Abstract
Recent data provide evidence that co-infection with human immunodeficiency virus type 1 (HIV-1) and human T lymphotropic virus type 2 (HTLV-2) delays progression to AIDS compared to isolated HIV-1 infection. These results were linked to expression of the HTLV-2 transcriptional activating gene known as Tax2. Preliminary studies in lymphocytic systems suggest that Tax2 is responsible for induction of CC-chemokines, which play a major role in innate immune responses against HIV-1. In this study, the effect of Tax2 on CC-chemokines (MIP-1α/CCL3, MIP-1β/CCL4, and RANTES/CCL5) in monocyte-derived macrophages (MDMs) was evaluated. An immortalized human monocytic cell line (U937) and donor-derived MDMs were used to evaluate these interactions. These cells were cultured in vitro, allowed to mature into macrophages for 14 d, and treated with Tax2 or Tax1 (the transcriptional activator of HTLV-1) at three concentrations (1, 10, and 100 pM) daily thereafter. Extracellular bacterial extract (EBE) lacking the vector and untreated samples served as controls. An additional group of donor-derived MDMs were transduced with an adenovirus vector that expressed either Tax2 or green fluorescent protein (GFP). Liposomal transfection agents alone were used as controls. Supernatants were collected from each sample on multiple days post-maturation and evaluated for MIP-1α, MIP-1β, and RANTES, by enzyme-linked immunosorbent assay. Analysis of variance and Tukey's Honestly Significant Difference tests were used to analyze the results. In all systems, cells exposed to either Tax2 or Tax1 expressed significantly (p<0.01) higher concentrations of CC-chemokines than controls. There was no significant difference in chemokine expression between Tax1-treated and Tax2-treated samples, between EBE-treated and EBE-untreated samples, or between GFP-transduced MDMs and controls. This suggests that HTLV-2 could alter innate immune responses in macrophagic reservoirs of HIV-1 in HIV-1/HTLV-2 co-infected individuals, and could guide the development of HIV-1 treatments.
Introduction
Members of the CC-chemokine family play a major role in innate immune responses against viral infections, including human immunodeficiency virus type 1 (HIV-1) (12). Particularly important are MIP-1α (CC motif ligand 3), MIP-1β (CC motif ligand 4), and RANTES (CC motif ligand 5), which bind the HIV-1 co-receptor CC-chemokine receptor type 5 (CCR5). These CC-chemokines have been previously correlated with innate resistance to HIV-1 infection, decreased viral loads in individuals already infected, and protection against disease progression to acquired immune deficiency syndrome (AIDS) (13,17,20,22,40,45,50).
It has been shown that the human T lymphotropic virus type 2 (HTLV-2) transcriptional activator, known as Tax2, is a potent mediator of CC-chemokine expression both in vivo and in vitro (10,38). This observation has clinical relevance during co-infection with HIV-1 and HTLV-2, based on recent data suggesting that HTLV-2 slows the decline of CD4+ cells and delays progression to AIDS in HIV-1-infected individuals (5,21,44). Co-infection with HIV-1 and human T lymphotropic virus type 1 (HTLV-1) does not appear to confer this same survival benefit, and may actually hasten the onset of AIDS (5,8), despite a slowed decline of CD4+ cells (7,23).
It is hypothesized that HTLV-2 infection of lymphocytes and macrophages could provide a source of chemokines that results in an antiviral response against HIV-1 infection. While previous literature provides strong preliminary evidence that Tax2 acts via chemokine induction in lymphocytic systems to downregulate HIV-1 replication (10–12,16,34,38), this study examined the effect of Tax2 on CC-chemokine expression in macrophages, a reservoir of HIV-1 that contributes significantly to its pathogenesis (6).
Materials and Methods
Experiments were carried out in two distinct cell culture systems: an immortalized human promonocytic cell line (U937), and monocyte-derived macrophages (MDMs) obtained from donated whole blood. The U937 cell line, derived from human histiocytic lymphoma, can be induced to become structurally and functionally identical to normal human macrophages (6). Blood samples from the donor were obtained following informed consent using a protocol that was approved by the Institutional Review Board for Human Investigation of the Milwaukee Veterans Affairs, Research Service Committee.
Cell line differentiation
Differentiation of U937 cells was accomplished by treatment with phorbol 12-myristate 13-acetate (PMA) at a concentration of 2500 pg/mL. Cells were incubated overnight with PMA in Iscove's modified Dulbecco's medium (IMDMC), 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin-glutamine (PSG) at 37°C and 5% CO2 while rotating. They were washed twice with IMDMC, plated in a 6-well plate at 100,000 cells/mL of IMDMC, and allowed to mature for 14 d in a humidified incubator at 37°C and 5% CO2. Half of the medium was removed every 3–4 d and replaced with fresh IMDMC to maintain cell viability. At day 8, the plates were washed once with IMDMC to remove any non-adherent cells.
Donor cell differentiation
To differentiate donor-derived cells, whole blood was collected in CPT/Vacutainer BD tubes (Becton Dickinson, San Jose, CA), and peripheral blood mononuclear cells (PBMCs) were isolated following the manufacturer's recommendations. The donor was confirmed to be HIV-1 and HTLV-1/-2 seronegative by enzyme-linked immunosorbent assay (ELISA). CD14+ cells were isolated using the Dynabeads FlowComp Human CD14 kit and a magnetic cell sorter (Invitrogen, Carlsbad, CA). Some monocytes were stored at −70°C for fluorescence-activated cell sorting (FACS) to evaluate cellular purity.
Monocytes were plated at a concentration of 125,000 cells/mL of RPMIAB (RPMI-1640, 10% human AB serum, 1% PSG, and 1% sodium pyruvate), and allowed to incubate for 14 d at 37°C and 5% CO2. Half of the medium was extracted and replaced on days 5 and 8 to preserve cell viability. On day 14 the wells were washed once with RPMI-1640 to remove any non-adherent cells. Medium was replaced with RPMIC (RPMI-1640, 10% FBS, 1% PSG, and 1% sodium pyruvate).
Evaluation of CD14 and CCR5 expression by FACS
Adherent U937 cells were scraped from the plate, washed with phosphate-buffered saline (PBS), and stained using mouse phycoerythrin (PE)-labeled anti-human CCR5 monoclonal antibody (CD195, clone 2D7; BD Biosciences, San Jose, CA), or PE-labeled mouse IgG2a kappa isotype control in staining buffer (PBS and 2% FBS).
Adherent donor-derived cells were detached using 100 μL of 0.05% trypsin-EDTA (Invitrogen) per well, washed with RPMIC, and double-stained with either fluorescein isothiocyanate (FITC)-labeled mouse IgG2a kappa anti-human CD14, or FITC-labeled mouse IgG2a kappa isotype control, and either PE-labeled mouse IgG2a kappa anti-human CD195 (CCR5), or PE-labeled mouse IgG2a kappa isotype control (all antibodies from BD Pharmingen, San Jose, CA). Stored monocytes were thawed and double-stained similarly. BD CompBeads (BD Biosciences) were stained with the antibodies to optimize fluorescence compensation setting for FITC and PE flow cytometric analyses.
Stained cells from both systems were investigated with flow cytometry using a LSR II Flow Cytometer (Becton Dickinson). Data were analyzed using FlowJo software version 7.6/9.0 (Tree Star Inc., Ashland, OR).
Treatment with recombinant Tax proteins
The BL21/DE3 strain of E. coli, containing the pET-Tax1 or the pET-Tax2 expression vectors, were generated as previously described (4). Proteins were analyzed for the presence of endotoxin at concentrations used in the experiments via the Limulus amebocyte lysate test (E-TOXATE; Sigma-Aldrich, St. Louis, MO). To reduce contamination of endotoxin in the purified proteins, all glassware was autoclaved for 1 h then heated in an oven at 175°C for 3 h. Solution transfers were performed with endotoxin-free devices. Extrabacterial extract (EBE) from E. coli lacking the vector, but processed identically, was used as background control.
Following differentiation, samples of U937 cells were treated daily with recombinant Tax2 at three concentrations (1, 10, or 100 pM). Triplicate donor-derived samples were treated with recombinant Tax2 or Tax1 (the transcriptional activating protein of HTLV-1) at the same three concentrations. Cells treated with EBE or without protein treatment were used as controls in both systems. Supernatants were collected at days 3, 5, 7, 10, and 13 post-maturation in the U937 system, and at days 1, 3, 5, 7, 10, 14, and 21 post-maturation in the donor-derived system. Samples were stored at −20°C and assayed for MIP-1α, MIP-1β, and RANTES by ELISA (DuoSet; R&D Systems, Hercules, CA).
Propagation and titration of recombinant adenovirus vectors
Recombinant replication-deficient adenoviruses expressing either Tax2 subtype B or green fluorescent protein (GFP) (Ad-Tax2 and Ad-GFP, respectively) (33) were propagated in HEK-293 grown in Dulbecco's modified Eagle medium (Invitrogen) (4.5 g/L d-glucose) with 10% FBS and 1% PSG (DMEMC) at 37°C and 5% CO2. Viral stocks were then titrated by end-point dilution as follows: low-passage-number HEK-293 cells were plated at 2×104 cells per well in a 96-well plate 24 h prior to infection. The culture medium was then removed and replaced with DMEM containing 2% FBS and 1% PSG (DMEM2%FBS) in which adenovirus vectors were serially diluted with uninfected controls receiving medium only. The plates were incubated for 10 days at 37°C and 5% CO2. Every 2–3 d, the media was changed with DMEMC to preserve cell viability. At the conclusion of the incubation period, the monolayers in the wells were microscopically examined for virus-induced cytopathic effect (CPE). The fraction of CPE-positive wells in each virus dilution was recorded, and the viral titer (pfu/mL) was calculated using the formula 10[1+Z (X − 0.5)] based on the Spearman-Karber method (18), where Z=log 10 of the starting dilution (=1 for a 10-fold dilution), and X=the sum of the fractions of CPE-positive wells.
Transduction of MDMs with adenovirus expressing Tax2 vector
MDMs lack the coxsackievirus-adenovirus receptor and express only small amounts of the α-V-β integrins necessary for effective binding and internalization of adenovirus (9,27,29). Therefore, to improve transduction of the Ad-Tax2 and Ad-GFP (adenovirus control) vectors into MDMs (30), lipofectamine plus reagents (Invitrogen) were utilized according to the manufacturer's instructions. MDMs (2.5×105 in 500 μL RPMIC) previously seeded in a 24-well plate were transduced with 50 μL/well of lipocomplexes (liposomal reagents bound to genetic material), containing lipofectamine plus reagent, and either Ad-Tax2 or Ad-GFP at a multiplicity of infection (MOI) of 10 in Opti-MEM I reduced serum medium (Invitrogen). An additional group was treated with only the lipofection reagents as a control, and all conditions were tested in triplicate. Cells were incubated for 14 d at 37°C and 5% CO2. The efficiency of transfection was calculated by fluorescence microscopy using the adenoviral control GFP beginning at 24 h and continuing through day 10 of incubation. To assess MIP-1α, MIP-1β, and RANTES expression, supernatants (250 μL) were taken and replaced with the same volume of RPMIC on days 1, 3, 5, 7, 10, and 14, stored at −20°C, and evaluated using ELISA.
Statistical analysis
One-way analysis of variance (ANOVA) was performed to assess the presence of differences in CC-chemokine expression between Tax2-treated, EBE-treated, and untreated U937 samples at each concentration during the course of sampling. Comparisons between each day were not possible due to sample size. Tukey's Honestly Significant Difference (HSD) test was used to determine between which variables the difference existed. Two-way ANOVA was performed to assess the presence of differences between Tax2-treated, Tax1-treated, EBE-treated, and untreated samples at each concentration at each day of sampling in the donor-derived system, as well as between Ad-Tax2-treated, Ad-GFP-treated, and liposomal controls at each day of sampling in the adenoviral transduction system. Tukey's HSD test was again used to determine between which variables differences existed.
Results
Cellular differentiation
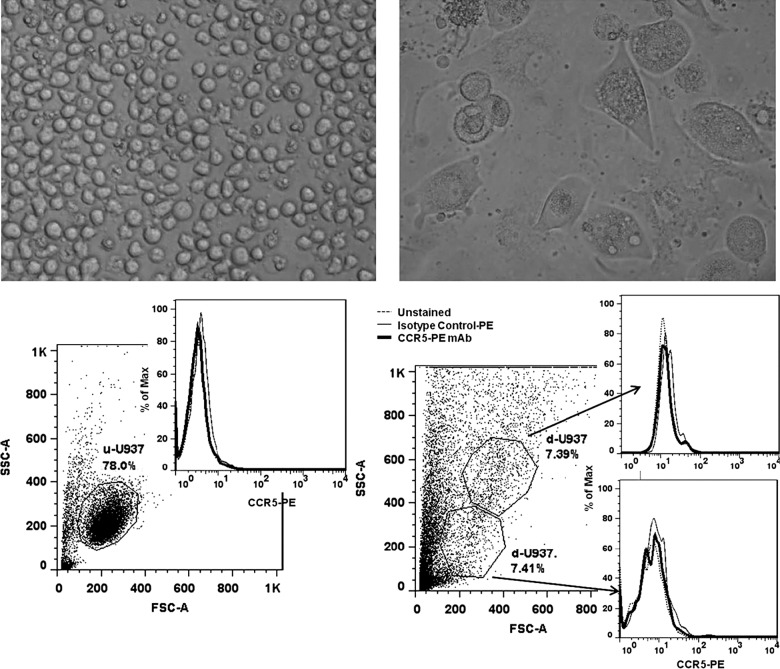
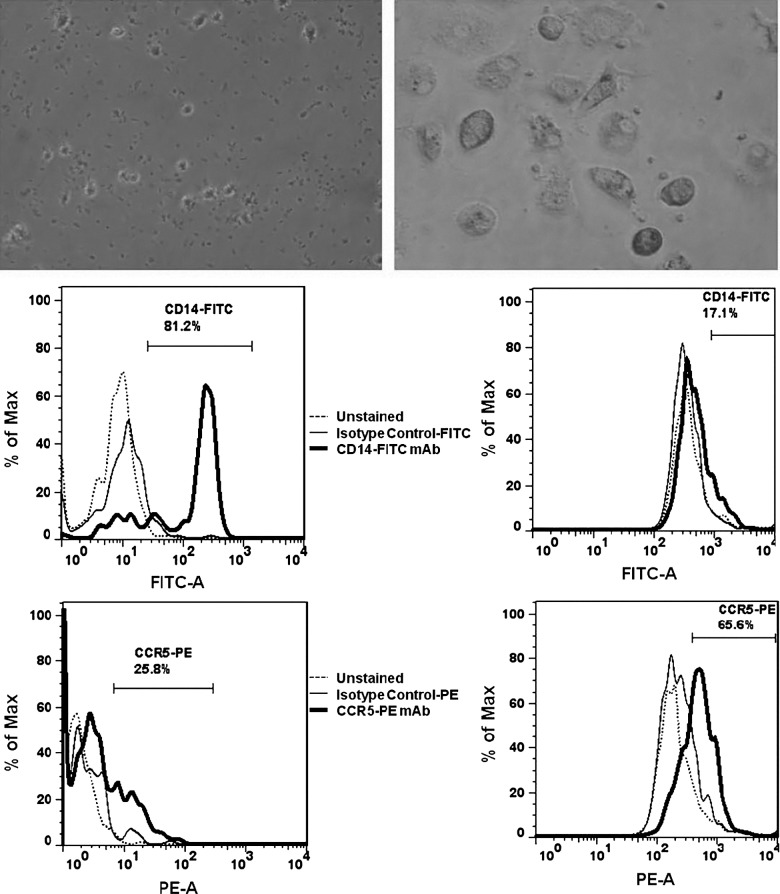
PMA treatment of non-adherent U937 cells resulted in mature, adherent U937 cells after 14 d in culture. However, neither undifferentiated U937 nor differentiated U937 cells expressed CCR5 co-receptors as determined by FACS (Fig. 1). Donor-derived CD14+ cells were found to be more than 80% pure as assessed by FACS. Prior to differentiation, 25.8% of cells expressed the CCR5 receptor, but following 14 d of in vitro incubation the number of CCR5-expressing cells increased to 65.6% (Fig. 2).
FIG. 1.
Undifferentiated, non-adherent U937 cells (top left), and differentiated, adherent U937 cells (top right) after PMA treatment and 14 d in culture. FACS analysis of undifferentiated U937 cells (u-U937, bottom left), and differentiated U937 cells (d-U937, bottom right) for CCR5 expression. Differentiated U937 cells failed to express detectable levels of CCR5.
FIG. 2.
Undifferentiated donor-derived cells after CD14+ isolation (top left), and differentiated donor-derived MDMs after 14 d in culture (top right). FACS analysis of undifferentiated donor-derived monocytes (bottom left), and differentiated MDMs (bottom right), for CD14 and CCR5 expression. A shift from CD14-predominant cells to CCR5-predominant cells was observed after differentiation, with 65.6% of differentiated MDMs staining positive for CCR5.
Chemokine expression in Tax-treated U937 cells and donor-derived MDMs
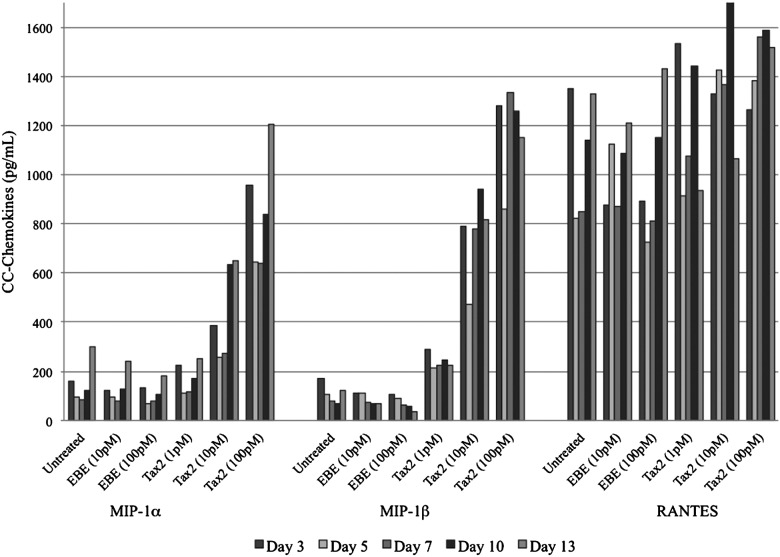
Proportionally increased expression of MIP-1α and MIP-1β occurred when differentiated U937 cells were cultured in the presence of Tax2 at 10 pM and 100 pM compared to treatment with Tax2 at 1 pM or controls (p<0.01; Fig. 3). There was no significant difference between Tax2 at 1 pM, EBE at any concentration, or untreated samples. RANTES expression was not significantly different between sample groups (Fig. 3).
FIG. 3.
Expression of CC-chemokines by differentiated, macrophage-like U937 cells incubated with Tax2. EBE-treated and untreated differentiated U937 cells were used as controls. Statistically significant differences in MIP-1α and MIP-1β expression were observed between Tax2 at 10 pM or 100 pM and controls (p<0.01). No significant differences were observed in RANTES expression between any groups.
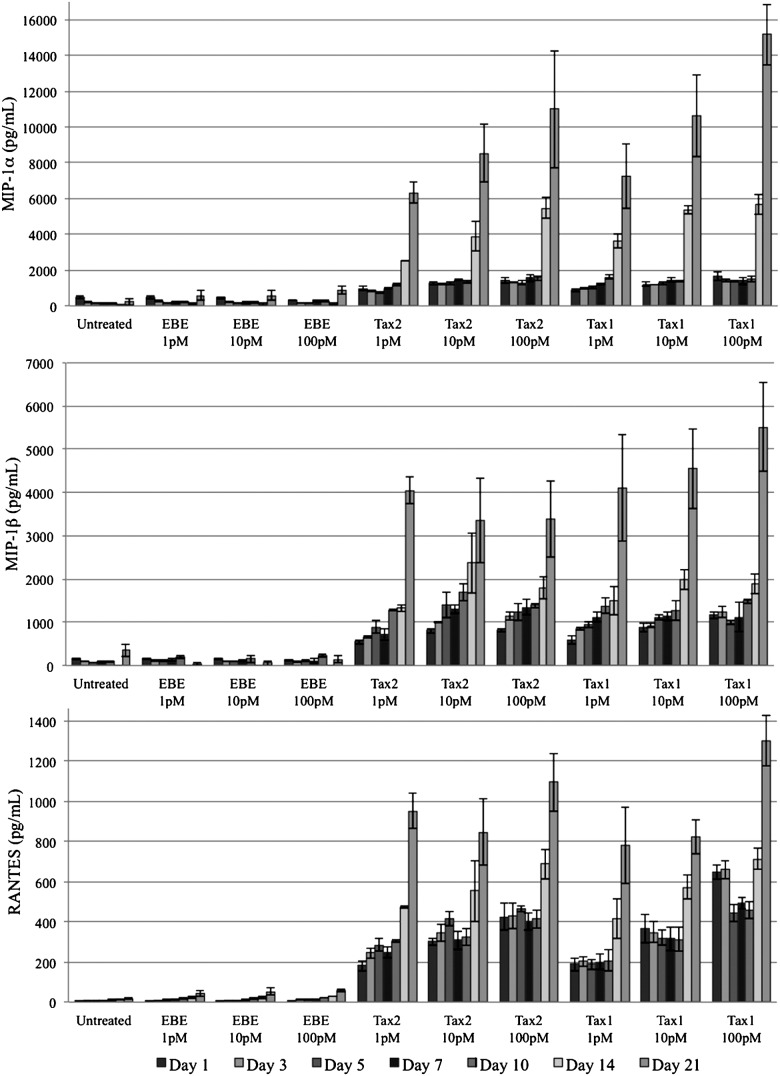
PBMC-derived macrophages expressed higher concentrations of the chemokines MIP-1α, MIP-1β, and RANTES when cultured in the presence of Tax2 or Tax1 at all tested concentrations compared to controls (Fig. 4). These results were significant (p<0.01) by ANOVA and Tukey's HSD at all measured time points post-treatment.
FIG. 4.
Expression of MIP-1α (top), MIP-1β (middle), and RANTES (bottom) by donor-derived macrophages. Concentrations of all CC-chemokines were significantly higher (p<0.01) in the presence of Tax2 or Tax1 compared to EBE-treated or untreated controls. There was no consistently significant difference between concentrations of any given protein, between Tax1- and Tax2-treated samples, or between EBE-treated and untreated samples.
There was no consistently significant difference between concentrations of any given protein, between Tax1- and Tax2-treated samples, or between EBE-treated and untreated samples. MIP-1α, MIP-1β, and RANTES expression began to increase significantly on day 10 post-treatment in both Tax-treated groups, and continued to progress accordingly through day 21 post-treatment. This corresponded to microscopic observations of cellular degradation. CC-chemokine expression in EBE-treated and in untreated groups did not increase significantly over time.
Transduction of MDMs
To additionally test the ability of Tax2 to induce the production of CC-chemokines in MDMs, constructed adenovirus vectors expressing Tax2 or GFP in the presence of liposomes were used to transduce MDMs. Lipocomplex-mediated transduction into MDMs was highly effective, resulting in 65–75% of cells expressing GFP as determined by fluorescence microscopy.
Effect of Tax2 transduction in MDMs on chemokine expression
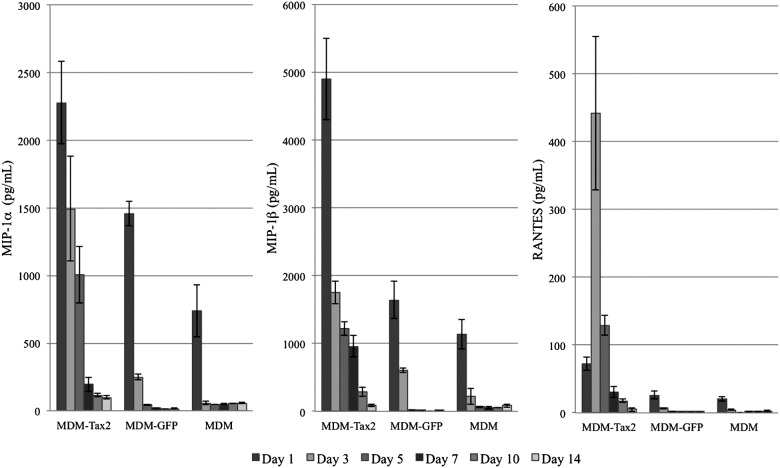
MDMs transduced with Ad-Tax2 demonstrated significant levels of MIP-1α (Fig. 5) compared to liposomal controls on day 1 (p<0.01), day 3 (p<0.01), and day 5 (p<0.05), and compared to MDMs transduced with Ad-GFP on day 3 (p<0.01) and day 5 (p<0.05). Significant levels of MIP-1β (Fig. 5) were also observed in MDMs transduced with Ad-Tax2 compared to liposomal controls on day 1 (p<0.01), day 3 (p<0.01), and day 5 (p<0.05), as well as compared to those transduced with Ad-GFP on day 1 (p<0.01), day 3 (p<0.05), and day 5 (p<0.05). No significant difference in MIP-1α or MIP-1β expression was observed on days 7, 10, or 14. MDMs transduced with Ad-Tax2 also demonstrated increased RANTES expression (Fig. 5), but this was only significant (p<0.01) on day 3 post-transduction in relationship to both Ad-GFP and liposomal treatments. No other day demonstrated statistically significant differences in RANTES expression between any of the groups studied.
FIG. 5.
Expression of CC-chemokines by donor-derived macrophages transduced with adenovirus vectors expressing Tax2 (MDM-Tax2), transduced with adenovirus vectors expressing green fluorescent protein (MDM-GFP), or treated with liposomal agents only (MDM). Statistically significant differences in MIP-1α and MIP-1β expression were observed between MDM-Tax2 and MDM on day 1 (p<0.01), day 3 (p<0.01), and day 5 (p<0.05). MDM-Tax2 expressed significantly higher levels of MIP-1α on day 3 (p<0.01) and day 5 (p<0.05), and MIP-1β on day 1 (p<0.01), day 3 (p<0.05), and day 5 (p<0.05) compared to MDM-GFP. No significant difference in MIP-1α or MIP-1β expression was observed on days 7, 10, or 14. MDM-Tax2 also demonstrated increased RANTES expression, but this was only significant (p<0.01) on day 3 in relationship to both MDM-GFP and MDM.
When data were grouped over the 14-day sampling period, however, significantly (p<0.01) elevated levels of MIP-1α, MIP-1β, and RANTES were each observed in the Ad-Tax2-treated group as compared to the Ad-GFP- and liposomal-treated groups. While MDMs transduced with Ad-GFP demonstrated more chemokine expression than those treated with liposomal agents alone, these differences were not statistically significant whether evaluated cumulatively or on any measured day.
Discussion
Similar to previous data in lymphocytic systems, this study also provides strong evidence that HTLV-2's Tax2 protein activates chemokine expression in macrophage systems. As demonstrated in both a macrophage cell line and donor-derived macrophages, the presence of Tax2 stimulates expression of CC-chemokines.
The addition of Tax2 to differentiated U937 cells resulted in a proportional increase in MIP-1α and MIP-1β expression. However, this relationship was not observed for RANTES expression. The difference is attributable to elevated baseline levels of this chemokine, as demonstrated in controls that released more than 500 pg/mL of RANTES on all measured days. PMA has previously been shown to stimulate RANTES expression in U937 cells (36), and was likely inducing RANTES in this case as well.
In the non-transduced donor-derived system, statistically significant differences in MIP-1α, MIP-1β, and RANTES were observed on every measured day between each Tax protein treatment and each control. This indicates that Tax2 likely is the means by which HTLV-2 stimulates chemokine expression. Although the mechanism of this induction was not examined, it is possible that it occurs via transactivation of chemokine promoters, as it occurs in CD8+ T cells (31).
Increased chemokine expression in the presence of Tax1 was consistent with previous studies in PBMCs, which demonstrated Tax1's induction of CC-chemokines (4) and other cytokines (1). This suggests that the difference in survival between co-infection with HIV-1/HTLV-1 compared to HIV-1/HTLV-2 is likely related to the pathogenicity of HTLV-1 (19), and the oncogenic potential of Tax1 (2), rather than the ability of HTLV-1 to induce chemokines.
The increased expression of MIP-1α, MIP-1β, and RANTES in Tax-treated groups beginning on day 10 post-treatment appeared to correspond with cellular demise. This could indicate large chemokine reservoirs within the PBMC-derived macrophages that were unaccounted for when measuring chemokine concentrations in the supernatant. While CC-chemokine reservoirs are not a well-established pattern of expression, other chemokines have been shown to be stored in endothelial reservoirs, and to be exocytosed upon stimulation (39). MIP-1α, MIP-1β, and RANTES could be processed in a similar manner within macrophages.
CCR5 expression in differentiated U937 cells was minimal or absent. Other studies have shown variability in CCR5 expression that can be attributed to subclonal variants or to poorly-understood effects of differentiating agents, such as PMA. In this case, the absence of CCR5 expression in PMA-treated U937 cells does not correspond to a lack of differentiation (3,37). Instead, cellular morphology and adherence to plastic culture plates can be used to indicate cellular differentiation (25,26). The unreliable expression of CCR5 in U937 cells limits the feasibility of employing this cell line in its differentiated state for investigations of HIV-1 infection.
To address the concern of possible lipopolysaccharide or related contamination in the recombinant protein preparation, we also employed the use of a replication-deficient (E1A-deleted) adenovirus vector delivery system. Adenoviral vectors have a high transduction efficiency, are capable of containing DNA inserts up to 8 kb, infect both replicating and differentiated cells, and do not integrate into the host genome; thus their expression is transient, no viral propagation occurs, and the infected cell does not die as a result of viral transfection (28,46). Therefore, it is not a surprise that the CC-chemokines were most heavily expressed in the first days after the recombinant adenovirus transduction.
Previous studies have reported transduction efficiencies of 10–80% with adenoviral vectors in human macrophages, with success rates varying significantly with cell culture conditions and MOI (14,24,42). To increase gene transfer efficiency (30), the liposomal agents lipofectamine and reagent were used to transduce the recombinant adenovirus. These agents were found to be very effective, as 65–75% of cells were transfected with the reporter Ad-GFP. Nevertheless, even when used without accompanying genetic material, these agents appeared to independently induce the production of CC-chemokines, particularly MIP-1α and MIP-1β at day 1, although no completely untreated controls were available for direct comparison. While previous studies in in vivo mouse models did demonstrate significant cytokine production in the presence of lipocomplexes, they failed to show any increase in cytokine production in the presence of liposomal agents alone (15,41,47). Studies in cell culture, however, suggest that liposomal agents themselves may stimulate various cytokines and interferons in a variety of cell types (32,48,49). The mechanism of cytokine induction by liposomal agents is not completely understood, but it has been proposed that the effects of Toll-like receptor signaling upon endocytosis causes activation of the nuclear factor-κB (NF-κB) pathway (43). This may have played a role in our experimental system. Furthermore, while the use of recombinant virus to infect mammalian cells is one of the preferred means to deliver exogenous genes to mammalian cells, the immunogenicity of adenovirus is a possible limitation. Recombinant adenovirus has also been reported to induce proinflammatory cytokine production (35,51). This could contribute to the elevated levels of chemokines seen at day 1 in MDMs transduced with Ad-GFP, although these were not statistically different from those treated with liposomal agents only.
Despite these influences, however, there were significantly elevated levels of CC-chemokines in MDMs transduced with Ad-Tax2 compared to those transduced with Ad-GFP or treated with liposomal agents alone. These effects persisted over several days (particularly in MIP-1α and MIP-1β), and maintained overall statistical significance in all three chemokines observed. This provides evidence that Tax2 alone is sufficient, independent of HTLV-2 infection, to induce CC-chemokine expression.
Further work will be needed to determine the exact nature of HIV-1/HTLV-2 interactions in these non-lymphocytic reservoirs of HIV-1. The increased expression of MIP-1α, MIP-1β, and RANTES, however, suggests that alterations in innate immune responses via stimulation of chemokine production and release may play an important role. By continuing to gain an overall understanding of how HTLV-2 functions in the regulation of T-cell decline during co-infection with HIV-1, new therapies could eventually be developed to treat HIV-1-infected individuals. These treatments would replicate this viral interaction, and could reduce the number of AIDS-related deaths in those infected with HIV-1.
Acknowledgments
This research was supported by grants from the VA Merit Review Program (BX000488-01), the Infectious Diseases Society of America's Medical Scholars Program, and the Department of Medicine of the Medical College of Wisconsin.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abrahao MH. Lima RG. Netto E. Brites C. Human lymphotropic virus type 1 coinfection modulates the synthesis of cytokines by peripheral blood mononuclear cells from HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/AID.2011.0192. [DOI] [PubMed] [Google Scholar]
- 2.Akagi T. Ono H. Shimotohno K. Characterization of T cells immortalized by Tax1 of human T-cell leukemia virus type 1. Blood. 1995;86:4243–4249. [PubMed] [Google Scholar]
- 3.Arcila ML. Sanchez MD. Ortiz B. Barrera LF. Garcia LF. Rojas M. Activation of apoptosis, but not necrosis, during Mycobacterium tuberculosis infection correlated with decreased bacterial growth: role of TNF-alpha, IL-10, caspases and phospholipase A2. Cell Immunol. 2007;249:80–93. doi: 10.1016/j.cellimm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Barrios CS. Abuerreish M. Lairmore MD. Castillo L. Giam CZ. Beilke MA. Recombinant human T-cell leukemia virus types 1 and 2 Tax proteins induce high levels of CC-chemokines and downregulate CCR5 in human peripheral blood mononuclear cells. Viral Immunol. 2011;24:429–439. doi: 10.1089/vim.2011.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beilke MA. Theall KP. O'Brien M, et al. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin Infect Dis. 2004;39:256–263. doi: 10.1086/422146. [DOI] [PubMed] [Google Scholar]
- 6.Brandriss MW. Schlesinger JJ. Chapman SE. Growth of measles virus in a human macrophagelike cell line: U937. Am J Pathol. 1982;109:179–183. [PMC free article] [PubMed] [Google Scholar]
- 7.Brites C. Alencar R. Gusmao R, et al. Co-infection with HTLV-1 is associated with a shorter survival time for HIV-1-infected patients in Bahia, Brazil. AIDS. 2001;15:2053–2055. doi: 10.1097/00002030-200110190-00023. [DOI] [PubMed] [Google Scholar]
- 8.Brites C. Sampalo J. Oliveira A. HIV/human T-cell lymphotropic virus coinfection revisited: impact on AIDS progression. AIDS Rev. 2009;11:8–16. [PubMed] [Google Scholar]
- 9.Burke B. Sumner S. Maitland N. Lewis CE. Macrophages in gene therapy: cellular delivery vehicles and in vivo targets. J Leukoc Biol. 2002;72:417–428. [PubMed] [Google Scholar]
- 10.Casoli C. Pilotti E. Bertazzoni U. Molecular and cellular interactions of HIV-1/HTLV coinfection and impact on AIDS progression. AIDS Rev. 2007;9:140–149. [PubMed] [Google Scholar]
- 11.Casoli C. Vicenzi E. Cimarelli A, et al. HTLV-II down-regulates HIV-1 replication in IL-2-stimulated primary PBMC of coinfected individuals through expression of MIP-1alpha. Blood. 2000;95:2760–2769. [PubMed] [Google Scholar]
- 12.Cocchi F. DeVico AL. Garzino-Demo A. Arya SK. Gallo RC. Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 13.Cocchi F. DeVico AL. Yarchoan R, et al. Higher macrophage inflammatory protein (MIP)-1alpha and MIP-1beta levels from CD8+ T cells are associated with asymptomatic HIV-1 infection. Proc Natl Acad Sci USA. 2000;97:13812–13817. doi: 10.1073/pnas.240469997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De SK. Venkateshan CN. Seth P. Gajdusek DC. Gibbs CJ. Adenovirus-mediated human immunodeficiency virus-1 Nef expression in human monocytes/macrophages and effect of Nef on downmodulation of Fcgamma receptors and expression of monokines. Blood. 1998;91:2108–2117. [PubMed] [Google Scholar]
- 15.Dow SW. Fradkin LG. Liggitt DH. Willson AP. Heath TD. Potter TA. Lipid-DNA complexes induce potent activation of innate immune responses and antitumor activity when administered intravenously. J Immunol. 1999;163:1552–1561. [PubMed] [Google Scholar]
- 16.Dragic T. Litwin V. Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 17.Ferbas J. Giorgi JV. Amini S, et al. Antigen-specific production of RANTES, macrophage inflammatory protein (MIP)-1alpha, and MIP-1beta in vitro is a correlate of reduced human immunodeficiency virus burden in vivo. J Infect Dis. 2000;182:1247–1250. doi: 10.1086/315849. [DOI] [PubMed] [Google Scholar]
- 18.Finney DJ. Statistical Methods in Biological Assay. Griffin; London: 1952. [Google Scholar]
- 19.Gallo RC. Research and discovery of the first human cancer virus, HTLV-1. Best Pract Res Clin Haematol. 2011;24:559–565. doi: 10.1016/j.beha.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Garzino-Demo A. Moss RB. Margolick JB, et al. Spontaneous and antigen-induced production of HIV-inhibitory beta-chemokines are associated with AIDS-free status. Proc Natl Acad Sci USA. 1999;96:11986–11991. doi: 10.1073/pnas.96.21.11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giacomo M. Franco EG. Claudio C, et al. Human T-cell leukemia virus type II infection among high risk groups and its influence on HIV-1 disease progression. Eur J Epidemiol. 1995;11:527–533. doi: 10.1007/BF01719304. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez E. Kulkarni H. Bolivar H, et al. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science. 2005;307:1434–1440. doi: 10.1126/science.1101160. [DOI] [PubMed] [Google Scholar]
- 23.Gudo ES. Bhatt NB. Bila DR, et al. Co-infection by human immunodeficiency virus type 1 (HIV-1) and human T cell leukemia virus type 1 (HTLV-1): does immune activation lead to a faster progression to AIDS? BMC Infect Dis. 2009;9:211. doi: 10.1186/1471-2334-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddada H. Lopez M. Martinache C. Ragot T. Abina MA. Perricaudet M. Efficient adenovirus-mediated gene transfer into human blood monocyte-derived macrophages. Biochem Biophys Res Commun. 1993;195:1174–1183. doi: 10.1006/bbrc.1993.2168. [DOI] [PubMed] [Google Scholar]
- 25.Harris P. Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985;37:407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 26.Hass R. Kohler L. Rehfeldt W, et al. Alterations in glycosylation and lectin pattern during phorbol ester-induced differentiation of U937 cells. Cancer Res. 1990;50:323–327. [PubMed] [Google Scholar]
- 27.Huang S. Endo RI. Nemerow GR. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaras M. Brun AC. Karlsson S. Fan X. Adenoviral vectors for transient gene expression in human primitive hematopoietic cells: applications and prospects. Exp Hematol. 2007;35:343–349. doi: 10.1016/j.exphem.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Kaner RJ. Worgall S. Leopold PL, et al. Modification of the genetic program of human alveolar macrophages by adenovirus vectors in vitro is feasible but inefficient, limited in part by the low level of expression of the coxsackie/adenovirus receptor. Am J Respir Cell Mol Biol. 1999;20:361–370. doi: 10.1165/ajrcmb.20.3.3398. [DOI] [PubMed] [Google Scholar]
- 30.Karmali PP. Chaudhuri A. Cationic liposomes as non-viral carriers of gene medicines: resolved issues, open questions, and future promises. Med Res Rev. 2007;27:696–722. doi: 10.1002/med.20090. [DOI] [PubMed] [Google Scholar]
- 31.Lewis MJ. Gautier VW. Wang XP. Kaplan MH. Hall WW. Spontaneous production of C-C chemokines by individuals infected with human T lymphotropic virus type II (HTLV-II) alone and HTLV-II/HIV-1 coinfected individuals. J Immunol. 2000;165:4127–4132. doi: 10.4049/jimmunol.165.7.4127. [DOI] [PubMed] [Google Scholar]
- 32.Li XL. Boyanapalli M. Weihua X. Kalvakolanu DV. Hassel BA. Induction of interferon synthesis and activation of interferon-stimulated genes by liposomal transfection reagents. J Interferon Cytokine Res. 1998;18:947–952. doi: 10.1089/jir.1998.18.947. [DOI] [PubMed] [Google Scholar]
- 33.Liu B. Liang MH. Kuo YL, et al. Human T-lymphotropic virus type 1 oncoprotein tax promotes unscheduled degradation of Pds1p/securin and Clb2p/cyclin B1 and causes chromosomal instability. Mol Cell Biol. 2003;23:5269–5281. doi: 10.1128/MCB.23.15.5269-5281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lusso P. HIV and the chemokine system: 10 years later. EMBO J. 2006;25:447–456. doi: 10.1038/sj.emboj.7600947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morelli AE. Larregina AT. Ganster RW, et al. Recombinant adenovirus induces maturation of dendritic cells via an NF-kappaB-dependent pathway. J Virol. 2000;74:9617–9628. doi: 10.1128/jvi.74.20.9617-9628.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moriuchi H. Moriuchi M. Fauci AS. Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol. 1997;158:3483–3491. [PubMed] [Google Scholar]
- 37.Moriuchi H. Moriuchi M. Fauci AS. Differentiation of promonocytic U937 subclones into macrophagelike phenotypes regulates a cellular factor(s) which modulates fusion/entry of macrophagetropic human immunodeficiency virus type 1. J Virol. 1998;72:3394–3400. doi: 10.1128/jvi.72.4.3394-3400.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oravecz T. Pall M. Norcross MA. Beta-chemokine inhibition of monocytotropic HIV-1 infection. Interference with a postbinding fusion step. J Immunol. 1996;157:1329–1332. [PubMed] [Google Scholar]
- 39.Oynebraten I. Barois N. Hagelsteen K. Johansen FE. Bakke O. Haraldsen G. Characterization of a novel chemokine-containing storage granule in endothelial cells: evidence for preferential exocytosis mediated by protein kinase A and diacylglycerol. J Immunol. 2005;175:5358–5369. doi: 10.4049/jimmunol.175.8.5358. [DOI] [PubMed] [Google Scholar]
- 40.Paxton WA. Martin SR. Tse D, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 41.Sakurai F. Terada T. Yasuda K. Yamashita F. Takakura Y. Hashida M. The role of tissue macrophages in the induction of proinflammatory cytokine production following intravenous injection of lipoplexes. Gene Ther. 2002;9:1120–1126. doi: 10.1038/sj.gt.3301784. [DOI] [PubMed] [Google Scholar]
- 42.Schneider SD. Rusconi S. Seger RA. Hossle JP. Adenovirus-mediated gene transfer into monocyte-derived macrophages of patients with X-linked chronic granulomatous disease: ex vivo correction of deficient respiratory burst. Gene Ther. 1997;4:524–532. doi: 10.1038/sj.gt.3300432. [DOI] [PubMed] [Google Scholar]
- 43.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Turci M. Pilotti E. Ronzi P, et al. Coinfection with HIV-1 and human T-cell lymphotropic virus type II in intravenous drug users is associated with delayed progression to AIDS. J Acquir Immune Defic Syndr. 2006;41:100–106. doi: 10.1097/01.qai.0000179426.04166.12. [DOI] [PubMed] [Google Scholar]
- 45.Ullum H. Cozzi Lepri A. Victor J, et al. Production of beta-chemokines in human immunodeficiency virus (HIV) infection: evidence that high levels of macrophage inflammatory protein-1beta are associated with a decreased risk of HIV disease progression. J Infect Dis. 1998;177:331–336. doi: 10.1086/514192. [DOI] [PubMed] [Google Scholar]
- 46.Warnock JN. Daigre C. Al-Rubeai M. Introduction to viral vectors. Methods Mol Biol. 2011;737:1–25. doi: 10.1007/978-1-61779-095-9_1. [DOI] [PubMed] [Google Scholar]
- 47.Whitmore M. Li S. Huang L. LPD lipopolyplex initiates a potent cytokine response and inhibits tumor growth. Gene Ther. 1999;6:1867–1875. doi: 10.1038/sj.gt.3301026. [DOI] [PubMed] [Google Scholar]
- 48.Yan W. Chen W. Huang L. Mechanism of adjuvant activity of cationic liposome: phosphorylation of a MAP kinase, ERK and induction of chemokines. Mol Immunol. 2007;44:3672–3681. doi: 10.1016/j.molimm.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Yoo JW. Hong SW. Kim S. Lee DK. Inflammatory cytokine induction by siRNAs is cell type- and transfection reagent-specific. Biochem Biophys Res Commun. 2006;347:1053–1058. doi: 10.1016/j.bbrc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 50.Zagury D. Lachgar A. Chams V, et al. C-C chemokines, pivotal in protection against HIV type 1 infection. Proc Natl Acad Sci USA. 1998;95:3857–3861. doi: 10.1073/pnas.95.7.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zsengeller Z. Otake K. Hossain SA. Berclaz PY. Trapnell BC. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J Virol. 2000;74:9655–9667. doi: 10.1128/jvi.74.20.9655-9667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]