Abstract
Working memory is essential to higher order cognition (e.g. fluid intelligence) and to performance of daily activities. Though working memory capacity was traditionally thought to be inflexible, recent studies report that working memory capacity can be trained and that offline processes occurring during sleep may facilitate improvements in working memory performance. We utilized a 48-h in-laboratory protocol consisting of repeated digit span forward (short-term attention measure) and digit span backward (working memory measure) tests and overnight polysomnography to investigate the specific sleep-dependent processes that may facilitate working memory performance improvements in the synucleinopathies. We found that digit span backward performance improved following a nocturnal sleep interval in patients with Parkinson's disease on dopaminergic medication, but not in those not taking dopaminergic medication and not in patients with dementia with Lewy bodies. Furthermore, the improvements in patients with Parkinson's disease on dopaminergic medication were positively correlated with the amount of slow-wave sleep that patients obtained between training sessions and negatively correlated with severity of nocturnal oxygen desaturation. The translational implication is that working memory capacity is potentially modifiable in patients with Parkinson's disease but that sleep disturbances may first need to be corrected.
Keywords: consolidation, sleep, working memory, training, Parkinson's disease, dementia with Lewy bodies
Introduction
Working memory is a dynamic cognitive ability in which information is actively stored and manipulated in the brain (Baddeley, 1992). Working memory is considered vital to higher order cognitive abilities such as planning (Phillips et al., 1999), problem solving (Logie et al., 1994), delayed goal execution (Brewer et al., 2010) and overall fluid intelligence (Engle et al., 1999; Shelton et al., 2010). For nearly half a century, working memory capacity, which is the amount of information one can temporarily store and manipulate, was considered to be a stable trait (Miller, 1956; Cowan, 2001). However, researchers are now finding that working memory capacity can be trained (Verhaeghen et al., 2004; Morrison and Chein, 2011) and that training working memory capacity might lead to a broad spectrum of cognitive enhancements (Chein and Morrison, 2010), perhaps even improved fluid intelligence (Jaeggi et al., 2008). The clinical implication is that working memory training might help patients who have cognitive impairments (Westerberg et al., 2007; Vogt et al., 2009). This study investigated whether sleep might enhance working memory training in patients with synucleinopathies such as Parkinson's disease, which is frequently associated with mild impairments in cognition and dementia with Lewy bodies (DLB), which is associated with more profound impairments to cognition (Martí et al., 2003; Metzler-Baddeley, 2007).
An unresolved question regards the mechanisms by which working memory performance is augmented with training (Shipstead et al., 2012). Neuroimaging studies have found cortical plasticity in response to training (Hempel et al., 2004), but do not indicate when or how such functional reorganization occurs. Because most training studies are conducted over a span of days, weeks or months, one intriguing possibility is that offline (i.e. operating in-between training events), sleep-dependent mechanisms are involved. Consistent with this idea, Kuriyama et al. (2008, 2011) experimentally interleaved sleep versus wake intervals between training events and found that a period of sleep is critical to facilitating working memory. However, no published study has attempted to identify the sleep-based physiological process(es) that promotes such enhancement.
One plausible offline mechanism for training working memory is slow-wave sleep. Slow-wave sleep has received increasing attention in recent years due to its link to offline memory reactivation (Wilson and McNaughton, 1994; Ji and Wilson, 2007) and subsequent consolidation (Fowler et al., 1973; Peigneux et al., 2004; Stickgold, 2005; Rasch et al., 2007). In addition to offline memory reactivation, slow-wave sleep has generally been linked to synaptic plasticity and cortical reorganization (Tononi and Cirelli, 2003; Takashima et al., 2006; Dang-Vu et al., 2010), thereby making it a candidate for facilitating working memory training.
This study investigated whether patients with neurodegenerative disease (specifically Parkinson's disease and DLB) can improve their working memory performance, and if so, whether slow-wave sleep or another sleep-dependent variable is associated with such improvements. Only one study has examined offline memory improvements in patients with Parkinson's disease and that study examined motor memory—not working memory—and did not use polysomnography (Marinelli et al., 2009). In this study, patients with Parkinson's disease and DLB stayed in an inpatient sleep laboratory for 48 consecutive hours, underwent two nights of polysomnography and completed eight verbally administered digit span forward and backward tests (Fig. 1).
Figure 1.
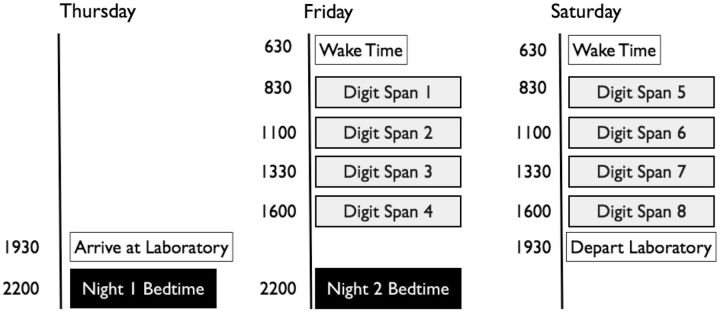
Example of the 48-h protocol focusing on the polysomnographic and digit span assessments. The timing of assessments was tailored to each patient depending on their typical bed and wake times.
The digit span task was chosen for several reasons. First, when administered verbally in Parkinsonian patients digit span avoids confounds that would likely be present in working memory tasks requiring motor function. Second, digit span was predicted to be a more amenable task (i.e. less fatiguing or stressful) in patients with Parkinson's disease and DLB than lengthy n-back or complex span measures, which was an important consideration in our 48-h inpatient procedure. Third, digit span is a validated measure of working memory (Shelton et al., 2009; Hill et al., 2010) and it is particularly appropriate for the present research because forward and backward subtests measure separate cognitive constructs (Reynolds, 1997; Groeger et al., 1999). While digit span forward is a simple span task that measures short-term memory (i.e. sustaining attention), digit span backward is a complex span task that measures working memory. This distinction is important because Kuriyama et al. (2011) found sleep-related enhancements in a healthy younger adult sample to a working memory task (n-back) but not for a simpler sustained attention task (psychomotor vigilance). Therefore, one would predict sleep-dependent facilitation of digit span backward but not digit span forward.
A final potentially relevant factor that could be implicated in working memory function in parkinsonism is dopamine. Dopamine release has been implicated in offline memory reactivation and consolidation (de Lima et al., 2011; Schicknick et al., 2012), general working memory capacity (Bäckman et al., 2006) and the ability to train working memory (Dahlin et al., 2008a; Brehmer et al., 2009; McNab et al., 2009). Because Parkinson's disease is characterized by the loss of dopamine-producing neurons (Lang and Lozano, 1998; Lotharius and Brundin, 2002) as well as working memory impairments (Cooper et al., 1991), we separately evaluated patients with Parkinson's disease who were not yet taking dopaminergic medication (Cooper et al., 1992) versus patients with Parkinson's disease actively taking dopaminergic medication.
Materials and methods
Participants
Fifty-four patients with idiopathic Parkinson's disease and 10 patients with DLB completed the 48-h laboratory protocol (Fig. 1) including two nights of polysomnography and eight digit span tests. We excluded one patient with idiopathic Parkinson's disease with a technical malfunction in the polysomnography recording on the first laboratory night, resulting in 53 cases with Parkinson's disease with complete data. Demographic information is presented in Table 1. Both patients with Parkinson's disease and DLB met consensually defined criteria for each condition. These are defined in detail elsewhere (Langston et al., 1992; McKeith et al., 2005), but can be briefly summarized here. As determined by a board-certified neurologist, Parkinson's disease was defined by the presence of at least two of the four cardinal signs of the disorder, including resting tremor, cogwheel rigidity, bradykinesia and postural reflex instability, with either resting tremor or bradykinesia required to be present. DLB was defined by the presence of dementia and at least two core features (Parkinsonism, recurrent visual hallucinations and fluctuating cognition) or one core feature and one suggestive feature of the disorder, including rapid eye movement sleep behaviour disorder, neuroleptic sensitivity, or reduced dopamine transporter uptake on neuroimaging.
Table 1.
Participant information across groups
| Demographic and clinical variables | PD patients (no dopaminergics) | PD patients (with dopaminergics) | DLB patients | P-value |
|---|---|---|---|---|
| Age | 64.64 (9.27) | 63.14 (9.16) | 70.00 (7.02) | 0.099 |
| % Male | 0.55 | 0.62 | 1.0 | 0.046 |
| % Caucasian | 0.91 | 1.0 | 1.0 | – |
| MMSE | 27.18 (3.09) | 27.67 (2.12) | 21.50 (5.10) | <0.001 |
| Disease duration (years) | 1.95 (0.99) | 6.68 (4.05) | 4.10 (4.08) | 0.001 |
| Hoehn and Yahr | 2.14 (0.60) | 2.14 (0.60) | 2.50 (0.47) | 0.21 |
| UPDRS | 18.00 (8.45) | 16.74 (8.63) | 26.75 (10.85) | 0.023 |
| Restless legs | 1.0 (1.41) | 1.76 (1.38) | 1.20 (0.79) | 0.18 |
| Levodopa dose (mg) | 0.0 (0.0) | 400.00 (295.70) | 255.00 (309.52) | <0.001 |
| Pergolide dose (mg) | 0.0 (0.0) | 2.01 (1.58) | 0.05 (0.16) | <0.001 |
| Combined dose (mg) | 0.0 (0.0) | 574.87 (323.82) | 258.35 (306.63) | <0.001 |
Standard deviations in parentheses. Where possible, F tests or χ2 tests were used to calculate P-values. Age, gender, race, Hoehn and Yahr and dosage were available for all 63 patients; MMSE (n = 60), disease duration (n = 61), UPDRS motor subscale (n = 53) data and restless legs questionnaire (n = 58) were available for the majority of patients. PD = Parkinson's disease.
Patients with Parkinson's disease were enrolled regardless of whether they carried a diagnosis of dementia, but most did not [94.3% scored 26 or higher on the Mini-Mental State Examination (MMSE)]. Additionally, patients were rated with the Hoehn and Yahr staging scale and the motor subscale of the Unified Parkinson's Disease Rating Scale (UPDRS). For patients receiving dopaminergic medication, these ratings were conducted while patients were taking their usual medication. We also assessed for the frequency of restless legs syndrome using the mean of responses on two self-report questions [ranging from never (0) to very often (3)] that have been used in two major studies of restless legs syndrome prevalence (Lavigne and Montplaisir, 1994; Phillips et al., 2000).
Within the Parkinson's disease group, 11 patients were taking no dopaminergic medication whereas 42 patients were taking one or more dopaminergic agents, including levodopa (n = 34), pramipexole (n = 16), ropinirole (n = 13) and bromocriptine (n = 1). To estimate dosage (Table 1) we used levodopa equivalents (Hobson et al., 2002), pergolide equivalents (Grosset and Drosset, 2006) and combined dopaminergic equivalents (Hobson et al., 2002). Because of the small sample size, we did not assess for medication effects in the patients with DLB (nlevodopa = 5, npramipexole = 1).
As demonstrated in Table 1, the groups differed significantly in MMSE and in UPDRS, indicating worse dementia and motor symptoms, respectively, in the patients with DLB relative to the patients with Parkinson's disease. The DLB group was also composed of a higher proportion of males relative to the Parkinson's disease groups. Furthermore, patients with Parkinson's disease not taking dopaminergic medication were more recently diagnosed than those actively taking dopaminergic medications.
Overnight polysomnography
Participants underwent two consecutive nights of conventional polysomnography using the Embla Flaga A10 system. Polysomnography consisted of central and occipital EEG, left and right monopolar electrooculography, single lead electrocardiography, respiratory airflow and effort and pulse oximetry. In addition, chin, leg and arm EMG was measured from placements above the mentalis, anterior tibialis and brachioradialis, respectively. This montage allowed us to examine sleep architecture, evaluate sleep efficiency and fragmentation, as well as identify severe nocturnal oxygen desaturation, breathing events (e.g. apnoeas) and periodic leg movements. Sleep stage analysis was conducted across 30 s epochs and was scored blind to the digit span data. We also monitored the patients' sleep continuously via a low-illumination, infra-red video system, which allowed us to determine the presence or absence of episodes of dream enactment behaviours during REM for each night.
Digit span measure
The digit span task is a classic measure from the Wechsler Adult Intelligence Scale-III (Wechsler, 1997), which has been validated against other working memory measures (e.g. Shelton et al., 2009), such as the n-back (i.e. the task used by previous working memory and sleep studies; Kuriyama et al., 2008, 2011). In the digit span task, the experimenter reads a string of digits aloud (e.g. 5 9 2 4 7) and the patient is instructed to immediately recall those digits either in the forward position (5 9 2 4 7), which is a measure of short-term memory/attention, or in the backward position (7 4 2 9 5), which is a measure of working memory (Reynolds, 1997). Digit span backward is a working memory task because the digits must be maintained in consciousness and also manipulated (re-ordered) prior to verbal output. Manipulation is not required for digit span forward.
The digit span test begins with trials that are two strings in length, and it increases in length by one digit following two correct trials. The test continues until the patient fails on two consecutive trials. Thus, via successive iterations, the test determines the maximum capacity of digits that a patient can recall in the forward or backward position. Each digit span trial included a different permutation of digits, thereby ensuring that any benefits we observed were not due to specific episodic memory for digit orders. Digit span forward was always administered prior to digit span backward.
Procedure
The 48-h protocol, an example of which is provided in Fig. 1, was conducted in a sound-attenuated, quasi-isolated sleep laboratory at Wesley Woods Geriatric Hospital. Patients were continuously monitored by a research technician and they did not leave the laboratory during the study. All meals and medications were provided at their customary times, and the technician ensured that patients maintained their usual bedtime and wake-up schedule during the study. Caffeinated beverages were not allowed. Between digit span tests, patients were allowed to watch television, read the newspaper and use the phone and Internet. Participants also underwent modified Maintenance of Wakefulness Testing to examine daytime alertness each day (see Bliwise et al., 2012, for full description of the protocol). These tests lasted 40 min regardless of whether sleep onset occurred, to allow for the examination of sleep duration, and we analysed each participant's sum of diurnal sleep in minutes. Digit span testing began ∼2 h after morning awakening and was conducted throughout the day approximately every 2–3 h.
Statistical analyses
Alpha was set to 0.05 for inferring statistical significance. Digit span performance was assessed as the number of correct digits prior to failing consecutive trials and was averaged across the four tests in each day. To examine the possible sleep-dependent process(es) that promote working memory improvements, we conducted partial correlations between sleep parameters and mean Day 2 digit span backward performance after controlling for mean Day 1 digit span backward performance. We adjusted for multiple testing in the correlational analyses using the Hochberg method (Norman and Steiner, 2000).
Results
Digit span improvement
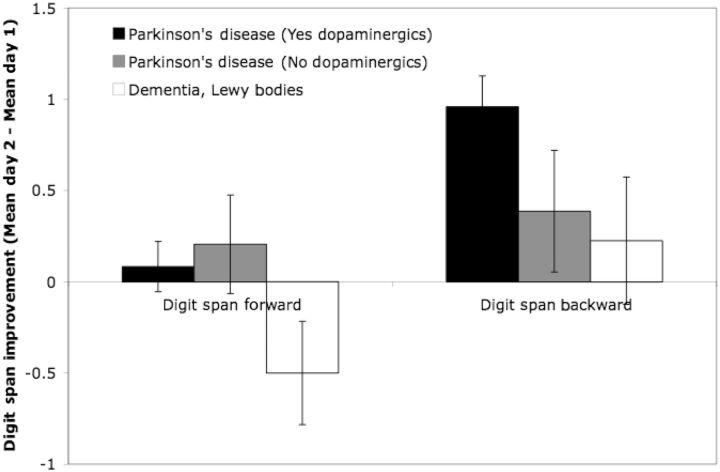
As illustrated in Fig. 2, the patients with Parkinson's disease demonstrated a significant performance improvement from Day 1 to Day 2 for digit span backward, t(52) = 5.21, P < 0.001, but not for digit span forward (t < 1). The significant digit span backward improvement replicated in both male, t(31) = 3.72, P = 0.001, and female patients with Parkinson's disease, t(20) = 3.731, P = 0.001. In contrast, the patients with DLB demonstrated no significant improvement in digit span backward (t < 1), but a significant decline in digit span forward, t(9) = 2.27, P < 0.05.
Figure 2.
Change in digit span forward and backward performance (Mean Day 2-Mean Day 1) across groups. Error bars reflect standard errors.
Within the Parkinson's disease group, digit span backward improvement from Day 1 to Day 2 was statistically significant in those taking dopaminergic medication, t(41) = 5.108, P < 0.001, but not in those not taking dopaminergic medication, t(10) = 1.418, P = 0.19 (Fig. 2) (cf. Cooper et al., 1992; Cools et al., 2001). This pattern is striking, considering that patients taking dopaminergic medication had longer disease duration than those not taking dopaminergic medication, t(49) = 3.80, P < 0.001 (Table 1). Such digit span backward improvements were neither time-of-day nor practice-dependent, which is evidenced by a lack of digit span backward improvement from the first test [mean (M)Test 1 = 5.12] to the last test (MTest 4 = 5.10) on Day 1 (t < 1). Similarly, on Day 2, there was not a significant digit span backward improvement from the first test (MTest 5 = 6.10) to the last test (MTest 8 = 5.81; t < 1). Critically, digit span backward performance improved significantly from Test 4 to Test 5, t(41) = 3.08, P = 0.004, which included an interval of nocturnal sleep. By contrast, digit span forward performance did not improve from Test 4 (M = 9.81) to Test 5 (M = 9.50) [t(41) = 1.08, P = 0.29]; there was also no significant digit span forward improvements across Day 1 [MTest 1 = 9.45; t(41) = 1.38, P = 0.18] or across Day 2 (MTest 8 = 9.64; t < 1). Therefore, the Day 1 to Day 2 digit span backward improvements observed (Fig. 2) appear to be dependent on processes that occur during nocturnal sleep rather than be dependent on time of day or practice.
Nocturnal sleep
Observed dream enactment in the sleep laboratory was significantly more likely in patients with DLB (90%) relative to patients with Parkinson's disease (30.2%) (P < 0.001, Fisher's exact test), which was consistent with the classification of rapid eye movement sleep behaviour disorder as one of the suggestive features of DLB (McKeith et al., 2005). Mean polysomnographic measures are presented in Table 2; there were no significant differences across groups.
Table 2.
Polysomnographic parameters averaged across both nights
| Sleep variables | PD patients (no dopaminergics) | PD patients (with dopaminergics) | DLB patients | P-value |
|---|---|---|---|---|
| Total sleep time (min) | 377.38 (70.98) | 325.47 (90.78) | 365.98 (88.07) | 0.14 |
| Sleep efficiency | 74.63 (13.78) | 68.78 (18.11) | 72.45 (13.06) | 0.54 |
| Sleep latency | 33.15 (27.36) | 52.33 (66.32) | 27.23 (29.41) | 0.35 |
| % Stage 1 | 0.09 (0.08) | 0.18 (0.14) | 0.15 (0.16) | 0.20 |
| % Stage 2 | 0.71 (0.09) | 0.62 (0.16) | 0.70 (0.17) | 0.15 |
| % Slow-wave sleep | 0.08 (0.07) | 0.08 (0.10) | 0.04 (0.05) | 0.41 |
| % REM | 0.12 (0.07) | 0.12 (0.08) | 0.12 (0.09) | 0.98 |
| AHI | 5.65 (9.59) | 5.53 (6.08) | 5.07 (7.04) | 0.98 |
| PLMS | 15.91 (18.28) | 17.97 (35.76) | 32.14 (25.07) | 0.42 |
| Lowest SaO2 | 86.00 (3.40) | 82.18 (7.20) | 84.45 (3.54) | 0.17 |
| SaO2 <90% (min) | 2.42 (2.75) | 3.12 (3.56) | 1.46 (1.80) | 0.33 |
F tests were used to calculate P-values.
AHI = apnoea–hypopnoea index; PD = Parkinson's disease; PLMS = periodic leg movements; SaO2 = oxygen saturation.
Correlations between sleep and digit span backward performance
Partial correlations between sleep parameters and digit span backward improvement (from Day 1 to Day 2) are presented in Table 3. As illustrated in Fig. 3A, patients with Parkinson's disease taking dopaminergic medication demonstrated a significant positive correlation between digit span backward improvement and Night 2 slow-wave sleep. Patients who demonstrated little or no slow-wave sleep (i.e. Night 2 slow-wave sleep per cent <1%) did not show significant digit span backward improvement [MDay 2–Day 1 = 0.55; t(10) =1.78, P = 0.11].
Table 3.
Partial correlations between sleep parameters (Nights 1 and 2) and Day 2 digit span backward performance (controlling for Day 1 digit span backward performance) within the patients with Parkinson's disease taking dopaminergic medication
| Sleep variables | Night 1 (pre-training) | Night 2 (during training) |
|---|---|---|
| Total sleep time | 0.059 | −0.049 |
| Sleep efficiency | 0.087 | −0.023 |
| Sleep latency | 0.079 | 0.023 |
| % Stage 1 | −0.068 | −0.156 |
| % Stage 2 | −0.160 | −0.195 |
| % Slow-wave sleep | 0.304 | 0.373* |
| % REM | 0.089 | 0.155 |
| AHI | −0.141 | −0.005 |
| PLMS index | 0.146 | 0.108 |
| Dream enactment | 0.094 | 0.061 |
| Lowest SaO2 | 0.112 | 0.188 |
| Sa02 <90% (min) | −0.018 | −0.318 |
*P < 0.05, after adjusting for multiple testing using the Hochberg method (Norman and Steiner, 2000).
AHI = apnoea–hypopnoea index; PLMS = periodic leg movements; SaO2 = oxygen saturation.
Figure 3.
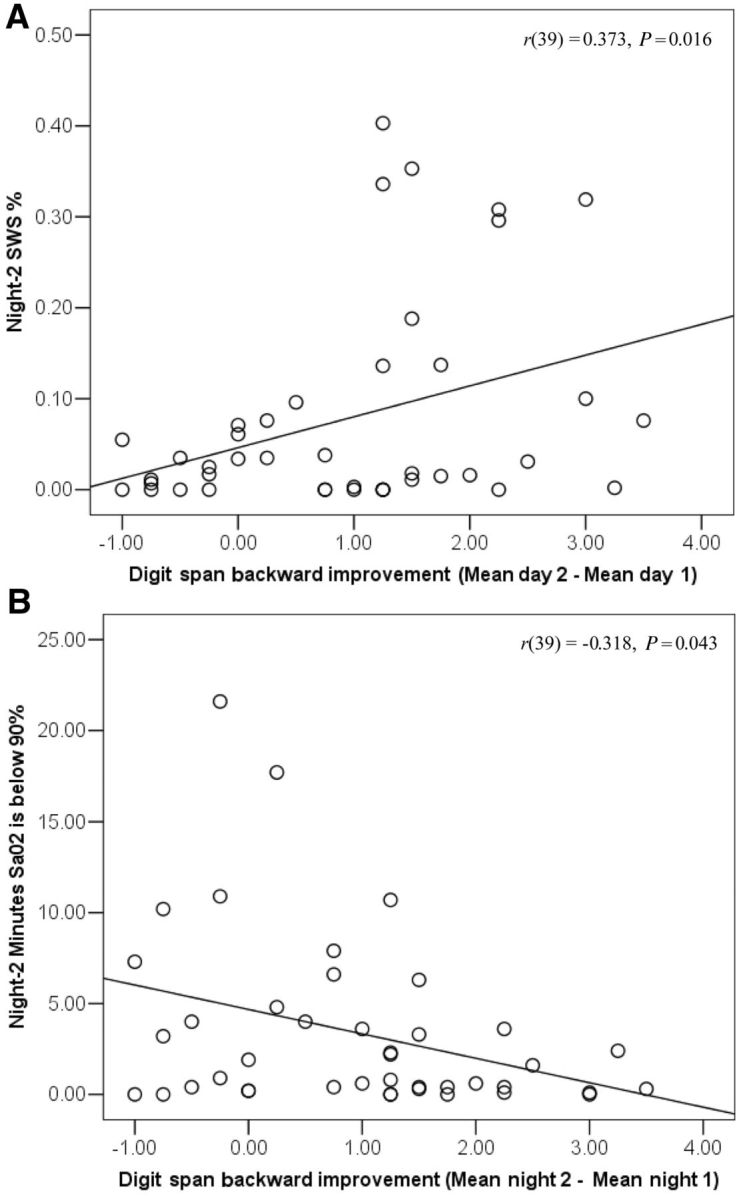
Scatterplots illustrating the relationship between mean digit span backward improvement (difference score used for illustrative purposes) and sleep parameters. (A) Night 2 slow-wave sleep (SWS) per cent; (B) severity of Night 2 night time oxygen desaturation in patients with Parkinson's disease on dopaminergic medication. P-values are uncorrected. The correlation is significant after Hochberg correction for Night 2 slow-wave sleep but not for Night 2 oxygen desaturation.
A close examination of Fig. 3A suggests that six patients with Parkinson's disease had higher slow-wave sleep than the other patients with Parkinson's disease, eliciting the question whether these patients differed on any relevant variables. The groups did not differ significantly on any Table 1 demographic variables, levodopa dose equivalence or any other medication classes (all P-values > 0.05). Patients with higher slow-wave sleep on Night 2 did not differ from the other patients with Parkinson's disease on length of diurnal sleep or amount of diurnal slow-wave sleep (all P-values > 0.10); they did show more Night 1 slow-wave sleep per cent [MHigher slow-wave sleep = 0.25, MLower slow-wave sleep = 0.06; t(40) = 5.83, P < 0.001], and less Night 1 Stage 2 sleep per cent [MHigher slow-wave sleep = 0.50, MLower slow-wave sleep = 0.66; t(40) = 2.08, P = 0.044].
The partial correlation between digit span backward improvement and the amount of time participants' Night 2 oxygen saturation levels were <90% (Fig. 3B) was not significant following Hochberg correction but had an uncorrected P-value < 0.05. Participants whose oxygen saturation were <90% for 5 or more min did not show a digit span backward improvement (MDay 2–Day 1 = 0.25; t < 1). Moreover, the patients with Parkinson's disease on dopaminergic medication with the highest quality sleep during Night 2, defined as those with relatively high levels of slow-wave sleep (at least 10%) and relatively brief oxygen desaturation (fewer than 5 min <90%), were those who showed the greatest digit span backward improvement (MDay 2–Day 1 = 1.97), t(8) = 8.58, P < 0.001, Cohen's d = 6.07. The above effects replicate when correlating final (Test 8) digit span backward performance (controlling for Test 1 performance) with Night 2 slow-wave sleep, r(39) = 0.420, P = 0.006 and Night 2 severe oxygen desaturation, r(39) = −0.375, P = 0.016 (significant following Hochberg adjustment).
No other sleep parameter including self-reported frequency of restless legs [r(34) = 0.102], nocturnal dream enactment, periodic leg movements, REM sleep per cent, or total sleep time (see Table 3 for full list), significantly correlated with digit span backward improvement in patients with Parkinson's disease taking dopaminergic medication. Furthermore, diurnal sleep that occurred during the Maintenance of Wakefulness Tests was not associated with nocturnal sleep or digit span performance. One possibility was that diurnal sleep would have a detrimental effect on nocturnal sleep but there were no significant negative correlations (all P-values > 0.10) between length of Day 1 diurnal sleep and Night 2 sleep parameters. Another possibility was that participants with diurnal sleep would have better digit span performance; however, there were no significant correlations between length of diurnal sleep and mean digit span backward or digit span forward performance (all P-values > 0.10). Additionally, digit span backward improvements (from Day 1 to Day 2) did not correlate with diurnal sleep, which was not surprising because of the low totals of slow-wave sleep during diurnal sleep (MDay 1 = 5.61 min, MDay 2 = 4.39 min).
We also considered whether the correlation between digit span backward and sleep parameters (e.g. slow-wave sleep %) simply reflects that individuals with relatively high levels of slow-wave sleep have better working memory. The data provided no support for this hypothesis. Mean digit span backward performance (collapsed across all tests) did not correlate significantly with mean slow-wave sleep (collapsed across both nights) [r(41) = 0.067] or mean time that nocturnal oxygen saturation levels were <90% [r(41) = −0.257]. The lack of a correlation between mean slow-wave sleep and mean digit span backward performance corresponds to the literature demonstrating few, if any, correlations between baseline night-time sleep parameters and baseline performance on neuropsychological batteries in older adults (Bliwise, 1989). This lack of correlation stands in contrast with Fenn and Hambrick's (2011) recent finding that baseline operation span performance (i.e. a working memory measure) predicts retention of episodic memories across sleep intervals in healthy younger adults. In other words, individual differences in baseline sleep measures may not explain individual differences in baseline cognitive measures (as in the present study), but individual differences in baseline cognitive measures may correlate with individual differences in cognitive improvements (Fenn and Hambrick, 2011).
The results have thus far suggested that digit span backward improvements across the 48-h protocol might be explained by the presence of dopaminergic medication, slow-wave sleep and nocturnal oxygen saturation, but not by other nocturnal sleep parameters (e.g. REM sleep) or by diurnal sleep. To determine the best predictor of digit span backward improvement in patients with Parkinson's disease we conducted a hierarchical regression analysis predicting Day 2 digit span backward performance. In Step 1, we controlled for Day 1 digit span backward performance. In Step 2 we entered duration of diurnal sleep and Night 2 REM sleep per cent. In Step 3, we entered (i) presence/absence of dopaminergic medication, (ii) amount of Night 2 slow-wave sleep and (iii) time in which oxygen saturation levels were <90% during Night 2 (the three factors in Step 3 did not correlate significantly with each other; all P-values > 0.10). Step 2 variables did not explain significant variance in digit span backward improvement (ΔR2 = 0.003, P = 0.775; both βs < 0.05, both P-values > 0.10). The Step 3 variables explained 19.35% of the remaining Day 2 digit span backward variance, F(3,46) = 3.67, P = 0.019. Of the Step 3 variables, slow-wave sleep (β = 0.16, P = 0.02), but not dopaminergic medication (β = 0.10, P = 0.18) or severity of nocturnal oxygen desaturation (β = −0.10, P = 0.17), significantly explained unique variance in digit span backward improvement. Similar results were observed when substituting dosage equivalents for presence/absence of dopaminergic medication as well as when excluding patients with Parkinson's disease with MMSE scores < 26 (n = 3). Thus, nocturnal slow-wave sleep during the training phase was the strongest predictor of digit span backward improvement.
Discussion
The most novel contribution of the present work regarded assessing the relationship between sleep and working memory performance improvements, which has previously received little attention in any population (Kuriyama et al., 2008, 2011; Scullin and McDaniel, 2010). Consistent with Kuriyama et al.'s (2008) study, the present results demonstrated a significant improvement in working memory performance only during the interval that included nocturnal sleep (i.e. between Tests 4 and 5); significant changes in working memory performance were not observed between daytime intervals (i.e. no improvement from Test 1 to Test 4 or from Test 5 to Test 8). This overnight working memory performance improvement that was observed in patients with Parkinson's disease perhaps bears some resemblance to the construct of ‘sleep benefit’ in such patients, a clinical construct that has been described for many years but one that has been difficult to demonstrate empirically (Factor et al., 1990; Currie et al., 1997; Merello et al., 1997; Högl et al., 1998; Bateman et al., 1999).
We expanded on previous sleep and working memory studies in three important ways. First, by separately examining patients with Parkinson's disease taking dopaminergic medication and patients with Parkinson's disease not taking dopaminergic medication we were able to provide converging evidence that dopamine might be important to working memory training (Dahlin et al., 2008a; Brehmer et al., 2009) and to offline sleep-specific cognitive improvements (de Lima et al., 2011; Schicknick et al., 2012). Second, by testing patients with DLB who have moderate dementia we were able to identify a patient population for whom sleep-dependent working memory improvements could no longer be detected. Third, and most importantly, by employing overnight polysomnography, we were able to identify potential roles for higher slow-wave sleep and improved nocturnal oxygen saturation in the facilitation of working memory training that might be amenable to future studies of targeted interventions.
We observed a possible negative correlation between severity of nocturnal oxygen desaturation and digit span backward improvement. Severity of nocturnal oxygen desaturation has been linked to cognitive impairments (Yamout et al., 2012), perhaps because such desaturation is indicative of sleep-disordered breathing (e.g. sleep apnoea). Kloepfer et al. (2009) found that sleep-dependent memory consolidation was impaired in patients who have moderate sleep apnoea (see also Djonlagic et al., 2012), which was consistent with our finding that severe nocturnal oxygen desaturation was linked to impaired offline working memory performance improvements. Though we did not observe a significant correlation between digit span backward improvement and apnoea–hypopnoea index (i.e. visually scored breathing events), this is likely to be due to our oxygen desaturation measure and apnoea–hypopnoea index not correlating significantly during Night 2 [r(41) = −0.008; these variables were correlated, as one would typically expect, during Night 1, r(41) = 0.503, P = 0.001]. Nocturnal hypoxia might be implicated, at least in part, in deficient working memory function, by virtue of the fact that nocturnal oxygen desaturation might reduce dopamine levels. Indeed, animal studies have found that intermittent hypoxia can cause reductions in extracellular dopamine levels (Decker et al., 2005) and working memory (Decker et al. 2003).
Though severity of nocturnal oxygen desaturation and presence of dopaminergic medication contributed to the observation of working memory performance improvements, slow-wave sleep was retained as the most significant contributor to working memory performance improvements. This novel finding converges with emerging research demonstrating that slow-wave sleep promotes synaptic plasticity, memory reactivation and cortical reorganization (Wilson and McNaughton, 1994; Tononi and Cirelli, 2003; Stickgold, 2005; Takashima et al., 2006; Rasch et al., 2007; Dang-Vu et al., 2010). Interestingly, slow-wave sleep is greatly reduced with increasing age and in dementia relative to healthy younger adults (Bliwise, 1993). The present study, which linked relatively preserved slow-wave sleep during training intervals to working memory performance improvements in a neurodegenerative condition, therefore raises the possibility that age-dependent slow-wave sleep reductions explain why working memory training is usually less effective in older adults than in younger adults (Dahlin et al., 2008b; Schmiedek et al., 2010). Future sleep and working memory studies may include a healthy adult control group to determine the extent to which Parkinson's disease is associated with impairments in sleep-dependent cognitive processes (cf. Marinelli et al., 2009, who reported Parkinson's disease impairments in motor memory consolidation).
An implication of the associations between sleep-dependent processes and working memory performance improvements is that behavioural studies of working memory need to assess the contributions of sleep. For example, training studies might pre-screen potential participants using overnight polysomnography and/or ambulatory pulse oximetry. A related approach is to continue to monitor sleep architecture and breathing in sleep during the training phase. Doing so allows for the examination of how fluctuations in sleep processes predict working memory capacity improvements. A third possible future approach would include experimentally correcting for sleep disturbances prior to working memory training. Physical exercise has been linked to increases in slow-wave sleep (Horne, 1981; Shapiro et al., 1981) and continuous positive airway pressure can eliminate sleep-disordered breathing. No study has examined whether continuous positive airway pressure promotes overnight, offline facilitation of working memory performance (cf. Kloepfer et al., 2009; Djonlagic et al., 2012).
Working memory capacity has traditionally been considered to be a stable trait, but the present results suggest that working memory capacity is potentially modifiable in some patients with Parkinson's disease, but not in patients with DLB who show more severe impairments on the MMSE. The observed performance improvements are striking because working memory capacity is degraded in patients with Parkinson's disease (Cooper et al., 1991; Altgassen et al., 2007), and cognitive impairments in this population may lead to impaired workplace functioning, worsened quality of life, increased risk for maintaining independent living and increased burden to caregivers. Training working memory after first correcting existing sleep disturbances may help alleviate some of the non-motor burdens associated with Parkinson's disease.
Funding
This work was supported by the National Institutes on Health (grant number R01 NS-050595 to D.L.B., F32 AG-041543 to M.K.S., KL2 RR-025009 to L.M.T., UL1 RR-025008 for the Atlanta Clinical and Translational Science Institute). M.K.S. was also partially supported by a Cottrell Fellowship from Emory University School of Medicine.
Acknowledgements
We are appreciative of Jill Shelton and Tyler Harrison for their helpful comments during the preparation of this article.
Glossary
Abbreviations
- DLB
dementia with Lewy bodies
- MMSE
Mini-Mental State Examination
- UPDRS
Unified Parkinson's Disease Rating Scale
References
- Altgassen M, Phillips L, Kopp U, Kliegel M. Role of working memory components in planning performance in Parkinson's disease. Neuropsychologia. 2007;45:2393–7. doi: 10.1016/j.neuropsychologia.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255:556–9. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li S, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav R. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Bateman DE, Levett K, Marsden CD. Sleep benefit in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:384–5. doi: 10.1136/jnnp.67.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL. Neuropsychological function and sleep. Clin Geriatr Med. 1989;5:381–94. [PubMed] [Google Scholar]
- Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- Bliwise DL, Trotti LM, Wilson AG, Greer SA, Wood-Silverio C, Juncos JJ, et al. Daytime alertness in Parkinson's disease: potentially dose-dependent, divergent effects by drug class. Mov Disord. doi: 10.1002/mds.25082. 2012. Advance Access published on Jul 2 2012, doi:10.1002/mds.25082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Bellander M, Fürth D, Karlsson S, Bäckman L. Working memory plasticity modulated by dopamine transporter genotype. Neurosci Lett. 2009;467:117–20. doi: 10.1016/j.neulet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Brewer GA, Knight JB, Marsh RL, Unsworth N. Individual differences in event-based prospective memory: evidence for multiple processes supporting cue detection. Mem Cognition. 2010;38:304–11. doi: 10.3758/MC.38.3.304. [DOI] [PubMed] [Google Scholar]
- Chein J, Morrison A. Expanding the mind's workspace: training and transfer effects with a complex working memory span task. Psychon B Rev. 2010;17:193–9. doi: 10.3758/PBR.17.2.193. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker R, Sahakian B, Robbins T. Mechanisms of cognitive set flexibility in Parkinson's disease. Brain. 2001;124:2503–12. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson's disease and its relationship to motor disability. Brain. 1991;114:2095–122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Doherty M, Jordan N, Tidswell P, Sullivan EV. Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson's disease. Brain. 1992;115:1701–25. doi: 10.1093/brain/115.6.1701. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Currie LJ, Bennett JP, Harrison MB, Trugman JM, Wooten GF. Clinical correlates of sleep benefit in Parkinson's disease. Neurology. 1997;48:1115–7. doi: 10.1212/wnl.48.4.1115. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Neely A, Larsson A, Bäckman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008a;320:1510–2. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Nyberg L, Bäckman L, Neely A. Plasticity of executive functioning in young and older adults: Immediate training gains, transfer, and long-term maintenance. Psychol Aging. 2008b;23:720–30. doi: 10.1037/a0014296. [DOI] [PubMed] [Google Scholar]
- Dang-Vu T, Schabus M, Desseilles M, Sterpenich V, Bonjean M, Maquet P. Functional neuroimaging insights into the physiology of human sleep. Sleep. 2010;33:1589–603. doi: 10.1093/sleep/33.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MJ, Hue GE, Caudle WM, Miller GW, Keating GL, Rye DB. Episodic neonatal hypoxia evokes executive dysfunction and regionally specific alterations in markers of dopamine signaling. Neurosci. 2003;117:417–25. doi: 10.1016/s0306-4522(02)00805-9. [DOI] [PubMed] [Google Scholar]
- Decker MJ, Jones KA, Solomon IG, Keating GL, Rye DB. Reduced extracellular dopamine and increased responsiveness to novelty: neurochemical and behavioral sequelae of intermittent hypoxia. Sleep. 2005;28:169–76. doi: 10.1093/sleep/28.2.169. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Presti-Torres J, Dornelles A, Scalco FC, Roesler R, Garcia VA, et al. Modulatory influence of dopamine receptors on consolidation of object recognition memory. Neurobiol Learn Mem. 2011;95:305–10. doi: 10.1016/j.nlm.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Djonlagic I, Saboisky J, Carusona A, Stickgold R, Malhotra A. Increased sleep fragmentation leads to impaired off-line consolidation of motor memories in humans. PLoS One. 2012;7:e34106. doi: 10.1371/journal.pone.0034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle R, Tuholski S, Laughlin J, Conway A. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J Exp Psychol Gen. 1999;128:309–31. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Factor SA, McAlarney T, Sanchez-Ramos JR, Weiner WJ. Sleep disorders and sleep effect in Parkinson's disease. Movement Disord. 1990;5:280–5. doi: 10.1002/mds.870050404. [DOI] [PubMed] [Google Scholar]
- Fenn KM, Hambrick DZ. Individual differences in working memory capacity predict sleep-dependent memory consolidation. J Exp Psychol Gen. 2011 doi: 10.1037/a0025268. Advance Access published on Sept 12 2011, doi:10.1037/a0025268. [DOI] [PubMed] [Google Scholar]
- Fowler MJ, Sullivan MJ, Ekstrand BR. Sleep and memory. Science. 1973;179:302–4. doi: 10.1126/science.179.4070.302. [DOI] [PubMed] [Google Scholar]
- Groeger JA, Field D, Hammond SM. Measuring memory span. Int J Psychol. 1999;34:359–63. [Google Scholar]
- Grosset KA, Drosset DG. Proposed dose equivalence for rapid switching between dopamine agonists in Parkinson's disease. Clin Ther. 2006;28:1063–4. doi: 10.1016/j.clinthera.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Hempel A, Giesel FL, Garcia Caraballo NM, Amann M, Meyer H, Wustenberg T, et al. Plasticity of cortical activation related to working memory during training. Am J Psychiat. 2004;161:745–7. doi: 10.1176/appi.ajp.161.4.745. [DOI] [PubMed] [Google Scholar]
- Hill B, Elliott E, Shelton J, Pella R, O'Jile J, Gouvier W. Can we improve the clinical assessment of working memory? An evaluation of the Wechsler Adult Intelligence Scale-Third Edition using a working memory criterion construct. J Clin Exp Neuropsyc. 2010;32:315–23. doi: 10.1080/13803390903032529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson DE, Lang AE, Martin WRW, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden onset sleep in Parkinson's disease: a survey by the Canadian Movement Disorders Group. JAMA. 2002;287:455–63. doi: 10.1001/jama.287.4.455. [DOI] [PubMed] [Google Scholar]
- Högl BE, Gómez-Arévalo G, García S, Scipioni O, Rubio M, Blanco M, et al. A clinical, pharmacologic, and polysomnographic study of sleep benefit in Parkinson's disease. Neurology. 1998;50:1332–9. doi: 10.1212/wnl.50.5.1332. [DOI] [PubMed] [Google Scholar]
- Horne JA. The effects of exercise upon sleep: a critical review. Biol Psychol. 1981;12:241–90. doi: 10.1016/0301-0511(81)90001-6. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA. 2008;105:6829–33. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci. 2007;10:100–7. doi: 10.1038/nn1825. [DOI] [PubMed] [Google Scholar]
- Kloepfer C, Riemann D, Nofzinger EA, Feige B, Unterrainer J, O'Hara R, et al. Memory before and after sleep in patients with moderate obstructive sleep apnea. J Clin Sleep Med. 2009;5:540–8. [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K, Honma M, Shimazaki M, Horie M, Yashiike T, Koyama S, et al. An N-methyl-d-aspartate receptor agonist facilitates sleep-independent synaptic plasticity associated with working memory capacity enhancement. Sci Rep. 2011;1:127. doi: 10.1038/srep00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama K, Mishima K, Suzuki H, Aritake S, Uchiyama M. Sleep accelerates the improvement in working memory performance. J Neurosci. 2008;28:10145–50. doi: 10.1523/JNEUROSCI.2039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's disease. First of two parts. N Engl J Med. 1998;339:1044–53. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Langston JW, Widner H, Goetz CG, Brooks D, Fahn S, Freeman T, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7:2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- Lavigne GJ, Montplaisir JY. Restless legs syndrome and sleep bruxism: prevalence and association among Canadians. Sleep. 1994;17:739–43. [PubMed] [Google Scholar]
- Logie R, Gilhooly K, Wynn V. Counting on working memory in arithmetic problem solving. Mem Cognition. 1994;22:395–410. doi: 10.3758/bf03200866. [DOI] [PubMed] [Google Scholar]
- Lotharius J, Brundin P. Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3:932–42. doi: 10.1038/nrn983. [DOI] [PubMed] [Google Scholar]
- Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, et al. Learning and consolidation of visuo-motor adaptation in Parkinson's disease. Parkinsonism Relat D. 2009;15:6–11. doi: 10.1016/j.parkreldis.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí M, Tolosa E, Campdelacreu J. Clinical overview of the synucleinopathies. Mov Disord. 2003;18(Suppl 6):S21–7. doi: 10.1002/mds.10559. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe DM, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, et al. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–2. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- Merello M, Hughes A, Colosimo C, Hoffman M, Starkstein S, Leiguarda R. Sleep benefit in Parkinson's disease. Mov Disord. 1997;12:506–8. doi: 10.1002/mds.870120405. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C. A review of cognitive impairments in dementia with Lewy bodies relative to Alzheimer's disease and Parkinson's disease with dementia. Cortex. 2007;43:583–600. doi: 10.1016/s0010-9452(08)70489-1. [DOI] [PubMed] [Google Scholar]
- Morrison A, Chein J. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon B Rev. 2011;18:46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- Miller G. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956;63:81–97. [PubMed] [Google Scholar]
- Norman GR, Steiner DL. Biostatistics: the bare essentials. Hamilton, Canada: B.C. Decker Inc.; 2000. [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Phillips LH, Wynn V, Gilhooly KJ, Della Sala S, Logie RH. The role of memory in the Tower of London task. Memory. 1999;7:209–31. doi: 10.1080/741944066. [DOI] [PubMed] [Google Scholar]
- Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160:2137–41. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. doi: 10.1126/science.1138581. [DOI] [PubMed] [Google Scholar]
- Reynolds C. Forward and backward memory span should not be combined for clinical analysis. Arch Clin Neuropsych. 1997;12:29–40. [PubMed] [Google Scholar]
- Schicknick H, Reichenbach N, Small K-H, Scheich H, Gundelfinger ED, Tischmeyer W. Dopamine modulates memory consolidation of discrimination learning in the auditory cortex. Eur J Neurosci. 2012;35:763–74. doi: 10.1111/j.1460-9568.2012.07994.x. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Lovden M, Lindenberger U. Hundred days of cognitive training enhance broad abilities in adulthood: findings from the COGITO study. Front Aging Neurosci. 2010;2:1–10. doi: 10.3389/fnagi.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin M, McDaniel M. Remembering to execute a goal: sleep on it! Psychol Sci. 2010;21:1028–035. doi: 10.1177/0956797610373373. [DOI] [PubMed] [Google Scholar]
- Shapiro CM, Bortz R, Mitchell D, Bartel P, Jooste P. Slow-wave sleep: a recovery period after exercise. Science. 1981;214:1253–54. doi: 10.1126/science.7302594. [DOI] [PubMed] [Google Scholar]
- Shelton JT, Elliott EM, Hill BD, Calamia MR, Gouvier WD. A comparison of laboratory and clinical working memory tests and their prediction of fluid intelligence. Intelligence. 2009;37:283–93. doi: 10.1016/j.intell.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton J, Elliott E, Matthews R, Hill B, Gouvier W. The relationships of working memory, secondary memory, and general fluid intelligence: working memory is special. J Exp Psychol Learn Mem Cogn. 2010;36:813–20. doi: 10.1037/a0019046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychol Bull. 2012;138:628–654. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–8. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Takashima A, Petersson K, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, et al. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci USA. 2006;103:756–61. doi: 10.1073/pnas.0507774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–50. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J, Basak C. A working memory workout: how to expand the focus of serial attention from one to four items in 10 hours or less. J Exp Psychol Learn Mem Cogn. 2004;30:1322–37. doi: 10.1037/0278-7393.30.6.1322. [DOI] [PubMed] [Google Scholar]
- Vogt A, Kappos L, Calabrese P, Stöcklin M, Gschwind L, Opwis K, et al. Working memory training in patients with multiple sclerosis - comparison of two different training schedules. Restor Neurol Neuros. 2009;27:225–35. doi: 10.3233/RNN-2009-0473. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale-Third Edition: Technical Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson M-L, Bartfai A, et al. Computerized working memory training after stroke – A pilot study. Brain Injury. 2007;21:21–29. doi: 10.1080/02699050601148726. [DOI] [PubMed] [Google Scholar]
- Wilson M, McNaughton B. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Yamout K, Goldstein F, Lah J, Levey A, Bliwise D. Neurocognitive correlates of nocturnal oxygen desaturation in a memory clinic population. J Clin Exp Neuropsy. 2012;34:325–332. doi: 10.1080/13803395.2011.642849. [DOI] [PMC free article] [PubMed] [Google Scholar]