Abstract
Objective
Cell surface localization and intracellular trafficking of ATP-binding cassette transporter A-1 (ABCA1) are essential for its function. However, regulation of these activities is still largely unknown. Brefeldin A (BFA), a uncompetitive inhibitor of brefeldin A-inhibited guanine nucleotide-exchange proteins (BIGs), disturbs the intracellular distribution of ABCA1, and thus inhibits cholesterol efflux. This study aimed to define the possible roles of BIGs in regulating ABCA1 trafficking and cholesterol efflux, and further to explore the potential mechanism.
Methods and Results
By vesicle immunoprecipitation, we found that BIG1 was associated with ABCA1 in vesicles preparation from rat liver. BIG1 depletion reduced surface ABCA1 on HepG2 cells and inhibited by 60% cholesterol release. In contrast, BIG1 over-expression increased surface ABCA1 and cholesterol secretion. With partial restoration of BIG1 through over-expression in BIG1-depleted cells, surface ABCA1 was also restored. Biotinylation and glutathione cleavage revealed that BIG1 siRNA dramatically decreased the internalization and recycling of ABCA1. This novel function of BIG1 was dependent on the guanine nucleotide-exchange activity and achieved through activation of ADP-ribosylation factor 1 (ARF1).
Conclusions
BIG1, through its ability to activate ARF1, regulates cell surface levels and function of ABCA1, indicating a transcription-independent mechanism for controlling ABCA1 action.
Keywords: BIG1, ABCA1, trafficking, cholesterol efflux
The ATP-binding cassette transporter (ABC) A1 belongs to the ABC superfamily of integral membrane proteins that are responsible for the ATP-powered translocation of cholesterol, phospholipids, and other substrates across membranes.1 The discovery that mutations in the ABCA1 gene cause Tangier disease and familial hypoalphalipoproteinemia clearly demonstrated ABCA1 as the key protein responsible for nascent high-density lipoprotein (HDL) particle and a critical molecule regulating an initial step of reverse cholesterol transport.2,3 Thus, ABCA1 has been considered as a potential target in the treatment of atherosclerotic vascular disease.4
ABCA1 mostly localizes to the plasma membrane (PM) and endocytic vesicles, and rapidly shuttles between the cell surface and intracellular compartments.5,6 The importance of its PM localization for proper ABCA1 function has been confirmed by a number of independent studies.7,8,9 Trafficking of ABCA1 between specific intracellular and PM sites is exquisitely controlled, and is essential for regulation of intracellular cholesterol. Decreasing ABCA1 internalization by deletion of its PEST sequence leads to decreased cholesterol efflux from late endosomal cholesterol pools.10 Over-expression of the small GTPase Rab8, which is involved in trafficking from the trans-Golgi network (TGN) to the PM and from the endosomes to the PM, facilitates ABCA1 surface expression and stimulates the delivery of cholesterol from late endosomal compartments to apoA-I.11,12 However, the processes regulating specific subcellular localization of ABCA1 are still largely unknown.
Brefeldin A (BFA), an uncompetitive inhibitor of ADP-ribosylation factor (ARF)-guanine nucleotide-exchange factor (GEF) activity, has been reported to disturb the dynamics and intracellular distribution of ABCA1, and thus inhibit cholesterol efflux.6,13,14 Through binding the Arf-GDP-ARF GEF complex, BFA locks it in a conformation that prevents nucleotide dissociation, resulting in specific blockade of ARF activation by a subset of its GEFs, and thus interferes reversibly with vesicular transport between the cell surface and intracellular compartments.15,16 There are three BFA-sensitive GEFs: BIG1, BIG2, and GBF1. BIG1 and BIG2 are 74% identical in amino acid sequence and 90% identical in Sec7 domains that are responsible for ARF activation. Both BIG1 and BIG2 are associated mainly with the regulation of trafficking through TGN and endosomes,17,18 whereas GBF1 functions primarily in transport between ER-Golgi intermediate and cis-Golgi compartments.19 Therefore, we hypothesized that BIGs might play a role in regulating ABCA1 transport and function.
Here, we present evidence that BIG1 is associated with ABCA1 in vesicles from rat liver. BIG1, but not BIG2, was required for trafficking and surface localization of ABCA1. Moreover, we demonstrated that BIG1 activity was crucial for ABCA1 function in cholesterol efflux. This study evaluated whether BIG1-mediated vesicle trafficking represents a transcription-independent mechanism for regulation of ABCA1 function.
Materials and Methods
A detailed Materials and Methods section is presented as an online supplement (available at http://atvb.ahajournals.org).
Vesicle immunoprecipitation
Membranes from rat liver were subjected to differential centrifugation. Fractions containing vesicles enriched in clathrin and AP-1 for vesicle IP by magnetic Dynabeads (Dynal).
Labeling and tracing of ABCA1
Cell surface proteins were biotinylated with EZ-Link Sulfo-NHS-SS-Biotin (Pierce) and isolated by incubating with streptavidin-agarose beads (Pierce). Biotinylated proteins remaining on the cell surface after internalization or recycling were cleaved by reduced glutathione (pH 8.0).
Results
Association of ABCA1 with BIG1 in vivo and in vitro
Because BFA, an inhibitor of BIG1 and BIG2 GEF activity, was reported to inhibit cholesterol efflux by disturbing intracellular trafficking of ABCA1,6,13,14,20 we hypothesized that BIGs might play a role in regulating ABCA1 transport and function. BIGs have been found in trans-Golgi compartments and colocalized with clathrin and AP-1.21,22 To test the hypothesis, membrane fractions containing vesicles enriched in clathrin and AP-1 from rat liver were immunoprecipitated with anti-BIG1 or BIG2 antibodies. ABCA1 was detected in the vesicle preparation by anti-BIG1 pull-down (Figure 1A), indicating colocalization of the two proteins in the same vesicle complex.
Figure 1. Association of ABCA1 with BIG1 in vivo and in vitro.
A, Membranes from rat liver were subjected to differential centrifugation. Markers of vesicles clathrin and AP-1 (adaptin γ) in the precipitates recovered by centrifugation at 19000g (F1) or 43000g (F2) were detected by Western blotting (A1). Samples from F2 were used for immunoprecipitation by anti-BIG1 antibody followed by Western blotting with antibodies against BIG1 or ABCA1 (A2), and electron microscopic image (A3, scale bar: 100 nm). B and C, HepG2 cells stimulated with (B) or without oxidized LDL (C, 50 µg/ml) were treated with atorvastatin (Ato, L: 2 µM, H: 10 µM) for 24 h. The expression of ABCA1 and BIG1 were detected. D, After BIG1 depletion by siRNA, HepG2 cells were incubated with oxidized LDL (50 µg/ml) and the expression of ABCA1 and BIG1 were detected. E, The expression of ABCA1 and BIG1 in HepG2 cells stimulated by apoA1 (10 µg/ml) for 8 h were detected. Results are representative of three independent experiments. Densitometric quantification was analyzed by ImageQuantTL software. α-tubulin was used as the loading control. Data are means ±S.E.M. #, $ p <0.05, **, ## p<0.01 and ***, ### p<0.001.
Sustained uptake of cholesterol by human macrophages upregulates ABCA1 expression.23, 24 ABCA1 levels in liver cells, but not macrophages, modulate susceptibility to atherosclerosis.25 To explore the possible mutual regulation of ABCA1 and BIG1 in hyperlipidemia, apoE−/− mice with their genetic abnormalities in lipid metabolism were used. As reported,26 atorvastatin dose-dependently reduced serum lipid content (TC, TG, and LDL-C) in apoE−/− mice fed a high fat diet (Figure S1A). Hepatic levels of ABCA1 and BIG1 were significantly higher in apoE−/− mice than WT mice, and were decreased by atorvastatin in a dose-dependent fashion (Figure S1B). The concurrent changes in amounts of BIG1 and ABCA1 in liver together with the co-immunoprecipitation (IP) suggests a close relationship between the two proteins in vivo.
Oxidized low-density lipoprotein (oxLDL) has been implicated in the pathogenesis of atherosclerosis, and reported to upregulate ABCA1 gene expression through activation of the nuclear liver X receptors.27 In HepG2 cells, ABCA1 and BIG1 protein expression increased significantly after addition of oxLDL. Upregulation as a result of oxLDL exposure was inhibited in a dose-dependent manner by atorvastatin (Figure 1B), but atorvastatin had no effect on the expression of BIG1 or ABCA1without oxLDL treatment (Figure 1C). In BIG1-depleted cells, oxLDL did not alter BIG1 expression, and the increase in ABCA1 by oxLDL stimulation was partly inhibited (Figure 1D). ApoA-I has been demonstrated also to increase ABCA1 levels by binding to and preventing degradation of the ABCA1 transporter.28,29 Treatment of cells with apoA-I increased ABCA1 without affecting BIG1 level (Figure 1E). These observations suggest that the expression of ABCA1 and BIG1 were modulated by cell lipid content, which differs from the apoA1-ABCA1 interaction.
Function of ABCA1 in HepG2 cells requires BIG1, but not BIG2
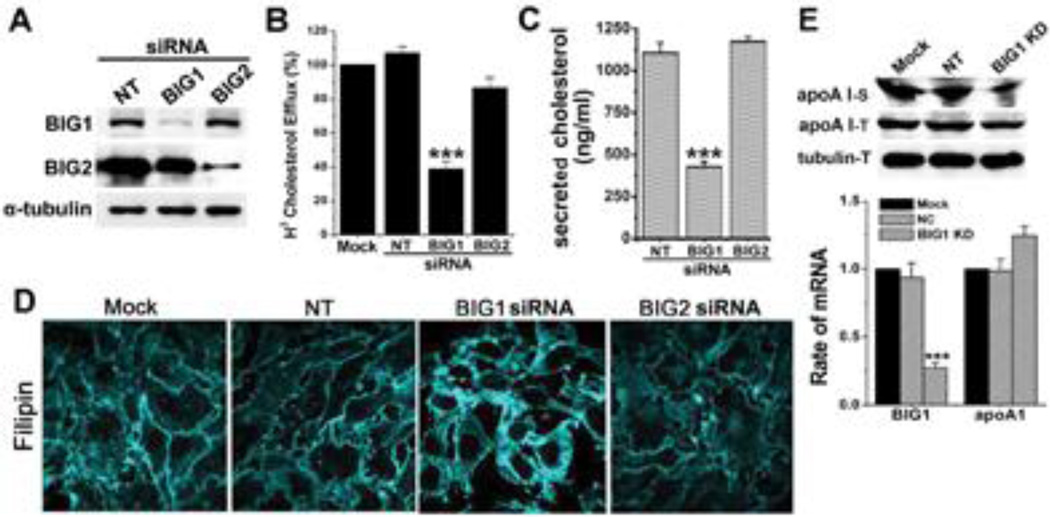
To explore roles of BIG1 and BIG2 in apoAI-ABCA1-dependent cholesterol efflux, we used siRNA to deplete BIG1 or BIG2 in HepG2 cells (Figure 2A). As shown in Figure 2B, only BIG1 siRNA reduced cholesterol efflux to apoA-I (about 60% decrease) by HepG2 cells. Similarly, decreased release of cellular cholesterol into HepG2 cell culture medium was observed only in BIG1-depleted cells by Amplex Red cholesterol Assay (Figure 2C). Cholesterol accumulation was evaluated by staining fixed cells with filipin, a specific marker for unesterified cholesterol. We observed a significant accumulation of filipin staining in BIG1-depleted cells compared to Mock, NT, or BIG2-depleted cells (Figure 2D), corresponding to the decreased efflux (Figure 2B). The amount of apoA I present in cells and in medium was also detected by Western blot. In BIG1-depleted cells, both cell associated and medium apoA I was decreased (Figure 2E). The mRNA level of apoA I was also measured by real-time RT-PCR. Our result revealed that apoA I mRNA slightly increased in BIG1-depleted cells but failed to reach statistic significance (Figure 2E, p> 0.05). Together, these findings are consistent with a role for BIG1 in ABCA1-mediated lipidation of apoA I.
Figure 2. BIG1, but not BIG2 depletion impaired the function of ABCA1.
A, HepG2 cells were incubated with vehicle (Mock), or with non-targeting control (NT), BIG1 or BIG2 siRNA for 72 h, followed by immuno-blotting to detect RNAi efficiency. B, HepG2 cells treated with vehicle, or NT, BIG1, or BIG2 siRNA were incubated with [H3]-labeled cholesterol followed by efflux to apoA-I (10 µg/ml). Mock was used as a control (set at 100%). n=3, ***P<0.001. C, After NT, BIG1, or BIG2 siRNA treatment, cholesterol content in condition medium was measured by the Amplex Red Cholesterol Assay Kit ( n=6, ***P<0.001). D, Cholesterol accumulation in HepG2 cells after siRNA was displayed by filipin labeling. The images were taken using a confocal microscope (Zeiss 710). E, HepG2 cells were incubated with vehicle (Mock), or with NT or BIG1 siRNA for 72 h. The levels of apoA I in total cell lysates (T) or culture medium (S) after TCA precipitation were detected by Western blotting. The levels of BIG1 and apoA1 mRNA were detected by real-time PCR. ***P<0.001 vs NT.
BIG1 is required for targeting of ABCA1 to the PM
Because localization of ABCA1 at the PM is vital for its lipid transport function, we hypothesized that BIG1 may play a role in targeting of ABCA1 to the PM. As shown in Figure 3A, surface ABCA1 of HepG2 or differentiated THP1 cells decreased significantly after BIG1-depletion, but total ABCA1 content was unchanged. To further verify our hypothesis, we over-expressed BIG1 in non-treated or BIG1-depleted cells. Expression of GFP-BIG1 (WT) increased cell surface ABCA1 without affecting the total content of ABCA1. In contrast, over-expression of HA-BIG1 (E793K), a dominant-negative mutant, which does not activate ARFs, had no effect on total amount or the surface level of ABCA1 (Figure 3B). In addition, over-expression of BIG1-WT partially reversed the reduction in surface ABCA1 in cells treated by BIG1 siRNA (Figure 3C). Consistent with increased content of cell surface ABCA1 following BIG1-WT expression, an increase in the amount of cholesterol in the culture medium was observed following BIG1-WT, but not HA-BIG1 (E793K) over-expression (Figure 3D).A reverse situation was seen in BFA-treated cells (Figure 3E). The medium cholesterol content correlated with the level of cell surface ABCA1. Differences between effects of BIG1-E793K over-expression and BIG1 depletion on surface ABCA1 expression and secreted cholesterol could be caused by low efficiency of plasmid transfection.
Figure 3. BIG1 regulated level of cell surface ABCA1.
A, After BIG1 or BIG2 depletion, surface proteins of HepG2 cells (left) or differentiated THP-1 cells (right) were biotinylated and separated by streptavidin beads. The levels of ABCA1 and BIG1 were detected by Western blot (S: cell surface proteins; T: total cell proteins). Densitometric quantification was analyzed by ImageQuantTL software. Data are means ± SEM (n=3). ***P<0.001 vs NT. B, HepG2 cells were over-expressed with empty vector of EGFP-C2 (EV), or BIG1 plasmids (WT: EGFP-wild type BIG1; E793K: HA-dominant negative BIG1). The surface proteins were isolated and analyzed as in Figure 3A. Data are means ± SEM (n=3). **P<0.01 vs EV. C, HepG2 cells treated with BIG1 siRNA were transiently over-expressed with HA-BIG1-WT followed by ABCA1 and BIG1 detection by Western blotting and densitometric quantification. Data are means ± SEM (n=3). ***P<0.001, #P<0.05. D and E, HepG2 cells over-expressing WT-BIG1 or BIG1-E793K (D), or treated with BFA (5 µg/ml, 8 h). The secreted cholesterol in condition medium were detected by Amplex Red analysis; n=3, *P<0.05.
BIG1 depletion inhibited ABCA1 internalization and recycling
ABCA1/apoA-I retroendocytosis was reported to contribute significantly to HDL formation when cells had accumulated excess cholesterol.30 To further elucidate the intracellular pathways of ABCA1 trafficking after BIG1-depletion, the internalization and recycling of surface ABCA1 were traced. Cell surface proteins were biotinylated and allowed to internalize for indicated time. Proteins remaining on the cell surface were cleaved by reduced glutathione. Internalized biotinylated proteins were isolated by streptavidin-agarose beads. As shown in Figure 4A, the internalized ABCA1 in BIG1-depleted cells was much less than in non-targeting control cells, indicating a dramatically decreased capability of internalization in BIG1-depleted cells. Furthermore, the amount of ABCA1 recycling back to the cell surface was also reduced by BIG1-depletion. Although both internalization and recycling were inhibited by BIG1 depletion, some ABCA1 was still transported between the PM and intracellular compartments (Figure 4B). Since that ABCA1 was reported to cycle intracellularly via a Rab4- or Rab8-mediated pathway,12,31 it is conceivable that BIG1 participates in only one part of the whole recycling process.
Figure 4. Effect of BIG1 depletion on internalization and recycling of surface ABCA1.
A, Internalized biotinylated ABCA1 (ABCA1-In) in the control of BIG1-depleted cells at indicated time points was separated, followed by Western blot detection. B, After internalization at 37°C for 1 hour, biotinylated proteins in the control or BIG1-depleted cells were recycled at 37°C for the indicated time. The intracellular remaining biotinylated ABCA1 (IC) or the total biotinylated ABCA1 (IC+S, including that on the surface) after recycling was detected. Transferrin receptor (TFR) was used as a control. Densitometric quantification normalized to TFR was analyzed by ImageQuantTL software. Data are means ± SEM (n=3). C, HeLa cells stably expressing WT-ABCA1-GFP were transfected with BIG1 siRNA, followed by filipin staining.
In HeLa cells without endogenous ABCA1, stably expressed ABCA1-GFP resides on the surface and also in intracellular endosomes (Figure S3). Depletion of endogenous BIG1 resulted in a loss of filipin labeling in endosome-like clusters and exhibited much greater accumulation of intracellular cholesterol (Figure 4C), suggesting that mobilization of cellular ABCA1-GFP through BIG1 contributed significantly to cholesterol release. Sucrose gradient fractionation also revealed that BIG1 depletion significantly affected the distribution of ABCA1 and late endosome marker Rab7, but had no effect on early endosome marker EEA1, recycling endosome marker transferrin receptor (TFR), or lysosome marker lamp2. ABCA1 in fractions 2, 3, and 6, in which enriched EEA1 and Rab7 were absent in BIG1-depleted cells indicating that the recycling of ABCA1 was partly eliminated (Figure S4).
Regulation of BIG1 in surface localization and function of ABCA1 was achieved through activation of ARF1
BIG1 functions as an activator of ARFs, which are major regulators of membrane remodeling and protein trafficking in eukaryotic cells. Our results from BIG1-E793K overexpression and BFA inhibition indicated that effects of BIG1 on the surface localization and function of ABCA1 were dependent on its GEF activity. To determine whether the activation of ARFs is truly indispensable for ABCA1 function, we compared the distribution of ARF (1, 3, 4, 5, and 6) between cytosol and membrane of HepG2 cells treated with non-targeting (NT) or specific BIG1 or BIG2 siRNA or vehicle alone (Mock). Only Arf1, which localizes primarily to the Golgi apparatus known to regulate both anterograde and retrograde vesicular traffic,32 was found to be decreased in membrane fractions after BIG1-depletion (Figure 5A). To assess the potential involvement of ARF1 in the BIG1-ABCA1 interaction, constructs of WT and two mutants of ARF1 with myc tags were prepared for transient over-expression. Cells over-expressing ARF1-Q71L, which is assumed to be constitutively active with GTP-bound, or WT-ARF1, showed increased surface ABCA1. In addition, ARF-Q71L stimulated a much greater surface distribution of ABCA1 than ARF1-WT. However, in cells expressing inactive ARF1-T31N, surface ABCA1 was not affected. No change was seen in total ABCA1 after plasmid transfection. Cholesterol secreted in the culture medium was also measured using the Amplex Red Cholesterol Assay Kit. An increased cholesterol content was found in cells over-expressing Arf1-WT and Arf1-Q71L (Figure 5C). There were no significant differences in the levels of total cell cholesterol.
Figure 5. The activation of ARF1 by BIG1 is required for the surface expression and function of ABCA1.
A, Proteins in cytosol and membrane fractions were isolated from HepG2 cells incubated with non-targeting control (NT), BIG1 or BIG2 siRNA, followed by immunoblotting with indicated antibodies. B, Surface ABCA1 from HepG2 cells transfected with plasmids of empty vector (EV), myc-ARF1-WT, or with Q71L or T31N ARF1 mutants were detected. C, Cellular cholesterol content and secreted cholesterol in medium were detected respectively after ARF1 WT or mutant over-expression. Data are means ± SEM, n=3, *P<0.05, **P<0.01.
Discussion
ABCA1 is widely expressed in nearly all tissues of the body, and is particularly abundant in the liver. Findings from studies of hepatic over-expression of ABCA1 suggested that the liver itself is a major source of cholesterol for both plasma HDL acceptors and nascent HDL particles, which mediate cholesterol efflux from peripheral cells.33,34 More important, results from liver-specific ABCA1-knockout mice demonstrated that hepatic ABCA1 is the single most important source of nascent apoA-I and maintains the majority of the plasma HDL pool.35 Studies using ABCA1-knockout bone marrow transplanted into wild-type mice demonstrated that ABCA1-mediated cholesterol efflux from macrophages did not significantly contribute to plasma HDL-C level.36 These combined results indicate that appropriate regulation of hepatic ABCA1 is critical for a selective increase in HDL cholesterol levels. It is thus important to understand how the expression and function of ABCA1 is regulated in hepatocytes. In our study, we used the human hepatoma cell line HepG2 as a model system, combined with rat liver and apoE−/− mice, to identify BIG1 as the novel regulator of ABCA1 and to demonstrate the underlying mechanism.
BFA, often used as an inhibitor of vesicle trafficking between the TGN and PM, inhibits the activity of ARF-GEFs and arrests ARFs in the GDP-bound form.37 It was reported that BFA disturbed the dynamics and intracellular distribution of ABCA1,6 and inhibited apoA1-mediated lipid efflux from macrophages and fibroblasts.20 Therefore, we hypothesized that BIGs, BFA-sensitive ARF-GEFs, might play a role in regulating ABCA1 transport and function. To identify BIGs involvement in the regulation of hepatic ABCA1 function, vesicles isolated from rat liver were immunoprecipitated with anti-BIG1 or BIG2 antibodies. Interestingly, ABCA1 was present in the anti-BIG1, but not anti-BIG2 antibodies, precipitated vesicles, indicating a potential interaction between the two proteins. It has been clearly established that regulation of ABCA1 expression is primarily via cellular cholesterol level through the nuclear receptor liver X receptor.38 To explore the possible regulation of BIG1 in hepatic ABCA1 function, the relationship between varying BIG1 and ABCA1 expression in liver was observed. In apoE−/− mice fed a high fat diet, increased hepatic ABCA1 and BIG1 were found along with high serum lipid content (TC, TG, and LDL-C). When serum lipids were decreased by atorvastatin treatment, both ABCA1 and BIG1 were down-regulated. Consistent with in vivo findings, high concentrations of oxLDL increased both ABCA1 and BIG1 proteins in HepG2 cells. Increases in ABCA1 and BIG1 were counteracted by atorvastatin treatment in a dose-dependent manner. BIG1 depletion, on the other hand, had no effect on total amount of ABCA1 but reduced the elevation of ABCA1 induced by oxLDL. Concurrently increased/decreased levels of BIG1 and ABCA1, together with the co-IP of ABCA1-BIG1 in hepatic vesicles, identified a novel interaction between the two proteins. Regulation of ABCA1 at the PM and endocytic compartments is important for lipid processing because the functionality of ABCA1 at these sites is considered essential for the initiation of cholesterol removal from cells.5,6 We showed that BIG1 over-expression increased levels of surface ABCA1 and facilitated HepG2 cells to discard excess cholesterol when exposed to ox-LDL. In BIG1-depleted cells with partial restoration of BIG1 through over-expression, ABCA1 localization at the PM was also restored, consistent with a role for BIG1 in the process. Recycling of ABCA1 from endocytic compartments to the PM was markedly reduced in BIG1-depleted cells. Because of these findings and the discovery of the interaction between BIG1 and ABCA1, it seems likely that BIG1 plays a significant role in the pathophysiology of atherosclerosis.
BIG1 is often found in trans-Golgi compartments and colocalizes with clathrin and AP-1.21,22 Silencing of BIG1 expression can halt protein trafficking at sites where it is required to activate ARFs and thereby initiate vesicle generation for the next stage of transport to the cell surface. Our previous studies revealed that BIG1 is required for integrity of the Golgi structure.39 Kinesin family member 21A, a plus-end-directed motor protein that moves cargo on microtubules away from TGN, was identified as a novel binding partner of BIG1. The newly recognized interaction integrates functions of BIG1 in local vesicle formation with longer-range transport processes toward the cell surface.40 Thus, it appears that BIG1 regulates ABCA1 cell surface expression via governing its internalization and trafficking. It has been clearly established that the PM is an important site at which ABCA1 plays an antiatherogenic role.7–9 Internalization and trafficking of ABCA1 is functionally important in mediating ABCA1 cell surface expression, as well as cholesterol efflux from intracellular cholesterol pools.41 Like the other cell surface proteins, once ABCA1 arrives at the TGN, it is sorted for delivery over long distances to the PM by large membrane carriers, in which BIG1 has been found to be involved.40 Consistent with the role of BIG1 in that process, our results from BIG1 depletion or over-expression revealed that BIG1 is required to maintain surface ABCA1 levels and cholesterol efflux to apoA-I. Further results from tracing the internalization and recycling of surface ABCA1 after BIG1-depletion revealed a dramatic inhibition of the capability of internalization and recycling. In HeLa cells stably expressing ABCA1-GFP, depletion of endogenous BIG1 resulted in loss of endosome-like filipin labeling clusters and exhibited much more intracellular cholesterol accumulation, consistent with the finding from internalization and recycling. However, some ABCA1 was still transported between the PM and intracellular compartments after BIG1 depletion, indicating that other pathways also participate in the recycling of ABCA1, such as the Rab4- or Rab8-mediated pathway.12,31
In the present study, the effects of BIG1 on cell-surface ABCA1 expression and cholesterol secretion is dependent on its GEF activity, since dominant-negative BIG1 over-expression did not increase surface ABCA1 levels and promote cholesterol secretion, as did WT BIG1. Previous studies reported that BIG1 preferentially functions as a GEF of class I ARFs (ARF1 and ARF3) by catalyzing replacement of ARF-bound GDP with GTP.42 Consistently, we found that BIG1 depletion decreased membrane binding by ARF1 in HepG2 cells, consistent with decreased ARF1 activity. Overexpression WT ARF1 resulted in increased cell-surface ABCA1, which was even more enhanced by the constitutively active ARF1(Q71L) mutant, suggesting that ARF1 activation is important for ABCA1 surface localization regulated by BIG1. Actually, ARF-like 7 has been found to be involved in transport between the perinuclear compartment and the PM, apparently linked to the ABCA1-mediated cholesterol secretion pathway.43 Nevertheless, the dominant negative ARF1 (T31N) exhibited no obvious suppression of surface ABCA1 or cholesterol secretion, perhaps due to low levels of the mutant proteins. Both BIG1 and ARF1 have been reported to function in the Golgi complex and clathrin-coated vesicles.21,22,44 The ABCA1/apoA-I complex is also endocytosed via the clathrin pathway.30 It appears that the newly synthesized ABCA1 is transported to the surface in the same manner. Clearly, further studies are required in order to evaluate these possibilities.
In summary, the present work identifies BIG1 as an important intracellular regulator that mediates cell-surface ABCA1 expression and facilitates cholesterol efflux to apoA-I by governing its intracellular trafficking. This novel function of BIG1 requires GEF activity to accelerate activation of ARF1. Interestingly, a xLxxKN motif serves as a Golgi exit signal and directs ABCA transporters to a post-Golgi vesicular sorting station where additional signals may be required for selective delivery of individual transporters to final subcellular destinations.45 Thus, BIG1 regulation may be related to xLxxKN motif, which requires further research. Our study represents a transcription-independent mechanism for regulating ABCA1 function.
Supplementary Material
Acknowledgements
Sources of Funding
This study was supported by National Natural Science Foundation of China (No. 31070924 and 81173056), Projects of International Cooperation and Exchanges, Science and Technology Planning Project of Guangdong Province, China (No. 1011420600004), and Research Fund for the Doctoral Program of Higher Education of China (No. 20100171110052). Edward Neufeld, Joel Moss and Martha Vaughan were supported by the Intramural Research Program, National Institutes of Health, NHLBI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other persons besides the authors have made substantial contributions to this manuscript.
Disclosures
None
References
- 1.Oram JF, Vaughan AM. ATP-binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–1043. doi: 10.1161/01.RES.0000250171.54048.5c. [DOI] [PubMed] [Google Scholar]
- 2.Bodzioch M, Orso E, Klucken J, et al. The gene encoding ATP-binding cassette transporter 1 is mutated in tangier disease. Nat Genet. 1999;22:347–351. doi: 10.1038/11914. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Chang E, Cherry AM, Bangs CD, Oei Y, Bodnar A, Bronstein A, Chiu CP, Herron GS. Human endothelial cell life extension by telomerase expression. J Biol Chem. 1999;274:26141–26148. doi: 10.1074/jbc.274.37.26141. [DOI] [PubMed] [Google Scholar]
- 4.Attie AD. ABCA1: At the nexus of cholesterol, hdl and atherosclerosis. Trends Biochem Sci. 2007;32:172–179. doi: 10.1016/j.tibs.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Neufeld EB, Stonik JA, Demosky SJ, Jr, Knapper CL, Combs CA, Cooney A, Comly M, Dwyer N, Blanchette-Mackie J, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr The ABCA1 transporter modulates late endocytic trafficking: Insights from the correction of the genetic defect in tangier disease. J Biol Chem. 2004;279:15571–15578. doi: 10.1074/jbc.M314160200. [DOI] [PubMed] [Google Scholar]
- 6.Neufeld EB, Remaley AT, Demosky SJ, Stonik JA, Cooney AM, Comly M, Dwyer NK, Zhang M, Blanchette-Mackie J, Santamarina-Fojo S, Brewer HB., Jr Cellular localization and trafficking of the human ABCA1 transporter. J Biol Chem. 2001;276:27584–27590. doi: 10.1074/jbc.M103264200. [DOI] [PubMed] [Google Scholar]
- 7.Singaraja RR, Visscher H, James ER, Chroni A, Coutinho JM, Brunham LR, Kang MH, Zannis VI, Chimini G, Hayden MR. Specific mutations in ABCA1 have discrete effects on ABCA1 function and lipid phenotypes both in vivo and in vitro. Circ Res. 2006;99:389–397. doi: 10.1161/01.RES.0000237920.70451.ad. [DOI] [PubMed] [Google Scholar]
- 8.Witting SR, Maiorano JN, Davidson WS. Ceramide enhances cholesterol efflux to apolipoprotein A-I by increasing the cell surface presence of ATP-binding cassette transporter a1. J Biol Chem. 2003;278:40121–40127. doi: 10.1074/jbc.M305193200. [DOI] [PubMed] [Google Scholar]
- 9.Singaraja RR, Kang MH, Vaid K, Sanders SS, Vilas GL, Arstikaitis P, Coutinho J, Drisdel RC, El-Husseini Ael D, Green WN, Berthiaume L, Hayden MR. Palmitoylation of ATP-binding cassette transporter A1 is essential for its trafficking and function. Circ Res. 2009;105:138–147. doi: 10.1161/CIRCRESAHA.108.193011. [DOI] [PubMed] [Google Scholar]
- 10.Chen W, Wang N, Tall AR. A pest deletion mutant of ABCA1 shows impaired internalization and defective cholesterol efflux from late endosomes. J Biol Chem. 2005;280:29277–29281. doi: 10.1074/jbc.M505566200. [DOI] [PubMed] [Google Scholar]
- 11.Linder MD, Uronen RL, Holtta-Vuori M, van der Sluijs P, Peranen J, Ikonen E. Rab8-dependent recycling promotes endosomal cholesterol removal in normal and sphingolipidosis cells. Mol Biol Cell. 2007;18:47–56. doi: 10.1091/mbc.E06-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linder MD, Mayranpaa MI, Peranen J, Pietila TE, Pietiainen VM, Uronen RL, Olkkonen VM, Kovanen PT, Ikonen E. Rab8 regulates ABCA1 cell surface expression and facilitates cholesterol efflux in primary human macrophages. Arterioscler Thromb Vasc Biol. 2009;29:883–888. doi: 10.1161/ATVBAHA.108.179481. [DOI] [PubMed] [Google Scholar]
- 13.Mendez AJ. Monensin and brefeldin A inhibit high density lipoprotein-mediated cholesterol efflux from cholesterol-enriched cells. Implications for intracellular cholesterol transport. J Biol Chem. 1995;270:5891–5900. doi: 10.1074/jbc.270.11.5891. [DOI] [PubMed] [Google Scholar]
- 14.Remaley AT, Schumacher UK, Stonik JA, Farsi BD, Nazih H, Brewer HB., Jr Decreased reverse cholesterol transport from Tangier disease fibroblasts. Acceptor specificity and effect of brefeldin on lipid efflux. Arterioscler Thromb Vasc Biol. 1997;17:1813–1821. doi: 10.1161/01.atv.17.9.1813. [DOI] [PubMed] [Google Scholar]
- 15.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: Insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mossessova E, Corpina RA, Goldberg J. Crystal structure of ARF1*sec7 complexed with brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol Cell. 2003;12:1403–1411. doi: 10.1016/s1097-2765(03)00475-1. [DOI] [PubMed] [Google Scholar]
- 17.Ishizaki R, Shin HW, Mitsuhashi H, Nakayama K. Redundant roles of BIG2 and BIG1, guanine-nucleotide exchange factors for ADP-ribosylation factors in membrane traffic between the trans-golgi network and endosomes. Mol Biol Cell. 2008;19:2650–2660. doi: 10.1091/mbc.E07-10-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen X, Xu KF, Fan Q, Pacheco-Rodriguez G, Moss J, Vaughan M. Association of brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) with recycling endosomes during transferrin uptake. Proc Natl Acad Sci U S A. 2006;103:2635–2640. doi: 10.1073/pnas.0510599103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Claude A, Chun J, Shields DJ, Presley JF, Melancon P. GBF1, a cis-golgi and vtcs-localized ARF-GEF, is implicated in ER-to-golgi protein traffic. J Cell Sci. 2006;119:3743–3753. doi: 10.1242/jcs.03173. [DOI] [PubMed] [Google Scholar]
- 20.Zha X, Gauthier A, Genest J, McPherson R. Secretory vesicular transport from the golgi is altered during ATP-binding cassette protein A1 (ABCA1)-mediated cholesterol efflux. J Biol Chem. 2003;278:10002–10005. doi: 10.1074/jbc.C300024200. [DOI] [PubMed] [Google Scholar]
- 21.Yamaji R, Adamik R, Takeda K, Togawa A, Pacheco-Rodriguez G, Ferrans VJ, Moss J, Vaughan M. Identification and localization of two brefeldin A-inhibited guanine nucleotide-exchange proteins for ADP-ribosylation factors in a macromolecular complex. Proc Natl Acad Sci U S A. 2000;97:2567–2572. doi: 10.1073/pnas.97.6.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X, Lasell TK, Melancon P. Localization of large ADP-ribosylation factor-guanine nucleotide exchange factors to different golgi compartments: Evidence for distinct functions in protein traffic. Mol Biol Cell. 2002;13:119–133. doi: 10.1091/mbc.01-08-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G. Molecular cloning of the human ATP-binding cassette transporter 1 (hABC1): Evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 24.Orso E, Broccardo C, Kaminski WE, et al. Transport of lipids from golgi to plasma membrane is defective in tangier disease patients and ABC1-deficient mice. Nat Genet. 2000;24:192–196. doi: 10.1038/72869. [DOI] [PubMed] [Google Scholar]
- 25.Brunham LR, Singaraja RR, Duong M, Timmins JM, Fievet C, Bissada N, Kang MH, Samra A, Fruchart JC, McManus B, Staels B, Parks JS, Hayden MR. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:548–554. doi: 10.1161/ATVBAHA.108.182303. [DOI] [PubMed] [Google Scholar]
- 26.Nachtigal P, Pospisilova N, Vecerova L, Micuda S, Brcakova E, Pospechova K, Semecky V. Atorvastatin increases endoglin, smad2, phosphorylated smad2/3 and eNOS expression in ApoE/LDLR double knockout mice. J Atheroscler Thromb. 2009;16:265–274. doi: 10.5551/jat.e745. [DOI] [PubMed] [Google Scholar]
- 27.Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7:161–171. doi: 10.1016/s1097-2765(01)00164-2. [DOI] [PubMed] [Google Scholar]
- 28.Arakawa R, Yokoyama S. Helical apolipoproteins stabilize ATP-binding cassette transporter A1 by protecting it from thiol protease-mediated degradation. J Biol Chem. 2002;277:22426–22429. doi: 10.1074/jbc.M202996200. [DOI] [PubMed] [Google Scholar]
- 29.Lu R, Arakawa R, Ito-Osumi C, Iwamoto N, Yokoyama S. ApoA-I facilitates ABCA1 recycle/accumulation to cell surface by inhibiting its intracellular degradation and increases HDL generation. Arterioscler Thromb Vasc Biol. 2008;28:1820–1824. doi: 10.1161/ATVBAHA.108.169482. [DOI] [PubMed] [Google Scholar]
- 30.Azuma Y, Takada M, Shin HW, Kioka N, Nakayama K, Ueda K. Retroendocytosis pathway of ABCA1/apoA-I contributes to HDL formation. Genes Cells. 2009;14:191–204. doi: 10.1111/j.1365-2443.2008.01261.x. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka AR, Kano F, Yamamoto A, Ueda K, Murata M. Formation of cholesterol-enriched structures by aberrant intracellular accumulation of ATP-binding cassette transporter A1. Genes Cells. 2008;13:889–904. doi: 10.1111/j.1365-2443.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 32.Casanova JE. Regulation of arf activation: The sec7 family of guanine nucleotide exchange factors. Traffic. 2007;8:1476–1485. doi: 10.1111/j.1600-0854.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 33.Wellington CL, Brunham LR, Zhou S, Singaraja RR, Visscher H, Gelfer A, Ross C, James E, Liu G, Huber MT, Yang YZ, Parks RJ, Groen A, Fruchart-Najib J, Hayden MR. Alterations of plasma lipids in mice via adenoviral-mediated hepatic overexpression of human ABCA1. J Lipid Res. 2003;44:1470–1480. doi: 10.1194/jlr.M300110-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Basso F, Freeman L, Knapper CL, Remaley A, Stonik J, Neufeld EB, Tansey T, Amar MJ, Fruchart-Najib J, Duverger N, Santamarina-Fojo S, Brewer HB., Jr Role of the hepatic ABCA1 transporter in modulating intrahepatic cholesterol and plasma HDL cholesterol concentrations. J Lipid Res. 2003;44:296–302. doi: 10.1194/jlr.M200414-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic ABCA1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest. 2005;115:1333–1342. doi: 10.1172/JCI23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haghpassand M, Bourassa PA, Francone OL, Aiello RJ. Monocyte/macrophage expression of ABCA1 has minimal contribution to plasma hdl levels. J Clin Invest. 2001;108:1315–1320. doi: 10.1172/JCI12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helms JB, Rothman JE. Inhibition by brefeldin A of a golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- 38.Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc Natl Acad Sci U S A. 2000;97:12097–12102. doi: 10.1073/pnas.200367697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen X, Hong MS, Moss J, Vaughan M. Big1, a brefeldin A-inhibited guanine nucleotide-exchange protein, is required for correct glycosylation and function of integrin beta1. Proc Natl Acad Sci U S A. 2007;104:1230–1235. doi: 10.1073/pnas.0610535104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen X, Meza-Carmen V, Puxeddu E, Wang G, Moss J, Vaughan M. Interaction of brefeldin A-inhibited guanine nucleotide-exchange protein (BIG) 1 and kinesin motor protein KIF21a. Proc Natl Acad Sci U S A. 2008;105:18788–18793. doi: 10.1073/pnas.0810104105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang MH, Singaraja R, Hayden MR. Adenosine-triphosphate-binding cassette transporter-1 trafficking and function. Trends Cardiovasc Med. 2010;20:41–49. doi: 10.1016/j.tcm.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Moss J, Vaughan M. Molecules in the arf orbit. J Biol Chem. 1998;273:21431–21434. doi: 10.1074/jbc.273.34.21431. [DOI] [PubMed] [Google Scholar]
- 43.Engel T, Lueken A, Bode G, Hobohm U, Lorkowski S, Schlueter B, Rust S, Cullen P, Pech M, Assmann G, Seedorf U. ADP-ribosylation factor (ARF)-like 7 (ARL7) is induced by cholesterol loading and participates in apolipoprotein AI-dependent cholesterol export. FEBS Lett. 2004;566:241–246. doi: 10.1016/j.febslet.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 44.Yu X, Breitman M, Goldberg J. A structure-based mechanism for arf1-dependent recruitment of coatomer to membranes. Cell. 2012;148:530–542. doi: 10.1016/j.cell.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beers MF, Hawkins A, Shuman H, Zhao M, Newitt JL, Maguire JA, Ding W, Mulugeta S. A novel conserved targeting motif found in ABCA transporters mediates trafficking to early post-golgi compartments. J Lipid Res. 2011;52:1471–1482. doi: 10.1194/jlr.M013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.