Abstract
Hallucinogenic drugs, such as lysergic acid diethylamide (LSD), mescaline and psilocybin, alter perception and cognitive processes. All hallucinogenic drugs have in common a high affinity for the serotonin 5-HT2A receptor. Metabotropic glutamate 2/3 (mGlu2/3) receptor ligands show efficacy in modulating the cellular and behavioral responses induced by hallucinogenic drugs. Here, we explored the effect of chronic treatment with the mGlu2/3 receptor antagonist 2S-2-amino-2-(1S,2S-2-carboxycyclopropan-1-yl)-3-(xanth-9-yl)-propionic acid (LY341495) on the hallucinogenic-like effects induced by LSD (0.24 mg/kg). Mice were chronically (21 days) treated with LY341495 (1.5 mg/kg), or vehicle, and experiments were carried out one day after the last injection. Chronic treatment with LY341495 down-regulated [3H]ketanserin binding in somatosensory cortex of wild-type, but not mGlu2 knockout (KO), mice. Head-twitch behavior, and expression of c-fos, egr-1 and egr-2, which are responses induced by hallucinogenic 5-HT2A agonists, were found to be significantly decreased by chronic treatment with LY341495. These findings suggest that repeated blockade of the mGlu2 receptor by LY341495 results in reduced 5-HT2A receptor-dependent hallucinogenic effects of LSD.
Keywords: Hallucinogenic drugs, Serotonin 5-HT2A receptor, Metabotropic glutamate 2 (mGlu2) receptor, Lysergic acid diethylamide (LSD), LY341495, G protein-coupled receptor (GPCR), schizophrenia, psychosis
1. Introduction
The serotonin [22] and glutamate [5] receptor systems play an important role in the mechanism of action of hallucinogenic drugs, such as lysergic acid diethylamide (LSD), mescaline or psilocybin. All hallucinogenic drugs have in common a high affinity for the serotonin 5-HT2A receptor [12], and this receptor has been shown to be necessary for some of their behavioral effects in rodents [13] and humans [24]. Metabotropic glutamate 2 and 3 (mGlu2/3) receptors have also been involved in the cellular and behavioral responses induced by hallucinogenic 5-HT2A receptor agonists. Thus, mGlu2/3 receptor orthosteric agonists, such as LY379268 and LY354740, reduce the cellular [10, 28], electrophysiological [17] and behavioral [7, 10] effects induced by hallucinogenic drugs. Similar findings have been reported for the mGlu2-selective allosteric agonist biphenyl-indanone A (BINA) [3]. Moreover, mice lacking mGlu2 receptor (mGlu2 knockout, KO), display reduced responses to hallucinogenic drugs, such as 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) and LSD [18].
Another point of interest is the effect of chronic administration of 5-HT2A ligands on expression and behavioral function of mGlu2/3 receptors. Chronic treatment with the hallucinogenic 5-HT2A agonist 1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane (DOB) reduces the behavioral effects induced by the mGlu2/3 agonist LY379268 in mice [2]. These include absence of effect of LY379268 on the 5-HT2A receptor-dependent head-twitch behavioral response, and lower effect of LY379268 on the hyperlocomotor behavioral response induced by phenyclidine (PCP). Chronic treatment with the atypical antipsychotic drug clozapine also affects mGlu2 mRNA expression and mGlu2/3 ligand binding in mouse cortical regions, an effect that is not observed in 5-HT2A-KO mice [10, 16]. Together, these findings suggest that chronic treatment with either hallucinogenic or antipsychotic 5-HT2A ligands modulates the expression of mGlu2/3 receptors. In this study, we investigated the effects of chronic treatment with the mGlu2/3 receptor antagonist LY341495 on the 5-HT2A receptor-dependent cellular and behavioral responses induced in mice by LSD. We measured LSD-dependent expression of c-fos, egr-1 and egr-2 in mouse somatosensory cortex, and head-twitch behavior. These cellular and behavioral responses have been previously shown to require expression of 5-HT2A receptor in cortical neurons [13].
2. Methods
2.1 Animals
Experiments were performed on adult (8–12 weeks old) male 129S6/SvEv mice. Animals were purchased from Taconic (Hudson, NY), and were housed at 12 h light/dark cycle (lights on, 8:00 to 20:00) at 23°C with food and water ad libitum. Mice were housed for at least 1 week before experimental use. mGlu2-KO mice [27] were obtained from the RIKEN BioResource Center, Japan (see [18] for details). For experiments involving wild-type and mGlu2-KO mice, all subjects were offspring of heterozygote breeding. The Institutional Animal Use and Care Committee at Mount Sinai School of Medicine approved all experimental procedures.
2.2. Radioligand binding
For effect of chronic treatment with LY341495 on [3H]LY341495 and [3H]ketanserin binding in mouse somatosensory cortex, mice were injected (i.p.) daily for 21 days with LY341495 (1.5 mg/kg). This dose was selected based on previous studies in rodents [7, 10]. Control mice were chronically (21 days) treated with vehicle (0.9% NaCl). Mice were sacrificed by cervical dislocation one day after the last injection with chronic LY341495, or vehicle, and bilateral somatosensory cortex (bregma 1.40 to −1.90 mm) was dissected and frozen at −80°C until processed for radioligand binding assays. Mouse somatosensory cortex plasma membrane preparations were obtained as previously reported [19], and stored at −80°C until assay.
[3H]LY341495 binding assays (0.0625–30 nM; eleven concentrations) were performed as previously reported [16]. Non-specific binding was determined in the presence of 1 mM L-glutamic acid (Tocris Cookson Inc., Minneapolis, MN). [3H]Ketanserin binding assays (0.0625–10 nM; ten concentrations) were performed as previously [16]. Non-specific binding was determined in the presence of 10 μM methysergide (Tocris Cookson Inc., Minneapolis, MN). [3H]Ketanserin binds with high affinity to both 5-HT2A and 5-HT2C receptors. Previous findings demonstrate that under these experimental conditions (i.e., 5-HT2 receptor ligand methysergide to define non-specific binding), specific [3H]ketanserin binding is abolished in somatosensory [13] and frontal [21] cortex of 5-HT2A knockout mice as compared to wild-type littermates.
[3H]-2S-2-amino-2-(1S,2S-2-carboxycyclopropan-1-yl)-3-(xanth-9-yl)-propionic acid ([3H]LY341495) was purchased from American Radiolabeled Chemicals, Inc. (Saint Louis, MO). [3H]Ketanserin was purchased from PerkinElmer Life and Analytical Sciences, Inc. (Waltham, MA). LY341495 was obtained from Tocris Cookson Inc. (Minneapolis, MN). All other chemicals were obtained from standard sources.
2.3. Head-twitch behavior
Head-twitch behavioral response was measured as previously reported (see [19]) one day after the last injection with chronic LY341495. LSD (0.24 mg/kg) was obtained from Sigma-Aldrich (Saint Louis, MO).
2.4. Reverse transcription quantitative PCR (RT-qPCR)
Mice were injected daily for 21 days with LY341495 (1.5 mg/kg), or vehicle (see above). Induction of c-fos, egr-1 and egr-2 by LSD (0.24 mg/kg) was measured one day after the last injection with chronic LY341495. Reverse transcription quantitative real-time PCR (RT-qPCR) experiments were performed as previously reported [18]. See [13] for primer sequences.
2.5. Statistical analysis
All graphs and statistical analyses were generated using GraphPad Prism 5.0b. Radioligand binding data were analyzed using a nonlinear curve fit. An extra-sum-of-squares (F-test) was used to determine statistical differences for simultaneous analyses of binding saturation curves. Differences in the maximum number of binding sites (Bmax) were assessed by unpaired Student’s t-test. Statistical significance of experiments involving two or more groups and two or more treatments was assessed by two-way ANOVA followed by Bonferroni’s post hoc test. Statistical significance of experiments involving three or more groups was assessed by one-way ANOVA followed by Bonferroni’s post hoc test. Statistical significance of experiments involving two groups was assessed by Student’s t-test. The level of significance was chosen at p = 0.05. All data are presented as mean ± SEM.
3. Results
3.1. Effect of chronic treatment with LY341495 on mGlu2/3 receptor binding
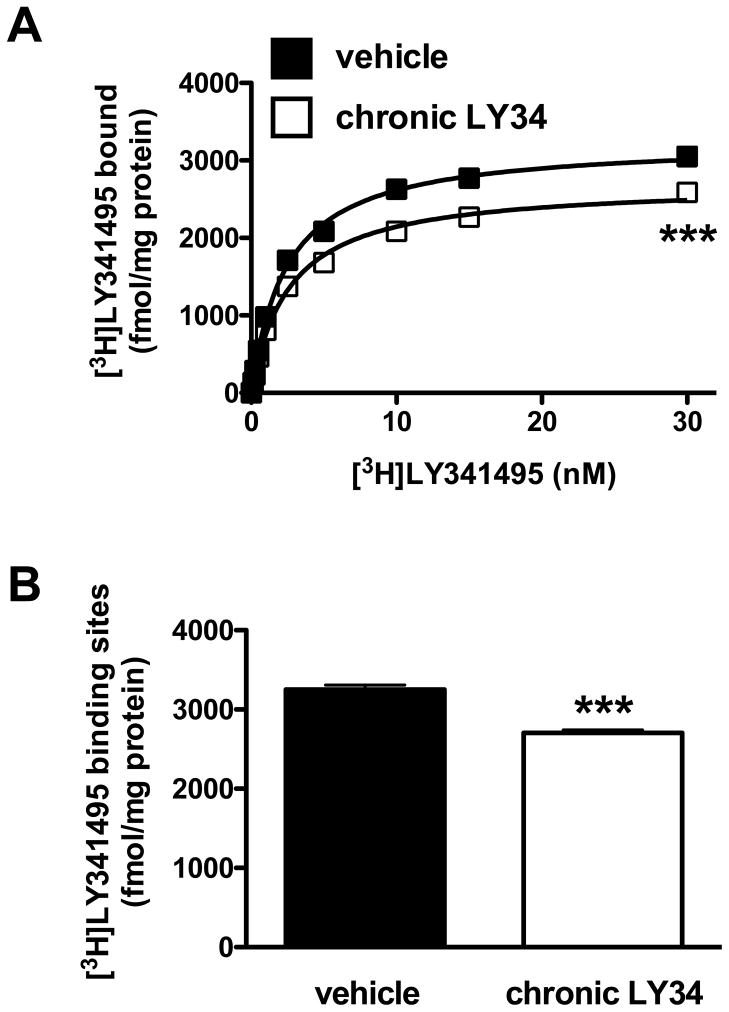
The simultaneous analysis of multiple saturation curves showed a significantly different [3H]LY341495 binding saturation curve in somatosensory cortex of mice chronically treated with LY341495 (F[2.116] = 99.75; p < 0.001), (Fig. 1A). Analysis of individual maximum number of binding sites (Bmax) demonstrated a lower density of mGlu2/3 receptors in mice chronically treated with LY341495 (t = 7.90, df = 10, p < 0.001; Student’s t-test), (Fig. 1B). The affinity (KD values) of [3H]LY341495 was not affected by chronic treatment by LY341495 (vehicle, 2.43 ± 0.15 nM; chronic LY341495, 2.61 ± 0.14 nM) (t = 0.87, df = 10, p > 0.05; Student’s t-test).
Fig. 1.
(A) [3H]LY341495 binding saturation curves in somatosensory cortex of wild-type mice one day after chronic treatment with LY341495 (LY34), or vehicle (n = 6). ***p < 0.001; F-test. (B) Maximum number of binding sites (Bmax) for [3H]LY341495 obtained from individual saturation curves. ***p < 0.001; Student’s t-test. Data are mean ± SEM.
3.2. Effect of chronic treatment with LY341495 on 5-HT2A receptor binding
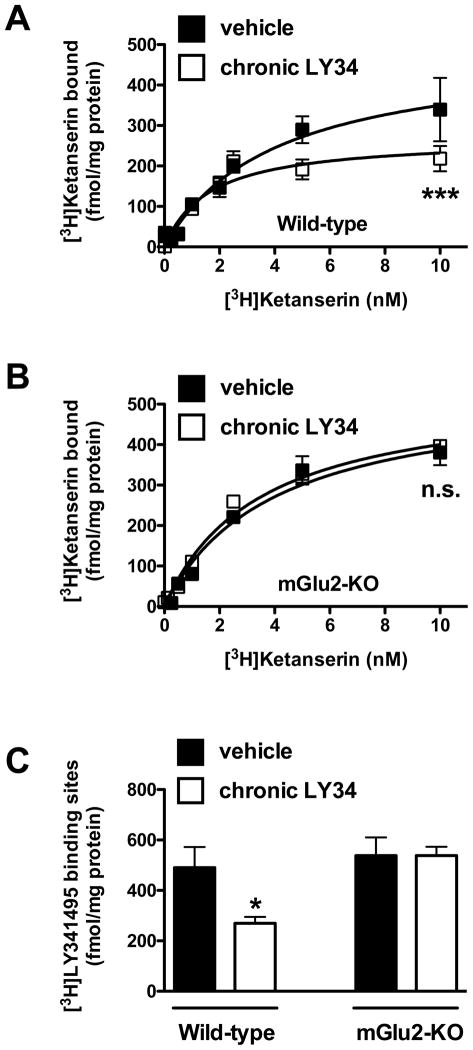
The simultaneous analysis of multiple saturation curves showed a significantly different [3H]ketanserin binding saturation curve in somatosensory cortex of wild-type mice (F[2,104] = 7.96; p < 0.001) (Fig. 2A), but not of mGlu2-KO mice (F[2.68] = 0.43; p > 0.05) (Fig. 2B), chronically treated with LY341495. Analysis of individual maximum number of binding sites (Bmax) indicated a significant effect of chronic treatment with LY341495 (F[1,14] = 5.41; p < 0.05) (Fig. 2C). Interestingly, post hoc analysis revealed that the maximum number of binding sites was decreased in wild type (p < 0.05), but not in mGlu2-KO (p > 0.05), mice (Fig. 2C). The affinity (KD values) for [3H]ketanserin was not affected by chronic treatment by LY341495 (vehicle—wild-type, 4.02 ± 1.43 nM; chronic LY341495—wild-type, 2.66 ± 0.44 nM; vehicle—mGlu2-KO, 3.94 ± 1.49 nM; chronic LY341495—mGlu2-KO, 3.45 ± 0.66) (F[1,14] = 0.10; p > 0.05).
Fig. 2.
(A) [3H]Ketanserin binding saturation curves in somatosensory cortex of wild-type mice one day after chronic treatment with LY341495 (LY34), or vehicle (n = 6). (B) [3H]Ketanserin binding saturation curves in somatosensory cortex of mGlu2-KO mice one day after chronic treatment with LY341495, or vehicle (n = 9). ***p < 0.001, n.s., not-significant; F-test. (C) Maximum number of binding sites (Bmax) for [3H]ketanserin obtained from individual saturation curves. ***p < 0.05; Bonferroni’s post hoc test of two-way ANOVA. Data are mean ± SEM.
3.3. Effect of chronic treatment with LY341495 on head-twitch behavior induced by LSD
Head-twitch behavior induced by LSD was decreased in mice chronically treated with LY341495 (t = 3.88, df = 8, Student’s t-test), (Fig. 3).
Fig. 3.
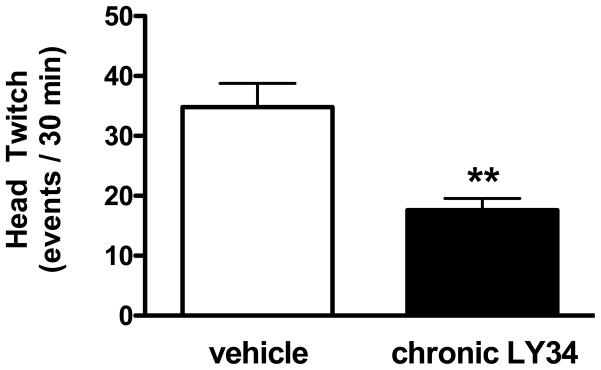
Head-twitch response induced by LSD one day after chronic LY341495 (LY34), or vehicle (n = 5). **p < 0.01; Student’s t-test.
3.3. Effect of chronic treatment with LY341495 on induction of expression of c-fos, egr-1 and egr-2 by LSD
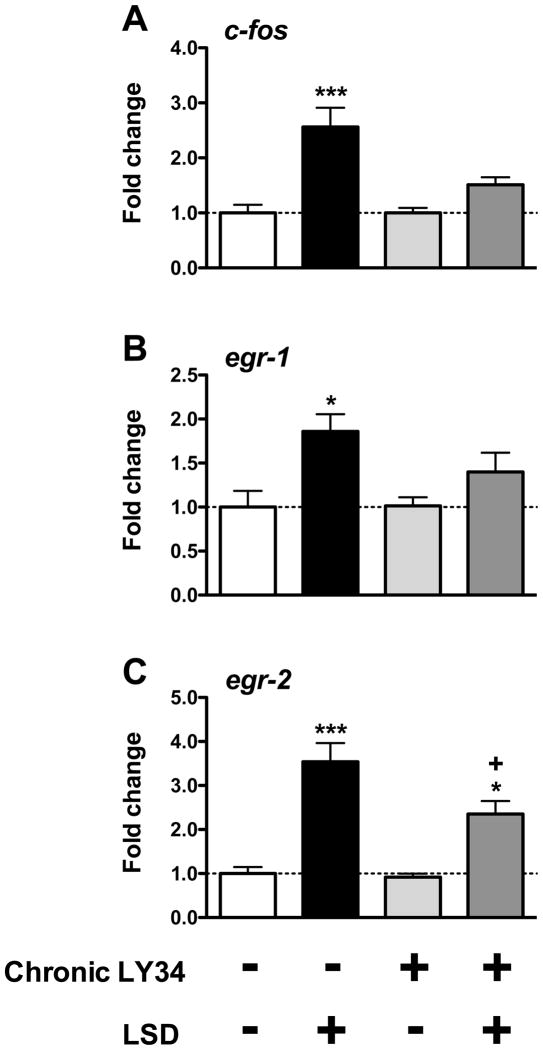
One-way ANOVA indicated that there is a significant effect of chronic treatment with LY341495 on induction of expression of c-fos by LSD mouse somatosensory cortex (F[3,20] = 12.65, p < 0.001), (Fig. 4A). Post hoc analysis revealed that a single dose of LSD induces expression of c-fos in mice chronically treated with saline (p < 0.001), but not in mice chronically treated with LY341495 (p > 0.05). Post hoc analysis also showed that expression of c-fos is not affected in mice chronically treated with LY341495 followed by a single dose of vehicle (p > 0.05).
Fig. 4.
Cellular response to LSD in mouse somatosensory cortex one day after chronic LY341495 (LY34) assayed by RT-qPCR (n = 6). Changes in expression levels of c-fos (A), egr-1 (B), and egr-2 (C) are reported as fold change over vehicle. *p < 0.05; **p < 0.001; Bonferroni’s post hoc test of one-way ANOVA as compared to vehicle. +p < 0.05; Bonferroni’s post hoc test of one-way ANOVA as compared to LSD
One-way ANOVA indicated that there is a significant effect of chronic treatment with LY341495 on induction of expression of egr-1 by LSD in mouse somatosensory cortex (F[3,20] = 5.09, p < 0.01), (Fig. 4B). Post hoc analysis revealed that a single dose of LSD induces expression of egr-1 in mice chronically treated with saline (p < 0.05), but not in mice chronically treated with LY341495 (p > 0.05). Post hoc analysis also showed that expression of egr-1 is not affected in mice chronically treated with LY341495 followed by a single dose of vehicle (p > 0.05).
One-way ANOVA indicated that there is a significant effect of chronic treatment with LY341495 on induction of expression of egr-2 by LSD mouse somatosensory cortex (F[3,20] = 20.87, p < 0.001), (Fig. 4C). Post hoc analysis revealed that a single dose of LSD induces expression of egr-2 in mice chronically treated with saline (p < 0.001), and in mice chronically treated with LY341495 (p < 0.05). Post hoc analysis also showed that induction of expression of egr-2 by LSD is lower in mice chronically treated with LY341495 as compared to that obtained in mice chronically treated with vehicle (p < 0.05). Expression of egr-2 is not affected in mice chronically treated with LY341495 followed by a single dose of vehicle (p > 0.05).
4. Discussion
This study demonstrates that chronic treatment with the mGlu2/3 receptor antagonist LY341495 down-regulates 5-HT2A receptor binding in mouse somatosensory cortex through a mechanism that requires expression of the mGlu2 receptor, as this effect is not observed in mGlu2-KO mice. The lower density of 5-HT2A receptor binding correlates with changes in hallucinogenic-like behavioral and cellular responses that require expression of 5-HT2A receptor in mouse brain cortex. Thus, chronic treatment with LY341495 decreases LSD-dependent head-twitch behavior, and LSD-dependent induction of expression of c-fos, egr-1 and egr-2 in mouse somatosensory cortex. Together, these findings suggest that persistent blockade of mGlu2 receptor-dependent signaling results in a lower density of 5-HT2A receptor, which, consequently, decreases the cellular signaling and behavioral responses induced by the hallucinogenic drug LSD.
Hallucinogenic drugs all bind with high affinity to and activate the 5-HT2A receptor [12]. However, closely related 5-HT2A receptor agonists, such a lisuride and ergotamine, do not induce hallucinogenic effects [12]. A potential explanation of why only certain 5-HT2A receptor agonists affect cognition, perception and mood is based on a pharmacological model termed functional selectivity (also known as agonist-trafficking and biased agonism) [11]. Based on this model, agonists stabilize a subset of receptor structural conformations that preferentially activate certain signaling pathways downstream. Previous findings suggest that each 5-HT2A receptor agonist elicits a distinct signal transduction response [13]. Further, it was reported that hallucinogenic drugs [such as DOI, DOB, 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM), mescaline, psilocin and LSD], and non-hallucinogenic drugs (such as R-lisuride, S-lisuride and ergotamine), induce a distinct transcriptome response that predicts their head-twitch behavioral effects in mice [13], and their hallucinogenic potential in human [8]. Among the transcriptome responses induced by hallucinogenic and non-hallucinogenic drugs, regulation of c-fos tracks with agonist activity at the 5-HT2A receptor, whereas induction of egr-1 and egr-2 predicts head-twitch behavior in mouse [13]. Our findings here suggest that chronic treatment with LY341495 reduces both the 5-HT2A receptor agonist-dependent expression of c-fos and the hallucinogenic-dependent expression of egr-1 and egr-2. This effect in mouse somatosensory cortex correlates with the profound decrease of 5-HT2A receptor binding induced by chronic treatment with LY341495.
A potential explanation for the mGlu2 receptor-dependent effect of chronic treatment with the mGlu2/3 receptor antagonist LY341495 on 5-HT2A receptor density in mouse somatosensory cortex is related to expression of 5-HT2A and mGlu2 as a G protein-coupled receptor (GPCR) heterocomplex. GPCRs have traditionally been considered to exist and function as monomeric structural units. However, during the last decade, increasing evidence suggest that GPCRs can form dimers or, potentially, higher-order oligomers [9]. There is also an extensive number of publications suggesting that GPCR heterocomplexes may exist. In this context, previous findings demonstrate that 5-HT2A and mGlu2 receptors interact in close molecular proximity as part of the same GPCR heteromeric complex [6, 10, 20]. One component of homeostasis in living cells is the functional desensitization with continuous GPCR stimulation. Mechanisms for receptor desensitization include uncoupling from G proteins, receptor endocytosis, and receptor degradation [25]. Agonist-dependent activation is generally required to initiate the molecular mechanisms responsible for GPCR desensitization and endocytosis. Interestingly, previous findings suggest that LY341495 behaves as a mGlu2 receptor inverse agonist, rather than as a neutral antagonist [6]. The heterotrimeric G protein subtypes activated by 5-HT2A and mGlu2 receptors are principally Gq/11 and Gi/o, respectively [12]. It has been shown that, in the presence of serotonin, the inverse agonist LY341495 not only decreases Gi/o-dependent signaling but also potentiates Gq/11-dependent signaling, and that this signaling crosstalk requires expression of 5-HT2A and mGlu2 as a GPCR heteromeric complex [6]. Together, these findings suggest that the molecular mechanism by which chronic treatment with LY341495 down-regulates 5-HT2A receptor density in mouse somatosensory cortex might be related to increases in serotonin-dependent 5-HT2A receptor-Gq/11 protein coupling through the 5-HT2A-mGlu2 receptor complex. However, further work is needed to validate this hypothesis in mice in which 5-HT2A and mGlu2 receptors are co-expressed but do not form a GPCR heteromer.
An interesting finding is the effect of chronic treatment with the mGlu2/3 antagonist LY341495 on [3H]LY341495 binding. A potential explanation would be the presence of LY341495 in the CNS one day after the chronic treatment. However, it is tempting to speculate that this is not the case since LY341495, which is water-soluble, will probably be washed out during the protocol of plasma membrane preparation. In the case of somatostatin and μ-opioid receptors [23], and β2-adrenergic and κ-opioid receptors [14], GPCR heteromerization regulates the agonist-induced endocytosis and redistribution of the receptors from the cell surface to intracellular compartments, suggesting that molecular proximity between GPCRs plays an important role in the localization and trafficking of these receptors. Based on these findings, the above mentioned crosstalk between 5-HT2A and mGlu2 as a GPCR heteromer with which LY341495 potentiates 5-HT2A receptor-dependent signaling (see [6]) may potentially be involved in the mechanisms responsible for down-regulation of mGlu2/3 receptors by chronic treatment with LY341495. Our findings in mGlu2-KO mice suggest that the mGlu2 receptor is necessary for the effects of chronic treatment with LY341495. However, since LY341495 binds with high affinity to both mGlu2 and mGlu3 receptors [10], further work is needed with mGlu3-KO mice to determine the potential role of the mGlu3 receptor in the effects of chronic treatment with LY341495 on 5-HT2A and mGlu2 receptor densities.
In conclusion, our data support the hypothesis that chronic blockade of mGlu2 receptor-dependent signaling down-regulates 5-HT2A receptor binding in mouse somatosensory cortex and its hallucinogenic-like cellular signaling and behavioral effects. Although hallucinogenic drugs as a model of schizophrenia have limitations [11, 12], our findings have potential relevance to develop new methods for modulating 5-HT2A receptor-dependent processes underlying non-drug-induced psychoses. Since the 5-HT2A receptor has been involved in depression and anxiety-related behaviors [26], these results may also have implications with regard to LY341495 use in rodent models of antidepressant responses [1, 4, 15].
Highlights.
Chronic LY341495 down-regulates 5-HT2A binding in mouse somatosensory cortex
This effect does not occur in mGlu2-KO mice
Chronic LY341495 decreases head-twitch behavior and cellular responses induced by LSD
These findings suggest that repeated blockade of mGlu2 reduces 5-HT2A-dependent effects of LSD
Acknowledgments
NIH R01 MH084894 (J.G.M.), NIDA P01 DA12923 (S.C.S.), Dainippon Sumitomo Pharma (J.G.M.), NARSAD (J.G.M.), the Maltz Family Foundation (J.G.M.) participated in the funding of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ago Y, Yano K, Araki R, Hiramatsu N, Kita Y, Kawasaki T, Onoe H, Chaki S, Nakazato A, Hashimoto H, Baba A, Takuma K, Matsuda T. Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology. 2012;65C:29. doi: 10.1016/j.neuropharm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Benneyworth MA, Smith RL, Sanders-Bush E. Chronic phenethylamine hallucinogen treatment alters behavioral sensitivity to a metabotropic glutamate 2/3 receptor agonist. Neuropsychopharmacology. 2008;33:2206. doi: 10.1038/sj.npp.1301600. [DOI] [PubMed] [Google Scholar]
- 3.Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72:477. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- 4.Bespalov AY, van Gaalen MM, Sukhotina IA, Wicke K, Mezler M, Schoemaker H, Gross G. Behavioral characterization of the mGlu group II/III receptor antagonist, LY-341495, in animal models of anxiety and depression. Eur J Pharmacol. 2008;592:96. doi: 10.1016/j.ejphar.2008.06.089. [DOI] [PubMed] [Google Scholar]
- 5.Field JR, Walker AG, Conn PJ. Targeting glutamate synapses in schizophrenia. Trends Mol Med. 2011;12:689. doi: 10.1016/j.molmed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, Mackerell AD, Jr, Brezina V, Sealfon SC, Filizola M, Gonzalez-Maeso J, Logothetis DE. Decoding the Signaling of a GPCR Heteromeric Complex Reveals a Unifying Mechanism of Action of Antipsychotic Drugs. Cell. 2011;147:1011. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 8.Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Maeso J. GPCR oligomers in pharmacology and signaling. Mol Brain. 2011;4:20. doi: 10.1186/1756-6606-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Maeso J, Sealfon SC. Agonist-trafficking and hallucinogens. Curr Med Chem. 2009;16:1017. doi: 10.2174/092986709787581851. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens Recruit Specific Cortical 5-HT(2A) Receptor-Mediated Signaling Pathways to Affect Behavior. Neuron. 2007;53:439. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci U S A. 2001;98:343. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koike H, Fukumoto K, Iijima M, Chaki S. Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res. 2012;238C:48. doi: 10.1016/j.bbr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 16.Kurita M, Holloway T, Garcia-Bea A, Kozlenkov A, Friedman AK, Moreno JL, Heshmati M, Golden SA, Kennedy PJ, Takahashi N, Dietz DM, Mocci G, Gabilondo AM, Hanks J, Umali A, Callado LF, Gallitano AL, Neve RL, Shen L, Buxbaum JD, Han MH, Nestler EJ, Meana JJ, Russo SJ, Gonzalez-Maeso J. HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci. 2012;15:1245. doi: 10.1038/nn.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marek GJ, Wright RA, Schoepp DD, Monn JA, Aghajanian GK. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J Pharmacol Exp Ther. 2000;292:76. [PubMed] [Google Scholar]
- 18.Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011;493:76. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neuroscience letters. 2011;493:76. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno JL, Muguruza C, Umali A, Mortillo S, Holloway T, Pilar-Cuellar F, Mocci G, Seto J, Callado LF, Neve RL, Milligan G, Sealfon SC, Lopez-Gimenez JF, Meana JJ, Benson DL, Gonzalez-Maeso J. Identification of Three Residues Essential for 5-HT2A-mGlu2 Receptor Heteromerization and its Psychoactive Behavioral Function. J Biol Chem. doi: 10.1074/jbc.M112.413161. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muguruza C, Moreno JL, Umali A, Callado LF, Meana JJ, Gonzalez-Maeso J. Dysregulated 5-HT2A receptor binding in postmortem frontal cortex of schizophrenic subjects. Eur Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2012.10.006. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer M, Koch T, Schroder H, Laugsch M, Hollt V, Schulz S. Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem. 2002;277:19762. doi: 10.1074/jbc.M110373200. [DOI] [PubMed] [Google Scholar]
- 24.Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology. 2012;37:630. doi: 10.1038/npp.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 27.Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 28.Zhai Y, George CA, Zhai J, Nisenbaum ES, Johnson MP, Nisenbaum LK. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology. 2003;28:45. doi: 10.1038/sj.npp.1300013. [DOI] [PubMed] [Google Scholar]