Abstract
Four methicillin-resistant coagulase-negative staphylococci (MRCoNS), one Staphylococcus haemolyticus and three Staphylococcus cohnii, from infections of humans collected via the Ministry of Health National Antimicrobial Resistance Surveillance Net (Mohnarin) program in China were identified as linezolid-resistant. These four isolates were negative for the 23S rRNA mutations, but positive for the gene cfr. Mutations in the gene for the ribosomal protein L3, which resulted in the amino acid exchanges Gly152Asp and Tyr158Phe, were identified in S. haemolyticus 09D279 and S. cohnii NDM113, respectively. In each isolate, the cfr gene was located on a plasmid of ca. 35.4 kb, as shown by S1 nuclease pulsed-field gel electrophoresis and Southern blotting experiments. This plasmid was indistinguishable from the previously described plasmid pSS-02 by its size, restriction pattern, and a sequenced 14-kb cfr-carrying segment. Plasmid pSS-02 was originally identified in staphylococci isolated from pigs. This is the first time that a cfr-carrying plasmid has been detected in MRCoNS obtained from intensive care patients in China. Based on the similarities to the cfr-carrying plasmid pSS-02 from porcine coagulase-negative staphylococci, a transmission of this cfr-carrying plasmid between staphylococci from pigs and humans appears to be likely.
Introduction
Linezolid is an important antimicrobial agent for the therapy of infections caused by gram-positive pathogens, especially methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Linezolid is available in almost 70 countries, and has been used to treat approximately four million patients since it has been approved for clinical use in the U.S.A. in 2000 [1]. Resistance to linezolid was first reported in a methicillin-resistant Staphylococcus aureus (MRSA) clinical isolate in 2001 [2]. Since then, the occurrence of linezolid-resistant staphylococci has been increasingly reported in Europe (e.g. in Ireland and Spain) and in the United States [3].
Resistance to oxazolininones can be based on mutations in the central loop of the 23SrRNA gene with the substitution G2576T occurring most frequently; substitutions for T2500A, T2504A and G2215A have also been found in staphylococcal isolates from clinical infections, while G2444T, G2447T, A2503G and T2504C have so far only been found among laboratory-derived Staphylococcus strains [3], [4]. Moreover, elevated linezolid MICs can also be associated with mutations in the genes for the ribosomal proteins L3 and L4, some regions of which interact closely with the linezolid binding site in the peptidyltransferase center [5], [6]. More recently, the transferable multiresistance gene cfr, originally identified in a bovine Staphylococcus sciuri isolate, was found to code for a RNA methyltransferase which modifies the adenine residue at position 2503 in the 23S rRNA and thereby confers resistance not only to oxazolidinones, but also to phenicols, lincosamides, pleuromutilins, and streptogramin A antibiotics (otherwise known as the PhLOPSA phenotype) [7]. To date, the cfr gene has been found in staphylococci from clinical cases isolated from Colombia, the United States, Italy, Spain, Ireland and Mexico [8], [9], [10], [11], [12], [13], [14], [15], [16].
In China, linezolid was first approved for use in clinical practice in 2007. Since then, there has been only one report of linezolid resistant methicillin-resistant coagulase-negative staphylococci, and this occurred in an intensive care unit (ICU) of a Chinese hospital [17]. In the respective study, the cfr gene was detected by PCR, but neither a plasmid location of the cfr gene could be confirmed nor the genetic environment of the cfr gene be determined. The present study was conducted to investigate four clinical linezolid-resistant Staphylococcus spp. isolates collected from the Ministry of Health National Antimicrobial Resistance Surveillance Net (Mohnarin) program in China for the presence and the location of the cfr gene, but also linezolid resistance-mediating mutations which may be present in the same isolates.
Materials and Methods
Bacterial isolates
Four linezolid-resistant coagulase-negative Staphylococcus isolates, including one Staphylococcus haemolyticus (09D279) and three Staphylococcus cohnii isolates (09D253, 09D363 and NDM113), were identified among 713 clinical staphylococcal isolates by growth on brain heart infusion (BHI) agar containing 6 µg/ml linezolid. All isolates were collected between 2009 and 2010 from 19 hospitals that participated in the Mohnarin program. These hospitals are located in 17 cities that are widely distributed across China. The four isolates (09D279, 09D253, 09D363 and NDM113) were obtained from blood cultures collected from four ICU patients, all of which were over 60 years of age. Further information about these patients, the underlying disease and possible antimicrobial pretreatment were unfortunately not available. The S. haemolyticus 09D279 collected in January 2010 and the S. cohnii isolates 09D253 collected in December 2009 and 09D363 collected in March 2010 were obtained from individual patients in hospital A in Shenyang, Liaoning province, whilst S. cohnii NDM113 collected in October 2010 was isolated from a patient in hospital B in Beijing. Two linezolid-susceptible isolates S. haemolyticus 09D044 and S. cohnii 09D071 (both with MIC values of 0.5 µg/ml) from hospital A were included as internal negative controls for the sequencing approaches. S. aureus RN4220 served as the recipient strain for transformation experiments.
Antimicrobial susceptibility testing
The antimicrobial susceptibilities of the four clinical isolates and the S. aureus RN4220 reference strain and its transformants were determined using the agar dilution method according to the recommendations given in document M100-S22 [18] of the Clinical and Laboratory Standards Institute (CLSI). S. aureus ATCC®29213 served as a quality strain for susceptibility testing.
DNA extraction and detection of linezolid resistance-mediating mutations and the multiresistance gene cfr
Whole-cell DNA from the Staphylococcus isolates was isolated using a commercial kit (TianGen, Beijing, China) according to the manufacturer's instructions. Plasmid DNA was extracted withthe QIAGEN plasmid extraction Midi Kit (Qiagen, Hilden, Germany) using a previously described modification [19]. To detect potential mutations involved in linezolid resistance, the genes rplC and rplD, coding for ribosomal proteins L3 and L4, respectively, and partial sequences of 23S rDNA were amplified and sequenced using previously described primers [20], [21]. The detection of the cfr gene also followed a previously described PCR assay [19].
Molecular typing and analysis of cfr-carrying plasmids
To determine their genetic relatedness, the three linezolid-resistant S. cohnii isolates were subjected to pulsed-field gel electrophoresis (PFGE) according to a protocol described previously [19]. To analyze the location of the cfr gene, S1 nuclease-PFGE and Southern blot analysis were performed. Briefly, whole-cell DNA of the four linezolid-resistant isolates embedded in agarose gel plugs was treated with S1 nuclease (TaKaRa, Dalian, China) and separated by PFGE alongside a standard low PFG marker (NEB, UK). Subsequently, Southern blot analysis was performed using a DNA probe specific for the cfr gene, which was non-radioactively labeled with a DIG High Prime DNA labeling and detection kit (Roche Diagnostics, Mannheim, Germany) as described previously [19]. In addition, the purified plasmids extracted from each of the four original strains were transformed into the S. aureus recipient strain RN4220 by electrotransformation [22]. Transformants were selected by incubation for 24 h on BHI agar supplemented with 10 µg/ml florfenicol. The transformants were screened for the presence of plasmids by S1-PFGE and their resistance phenotypes were determined. The sizes of the cfr-carrying plasmids extracted from the transformants were estimated by calculation of the sums of the different fragment sizes obtained after BglII digestion.
DNA sequencing
The partial nucleotide sequences of the cfr-carrying plasmids extracted from the transformants were determined by primer walking (Invitrogen, Beijing, China), or a modified random primer sequencing walking strategy [19]. The sequences obtained were annotated using the VectorNTI program (Invitrogen, Carlsbad, CA), and the predicated coding sequences were identified using GLIMMER software. The DNA sequences and deduced amino acid sequences were compared to those deposited in GenBank using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast).
Nucleotide Sequence Accession Number
The nucleotide sequences of a 13,976-bp fragment of cfr-carrying plasmid have been deposited in GenBank with the accession number JX827253.
Results and Discussion
Identification of linezolid resistance-mediating mutations and the gene cfr
All four linezolid-resistant staphylococcal isolates were PCR-positive for the cfr gene, and the sequences of the cfr amplicons obtained from these isolates were identical to one another and to the corresponding cfr sequence of plasmids pSCFS1 (GenBank acccession number AJ579365) in S. sciuri, pSS-01 (JQ041372) in S. cohnii, pSS-02 (JF834910) in Staphylococcus saprophyticus, pSCFS6 (AM408573) in Staphylococus warneri and pSCFS7 (FR675942) in ST8-MRSA-IVa/USA300, and also shared 99.9% sequence identity with cfr from pSCFS3 in S. aureus (AM086211). In addition, all four cfr-carrying staphylococcal isolates showed wild-type sequences of the 23S rDNA and the gene rplD for the L4 protein through PCR. Alterations were detected in the gene rplC, which resulted in amino acid substitutions Gly152Asp and Tyr158Phe in the L3 proteins of S. haemolyticus 09D279 and S. cohnii NDM113, respectively. The Gly152Asp substitution in the L3 protein has been implicated indirectly in reducing the affinity of oxazolidinones for its target through perturbation of bases 2505 and 2506 in the coding sequence [23]. In addition, in this study, the Tyr158Phe substitution in the L3 protein of S. cohnii NDM113 involved a residue that was located in close proximity to the residues Gly155 and Ala157, which were previously found to be associated with linezolid resistance. Substitutions at these positions were reported to cause resistance by abolishing linezolid binding to its target [23], [24]. Thus, the presence of the cfr gene and L3 substitutions in both S. haemolyticus 09D279 and S. cohnii NDM113 may act synergistically.
Antimicrobial resistance patterns, PFGE analysis, and plasmid analysis
All four cfr-carrying isolates exhibited resistance to linezolid, chloramphenicol, and clindamycin, and showed elevated MICs to florfenicol, tiamulin, quinupristin/dalfopristin and virginiamycin M1, all of which are consistent with the resistance phenotype caused by the cfr gene. Additionally, these isolates were resistant to oxacillin, cefoxitin, and levofloxacin, but susceptible to rifampicin, tigecycline, teicoplanin, and vancomycin (Table 1). All four oxacillin-resistant isolates harbored the mecA gene and were therefore considered to be methicillin-resistant coagulase-negative staphylococci (MRCoNS). The MIC results showed that the three S. cohnii isolates exhibited resistance to erythromycin and susceptibility to sulfamethoxazole-trimethoprim, while the S. haemolyticus 09D279 isolate was susceptible to erythromycin but resistant to trimethoprim-sulfamethoxazole (Table 1). The PFGE results suggested that the three S. cohnii isolates represented two different clones, with isolates 09D253 and 09D363 recovered from different patients in hospital A belonging to the same clone (data not shown).
Table 1. Antimicrobial susceptibility profiles of linezolid-resistant clinical strains.
| Staphylococcal isolates | MIC (in µg/ml)* | ||||||||||||||||
| LZD | CHL | CLI | FFC | TIA | VM1 | Q-D | OXA | FOX | LEV | ERY | RIF | TGC | SXT | GEN | VAN | TEC | |
| S. haemolyticus 09D279 | 8 | 256 | 32 | 256 | 256 | 32 | 2 | ≥512 | 256 | 16 | 0.25 | 0.008 | 0.125 | 64 | 8 | 0.5 | 4 |
| S. cohnii 09D253 | 32 | 128 | ≥512 | 256 | 128 | >128 | 4 | 1 | 8 | 8 | ≥512 | 0.016 | 0.125 | 0.125 | 1 | 0.5 | 2 |
| S. cohnii 09D363 | 32 | 128 | ≥512 | 256 | 128 | 128 | 4 | 0.5 | 8 | 8 | ≥512 | 0.008 | 0.125 | 0.125 | 2 | 0.5 | 2 |
| S. cohnii NDM113 | 32 | 128 | 256 | 256 | 128 | 128 | 8 | 128 | 32 | 8 | 256 | 0.008 | 0.25 | 0.125 | 32 | 0.5 | 1 |
| S. aureus RN4220 | 2 | 8 | 0.125 | 1 | 0.5 | 0.5 | 0.25 | 0.125 | 1 | 0.25 | 0.125 | 0.008 | 0.125 | 0.125 | 0.125 | 2 | 0.5 |
| S. aureus RN4220 + pSS-02-like (09D279) | 8 | >256 | >256 | 128 | 64 | 4 | 2 | 0.125 | 1 | 0.25 | 0.125 | 0.008 | 0.125 | 0.125 | 0.125 | 2 | 0.5 |
| S. aureus RN4220 + pSS-02-like (09D253) | 8 | >256 | >256 | 128 | 64 | 4 | 2 | 0.125 | 1 | 0.25 | 0.125 | 0.008 | 0.125 | 0.125 | 0.125 | 2 | 0.5 |
| S. aureus RN4220 + pSS-02-like (09D363) | 8 | >256 | >256 | 128 | 64 | 4 | 1 | 0.125 | 1 | 0.25 | 0.25 | 0.008 | 0.125 | 0.125 | 0.125 | 2 | 0.5 |
| S. aureus RN4220 + pSS-02-like (NDM113) | 8 | >256 | >256 | 128 | 64 | 4 | 2 | 0.125 | 1 | 0.25 | 0.125 | 0.008 | 0.125 | 0.125 | 0.25 | 2 | 0.5 |
| S. aureus RN4220 + pSS-02 | 8 | >256 | >256 | 256 | 64 | 4 | 2 | 0.12 | 1 | 0.25 | 0.125 | 0.016 | 0.125 | 0.125 | 0.25 | 2 | 0.5 |
LZD, linezolid; CHL, chloramphenicol, CLI, clindamycin; FFC, florfenicol; TIA, tiamulin; VM1, virginiamycin M1; Q-D, quinupristin/dalfopristin; OXA, oxacillin; FOX, cefoxitin; LEV, levofloxacin; ERY, erythromycin; RIF, rifampicin; TGC, tigecycline; SXT, sulfamethoxazole-trimethoprim; GEN, gentamicin; VAN, vancomycin; TEC, teicoplanin.
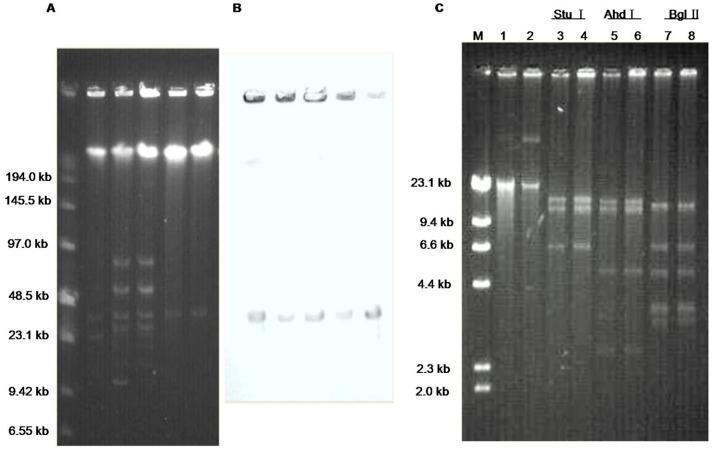
Although the cfr gene can also be located in chromosomal DNA, it is mostly plasmid-borne [25]. S1 nuclease-PFGE analysis revealed that each of the four isolates harbored multiple plasmids of different sizes. The two clonally related S. cohnii isolates displayed the similar plasmid profile (Figure 1A). In addition, we found that the cfr probe hybridized to a plasmid of ca. 35.4 kb in all four clinical isolates (Figure 1B). Electrotransformation into S. aureus RN4220 was successful and transformants carrying only the 35.4-kb plasmid were obtained from each of the four MRCoNS isolates. A similar-sized plasmid, designated pSS-02, has previously been identified in S. sciuri and S. saprophyticus isolates of porcine origin [19].
Figure 1. Identification and characterization of the pSS-02-like cfr-carrying plasmid found in the four MRCoNS isolates in comparison to the original plasmid pSS-02.
(A) S1 nuclease-PFGE (B) Southern blot hybridization with the cfr probe. Lane 1:S. haemolyticus 09D279, Lane 2: S. cohnii 09D253, Lane 3: S. cohnii 09D363; Lane 4: S. cohnii NDM113; Lane 5: RN4220 + pSS-02. (C) Restriction digests of the pSS-02-like plasmid extracted from transformants originating from S. haemolyticus 09D279 (lanes 1, 3, 5, and 7) and the original plasmid pSS-02 extracted from transformants originating from S. saprophyticus 2-87 (lanes 2, 4, 6, and 8). The restriction enzymes used to digest the plasmids are indicated above the respective lanes.
MIC determination of these transformants identified the same resistance pattern, namely resistance to linezolid, chloramphenicol and clindamycin, and presented elevated MICs of florfenicol, tiamulin, quinupristin/dalfopristin and virginiamycin M1 in all transformants (Table 1). This resistance phenotype is indicative for the presence and the functional activity of the cfr gene. In addition, the transformants that carried these cfr plasmids had identical MIC values when compared with the previously described RN4220 transformants harboring plasmid pSS-02 (Table 1). Moreover, indistinguishable AhdI, BglII, EcoRI, StuI and XhoI restriction patterns were observed in both pSS-02 and the cfr-carrying plasmids extracted from transformants in this study. Some of the restriction patterns are shown in Figure 1C. Taken together, the aforementioned observations strongly suggest that a plasmid which is at least very closely related to plasmid pSS-02 of porcine origin, was also present in these four clinical MRCoNS isolates from China.
Genetic environment of the cfr gene on plasmid pSS-02
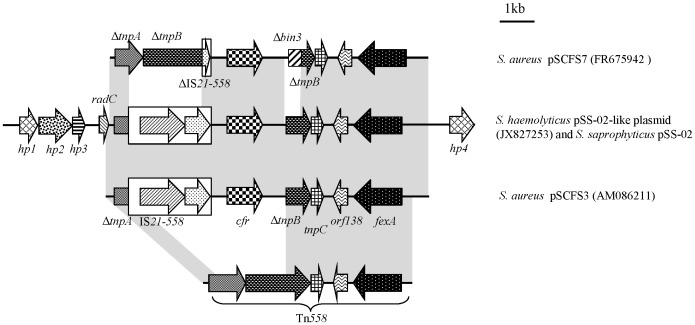
To gain insight into the genetic environment of the cfr gene on the pSS-02-like plasmid extracted from a S. haemolyticus 09D279 transformant (patient origin), a 13,976 bp segment encompassing the cfr gene was sequenced. Of plasmid pSS-02 from porcine S. saprophyticus, a 8.5-kb segment including the cfr gene had been sequenced. For a better comparison, sequence analysis of the original plasmid pSS-02 had been extended to match the sequence of the pSS-02-like plasmid from human S. haemolyticus 09D279. A comparison of these two almost 14-kb fragments revealed 100% nucleotide sequence identity (Figure 2). Subsequently, this segment was sequenced from plasmids of the remaining three clinical S. cohnii isolates and shown to be also identical to the aforementioned two sequences. The detection of identical cfr-carrying segments on plasmids isolated from CoNS of porcine and MRCoNS of human origin provided further confirmation that plasmid pSS-02 or a closely related derivative is exchanged between animal and human staphylococci.
Figure 2. Schematic representation of the genetic environment of the cfr gene in pSS-02-like, pSS-02, pSCFS3 and pSCFS7 plasmids.
Arrows indicate the positions and directions of gene transcription. Regions exhibiting >99% homology are marked with gray shading. Δ indicates a truncated gene. The distance scale (in kilobases) is displayed in the upper right-hand corner.
In this 14-kb segment from pSS-02, a 9.5-kb region containing an IS21-558 insertion sequence and the cfr gene, were integrated into a Tn558 element, thereby truncating the transposase genes tnpA and tnpB. This 9.5-kb region resembled closely (99.8% identity; 9487/9503 bp) the corresponding region of the ca. 34.7-kb pSCFS3 (GenBank accession number AM086211), which originated from bovine and porcine Staphylococcus lentus and porcine S. aureus (including one MRSA ST398) from Germany; while differed slightly from the corresponding region of the ca. 45-kb plasmid pSCFS7, which originated from a linezolid-resistant ST8-MRSA-IVa/USA300 recovered from a skin scalp abscess of Irish male (Figure 2) [10], [26]. Moreover, we previously reported that pSS-02 might be similar to pSCFS3 based on their similar plasmid sizes, BglII digest fragment patterns, and the absence of other antimicrobial resistance genes [19]. Immediately upstream of the truncated tnpA sequence in pSS-02, we found the radC gene that encoded an 109 amino acid DNA repair protein that shared 94.5% identity with the 114 amino acid RadC protein from the S. aureus plasmid pSK73 (GenBank accession number GQ915269). Three open reading frames encoding hypothetical proteins were identified upstream of the radC gene and another downstream of the fexA gene on Tn558. The observation that similar plasmids (pSS-02 and pSCFS3) were found in porcine isolates of S. sciuri and S. saprophyticus, and clinical samples of S. cohnii and S. haemolyticus in China, as well as bovine and porcine S. lentus and porcine S. aureus in Europe, provides further confirmation of how widely these plasmids are disseminated.
In conclusion, this is the first report of four clinical linezolid-resistant MRCoNS in which a cfr-carrying plasmid previously found in staphylococci from food producing animal was detected. This finding has important implications as it showed that closely related – if not identical – plasmids can be exchanged between CoNS from animals and MRCoNS from humans and that these MRCoNS can be involved in severe infections in humans. When and under which conditions this plasmid transfer has occurred remains to be answered. An additional concern is that S. cohnii and S. haemolyticus isolates that harbor this cfr-carrying plasmid could act as reservoirs for the cfr gene in nosocomial environments. Although a very low prevalence (0.55%, 4/713) of the cfr gene was observed in the clinical staphylococcal isolates from the Mohnarin study 2009–2010, continued surveillance of the dissemination of the cfr gene in Gram-positive bacteria from hospital patients is urgently needed in China.
Funding Statement
This work was supported by grants from the National Science and Technology Major Project (No.2012ZX09303005-003) and National Natural Science Foundation of China (No.31001087). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Leach KL, Brickner SJ, Noe MC, Miller PF (2011) Linezolid, the first oxazolidinone antibacterial agent. Ann N Y Acad Sci 1222: 49–54. [DOI] [PubMed] [Google Scholar]
- 2. Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, et al. (2001) Linezolid resistance in a clinical isolate of Staphylococcus aureus . Lancet 358: 207–208. [DOI] [PubMed] [Google Scholar]
- 3. Kosowska-Shick K, Julian KG, McGhee PL, Appelbaum PC, Whitener CJ (2010) Molecular and epidemiologic characteristics of linezolid-resistant coagulase-negative staphylococci at a tertiary care hospital. Diagn Microbiol Infect Dis 68: 34–39. [DOI] [PubMed] [Google Scholar]
- 4. Liakopoulos A, Neocleous C, Klapsa D, Kanellopoulou M, Spiliopoulou I, et al. (2009) A T2504A mutation in the 23S rRNA gene responsible for high-level resistance to linezolid of Staphylococcus epidermidis . J Antimicrob Chemother 64: 206–207. [DOI] [PubMed] [Google Scholar]
- 5. Bosling J, Poulsen SM, Vester B, Long KS (2003) Resistance to the peptidyltransferase inhibitor tiamulin caused by mutation of ribosomal protein l3. Antimicrob Agents Chemother 47: 2892–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wolter N, Smith AM, Farrell DJ, Schaffner W, Moore M, et al. (2005) Novel mechanism of resistance to oxazolidinones, macrolides, and chloramphenicol in ribosomal protein L4 of the pneumococcus. Antimicrob Agents Chemother 49: 3554–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B (2006) The Cfr rRNA methyltransferase confers resistance to Phenicols, Lincosamides, Oxazolidinones, Pleuromutilins, and Streptogramin A antibiotics. Antimicrob Agents Chemother 50: 2500–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mendes RE, Deshpande LM, Castanheira M, DiPersio J, Saubolle MA, et al. (2008) First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob. Agents Chemother 52: 2244–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendes RE, Deshpande L, Rodriguez-Noriega E, Ross JE, Jones RN, et al. (2010) First report of staphylococcal clinical isolates in Mexico with linezolid resistance caused by cfr: evidence of in vivo cfr mobilization. J Clin Microbiol 48: 3041–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shore AC, Brennan OM, Ehricht R, Monecke S, Schwarz S, et al. (2010) Identification and characterization of the multidrug resistance gene cfr in a Panton-Valentine leukocidin-positive sequence type 8 methicillin-resistant Staphylococcus aureus IVa (USA300) isolate. Antimicrob Agents Chemother 54: 4978–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bongiorno D, Campanile F, Mongelli G, Baldi MT, Provenzani R, et al. (2010) DNA methylase modifications and other linezolid resistance mutations in coagulase-negative staphylococci in Italy. J Antimicrob Chemother 65: 2336–2340. [DOI] [PubMed] [Google Scholar]
- 12. Mendes RE, Deshpande LM, Farrell DJ, Spanu T, Fadda G, et al. (2010) Assessment of linezolid resistance mechanisms among Staphylococcus epidermidis causing bacteraemia in Rome, Italy. J Antimicrob Chemother 65: 2329–2335. [DOI] [PubMed] [Google Scholar]
- 13. Morales G, Picazo JJ, Baos E, Candel FJ, Arribi A, et al. (2010) Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus . Clin Infect Dis 50: 821–825. [DOI] [PubMed] [Google Scholar]
- 14. Sánchez-García M, De la Torre MA, Morales G, Peláez B, Tolón MJ, et al. (2010) Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA 303: 2260–2264. [DOI] [PubMed] [Google Scholar]
- 15. Seral C, Sáenz Y, Algarate S, Duran E, Luque P, et al. (2011) Nosocomial outbreak of methicillin- and linezolid-resistant Staphylococcus epidermidis associated with catheter-related infections in intensive care unit patients. In J Med Microbiol 301: 354–358. [DOI] [PubMed] [Google Scholar]
- 16. Gopegui ER, Juan C, Zamorano L, Pérez JL, Oliver A (2012) Transferable multidrug resistance plasmid carrying cfr associated with tet(L), ant(4′)-Ia, and dfrK genes from a clinical methicillin-resistant Staphylococcus aureus ST125 strain. Antimicrob Agents Chemother 56: 2139–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai JC, Hu YY, Zhang R, Zhou HW, Chen GX (2012) Linezolid-resistant clinical isolates of methicillin-resistant coagulase-negative staphylococci and Enterococcus faecium from China. J Med Microbiol 61: 1568–1573. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute (2012) Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute.
- 19. Wang Y, Zhang W, Wang J, Wu C, Shen Z, et al. (2012) Distribution of the multidrug resistance gene cfr in Staphylococcus species isolates from swine farms in China. Antimicrob Agents Chemother 56: 1485–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong T, Li X, Wang J, Sloan C, Cicogna C (2007) Sequential linezolid-resistant Staphylococcus epidermidis isolates with G2576T mutation. J Clin. Microbiol 45: 3277–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller K, Dunsmore CJ, Fishwick CW, Chopra I (2008) Linezolid and tiamulin cross-resistance in Staphylococcus aureus mediated by point mutations in the peptidyltransferase center. Antimicrob Agents Chemother 52: 1737–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schenk S, Laddaga RA (1992) Improved method for electroporation of Staphylococcus aureus . FEMS Microbiol Lett 73: 133–138. [DOI] [PubMed] [Google Scholar]
- 23. Locke JB, Hilgers M, Shaw KJ (2009) Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700). Antimicrob Agents Chemother 53: 5265–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Locke JB, Hilgers M, Shaw KJ (2009) Mutations in ribosomal protein L3 are associated with oxazolidinone resistance in staphylococci of clinical origin. Antimicrob Agents Chemother 53: 5275–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Long KS, Vester B (2012) Resistance to linezolid caused by modifications at its binding site on the ribosome. Antimicrob Agents Chemother 56: 603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kehrenberg C, Schwarz S (2006) Distribution of florfenicol resistance genes fexA and cfr among chloramphenicol-resistant staphylococcus isolates. Antimicrob Agents Chemother 50: 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]