatase substrate tablets (Sigma).
Statistical Analyses
Statistical analyses were performed using GraphPad Prism5 software (La Jolla, CA). Comparisons between multiple groups were done using ANOVA, followed by Tukey's multiple means comparison test. A student's t test was done to compare individual groups and a single sample t test was done for samples with a variance of zero. Survival differences were compared using Kaplan-Meier survival curves, followed by log rank test. Statistical significance was defined as p < 0.05.
Results
Suboptimal gentamicin treatment decreases mean survival time during a Schu S4 infection
A suboptimal gentamicin dose and administration time was established to allow for evaluation of immune augmentation of chemotherapy with an MPF-CLDC post-exposure vaccine. Four gentamicin doses (40, 20, 10, and 5 mg/kg) were administered to mice at 6, 12, 24, or 48 h post-infection, and given daily for 10 days. In agreement with previous studies, 40 mg/kg of gentamicin was completely protective regardless of administration time and 5 mg/kg did not significantly extend survival at any of the time points administered (not shown) [5]. The 20 mg/kg dose showed complete protection when administered at 12 and 24 h post-infection, but showed decreased protection when administered at 48 h post-infection (not shown). Gentamicin at 10 mg/kg failed to completely protect regardless of the time administered (Fig. 1). Animals receiving 10 mg/kg of gentamicin, administered 24 h post-infection, had an extended mean survival time of several days but all animals eventually reached the pre-determined endpoints for euthanasia. The 10 mg/kg dose of gentamicin given 24 h post-infection was selected for the suboptimal gentamicin treatment model.
Figure 1.
Gentamicin (10 mg/kg) given 24 h post-infection is a suboptimal antibiotic dose and administration time. Following i.n. challenge with 50 CFU of Ft Schu S4, BALB/c mice (n=5) were given 10 mg/kg of gentamicin (i.p.) as a time-delayed treatment, starting either 6, 12, 24, or 48 h post-infection. Gentamicin was continued daily for 10 days and survival was monitored for 25 days. Untreated controls received PBS alone. Data are representative of two independent experiments.
Protection against Ft Schu S4 is MPF mediated
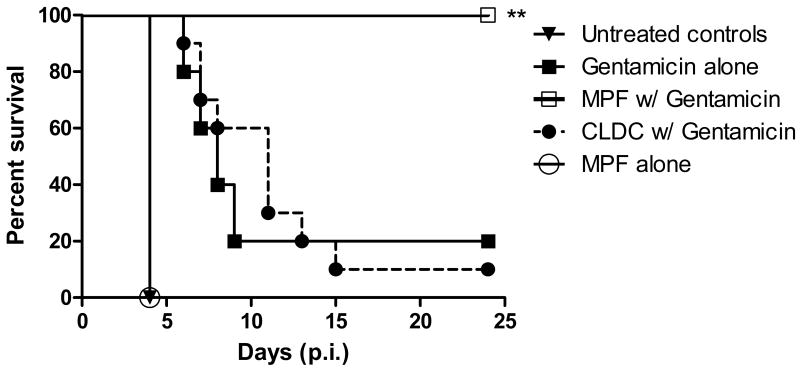
Augmentation of suboptimal gentamicin treatment was evaluated by administering MPF (10 μg), CLDC (100 μg/ml), or a combination of MPF (10 μg) and CLDC (100 μg/ml) at days 1 and 4 post-infection. MPF or MPF-CLDC (not shown) given along with 10 days of suboptimal gentamicin therapy, provided 100% protection (Fig. 2). In contrast, CLDC with gentamicin provided no measurable protective effect compared to the gentamicin alone control groups. Post-exposure vaccination with MPF or MPF-CLDC (not shown) did not provide protection in the absence of gentamicin. The average weight of animals (in grams) per cage correlated with protection elicited by the treatment regimens (Supplemental Fig. 1). Animals receiving MPF along with gentamicin treatment reached their minimum weight between days 7 and 9 post-infection and quickly recovered with a rapid weight gain. Untreated controls, CLDC-gentamicin, and gentamicin alone groups did not show an increase in weight prior to reaching pre-determined endpoints except in the circumstance where an outlier survived treatment long term.
Figure 2.
MPF augments a suboptimal gentamicin regime. Mice (n=10) were infected with 50 CFU of Ft Schu S4 (i.n.) and gentamicin (10 mg/kg) was given starting 24 h post-infection. MPF (10 μg) or CLDC (100 μg/ml) was given i.p. on days 1 and 4 post-infection along with the gentamicin treatment. Untreated controls were given D5W i.p. in lieu of immunotherapy. Survival was monitored for 25 days. Data are representative of three independent experiments. Statistical significance was determined using a Mantel Cox log rank test. **p < 0.001.
MPF treatment reduces bacterial burden
We also investigated whether combination therapy reduced bacterial burden and dissemination to target organs. Lungs, liver, and spleen from all treatment groups were harvested at 1, 4, 7, 10, 20, and 40 days post-infection and bacterial burden assessed. Four days post-infection, all treatment groups showed a significant decrease in bacteria in the liver, spleen, and lungs when compared to untreated controls (p < 0.001) (Fig. 3a). All untreated controls reached predetermined endpoints at day 4 following challenge. Therefore, subsequent time points were compared to animals receiving only gentamicin.
Figure 3.
MPF immunotherapy significantly reduces the dissemination and accumulation of Ft Schu S4. Mice were challenged i.n. with 50 CFU of Ft Schu S4 and euthanized at days 4, 7, 10, and 20 post-infection. Lungs, liver, and spleen were harvested and processed to determine bacterial burden. Bacterial burden was assessed on day 4 (A), day 7 (B), day 10 (C), and day 20 post-infection (D). At day 20, only MPF treated groups remained. The total number of animals present in each group is represented to the right of each treatment group. Data are representative of two independent experiments. A one-way Anova with Tukey's multiple comparison test was done to determine significance between treatment groups. Bars represent groups mean ±SD. **p < 0.001. *p < 0.05.
At day 7, MPF treated groups showed a significant (p<0.05) reduction in bacterial burden in the liver and spleen but not in the lungs (Fig. 3b). By day 10, the liver showed almost complete bacterial clearance in MPF treated groups, while the lungs and spleen showed variable reduction in bacteria compared to gentamicin treated groups (Fig. 3c). At day 20 (Fig. 3d) and day 40 post-infection (not shown) all groups not receiving MPF treatments had reached endpoints. At day 20, all surviving animals (n = 20 total) had low levels of bacteria still present in at least one organ. By day 40 post-infection, most animals had completely cleared the infection. CLDC with MPF was not found to significantly reduce bacterial burden compared to MPF alone. Thus, the addition of CLDC did not increase protection or reduce bacterial burden compared to groups receiving only MPF. Subsequent experiments examined the effects of MPF in the absence of CLDC.
MPF protection is antibody mediated
Previous studies suggested that the protective effects of Ft membrane antigens were dependent on humoral immunity [20, 22, 30]. Thus, we hypothesized that the protective effects of MPF in our model were mediated by antibodies. To test this hypothesis, MPF immune serum was generated as described in materials and methods and transferred to mice infected with Ft Schu S4 and treated with the subtherapeutic gentamicin regime. We observed that complete resolution of a Ft Schu S4 infection occurred with transfer of MPF immune serum to mice that were receiving gentamicin (Fig. 4). Control mice given serum from non-vaccinated mice along with gentamicin treatment showed no significant difference in survival compared to mice treated with gentamicin alone (Fig. 4 and data not shown). These data are consistent with the hypothesis that the protective effects of MPF immunization were mediated primarily by anti-MPF antibodies.
Figure 4.
Anti-MPF antibodies are protective against a Ft Schu S4 infection. Anti-MPF immune sera for passive transfer was generated by giving naïve mice two MPF boosts (10 μg, i.p.), 3 days apart, and collecting sera 7 days after the first injection. Ft Schu S4 infected mice (n=5, 50 CFU i.n.) were given anti-MPF immune sera (300 μl) i.p. either alone, or in addition to daily gentamicin but in lieu of MPF treatment at days 1 and 4 post-infection. Controls groups received naïve sera in addition to the gentamicin treatment. Data are representative of two independent experiments. Statistical significance was determined using a Mantel Cox log rank test. **p < 0.001.
The titers of anti-MPF immune sera generated in BALB/cJ mice by two MPF immunizations alone (Group 1 mice) were compared to anti-MPF titers generated in mice that were infected with Ft Schu S4 and then immunized with MPF and treated with gentamicin (Group 2 mice). In both groups of animals, there were high titers of IgM, IgG3 and IgG2a antibodies (Fig. 5). The IgM titer against MPF was 1:3,200 in both Groups 1 and 2 at day 7 after immunization. The IgG3 titer to MPF was 1:800 and 1:400 in Group 1 and Group 2 mice, respectively, while the IgG2a titers were 1:400 and 1:200 in Group 1 and Group 2 mice. The only significant difference in titers was found with IgG1, which was significantly higher in Group 1 mice (1:1,600) versus Group 2 mice (1:50). The IgG2b titers were at or below the level of detection in our assay for both groups of mice (data not shown). Western blot analysis revealed that the IgM response in both groups of mice was primarily directed against the LPS component of MPF, while the IgG response appeared to be directed to a limited number of MPF membrane proteins (Fig. 6). This suggests that the MPF proteins are quite immunogenic even when administered without an adjuvant.
Figure 5.
MPF immune serum from uninfected mice has similar immunoglobulin titers to serum from infected mice. Immune serum was generated by giving naïve mice two MPF boosts (10 μg, i.p.), 3 days apart, and collecting sera 7 days after the first injection. The IgM, IgG3, and IgG2a titers were quantified via ELISA and compared to the titers of sera collected 7 days post-infection from mice with Ft Schu S4 who received gentamicin and MPF treatment. Data are representative of three independent experiments. A student's t test was done to determine statistical significance between groups. Bars represent groups mean ±SD. **p < 0.001.
Figure 6.
Anti-MPF antibodies are directed to LPS and membrane proteins. Western blots of MPF (5 μg) with anti-serum from naïve mice receiving 2 MPF injections or from mice infected with Ft Schu S4, 7 days post-infection that received daily gentamicin and 2 MPF injections. Lanes 2-4 show anti-mouse IgG reactivity and lanes 5-7 show anti-mouse IgM reactivity. The 5 most dominant reactive bands in lanes 3 and 4 (*) have apparent masses of 46, 20, 18, 15, and 11 kD respectively. Lane 8 is anti-Ft LVS LPS reactivity. Lanes 2 and 5 contain naïve sera as a control. Lanes 3 and 6 are immune sera from uninfected donor mice. Lanes 4 and 7 are from mice infected with Ft Schu S4 at day 7 post-infection. Lane 1 is a molecular weight marker. Antiserum was pooled from 5 mice for each group.
Discussion
Antibiotic treatment of tularemia is generally successful when administered soon after infection [5-9, 31]. However, patient responses vary and disease relapse is known to occur [3, 12, 13]. Therefore, we used a combination treatment model to evaluate the benefit of adding antigen specific vaccination to antibiotic therapy as a means to improve the control of acute tularemia. Using this model we demonstrated that post-exposure vaccination with MPF from Ft LVS augmented the effectiveness of chemotherapy and generated 100% survival in an otherwise rapidly lethal pulmonary infection. Our studies are unique in that they demonstrate for the first time that a post-exposure vaccination approach can augment the effectiveness of antimicrobial therapy against a rapidly lethal strain of Ft.
In the present studies MPF immunization alone did not allow animals to recover from infection, but did show synergistic activity when combined with gentamicin. Gentamicin has high bactericidal activity against both intracellular and extracellular bacteria, but slowly accumulates in eukaryotic cells and only becomes detectable in intracellular vacuoles after 48 h [32]. Thus in our model, gentamicin given daily starting 24 h post-infection would not be expected to start accumulating in phagocytic vesicles until several days after initiation of treatment. However, the gentamicin could have suppressed replication of extracellular bacteria and thereby slowed dissemination from the lungs to distal organs. This possibility was supported by our bacterial burden data that showed a significant reduction in bacterial loads at day 4 post-infection even with the gentamicin alone group. Interestingly, at the day 4 time point the post-exposure vaccination did not have an impact on bacterial load as compared to the gentamicin alone. At days 7 and 10, however, several of the animals in the gentamicin alone group had succumbed to the infection and those that were still alive had consistently higher bacterial loads, in the liver and spleen as compared to animals receiving combination therapy. The day 10 time point also correlated with the stage at which animals receiving the combination therapy began to gain weight. Thus, MPF vaccination plus gentamicin enhanced the reduction of the bacterial burdens in the liver and spleen between days 4 and 10. This suggested that at later time points, specific immunity to MPF antigens suppressed bacterial dissemination and reduced the bacterial burden.
The passive transfer of anti-MPF immune serum to mice infected with Ft Schu S4 restored complete protection when given with gentamicin therapy. This sera contained high IgM and IgG titers to MPF, of similar magnitude to those generated during the course of active infection. These data are consistent with the work of Huntley et al. that showed robust Th1 and Th2 responses to outer membrane proteins from Ft [24]. Likewise, others demonstrated the important protective effects of humoral immune responses to Ft infections via passive transfer experiments (3, 6, 16). Recently, Mara-Koosham et al. demonstrated that sera or purified IgG from LVS vaccinated rats reduced the severity and duration of disease when transferred to rats infected with Ft Schu S4 [21]. The data show reactive antibodies to Ft LPS and a limited number of proteins. MPF contains over 250 proteins [33] as well as phospholipids and LPS (unpublished data)[34]. Thus, the antibody response required for the protection observed was focused to select antigens.
A possible mechanism that would explain our current result is that antibody opsonization of Ft could provide for more efficient phagocytic uptake and clearance of the organism. Specifically, immune antibodies and the Fcγ receptor are known to influence the phagocytosis of Francisella [35] and the work of Geier et al. revealed increased uptake of Ft Schu S4 by macrophages following opsonization with anti-LPS IgG antibody [36]. An alternative mechanism is complement mediated lysis of Ft, however, virulent Ft is noted to be resistant to this effect [37]. Thus, the activation of complement and direct bacterial lysis is not likely to contribute to the sustained protection and bacterial clearance induced by post-exposure vaccination with MPF. Instead, enhanced uptake and clearance by activated neutrophils or macrophages is a more likely explanation for the MPF vaccination effect. While additional studies will be required to more fully explore the protective mechanisms of MPF post-exposure immunization, our findings suggest a new approach to improving antibiotic therapy of acute tularemia and the importance of antibodies for this protection.
Supplementary Material
Highlights.
These studies provide direct evidence for immunotherapy to treat tularemia.
Post-exposure vaccination augments suboptimal antibiotic therapy of tularemia.
Post-exposure vaccination reduces the load of F. tularensis in distal organs.
Passive transfer of anti-F. tularensis antibodies augments treatment of tularemia.
Acknowledgments
We would like to thank Dr. Jeannine Petersen from the Centers of Disease Control and Prevention for providing F. tularensis Schu S4 and LVS stocks. We would also like to thank Juvaris BioTherapeutics, Inc. for providing CLDC, as well as thank Angie Henderson for flow cytometry support. Funding was provided by the Rocky Mountain Regional Centers for Excellence grant number U54AIO65357-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saslaw S, Eigelsbach HT, Wilson HE, Prior JA, Carhart S. Tularemia vaccine study. I. Intracutaneous challenge. Arch Intern Med. 1961;107:689–701. doi: 10.1001/archinte.1961.03620050055006. [DOI] [PubMed] [Google Scholar]
- 2.Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. Tularemia vaccine study. II. Respiratory challenge. Arch Intern Med. 1961;107:702–14. doi: 10.1001/archinte.1961.03620050068007. [DOI] [PubMed] [Google Scholar]
- 3.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, et al. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285(21):2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 4.Petersen JM, Schriefer ME. Tularemia: emergence/re-emergence. Vet Res. 2005;36(3):455–67. doi: 10.1051/vetres:2005006. [DOI] [PubMed] [Google Scholar]
- 5.Mason WL, Eigelsbach HT, Little SF, Bates JH. Treatment of tularemia, including pulmonary tularemia, with gentamicin. Am Rev Respir Dis. 1980;121(1):39–45. doi: 10.1164/arrd.1980.121.1.39. [DOI] [PubMed] [Google Scholar]
- 6.Piercy T, Steward J, Lever MS, Brooks TJ. In vivo efficacy of fluoroquinolones againstsystemic tularaemia infection in mice. J Antimicrob Chemother. 2005;56(6):1069–73. doi: 10.1093/jac/dki359. [DOI] [PubMed] [Google Scholar]
- 7.Steward J, Piercy T, Lever MS, Simpson AJ, Brooks TJ. Treatment of murine pneumonic Francisella tularensis infection with gatifloxacin, moxifloxacin or ciprofloxacin. Int J Antimicrob Agents. 2006;27(5):439–43. doi: 10.1016/j.ijantimicag.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Russell P, Eley SM, Fulop MJ, Bell DL, Titball RW. The efficacy of ciprofloxacin and doxycycline against experimental tularaemia. J Antimicrob Chemother. 1998;41(4):461–5. doi: 10.1093/jac/41.4.461. [DOI] [PubMed] [Google Scholar]
- 9.Scheel O, Reiersen R, Hoel T. Treatment of tularemia with ciprofloxacin. Eur J Clin Microbiol Infect Dis. 1992;11(5):447–8. doi: 10.1007/BF01961860. [DOI] [PubMed] [Google Scholar]
- 10.Harrell RE, Jr, Whitaker GR. Tularemia: emergency department presentation of an infrequently recognized disease. Am J Emerg Med. 1985;3(5):415–8. doi: 10.1016/0735-6757(85)90201-3. [DOI] [PubMed] [Google Scholar]
- 11.Risi GF, Pombo DJ. Relapse of tularemia after aminoglycoside therapy: case report and discussion of therapeutic options. Clin Infect Dis. 1995;20(1):174–5. doi: 10.1093/clinids/20.1.174. [DOI] [PubMed] [Google Scholar]
- 12.Miller SD, Snyder MB, Kleerekoper M, Grossman CH. Ulceroglandular tularemia: a typical case of relapse. Henry Ford Hosp Med J. 1989;37(2):73–5. [PubMed] [Google Scholar]
- 13.Penn RL, Kinasewitz GT. Factors associated with a poor outcome in tularemia. Arch Intern Med. 1987;147(2):265–8. [PubMed] [Google Scholar]
- 14.Kaya A, Uysal IO, Guven AS, Engin A, Gulturk A, Icagasioglu FD, et al. Treatment failure of gentamicin in pediatric patients with oropharyngeal tularemia. Med Sci Monit. 2011;17(7):CR376–80. doi: 10.12659/MSM.881848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurin M, Mersali NF, Raoult D. Bactericidal activities of antibiotics against intracellular Francisella tularensis. Antimicrob Agents Chemother. 2000;44(12):3428–31. doi: 10.1128/aac.44.12.3428-3431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacifici GM. Clinical pharmacokinetics of aminoglycosides in the neonate: a review. Eur J Clin Pharmacol. 2009;65(4):419–27. doi: 10.1007/s00228-008-0599-y. [DOI] [PubMed] [Google Scholar]
- 17.Pammit MA, Budhavarapu VN, Raulie EK, Klose KE, Teale JM, Arulanandam BP. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob Agents Chemother. 2004;48(12):4513–9. doi: 10.1128/AAC.48.12.4513-4519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onyeji CO, Bui KQ, Nicolau DP, Nightingale CH, Bow L, Quintiliani R. Influence of adjunctive interferon-gamma on treatment of gentamicin- and vancomycin-resistant Enterococcus faecalis infection in mice. Int J Antimicrob Agents. 1999;12(4):301–9. doi: 10.1016/s0924-8579(99)00055-2. [DOI] [PubMed] [Google Scholar]
- 19.Propst KL, Troyer RM, Kellihan LM, Schweizer HP, Dow SW. Immunotherapy markedly increases the effectiveness of antimicrobial therapy for treatment of Burkholderia pseudomallei infection. Antimicrob Agents Chemother. 2010;54(5):1785–92. doi: 10.1128/AAC.01513-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drabick JJ, Narayanan RB, Williams JC, Leduc JW, Nacy CA. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am J Med Sci. 1994;308(2):83–7. doi: 10.1097/00000441-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Mara-Koosham G, Hutt JA, Lyons CR, Wu TH. Antibodies Contribute to Effective Vaccination against Respiratory Infection by Type A Francisella tularensis Strains. Infect Immun. 2011;79(4):1770–8. doi: 10.1128/IAI.00605-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179(1):532–9. doi: 10.4049/jimmunol.179.1.532. [DOI] [PubMed] [Google Scholar]
- 23.Fulop M, Manchee R, Titball R. Role of lipopolysaccharide and a major outer membrane protein from Francisella tularensis in the induction of immunity against tularemia. Vaccine. 1995;13(13):1220–5. doi: 10.1016/0264-410x(95)00062-6. [DOI] [PubMed] [Google Scholar]
- 24.Huntley JF, Conley PG, Rasko DA, Hagman KE, Apicella MA, Norgard MV. Native outer membrane proteins protect mice against pulmonary challenge with virulent type A Francisella tularensis. Infect Immun. 2008;76(8):3664–71. doi: 10.1128/IAI.00374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huntley JF, Conley PG, Hagman KE, Norgard MV. Characterization of Francisella tularensis outer membrane proteins. J Bacteriol. 2007;189(2):561–74. doi: 10.1128/JB.01505-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ireland R, Olivares-Zavaleta N, Warawa JM, Gherardini FC, Jarrett C, Hinnebusch BJ, et al. Effective, broad spectrum control of virulent bacterial infections using cationic DNA liposome complexes combined with bacterial antigens. PLoS Pathog. 2010;6(5):e1000921. doi: 10.1371/journal.ppat.1000921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandler JC, Molins CR, Petersen JM, Belisle JT. Differential chitinase activity and production within Francisella species, subspecies, and subpopulations. J Bacteriol. 2011;193(13):3265–75. doi: 10.1128/JB.00093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson A, Propst K, Kedl R, Dow S. Mucosal immunization with liposome-nucleic acid adjuvants generates effective humoral and cellular immunity. Vaccine. 2011;29(32):5304–12. doi: 10.1016/j.vaccine.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for Immunoassays. J Immunol Methods. 1998;221(1-2):35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 30.Fulop M, Mastroeni P, Green M, Titball RW. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine. 2001;19(31):4465–72. doi: 10.1016/s0264-410x(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 31.Parker RT, Lister LM, Bauer RE, Hall HE, Woodward TE. Use of chloramphenicol (chloromycetin) in experimental and human tularemia. J Am Med Assoc. 1950;143(1):7–11. doi: 10.1001/jama.1950.02910360009004. [DOI] [PubMed] [Google Scholar]
- 32.Tulkens P, Trouet A. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem Pharmacol. 1978;27(4):415–24. doi: 10.1016/0006-2952(78)90370-2. [DOI] [PubMed] [Google Scholar]
- 33.Chandler JC. The Surface Proteome of Francisella tularensis. Colorado State University; 2011. [Google Scholar]
- 34.Hood AM. Virulence factors of Francisella tularensis. J Hyg (Lond) 1977;79(1):47–60. doi: 10.1017/s0022172400052840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balagopal A, MacFarlane AS, Mohapatra N, Soni S, Gunn JS, Schlesinger LS. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74(9):5114–25. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geier H, Celli J. Phagocytic receptors dictate phagosomal escape and intracellular proliferation of Francisella tularensis. Infect Immun. 2011;79(6):2204–14. doi: 10.1128/IAI.01382-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clay CD, Soni S, Gunn JS, Schlesinger LS. Evasion of complement-mediated lysis and complement C3 deposition are regulated by Francisella tularensis lipopolysaccharide O antigen. J Immunol. 2008;181(8):5568–78. doi: 10.4049/jimmunol.181.8.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.