Abstract
Neurocognitive dysfunction is a central feature of schizophrenia and is observed during all phases of the illness. Because schizophrenia is known to run in families, studying neurocognitive function in first-degree, nonpsychotic relatives has been a widely utilized strategy for almost 50 years for understanding presumed “genetic risk.” Studying nonpsychotic relatives (“familial high-risk” or (FHR)) allows for identification of cognitive vulnerability markers independent of confounds associated with psychosis. Prior meta-analyses have elucidated the level and pattern of cognitive deficits in the premorbid, prodromal and post-onset periods of psychosis, and in relatives regardless of age. However, no prior quantitative analyses have specifically focused on studies of young first-degree relatives of individuals with schizophrenia who have not passed through the peak age illness risk (< age 30). The English language literature of neuropsychological studies of first-degree relatives for schizophrenia was identified through May 15th, 2011. From 33 studies, 28 studies met our criteria for quantitative review, utilizing >70 individual tests and 250 variables. In general, young FHR individuals demonstrated deficits with a moderate level of severity compared with healthy controls. The largest average effect sizes (ESs), based on tests given in at least 3 independent studies, were on estimates of Full Scale IQ (d=−0.777), followed by Vocabulary (d=−0.749) and single word reading tests (d=−0.698) (often used as estimates of IQ). Measures of sustained attention, working memory and others had more modest ESs. Deficits were milder than in established schizophrenia, but at least as severe as in clinical high-risk (HR) or putatively prodromal participants and in older relatives examined in prior meta-analyses. Additionally, while assessed from a more limited literature, youth at FHR for schizophrenia tended to show worse neurocognitive functioning than those at FHR for affective psychosis. This suggests that genetic risk for schizophrenia as reflected in a positive FHR carries an especially heavy impact on cognitive ability.
Keywords: schizophrenia, psychosis, familial high risk, neurocognition, meta-analysis
Introduction
The view of schizophrenia as a neurodevelopmental disorder, originating at conception or during pregnancy, is increasingly well accepted, and provides a framework for considering why genetic high-risk (GHR) studies are valuable for identifying developmental dysfunctions (Murray & Lewis, 1987; Weinberger, 1987). That is, GHR studies allow a defined selection process for ascertaining non-psychotic subjects at any age to test the hypothesis that they carry genetic liability for the illness of schizophrenia, expressed in a range of phenotypes reflecting the underlying disorder. Those phenotypes (e.g., working memory problems, smaller hippocampi) can be studied at different ages to evaluate developmental effects, and in different sub-populations (e.g., those with higher vs. lower genetic loading) to study the specific subgroup expression of the phenotypes. This approach has been employed for over half a century, and has been one of the most fruitful ways of identifying components of the vulnerability to schizophrenia. The GHR approach complements studies of premorbid functioning in those who later develop schizophrenia by focusing prospectively on those with a positive family history (FH) of schizophrenia in first-degree relatives.
Given the substantial evidence that, on average, youth who later develop schizophrenia display premorbid IQ half a standard deviation lower than their peers (Woodberry, Giuliano, & Seidman, 2008), it is now accepted that the neurocognitive disorder of schizophrenia often precedes the frank illness by many years. Such neurodevelopmental problems in childhood represent relatively mild precursors to the neurocognitive dysfunction in schizophrenia that is regarded as a core component of the illness (Seidman, 1983; Green, 1986; Nuechterlein & Dawson, 1986; Heinrichs, & Zakzanis, 1998). In fact, cognitive impairment was considered a primary feature of the disorder when first described by Kraepelin and Bleuler (Kraepelin, 1919; Bleuler, 1919). Among people with schizophrenia, neuropsychological problems play an important role in level of functional outcome. (Green, 1996; Green, Kern, Braff, & Mintz, 2000). Additionally, antipsychotic medications, despite a fairly effective impact on reducing positive symptoms, provide only modest improvements in neurocognition, pointing to the relative independence of these two aspects in individuals with this disorder (Mishra & Goldberg, 2004; Keefe, Perkins, Silva, & Lieberman, 1999; Harvey & Keefe 2001). Therefore, understanding neurocognition in the developmental risk for schizophrenia enhances understanding of the etiology and pathophysiology of the illness and possible paths to improvements in treatment.
There are several study populations in which neurocognitive deficits are assessed as a potential risk factor for schizophrenia: biological relatives at GHR (including siblings and offspring of individuals with schizophrenia); clinical HR (putatively “prodromal”) cases defined largely by symptoms; schizophrenia spectrum cases; psychometrically “at-risk” subjects identified using questionnaires; prospective birth cohorts; and follow-back studies of individuals with schizophrenia. All of these approaches have made significant contributions to identifying and understanding the neurocognitive risk factors for schizophrenia, and here we will focus on a review of the findings of the genetic (“familial”) FHR approach. We use the term “familial” HR because it more conservatively reflects ascertainment of persons at risk based on close family relatedness, rather than the traditional “genetic” HR nomenclature. The term FHR better reflects the fact that family relatedness could include both biological and non-biological environmental factors (e.g., obstetric complications, psychological trauma) (e.g., FHR), and not only the action of susceptibility genes transmitted in families (GHR). Nevertheless, the FHR approach retains the possibility of capturing the genetic factors long thought to influence the occurrence of schizophrenia, including their interaction with other environmental factors.
The FHR approach builds upon the knowledge that the best-known risk factors for schizophrenia are genetic influences. Heritability of the disorder has been estimated to range from 60–90% (Gottesman, 1991). Nonpsychotic first-degree relatives of individuals with schizophrenia on average share 50% of genes with their ill relatives, but are typically free of confounds associated with psychosis (e.g., positive symptoms and medication) that could affect cognition. Therefore, relatives provide a unique population in which to study genetic risk for schizophrenia over the life course. Previous meta-analyses have found that adult relatives show deficits in declarative and working memory, sustained attention, verbal fluency, perceptualmotor speed, and certain executive functions (Sitskoorn, 2004; Snitz, 2006), lending support to other qualitative reviews that have highlighted problems in attentional processing (Nuechterlein, 1986; Cornblatt, & Keilp, 1994). These data provide evidence for a model of neurocognitive impairment defined by a common difficulty in high-load executive control processing. Studies also find that impairments in memory and executive control are stable over the life course (Faraone, Seidman, Kremen, Toomey, Pepple, & Tsuang, 1999) and are related to the amount of genetic loading (Faraone, Seidman, Kremen, Toomey, Pepple, & Tsuang, 2000), and therefore, index genetic liability.
Studies of relatives older than the age of peak risk for schizophrenia (roughly > age 30) allow for investigation of aspects of the syndrome that are independent of psychosis. A large number of FHR studies have analyzed older populations who have passed through the period of highest risk for schizophrenia without developing the disorder, and these data have been well-reviewed elsewhere (Faraone, Green, Seidman, & Tsuang, 2001; Kremen & Hoff, 2004; Sitskoorn, Aleman, Ebisch, Appels, & Kahn, 2004; Kremen, Seidman, Pepple, Lyons, Tsuang, & Faraone, 1994; Seidman, 1997; Snitz, McDonald, & Carter, 2006). Assessment of younger HR relatives (< age 30) provides an opportunity to examine the neurocognitive differences present prior to illness, and thus enables an assessment of neurocognitive risk factors that may have predictive utility (i.e., for transition to psychosis or associations with functioning).
Previous studies of this younger epoch have produced extensive neurocognitive data and have been summarized in Seidman 2006, Keshavan 2010 and Niemi 2003. These studies are based on different sample sizes, individuals at different ages, numerous neuropsychological tests, and various means of ascertaining HR and control samples, thereby limiting definitive conclusions. However, reasonably strong evidence for deficits in certain neurocognitive functions among those at FHR for schizophrenia have been found in concept formation and abstract reasoning, attention and working memory (sustained attention and vigilance, perceptual-motor speed), verbal-linguistic ability, general intelligence, and declarative memory. Impairments observed among young HR individuals are similar to those found in older relatives in family studies and are less severe than those observed in individuals with schizophrenia. In this paper we provide a quantitative and qualitative review of young first-degree relatives < age 30 and go beyond the existing qualitative reviews of the literature of this age group (Niemi, Suvisaari, Tuulio-Henriksson, & Lonnqvist, 2003; Seidman, Giuliano, Smith, Stone, Glatt, Meyer, et al. 2006; Keshavan, Kulkarni, Bhojraj, Francis, Diwadkar, Montrose, et al., 2010).
Methods
Literature Search
Neuropsychological studies of youth at FHR for schizophrenia were identified through a MEDLINE (PubMed) search using the combined search terms “genetic high-risk” and “schizophrenia.” Studies were also identified by reviewing the references of relevant articles identified through the search, as well as articles referenced by recent reviews (Niemi et al., 2003; Seidman, et al. 2006; Keshavan, et al. 2010). The search cutoff date for all articles was May 15th, 2011.
Inclusion/Exclusion Criteria
Inclusion criteria for the current review specifies studies that: (a) are written in English, (b) are published in a journal or on-line, (c) provide neuropsychological performance data for individuals at FHR for schizophrenia, (d) include data for a healthy control group, and (e) present data for a FHR group with subjects < age 30. Additionally, the current review specifically focuses on individuals at FHR, therefore studies of ultra-high risk/prodromal groups (defined by additional clinical symptoms) were not included unless there was a clearly defined FHR subgroup with adequate data as specified above. The studies that were identified as relevant from this literature search are described in Table 1. If it was not possible to compute effect sizes (ESs) based on data presented, the paper was not included in supplemental table 1 or table 2, but an attempt was made to address the impact of these findings qualitatively in the text of the results and discussion sections.
Table 1.
All family high-risk studies producing neuropsychological data in individuals < age 30
| Cohort by chronological order |
Ages of testing | Sample size* | Group descriptions | Study design |
Unique study aspects |
|---|---|---|---|---|---|
| New York Infant Study (Fish 1963) | 0, 3 days, 1–4 months, 2, 10, and 20–22 years | HR-SCZ=12, CC=12 | HR-SCZ= offspring of mothers with SCZ (DSM-I). | Longitudinal | Very young infants |
| Copenhagen High Risk Study (Mednick 1968) | Age range 9–20 yrs, mean 15.1 yrs at enrollment; additional assessment ages ~19–30 | HR-SCZ=207, CC=104 | HR-SCZ= offspring of mothers w/ SCZ. HR and CC matched on: sex of sick parent, sex of child, race, multiple birth status, pregnancy number, social class, mothers age, mothers height, and father’s height. | Longitudinal | |
| Landau 1972 | Not listed | HR-PSY=70, CC=181 | HR-PSY= children of psychotic patients (all parents married); CC=neither parent ever mental health inpatient. HR and CC matched on country of origin, neighborhood, age of parents and children, # of children in family. | Cross-sectional | |
| Massachusetts Mental Health Center Intervention Project (Grunebaum 1974, Cohler 1977) | Age range= 1–6 years at enrollment, mean age 5.1 | HR-PSY=68, HR-SCZ=16, CC=68 | HR-PSY= children of mothers hospitalized for psychosis. CC=children of mothers w/out hx of psychiatric illness in themselves or immediate family. Matched on age, parity, social class, education, religion of mother, age of child, sex of child, and ordinal position of child. HR-PSY incl. SCZ=16, SCZ-affective=15, MDD=10, BD=4, “borderline SCZ”=5. | Cross-sectional | Intervention Study |
| Waterloo-McMaster High Risk Project (Asarnow 1977, 1978) | Age range 15–19 yrs. Mean ages: HR-SCZ=16.1, FC=16.1, CC=15.4 | HR-SCZ=9, FC=10, CC=10 | Maternal dx of SCZ. FC= foster control; HR-SCZ in this study were also foster children. | Cross-sectional | Sample includes HR-SCZ and control foster children. |
| Danish Cohort (Orvaschel 1979) | HR-SCZ=72, HR-PSY=72, HR-OTHER=72, CC=72 | HR-SCZ= children of parent with SCZ with hx of psychiatric hospitalization, HR- OTHER= children of parent with hx of hospitalization with other non-psychotic psychiatric disorder, CC=children of parents with no record of psychiatric illness. | Cross-sectional | Includes comparisons with children of parent with other non-psychotic, psychiatric disorder. | |
| New York Longitudinal High Risk Project—Sample A (Rutschmann 1980, Cornblatt 1984, Ott 1998, Wolf 2002) | 1st round ages 7–12; 6 waves of testing | HR-SCZ=80, HR-AFF=25, CC=100 | HR-SCZ= children of SCZ/schizoaffective parents; HR-AFF= children of parents with affective disorders (both identified through hospital records); CC=children of parents with no hx of psychiatric tx. | Longitudinal | Followed well into adulthood. |
| Minnesota High Risk Study (Nuechterlein 1983) | Age range 9–16 at time of initial testing | HR-PSY=24, HR-OTHER=20, HA=14, CC=125 | HR-PSY= children of psychotic mothers, HR-OTHER=children of mothers with other non-psychotic psychiatric disorders, HA=hyperactive children, CC=community controls. Matched on public school classroom, sex, age, peer evaluation level, reading achievement vocabulary and comprehension scores, and SES. SCZ classification based on the RDC. | Cross-sectional | Includes comparisons with children with ADHD as well as children of mothers with other non-psychotic, psychiatric disorders. |
| Birmingham High Risk Cohort (Hallett 1983, Hallett 1986) | Mean ages: HR-SCZ=12.5, CC=12.8 (Hallett 1983); Mean ages: HR-SCZ=15.1, CC=16.1 (Hallett 1986) | HR-SCZ=13, CC=13 | HR-SCZ=offspring of 1 parent dx w/ SCZ; CC= parents and CC free of psychiatric hx. Matched on age and verbal IQ. | Longitudinal | Focus on brain asymmetry. |
| New York Longitudinal High Risk Project—Sample B (Cornblatt 1984, Erlenmeyer-Kimling 1987, Dworkin 1993, Ott 1998, Wolf 2002) | 1st round ages 7–12; 6 waves of testing | HR-SCZ=44, HR-Aff=40, CC=66 | Same as NYHRP-Sample A above | Longitudinal | Followed well into adulthood. |
| St. Louis Risk Research Project (Worland 1984) | Mean ages: HR-SCZ=9.5; HR-BD=10.3; C-PI=9.1; CC=9.2 | HR-SCZ=100; HR-BD=60; C-PI=78; CC=130 | SCZ dx w/ DSM-II, later re-dx with DSM-III. C-PI=children of parents with a physical illness | Cross-sectional | Compares HR-SCZ and HR-BD. |
| Stony Brook High Risk Project (Neale 1984, Weintraub 1987) | 6–16 age range | HR-SCZ=72, HR-Aff=53, CC=52 | HR-SCZ= at least one parent with SCZ, HR-AFF= parent with MDD or BD, CC= parents free of psychopathology. | Longitudinal | |
| Rochester Longitudinal Study (Sameroff 1984, 1987) | Assessments at birth, 4 mos, 12 mos, 30 mos and 48 mos | HR-SCZ=29, HR-MDD=58, HR-Pers=40, CC=57 | HR-SCZ= mother dx w/ SCZ, HR-MDD= mother dx w/ depression, HR-Pers=mother dx w/ personality disorder. CC matched on age, race, SES, marital status and parity. CC was free of any mental disorder. | Longitudinal | |
| Israeli High Risk Cohort Study (Lifshitz, 1985, Sohlberg 1985, Mirsky 1995) | Assessed at ages 11, 17, 26 and 32 | HR-SCZ=50, CC=50 | HR-SCZ=parental SCZ based on dx DSM-III in hospital records | Longitudinal | Examines the effect of growing up in town vs kibbutz environment. |
| Emory University Study (Goodman 1987) | Age range at enrollment, 0–5 years; 3 longitudinal assessments, each 1 year apart. | HR-SCZ=61, HR-DEP=33, CC=33 | Children of mothers w/ DSM-III dx schizophrenia (schizoaffective and schizophreniform excluded), and depression (MDD or dysthymia); control mothers (no hx of disorders in themselves, their husbands or their first degree relatives). | Cross-sectional | Largely African-American, low SES population. |
| Maier 1994 | Age range=17–30. HR-SCZ mean=24.0; CC mean=22.8 | HR-SCZ=45; CC=68 | HR-SCZ= 1st deg relatives of SCZ pts w/out a psychiatric lifetime diagnosis (DSM-III-R), CC=no lifetime hx of any psychiatric disorder. CC matched on age and sex. | Cross-sectional | |
| Jerusalem Infant Development Study (Hans 1999) | Childhood follow-up: age range 8–12, mean age 10.3. Adolescent follow-up: age range 14–21; mean age 17.6 | HR-SCZ=24; HR-OTHER=25, CC=16 | Dx based on the RDC. HR-SCZ=children of psychotic parent, HR-OTHER=children of parent w/ other nonpsychotic psychiatric disorders, CC=children of parent w/ no mental illness. | Longitudinal | |
| New England Family Study NCPP High Risk Cohort (Goldstein 2000) | 4, 8 months, 1, 4, 7 years | HR-SCZ=115, HR-Aff=127, CC=163 | CC= comparable to HR on parent’s age, ethnicity, study site, # of offspring in NCPP, payment status (public or private), offspring’s age, sex, and hx of PPCs; CC parents free of hx of psychiatric tx. | Longitudinal | Study began during pregnancy. Pregnancy data available |
| Philadelphia NCPP (Cannon 2000, Niendam 2003) | 4, 8 months, 1, 4, 7 years | HR-SCZ=33, CC=201 (controls in Cannon 2000=5,127) | HR-SCZ= siblings of cases w/ chart-review-based DSM-IV dx of schizophrenia or schizoaffective disorder. CC not tx in greater Philadelphia-area public mental health facility as an adult. Niendam 2000 CC matched on age, sex, SES and ethnicity. | Longitudinal | Study began during pregnancy. Pregnancy data available |
| Edinburgh High Risk Study (Cosway 2000, Byrne 2003, Marjoram 2006) | Mean age 21.2 at 1st assessment; range 16–25 | HR-SCZ=157; CC=34 | HR-SCZ= at least one 1st or 2nd deg relatives with SCZ (2nd deg only=51, 1st and 2nd deg=82, 2+ 1st deg relatives=19); CC= no fam hx of psychotic disorder. Matched on age and sex. | Longitudinal | Varying genetic load for HR-SCZ group. Has neuroimaging data. |
| Schreiber 2003 | HR-SCZ mean=12.1, range 7.8–19.8; CC mean=12.3, range 8.0–19.4 | HR-SCZ=23; CC=61 | HR-SCZ= offspring of at least one SCZ parent; CC=no family hx of psychiatric illness. | Cross-sectional | |
| Colorado Cohort (Davalos 2004) | Age range at enrollment =6–15 yrs. Mean ages: HR-SCZ=10.2, CC=10.5 | HR-SCZ=51, CC=51 | HR-SCZ=at least one parent meeting DSM-IV criteria for SCZ, CC= free from family hx of psychosis out to 3rd deg relatives. Matched for age and gender. | Cross-sectional | |
| Swedish High Risk Study (Schubert 2005) | Age at adult young follow-up (when testing occurred)=mean 22.3 yrs | HR-SCZ=28, HR-SPS=38, HR-AP=36, HR-APS=36, CC=88 | HR-SCZ=mothers w/ SCZ, HR-SPS=mothers w/ SCZ-spectrum dx, HR-AP=mothers w/ affective psychoses dx, HR-APS=mothers w/ affective psychoses spectrum dx, CC= no maternal or paternal hx of psychosis. CC matched on prenatal clinic, age, parity, social class, marital status in pregnancy. Maternal dx based on the RDC. | Longitudinal | Comparison with HR-SCZ and HR-AP |
| Harvard Adolescent High Risk Study (Seidman 2006) | Age range 13–25 | HR-SCZ=35; HR-AP=6; CC=54 | HR-SCZ= sibs & children of patients with SCZ or schizoaffective-depressed type, HR-AP= children & sibs of patients w/ affective psychosis, CC= children & sibs of community controls. | Longitudinal but only Cross-sectional data reported so far. | Comparison with HR-SCZ and HR-AP. Has neuroimaging data. |
| Klemm 2006 | Age range=12–20; Mean ages: HR-SCZ=16.0, CC=16.2 | HR-SCZ=32, CC=32 | HR-SCZ= children or siblings of SCZ in or outpts. CC had no 1st or 2nd deg rels with SCZ sx. Matched for age, sex, education level. Subjects excluded if dx w/ schizotypal personality, affective, neurological or eating disorders. | Cross-sectional | |
| Washington University High Risk study (Bonner-Jackson 2007, Delawalla 2008) | Mean ages: CC=21.3, CC-sibs=20.8, SCZ=22.1, HR-SCZ=20.9 | SCZ= 27, HR-SCZ=31, CC=39, CC-sibs=42 | HR-SCZ and CC-sibs= no hx of Axis I psychotic disorder, incl BD. CC= no hx of Axis I psych disorder, no 1st deg rel. w/ a psychotic disorder. | Longitudinal | Has neuroimaging data. |
| Palau Early Psychosis Study (Myles-Worsley 2007) | Age range 14–19, mean age 16.5 | HR-PSY+=44, HR-PSY−=54, CC+=113, CC−=99 | HR-PSY=adolescents w/ affected parent or 2 or more affected siblings; CC had no 1st, 2nd or 3rd deg. relative w/ psychotic disorder. Subject sx level measured by the Youth Psychosis At Risk Questionnaire. | Cross-sectional | Combines FHR and clinical HR approaches. |
| Groom 2008 | Mean ages=SZ=19.2, HR-SCZ=17.5, C-ADHD=15.7, CC=17.2 | SZ=30, HR-SZ=36, C-ADHD=27, CC=72 | HR-SCZ= siblings of individuals with SCZ-spectrum psychosis w/ no hx of psychosis, C-ADHD=controls with dx of ADHD, CC=free of SCZ or SCZ prodrome, no 1st deg rels w/ SCZ or prodrome. | Cross-sectional | Includes comparison to ADHD controls. |
| Istanbul Cohort (Fis 2008) | Age range 8–15 | HR-SCZ=30, CC=30 | HR-SCZ= offspring of parent attending outpt psychiatry w/ dx of SCZ based on DSM-IV criteria, CC= offspring of parents free of any mental disorder. Matched on age and sex. | Cross-sectional | |
| University of Pittsburgh (Bhojraj 2009, Eack and Mermon 2009) | Mean ages= HR-SCZ=16.6, CC=16.5 | HR-SCZ=70, CC=63 | HR-SCZ= 1st or 2nd deg relative of SCZ proband (50 1st deg and 20 2nd deg). CC= Age- and gender matched. | Longitudinal | Has neuroimaging data. |
| Eastern Quebec Multigenerational Families High-Risk Study (Maziade 2009) | Mean ages: HR=17.3, SD 4.3; CC=17.3, SD=4.3 | HR-SCZ=22, HR-BP=23, CC=45 | HR= offspring of SCZ indiv w/ 1 1st-degree relative w/ DSM-IV-TR SCZ or BP and at least 4 affected individuals w/ same disorder w/in multigen. family. Ave # of family members affected=6. HR excluded if dxed w/ psychotic disorder, BP or MDD, CC also excl if ADHD or fam hx of BP or SCZ. Controls matched on age and gender. | Cross-sectional | Densely affected SCZ and BP families; comparison of HR-SCZ and HR-BP individuals. |
| Mapping Cortical Circuit Maturation in High Risk Adolescents study (UNC) (Gibson 2010) | Age range=9–18 yrs | HR-SCZ=23, CC=31 | HR-SCZ= 1st deg relative w/ SCZ or Schizoaffective Disorder by DSM-IV-TR. CC= no 1st deg. relative w/ psychotic disorder by DSM-IV-TR. | Cross-sectional | Has neuroimaging data. |
| Eastern Turkey Cohort (Ozan 2010) | Age range 8–17 | HR-SCZ=30, CC=37 | HR-SCZ=parent dx w/ DSM-IV SCZ, CC=parent w/out dx of SCZ or affective psychosis. Excluded: children w/ lifetime dx of ADHD, or conduct, substance, mood or psychotic disorders. Matched on age, sex and yrs of education. | Cross-sectional |
Sample size is sample size at study entry; for those studies that did multiple, longitudinal assessments, sample sizes changed over the course of follow-up.
HR-SCZ= familial HR for schizophrenia; HR-BD=familial HR for bipolar disorder (either w/out psychosis or not specified); HR-AP=familial HR for affective psychoses; HR-PSY=familial HR for psychotic disorder (type of psychotic disorder (SCZ vs AP) not specified); HR-Aff=familial HR for affective disorder; HR-MDD= familial HR for major depressive disorder; CC= community controls; C-PI= controls with parental physical illness, C-ADHD= Controls with Attention-Deficit/Hyperactivity Disorder; FC=Foster care controls; HA= Hyperactive Children; HR-Pers=familial at high-risk for personality disorders.; HR-Dep= familial HR for depression; HR-APS= familial HR for affective psychosis spectrum disorders; HR-OTHER=familial HR for other non-psychotic psychiatric disorder.
Sample and Procedures
Statistical Analyses
For each neurocognitive test, we computed the standardized mean difference between the high-risk for schizophrenia (HR-SCZ) and control group using Cohen’s method (Cohen, 1988), and then, divided by the pooled standard deviation (SD) (Lipsey, & Wilson, 2001) to calculate the ES. If the mean and SD was not listed in the paper, ESs were calculated from statistical results that were listed, such as F values and Chi-Sq values. Cohen’s (1988) criteria were used to define the ESs as small (d = 0.2), medium (d = 0.5), and large (d = 0.8).
Dependent Variables
A wide variety of neuropsychological tests were used across the studies reviewed. We categorized the tests into broadly defined and putatively separable cognitive domains to provide a general organizational framework for this review (Lezak, 1995). While we recognize that the “lumping and splitting” of complex neuropsychological tests into categories is imperfect, it does have heuristic value. Nine cognitive domains are presented in supplemental table 1 (S1) to organize the findings, though we readily acknowledge that this is but one of a number of classification systems that could have been used, as several cognitive functions can be recruited for a particular task. These nine cognitive domains include: general intelligence, attention, executive functioning, verbal/linguistic ability, visual spatial ability, social cognition, declarative memory, motor functioning and cerebral asymmetry. Some domains, such as “attention” are comprised of a number of “subdomains”. For specific test scores that appeared in at least 3 independent studies, we averaged the scores and report them in table 2.
Table 2. Average Effect Sizes Characterizing Group Differences Between Family High Risk Participants and Controls.
A negative value denotes poorer performance in the FHR group.
| Cognitive Domain | # of studies | Average ES |
|---|---|---|
| Full Scale IQ: General Cognitive Ability | 13 | −0.777 |
| Attention | ||
| Digit symbol | 7 | −0.546 |
| d2 concentration (cancellations) | 3 | −0.528 |
| Trail making B | 5 | −0.498 |
| Trail making A | 5 | −0.465 |
| Arithmetic | 4 | −0.343 |
| Stroop interference score | 4 | −0.303 |
| Continuous Performance Test (CPT) – Identical Pairs (IP)- numbers d’ | 4 | −0.282 |
| Digit span forward | 5 | −0.279 |
| Digit span total or scale score | 7 | −0.266 |
| Continuous Performance Test (CPT) – Identical Pairs (IP)- shapes d’ | 4 | −0.262 |
| Digit span backward | 5 | −0.208 |
| Executive Function | ||
| Picture arrangement | 3 | −0.524 |
| WCST perseverative errors/responses | 3 | −0.500 |
| WCST total errors | 4 | −0.339 |
| WCST completed categories | 4 | −0.282 |
| Verbal/linguistic | ||
| Vocabulary | 5 | −0.749 |
| NART & WRAT Single Word Reading | 3 | −0.698 |
| Verbal IQ score | 5 | −0.600 |
| Comprehension | 5 | −0.412 |
| Letter fluency | 5 | −0.377 |
| Similarities | 3 | −0.371 |
| Information | 5 | −0.344 |
| Total verbal fluency | 3 | −0.168 |
| Visual spatial ability | ||
| Performance IQ | 5 | −0.652 |
| Block design | 6 | −0.472 |
| Verbal Declarative memory | ||
| Rey Auditory Verbal Learning (RAVLT) Delayed Recall | 3 | −0.636 |
| RAVLT Trials 1–5 sum | 3 | −0.584 |
| Logical Memory (Wechsler Memory Scales) Story recall | 3 | −0.403 |
Results
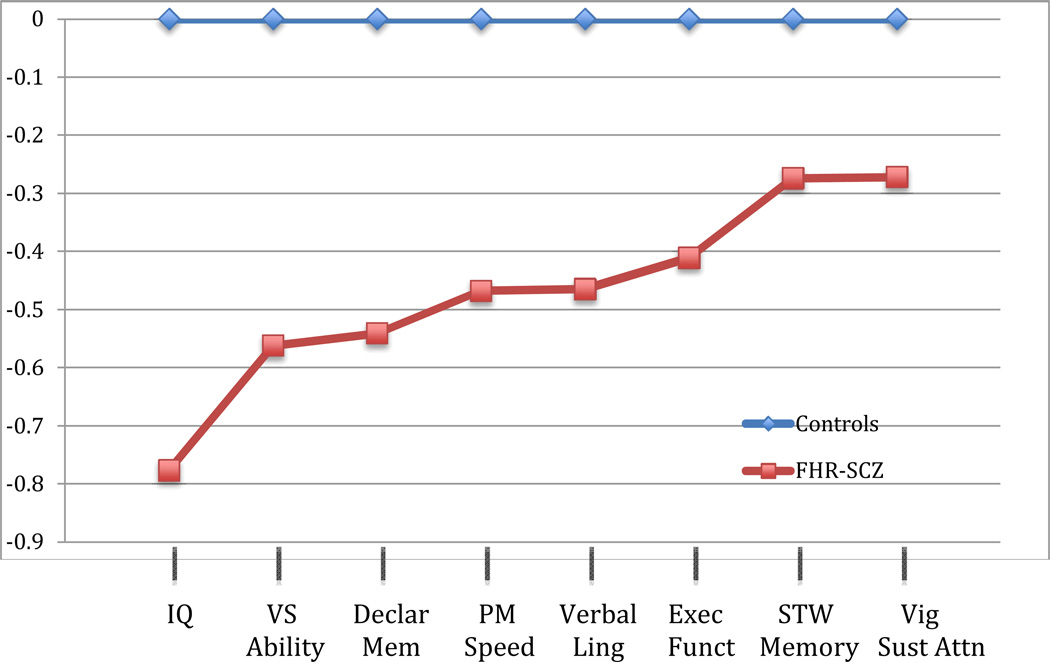
Table 1 provides additional information regarding the 33 studies included in this review, such as sample size, ages at testing, FHR and control group recruitment, and other unique aspects of the study design. Supplemental table 1 lists more than 250 ES calculations reflecting the various measures from more than 70 tests used. Figure 1 shows the average ESs, by cognitive domain, comparing individuals at familial HR for schizophrenia to those not at HR.
Figure 1.
Average effect sizes by domain comparing those at family high-risk for schizophrenia to controls
IQ: intelligence quotient, full scale and estimated; VS ability: Visual Spatial Ability, averaged tests include: Performance IQ and Block Design; Declar Mem: Declarative memory, averaged tests include: Story Recall, RAVLT Delayed Recall, RAVLT T1-T5; PM speed: perceptual motor speed, averaged tests include: Digit Symbol, Stroop interference score, Trail Making Time to Execute Part A, Trail Making Time to Execute Part, d2 Concentration Test; Verbal Ling: Verbal Linguistic Ability, averaged tests include: Verbal IQ Score, Comprehension, Information, Similarities, NART & WRAT Reading, Total Fluency, Letter Fluency, and Vocabulary; Exec Funct: Executive Functioning, averaged tests include: WCST Perseverative Errors/Responses, WCST Total Errors, WCST Completed Categories, Picture Arrangement; STW Memory: short-term working memory, averaged tests include: Arithmetic, Total Digit Span, Digit Span Forward, Digit Span Backward; Vig Sust Attn: Vigilance and Sustained Attention, averaged tests include: CPT-IP numbers d’ and CPT-IP shapes d’
Neurocognitive Domains and Tests
1. General Intelligence
It has been well established that on average individuals who will develop schizophrenia show lower general intelligence, as measured by IQ, in childhood (d = −0.54; Woodberry et al., 2008). However, the ES calculated in this meta-analysis did not distinguish individuals with FHR for schizophrenia from those without a positive family history.
Overall, there were sixteen FHR studies of “IQ” and thirteen that could be used for quantitative analysis (see S1 table). The range of ESs was from −0.225 to −1.583, with an average unweighted ES of −0.777. In general, this review supports the finding that full scale IQ is on average lower among FHR individuals compared to healthy controls. It also suggests that the effect of FH on IQ is even stronger than simple premorbid assessment demonstrates, raising the possibility that FH of schizophrenia has a particularly strong association with neurocognition. It is important to note that some of the larger ESs reviewed involved a comparison group with a higher than average mean IQ, such as the Dworkin et al. study (Dworkin, Cornblatt, Friedmann, Kaplansky, Lewis, Rinaldi, et al., 1993), where the mean for controls was 111.3 in childhood, and the Fis study (Fis, Cetin, Erturk, Erdogan, Dedeoglu & Yazgan, 2008) in which the mean for controls was 114.9; these higher control average IQs could potentially inflate the ESs and they do contribute to the largest ESs for IQ seen in this review
A number of studies evaluated FHR youth at more than one time point and these are relevant for understanding the stability of deficits in the premorbid period. Dworkin and colleagues from the New York High Risk Project (NYHRP) made assessments in childhood and adolescence (Dworkin et al., 1993). At both ages, FHR-SCZ exhibited significantly lower IQ compared to controls, with an ES of −1.28 in childhood and −1.31 in adolescence. In the Emory HR Project, at the time of first testing there was a significant difference in IQ between FHR and normal risk children, with a moderate ES of −0.544, while no significant difference in IQ at the second and third study assessments occurring in successive years (Goodman, 1987). In the St. Louis HR Project, children were tested at two time points and FHR children showed impairment relative to controls in FSIQ at both time points (Worland, Weeks, Janes & Strock, 1984). Thus most of the longitudinal studies demonstrated that IQ deficits among FHR individuals were relatively stable (consistent with Woodberry, et al., 2008); (although among those who develop schizophrenia, the prodromal period is likely to be associated with a decline in cognition, which was not measured in these studies).
2. Attention
Attention has long been considered a central problem among patients with schizophrenia (Nuechterlein, 1984; Cornblatt & Keilp, 1994). Articles reviewed here reported on several tests used to examine different domains of attention, including perceptual-motor speed, short-term/working memory, vigilance and sustained attention. There were dozens of studies of this domain with many permutations of variables and measurement presentations.
Perceptual/motor speed
Across the perceptual motor speed domain, overall, there were fourteen studies included. The average ES of tests given in three or more studies (digit symbol, trail making A and B, Stroop, and d2 concentration) was −0.468, in the moderate range.
The digit symbol task generally differentiated between FHR and normal risk groups with a moderate ES, ranging from −1.065 to −0.271 (Asarnow, Steffy, MacCrimmon & Cleghorn, 1978; Seidman et al., 2006). While the study by Asarnow and colleagues reported the largest ES comparing FHR-SCZ individuals to controls, this was not found to be statistically significant, most likely due to a small sample size (Asarnow, 1978). Trail making, part B (involving more complex set shifting than part A) was found to distinguish more effectively between FHR individuals and controls in four of the five studies, although impairment in the FHR group produced a larger ES in Part A than B in Ozan et al. (Ozan, Deveci, Oral, Karahan, Oral, Aydın et al., 2010).
There are many other tests that have been employed across different studies to assess perceptual motor speed, including tests of reaction time (RT), visual search, speed of comprehension, and the Stroop test. In the Schreiber et al. study, both simple and warned RTs were slower in the FHR than control group (Schreiber, Stolz-Born, Heinrich, Kornhuber & Born, 1992). Maier and colleagues measured the effect of modality shifts and regular and irregular presentation of stimuli on RTs and found that while schizophrenia patients showed longer regular, irregular, crossover and modality-shift RTs compared to controls, siblings of these patients only showed impairment for crossover RTs (Maier, Franke, Kopp, Hardt, Hain & Rist, 1994). Neale et al. employed a visual search task to assess the ability to ignore irrelevant input and to maintain attention. FHR-SCZ and FHR-depressed children showed slower visual search times than controls, with impairment becoming more pronounced as attention was required to be maintained for a longer time (Neale, Winters, & Weintraub, 1984).
Overall, FHR individuals show impairments in perceptual-motor aspects of attention, most consistently in the digit symbol subtest and trail making tasks, and variably in other measures including the Stroop test, and tests of RT and visual search. This is consistent with the literature indicating that perceptual motor speed is one of the most severe deficits in established illness, and further indicates it is associated with FHR for schizophrenia.
Short term or working memory
Across the short term or working memory (WM) domain there were many studies, with the Digit Span test used most often (n=10), followed by the mental arithmetic subtest of the Wechsler Intelligence Scale for Children (WISC) as the second most frequently administered task (n=4).
Performance on the digit span task resulted in ESs ranging from −0.032 to −0.467 (Fis et al., 2008; Neale, 1984). Surprisingly, there was no appreciable difference between digits forward and backward, despite the fact that digits forward is thought to require maintenance in WM, whereas digits backwards requires maintenance and manipulation. The NYHRP used the “Visual-Aural Digits Span” which presents digits in alternating aural and visual modes. ESs could not be computed from published data (Cornblatt & Erlenmeyer-Kimling, 1984), however the authors note that FHR subjects performed significantly worse than controls on the “ visual stimulus/written response” conditions.
Generally, differences between FHR and control groups on the arithmetic subtest of the WISC yield small ESs, with a range from −0.263 to −0.473 (Landau et al., 1972; Fis et al., 2008). However, while ESs could not be calculated for the data presented in the Copenhagen HR study, arithmetic was one of the only subtests in the WISC which, along with digit symbol, significantly differentiated between FHR and control groups (Mednick & Schulsinger, 1985).
A number of investigators hypothesized that distraction would augment the WM deficit in FHR youth. In the NYHRP, distraction produced a large decrement in performance on the Attention Span Test, although it did not differentiate between FHR and control groups, perhaps because the task became too difficult for both groups in the tested age range (Erlenmeyer-Kimling, et al., 1982). Two other WM tasks administered in the NYHRP include: the short-term memory lag test (STM-lag test) and the Information Overload Test (IOT) (Rutschmann, Cornblatt & Erlenmeyer-Kimling, 1980; Cornblatt, et al., 1984). In the STM-lag test, FHR individuals showed lower correct response rates and memory strength for the Consonant Vowel Consonant trigrams (Rutschmann, et al., 1980). In the IOT, interesting results were found. While there were no differences between FHR and control groups in ability to identify target stimuli in the absence of distraction, FHR individuals showed significant impairments when distraction was introduced, more specifically in both identifying target stimuli as well as answering questions about the distracter story (Erlenmeyer-Kimling, et al., 1984).
Davalos and colleagues in the Colorado HR cohort assessed WM with counting and sentence span tasks, tapping the ability to hold information “on-line.” The Colorado study found that both memory span tasks significantly distinguished between FHR subjects and controls, with ESs for the counting and sentence spans of −0.548 and −0.478, respectively (Davalos, Compagnon, Heinlein & Ross, 2004). Maziade and colleagues employed a different form of the memory span test, total spatial span, and found that FHR subjects performed significantly worse than controls, with a raw ES of −0.44, which increased to −0.66 after controlling for age, gender and overall IQ (Maziade, Rouleau, Gingras, Boutin, Paradis & Jomph, 2009).
Thus, it appears that more complex short-term or WM tasks requiring greater loads (memory span rather than simple digit span), often augmenting “load” by distraction, yield larger and more consistent impairments in FHR individuals.
Vigilance and sustained attention
Across the vigilance and sustained attention domain there were eight studies using five forms of vigilance tasks for which we could quantify ESs. The range of ESs among these studies was quite variable from +0.559 to −0.609. Vigilance and sustained attention was assessed with the continuous performance test, (CPT; Rosvold, Mirsky, Sarason, Bransome, & Beck, 1956). The CPT is a methodology rather than a “test”, and several different forms of the CPT with varying difficulty and methodologies have been employed by various FHR studies.
The NYHRP used different forms of the CPT by study round and sample, including the CPT playing card, CPT double-digit (simple and more complex) and CPT-Identical Pairs (CPT-IP) (details regarding the round and sample of the NYHRP are provided in Table 1). In the NYHRP, Round 1, children ages 7–12 were assessed with the playing card CPT, and FHR children showed a significantly lower mean number of hits and were significantly more likely to respond to irrelevant card sequences compared with controls. However, control and FHR subjects were not significantly different on the number of false alarms, suggesting that differences in performance between the groups were due to deficits among FHR in discriminability (“vigilance”) rather than in response bias (the subject’s tendency to respond liberally or conservatively) (Rutschmann, Cornblatt, & Erlenmeyer-Kimling, 1977). The Double-Digit CPT Task A and B were administered in Sample B of the NYHRP. Task A, a simple vigilance task, did not show significant differences between FHR and non-HR groups, while the more complex Task B showed deficits in discriminability among FHR subjects (Rutschmann, Cornblatt, & Erlenmeyer-Kimling, 1986). In Rounds 2 through 4 in Sample B, the NYHRP used the CPT-IP, a demanding WM CPT (“one-back”). Over these three rounds of testing, FHR subjects showed significantly poorer discriminability compared to controls and to individuals at FHR for affective disorders, replicating the earlier finding in the double-digit Task B CPT (Erlenmeyer-Kimling & Cornblatt, 1992).
Overall, results from CPTs show deficits in discriminability among FHR individuals, which tend to become apparent in more complex versions of the CPT (Cornblatt, 1984). Indeed, differing conclusions as to whether FHR individuals show deficits in sustained attention may be due to an inability of simpler versions of the CPT to identify more subtle deficits. Several studies using simple CPTs (Asarnow, Steffy, MacCrimmon & Cleghorn, 1977; Cohler, Grunebaum, Weiss, Gamer & Gallant, 1977) found no difference between FHR and controls, or only found significant differences using a more complex version of the CPT (Grunebaum, Weiss, Gallant, & Cohler 1974; Nuechterlein, 1983). However, not all complex CPTs find deficits in FHR subjects, as two analyses using complex CPTs in the Edinburgh HR cohort did not find a significant difference in performance between FHR and controls (Byrne, Hodges, Grant, Owens, & Johnstone, 1999; Cosway, Byrne, Hodges, Grant, Morris, et al., 2002), nor were they found in Seidman et al., (2006).
The results of this review of this domain suggest that FHR individuals exhibit some impairment in various domains of attention and WM ranging from average ESs of −0.208 (for backwards digit span) to −0.546 (for digit symbol). Consistency in impairment among FHR individuals across attention domains has led some researchers to group attention tasks into a broader index of attention. This was done in the NYHRP, and while controls did not show significant correlation across time points, suggesting that their attention capacities showed “random fluctuations”, FHR subjects showed stable deficits in attention performance over time (Erlenmeyer-Kimling, 1982). Additionally, it has been found in several attention tasks that more difficult or complex versions (CPT-IP and tasks with distraction) are better at distinguishing FHR from control groups.
3. Executive functioning (EF)
Executive functioning (EF) is particularly important to models of brain dysfunction in schizophrenia as EFs are known to be strongly associated with prefrontal cortical function, a key component of schizophrenia pathophysiology. There is well-known difficulty in precisely defining EFs, although most investigators would agree that EFs involve “higher” cognitive functions. For example, Sergeant, Geurts and Oosterlaan (2002) note that there are at least “33 definitions of EF” (p. 3). There is a reasonable consensus in the field that EFs are self-regulatory functions incorporating the ability to inhibit, shift set, plan, organize, use WM, problem solve and maintain set for future goals (Pennington & Ozonoff, 1996; Sergeant, 2002). While EFs are distinct from other mental functions such as perception or memory, there is substantial overlap with some components of learning and memory, especially processes involved with encoding and retrieval (Pennington, 1996).
Deficits in EF are assessed using many different cognitive tasks, and in the FHR literature, only a small number of these tasks have been explicitly used, as many of these studies began prior to the era (the 1980’s) when EF was becoming understood in human neuropsychology. What we present here are the mainly conceptual tasks, including the object sorting and picture arrangement tasks, and the Wisconsin Cart Sort Test (WCST). Other tasks involving EF, such as working memory, are presented in other sections (see the “attention” section above). Across the EF domain, overall, there were eleven studies with measures that we could quantify; ESs ranged widely, from +0.672 to −1.731 (Maziade, 2009; Asarnow, 1978).
The WCST is a commonly used measure of EF and assesses aspects of concept formation and response flexibility (Lezak, 1995). The WCST has been administered in the NY, Turkish, Swedish and Quebec Multiplex HR cohorts, among others (Wolf, Cornblatt, Roberts, Shapiro, & Erlenmeyer-Kimling, 2002; Ozan, 2010; Schubert & McNeil, 2005; Maziade, 2009; Klemm, Schmidt, Knappe, & Blanz, 2006). A key dimension, lack of ability to adapt to a new card sorting strategy (perseveration), has been found in individuals with schizophrenia (Wolf, 2002). Studies have found that FHR individuals make significantly more total errors (Ozan, 2010; Wolf, 2002), as well as perseverative errors and responses (Wolf, 2002; Klemm, 2006) compared with healthy controls. The Swedish HR study, however, did not find elevated total errors or perseverative responses in FHR offspring (Schubert, 2005). FHR individuals also show deficits in maintaining set, number of categories completed and percent of correct trials, with moderate ESs generally distinguishing HR from control groups (Ozan, 2010; Klemm, 2006; Maziade, 2009).
Two other tests of EF include the Progressive Figures and Color Form tests of the Reitan-Indiana Neuropsychological Test Battery, and the Tower of London (TOL) Task. FHR subjects in the Turkish cohort took significantly longer to complete the color form and progressive figures tasks compared to healthy controls (Fis, 2008). In the Quebec HR cohort, the FHR group had a significantly fewer number of problems solved in minimum moves on the TOL task, as well as more rule violations; even after controlling for overall IQ, FHR subjects still showed a significant deficit in problem solving on the TOL with a moderate ES (d=−0.50) (Maziade, 2009).
The preponderance of the evidence regarding FHR performance on EF tasks suggests that individuals at FHR for schizophrenia exhibit deficits compared to healthy controls. More specifically, FHR individuals show problems of flexibility in set shifting as measured by the WCST, and may exhibit problem-solving deficits independent of overall IQ, at least in the context of a dense family history of schizophrenia (Maziade, 2009).
4. Verbal/linguistic ability
Across the verbal/linguistic domain there were fifteen studies and forty-five measures that we could quantify. The range of ESs was from +0.242 to −1.153. The extent of impairment in verbal linguistic ability among FHR individuals is variable and depends on the assessment administered. For example, the WISC and WAIS include a vocabulary subtest that has been found to be consistently impaired among FHR individuals in several HR cohorts, with a mean ES of −0.749 (Byrne, 2003; Niendam, 2003; Seidman, 2006; Davalos, 2003). However, results for comprehension and information subtests of the WISC are less consistent: studies by Fis and Schreiber found significant deficits with moderate ESs, but Niendam did not find significant group differences in the Philadelphia cohort of the National Collaborative Perinatal Project (NCPP) (Niendam, 2003). Two single word-reading tasks, the National Adult Reading Test (NART) and the Wide Range Achievement Test (WRAT), generally yield deficits of moderate ESs between FHR and controls (Marjoram, Miller, McIntosh, Owens, Johnstone, & Lawrie, 2006; Byrne, et al., 2003; Seidman, et al., 2006).
The University of Pittsburgh HR study assessed verbal ability using measures of verbal fluency, including total, letter, and category fluency (Bhojraj, Franci, Rajarethinam, Eack, Kulkarni, Prasad, et al., 2009); in the Edinburgh HR cohort, verbal fluency was assessed using letters and categories (Byrne, 2003). On average, results were not significantly different between risk groups, and ESs were generally small to moderate (with the largest found in Ozan et al., d=−0.575). Results for letter and category fluency were variable across studies, with Bhojraj finding significant group differences on letter, but not category fluency, while Byrne found significant differences on category, but not letter fluency (Bhojraj, et al., 2009; Byrne, et al., 2003).
When we average across verbal tasks administered in at least three studies, we find a mean ES of −0.465, suggesting that on average FHR individuals show a nearly moderately sized deficit in verbal ability; additionally, certain tasks, such as the single word reading tasks and verbal IQ, showed larger ESs.
5. Visual spatial ability
Across the visual-spatial domain, overall, there were thirteen studies and twenty ESs that we could quantify from the eight different tasks that were administered. The range of ESs was from −0.115 to −1.262, with an average unweighted ES for tasks administered across at least 3 studies of −0.562.
In the first round of cognitive assessment at ages 8–11 in the Israeli HR Cohort, FHR subjects performed significantly worse than controls on the Spatial Relations portion of the Thurstone Primary Mental Abilities Index, while there were no differences between groups on the verbal aspects of this index. And while there were no FHR group differences on overall score on the Bender-Gestalt, a test of visuoconstructive skills in which subjects are asked to reproduce designs on nine cards (Groth-Marnat, 2009), FHR subjects performed more poorly on specific cards within the test. Additionally, the Taylor Perceptual Closure Scale, which taps into visuospatial skills by asking subjects to copy and fill in drawings that include small gaps in each figure, also suggested some deficits in visual spatial abilities among FHR individuals in this cohort (Sohlberg, 1985).
The Block Design is a WISC subtest that involves conceptual-level spatial perception and motor skills (Lezak, 1995). A study in a cohort of Turkish children found significant impairment in FHR participants on the Block Design task, as well as the Object Assembly test, another measure of visual spatial ability (Fis, et al., 2008). Additionally the Edinburgh HR Study found a significant deficit in Block Design performance at both the first and second study assessments (Cosway, et al., 2000). However, some cohorts have not shown a significant difference between FHR and normal risk groups in performance on this task (Davalos, et al., 2004; Schubert, et al., 2005; Niendam, et al., 2003).
A deficit in performance IQ has been found in individuals with schizophrenia (Aylward, Walker & Bettes, 1984), and the current review suggests that some of these impairments in visuospatial ability may extend to those at familial HR for schizophrenia. The most commonly assessed measures of visuospatial skills, including performance IQ and, more specifically, the block design WISC subtest, showed an average moderately large ES comparing FHR and non HR individuals; there was a range of ESs among the studies examined, spanning small to large ES.
6. Social cognition
Across the social cognition domain, there were nine studies and eleven measures that we could quantify. The range of ESs was from −0.030 to −0.725 (Davalos, et al., 2004; Eack, et al., 2010). Social cognition is an emerging and important area of focus among FHR studies. Social functioning among youth at FHR for schizophrenia has been found to be impaired, including deficits in peer relationships and poor social adjustment (Glatt, Stone, Faraone, Seidman & Tsaung, 2006; Hans, Auerbach, Asarnow, Styr, & Marcus, 2000; Tarbox & Pogue-Geile, 2008). Tasks assessing social cognition seek to quantitatively measure the cognitive processes underlying these problems in social functioning.
Theory of mind (ToM), the ability to make assumptions about the mental states of others, is one area of assessment: in the Edinburgh HR Study, ToM was examined with a Hinting Task, Self-Monitoring drawing task and cartoon picture sequences. FHR subjects were categorized as ever vs never reporting any transient symptoms of psychosis on the Present State Exam interview. Overall, this study did not find any differences between either symptomatic or non-symptomatic FHR groups versus healthy controls on the ToM tasks; however, the FHR symptomatic group performed worse on certain questions in the cartoon picture sequences task, specifically when asked to make inferences about cheating and reciprocity (Marjoram, et al., 2006).
A recent study in the Mapping Cortical Circuit Maturation in HR Adolescents cohort examined social cognition with the Reading the Mind in the Eyes Task, and found no significant differences between FHR and control groups (Gibson, Penn, Prinstein, Perkins & Belger, 2010). A study in the Colorado HR cohort did not find any difference between FHR and normal risk groups in accuracy of identifying faces as happy, sad, angry or fearful (Davalos, et al., 2004). Speed and accuracy of emotion recognition was also assessed in the Pittsburgh HR Study using the Penn Emotional Recognition Test-40. FHR subjects showed deficits in speed for identifying neutral and emotional faces, but interestingly, showed no deficits in accuracy of identifying emotional faces; rather, deficits occurred as FHR subjects were more likely to attribute negative emotions to neutral faces (Eack, Mermon, Montrose, Miewald, Gur, Gur, et al., 2010). This finding is consistent with the finding that individuals with schizophrenia are more likely to selectively attend to negative stimuli (Bentall, Corcoran, Howard, Blackwood & Kinderman 2001; Phillips & Seidman, 2008). Further work in this emerging area is warranted.
While results comparing FHR and non-HR individuals on tasks of social cognition have so far been mixed, this domain represents a promising area of study in HR research. Deficits in social cognition may contribute to functional impairment in individuals with schizophrenia, and, as with the historically more comprehensively studied domains of cognition, understanding dysfunction in this area in FHR individuals will offer a deeper understanding of the relationship between social cognition and schizophrenia.
7. Verbal Declarative Memory
Across the declarative memory domain there were nine studies and thirty-three measures that we could quantify, with ESs ranging from +0.140 to −0.792 (Maziade, et al., 2009).
Declarative memory allows individuals to recount facts and events subject to conscious recollection, verbal reflection, and explicit expression (Eichenbaum, 1997) and has been consistently found to be impaired among individuals with schizophrenia (Stone & Hsi, 2011). A commonly used test of declarative verbal memory is the Rey Auditory Verbal Learning Test (RAVLT), and with this test, the Edinburgh HR study found deficits in the FHR group in the first trial and total number of words recalled over 5 learning trials (Byrne, et al., 2003). Studies by Ozan and Groom also identified deficits among FHR individuals in total trials 1–5, with moderate ESs ranging from −0.546 to −0.618 (Ozan, et al., 2010; Groom, Jackson, Calton, Andrew, Bates, Liddle, et al., 2008). Deficits were also found among these cohorts in delayed recall (with mean d of −0.636) (Maziade, et al., 2009; Ozan, et al., 2010; Byrne, et al., 2003).
The Rivermead Behavioral Memory Test (RBMT) was also used in the Edinburgh HR Study and found to be significantly impaired in both immediate and delayed forms among FHR-SCZ individuals (Byrne, et al., 2003). Schubert et al. assessed declarative memory in the Swedish HR study using the Word Pairs Task found that immediate and delayed recall was impaired in the FHR-SCZ group (FHR-AFF individuals were also impaired on these tasks, but not significantly so) (Schubert, et al., 2005). Thus, verbal declarative memory appears to be consistently impaired in FHR individuals.
8. Motor functioning
Across the motor functioning domain, there were three studies and four measures that we could quantify, with ESs ranging from 0.022 to −0.147 (Seidman, et al., 2006; Maziade, et al. 2009). In this review, we are only focusing on results of motor performance measures, rather than a broader domain of motor functioning captured in neurological exams. Early studies by Fish and colleagues of individuals with childhood-onset schizophrenia (Fish, 1977) led to the development of the theory of pandysmaturation, a neurointegrative disorder that includes problems with motor and visual motor development. Perceptual-motor functioning was assessed in the Israeli HR Study, in which FHR subjects performed worse than controls on a mirror drawing task, but not on an individual rhythm task (Lifshitz, et al., 1985). The Swedish HR study did not find a significant difference between offspring of mothers with schizophrenia and normal risk offspring in the finger-tapping test (Schubert, et al., 2005). The Jerusalem Infant Development Study also assessed motor performance with the mirror drawing and Purdue Pegboard tasks, but comparisons between the FHR and controls groups are not reported (Hans, Marcus, Nuechterlein, Asarnow, Styr & Auerbach, 1999; Marcus, Auerbach, Wilkinson, & Burack, 1981). Seidman 2006, in a combined cohort of the Harvard Adolescent HR study and the Hillside Family Study, did not find any significant impairment in speed of performance on the right or left handed grooved pegboard task (Seidman, et al., 2006). Overall, results from motor performance tests in this review were variable and somewhat inconclusive regarding FHR-SCZ impairment.
9. Cerebral asymmetry
Across the cerebral asymmetry domain reviewed here, there were two studies and eleven measures that we could quantify, with ESs ranging from +0.465 to −0.560 (Hallet, Quinn, & Hewitt, 1986). An important hypothesis regarding the pathophysiology of schizophrenia focuses on disturbed brain lateralization (Sommer, Ramsey, Kahn, Aleman & Bourma, 2001), and problems with intrahemispheric communication (Green, 1978), and it has been hypothesized that the presence of certain positive symptoms, such as auditory hallucinations, may be related to increased interference from the right hemisphere into the language processing of the left (Green, Hallett & Hunter, 1982). Dysfunctional language lateralization was tested in the Birmingham HR Project using the verbal dichotic listening task. Normal-risk subjects usually show a right-ear advantage on this task; however, FHR individuals in this study did not show this advantage, implying a more symmetrical representation of language. Interestingly, this study found that only FHR males exhibited this abnormal language dominance. This study also administered a story comprehension test and auditory recall test and found that while the risk groups did not differ on absolute left or right ear recall, the FHR group performed worse on binaural recall score, suggesting a deficit in interhemispheric communication (Hallett, et al., 1986).
Another source of information regarding hemispheric dominance is handedness. Studies in the Swedish and Edinburgh HR Cohort found no significant differences in the percentage of left-handed individuals between FHR and control groups (Schubert, et al., 2005; Byrne, et al., 1999). The Consortium of Longitudinal Studies on Schizophrenia Risk also did not find any FHR group differences on any measure of hand preference tasks (Erlenmeyer-Kimling, Hans, Ingraham Marcus, Wynne, Rehman, et al., 2005). Thus, results from studies examining cerebral asymmetry have provided mixed results. Generally, differences in hand dominance have not been found to be significant, but more subtle tests of language dominance and interhemispheric communication have shown some differences between FHR and control groups.
Discussion
1. Overall Group Difference
This review covered an extensive literature beginning in the 1960’s through May 2011 examining neurocognitive differences between FHR youth (< age 30) compared with healthy control subjects. At least 30 cohorts, using greater than 70 individual tests, with at least 250 comparisons yielding ESs (see Supplemental Table 1) were reviewed and quantified. Scores that appeared in at least 3 study cohorts were averaged, originating from more than 20 different tests (Table 2). Other study reports were described qualitatively, as data may have been presented in a manner precluding calculation of ESs. As has been shown in a number of meta-analyses of FHR studies regardless of age (Snitz et al., 2006), FHR individuals as a group demonstrated a widespread pattern of neurocognitive deficits characterized by a moderate level of severity compared with healthy controls. Based on our strategy for averaging ESs, the largest ESs were demonstrated on estimates of Full Scale IQ (d=−0.777), followed by Vocabulary (d=0.749) and single word reading tests (d=- 0.698).
2. Robustness of deficits
This review suggests that neurocognitive deficits associated with a positive FH of schizophrenia are robust as they have been demonstrated in many different studies, countries, eras, and tests. Across different age groups, when the participants remain largely clinically stable, the deficits are reasonably stable. Studies of FHR adolescents, a subgroup of whom subsequently develop psychosis, demonstrate increasing deficits in some domains prior to developing psychosis (Pittsburgh HR, Edinburgh HR). Of importance is the observation that IQ was measured most often (13 studies went into our average ES of −0.777). This ES is somewhat larger that the ES calculated in a meta-analysis of 18 studies of IQ in premorbid periods prior to the onset of schizophrenia (d = −0.540) (Woodberry et al., 2008). This suggests that genetic risk for schizophrenia as reflected in positive FHR carries an especially heavy impact on general cognitive ability. This also affirms the observations made by Seidman and colleagues that neurocognitive impairments in premorbid children (Seidman, Agnew-Blais, Cherkerzian, Goldstein, Tsuang, & Buka 2011) and prodromal adolescents (Seidman, Giuliano, Meyer, Addington, Cadenhead, Cannon, et al., 2010) who later develop schizophrenia, are worse when there is a positive FH.
3. Central Cognitive Deficit?
The strength of the difference between FHR and controls on IQ raises the question as to whether the IQ deficit is the central genetically transmitted deficit. There is certainly strong evidence for the genetic transmission of IQ and schizophrenia (Toulopoulou, Goldberg, Mesa, Picchoni, Rijsdijk, Stahl, et al. 2010). In order to determine whether IQ accounts for the other (e.g., memory, attention) deficits, studies would need to systematically control for IQ in their matching or statistical designs, and that has not often been done. Further research on this question is important to carry out. Moreover, given the degree of heterogeneity among the FHR samples across most cognitive domains, it is hard to impute a single core cognitive deficit across time. A recent longitudinal birth cohort study by Reichenberg and colleagues (Reichenberg, Caspi, Harrington, Hout, Keefe, Murray, et al. 2010) is instructive to consider in this regard. They found that children who developed schizophrenia in adulthood showed both pre-pubertal developmental deficits (i.e., static impairments on measures of verbal and visual knowledge, reasoning, and conceptualization) and developmental lags (i.e., slowed growth relative to healthy comparison subjects on tests measuring processing speed, attention, working memory, and visual-spatial problem-solving). In short, different cognitive functions were observed to follow different developmental courses between childhood and early adolescence.
Finally, it is important to note that the IQ measures are typically made up of other more elemental measures such as processing speed, working memory, language ability and visual-spatial ability, all of which individually have been shown to be impaired in FHR samples. At this point, most investigators have not addressed this complex issue and thus, there is no clear resolution. Future research should address this and consider using more elemental designs of cognitive neuroscience that may help tease apart “molecular” and “molar” contributions to cognitive deficit.
4. Using Cognitive Measures to Predict Future Psychosis
One of the ultimate goals of several of the FHR studies discussed here is not only to assess neurocognitive impairment FHR individuals, but to also identify differences between FHR youth who do and who do not go on to develop schizophrenia. A smaller subset of the studies in this review have the longitudinal design and duration of follow-up to identify such individuals, and include the New York Infant Development study, NYHRP, the Edinburgh HR, Copenhagen and Israeli HR Projects, and the Jerusalem Infant Development study. FHR individuals who transitioned to schizophrenia generally showed more severe deficits in cognition than those who had not transitioned to that point. In the Israeli HR study, nine individuals in the FHR group developed schizophrenia or a schizophrenia spectrum disorder, compared with zero individuals in the control group. Eight of these nine (88.9%) HR individuals fell into the category of “poor neurobehavioral” index performance, which included items related to neurocognition such as poor attention. Less than half (40.5%) of the FHR sample who did not develop schizophrenia fell into this category (Marcus, Hans, Nagler, Auerbach, Mirsky & Aubrey 1987). In the Edinburgh HR cohort, only performance on total trials in the RAVLT was a significant predictor among FHR individuals of who would development psychosis, and was associated with a sensitivity of 61.1% and a specificity of 32.8% (Johnstone, Klaus, Ebmeier, Miller, Owns & Lawrie, 2005). The Jerusalem HR Study also found that development of schizophrenia among FHR individuals was associated with impaired neurobehavioral functioning in childhood and adolescence (Hans, 1999). 15.2% of the FHR offspring in the NYHRP developed schizophrenia-related psychoses, and verbal memory and motor skills were sensitive predictors of disorder (83.3% and 75.0%, respectively), with associated specificities of 71.6% and 73.6% (Erlenmeyer-Kimling, Rock, Roberts, Janal, Kestenbaum, Cornblatt et al., 2000). However, in the Copenhagen HR Cohort, estimated FSIQ was not found to be predictive of schizophrenia or schizophrenia-spectrum disorders (Carter, Parnas, Urfer-Parnas, Watsone & Mednick, 2011).
Taken together, findings from our quantitative review indicate that modest neurocognitive deficits are broadly present in the modal FHR individual and, on average, are more severe among those who eventually transition to schizophrenia. Because the premorbid deficits are not as severe as those in first episode schizophrenia these findings also suggest that cognitive impairment likely increases from some point in the prodromal period until the first episode, usually measured by first hospitalization. This supports the notion of progressive decline with illness evolution (or at least increased lags in growth), at least up to the phase of established illness that appears to be marked by largely stable deficits.
5. Specificity
Several of the studies we examined in this review included some form of comparison group that was FHR for another type of psychiatric disorder, including affective disorders, affective psychoses, depression, and personality disorders (Rutschmann, et al., 1986; Goodman, 1987; Sameroff, Barocas, & Seifer, 1984; Seidman, et al., 2006). Given the range of psychiatric comparison groups, it is difficult to draw broad conclusions regarding the specificity of neurocognitive deficits among those FHR-SCZ versus other disorders. Some studies have found deficits specific to FHR-SCZ subjects: for example, discriminability on the doubledigit CPT in the NYHRP was found only in the FHR-SCZ group but not among those FHR for affective disorders (Rutschmann, et al., 1986). Seidman and colleagues found that the FHR-AP group was impaired compared with controls on verbal ability, visual spatial skills and EF/working memory, but less impaired than the FHR-SCZ group (although the FHR-SCZ vs FHR-AP difference was not statistically significant) (Seidman, et al., 2006). In other instances, as in the Emory cohort, FHRSCZ subjects showed significantly lower IQ at initial testing than HR-DEP or controls (Goodman, 1987). Maziade 2009 compared FHR offspring of densely affected SCZ and bipolar disorder families and found a pattern similar to Seidman et al. 2006 of a trend of worsening impairment from controls, to FHR-BP to FHR-SCZ (including global IQ, Rey-Osterrieth Complex Figure immediate recall, motor coordination and stroop interference score); however, this study also found that FHR-BP individuals performed worse than controls on certain tasks, such as CPT omissions, declarative memory, and letter and category fluency tests, suggesting for at least those individuals with multiplex high-risk families, a linear trend of worsening impairment compared to schizophrenia FHR may not be clear (Maziade, et al., 2009).
Ideally, the HR psychiatric comparison group selected should provide the most appropriate control for the particular area of neurocognition being assessed; for example, attention deficits could be examined with an ADHD control group, or impairment in schizophrenia versus affective disorders could be examined while controlling for psychosis with an affective psychosis comparison group.
6. Limitations
Our results should be interpreted in the context of the limitations of meta-analytic procedures, and the weaknesses in the extant literature. For instance, it can be difficult to generalize across studies with varying control and case selection strategies. For example, a FHR group with a higher density of affected relatives, or a control group including offspring of parents free of any psychiatric disorder, versus those free of only psychotic disorders, have different interpretations, and an average ES across these studies could obscure such nuances.
Secondly, the use of healthy control comparison groups sets up a contrast that is most likely to identify deficits associated with general psychopathology, and not necessarily deficits most highly associated with psychosis in general or schizophrenia-spectrum disorders in particular. There are ample examples in the adult literature illustrating that when psychiatric contrasts groups are included, ESs are notably attenuated or negligible. While several of the studies we reviewed included a group at high risk for a non-psychotic psychiatric disorder (Erlenmeyer-Kimling, et al. 1987; Weintraub, 1987; Worland, et al., 1984; Goodman, 1987; Maziade et al. 2009), this was not the majority of studies, and future research would likely benefit from including both a normatively developing control group and a contrast comparison group made up of other disorders.
This quantitative analysis, like those meta-analyses published previously, aggregated measures within domains. By combining different neuropsychological measures, some effects may have been diluted by reducing the specificity of the aggregated ESs. Given the limited number of studies with the same measures, however, aggregation was necessary to increase reliability of the findings. As this literature further develops, future meta-analyses can provide a more detailed examination of individual neuropsychological measures, including those tapping the specific aspects or subprocesses of dimensions gaining more recent attention such as social cognition (e.g., emotion perception, theory of mind, attribution).
Most traditional neuropsychological tests are multidimensional, and thus not sufficiently independent of one another to be useful in assaying discrete cognitive domains and processes or neural systems (Dickinson & Gold, 2008). As noted by Snitz and colleagues (Snitz et al., 2006), there are no well-established conventions in the field for this task, nor is there firm data supporting the independence of cognitive dimensions and tests. That is, with large portions of overall variance in a typical neuropsychological battery being common variance, from a psychometric standpoint it may be unreasonable to expect clear independent signals for differential characterization of illness effects.
An additional limitation of this review was that there were several historic and important FHR studies, including the NYHRP and the Jerusalem Infant Development study, for which ESs for individual tests comparing FHR and control groups could not be calculated from published data. Because of this, important contributions to the field, such as significant findings regarding poorer FHR performance on the CPT-IP in the NYHRP, could not be quantitatively presented here, which could result in a smaller average ES for the attention domain than would be calculated if the NYHRP CPT-IP findings were incorporated. Because only a subgroup of the FHR group in a given FHR study would be expected to develop schizophrenia in adulthood, several important FHR studies have focused on presenting deviant subgroup analyses, rather than averaging those at FHR and comparing to healthy controls (Marcus, et al., 1981). This strategy, while very important, obscures some standard group differences.
Conclusions
Quantitative analysis from 30 studies yielded medium to large impairments across nine neurocognitive domains. Mild cognitive deficits are reliably and broadly present in young FHR individuals, falling at a level that is intermediate between healthy individuals and those diagnosed with schizophrenia. Future prospective, longitudinal studies that may elucidate differential trajectories of cognitive change have the potential to inform the optimal timing of early intervention strategies. Careful characterization of heterogeneous FHR samples will contribute to refinements in the comparative quantification of presumably modest cognitive changes over time.
Supplementary Material
Acknowledgments
This research was supported, in part, by NIMH Grants (T32MH017119, J Agnew-Blais) U01 MH081928, P50MH080272 and SCDMH82101008006 (LJ Seidman).
References
- Aylward E, Walker E, Bettes B. Intelligence in schizophrenia: meta-analysis of the research. Schizophrenia Bulletin. 1984;10:430–459. doi: 10.1093/schbul/10.3.430. [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Steffy RA, MacCrimmon DJ, Cleghorn JM. An attentional assessment of foster children at risk for schizophrenia. Journal of Abnormal Psychology. 1977;86:267–275. doi: 10.1037//0021-843x.86.3.267. [DOI] [PubMed] [Google Scholar]
- Asarnow JR, Steffy RA, MacCrimmon DJ, Cleghorn JM. An attentional assessment of foster children at risk for schizophrenia. In: Wynne LC, Cromwell RL, Matthysse S, editors. The Nature of Schizophrenia: New Approaches to Research and Treatment. New York, NY: John Wiley; 1978. pp. 339–358. [Google Scholar]
- Bentall RP, Corcoran R, Howard R, Blackwood N, Kinderman P. Persecutory delusions: a review and theoretical integration. Clinical Psychology Review. 2001;21:1143–1192. doi: 10.1016/s0272-7358(01)00106-4. [DOI] [PubMed] [Google Scholar]
- Bhojraj TS, Franci AN, Rajarethinam R, Eack S, Kulkarni S, Prasad KM, Montrose DM, Dworakowski D, Diwadkar V, Keshavan MS. Verbal fluency deficits and altered lateralization of language brain areas in individuals genetically predisposed to schizophrenia. Schizophrenia Research. 2009;11:202–208. doi: 10.1016/j.schres.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York, NY: International Universities Press; 1911. [Google Scholar]
- Bonner-Jackson A, Csernansky JG, Barch DM. Levels-of-processing effects in first degree relatives of individuals with schizophrenia. Biological Psychiatry. 2007;61:1141–1147. doi: 10.1016/j.biopsych.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Byrne M, Hodges A, Grant E, Owens D, Johnstone E. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS) Psychological Medicine. 1999;29:1161–1173. doi: 10.1017/s0033291799001002. [DOI] [PubMed] [Google Scholar]
- Byrne M, Clafferty R, Cosway R, Grant E, Hodges A, Whalley HC, Lawrie ST, Carter JW, Parnas J, Urfer-Parnas A, Watson J, Mednick SA. Intellectual functioning and the long-term course of schizophrenia-spectrum illness. Psychological Medicine. 2011;41:1223–1237. doi: 10.1017/S0033291710001820. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Megginson Hollister J, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: A prospective cohort story. Schizophrenia Bulletin. 2000;26:379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- Carter JW, Parnas J, Urfer-Parnas A, Watson J, Mednick SA. Intellectual functioning and the long-term course of schizophrenia spectrum illness. Psychological Medicine. 2011;41:1223–1237. doi: 10.1017/S0033291710001820. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cohler BJ, Grunebaum HU, Weiss JL, Gamer E, Gallant DH. Disturbance of attention among schizophrenic, depressed and well mothers and their young children. Journal of Child Psychology and Psychiatry. 1977;18:115–135. doi: 10.1111/j.1469-7610.1977.tb00424.x. [DOI] [PubMed] [Google Scholar]
- Cornblatt B, Erlenmeyer-Kimling L. Early attentional predictors of adolescent’s behavioral disturbances in children at risk for schizophrenia. In: Watt NF, Anthony EJ, Wynne LC, Rolf JE, editors. Children at Risk for Schizophrenia: A Longitudinal Perspective. New York, NY: Cambridge University Press; 1984. pp. 198–211. [Google Scholar]
- Cornblatt B, Keilp J. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophrenia Bulletin. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Abukmeil SS, Lawrie SM, Miller P, Johnstone EC. Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychological Medicine. 2000;30:1111–1121. doi: 10.1017/s0033291799002585. [DOI] [PubMed] [Google Scholar]
- Cosway R, Byrne M, Clafferty R, Hodges E, Grant J, Morris J, Abukmeil SS, Lawrie SM, Miller P, Owens DGC, Johnstone EC. Sustained attention in young people at high risk for schizophrenia. Psychological Medicine. 2002;32:277–286. doi: 10.1017/s0033291701005050. [DOI] [PubMed] [Google Scholar]
- Cunningham DG, Johnstone EC. Neuropsychology, genetic liability and psychotic symptoms in those at high risk for schizophrenia. Journal of Abnormal Psychology. 2003;112:38–48. [PubMed] [Google Scholar]
- Davalos D, Compagnon N, Heinlein S, Ross R. Neuropsychological deficits in children associated with increased familial risk for schizophrenia. Schizophrenia Research. 2004;67:123–130. doi: 10.1016/S0920-9964(03)00187-7. [DOI] [PubMed] [Google Scholar]
- Delawalla Z, Csernansky JG, Barach DM. Prefrontal cortex function in nonpsychotic siblings of individuals with schizophrenia. Biological Psychiatry. 2008;63:490–497. doi: 10.1016/j.biopsych.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Cornblatt BA, Friedmann R, Kaplansky LM, Lewis JA, Rinaldi A, Shilliday C, Erlenmeyer-Kimling L. Childhood precursors of affective vs. social deficits in adolescents at risk for schizophrenia. Schizophrenia Bulletin. 1993;19:563–577. doi: 10.1093/schbul/19.3.563. [DOI] [PubMed] [Google Scholar]
- Eack SM, Mermon DE, Montrose DM, Miewald J, Gur RE, Gur RC, Sweeney JA, Keshavan MS. Social cognition deficits among individuals at familial high risk for schizophrenia. Schizophrenia Bulletin. 2009;36:1081–1088. doi: 10.1093/schbul/sbp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Declarative memory: Insights from cognitive neurobiology. Annual Review of Psychology. 1997;48:547–572. doi: 10.1146/annurev.psych.48.1.547. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L. Neurological, electrophysiological and attention deviations in children at risk for schizophrenia. In: Henn FA, Nasrallah HA, editors. Schizophrenia as a brain disease. New York, NY: Oxford University Press; 1982. pp. 61–98. [Google Scholar]
- Erlenmeyer-Kimling L, Cornblatt B. The New York High-Risk Project: A follow-up report. Schizophrenia Bulletin. 1987;13:451–461. doi: 10.1093/schbul/13.3.451. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Cornblatt BA. Summary of attentional findings in the New York High-Risk Project. Journal of Psychiatric Research. 1992;26:405–426. doi: 10.1016/0022-3956(92)90043-n. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Rock D, Roberts SA, Janal M, Kestenbaum C, Cornblatt B, Adamo UH, Gottesman II. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. American Journal of Psychiatry. 2000;157:1416–1422. doi: 10.1176/appi.ajp.157.9.1416. [DOI] [PubMed] [Google Scholar]
- Erlenmeyer-Kimling L, Hans S, Ingraham L, Marcus J, Wynne L, Rehman A, Roberts SA, Auerbach J. Handedness in children of schizophrenia parents: Data from the three high-risk studies. Behavioral Genetics. 2005;35:351–358. doi: 10.1007/s10519-005-3227-y. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Green A, Seidman LJ, Tsuang MT. “Schizotaxia”: clinical implications and new directions for research. Schizophrenia Bulletin. 2001;27:1–18. doi: 10.1093/oxfordjournals.schbul.a006849. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: A four-year follow-up study. Journal of Abnormal Psychology. 1999;108:176–181. doi: 10.1037//0021-843x.108.1.176. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Seidman LJ, Kremen W, Toomey R, Pepple J, Tsuang MT. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: the effect of genetic loading. Biological Psychiatry. 2000;48:120–126. doi: 10.1016/s0006-3223(99)00263-2. [DOI] [PubMed] [Google Scholar]
- Fis NP, Cetin FC, Erturk M, Erdogan E, Dedeoglu C, Yazgan Y. Executive dysfunction in Turkish children at high risk for schizophrenia. European Child & Adolescent Psychiatry. 2008;17:424–431. doi: 10.1007/s00787-008-0684-x. [DOI] [PubMed] [Google Scholar]
- Fish B. The maturation of arousal and attention in the first months of life: a study of variations in ego development. Journal of the American Academy of Child Psychiatry. 1963;2:253–270. doi: 10.1016/s0002-7138(09)61972-5. [DOI] [PubMed] [Google Scholar]
- Fish B. Neurobiologic antecedents of schizophrenia in children. Evidence for an inherited, congenital neurointegrative defect. Archives of General Psychiatry. 1977;34:1297–1313. doi: 10.1001/archpsyc.1977.01770230039002. [DOI] [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Prinstein MJ, Perkins DO, Belger A. Social skill and social cognition in adolescents at genetic risk for psychosis. Schizophrenia Research. 2010;122:179–184. doi: 10.1016/j.schres.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Stone WS, Faraone SV, Seidman LJ, Tsuang MT. Psychopathology, personality traits and social development among young first-degree relatives of patients with schizophrenia. British Journal of Psychiatry. 2006;189:337–345. doi: 10.1192/bjp.bp.105.016998. [DOI] [PubMed] [Google Scholar]
- Goldstein J, Seidman L, Buka SL, Horton NJ, Donatelli JL, Rider RO, Tsuang MT. Impact of genetic vulnerability and chronic exposure to hypoxia on overall intelligence by age 7 in offspring at high risk for schizophrenia compared with affective psychosis. Schizophrenia Bulletin. 2000;26:323–334. doi: 10.1093/oxfordjournals.schbul.a033456. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Emory University project on children of disturbed parents. Schizophrenia Bulletin. 1987;13:411–423. doi: 10.1093/schbul/13.3.411. [DOI] [PubMed] [Google Scholar]
- Gottesman I. Schizophrenia Genesis: The Origin of Madness. New York, NY: Freeman; 1991. [Google Scholar]
- Green P. Defective interhemispheric transfer in schizophrenia. Journal of Abnormal Psychology. 1978;87:472–480. doi: 10.1037//0021-843x.87.5.472. [DOI] [PubMed] [Google Scholar]
- Green P, Hallett S, Hunter M. Abnormal interhemispheric integration and hemispheric specialization in schizophrenics and high-risk children. In: Flor-Henry P, Gruzelier J, editors. Laterality and Psychopathology. Elsevier: Amsterdam; 1982. [Google Scholar]
- Green M. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green M, Kern R, Braff D, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Jackson GM, Calton TG, Andrew HK, Bates AT, Liddle PF, Hollis C. Cognitive deficits in early-onset schizophrenia spectrum patients and their non-psychotic siblings: A comparison with ADHD. Schizophrenia Research. 2008;99:85–95. doi: 10.1016/j.schres.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Groth-Marnat G. Handbook of Psychological Assessment. Hoboken, New Jersey: John Wiley & Sons; 2009. [Google Scholar]
- Grunebaum H, Weiss JL, Gallant D, Cohler BJ. Attention in young children of psychotic mothers. American Journal of Psychiatry. 1974;131:887–891. doi: 10.1176/ajp.131.8.887. [DOI] [PubMed] [Google Scholar]
- Hallet S, Green P. Possible defects of interhemispheric integration of children of schizophrenics. Journal of Nervous and Mental Disease. 1983;171:421–425. doi: 10.1097/00005053-198307000-00005. [DOI] [PubMed] [Google Scholar]
- Hallet S, Quinn D, Hewitt J. Defective interhemispheric integration and anomalous language lateralization in children at risk for schizophrenia. Journal of Nervous and Mental Disease. 1986;174:418–427. doi: 10.1097/00005053-198607000-00006. [DOI] [PubMed] [Google Scholar]
- Hans SL, Marcus J, Nuechterlein KH, Asarnow RF, Styr B, Auerbach JG. Neurobehavioral deficits at adolescence in children at risk for schizophrenia: the Jerusalem Infant Development Study. Archives of General Psychiatry. 1999;56:741–748. doi: 10.1001/archpsyc.56.8.741. [DOI] [PubMed] [Google Scholar]
- Hans SL, Auerbach JG, Asarnow JR, Styr B, Marcus J. Social adjustment of adolescents at risk for schizophrenia: the Jerusalem Infant Development Study. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1406–1414. doi: 10.1097/00004583-200011000-00015. [DOI] [PubMed] [Google Scholar]
- Harvey P, Keefe R. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. American Journal of Psychiatry. 2001;158:176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- Heinrichs R, Zakzanis K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Ebmeier KP, Miller P, Owens DGC, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. British Journal of Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Keefe R, Perkins D, Silva S, Lieberman J. The effect of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophrenia Bulletin. 1999;25:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Diwadkar V, Montrose D, Rajarethinam R, Sweeney J. Premorbid indicators and risk for schizophrenia: a selective review and update. Schizophrenia Research. 2005;79:45–57. doi: 10.1016/j.schres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Kulkarni S, Bhojraj T, Francis A, Diwadkar V, Montrose D, Seidman LJ, Sweeney J. Premorbid cognitive deficits in young relatives of schizophrenia patients. Frontiers in Human Neuroscience. 2010;62:1–14. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm S, Schmidt B, Knappe S, Blanz B. Impaired working speed and executive functions as frontal lobe dysfunctions in young first-degree relatives of schizophrenic patients. European Child and Adolescent Psychiatry. 2006;15:400–408. doi: 10.1007/s00787-006-0547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and Paraphrenia. Edinburgh: E & S Livingstone; 1919. [Google Scholar]
- Kremen W, Hoff A. Neurocognitive deficits in biological relatives of individuals with schizophrenia. In: Stone W, Faraone S, Tsuang M M, editors. Early Clinical Intervention and Prevention in Schizophrenia. Totowa, NJ: Humana Press; 2004. pp. 133–154. [Google Scholar]
- Kremen W, Seidman L, Pepple J, Lyons M, Tsuang M, Faraone S. Neuropsychological risk indicators for schizophrenia: a review of family studies. Schizophrenia Bulletin. 1994;20:96–108. doi: 10.1093/schbul/20.1.103. [DOI] [PubMed] [Google Scholar]
- Landau R, Harth P, Othnay N, Shafhertz C. The influence of psychotic parents on their children’s development. American Journal of Psychiatry. 1972;129:38–43. doi: 10.1176/ajp.129.1.38. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3rd ed. New York: Oxford University Press; 1995. [Google Scholar]
- Lifshitz M, Kugelmass S, Karov M. Perceptual-motor and memory performance of high-risk children. Schizophrenia Bulletin. 1985;11:74–84. doi: 10.1093/schbul/11.1.74. [DOI] [PubMed] [Google Scholar]
- Lipsey MW, Wilson DB. Applied Social Research Methods Series. Vol. 49. Thousand Oaks, CA: SAGE Publications; 2001. Practical Meta-Analysis. [Google Scholar]
- Maier W, Franke P, Kopp B, Hardt J, Hain CH, Rist F. Reaction time paradigms in subjects at risk for schizophrenia. Schizophrenia Research. 1994;13:35–43. doi: 10.1016/0920-9964(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Marcus J, Auerbach J, Wilkinson L, Burack CM. Infants at risk for schizophrenia: The Jerusalem Infant Development Study. Archives of General Psychiatry. 1981;38:703–713. doi: 10.1001/archpsyc.1981.01780310103011. [DOI] [PubMed] [Google Scholar]
- Marcus J, Hans SL, Nagler S, Auerbach JG, Mirsky AF, Aubrey A. Review of the NIMH Israeli Kibbutz-City Study and the Jerusalem Infant Development Study. Schizophrenia Bulletin. 1987;13:425–438. doi: 10.1093/schbul/13.3.425. [DOI] [PubMed] [Google Scholar]
- Marjoram D, Miller P, McIntosh AM, Cunningham Owens DG, Johnstone EC, Lawrie S. A neuropsychological investigation into ‘Theory of Mind’ and enhanced risk of schizophrenia. Psychiatry Research. 2006;144:29–37. doi: 10.1016/j.psychres.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Maziade M, Rouleau N, Gingras N, Boutin P, Paradis M, Jomphe V, Boutin J, Le´tourneau K, Gilbert E, Lefebvre E, Dore M, Marino C, Battaglia M, Me´rette C, Roy M. Shared neurocognitive dysfunctions in young offspring at extreme risk for schizophrenia or bipolar disorder in Eastern Quebec multigenerational families. Schizophrenia Bulletin. 2009;35:919–930. doi: 10.1093/schbul/sbn058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky AF, Ingramham LJ, Kugelmass S. Neuropsychological assessment of attention and its pathology in the Israeli cohort. Schizophrenia Bulletin. 1995;21:193–204. doi: 10.1093/schbul/21.2.193. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Schulsinger F. Some premorbid characteristics relating to breakdown in children with schizophrenic mothers. In: Rosenthal D, Kety SS, editors. The Transmission of Schizophrenia. Oxford, UK: Pergamon Press; 1968. pp. 267–291. [Google Scholar]
- Mishara A, Goldberg T. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: opening a closed book. Biological Psychiatry. 2004;55:1013–1022. doi: 10.1016/j.biopsych.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Murray R, Lewis S. Is schizophrenia a neurodevelopmental disorder? BMJ (Clin Research Ed) 1987;295:681–682. doi: 10.1136/bmj.295.6600.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles-Worsley M, Ord LM, Ngiralmau H, Weaver S, Blailes F, Faraone SV. The Palau Early Psychosis Study: Neurocognitive functioning in high-risk adolescents. Schizophrenia Research. 2007;89:299–307. doi: 10.1016/j.schres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Neale JM, Winters KC, Weintraub S. Information-processing deficits in children at high risk for schizophrenia. In: Watt NF, Anthony EJ, Wynne LC, Rolf JE, editors. Children at Risk for Schizophrenia: A Longitudinal Perspective. New York, NY: Cambridge University Press; 1984. pp. 264–277. [Google Scholar]
- Niemi L, Suvisaari J, Tuulio-Henriksson A, Lonnqvist J. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophrenia Research. 2003;60:239–258. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- Niendam T, Bearden C, Rosso I, Sanchez LE, Hadley T, Nuechterlein KH, Cannon TD. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. American Journal of Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH. Signal detection in vigilance tasks and behavioral attributes among offspring of schizophrenic mothers and among hyperactive children. Journal of Abnormal Psychology. 1983;92:4–28. doi: 10.1037//0021-843x.92.1.4. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K, Dawson M. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophrenia Bulletin. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Mednick S, Schulsinger F, Rock D. The children of psychiatrically disturbed parents: differences as a function of the sex of the sick parents. Archives of General Psychiatry. 1979;36:691–695. doi: 10.1001/archpsyc.1979.01780060081009. [DOI] [PubMed] [Google Scholar]
- Ott SL, Spinelli S, Rock D, Roberts S, Amminger GP, Erlenmeyer-Kimling L. The New York High-Risk Project: social and general intelligence in children at risk for schizophrenia. Schizophrenia Research. 1998;4(3):1–11. doi: 10.1016/s0920-9964(98)00010-3. [DOI] [PubMed] [Google Scholar]
- Ozan E, Deveci E, Oral M, Karahan U, Oral E, Aydın N, Kırpınar I. Neurocognitive functioning in a group of offspring genetically at high-risk for schizophrenia in Eastern Turkey. Brain Research Bulletin. 2010;82:218–223. doi: 10.1016/j.brainresbull.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37:51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Phillips LK, Seidman LJ. Emotion processing in persons at risk for schizophrenia. Schizophrenia Bulletin. 2008;34:888–903. doi: 10.1093/schbul/sbn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, Poulton R, Moffitt TE. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. American Journal of Psychiatry. 2010;167:160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 2d ed. Tucson, Arizona: Neuropsychology Press; 1993. [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Jr, Beck LH. A continuous performance test of brain damage. Journal of Consulting Psychology. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rutschmann J, Cornblatt BA, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia. Archives of General Psychiatry. 1977;34:571–575. doi: 10.1001/archpsyc.1977.01770170081007. [DOI] [PubMed] [Google Scholar]
- Rutschmann J, Cornblatt BA, Erlenmeyer-Kimling L. Auditory recognition memory in adolescents at risk for schizophrenia: report on a verbal continuous recognition task. Psychiatric Research. 1980;3:151–161. doi: 10.1016/0165-1781(80)90032-3. [DOI] [PubMed] [Google Scholar]
- Rutschmann J, Cornblatt BA, Erlenmeyer-Kimling L. Sustained attention in children at risk for schizophrenia: findings with two visual continuous performance tests in a new sample. Journal of Abnormal Child Psychology. 1986;14:365–385. doi: 10.1007/BF00915432. [DOI] [PubMed] [Google Scholar]
- Sameroff AJ, Barocas R, Seifer R. Children at risk for schizophrenia: A longitudinal perspective. New York: Cambridge University Press; 1984. The early development of children born to mentally ill women. Children at risk for schizophrenia: A longitudinal perspective; pp. 482–514. [Google Scholar]
- Sameroff A, Seifer R, Zax M, Barocas R. Early indicators of developmental risk: Rochester Longitudinal Study. Schizophrenia Bulletin. 1987;13:383–394. doi: 10.1093/schbul/13.3.383. [DOI] [PubMed] [Google Scholar]
- Schreiber H, Stolz-Born G, Heinrich H, Kornhuber HH, Born J. Attention, cognition, and motor perseveration in adolescents at genetic risk for schizophrenia and control subjects. Psychiatric Research. 1992;44:125–140. doi: 10.1016/0165-1781(92)90047-7. [DOI] [PubMed] [Google Scholar]
- Schubert E, McNeil T. Neuropsychological impairment and its neurological correlates in adult offspring with heightened risk for schizophrenia and affective psychosis. American Journal of Psychiatry. 2005;162:758–766. doi: 10.1176/appi.ajp.162.4.758. [DOI] [PubMed] [Google Scholar]
- Seidman LJ. Schizophrenia and brain dysfunction: An integration of recent neurodiagnostic findings. Psychological Bulletin. 1983;94:195–238. [PubMed] [Google Scholar]
- Seidman LJ. Clinical neuroscience and epidemiology in schizophrenia. Harvard Review of Psychiatry. 1997;3:338–342. doi: 10.3109/10673229709030562. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Smith CW, Stone WS, Glatt SJ, Meyer E, Faraone SV, Tsuang MT, Cornblatt B. Neuropsychological functioning in adolescents and young adults at genetic risk for schizophrenia and affective psychoses: Results from the Harvard and Hillside Adolescent High Risk Studies. Schizophrenia Bulletin. 2006;32:507–524. doi: 10.1093/schbul/sbj078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, Bearden CE, Christensen BK, Hawkins K, Heaton R, Keefe RSE, Heinssen R, Cornblatt B. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: Relationship to family history and conversion to psychosis. Archives of General Psychiatry. 2010;67(6):578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Agnew-Blais J, Cherkerzian S, Goldstein JM, Tsuang MT, Buka SL. Specificity of neuropsychological impairment in schizophrenia vs bipolar psychosis from premorbid period to chronic psychosis phase. Schizophrenia Bulletin. 2011 (abstract). [Google Scholar]
- Sergeant JA, Geurts HM, Oosterlaan J. How specific is a deficit of executive functioning for Attention-Deficit/Hyperactivity Disorder? Behavioural Brain Research. 2002;130:3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- Sitskoorn M, Aleman A, Ebisch S, Appels M, Kahn R. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophrenia Research. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Snitz B, McDonald A, Carter C. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohlberg SC. Personality and neuropsychological performance of high-risk children. Schizophrenia Bulletin. 1985;11:48–60. doi: 10.1093/schbul/11.1.48. [DOI] [PubMed] [Google Scholar]
- Sohlberg SC, Yaniv S. Social adjustment and cognitive performance of high-risk children. Schizophrenia Bulletin. 1985;11:61–65. doi: 10.1093/schbul/11.1.61. [DOI] [PubMed] [Google Scholar]
- Sommer I, Ramsey N, Kahn R, Aleman A, Bourma A. Handedness, language lateralization and anatomical asymmetry in schizophrenia: Meta-analysis. British Journal of Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Stone WS, Hsi X. Declarative memory deficits and schizophrenia: problems and prospects. Neurobiology of Leaning and Memory. 2011 doi: 10.1016/j.nlm.2011.04.006. In Press. [DOI] [PubMed] [Google Scholar]
- Tarbox SI, Pogue-Geile MF. Development of social functioning in preschizophrenia children and adolescents: a systematic review. Psychological Bulletin. 2008;134:561–583. doi: 10.1037/0033-2909.34.4.561. [DOI] [PubMed] [Google Scholar]
- Toulopoulou T, Goldberg TE, Mesa IR, Picchioni M, Rijsdijk F, Stahl D, Cherny SS, Sham P, Faraone SV, Tsuang M, Weinberger DR, Seidman LJ, Murray RM. Impaired intellect and memory: a missing link between genetic risk and schizophrenia? Archives of General Psychiatry. 2010;67:905–913. doi: 10.1001/archgenpsychiatry.2010.99. [DOI] [PubMed] [Google Scholar]
- Winters KC, Stone AA, Weintraub S, Neale JM. Cognitive and attentional deficits in children vulnerable to psychopathology. Journal of Abnormal Child Psychololgy. 1981;9:435–453. doi: 10.1007/BF00917794. [DOI] [PubMed] [Google Scholar]
- Wolf LE, Cornblatt BA, Roberts SA, Shapiro BM, Erlenmeyer-Kimling L. Wisconsin Card Sorting deficits in the offspring of schizophrenics in the New York High Risk Project. Schizophrenia Research. 2002;57:173–182. doi: 10.1016/s0920-9964(01)00301-2. [DOI] [PubMed] [Google Scholar]
- Weinberger D. Implications of normal brain development for the pathogenesis of schizophrenia. Archives of General Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. American Journal of Psychiatry. 2008;165(5):579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- Worland J, Weeks D, Janes C, Strock B. Intelligence, classroom behavior, and academic achievement in children at high and low risk for psychopathology: a structural equation analysis. Journal of Abnormal Child Psychology. 1984;12:437–454. doi: 10.1007/BF00910658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.