Abstract
Objectives
We explored the association of noncoronary cardiac abnormalities with coronary artery dilation and with laboratory inflammatory markers early after Kawasaki disease (KD) diagnosis.
Background
Left ventricular (LV) dysfunction, mitral regurgitation (MR), and aortic root dilation occur early after diagnosis; their associations with coronary artery dilation and inflammatory markers have not been well-described.
Methods
Centrally interpreted echocardiograms were obtained at KD diagnosis and 1 and 5 weeks after diagnosis on 198 subjects in the National Institutes of Health-sponsored Pediatric Heart Network KD pulsed steroid trial. Regression models were constructed to investigate the relationships among early LV dysfunction, MR, and aortic root dilation with coronary artery dilation and laboratory inflammatory markers.
Results
At diagnosis, LV systolic dysfunction was present in 20% of subjects and was associated with coronary artery dilation, seen in 29% (p = 0.004). Although LV dysfunction improved rapidly, LV dysfunction at diagnosis predicted greater odds of coronary artery dilation at 1 and 5 weeks after diagnosis (5-week odds ratio: 2.7, 95% confidence interval: 1.2 to 6.3). At diagnosis, MR was present in 27% of subjects and aortic root dilation was present in 8%; each was associated with larger coronary artery size at diagnosis. Left ventricular dysfunction was associated with higher erythrocyte sedimentation rate and, at diagnosis only, lower serum albumin; MR was associated with higher erythrocyte sedimentation rate and lower albumin at all times. Aortic root size had little association with inflammatory markers.
Conclusions
Noncoronary cardiac abnormalities are associated with coronary artery dilation and laboratory evidence of inflammation in the first 5 weeks after KD, suggesting a shared inflammatory mechanism. (Trial of Pulse Steroid Therapy in Kawasaki Disease [A Trial Conducted by the Pediatric Heart Network]; NCT00132080)
Keywords: coronary artery dilation, Kawasaki disease, ventricular dysfunction
Kawasaki disease (KD) is an acute, self-limited febrile illness of unknown etiology. Although coronary artery sequelae are responsible for the major morbidity and mortality of KD, acute KD is often accompanied by noncoronary cardiac abnormalities, including left ventricular (LV) dysfunction, valvar regurgitation, pericardial effusion, and aortic root dilation (1-7). Both the vasculitis that leads to coronary artery sequelae and these noncoronary cardiac abnormalities might be consequences of an initial immune-mediated response (1,8-11). Although prior studies have confirmed a relationship between inflammatory changes in acute KD and the risk of coronary artery dilation (12-18), no consistent relation has been found between LV dysfunction, valvar regurgitation, and aortic root dilation and coronary artery dilation or systemic inflammatory markers (4-7,9,19-21). The existence of such a relation might have mechanistic implications for understanding KD and its consequences.
We sought to analyze the relationship between echocardiographic evidence of noncoronary cardiac abnormalities with coronary artery dilation and echocardiographic evidence of noncoronary cardiac abnormalities with markers of systemic inflammation during the first 5 weeks after KD diagnosis. We hypothesized that: 1) noncoronary cardiac abnormalities and coronary artery abnormalities are correlated early after KD diagnosis; and 2) LV systolic dysfunction, mitral regurgitation (MR), and aortic root dilation in acute KD are associated with laboratory evidence of systemic inflammation.
Methods
The National Heart, Lung, and Blood Institute-sponsored Pediatric Heart Network multicenter, prospective, randomized trial of pulsed steroid therapy in primary treatment of KD produced a well-characterized database of clinical data and centrally interpreted echocardiographic findings at the time of KD diagnosis (baseline) and at 1 and 5 weeks after diagnosis (22). We used the KD trial database to analyze the relationships of LV systolic dysfunction, the incidence of MR, and aortic root dimension with coronary artery dilation and with systemic inflammatory markers. Trial eligibility, enrollment, and procedures have been reported (22). Briefly, between December 2002 and December 2004, 199 consenting subjects from 8 North American centers who met specific study criteria for KD (1,23) and were between days 4 and 10 of illness were enrolled at the time of KD diagnosis. One subject withdrew shortly after consent, and no echocardiograms were submitted. Mean age of the 198 remaining subjects was 3.3 ± 2.2 years (16% under 1 year), and 63% were boys. Two subjects who met KD diagnostic criteria were later identified as having had herpes simplex virus and Ebstein-Barr virus, respectively; these subjects remain in the dataset, although their exclusion did not affect the results. The study was approved by the institutional review board or ethics committee of each center.
Echocardiographic methods
Echocardiograms were performed with pre-specified protocol guidelines (22); children unable to cooperate were sedated according to local practice. All baseline echocardiograms were performed within 48 h of trial randomization. Follow-up echocardiograms were performed 1 week (mean 7.8 ± 1.8 days, n = 198) and 5 weeks (mean 36. ± 3 4.3 days, n = 195) after randomization. Echocardiograms were centrally interpreted by a single core laboratory reviewer who was blinded to subject identity, treatment group, and illness day. Measurements of LV dimensions, LV fractional shortening (FS), aortic root dimension, and coronary artery segment diameters were made. The severity of valvar regurgitation was qualitatively assessed by color Doppler imaging, with notation made if there was at least mild MR or aortic regurgitation. A pericardial effusion was considered present if its maximal dimension was at least 1 mm in any imaging plane. LVFS, aortic root dimension, and the incidence of valvar regurgitation and of pericardial effusion were reported as part of the trial results (22).
Echocardiographic measurements of LV dimensions, aortic root dimension, and proximal left anterior descending and right coronary artery segments were transformed to z-scores (SD U) on the basis of body surface area (BSA) (12). Maximum coronary artery z-score was defined as the greater of the z-scores relative to BSA of either the proximal left anterior descending or right coronary artery segment. Coronary artery dilation was defined as present if the maximum coronary artery z-score of the subject was >2. Normal values for LVFS are age-dependent (24). For this reason, LV systolic dysfunction was defined as an age-adjusted LVFS z-score < −2 (i.e., a LVFS measurement that was more than 2 SDs below the mean for age).
Statistical methods
Data from both KD trial arms were combined for this study analysis, because there were no differences in echocardiographic parameters between treatment groups (22). An exploratory analysis was performed to assess whether baseline LVFS varied according to timing of the baseline echocardiogram with respect to intravenous immune globulin (IVIG) infusion (i.e., whether the echocardiogram was performed before, the day of, or after IVIG); because no correlation was found, this was not explored further (Online Appendix).
Longitudinal mixed model regression analyses were conducted that examined the association between LVFS z-score and continuous maximum coronary artery z-score and the association between aortic root size and maximum coronary artery z-score. Interactions with study visit (time) were fit to assess the existence of differential correlations by time.
Logistic regression was used to determine whether the presence versus absence of baseline LV dysfunction, MR, or aortic root dilation could predict those subjects who had coronary artery dilation (defined as z-score >2) at baseline and at 1- and 5-week follow-up.
Multivariate longitudinal mixed regression models were constructed for 3 outcomes: LVFS z-score, aortic root z-score, and the presence of MR to estimate associations with laboratory measures of inflammation: white blood count (WBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), albumin concentration, platelet count, hemoglobin concentration, hematocrit, and neutrophil count. Several clinical variables, including time since randomization (study visit), sex, and age, were also evaluated as potential correlates. Interactions between study visit and laboratory measures were examined. For all analyses, we considered p < 0.05 to represent a significant association.
Results
LV systolic dysfunction and coronary artery dilation in acute KD
At baseline, mean LVFS z-score was significantly lower than expected for a normal age-matched population, and LV dysfunction (age-adjusted LVFS z-score <−2) was present in 20% of subjects (Tables 1 and 2). Left ventricular function improved rapidly over time; among subjects defined as having LV dysfunction at baseline, 89% had normal LV systolic function by Week 5. Baseline LV end-diastolic and end-systolic dimension z-scores were higher than normal, indicating ventricular dilation (Table 1).
Tabel 1.
Echocardiographic Parameters by Time Since Randomization
| Parameter | Baseline | Week 1 | Week 5 | p Value |
|---|---|---|---|---|
| LVFS (%) | 36.0 ± 6.2 (189) | 38.7 ± 5.0 (186) | 37.8 ± 5.4 (185) | NS |
| LVFS z-score | −0.32 ± 2.22* (189) | 0.70 ± 1.65† (186) | 0.38 ± 1.93† (185) | <0.001 |
| LV end-diastolic dimension z-score | 0.54 ± 1.16† (189) | 0.28 ± 1.20‡ (186) | 0.31 ± 1.14† (185) | 0.007 |
| LV end-systolic dimension z-score | 0.45 ± 1.54† (189) | −0.20 ± 1.34* (185) | 0.04 ± 1.32 (185) | <0.001 |
| Aortic root z-score | 0.81 ± 0.85† (162) | 0.94 ± 0.82† (169) | 0.84 ± 0.87† (175) | 0.129 |
| Maximum coronary artery z-score | 1.65 ± 1.48† (195) | 1.73 ± 1.83† (190) | 1.35 ± 1.81† (190) | <0.001 |
Values are mean ± SD (number of observations). Differs significantly from normal population mean,
p < 0.05;
p < 0.001;
p < 0.01. The p value from longitudinal model assessing linear change over time.
Table 2.
Percentage of Subjects With Abnormal Echocardiographic Values
| Parameter | Baseline | Week 1 | Week 5 |
|---|---|---|---|
| LVFS z-score <−2 | 20% (189) | 4% (186) | 10% (185) |
| Aortic root z-score >2 | 8% (162) | 9% (169) | 10% (175) |
| Maximum coronary artery z-score >2 | 29% (195) | 29% (190) | 18% (190) |
| Mitral regurgitation | 27% (193) | 15% (197) | 9% (195) |
| Aortic regurgitation | 1% (191) | 1% (197) | 1% (195) |
| Pericardial effusion | 2% (195) | 3% (195) | 0% (195) |
LVFS = left ventricular fractional shortening.
Longitudinal mixed regression modeling found a weak but significant inverse association between FS z-score and maximum coronary artery z-score across all time points: for each unit increase in FS z-score, coronary artery z-score decreased 0.05 U (n = 559 observations on 198 subjects, estimated slope ± SE −0.051 ± 0.024, p = 0.033) (i.e., better LV systolic function was associated with smaller coronary artery z-scores). There were also significant but weak correlations between LV end-diastolic and end-systolic dimensions with maximal coronary artery z-score at baseline; these correlations were weaker or nonexistent at follow-up.
Patients with LV dysfunction at baseline, compared with those with normal LV function, had greater odds of having coronary artery dilation at baseline (odds ratio [OR]: 2.9, 95% confidence interval [CI]: 1.4 to 6.2, p = 0.004) and at 1 week (OR: 2.5, 95% CI: 1.1 to 6.3, p = 0.016) and 5-week follow-up (OR: 2.7, 95% CI: 1.2 to 6.1, p = 0.019) (Fig. 1). Thus, the presence of LV dysfunction at baseline predicted baseline and early convalescent (1- and 5-week) coronary artery dilation.
Figure 1. Coronary Artery Dilation by Initial LVFS z-Score.
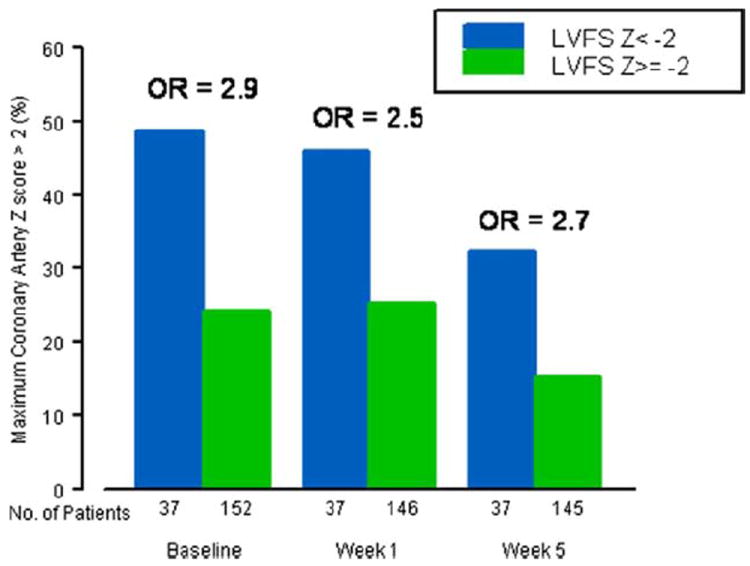
Odds ratio (OR): p = 0.002 at baseline; p = 0.016 at Week 1; p = 0.019 at Week 5. The ORs shown here are from logistic regression models with maximum coronary artery z-score >2 as the outcome. LVFS = left ventricular fractional shortening.
As noted in Newburger et al. (22), there were 4 patients who developed coronary artery dilation >4 mm in any coronary artery segment (“aneurysm”), excluding potentially pre-existing, isolated left main coronary artery abnormalities in 2 patients; none developed “giant” (>8 mm) aneurysms. Three of 4 patients who developed aneurysms had severe baseline LV dysfunction, with FS z-scores of −3.8, −5.8, −2.9, and 1.4. Increased baseline LVFS z-score was protective against development of a coronary artery aneurysm (OR: 0.62/U increase in z-score, 95% CI: 0.40 to 0.97, p = 0.035).
Association of LV systolic dysfunction with laboratory and clinical markers
Table 3 presents a multivariate model for LVFS z-score incorporating the 3 independent correlates of LV systolic dysfunction: higher ESR (at all time points; p = 0.007), higher WBC (at 1- and 5-week follow-up; p = 0.047 and p = 0.048, respectively), and lower serum albumin concentration (at baseline; p = 0.028). CRP was inversely correlated with LVFS z-score in univariate analysis (p = 0.044), but because CRP and albumin were correlated in this dataset, CRP did not remain as an independent correlate of LVFS z-score in the multivariate model.
Table 3.
Multivariate Model for LVFS z-Score
| Variable | Slope ± SE | p Value |
|---|---|---|
| ESR, mm/h | −0.009 ± 0.003 | 0.007 |
|
| ||
| WBC (103/mm3) × visit | 0.033 | |
| Week 0 | 0.016 ± 0.03 | 0.561 |
| Week 1 | 0.079 ± 0.04 | 0.047 |
| Week 5 | 0.11 ± 0.06 | 0.048 |
|
| ||
| Albumin (g/dl) × visit | 0.008 | |
| Week 0 | 0.51 ± 0.23 | 0.028 |
| Week 1 | −0.47 ± 0.34 | 0.172 |
| Week 5 | −0.66 ± 0.42 | 0.117 |
The model is based on analysis of 507 echocardiograms from 3 time points (visits). Due to the presence of interactions between laboratory parameters and visit, main effect estimates for these terms in the model are not shown.
LVFS = left ventricular fractional shortening.
Presence of MR and coronary artery dilation
At least mild MR was noted in 27% of subjects on their baseline echocardiogram; the incidence of MR rapidly decreased by 1- and 5-week follow-up (Table 2) (p < 0.0001). No subject had severe MR. Subjects with MR at baseline had greater baseline coronary artery size when compared with those subjects without baseline MR (mean maximum coronary artery z-score 1.99 ± 1.57 vs. 1.51 ± 1.42, respectively, p = 0.04), as seen in Figure 2. Unlike LV dysfunction at baseline, the presence of MR at baseline did not predict coronary artery dilation or the presence of coronary artery aneurysms at follow-up.
Figure 2. Maximum Coronary Artery z-Score at Baseline by Mitral Regurgitation Status at Baseline.
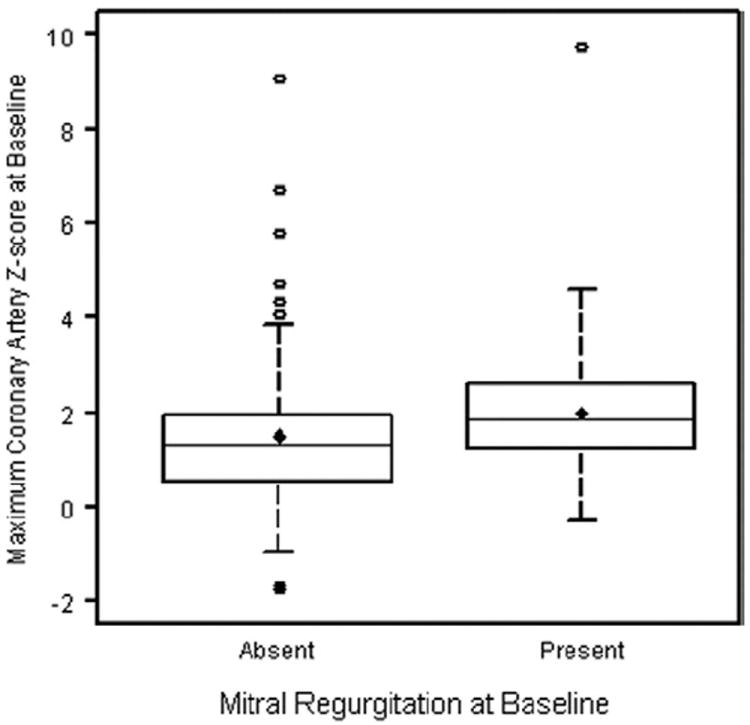
The diamond denotes the mean, lower and upper edges of the box denote the 25th and 75th quartiles, and the center line in the box denotes the median. p = 0.04.
Association of the presence of MR with laboratory and clinical markers
Table 4 presents the multivariate model for the presence of MR versus clinical/laboratory data. Elevated ESR (p = 0.020), lower albumin (p = 0.003), and female sex (p = 0.019) were independently correlated with the presence of MR, regardless of study visit. After adjustment for laboratory values, there remained a trend toward an inverse association between the presence of MR and study visit (p = 0.068). Mitral regurgitation did not correlate with CRP, WBC, or degree of anemia.
Table 4.
Multivariate Model for Presence Versus Absence of at Least Mild Mitral Regurgitation
| Variable | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Visit | 0.068 | ||
| Week 0 vs. Week 1 | 1.70 | 1.04–2.79 | 0.035 |
| Week 0 vs. Week 5 | 1.14 | 0.52–2.51 | 0.739 |
| Week 1 vs. Week 5 | 1.49 | 0.34–1.32 | 0.248 |
|
| |||
| Female | 2.02 | 1.12–3.66 | 0.019 |
|
| |||
| ESR, mm/h | 1.48* | 1.06–2.07 | 0.020 |
|
| |||
| Albumin, g/dl | 0.40 | 0.22–0.73 | 0.003 |
Model is based on analysis of 527 echocardiograms from 3 time points (visits).
Per 50-U increase in erythrocyte sedimentation rate (ESR).
CI = confidence interval.
Aortic root and coronary artery dilation in acute KD
Mean aortic root z-scores were significantly higher than normal at baseline and at 1- and 5-week follow-up, with 8%, 9%, and 10% of subjects found to have aortic root z-scores at or above 2 at baseline and 1 and 5 weeks, respectively (Tables 1 and 2). Aortic root z-scores did not vary significantly between study visits.
Longitudinal mixed regression modeling of the association between aortic root and coronary artery size at all time points revealed a moderate association between maximum coronary artery z-score and aortic root z-score: for every 1-U increase in aortic root z-score, maximum coronary artery z-score increased by 0.3 U (n = 505 observations on 193 subjects, estimated slope ± SE: 0.30 ± 0.08, p = 0.0003). There was no interaction with study visit, indicating that the association exists at all time points.
Association of aortic root size versus laboratory and clinical markers
In a multivariate model constructed exploring associations of aortic root size, there were significant independent effects of time (aortic root z-score highest at baseline; p = 0.017) and sex (aortic root z-score higher for boys, 1.04 ± 0.07 vs. 0.52 ± 0.09 for girls; p < 0.0001). There were 2 marginal associations between aortic root size and laboratory inflammatory markers: there was a weak positive correlation between albumin and aortic root z-score at baseline only (p = 0.052), and there was a trend toward a positive correlation between ESR and aortic root size (p = 0.093), with a 0.2-U increase in aortic root z-score per each 100-mm/h increase in ESR.
Discussion
We found that noncoronary cardiac abnormalities, including early LV systolic dysfunction, the presence of MR, and aortic root dilation, are common in acute KD and are associated with coronary artery dilation during the first 5 weeks after KD diagnosis. Acute LV systolic dysfunction and the presence of MR are associated with laboratory evidence of inflammation, including elevated ESR and decreased serum albumin, whereas aortic root dilation shows little correlation with these markers. These findings suggest that noncoronary cardiac abnormalities and coronary artery dilation in acute KD share a common but complex etiologic mechanism that relates to systemic inflammation. Further investigation of this mechanism might help in understanding the long-term consequences of KD, which might not be isolated to those subjects with significant residual coronary artery dilation (25).
Histological evidence of cardiac inflammation has been demonstrated in almost all subjects with acute KD when assessed by endomyocardial biopsy (26), and in a majority of subjects when assessed by WBC-labeled radionuclide studies (27-29); acute LV dysfunction has also been reported (4,5,28-30). We detected a 20% incidence of acute LV dysfunction; prior investigators detected a higher incidence of LV dysfunction (5), but this and prior studies were based on different definitions of ventricular dysfunction or used ventricular function assessment techniques not widely available in clinical practice. Acute LV dysfunction in KD is typically transient, as opposed to late onset or chronic LV dysfunction that might result from coronary insufficiency and/or myocardial fibrosis. We detected a rapid improvement in LV function, similar to prior studies (4,5), within 1 week of IVIG therapy. Rapid improvement in LV function is unique when compared with other etiologies of myocarditis in which function improves more slowly and less consistently. This rapid LV function improvement has led some investigators to postulate that acute LV dysfunction in KD is modulated by immune-mediated processes related to neutralization of circulating toxins or activated cytokines (5).
Prior studies have failed to consistently establish an association between LV dysfunction and coronary artery dilation, despite the high prevalence of LV dysfunction early after KD diagnosis. Hsu et al. (29) found that patients with carditis (as diagnosed by abnormal radionuclide-labeled WBC scans) had lower ejection fractions and larger coronary arteries when assessed within 1 week of KD diagnosis. In contrast, Anderson et al. (31) and Newburger et al. (4) found no relationship between LV dysfunction and coronary artery dilation in acute KD. These 2 studies used Japanese Ministry of Health criteria for coronary abnormalities (23) rather than the more sensitive BSA-adjusted coronary artery z-scores (32) used in our current study; this might explain why we detected an association between LV dysfunction and coronary artery dilation while prior studies did not.
We found ESR, WBC, and albumin to be associated with LV dysfunction at some or all time points in the first 5 weeks of illness. Few prior studies have demonstrated any association between systemic inflammatory indexes and LV dysfunction. Newburger et al (4) did not detect any relation between LV function and laboratory inflammatory markers. Moran et al. (5) also found no association between baseline levels of acute phase reactants and LV contractility but did demonstrate concordance between clinical and myocardial response to IVIG therapy. Yoshikawa et al. (33) found that LV dysfunction was associated with an increase in CRP in 3 of 4 KD subjects assessed but only after IVIG infusion. Again, these differences might be attributable to differing methods of LV function assessment.
Mild or moderate MR occurred in approximately one-quarter of subjects and was associated with laboratory evidence of acute inflammation. To the best of our knowledge, our study is the first to demonstrate an association between acute MR and laboratory inflammatory markers.
Our incidence of acute MR (27%) was similar to the 23% reported by Giddings et al. (20) but lower than the 47% reported by Suzuki et al. (34). These differences might be because different treatment eras were examined or because qualitative rather than quantitative definitions of MR were employed. Mechanisms for MR in acute KD might also be different from mechanisms for MR present after the acute KD episode (35). Our study did not investigate the mechanism of MR or its persistence beyond 5 weeks after acute KD.
Aortic root dilation was associated with coronary artery dilation early after KD diagnosis. The degree of aortic root dilation that we found early after KD diagnosis was similar to that reported by Ravekes et al. (7). They did not detect any association between aortic root dilation and coronary artery dilation, but BSA-adjusted coronary artery z-scores were not examined. Our study and Ravekes et al. (7) showed that the degree of aortic root dilation remained constant between Weeks 1 and 5 after KD diagnosis. We could not confirm their finding that aortic root dilation persisted 1 year after KD, because our patients were not followed beyond Week 5.
Our finding of a weak correlation between larger baseline aortic root size and higher serum albumin (in contrast to the correlation between baseline LV dysfunction and lower serum albumin) is consistent with there being a different temporal course and/or etiology for these 2 findings. These differences suggest that LV dysfunction, MR, aortic root dilation, and coronary artery dilation might share slightly different inflammatory mechanisms. As noted by Ravekes et al. (7), prior pathologic studies have suggested that microvascular vasculitis (relating to coronary artery dilation) occurs within 10 days of KD onset, whereas vasculitis involving larger arteries (potentially relating to aortic root dilation) occurs on Days 12 to 25 into the course of KD. An alternate explanation for the temporal differences of aortic root and coronary artery dilation might be a limited capacity for great artery remodeling, perhaps because there is more connective tissue and less muscularization of the aortic wall. Further research into these differences might help in understanding the long-term vascular sequelae of KD.
We found that acute MR was more common in girls with KD, whereas aortic root size adjusted for BSA was larger in boys. These observations have not been demonstrated previously, although male sex has been associated with “higher-risk” KD. It is unclear whether sex differences represent differing genetic susceptibilities to inflammatory responses in acute KD or whether these differences are spurious. This is another area that might relate to potential long-term cardiovascular effects of KD.
Although 20% of subjects had baseline LV dysfunction, no subject was coded by site investigators to have congestive heart failure, perhaps because this would have been difficult to differentiate from symptoms like tachycardia and tachypnea due to fever, anemia, and/or dehydration. Also, because identification of congestive heart failure was not a trial aim, coding for congestive heart failure could have been incomplete. Similarly, many acute KD patients have heart murmurs due to fever and anemia; the presence of an MR murmur was not assessed during this trial.
Study limitations
Because the randomized trial of pulsed steroids in KD was designed to evaluate acute changes associated with treatment, long-term follow-up is not available. All patients in this study were treated with IVIG within the first 10 days of illness onset, and there were few patients with coronary artery aneurysms; it is possible that the associations between cardiac and laboratory parameters might have been even stronger in a sicker group of children. Finally, this study has reported correlations between parameters but cannot establish causality.
Conclusions
Noncoronary cardiac abnormalities are associated with coronary artery dilation and with laboratory evidence of inflammation in the first 5 weeks after KD diagnosis. Future studies should explore shared inflammatory mechanisms that might underlie these associations and help in understanding the long-term consequences of KD.
Supplementary Material
Acknowledgments
This work was supported by U01 Grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI. Dr. Shirali is a consultant and advisory board member for Philips Medical Systems. All other authors have reported that they have no relationships to disclose. Thomas Graham Jr, MD, served as Guest Editor for this paper.
Abbreviations and Acronyms
- BSA
body surface area
- CI
confidence interval
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- FS
fractional shortening
- IVIG
intravenous immune globulin
- KD
Kawasaki disease
- LV
left ventricular
- MR
mitral regurgitation
- OR
odds ratio
- WBC
white blood count
Footnotes
APPENDIX
For supplementary data, please see the online version of this article.
References
- 1.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–33. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 2.Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978;61:100–7. [PubMed] [Google Scholar]
- 3.Rowley AH, Shulman ST. Kawasaki syndrome. Clin Microbiol Rev. 1998;11:405–14. doi: 10.1128/cmr.11.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newburger JW, Sanders SP, Burns JC, Parness IA, Beiser AS, Colan SD. Left ventricular contractility and function in Kawasaki syndrome. Effect of intravenous gamma-globulin. Circulation. 1989;79:1237–46. doi: 10.1161/01.cir.79.6.1237. [DOI] [PubMed] [Google Scholar]
- 5.Moran AM, Newburger JW, Sanders SP, et al. Abnormal myocardial mechanics in Kawasaki disease: rapid response to gamma-globulin. Am Heart J. 2000;139:217–23. doi: 10.1067/mhj.2000.101221. [DOI] [PubMed] [Google Scholar]
- 6.Akagi T, Kato H, Inoue O, Sato N, Imamura K. Valvular heart disease in Kawasaki syndrome: incidence and natural history. Am Heart J. 1990;120:366–72. doi: 10.1016/0002-8703(90)90081-8. [DOI] [PubMed] [Google Scholar]
- 7.Ravekes WJ, Colan SD, Gauvreau K, et al. Aortic root dilatation in Kawasaki disease. Am J Cardiol. 2001;87:919–22. doi: 10.1016/s0002-9149(00)01541-1. [DOI] [PubMed] [Google Scholar]
- 8.Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364:533–44. doi: 10.1016/S0140-6736(04)16814-1. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi M. Myocarditis in Kawasaki syndrome. A minor villain? Circulation. 1989;79:1398–400. doi: 10.1161/01.cir.79.6.1398. [DOI] [PubMed] [Google Scholar]
- 10.Eberhard E, Andersson U, Laxer RM, Rose V, Silverman ED. Evaluation of the cytokine response in Kawasaki disease. Pediatr Infect Dis J. 1995;14:199–203. doi: 10.1097/00006454-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Matsubara T, Ichiyama T, Furukawa S. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clin Exp Immunol. 2005;141:381–7. doi: 10.1111/j.1365-2249.2005.02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCrindle BW, LI JS, Minich LL, et al. for the Pediatric Heart Network Investigators. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. 2007;116:174–9. doi: 10.1161/CIRCULATIONAHA.107.690875. [DOI] [PubMed] [Google Scholar]
- 13.Nakano H, Ueda K, Saito A, et al. Scoring method for identifying patients with Kawasaki disease at high risk of coronary artery aneurysms. Am J Cardiol. 1986;58:739–42. doi: 10.1016/0002-9149(86)90348-6. [DOI] [PubMed] [Google Scholar]
- 14.Koren G, Lavi S, Rose V, Rowe R. Kawasaki disease: review of risk factors for coronary aneurysms. J Pediatr. 1986;108:388–92. doi: 10.1016/s0022-3476(86)80878-2. [DOI] [PubMed] [Google Scholar]
- 15.Burns JC, Glode MP, Clarke SH, Wiggins J, Hathaway WE. Coagulopathy and platelet activation in Kawasaki syndrome: identification of patients at high risk for development of coronary artery aneurysms. J Pediatr. 1983;105:206–11. doi: 10.1016/s0022-3476(84)80114-6. [DOI] [PubMed] [Google Scholar]
- 16.Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr. 1992;121:924–6. doi: 10.1016/s0022-3476(05)80343-9. [DOI] [PubMed] [Google Scholar]
- 17.Beiser AS, Takahashi M, Baker AL, Sundel RP, Newburger JW for the US Multicenter Kawasaki Disease Study Group. A predictive instrument for coronary artery aneurysms in Kawasaki disease. Am J Cardiol. 1998;81:1116–20. doi: 10.1016/s0002-9149(98)00116-7. [DOI] [PubMed] [Google Scholar]
- 18.Schiller B, Elinder G. Inflammatory parameters and soluble cell adhesion molecules in Swedish children with Kawasaki disease: relationship to cardiac lesions and intravenous immunoglobulin treatment. Acta Paediatr. 1999;88:844–8. doi: 10.1080/08035259950168766. [DOI] [PubMed] [Google Scholar]
- 19.Hirashi S, Yashiro K, Oguchi K, Kusano S, Ishii K, Nakazawa K. Clinical course of cardiovascular involvement in the mucocutaneous lymph node syndrome. Relation between clinical signs of carditis and development of coronary arterial aneurysm. Am J Cardiol. 1981;47:323–30. doi: 10.1016/0002-9149(81)90404-5. [DOI] [PubMed] [Google Scholar]
- 20.Giddings SS, Duffy CE, Pajcic S, Berdusis K, Shulman ST. Usefulness of echocardiographic evidence of pericardial effusion and mitral regurgitation during the acute stage in predicting development of coronary arterial aneurysms in the late stage of Kawasaki disease. Am J Cardiol. 1987;60:76–9. doi: 10.1016/0002-9149(87)90988-x. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi D, Saji T, Takatsuki S, Fujiwara M. Abnormal tissue Doppler images are associated with elevated plasma brain natriuretic peptide and increased oxidative stress in acute Kawasaki disease. Circ J. 2007;71:357–62. doi: 10.1253/circj.71.357. [DOI] [PubMed] [Google Scholar]
- 22.Newburger JW, Sleeper LA, McCrindle BW, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356:663–75. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 23.Research Committee on Kawasaki Disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki Disease. Tokyo, Japan: Ministry of Health and Welfare; 1984. [Google Scholar]
- 24.Colan SD, Parness IA, Spevak PJ, Sanders SP. Developmental modulation of myocardial mechanics: age- and growth-related alterations in afterload and contractility. J Am Coll Cardiol. 1992;19:619–29. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 25.Gordon JB, Kahn AM, Burns JC. When children with Kawasaki Disease grow up: myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54:1911–20. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yutani C, Go S, Kamiya T, et al. Cardiac biopsy of Kawasaki Disease. Arch Pathol Lab Med. 1981;105:470–3. [PubMed] [Google Scholar]
- 27.Kao CK, Hsieh KS, Chen YC, Wang YL, Wang SJ. Relationships between coronary artery dilatation and severity of carditis detected by two-dimensional echocardiography and [99mTc] HMPAO-labeled white blood cell heart scan in children with Kawasaki disease. Pediatr Radiol. 1994;24:41–4. doi: 10.1007/BF02017659. [DOI] [PubMed] [Google Scholar]
- 28.Kao CH, Hsieh KS, Wang YL, Wang SJ, Yeh SH. The detection of ventricular dysfunction and carditis in children with Kawasaki disease using equilibrium multigated blood pooling ventriculography and Tc-99m-HMPAO-labelled WBC heart scans. Nucl Med Commun. 1993;14:539–43. doi: 10.1097/00006231-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Hsu H, Fu Y, Tsai S, Yen R, Hwang B. Usefulness of Tc-99m HMPA O-labeled WBC heart scan to predict impaired ventricular function and coronary artery dilation in children with Kawasaki disease. Int J Cardiol. 2003;92:65–9. doi: 10.1016/s0167-5273(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 30.Chung KJ, Brandt L, Fulton DR, Kreidberg MB. Cardiac and coronary arterial involvement in infants and children from New England with mucocutaneous lymph node syndrome (Kawasaki disease). Angiocardiographic-echocardiographic correlations. Am J Cardiol. 1982;50:136–42. doi: 10.1016/0002-9149(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 31.Anderson TM, Meyer RA, Kaplan S. Long-term echocardiographic evaluation of cardiac size and function in patients with Kawasaki disease. Am Heart J. 1985;110:107–15. doi: 10.1016/0002-8703(85)90523-x. [DOI] [PubMed] [Google Scholar]
- 32.De Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254–8. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]
- 33.Yoshikawa H, Nomura Y, Masuda K, et al. Four cases of Kawasaki syndrome complicated with myocarditis. Circ J. 2006;70:202–5. doi: 10.1253/circj.70.202. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki A, Kamiya T, Tsuchiya K, et al. Tricuspid and mitral regurgitation detected by color flow Doppler in the acute phase of Kawasaki Disease. Am J Cardiol. 1988;61:386–90. doi: 10.1016/0002-9149(88)90950-2. [DOI] [PubMed] [Google Scholar]
- 35.Mishima A, Asano M, Saito T, et al. Mitral regurgitation caused by ruptured chordae tendineae in Kawasaki disease. J Thorac Cardiovasc Surg. 1996;111:895–6. doi: 10.1016/s0022-5223(96)70352-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


