Abstract
Reactive oxygen species (ROS) play a central role in oxidative stress, which leads to the onset of diseases, such as cancer. Furthermore, ROS contributes to the delicate balance between tumor cell survival and death. However, the mechanisms by which tumor cells decide to elicit survival or death signals during oxidative stress are not completely understood. We have previously reported that ROS enhanced tumorigenic functions in prostate cancer cells, such as transendothelial migration and invasion, which depended on CXCR4 and AKT signaling. Here, we report a novel mechanism by which ROS facilitated cell death through activation of AKT. We initially observed that ROS increased expression of phosphorylated AKT (p-AKT) in 22Rv1 human prostate cancer cells. The tumor suppressor PTEN, a negative regulator of AKT signaling, was rendered catalytically inactive through oxidation by ROS, although the expression levels remained consistent. Despite these events, cells still underwent apoptosis. Further investigation into apoptosis revealed that expression of the tumor suppressor pVHL increased, and contains a target site for p-AKT phosphorylation. pVHL and p-AKT associated in vitro, and knockdown of pVHL rescued HIF1α expression and the cells from apoptosis. Collectively, our study suggests that in the context of oxidative stress, p-AKT facilitated apoptosis by inducing pVHL function.
Keywords: ROS, p-AKT, HIF1α, pVHL, apoptosis, prostate cancer
Introduction
Malignant transformation of cancer cells is driven by the inactivation of tumor suppressors and/or the overwhelming activation of mitogenic molecules, due to aberrant regulation of various signaling pathways that encourage survival functions. One such signaling molecule is protein kinase B (AKT). In cancer cells, AKT is an established molecule that regulates cellular functions that encourages malignancy, such as cell survival, proliferation and migration [1]. Upstream of AKT, phosphinositide 3-kinase (PI3K) enzymatically converts phosphatidylinositol 3,4-triphosphase (PIP2) to phosphatidylinositol 3,4,5-triphosphase (PIP3), which recruits and activates PDK, thereby activating AKT by phosphorylation. Under normal physiologic conditions, AKT is activated by signaling receptors such as receptor tyrosine kinases (RTK) and G-protein coupled receptors (GPCR). AKT can also be constitutively active, independent of receptor signaling, due to mutations and/ or constitutively active upstream signaling molecules [2]. AKT signaling provides protection from apoptosis and encourages uncontrolled cell-cycle progression. These functions are innate to AKT in normal energy metabolism, which makes it favorable to tumor cell survival [3]. However, an emerging concept is that AKT may also sensitize cells to senescence and apoptosis [4].
Reactive oxygen species (ROS) are oxygen-derived free radicals, such as hydroxyl radicals, peroxides and superoxides, that are endogenously produced in the mitochondria by complexes I and III; ROS is also produced in the cytosol by NAD(P)H oxidase systems and enzymes, such as xanthine oxidases, cytochrome p450 and cyclooxygenases [5]. Cellular damage is a major consequence induced by ROS accumulation, which is a natural byproduct of energy metabolism. Cellular insults by ROS include DNA lesions, protein oxidation and lipid peroxidation [6]. Conversely, ROS induces tumorigenic functions such as proliferation and metastasis [7]. The delicate balance of ROS concentration that determines the lifespan of the cell remains unknown [7]. Concerning oxidative stress, hypoxic inducible factor 1α (HIF1α) has long been involved in targeting cells for apoptosis in response to ROS accumulation [7].
The HIF1α complex of proteins enhances expression of targeted genes that adjust cells to oxidative stress and/or cell survival. The complex consists of a regulatory HIF1α subunit and the constitutively expressed HIF1β subunit, both members of the basic helix-loop-helix (bHLH) and PER-ARNT-SIM (PAS) families of transcription factors [8]. A hydroxylated HIF1α binds to the tumor suppressor von Hippel–Lindau protein (pVHL), which contains E3 ligase activity. The binding of HIF1α and pVHL facilitates ubiquitination of HIF1α and subsequent proteasomal degradation of the complex [9]. For this reason, pVHL has been recognized as a tumor suppressor [10]. These collective events implicate ROS in apoptosis. Reports have shown that ROS plays a critical role in determining the cell cycle and senescence in cells. For example, mouse embroyic fibroblast developed long telomeres when grown at ambient oxygen levels [6]. Likewise, human diploid cells underwent senescence at low oxygen conditions [11].
Currently, chemotherapeutic agents are used to induce ROS-mediated apoptosis in tumor cells, thereby decreasing malignancy. A number of anticancer drugs exert their effect by causing DNA damage which leads to apoptosis induction [12]. Hydrogen peroxide and super oxide anion (O2−) participate in apoptosis and DNA damage induced by some anticancer drugs, however, the precise mechanism of apoptosis via ROS formation remains to be clarified. For instance, Jacaranone and other small molecule compounds have induced apoptosis through oxidative stress [13, 14], along with concomitant downregulation of AKT signaling and expression. Downregulation of AKT is the canonical concept of ROS-mediated apoptosis. However, Noguiera et al. described that increased expression of phosphorylated AKT (p-AKT) determined replicative senescence of mammalian cells in culture and mediated apoptosis induced by oxidative stress [4]. What differed in this report from long-standing reports on AKT and apoptosis was that activation, not inhibition or downregulation, sensitized cell to apoptosis. Moreover, rapamycin, which is usually cytostatic, sensitized cells to ROS-mediated cell death through activation of AKT [4]. Further, increased p-AKT deficiency exerted resistance to senescence induced by oxidative stress [4].
In this study, we observed that prostate cancer cells responded to ROS by inducing apoptosis, despite increased expression of p-AKT. Several signatures of apoptosis were observed, including decreased HIF1α expression. Further studies revealed that ROS-mediated decrease of HIF1α correlated with increased pVHL expression. We then investigated a correlation between ROS-mediated activation of AKT and pVHL expression. We found that activated, not downregulated, AKT enhanced pVHL expression, thereby targeting cells for apoptosis. Finally, downregulation of pVHL rescued cells from apoptosis. Collectively, these findings may change the paradigm of AKT expression in tumor apoptosis, as it is one of the most targeted molecules in chemotherapeutics.
Materials and Methods
Cell Culture, Antibodies, and Reagents
Human prostate cancer cell line 22Rv1 was obtained from American Type Culture Collection (ATCC) and maintained in complete RPMI 1640 media (10% Fetal Bovine Serum (FBS), 1% nonessential amino acids and 1% antibiotic-antimycotic), or starvation media (RPMI only), at 37°C and 5% CO2. Cells were maintained at 60% to 80% confluency. Hydrogen peroxide (H2O2) was used as our model of ROS (Acros Organics). N-acetyl-cysteine (NAC) was from Sigma Aldrich; cobalt chloride (CoCl2) and N-ethylmaleimide (NEM) were from EMD Chemicals; LY294002 was from Cayman Chemicals. Cell culture supplies were from MediaTech and the following human antibodies were from Cell Signaling: anti-pVHL, anti-PTEN, anti-AKT, anti-phospho-AKT (p-AKT) and anti-cleaved-PARP; anti-HIF1α was from BD Bioscience; anti-α-Tubulin was from Santa Cruz Biotech.
Proliferation (Viability) Assay
Cell proliferation was assessed by a MTT dye conversion assay at 570 nm following manufacturer’s instructions (Trevigen). In triplicates, 1 × 103 cells/well were seeded in a 96-well flat-bottomed plate. Cells were serum starved for 4 hours prior to treatments in RPMI at 37 °C in 5% CO2. At each time point (24 and 48 hours), the treatments were replaced with 100 µL of RPMI media and then incubated with 10 µL of MTT reagent for 2 hours at 37°C, followed by 100 µL of detergent reagent at 37 °C for 2 hours. Proliferation (viability) was measured at 570 nm using a microplate reader (Bio-Tek Synergy HT). Results were quantified using GraphPad Prism 5 statistical software.
Apoptosis Assay
Annexin-V Apoptosis Detection Kit Plus (MBL) was used to quantify the levels of apoptosis in samples, according to the manufacturer's specifications. Briefly, cells were trypsinized, centrifuged and resuspended in 500µl of 1X binding buffer prior to adding Annexin V-FITC. After 15 minutes of incubation, apoptosis was analyzed by flow cytometry (Accuri C6) or microplate reader at 488nm ex/578nm em (Bio-Tek Synergy HT) for the detection of Annexin V-FITC.
Western Blot Analysis
3×105 cells were harvested in lysis buffer (Cell Signaling) as previously described [15]. Equal concentrations of total cell lysate were resolved by 10% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane. Nonspecific binding sites were blocked with 5% nonfat dry milk/0.1% Tween 20/1XTBS, followed by an incubation with primary antibodies for the proteins of interest in 3% Bovine Serum Albumin - Tris-buffered saline/Tween 20 (BSA-TBS/T; p-AKT, AKT, PTEN, HIF1α, cleaved-PARP). Protein complexes were detected with horseradish peroxidase-conjugated secondary antibodies (JacksonImmuno Research) and enhanced chemiluminescence reagents (Pierce). Exposed films were developed using an automated X-ray processor (Kodak X-OMAT M35A Processor).
Western Blot Analysis of Alkylated PTEN
The oxidation state of PTEN was investigated using alkylating agents, as described by our laboratory and Lee et al. [7, 16]. Briefly, 1×106 cells/well were treated with 0.25mM H2O2 and scraped into alkylating lysis buffer (20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L Na2EDTA, 1 mmol/L EGTA, 1% Triton, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L b-glycerophosphate, 1 mmol/L Na3VO4, 1 mg/mL leupeptin, 1mmol/L PMSF, 2% SDS and 40 mM N-ethylmaleimide (NEM)). Cell lysates were sonicated, and equal amounts of protein (60 µg) were incubated at room temperature for 30 minutes. Total protein lysates were diluted in sample buffer without β-mercaptoethanol, resolved by 10% SDS-PAGE and transferred to a PVDF membrane. Reduced and non-reduced forms of PTEN were detected as described above.
PCR Amplification
Total RNA was isolated with Total RNA Kit-I (Omega Bio-TeK), as described by the manufacturer. Total RNA was reversed transcribed with M-MLV Transcriptase (Promega) to generate cDNA for PCR amplification. Specific sense and antisense primers for PTEN, HIF1α and housekeeping gene L19, were synthesized by Integrated DNA Technologies as follows: PTEN (forward GGA CGA ACT GGT GTA ATG ATA TG; reverse TCT ACT GTT TTT GTG AAG TAC AGC), HIF1α (forward ACA TAA AGT CTG CAA CAT GGA AGG; reverse TTG ATG GGT GAG GAA TGG GTT C) and L19 (forward GAA ATC GCC AAT GCC TC; reverse TCT TA ACC TC GAG CCT CA).
Short Interfering RNA Transfection
Transient transfection of pVHL specific short interfering RNA (siRNA; Santa Cruz) was carried out in 22Rv1 cells using JetPrime transfection reagent (Polyplus Transfection). Briefly, cells (5×105) were plated in 100-mm dishes and transfected with various concentrations of respective siRNA in 20% FBS/RPMI at 37°C and 5% CO2 for 18 hours. Transfected cells were serum starved, treated and then harvested for western blot analysis.
Phosphatase Activity Assay
Treated cells (1×106 cells/well) were harvested in 1X lysis buffer (Cell Signaling). 500µg of total protein were incubated with a PTEN specific primary antibody overnight at 4°C, then immunoprecipitated with protein A/G agarose beads (Santa Cruz) for 4 hours at 4°C. Samples were centrifuged for 10 minutes at 10,000 rpm, the supernatant was collected and the bead pellet was then resuspended in 40µL of 1X lysis buffer. Western blot analysis was performed on total protein lysate, supernatant and bead pellets for PTEN. The PIP phosphatase assay, as described by Maehama et al. was adapted to a 96-well format [17]. PIP3 lipid (Echelon, Salt Lake City, UT) was combined with 10µL of immunoprecipitated PTEN and allowed to dephosphorylate PIP3 to PIP2 at 37°C for 2 hours. 100µL of malachite green was added to react with free phosphates, representing the successful dephosphorylation of PIP3, and the absorbance for the phosphatase products was measured at 620 nm with a microplate reader at increasing time intervals. Each data point was assayed in triplicate, and all experiments were repeated at least three times.
Statistics and Quantifications
Data are presented as the mean ±SE of at least 3 independent experiments and were analyzed by 2-way ANOVA or Student's t test. All statistical analyses were done, and all graphs generated, using GraphPad Prism 5.0 software (GraphPad).
Results
H2O2 accumulation induced phospho-AKT expression
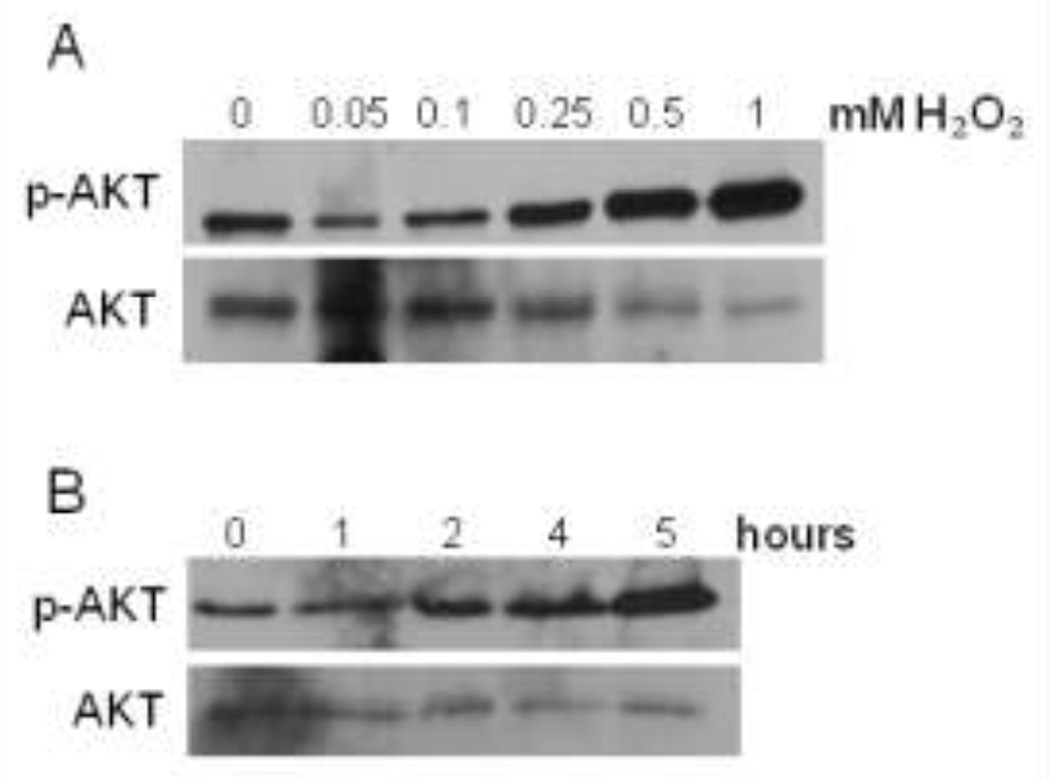
In the following study, we used H2O2 as our model of ROS and human prostate cancer cells, 22Rv1, was a tumor model. We have previously observed that H2O2 induced expression of p-AKT in DU145 prostate cancer cells, suggesting activation of survival mechanisms and functions [7]. Likewise, we observed a gradual increase in p-AKT expression in a concentration dependent manner in 22Rv1 cells (Fig. 1a). We chose 0.25 mM of H2O2 as a median concentration and decided to treat cells at various time points. We found that exposing cells to 0.25 mM H2O2 at increasing time points correlated with increasing p-AKT expression (Fig. 1b). For subsequent experiments, we chose 0.25 mM H2O2 for 4 hours as our treatment.
Figure 1. H2O2 induced the expression of p-AKT in 22Rv1 cells.
Serum-starved cells were treated with various concentrations of H2O2 for 2 hours (A), or 0.25mM H2O2 at various time points (B). Forty micrograms (µg) of total protein were analyzed for total (AKT) and phosphorylated AKT (p-AKT) expression by western blot analysis using specific antibodies. Total AKT, served as a loading control.
H2O2 inhibited PTEN catalytic function
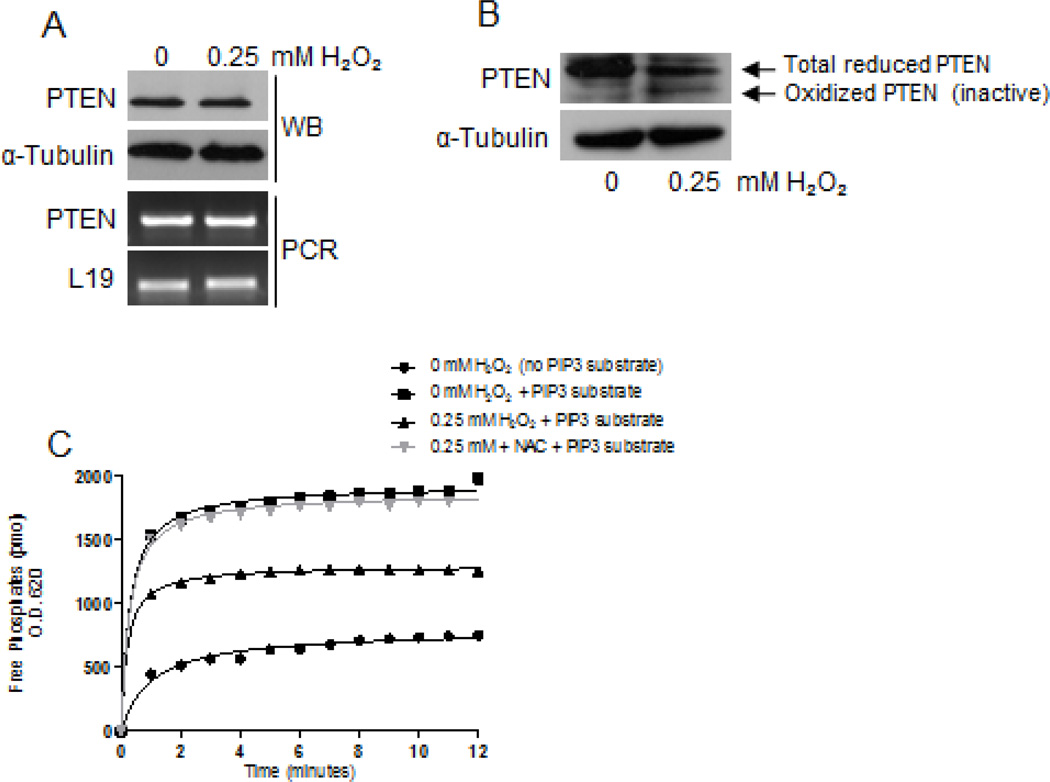
The tumor suppressor PTEN negatively regulates the PI3K/AKT pathway by converting PIP3 to PIP2, thus removing the signal that would activate AKT [18]. We investigated whether ROS-mediated p-AKT expression was due to decreased expression of PTEN. H2O2 did not alter the expression of PTEN at the mRNA or at the protein level; expression levels remained consistent (Fig. 2a). Previous reports have shown that ROS accumulation oxidized PTEN catalytic domain by forming a disulfide bond between amino acids cysteine(s) 71 and 124 within the active site, thus inactivating its phosphatase function [16]. We determined whether H2O2 oxidizes PTEN, thereby inactivating its catalytic function, since there was no change in PTEN expression levels. By alkylation method [7], we found increased expression of the oxidized form of PTEN (a low molecular weight band) in cells treated with H2O2 compared to untreated cells (Fig. 2b). To further confirm loss of PTEN catalytic function, we treated cells with H2O2 and analyzed its activity by performing a phosphatase assay. Following treatments, equal amounts of immunoprecipitated PTEN was allowed to convert PIP3 (substrate) to PIP2, which produced free phosphates. The free phosphates were allowed to react with malachite green dye as the functional output of phosphatase activity. H2O2-treated cells demonstrated a moderate level of activity, which increased in the presence of ROS scavenger NAC, or the absence of 0.25mM H2O2 (Fig. 2c). Although the expression levels didn't change, H2O2 inactivated PTEN catalytically; this may have contributed to increased expression of p-AKT.
Figure 2. H2O2 inhibited PTEN catalytic activity in 22Rv1 cells.
(A) Serum-starved cells were treated with H2O2 for 4 hours. Total protein (40µg) was analyzed for PTEN expression by western blot analysis using a specific antibody; α-Tubulin served as a loading control. Total RNA (2µg) was isolated and reverse transcribed to cDNA for PCR amplification using primers specific for PTEN; L19 served as a loading control. (B) Serum-starved cells were treated with H2O2 for 4 hours, followed by alkylation as previously described [7]. Total protein lysate (60µg) was analyzed for oxidized and reduced forms of PTEN by western blot analysis using a specific antibody; α-Tubulin served as a loading control. (C) Equal concentrations of total protein lysate were allowed to dephosphorylate PIP3 to PIP2, prior to the quantitation for free phosphates via a phosphatase assay.
H2O2 induced apoptosis in 22Rv1 cells
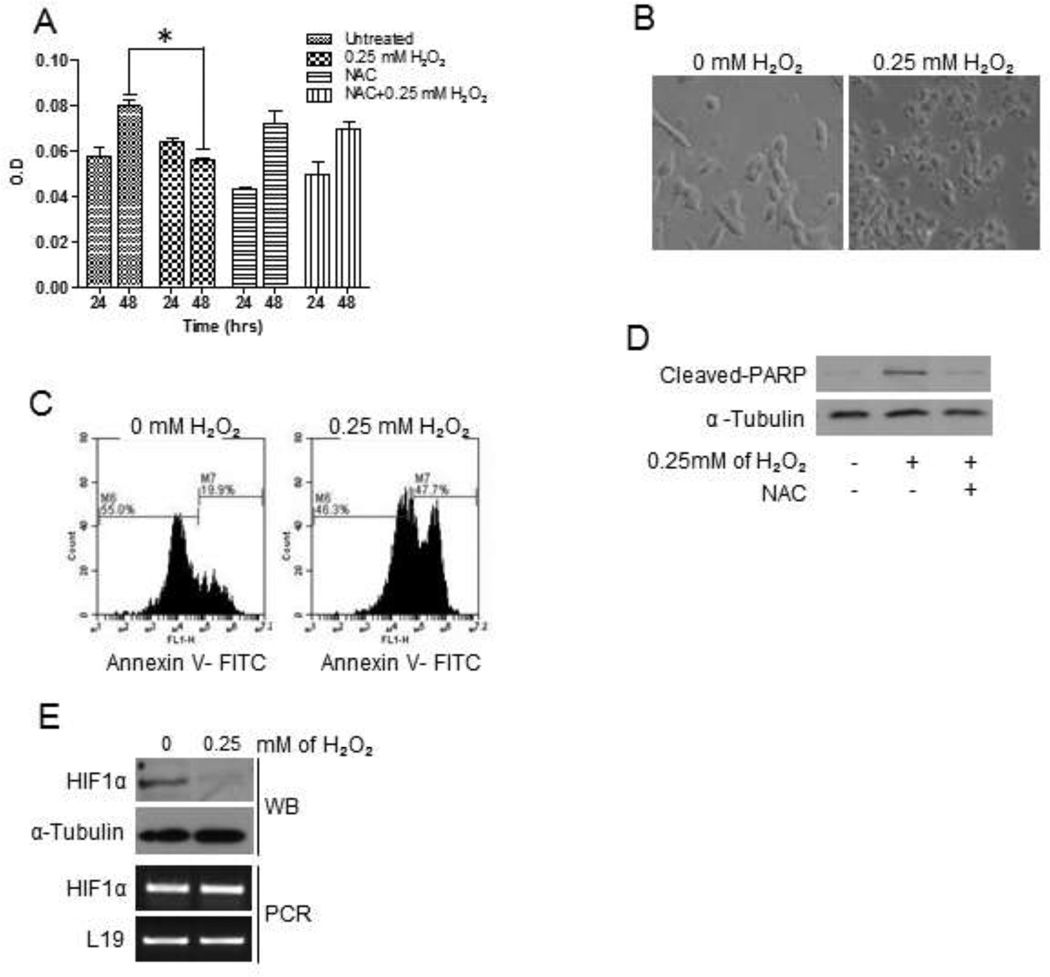
The activation of oncogenic pathways, such as AKT, and inactivation of tumor suppressor molecules, such as PTEN, are strong indicators for cell proliferation, growth and tumor cell progression [19]. Hence, we determined whether the observed H2O2-mediated increase in p-AKT expression and inactivation of PTEN led to increase cell viability, by performing a MTT assay. Surprisingly, we found that H2O2-treated cells decreased in cell viability compared to untreated control cells (Fig. 3a). Furthermore, cells treated with NAC, a ROS scavenger and 0.25 mM H2O2 showed similar viability levels compared to untreated control (Fig. 3a). The decrease in viability was unexpected; therefore, we investigated whether cells may have entered cell death. Bright field microscopy revealed that cells were phenotypically smaller, condensed and granular in the presence of H2O2, compared to untreated control cells (Fig. 3b), which was indicative of apoptosis [20]. Cell death in 22Rv1 cells was confirmed by an Annexin-V assay, where 47% of H2O2-treated 22Rv1 cells were positive for apoptosis, compared to 19.9% of untreated cells (Fig. 3c). We further substantiated apoptosis in 22Rv1 cells by analyzing for cleaved-PARP, an apoptotic marker that indicates caspase-3 activation, and HIF1α, a cell survival marker [21]. Hydrogen peroxide-treated cells expressed cleaved-PARP, which was abrogated by NAC (Fig. 3d). Furthermore, treating cells with 0.25 mM H2O2 reduced HIF1α expression at the protein level compared to untreated control; there was no change in HIF1α mRNA levels, indicating that H2O2 regulated HIF1α at the translational level and not at transcription (Fig 3e).
Figure 3. H2O2 induced apoptosis in 22Rv1 cells.
(A) Serum-starved cells were treated with H2O2 for 4 hours. MTT assays were performed at each time point following the manufacturer’s protocol. Experiments were repeated thrice, in triplicate, and the data represents the averages of three independent experiments. (B) Serum-starved cells were untreated or treated with H2O2 for 4 hours. Light micrographs were taken at 20X magnification using a Zeiss Axiovert 200M microscope. Serum-starved cells were treated with H2O2 for 4 hours prior to analyzing for (C) Annexin-V by flow cytometry, and (D) cleaved-PARP by western blot analysis using a specific antibody; α-Tubulin served as a loading control. (E) Serum-starved cells were treated with H2O2 for 4 hours. Total protein lysate (100µg) was analyzed for HIF1α expression by western blot analysis using a specific antibody; α-Tubulin served as a loading control. Total RNA (2µg) was isolated and reverse transcribed to cDNA for PCR amplification using primers specific for HIF1α; L19 served as a loading control.
H2O2 inhibited HIF1α expression through pVHL
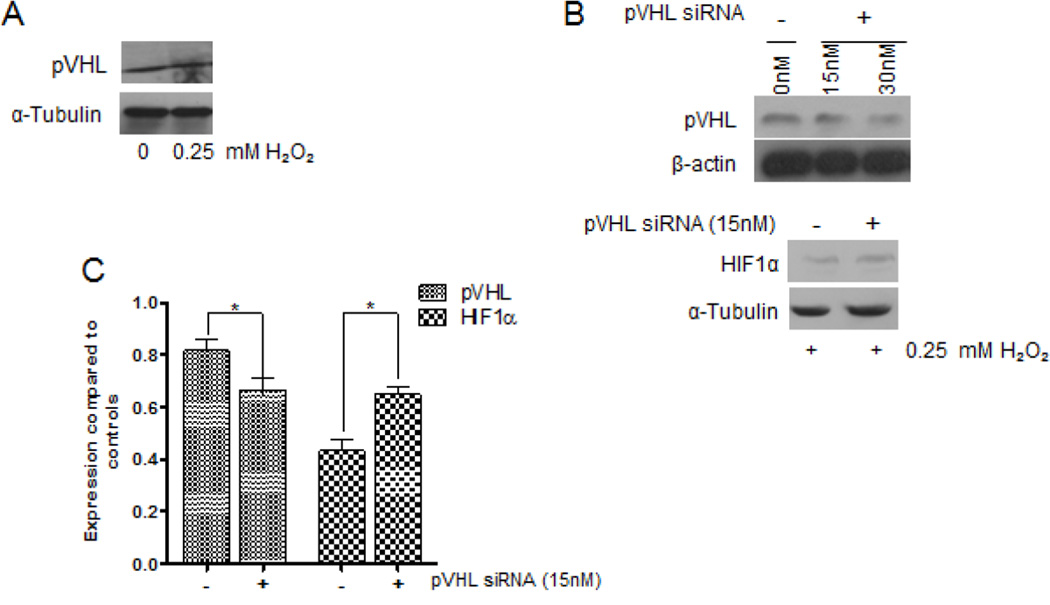
HIF1α protein is regulated by the tumor suppressor pVHL, which contains E3 ligase activity that tags HIF1α for proteasomal degradation [22]. Therefore, we determined whether H2O2-mediated reduction of HIF1α expression correlated with pVHL activity. The expression levels of pVHL did not change in the presence of 0.25 mM H2O2, compared to control (Fig 4a). We decreased pVHL expression by siRNA to determine whether HIF1α would be affected. We investigated three concentrations of pVHL siRNA: 15nM, 30nM and 50nM. We saw diminished knockdown at 15nM and 30nM, and complete knockdown at 50nM (data not shown); however the cells would easily contaminate at 30nM or become unhealthy at 50nM. Subsequently, we decided to use 15nM. Upon treating pVHL-knockdown cells with 0.25 mM H2O2, we observed an increase on HIF1α expression compared to untreated cells (Fig 4b & c), indicating that expression of HIF1α and pVHL are inversely correlated. These results suggest that ROS mediates apoptosis and degrades HIF1α through pVHL.
Figure 4. H2O2-mediated degradation of HIF1α was through pVHL in 22Rv1 cells.
(A) Serum-starved cells were treated with combinations of 0.25mM H2O2 or 10µM of LY294002 for 4 hours. Total protein lysate (100µg) was analyzed for pVHL expression by western blot analysis using a specific antibody; α-Tubulin served as a loading control. (B) Cells were transfected with 15nM, 30nM or 50nM (data not shown) of pVHL-specific siRNA, or the proper controls at 37°C. Twenty-four hours post-transfection, 100µg of total protein lysate was isolated from serum-starved cells and analyzed for pVHL and HIF1α expression by western blot analysis; α-Tubulin and β-actin served as loading controls. (C) A densitometric analysis of the relative expression of pVHL and HIF1α, compared to respective controls. Experiments were done in triplicate.
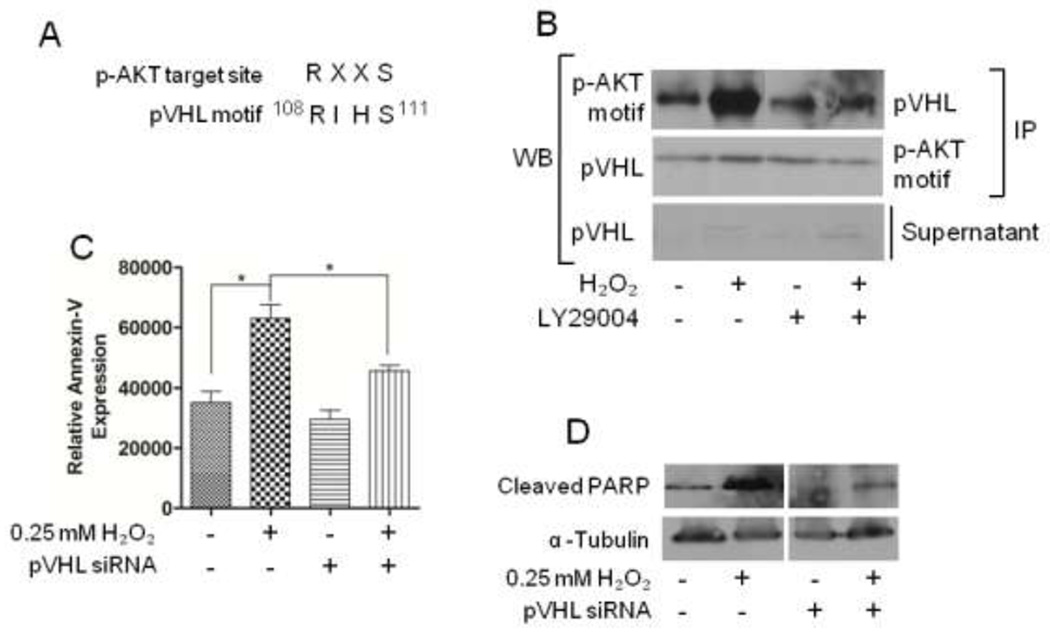
pVHL activity depended on p-AKT
Previous studies have shown that pVHL activity was involved in directing apoptosis [23]. Roe et al. demonstrated that phosphorylation of pVHL at serine 111 (S111) led to an increase in function [24]. Interestingly, S111 is a residue within a consensus target motif for p-AKT (Fig 5a) and sought to determine whether p-AKT is involved in pVHL activity. Cells were treated with combinations of H2O2 and LY294002, after which pVHL was immunoprecipitated. A western blot analysis was performed using an monoclonal antibody specific for the phosphorylated substrate consensus sequence, RXXS. We found increased expression of pVHL at the phosphorylated AKT consensus motif in cells treated with 0.25 mM H2O2 compared to untreated control cells (Fig 5b). This increase diminished when AKT was inhibited with LY294002 alone, and in combination with 0.25 mM H2O2 (Fig. 5b).
Figure 5. AKT phosphorylates pVHL under oxidative (H2O2) stress.
(A) A comparison between the p-AKT target consensus sequence and the putative p-AKT target site found in pVHL. (B) Serum-starved cells were treated with combinations of 0.25mM H2O2 or 10µM of LY294002 for 4 hours. Total protein (500µg) was immunoprecipitated with the indicated primary antibodies, and western blot analysis was performed to detect pVHL or p-AKT-RXXS. (C) Graph represents quantitation of annexin-V fluorescence expression. (D) Cleaved-PARP expression was analyzed by western blot analysis using a specific antibody; α-Tubulin served as a loading control.
Increased pVHL activity enhances apoptosis [23]. Therefore, we determined whether diminished pVHL would rescue cells from apoptosis. Cells were incubated with siRNA specific for pVHL, prior to treating with H2O2 and assaying for signatures of apoptosis. Downregulating pVHL prevented apoptosis, as observed by reduced Annexin-V (Fig. 5c) and cleaved-PARP (Fig. 5d); H2O2 did not direct the cells towards apoptosis.
Discussion
In this study, we propose a regulatory mechanism by which cells die in response to ROS accumulation that uses AKT. In literature, ROS generation initiates signaling cascades, such as the AKT pathway, that can enhance or inhibit survival mechanisms [4, 25]. We initially observed that ROS induced p-AKT expression in 22Rv1 prostate cancer cells, which was consistent with previously published reports [7, 25]. Herein, and in previous data, we have shown that ROS-mediated upregulation of p-AKT expression was not due to diminished levels of PTEN expression [7]. Lee et al. described that H2O2 induced disulfide bonding within the active sites of PTEN, rendering it inactive [16]. We observed catalytic inactivation of PTEN, where it failed to convert PIP3 to PIP2 in the presence of H2O2, thus allowing increased expression of p-AKT. Likewise, Ha et al. demonstrated that ROS accumulation inactivated PTEN catalytic function and promoted AKT expression in hepatocellular carcinoma [26]. Considering the effects of ROS on PTEN function, it is plausible to suggest that ROS may indirectly regulate p-AKT through catalytic inactivation of PTEN.
The development and progression of cancer cells are generally due to the inactivation of tumor suppressor genes, such as PTEN, and the activation of oncogenes, such as AKT. We observed this effect upon treatment with ROS, thereby suggesting that cells would progress towards cell survival [7]. On the contrary, cells treated with H2O2 demonstrated a decrease in cell viability compared to control, and demonstrated several signatures of apoptosis, such as an increase in extracellular annexin-V, increase in cleaved-PARP and decreased HIF1α. Indeed, ROS accumulation leads to apoptosis, but it was interesting to observe a gradual increase in expression of survival molecule p-AKT, suggesting that expressed p-AKT may have more of a role in apoptosis than attributed.
The stabilization of HIF1α is dependent on the activity of the E3 protein ligase, pVHL, which targets HIF1α towards proteasomal degradation. Thus, a decrease in HIF1α inversely correlates with an increase in pVHL activity. We observed that silencing pVHL restored HIF1α expression in the presence of ROS. These results support Park et al. where cellular toxicity by zinc ions inhibited HIF1α prolyl hydroxylase (PHD) activity, and blocked pVHL binding to HIF1α to induce its degradation in prostate cancer cells [27]. An increase in pVHL expression depends on phosphorylation by various molecules, such as glycogen synthase kinase 3 (GSK3) and checkpoint kinase 2 (chk2) [24, 28]. Notably, Roe et al. demonstrated that phosphorylation of pVHL at serine111 (S111) by chk2 led to increased activity, and subsequently, apoptosis [24]. The S111 residue in pVHL is within a motif (108RIHS111) that is homologous to a consensus p-AKT substrate motif (RXXS) [29]. Several targets of p-AKT, such as BAD, MDM2 and FOXO1, contain the RXXS motif and are phosphorylated by p-AKT [30–32] (Table 1). This led us to believe that phosphorylation of this putative AKT-substrate site on pVHL may facilitate pVHL-mediated activity, and subsequently, apoptosis. Accordingly, we observed that knockdown of pVHL prevented ROS-mediated cell death.
Table 1.
A comparison of the AKT target consensus sequence, RXXS, to known AKT target motif within proteins.
| Phospho-AKT motif | |
|---|---|
| BAD | |
| HDM2 | |
| FOXO1 |
The idea of AKT positively influencing a tumor suppressor is a conflicting concept, but has been described. For instance, Hinton et al. demonstrated that the tumor suppressor BRCA1 contained a phosphorylation site for AKT, which enhanced BRCA1 localization to the nucleus and BRCA1-dependent transcriptional activity [33]. Nogueira et al. has shown that p-AKT mediated tumor suppressive functions by inhibiting FOXO, which led to apoptosis in a ROS dependent manner [4]. Another mechanism by which AKT may exert its tumor suppressive functions is by inducing the activity of apoptin, a pro-apoptotic molecule [34].
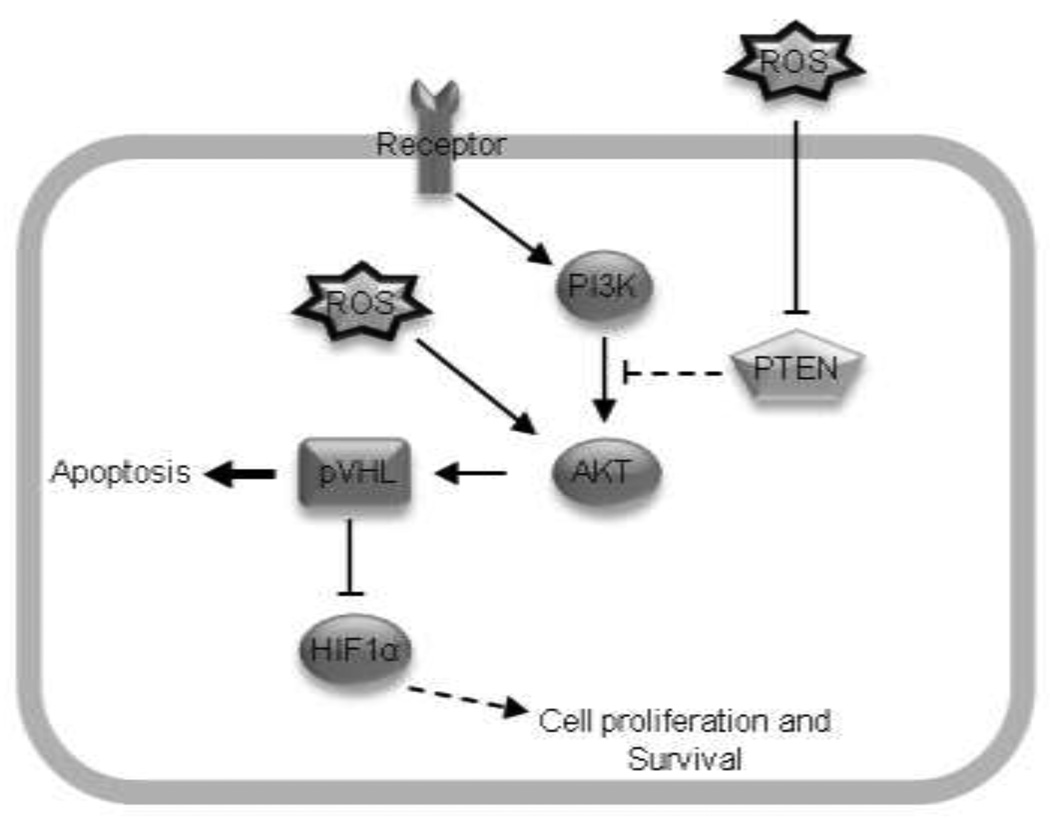
AKT functioning as an anti-survival molecule is novel and emerging. Most studies view AKT as a cell survival molecule that functions to prevent apoptosis. However, the emerging notion of AKT working as a pro-apoptotic molecule suggests that AKT should not be exclusively labeled as a survival molecule, and may contribute to chemotherapeutics (Fig. 6). Taken together, our study supports the emerging role of p-AKT in promoting apoptosis and tumor suppressive functions [35]. However, additional studies are warranted to fully elucidate the divergent role of ROS on AKT-mediated cell survival and death in tumors.
Figure 6. Proposed schematic for p-AKT and pVHL in mediating apoptosis.
Putatively, the accumulation of ROS promoted AKT activation with concomitant inhibition of PTEN catalytic activity. Increased p-AKT phosphorylated pVHL, which targeted HIF1α for proteasomal degradation. Collectively, these activities led to apoptosis.
Acknowledgments
Research in this laboratory is supported, in part, by National Institutes of Health grants F31CA153908 (MAC), G12RR003062-22 (CVH) and P20MD002285 (CVH and VOM), and the American Association for the Advancement of Science (AAAS) Women's International Research Collaborations (WIRC) for Minority Serving Institutions (MSIs), a National Science Foundation grant.
References
- 1.Gunn RM, Hailes HC. Insights into the PI3-K-PKB-mTOR signalling pathway from small molecules. J Chem Biol. 2008;1:49–62. doi: 10.1007/s12154-008-0008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies MA, Stemke-Hale K, Tellez C, Calderone TL, Deng W, Prieto VG, Lazar AJ, Gershenwald JE, Mills GB. A novel AKT3 mutation in melanoma tumours and cell lines. British Journal of Cancer. 2008;99:1265–1268. doi: 10.1038/sj.bjc.6604637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 4.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, Unterman T, Hay N. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008;14:458–470. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Chetram MA, Don-Salu-Hewage AS, Hinton CV. ROS enhances CXCR4-mediated functions through inactivation of PTEN in prostate cancer cells. Biochem Biophys Res Commun. 2011 doi: 10.1016/j.bbrc.2011.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nature Cell Biology. 2000;2:423–427. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 10.Shen C, Kaelin WG., Jr The VHL/HIF axis in clear cell renal carcinoma. Seminars in Cancer Biology. 2012 doi: 10.1016/j.semcancer.2012.06.001. PMID: 22705278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature Cell Biology. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang HL, Fang LW, Lu SP, Chou CK, Luh TY, Lai MZ. DNA-damaging reagents induce apoptosis through reactive oxygen species-dependent Fas aggregation. Oncogene. 2003;22:8168–8177. doi: 10.1038/sj.onc.1206979. [DOI] [PubMed] [Google Scholar]
- 13.Massaoka MH, Matsuo AL, Figueiredo CR, Farias CF, Girola N, Arruda DC, Scutti JA, Romoff P, Favero OA, Ferreira MJ, et al. Jacaranone induces apoptosis in melanoma cells via ROS-mediated downregulation of Akt and p38 MAPK activation and displays antitumor activity in vivo. PLoS One. 2012;7:e38698. doi: 10.1371/journal.pone.0038698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu CC, Wu PJ, Hsu JL, Ho YF, Hsu LC, Chang YJ, Chang HS, Chen IS, Guh JH. Ardisianone, a natural benzoquinone, efficiently induces apoptosis in human hormone-refractory prostate cancers through mitochondrial damage stress and survivin downregulation. The Prostate. 2012 doi: 10.1002/pros.22548. [DOI] [PubMed] [Google Scholar]
- 15.Chetram MA, Odero-Marah V, Hinton CV. Loss of PTEN permits CXCR4-mediated tumorigenesis through ERK1/2 in prostate cancer cells. Mol Cancer Res. 2011;9:90–102. doi: 10.1158/1541-7786.MCR-10-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Yang K, Kwon J, Lee C, Jeong W, Rhee S. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 17.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Jiang X. PTEN: a default gate-keeping tumor suppressor with a versatile tail. Cell Res. 2008;18:807–816. doi: 10.1038/cr.2008.83. [DOI] [PubMed] [Google Scholar]
- 19.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bortner CD, Cidlowski JA. Apoptotic volume decrease and the incredible shrinking cell. Cell Death Differ. 2002;9:1307–1310. doi: 10.1038/sj.cdd.4401126. [DOI] [PubMed] [Google Scholar]
- 21.Janicke RU, Ng P, Sprengart ML, Porter AG. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. The Journal of biological chemistry. 1998;273:15540–15545. doi: 10.1074/jbc.273.25.15540. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Kim WY. Two sides to every story: the HIF-dependent and HIF-independent functions of pVHL. J Cell Mol Med. 2011;15:187–195. doi: 10.1111/j.1582-4934.2010.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nyhan MJ, O'Sullivan GC, McKenna SL. Role of the VHL (von Hippel-Lindau) gene in renal cancer: a multifunctional tumour suppressor. Biochem Soc Trans. 2008;36:472–478. doi: 10.1042/BST0360472. [DOI] [PubMed] [Google Scholar]
- 24.Roe JS, Kim HR, Hwang IY, Ha NC, Kim ST, Cho EJ, Youn HD. Phosphorylation of von Hippel-Lindau protein by checkpoint kinase 2 regulates p53 transactivation. Cell Cycle. 2011;10:3920–3928. doi: 10.4161/cc.10.22.18096. [DOI] [PubMed] [Google Scholar]
- 25.Kumar B, Koul S, Khandrika L, Meacham R, Koul H. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 26.Ha HL, Yu DY. HBx-induced reactive oxygen species activates hepatocellular carcinogenesis via dysregulation of PTEN/Akt pathway. World journal of gastroenterology : WJG. 2010;16:4932–4937. doi: 10.3748/wjg.v16.i39.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SE, Park JW, Cho YS, Ryu JH, Paick JS, Chun YS. HIF-1alpha promotes survival of prostate cells at a high zinc environment. The Prostate. 2007;67:1514–1523. doi: 10.1002/pros.20641. [DOI] [PubMed] [Google Scholar]
- 28.Hergovich A, Lisztwan J, Thoma CR, Wirbelauer C, Barry RE, Krek W. Priming-dependent phosphorylation and regulation of the tumor suppressor pVHL by glycogen synthase kinase 3. Molecular and cellular biology. 2006;26:5784–5796. doi: 10.1128/MCB.00232-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. The EMBO journal. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 30.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 31.Ashcroft M, Ludwig RL, Woods DB, Copeland TD, Weber HO, MacRae EJ, Vousden KH. Phosphorylation of HDM2 by Akt. Oncogene. 2002;21:1955–1962. doi: 10.1038/sj.onc.1205276. [DOI] [PubMed] [Google Scholar]
- 32.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. The Journal of biological chemistry. 1999;274:17179–17183. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 33.Hinton CV, Fitzgerald LD, Thompson ME. Phosphatidylinositol 3-kinase/Akt signaling enhances nuclear localization and transcriptional activity of BRCA1. Experimental cell research. 2007;313:1735–1744. doi: 10.1016/j.yexcr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddika S, Panigrahi S, Wiechec E, Wesselborg S, Fischer U, Schulze-Osthoff K, Los M. Unscheduled Akt-triggered activation of cyclin-dependent kinase 2 as a key effector mechanism of apoptin's anticancer toxicity. Molecular and cellular biology. 2009;29:1235–1248. doi: 10.1128/MCB.00668-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Los M, Maddika S, Erb B, Schulze-Osthoff K. Switching Akt: from survival signaling to deadly response. Bioessays. 2009;31:492–495. doi: 10.1002/bies.200900005. [DOI] [PMC free article] [PubMed] [Google Scholar]