Abstract
On average, older people remember less and walk more slowly than do younger persons. Some researchers argue that this is due in part to a common biologic process underlying age-related declines in both physical and cognitive functioning. Only recently have longitudinal data become available for analyzing this claim. We conducted a systematic review of English-language research published between 2000 and 2011 to evaluate the relations between rates of change in physical and cognitive functioning in older cohorts. Physical functioning was assessed using objective measures: walking speed, grip strength, chair rise time, flamingo stand time, and summary measures of physical functioning. Cognition was measured using mental state examinations, fluid cognition, and diagnosis of impairment. Results depended on measurement type: Change in grip strength was more strongly correlated with mental state, while change in walking speed was more strongly correlated with change in fluid cognition. Examining physical and cognitive functioning can help clinicians and researchers to better identify individuals and groups that are aging differently and at different rates. In future research, investigators should consider the importance of identifying different patterns and rates of decline, examine relations between more diverse types of measures, and analyze the order in which age-related declines occur.
Keywords: aging, cognition, correlated change, longitudinal analysis, meta-analysis, physical functioning, systematic review
On average, older people walk more slowly, have less muscle strength, have poorer memory and reasoning abilities, and are slower to respond on speeded cognitive tasks relative to younger adults and to themselves when they were younger. Physical and cognitive functioning are both indicators of biologic aging—the progressive generalized impairment of functioning that occurs when people grow older. The aging process is often characterized by a loss of adaptive response to life challenges and an increasing vulnerability to pathology (i.e., aging-associated diseases) and functional limitations. With respect to cognition, fluid cognition (the ability to learn new processes and form new memories) has been shown to decline from midlife onwards as a result of normal aging (1, 2), while cognitive decline of functional or clinical significance (including diagnosis of dementia or Alzheimer's disease) is generally not detected until much later in life, often as a result of significant neurologic morbidity (such as cerebrovascular disease) (3). Age-related declines in physical function are also observed even in the absence of disease from midlife onwards as a result of normative age-related changes in the musculoskeletal system and other body systems on which these measures depend (4). Given that changes in cognitive and physical functioning probably result from interactions among aging and disease, their effects may not be fully distinguishable (5). As people age, some individuals experience abnormal declines in physical and cognitive functioning that increase their risk of dependence and premature death (6–9). There are, however, remarkable individual differences in rates of age-associated decline and in the age at which these declines begin to accelerate.
Although changes in physical and cognitive functioning are almost universal in older adulthood, there is debate in the literature regarding the nature and degree of interdependency across these domains and whether a common causal mechanism is responsible for declines in both. On the basis of cross-sectional evidence, Christensen et al. (10) found the link between age-related differences across physical and cognitive domains to be very strong, with the suggestion that declines in both domains are driven by a unifying process. A common causal account of aging has been that the process operates at various levels, from brain circuitry (11) and brain pathology (12) to the loss of molecular fidelity (13). However, as noted, evidence for this “common cause” hypothesis relies primarily on cross-sectional studies, with the problematic assumption that between-person age differences provide valid proxy estimates for within-person aging-related change. Highlighting the confounding created by age heterogeneity, Hofer et al. (14, 15) argued that longitudinal data are necessary for rigorously examining multivariate associations among aging-associated outcomes. To date, a relatively few studies, differing in design, measurement, and statistical analyses, have examined longitudinal associations between physical and cognitive functioning.
We systematically reviewed longitudinal studies that investigated the association between objective measures of physical and cognitive functioning in general community-dwelling population samples aged 40 years or older. In this paper, we focus on the relation between individual differences in physical and cognitive functioning both at baseline (intercepts) and over time (slopes). We performed random-effects meta-analyses of published data to obtain estimates of the interdependency between changes in physical and cognitive functioning. We also examined the degree to which sex, type of measurement (e.g., grip strength, walking speed, executive functioning, verbal memory), heterogeneity in samples (e.g., length of follow-up, age) or measurement, and geographic region modified these relations. To our knowledge, this is the first systematic review of the longitudinal association between changes in physical functioning and cognitive functioning.
MATERIALS AND METHODS
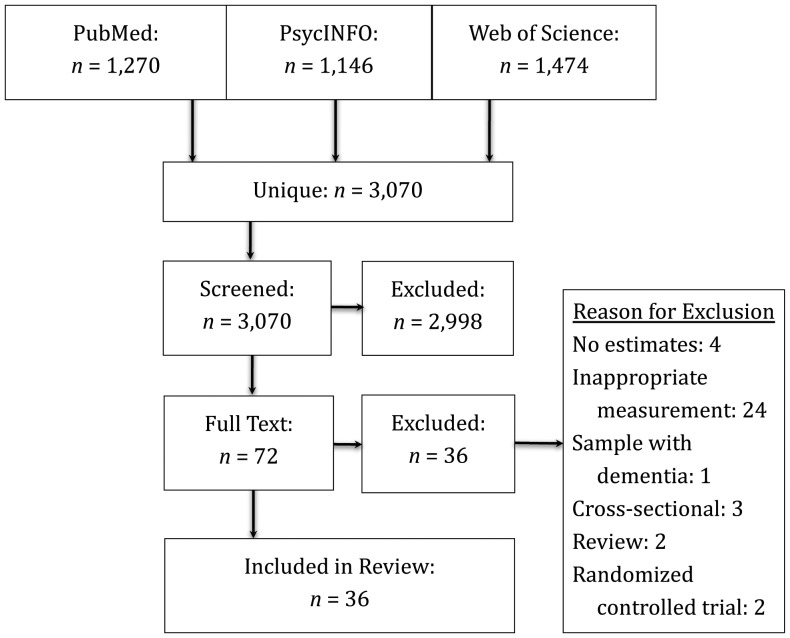
We used the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) to create the protocol for this systematic review (16). A comprehensive literature search for studies that longitudinally examined the association between physical change and cognitive change in older adulthood was performed using PubMed, Web of Science, and PsycINFO from January 2000 to October 2011 (Figure 1). This study follows up on a previous literature review (published in 2003), which highlighted the need for this form of research (14). After the previous review, the number of longitudinal studies rose substantially. The search strategy focused on 3 key elements: physical functioning, cognitive functioning, and older adult populations. Only English-language papers were reviewed.
Figure 1.
Strategy used to select recently published articles (2000–2011) for a review of the relations between rates of change in physical functioning and cognitive functioning in community-dwelling population samples aged ≥40 years.
Eligibility criteria
We included human studies that 1) used data drawn from community-dwelling population samples aged 40 years or older, 2) analyzed individual-level data, 3) included objective measurements of both physical and cognitive functioning, 4) measured explanatory factors coincident with or prior to the outcome factors, 5) reported effect estimates of the association between physical and cognitive functioning, and 6) included longitudinal information about physical functioning and/or cognition. We excluded studies in which 1) the outcome was assessed in childhood or early adulthood, 2) data were derived from animal models, 3) original data were not reported (e.g., review articles), and 4) data from clinical samples were reported. Finally, we excluded intervention studies (where the physical or cognitive variable was manipulated).
Study selection and data collection
Three authors independently reviewed the resultant list of unique citations to assess eligibility for inclusion; of the original 3,070 citations, they identified 72 candidates eligible for review. Of these, 36 were included in the final analysis, with most of the excluded studies (n = 24) having failed to use appropriate (objective) measures (Figure 1). We used a standardized form to extract data from our final sample (n = 36). Included in the form was information related to study design (waves of data collection, years between measurements, total time between baseline and final follow-up, response rate, study exclusion criteria, variables used); sample characteristics (mean sample age, sex distribution, community vs. institutional setting, geographic region); measurements (tools employed for objective measurement of physical and cognitive functioning); and statistical methods (analytical approach, covariates, effects, and fully adjusted estimates of the statistical significance and reliability of study estimates). We did not formally assess study quality because, unlike the case with randomized controlled trials, validated quality criteria were not available (17). However, in meeting our inclusion criteria, all included studies were considered to be adequately methodologically rigorous. Information was gathered from tables and figures as well as the text of manuscripts. When the reviewers disagreed with regard to the extracted models and details of performance (approximately 5% of studies at each stage of analysis), consensus was reached through discussion.
Data analysis
Three types of associations characterize most relations examined in these studies: 1) cross-sectional (between-person differences), 2) effects of baseline characteristics in one outcome on change in another (baseline effects on within-person change), and 3) change in one form of functioning regressed or correlated with change in the other (correlated within-person change). Studies not conforming to one of these 3 types tended to be unique and are discussed at greater length in the Results section. In studies modeling the relation of baseline characteristics with change (point 2 above), we employed 2 approaches: 1) using follow-up observations indicating observed declines (for which we present beta coefficients) and 2) using later diagnosis (of dementia or physical impairment) to infer decline (with results presented as hazard ratios).
A pooled estimate of the association between each measure of physical functioning and cognitive functioning was calculated using meta-analytic techniques for regression coefficients, odds ratios, and risk ratios. Estimates and 95% confidence intervals are presented. I2 was used to evaluate the percentage of total variation that was due to between-study heterogeneity. Because of small sample sizes, we used α = 0.10 to designate statistical significance. To maintain sample comparability, we reduced heterogeneity in 2 ways: first by separating fundamentally different types of analysis and then by standardizing the estimates (for which we present standardized beta coefficients).
Heterogeneity between studies may be due to variation in the explanatory or outcome measures, the age and sex of participants, the number of and time interval between follow-up assessments, geographic region, apolipoprotein E ɛ4 allele (APOE4) status, and age. When possible, we analyzed the roles of these sources of heterogeneity in explaining differences in results by means of subgroup analysis. We analyzed publication bias using funnel plots, which showed no evidence for publication bias in any of the risk estimates, intercepts, or slope estimates. All analyses were performed using Stata 11/IC (StataCorp LP, College Station, Texas); graphics were created using Excel (Microsoft Corporation, Seattle, Washington).
RESULTS
Study characteristics
The samples included in these analyses (see Table 1) varied substantially from studies with small numbers of homogeneous participants (minimum n = 60) to larger samples that were heterogeneous in terms of age, geography, genetics, and culture (maximum n = 17,333). The shortest follow-up time was 1 year, the longest 20; and the ages of the subjects ranged from 41 years to over 100 years. The samples were concentrated in the United States (n = 20) and continental Europe (n = 9), with the remaining studies observing respondents in the United Kingdom (n = 3), Canada (n = 2), Australia (n = 1), and Hong Kong (n = 1). One-fifth of the studies were sex-homogeneous, and four-fifths were age-heterogeneous (defined as an age range greater than 9 years or a standard deviation greater than 3 years). Baseline response rates ranged from 47% to 91%; however, only 41% of the published articles reported this statistic. When it was reported, retention was good, with 46% to 80% of the sample being retained between waves; however, only 24% of the articles reported this statistic. Only 22% of study investigators used methods to reduce bias from missing data; the rest either did not report how they dealt with missing data or excluded persons with missing data from the analysis. When it was discussed, most of the attrition tended to be due to mortality.
Table 1.
Characteristics of Studiesa Included in a Review of the Relations Between Rates of Change in Physical and Cognitive Functioning in Older Adults
| First Author, Year (Reference No.) | Region | Study | No. of Participants | % Male | Length of Follow-up, years | Baseline Age Range, years | Cognitive Measure | Physical Measure | Main Result |
|---|---|---|---|---|---|---|---|---|---|
| Abbott, 2004 (49) | Hawaii, United States | Honolulu Heart Program | 2,257 | 100 | 7 | 71–93 | Diagnostic criteria | Walking speed | Incidence of dementia was higher in persons who walked 10 feet (3 m) in 6 seconds or more relative to those who walked 10 feet in 3 seconds or less. |
| Aichberger, 2010 (25) | Europe (EU countries) | Survey of Health, Ageing, and Retirement in Europe | 17,333 | 46 | 2.5 | ≥50 | Fluid cognition | Grip strength | Grip strength at baseline was correlated with baseline fluid cognition but not change in fluid cognition. |
| Alfaro-Acha, 2006 (37) | Southwestern United States | Hispanic EPESE | 2,160 | 43 | 7 | ≥65 | MSEs | Grip strength | Lower grip strength predicted significant declines in mental state. |
| Alfaro-Acha, 2007 (52) | Southwestern United States | Hispanic EPESE | 2,070 | 43 | 8 | ≥65 | MSEs | Walking speed | Slow walkers were more likely to experience greater decline in mental state over 7 years, but walking speed was not correlated with mental state at baseline. |
| Atkinson, 2007 (54) | Southeastern United States | Health ABC Study | 2,341 | 48 | 3 | 65–80 | MSEs | Walking speed, grip strength, chair stands | Mental state was correlated with increased decline in walking speed. The effect was much reduced when vascular risk factors were included in the model. |
| Atkinson, 2010 (32) | United States | Women's Health Initiative Memory Study | 1,793 | 0 | 6 | 70–79 | MSEs, fluid cognition | Walking speed | Mental state and fluid cognition at baseline were associated with changes in physical functioning. Physical function at baseline was not associated with changes in cognition. Changes in cognition were associated with changes in physical functioning. |
| Auyeung, 2011 (31) | Hong Kong | Chinese University of Hong Kong Study | 2,737 | 55 | 4 | ≥65 | MSEs, diagnostic criteria | Grip strength | Weaker grip strength was correlated with declines in mental state. Muscle strength was associated with slowed global cognitive decline and decreased diagnostic risk. |
| Boyle, 2009 (18) | Midwestern United States | Rush Memory and Aging Study | 970 | 25 | 3.6 | 54–100 | Fluid cognition, diagnostic criteria | Grip strength, muscle strength | Muscle strength was associated with risk of mild cognitive impairment. |
| Buchman, 2011 (44) | Midwestern United States | Rush Memory and Aging Study | 836 | 26 | 4.5 | 54–100 | Fluid cognition | Walking speed | Fluid cognition at baseline was associated with incident mobility impairment and mobility decline. |
| Buracchio, 2010 (34) | Northwestern United States | Oregon Brain Aging Study | 204 | 42 | 19 | ≥65 | MSEs | Walking speed | Persons with incident mild cognitive impairment experienced a more rapid decline in gait speed up to 12 years prior. In contrast, declines in finger-tapping occurred after the incidence of mild cognitive impairment. |
| Charles, 2006 (28) | Hawaii, United States | Honolulu-Asia Aging Study | 3,519 | 100 | 20 | 46–68 | MSEs | Grip strength | Decline in grip strength was related to mental state at baseline. |
| Christensen, 2000 (55) | Australia | Queanbeyan Community Study | 425 | 48 | 3.5 | 70–93 | Fluid cognition, crystallized cognition | Grip strength | Grip strength at baseline did not predict rate of change in fluid cognition. Changes in grip strength did correlate moderately. |
| Deary, 2011 (30) | United Kingdom | Lothian Birth Cohort 1921 | 550 | 43 | 8 | 79 | Fluid cognition | Grip strength | Baseline fluid cognition and grip strength were correlated, but no evidence emerged regarding correlations with changes in either grip strength or cognition. |
| Deshpande, 2009 (47) | Italy | Invecchiare in Chianti Study | 660 | 47 | 3 | ≥65 | MSEs | Walking speed | Walking speed (especially "walks as fast as possible") was correlated with declines in mental state, though walking at a usual pace was not. |
| Gatz, 2010 (35) | Sweden | Swedish Adoption/Twin Study of Aging | 60 | 23 | 9 | 57–88 | Diagnostic criteria | Grip strength | Persons who developed preclinical dementia in 3 years performed worse on grip strength measures at baseline than did their twins who did not develop preclinical dementia. |
| Giltay, 2009 (24) | Europe (EU countries) | Seven Countries Study | 857 | 100 | 10 | 45–64 | Diagnostic criteria | Lung function | Midlife lung function was associated with the risk of dementia in APOE4 noncarriers but not in APOE4 carriers, where it was inversely related to risk of dementia. Lowered lung function predicted increased risk of dementia. |
| Guo, 2007 (51) | Sweden | Swedish Adoption/Twin Study of Aging | 1,291 | 0 | 29 | 44–66 | Diagnostic criteria | Lung function | Better respiratory function was associated with lower risk of dementia. |
| Inzitari, 2007 (71) | Northeastern United States | Health ABC Study | 2,276 | 47 | 5 | 70–79 | Fluid cognition | Walking speed | Gait speed at baseline predicted changes in fluid cognition. |
| Inzitari, 2007 (45) | Italy | Italian Longitudinal Study of Aging | 1,052 | 67 | 3 | 70–79 | MSE, fluid cognition | Walking speed | Fluid cognition predicted 3-year risk of decline in motor performance. |
| Kuh, 2009 (48) | United Kingdom | National Survey of Health and Development | 2,135 | 49 | 10 | 53 | Fluid cognition, crystallized cognition | Grip strength, chair stands, flamingo stands | Higher childhood cognition at age 11 years and fewer declines in fluid cognition between ages 43 and 53 years were associated with balance and chair rising speed at age 53 years. The association between fluid cognition and grip strength was inconsistent. |
| Larson, 2006 (27) | Northwestern United States | Adult Changes in Thought Study | 1,740 | 38 | 6.2 | ≥65 | Diagnostic criteria | Grip strength, walking speed, chair stands, flamingo stands | In sedentary older adults, baseline physical functioning reduced risk of subsequent dementia. The association was nonsignificant among persons who exercised more. |
| MacDonald, 2011 (72) | Canada | Victoria Longitudinal Study | 1,043 | 34 | 6 | 55–85 | Fluid cognition | Grip strength, lung function | Higher grip strength and lung function ameliorated the risk of declines in fluid cognition. |
| Marquis, 2002 (73) | Northwestern United States | Oregon Brain Aging Study | 108 | 37 | 6 | ≥65 | Diagnostic criteria | Walking speed | Walking speed predicted the onset of cognitive impairment. |
| Payette, 2011 (46) | Canada | Nutrition and Successful Aging Cohort Study | 1,741 | 48 | 3 | 68–82 | MSEs | Walking speed, flamingo stands, chair stands | Physical function declined over the years more than did mental state. Although the 2 factors declined contemporaneously, they did not accelerate together within individuals. |
| Pugh, 2007 (19) | Southwestern United States | Hispanic EPESE | 1,682 | 42 | 6 | ≥65 | MSEs | Walking speed, chair stands | Mental state at baseline predicted changes in lower extremity functioning. |
| Raji, 2002 (33) | Southwestern United States | Hispanic EPESE | 2,068 | 41 | 2 | ≥65 | MSEs | Grip strength | Mental state at baseline predicted later grip strength. |
| Raji, 2005 (21) | Southwestern United States | Hispanic EPESE | 2,381 | 43 | 8.25 | ≥65 | MSEs | Walking speed, flamingo stands, chair stands | Mental state at baseline predicted steeper declines in grip strength. |
| Richards, 2005 (29) | United Kingdom | National Survey of Health and Development | 1,778 | 50 | 10 | 53 | Fluid cognition, crystallized cognition | Lung function | Fluid cognition was correlated with baseline lung function. |
| Rivera, 2008 (22) | Northeastern United States | Women's Health and Aging Study | 474 | 0 | 1 | ≥65 | MSEs | Grip strength, walking speed | Cognitive impairment (MSE) predicted incidence of severe mobility difficulties. |
| Sattler, 2011 (26) | Germany | Interdisziplinäre Längsschnittstudie des Erwachsenenalters | 381 | 51 | 12 | 41–44; 61–64 | Fluid cognition | Grip strength, flamingo stands | Flamingo stands were correlated with baseline fluid cognition but did not predict changes in fluid cognition. |
| Soumare, 2009 (23) | France | Three-City Study | 3,769 | 38 | 7 | 65–85 | MSEs, fluid cognition | Walking speed | Baseline walking speed was correlated with cognition at baseline. Changes in walking speed were correlated with baseline cognition. |
| Tabbarah, 2002 (20) | Northeastern United States | MacArthur Research Network on Successful Aging Community Study | 488 | 42 | 7 | 70–80 | Fluid cognition | Grip strength, walking speed, chair stands, flamingo stands | Physical functioning at baseline and changes in physical functioning were correlated with fluid cognition, regardless of how fluid cognition was measured. |
| Taekema, 2010 (74) | Netherlands | Leiden 85+ Study | 555 | 35 | 4 | 85 | MSEs | Grip strength | MSE at baseline predicted baseline grip strength and changes in grip strength. |
| Wang, 2006 (50) | Northwestern United States | Adult Changes in Thought Study | 2,288 | 40 | 9 | ≥65 | Diagnostic criteria | Grip strength, walking speed, chair stands, flamingo stands | Lower levels of physical function were correlated with increases in risk of dementia and Alzheimer's disease. |
| Watson, 2010 (53) | Northeastern United States | Health ABC Study | 865 | 50 | 5 | 70–79 | Fluid cognition | Walking speed | Fluid cognition was associated with decline in gait speed. |
| Weuve, 2011 2(36) | Northeastern United States | Normative Aging Study | 864 | 100 | 12 | ≥50 | MSEs, fluid cognition | Lung function | Lung function did not correlate with declines in fluid cognition or MSEs. |
| Mean value | 1,857 | 45 | 7.41 |
Abbreviations: APOE4, apolipoprotein E ɛ4 allele; EPESE, Established Populations for the Epidemiologic Study of the Elderly; EU, European Union; Health ABC, Health, Aging and Body Composition; MSE, mental state examination.
a English-language articles published between 2000 and 2011.
Physical and cognitive functioning were examined as study outcomes and main independent variables. Most studies (78%) used cognition as the main outcome; fewer (35%) used physical functioning as an outcome of interest (14% estimated both directions). Forty-three percent of studies included some estimate of the risk of cognitive impairment (including Alzheimer's disease), while 68% estimated changes in cognition or physical functioning.
Measures of physical and cognitive functioning
Physical functioning was measured using a variety of indicators, including grip strength (51% of studies), walking speed (54%), chair stands (22%), flamingo stand times (22%), and lung function (14%). Eleven percent used composite scores, and 41% analyzed more than one measure of physical functioning—for example, muscle strength (axial strength; arm and leg abduction, flexion, and extension; grip and pinch strength (18)) or lower extremity function (flamingo stands, chair stands, and walking speed (19)). Three studies used these objective measures to code physical impairment, defined alternatively as loss of ambulation, mobility impairment, and motor performance decline.
In assessing cognition, researchers used measures of fluid cognition, measures of crystallized cognition, and mental state examinations (MSEs). In some cases, MSEs were further used in combination with administrative data to indicate mild cognitive impairment and other clinical diagnostic outcomes, including dementia or Alzheimer's disease (3). Cognition was measured using MSEs 46% of the time, fluid cognition 43% of the time, and diagnostic measures of cognition 27% of the time. A variety of tests were used as indicators of fluid cognition, including verbal recall, letter cancellation, Raven's Progressive Matrices (Harcourt Assessment, Inc., San Antonio, Texas), processing speed, executive function, etc. When measuring fluid cognition, researchers used a vast array of measures; these were included either as single indicators or as part of a global measure of cognition. Most studies included only 1 measure of fluid cognition, sometimes accompanied by a measure of mental state. Our analyses were separated by domain but not by measure within domain; thus, in cases where multiple measures were presented, we used estimates based on global measures of fluid cognition. In the single study that tested the differences between multiple indicators of fluid cognition, the authors suggested that the choice of indicator had little bearing on the associations presented (20). Studies using MSEs employed a number of tests to measure mental state, including, most often (88% of studies), the Mini-Mental State Examination (MMSE) or its modified version (the 3MS). Of studies that used diagnostic measures of cognition, 44% were based on diagnoses of Alzheimer's disease and 33% were based on clinical or research-based diagnoses of mild cognitive impairment. Clinical outcomes were often retrieved from clinical reports and administrative data. When researchers created a diagnostic measure of cognition, they used arbitrary cutpoints (e.g., MMSE score <21 (21) or MMSE score <24 (22)). Because crystallized cognition is relatively stable across the life span, we excluded it from our analysis. Studies using MSEs identified these measures as alternatively indicating global cognition (23) or cognitive impairment (24). Because of the wide range of different measures of cognition and physical functioning used in different studies and the differences between these measures, we do not provide overall estimates of physical functioning or cognition but rather provide summary estimates for the association between each measure of physical function and each measure of cognition.
Adjustment for confounding variables
The included studies adjusted their statistical models using covariates that fall broadly within 8 domains: health status (body mass, self-assessed health, depressive symptoms, blood pressure, cholesterol), behavior (smoking, alcohol intake, physical activity), clinical conditions (cardiovascular disease, diabetes, hypertension, chronic obstructive pulmonary disease), social factors (education, socioeconomic status, marital status), functioning (visual acuity, ability to stand for 15 minutes), demographic factors (age, sex), and genetic factors (APOE4 status).
Researchers highlighted a number of potential covariates with varying degrees of impact. Physical activity or exercise was significantly associated with both physical and cognitive functioning (25, 26), and it attenuated the relation between physical and cognitive functioning in one study (27). However, exposure to pesticides, solvents, and metals (28) and polypharmacy or suboptimal prescribing (19) did not attenuate the relation between grip strength and cognitive function. Two studies adjusted for childhood intelligence (measured at age 8 years in one study, using the Alice Heim general ability test (29), and at age 11 years in the other study, using the Moray House test (30)), and both found that this adjustment attenuated the association between physical and cognitive functioning in adulthood. Though results for socioeconomic position (SEP) were not often reported, adjusting for SEP attenuated estimates when it was analyzed (21, 29). Finally, including measurements of body size often attenuated the results.
Relations between physical and cognitive functioning
Cross-sectional correlations (intercepts)
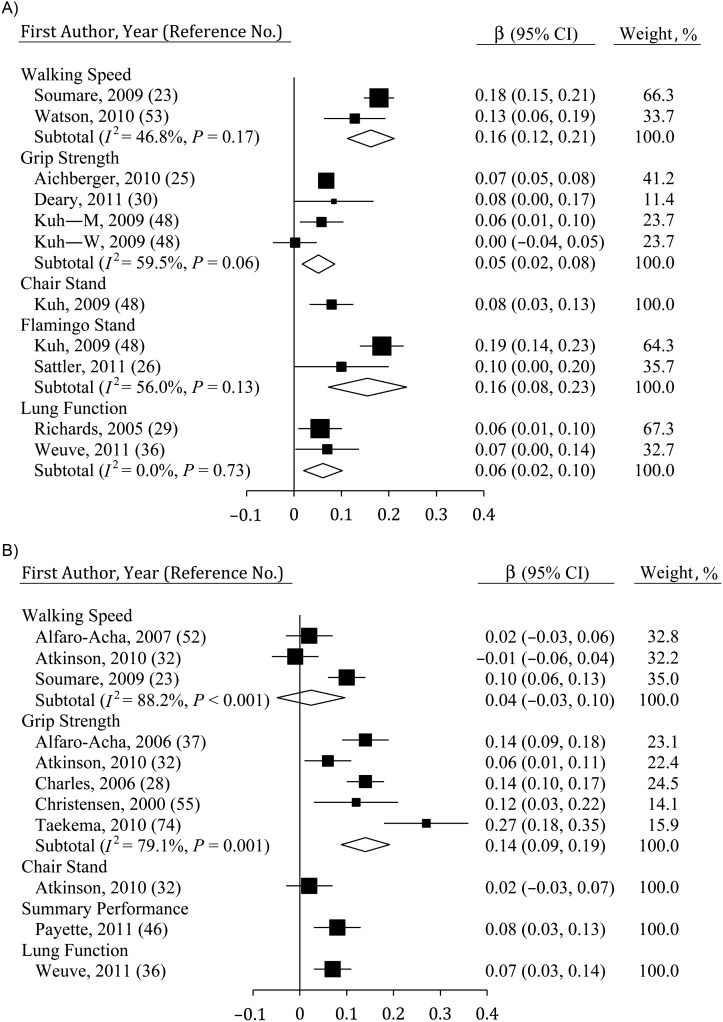
In the studies evaluated here, cross-sectional, between-person associations were evident between measures of physical function and both fluid cognition (Figure 2A) and MSE (Figure 2B). Grip strength (estimate size (β) = 0.05, 95% confidence interval (CI): 0.02, 0.08; n = 4), walking speed (β = 0.16, 95% CI: 0.12, 0.21; n = 2), flamingo stands (β = 0.16, 95% CI: 0.08, 0.23; n = 2), chair stands (β = 0.08, 95% CI: 0.03, 0.13; n = 1), and lung function (β = 0.06, 95% CI: 0.02, 0.10; n = 2) were all significantly correlated with fluid cognition at baseline. However, results for walking speed were significantly stronger than were estimates for grip strength (P < 0.05). Study heterogeneity was observed when linking grip strength to fluid cognition at baseline (I2 = 59.5%, P < 0.10), due largely to nonsignificant estimates among women in 1 study. In contrast, for MSE, a consistent relation was observed only for grip strength (β = 0.14, 95% CI: 0.09, 0.19; n = 5). Limited evidence did show a significant relation using a summary performance measure (β = 0.08, 95% CI: 0.03, 0.13; n = 1) and lung function (β = 0.07, 95% CI: 0.03, 0.14; n = 1). Study heterogeneity was significant for relations between MSE and both grip strength and walking speed (P ≤ 0.001).
Figure 2.
Average standardized regression coefficients (β) obtained when baseline physical function was regressed on cognition at baseline using A) fluid cognition and B) mental state examinations among persons aged ≥40 years, 2000–2011. Data from the study by Kuh et al. (48) were separated by sex (M, men; W, women). Individual studies are represented by squares; subtotals provide within-group variance-weighted averages and are represented by diamonds. Bars, 95% confidence interval (CI).
Effect of baseline status on within-person change
Change in cognition
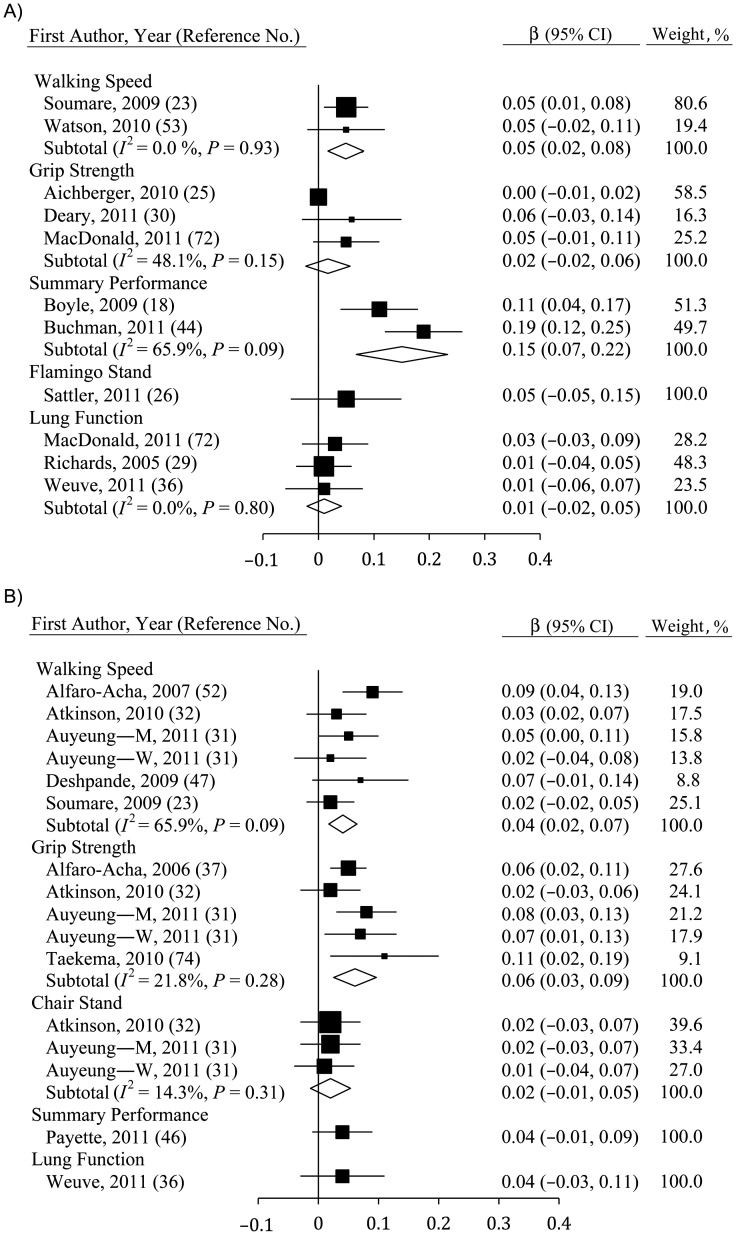
Some measures of physical function at baseline were correlated with changes in fluid cognition (Figure 3A) and MSE (Figure 3B). For fluid cognition, only walking speed (β = 0.05, 95% CI: 0.02, 0.08; n = 2) and summary performance measures (β = 0.15, 95% CI: 0.07, 0.22; n = 2) yielded a significant association. Indeed, the relation between summary performance measures and change in cognition was stronger than was any other measure of physical function (P < 0.10), though there was substantial heterogeneity in this estimate (I2 = 65.9%, P < 0.10). In contrast, effects were consistently null for grip strength, for lung function, and for flamingo stands, which were only used in 1 study (26). One study additionally showed that changes in fluid cognition were correlated with between-person differences in flamingo stands and chair stands at follow-up (31).
Figure 3.
Average standardized regression coefficients (β) for the association between change in cognition and baseline physical functioning derived using A) fluid cognition and B) mental state examinations among persons aged ≥40 years, 2000–2011. Data from the study by Auyeung et al. (32) were separated by sex (M, men; W, women). Individual studies are represented by squares; subtotals provide within-group variance-weighted averages and are represented by diamonds. Bars, 95% confidence interval (CI).
Baseline grip strength (β = 0.06, 95% CI: 0.03, 0.09; n = 5) and walking speed (β = 0.04, 95% CI: 0.02, 0.07; n = 6) were associated with declines in MSE score, though the relation with grip strength was slightly stronger (not significant). Baseline measures of chair stands, flamingo stands, summary performance measures, and lung function were not significant predictors of change in cognition over time, though small samples resulted in limited statistical power.
Change in physical functioning
Fewer studies reported analysis of the relation between baseline cognition and changes in physical function (see Figure 4A for fluid cognition, Figure 4B for MSE). However, fluid cognition was correlated with changes in walking speed (β = 0.09, 95% CI: 0.02, 0.15; n = 2) in 2 studies (20, 32). In contrast, no significant relation emerged for fluid cognition and grip strength, flamingo stands, or chair stands. Results were also mixed for MSE, which produced the sole significant result with change in a summary performance measure (33).
Figure 4.
Average standardized regression coefficients (β) for the association between change in physical function and baseline cognition derived using A) fluid cognition and B) mental state examinations among persons aged ≥40 years, 2000–2011. Individual studies are represented by squares; subtotals provide within-group variance-weighted averages and are represented by diamonds. Bars, 95% confidence interval (CI).
Models of risk at follow-up
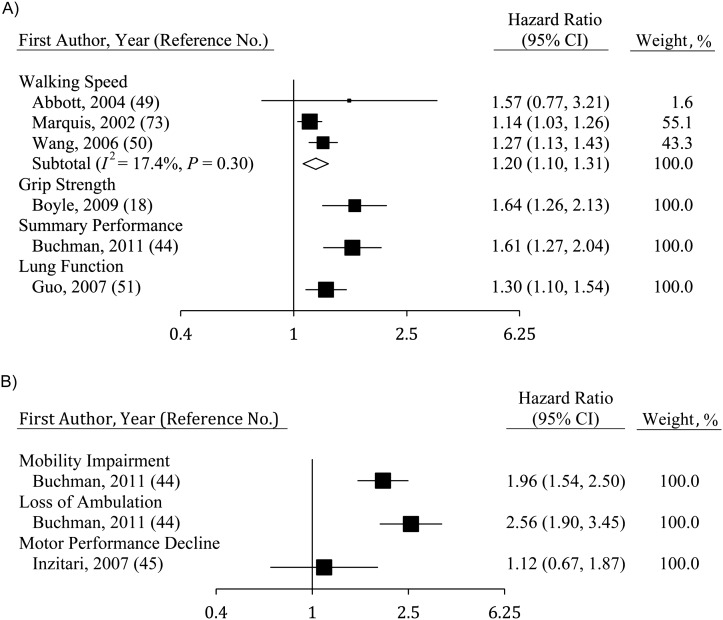
The risk of cognitive impairment (Figure 5A) was associated with walking speed (hazard ratio (HR) = 1.20, 95% CI: 1.10, 1.31; n = 3), grip strength (HR = 1.64, 95% CI: 1.26, 2.13; n = 5), a summary performance measure (HR = 1.61, 95% CI: 1.27, 2.04; n = 1), and lung function (HR = 1.30, 95% CI: 1.10, 1.54; n = 1). However, some results were not included in Figure 5A due to a lack of comparability in measures used. Analyses using the risk of impaired scores on MSE at follow-up showed much stronger relations with baseline walking speed (odds ratio (OR) = 2.32, 95% CI: 1.15, 4.90) than those described above (34). Sattler et al. (26) used odds ratios to estimate risk and found that grip strength was not related to cognitive impairment (OR = 1.00, 95% CI: 0.99, 1.01), while relations between flamingo stands and cognitive impairment were substantial (OR = 2.86, 95% CI: 1.56, 5.26). Results from Giltay et al. (24), who stratified their analyses by APOE4 status, showed that lung function predicted the onset of dementia among persons with the APOE4 allele but not among noncarriers. Finally, Gatz et al. (35), whose study was unique in its capability to control for heritability by using the Swedish Twin Registry and assessments of both monozygotic and dizygotic twins, found support for an increased risk by showing that persons with lowered grip strength developed dementia faster than their twins did.
Figure 5.
Hazard ratios for development of physical and cognitive impairment over time, estimated using baseline indicators of functioning, among persons aged ≥40 years, 2000–2011. A) Impact of physical functioning at baseline on the risk of cognitive impairment; B) impact of baseline cognition on the risk of physical impairment. Individual studies are represented by squares; the subtotal provides a within-group variance-weighted average and is represented by a diamond. Bars, 95% confidence interval (CI).
The risk of physical impairment (Figure 5B) was significantly related to lowered fluid cognition when mobility impairment (HR = 1.96, 95% CI: 1.54, 2.50) or loss of ambulation (HR = 2.56, 95% CI: 1.90, 3.45) was used to indicate physical impairment, though not when motor performance declines were used. Modifying risk factors were not assessed because of the small number of samples included.
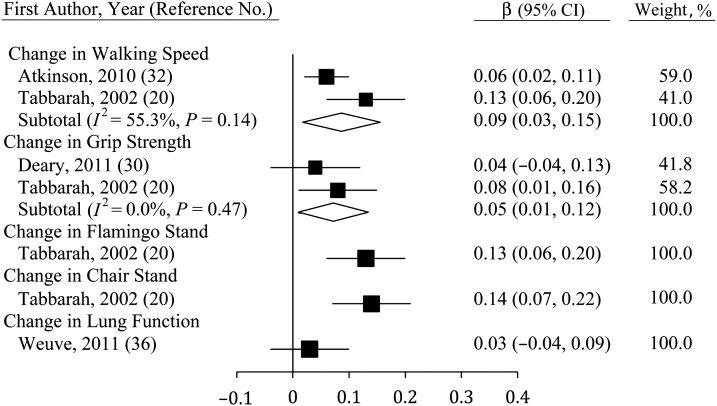
Correlating changes in physical functioning with changes in cognition
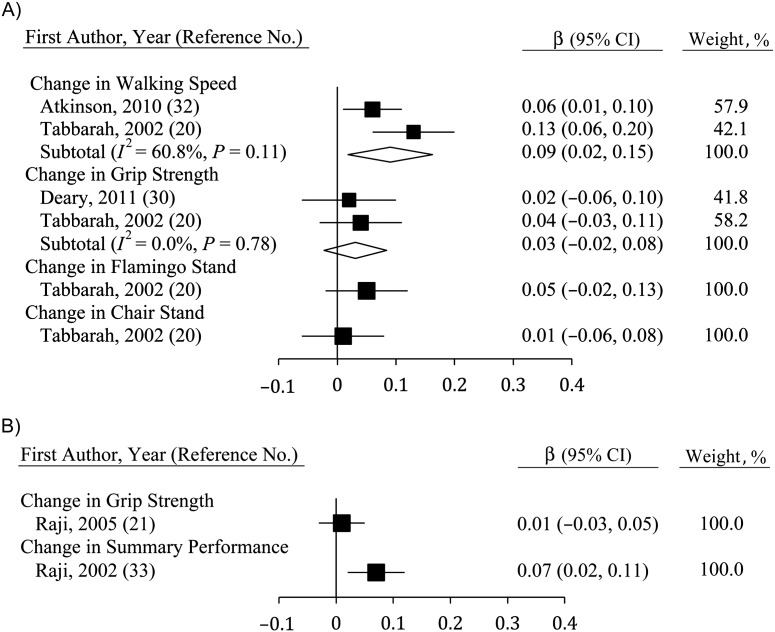
Studies correlating changes in physical function and changes in fluid cognition (Figure 6) were few but showed consistent and significant relations for walking speed (β = 0.09, 95% CI: 0.03, 0.15; n = 2) and grip strength (β = 0.05, 95% CI: 0.01, 0.12; n = 2). Single-study estimates for flamingo stands and chair stands were also significant (20), though this was not true for lung function (36). The sole study to correlate changes in lung function with changes in MSE found no significant association (36).
Figure 6.
Average standardized regression coefficients (β) for change in fluid cognition derived using change in physical function among persons aged ≥40 years, 2000–2011. Individual studies are represented by squares; subtotals provide within-group variance-weighted averages and are represented by diamonds. Bars, 95% confidence interval (CI).
Modifying factors
Two studies in our review analyzed data from male and female participants separately, noting that their associations were sufficiently different for this to be necessary (31, 37). We found that percent male, number of follow-ups, years observed, and age were not significant modifiers of any relations presented. This may have been partly due to small sample size. The one study to stratify analyses by APOE4 status revealed an interaction between APOE4 status and lung function when modeling cognitive decline (24).
DISCUSSION
In this study, we reviewed an emerging body of literature that uses longitudinal data to examine the relation between objective measures of physical and cognitive functioning in later life. Consistent with cross-sectional research, we found an association between baseline physical functioning and cognitive functioning. Physical functioning at baseline was also correlated with longitudinal changes in cognition, but the inverse correlation of baseline cognition with longitudinal changes in physical functioning was only marginally significant, perhaps because of the limited number of studies examining this association. Further, all but one of the studies reporting correlations among slopes of change in physical functioning and change in cognition found a significant association. However, we also found that the degree of change depended on the particular measures of physical and cognitive functioning: Grip strength was associated with changes in MSE, while walking speed was correlated with changes in fluid cognition. Here we highlight areas for further development and critique the methodological approaches used to date.
Possible common-cause mechanisms
There has been an increasing push to understand and identify persons at risk of decline prior to the onset of significant forms of disease (38). We found that baseline physical and cognitive functioning were correlated with poorer cognitive and physical functioning at follow-up, respectively; other researchers have suggested that both are predictors of health and mortality in later life (6–9). As such, understanding the link between physical and cognitive functioning is of substantive interest. However, we also found that not all measures of physical and cognitive functioning were equally associated. Walking requires motor coordination and balance, which are regulated by the cerebellum and are also linked to loss of strength and impaired coordination (11). Importantly, the cerebellum also connects to cortical associative areas supporting higher mental function, including the prefrontal cortex, which regulates several aspects of fluid cognitive skills. Consistent with this, evidence suggests that brain white-matter integrity, which affects connectivity between brain systems, is associated with information processing speed (12) and with balance and gait speed (39). Variations in this more general state of brain integrity may also underlie the association observed here between strength and mental state, in a manner consistent with our findings regarding the order of decline, and the clinical severity of measures. In their influential paper on the common-cause hypothesis of cognitive aging, Christensen et al. (10) focused on such “brain-aging” mechanisms and noted possible candidates, including white-matter changes and telomere shortening. Our summary results provide some support for such theses, suggesting that such commonalities may be important for some outcomes. However, our findings were not sufficiently strong (standardized beta coefficients on the order of 0.05–0.15) or consistent (some measures were associated, while others were not) to support a common-cause argument (40).
Operationalization and measurement
There were differences between studies in the way that measures of physical functioning were used and in the theoretical constructs they were thought to measure. For example, we excluded some studies that used measures of physical functioning in combination with self-reported measures of perceived frailty (e.g., weight loss, exhaustion, weakness) as indicators of “frailty” (41–43). There were also inconsistencies in how objective measures of physical functioning were operationalized. Different measures of physical functioning were combined to create scales that indicated lower extremity functioning (19), muscular strength (18), mobility (42, 44, 45), or overall performance (33, 46). This is potentially problematic, because our findings suggested that measures of physical functioning differ in their sensitivity to cognitive change, a result that many investigators acknowledged. For instance, Deshpande et al. (47) argued that walking as quickly as possible is more strongly correlated with cognition than is walking at a usual or comfortable speed. Kuh et al. provided a possible explanation for differences in the associations of cognition with different measures of physical functioning, by suggesting that the “differential patterns of association found are consistent with the degree to which each is dependent on central nervous system function” (48, p. 38).
The included studies used a heterogeneous set of cognitive tests that bridged different cognitive domains. For the majority of studies (89%), measures of absolute decline were used, although for a substantial minority, rates of onset of postdecline diagnosis (for instance, a diagnosis of dementia for a sample of nondemented persons) were used in conjunction with or instead of cognition (27%). These usually included risk of mild cognitive impairment (18, 26), dementia (24, 49, 50), and Alzheimer's disease (18, 42, 51), though some studies used hazard models to predict risk of poor functioning defined as lower scores for fluid cognition (36) or MSE (34).
In all cases, measures of physical and cognitive functioning were correlated, though the strength and consistency of those relations depended on the particular measures used. Many studies used an indicator of MSE, such as the MMSE (21, 37, 45, 47, 52), while some provided estimates for general fluid cognition (41, 44, 53) and in some cases for cognitive domains, including memory (25, 29, 41, 53), fluency (23, 25), and executive functioning (23, 53, 54). In a few cases, analyses included a measure of crystallized intelligence (29, 31, 55). While we were unable to differentiate between different domains of fluid cognition, Tabbarah et al. (20) made a convincing argument that different domains of fluid cognition are not substantively distinct.
Potential limitations
This review had some potential limitations that may affect the generalizability of our results. For example, we limited our review to studies published between January 2000 and October 2011. We did not review studies published prior to 2000 to avoid redundancy, as this literature has been examined previously (14) and because the number of studies examining the longitudinal relation between physical and cognitive change, virtually nonexistent prior to 2003, increased substantially after 2005. We also included only studies written in English. By replicating our searches without this language restriction, we estimated that this restriction was unlikely to have reduced the total number of studies identified by more than 5%. While this is likely to have been a conservative estimate (since removing the language restriction enabled us to identify papers in other languages only if they gave key words or an abstract in English, given that our search terms were in English), we do not believe that this restriction will have introduced significant bias.
We used fluid cognition, MSE, and diagnostic measures of cognition to characterize different measures of cognition, although measures used to indicate any of these varied between studies. At least one study characterizing the differences between specific indicators noted no difference in the estimates using various indicators of fluid cognition (20). Moreover, others alternatively called MSE an indicator of global cognition (23) and an indicator of impairment (24). Finally, our use of diagnostic measures of cognition as an outcome for cognitive change involved collapsing across several distinct diagnostic outcomes (e.g., unspecified dementia, mild cognitive impairment, Alzheimer's disease) that were used in the reviewed studies. This heterogeneity may have introduced some error into our estimates for studies using diagnostic measures of cognition as their outcome; however, our results did not depend on which diagnosis was used. Despite these potential limitations, analyses by subtype of measures within the general cognitive concepts (diagnostic measures of cognition, MSE, and fluid cognition) provided substantively similar results, and thus we felt that these groupings were acceptable for our purposes.
We were unable to examine whether physical functioning declined first or cognition declined first, because of insufficient data. In our review, most studies viewed cognition as the outcome, while others used physical functioning. However, Kuh et al. (48) found that midlife changes in fluid cognition predicted levels of physical performance 10 years after onset of cognitive impairment, and Buracchio et al. (34) found that walking speed declined 12 years before onset of cognitive impairment. We found that walking speed was more strongly and consistently correlated with fluid cognition (which declines as early as midlife (1, 2)) than with MSE (which declines much later (3)), while grip strength was more strongly and consistently correlated with MSE than with fluid cognition. Such a result might suggest that measures are differently susceptible to change in a way that might depend on age. Further longitudinal analysis using repeat measurements of physical and cognitive functioning is necessary to unravel such complex relations.
Finally, the relatively small number of studies investigating any one specific relation, due in part to the diversity of measures used, was a limitation of our analyses. Thus, we were often unable to pursue analysis of potential modifying factors because of limited statistical power. This highlights the importance of trying to standardize the analyses undertaken in future studies so that results are more comparable.
Relevance to public health
There is a growing body of literature examining the impact of physical exercise interventions on ameliorating cognitive decline in persons with cognitive impairment and examining the impact of cognitive training for reducing fall risk (56). The results of this literature broadly support the effectiveness of physical intervention for improving cognitive function and the effectiveness of cognitive intervention for improving aspects of physical functioning. However, in addition to confirming the longitudinal interrelation of physical and cognitive change, our results suggest that interventions may vary in their capacity to effect change depending on the outcomes used to measure effectiveness. Some authors support this differential view, noting that involvement in resistance training may provide stronger benefit to fluid cognition than involvement in balance interventions (57).
Methodological considerations
The majority of studies used age-heterogeneous samples, which can be problematic for a number of reasons. In particular, when not carefully analyzed, age-heterogeneous samples can increase bias in results by conflating between-person age differences with within-person change when models are misspecified (58, 59). Additionally, because of systemic changes related to morbidity and mortality, it is important to consider health and terminal decline processes in understanding the effects of particular biologic and health-related changes on physical and cognitive functioning. With measures of physical functioning, such as grip strength, there are additional problems because grip strength varies interactively by age and sex (4, 60, 61); thus, simply adjusting for age and sex may not be sufficient. Similarly, the secular increases in both height and childhood cognition across birth cohorts are likely to cause substantial bias in the intercepts and possibly in the slopes. Since the findings on within-person change presented here were significant on average, this is unlikely to have modified the results shown. However, in future research, investigators should consider the role of secular differences in birth cohorts when considering the relations between physical and cognitive functioning.
Mediating factors
Many of the reviewed studies included adjustment for SEP, along with demographic and health factors that are strongly correlated with SEP at baseline. There were sex differences in the relation between grip strength and fluid cognition at midlife in one case where it was explicitly analyzed (31). When indicators of individual SEP were included, relations were often not reported, though when reported the addition of SEP to models attenuated results. Parental SEP is known to predict both physical and cognitive development (62). Moreover, a person's own SEP is strongly correlated with cognitive reserve (63) and physical functioning in later life (64, 65). However, we also found that 2 studies including measures of childhood cognition found no results (29, 30), consistent with evidence that SEP is associated with prior cognition. Better conceptualization of the fundamental role that SEP plays in late-life physical and cognitive functioning may help us understand why some people experience faster declines in cognition and physical function than do others.
Depression is correlated with cognitive decline and cognitive impairment in later life (66) and has also been shown to be associated with physical functioning (67). In our review, 11 studies adjusted for depression. Depression was usually measured with the Centers for Epidemiologic Studies Depression Scale, which was sometimes dichotomized (37, 52). Depression was rarely considered in studies using physical function as the outcome, and when included these results were generally not discussed (53). Nevertheless, when they were included in Atkinson et al.'s analyses of cognition, “depressive symptoms had the greatest impact on reducing the association between [cognition] and gait speed decline” (54, p. 848). Depression may play a complex role in aging, acting as both an outcome of physical and cognitive aging and a cause of declines in capability. Future research should include investigation of the role that depression plays in linking physical functioning to cognition and in hastening age-related decline.
Risky health behaviors (excessive alcohol intake, low physical activity, high body mass, and smoking) provide a mechanism through which individuals may modify their own risk of age-related decline across multiple domains of functioning. Seventeen studies included some analysis of health behaviors and generally found mixed results. Alcohol intake was not correlated with worse performance (20, 30). Results for physical activity were mixed, though generally suggestive that physical activity plays a role in explaining the relation between cognitive functioning and physical functioning (25, 44, 46). Though body mass was examined in 17 studies, it was not found to play a consistent role in explaining associations between cognition and physical functioning. Finally, results for smoking were overwhelmingly negative (18, 36, 44, 51) or were not reported (46, 47, 54). In future research, investigators may consider the role that smoking plays in relation to physical activity and the overall role of a healthy lifestyle, combining information across multiple domains. Our results might provide support for the view that some common processes of oxidative and nitrosative stress (13) and changes in vulnerability to pathology (40), in conjunction with external factors such as smoking (68), may increase the risk of declines in both physical and cognitive functioning.
Recommendations for future analysis
We have identified several substantial gaps in this literature on the association between age-associated physical functioning and cognitive functioning related to measurement, gaps in analysis, reporting of results, and statistical analysis. First, few studies considered potential explanatory variables that might account for joint change in physical and cognitive functioning. Similarly, few studies included analysis of lung function, chair rises, or flamingo stands. Finally, we found little discussion of the order of declines, with only 1 study explicitly evaluating whether changes in the rate of decline in physical function preceded future cognitive impairment (34). Investigators should seek to incorporate more repeat measures of physical and cognitive functioning in order to identify which measures change first. The relations identified here were not consistent. Researchers should consider the possibility that common declines occur as a result of external factors that hasten aging (68). Finally, studies should incorporate a wider range of physical and cognitive measures. In many studies, single measures of cognition (e.g., the MMSE) or physical function (e.g., grip strength) are used; however, future research will require repeated measurement across multiple domains of physical and cognitive functioning to analyze these complex relations.
Other factors remain important when completing future research. Consistency is important when reporting information about data and their analyses. For instance, a number of studies only included P values or characterized their estimates as nonsignificant. Similarly, authors rarely reported estimates of their response rates at baseline or their rates of attrition from these effective samples afterwards. Better cognizance about statements detailing reporting standards for systematic reviews, including the STROBE statement (69), may be helpful when reporting results from single-sample analyses. Finally, analytic methods for understanding longitudinal data have progressed significantly. These methods can be used to correct for bias resulting from incomplete observations (due to attrition or mortality selection), permit models based on alternative temporal axes (e.g., age, birth cohort, proximity to death), and can be used for fitting alternative models of change (random change-points, mixed declines). Such methods (including mixed-effects regression and structural equation modeling) are necessary for separating between-person differences from within-person changes in the analysis of longitudinal studies, especially when data include multiple waves of age-heterogeneous data (81% of our studies) (70).
Conclusion
Staying alert and keeping physically able are important for healthy aging and maintenance of functional independence. As the global population ages and health-care cost projections increase, there is keen interest in maximizing years of health and minimizing years of disability for older adults. Cross-sectional evidence for a strong association between age-related differences in physical and cognitive functioning prompted a “common cause” theory of aging, positing a single aging process that manifests in joint within-individual declines in physical and cognitive ability. This systematic review provides an evaluation of the evidence from longitudinal studies, permitting testing of the link between physical and cognitive functioning without the fundamental confounds of cross-sectional studies. In general, we found evidence that changes in physical and cognitive functioning are associated, but these associations were not sufficiently strong and consistent to provide conclusive evidence for “common cause” aging. Not all declines occurred together, and not all measures had equal impact. Future studies that delineate the causal order of physical and cognitive change by examining in greater detail the individual rates of change and variation and the temporal ordering of individual changes in the acceleration of physical and cognitive change using appropriate measures will contribute to the advancement of current knowledge related to trajectories of healthy and pathologic aging.
ACKNOWLEDGMENTS
Author affiliations: Laboratory for Integrative Lifespan Research, Department of Psychology, Faculty of Social Sciences, University of Victoria, Victoria, British Columbia, Canada (Sean Clouston, Scott M. Hofer, Paul Brewster); MRC Unit for Lifelong Health and Ageing, London, United Kingdom (Diana Kuh, Marcus Richards, Rebecca Hardy, Rachel Cooper, Sean Clouston); MRC National Survey of Health and Development, Institute of Epidemiology and Health Care, University College London, London, United Kingdom (Diana Kuh, Marcus Richards, Rachel Cooper, Rebecca Hardy); and Section of Social and Behavioral Sciences, College of Dental Medicine, Columbia University, New York, New York (Marcie S. Rubin).
This work was supported by the Medical Research Council (grant U123092724; Diana Kuh, Marcus Richards, Rebecca Hardy, and Rachel Cooper); by the Canadian Institutes of Health Research—Institute of Aging (grant CUK103284; Sean Clouston, Scott Hofer, and Marcie Rubin); by doctoral funding from the Canadian Institutes of Health Research (Paul Brewster); by New Dynamics of Ageing, a United Kingdom cross-council research program (HALCyon grant RES-353-25-0001; Rachel Cooper); and by grants for the Integrative Analysis of Longitudinal Studies on Aging from the US National Institute on Aging (grant AG026453; Scott Hofer).
Conflict of interest: none declared.
REFERENCES
- 1.Singh-Manoux A, Kivimaki M, Glymour MM, et al. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. Br Med J. 2012;344(d7622):1–8. doi: 10.1136/bmj.d7622. doi:10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards M, Shipley B, Fuhrer R, et al. Cognitive ability in childhood and cognitive decline in mid-life: longitudinal birth cohort study. Br Med J. 2004;328(7439):552–557. doi: 10.1136/bmj.37972.513819.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry. 2010;25(2):111–120. doi: 10.1002/gps.2306. [DOI] [PubMed] [Google Scholar]
- 4.Cooper R, Hardy R, Sayer AA, et al. Age and gender differences in physical capability levels from mid-life onwards: the harmonisation and meta-analysis of data from eight UK cohort studies. PLoS One. 2011;6(11):1–14. doi: 10.1371/journal.pone.0027899. doi:10.1371/journal.pone.0027899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumenthal HT. The aging-disease dichotomy: true or false? J Gerontol A Biol Sci Med Sci. 2003;58(2):138–145. doi: 10.1093/gerona/58.2.m138. [DOI] [PubMed] [Google Scholar]
- 6.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. Br Med J. 2010;341:c4467. doi: 10.1136/bmj.c4467. http://dx.doi.org/10.1136/bmj.c4467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40(1):14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewey ME, Saz P. Dementia, cognitive impairment and mortality in persons aged 65 and over living in the community: a systematic review of the literature. Int J Geriatr Psychiatry. 2001;16(8):751–761. doi: 10.1002/gps.397. [DOI] [PubMed] [Google Scholar]
- 10.Christensen H, Mackinnon A, Korten A, et al. The “common cause hypothesis” of cognitive aging: evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol Aging. 2001;16(4):588–599. doi: 10.1037//0882-7974.16.4.588. [DOI] [PubMed] [Google Scholar]
- 11.Baillieux H, Smet HJD, Paquier PF, et al. Cerebellar neurocognition: insights into the bottom of the brain. Clin Neurol Neurosurg. 2008;110(8):763–773. doi: 10.1016/j.clineuro.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Penke L, Maniega SM, Murray C, et al. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci. 2010;30(22):7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farooqui T, Farooqui AA. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev. 2009;130(4):203–215. doi: 10.1016/j.mad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Hofer SM, Berg S, Era P. Evaluating the interdependence of aging-related changes in visual and auditory acuity, balance, and cognitive functioning. Psychol Aging. 2003;18(2):285–305. doi: 10.1037/0882-7974.18.2.285. [DOI] [PubMed] [Google Scholar]
- 15.Hofer SM, Flaherty BP, Hoffman L. Cross-sectional analysis of time-dependent data: mean-induced association in age-heterogeneous samples and an alternative method based on sequential narrow age-cohort samples. Multivariate Behav Res. 2006;41(2):165–187. doi: 10.1207/s15327906mbr4102_4. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):1–6. doi: 10.1371/journal.pmed.1000097. doi:10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jüni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282(11):1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 18.Boyle PA, Buchman AS, Wilson RS, et al. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66(11):1339–1344. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pugh MJ, Palmer RF, Parchman ML, et al. Association of suboptimal prescribing and change in lower extremity physical function over time. Gerontology. 2007;53(6):445–453. doi: 10.1159/000119460. [DOI] [PubMed] [Google Scholar]
- 20.Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2002;57(4):M228–M235. doi: 10.1093/gerona/57.4.m228. [DOI] [PubMed] [Google Scholar]
- 21.Raji MA, Kuo Y-F, Snih SA, et al. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc. 2005;53(9):1462–1468. doi: 10.1111/j.1532-5415.2005.53457.x. [DOI] [PubMed] [Google Scholar]
- 22.Rivera JA, Fried LP, Weiss CO, et al. At the tipping point: predicting severe mobility difficulty in vulnerable older women. J Am Geriatr Soc. 2008;56(8):1417–1423. doi: 10.1111/j.1532-5415.2008.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soumare A, Tavernier B, Alperovitch A, et al. A cross-sectional and longitudinal study of the relationship between walking speed and cognitive function in community-dwelling elderly people. J Gerontol A Biol Sci Med Sci. 2009;64(10):1058–1065. doi: 10.1093/gerona/glp077. [DOI] [PubMed] [Google Scholar]
- 24.Giltay EJ, Nissinen A, Giampaoli S, et al. Apolipoprotein E genotype modifies the association between midlife lung function and cognitive function. Dement Geriatr Cogn Disord. 2009;28(5):433–441. doi: 10.1159/000255600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aichberger MC, Busch MA, Reischies FM, et al. Effect of physical inactivity on cognitive performance after 2.5 years of follow-up: longitudinal results from the Survey of Health, Ageing, and Retirement (SHARE) J Gerontopsychol Geriatr Psychiatry. 2010;23(1):7–15. [Google Scholar]
- 26.Sattler C, Erickson KI, Toro P, et al. Physical fitness as a protective factor for cognitive impairment in a prospective population-based study in Germany. J Alzheimers Dis. 2011;26(4):709–718. doi: 10.3233/JAD-2011-110548. [DOI] [PubMed] [Google Scholar]
- 27.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144(2):73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 28.Charles LE, Burchfiel CM, Fekedulegn D, et al. Occupational and other risk factors for hand-grip strength: the Honolulu-Asia Aging Study. Occup Environ Med. 2006;63(12):820–827. doi: 10.1136/oem.2006.027813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards M, Strachan D, Hardy R, et al. Lung function and cognitive ability in a longitudinal birth cohort study. Psychosom Med. 2005;67(4):602–608. doi: 10.1097/01.psy.0000170337.51848.68. [DOI] [PubMed] [Google Scholar]
- 30.Deary I, Johnson W, Gow A, et al. Losing one's grip: a bivariate growth curve model of grip strength and nonverbal reasoning from age 79 to 87 years in the Lothian Birth Cohort 1921. J Gerontol B Psychol Sci Soc Sci. 2011;66(6):699–707. doi: 10.1093/geronb/gbr059. [DOI] [PubMed] [Google Scholar]
- 31.Auyeung T, Lee J, Kwok T, et al. Physical frailty predicts future cognitive decline—a four-year prospective study in 2737 cognitively normal older adults. J Nutr Health Aging. 2011;15(8):690–694. doi: 10.1007/s12603-011-0110-9. [DOI] [PubMed] [Google Scholar]
- 32.Atkinson HH, Rapp SR, Williamson JD, et al. The relationship between cognitive function and physical performance in older women: results from the Women's Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2010;65(3):300–306. doi: 10.1093/gerona/glp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raji MA, Ostir GV, Markides KS, et al. The interaction of cognitive and emotional status on subsequent physical functioning in older Mexican Americans: findings from the Hispanic Established Population for the Epidemiologic Study of the Elderly. J Gerontol A Biol Sci Med Sci. 2002;57(10):M678–M682. doi: 10.1093/gerona/57.10.m678. [DOI] [PubMed] [Google Scholar]
- 34.Buracchio T, Dodge HH, Howieson D, et al. The trajectory of gait speed preceding MCI. Arch Neurol. 2010;67(8):980–986. doi: 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gatz M, Reynolds CA, Finkel D, et al. Dementia in Swedish twins: predicting incident cases. Behav Genet. 2010;40(6):768–775. doi: 10.1007/s10519-010-9407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weuve J, Glymour MM, Hu H, et al. Forced expiratory volume in 1 second and cognitive aging in men. J Am Geriatr Soc. 2011;59(7):1283–1292. doi: 10.1111/j.1532-5415.2011.03487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alfaro-Acha A, Snih SA, Raji MA, et al. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2006;61(8):859–865. doi: 10.1093/gerona/61.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bain LJ, Barker W, Loewenstein DA, et al. Towards an earlier diagnosis of Alzheimer disease (Proceedings of the 5th MCI Symposium, 2007) Alzheimer Dis Assoc Disord. 2008;22(2):99–110. doi: 10.1097/WAD.0b013e3181630b93. [DOI] [PubMed] [Google Scholar]
- 39.Starr JM, Leaper S, Murray A, et al. Brain white matter lesions detected by magnetic resonance imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74(1):94–98. doi: 10.1136/jnnp.74.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayflick L. The not-so-close relationship between biological aging and age-associated pathologies in humans. J Gerontol A Biol Sci Med Sci. 2004;59(6):B547–B550. doi: 10.1093/gerona/59.6.b547. [DOI] [PubMed] [Google Scholar]
- 41.Boyle PA, Buchman AS, Wilson RS, et al. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J Am Geriatr Soc. 2010;58(2):248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchman AS, Leurgans SE, Boyle PA, et al. Combinations of motor measures more strongly predict adverse health outcomes in old age: the Rush Memory and Aging Project, a community-based cohort study. BMC Med. 2011;9(42):1–11. doi: 10.1186/1741-7015-9-42. doi:10.1186/1741-7015-1189-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raji MA, Al Snih S, Ostir GV, et al. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010;65(11):1228–1234. doi: 10.1093/gerona/glq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchman AS, Boyle PA, Leurgans SE, et al. Cognitive function is associated with the development of mobility impairments in community-dwelling elders. Am J Geriatr Psychiatry. 2011;19(6):571–580. doi: 10.1097/JGP.0b013e3181ef7a2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inzitari M, Baldereschi M, Di Carlo A, et al. Impaired attention predicts motor performance decline in older community-dwellers with normal baseline mobility: results from the Italian Longitudinal Study on Aging (ILSA) J Gerontol A Biol Sci Med Sci. 2007;62(8):837–843. doi: 10.1093/gerona/62.8.837. [DOI] [PubMed] [Google Scholar]
- 46.Payette H, Gueye NR, Gaudreau P, et al. Trajectories of physical function decline and psychological functioning: the Quebec Longitudinal Study on Nutrition and Successful Aging (NuAge) J Gerontol B Psychol Sci Soc Sci. 2011;66(S1):i82–i90. doi: 10.1093/geronb/gbq085. [DOI] [PubMed] [Google Scholar]
- 47.Deshpande N, Metter EJ, Bandinelli S, et al. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009;38(5):509–514. doi: 10.1093/ageing/afp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuh D, Cooper R, Hardy R, et al. Lifetime cognitive performance is associated with midlife physical performance in a prospective national birth cohort study. Psychosom Med. 2009;71(1):38–48. doi: 10.1097/PSY.0b013e31818a1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbott RD, White LR, Ross GW, et al. Walking and dementia in physically capable elderly men. JAMA. 2004;292(12):1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Larson EB, Bowen JD, et al. Performance-based physical function and future dementia in older people. Arch Intern Med. 2006;166(10):1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 51.Guo X, Waern M, Sjögren K, et al. Midlife respiratory function and incidence of Alzheimer's disease: a 29-year longitudinal study in women. Neurobiol Aging. 2007;28(3):343–350. doi: 10.1016/j.neurobiolaging.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Alfaro-Acha A, Al Snih S, Raji MA, et al. Does 8-foot walk time predict cognitive decline in older Mexican Americans? J Am Geriatr Soc. 2007;55(2):245–251. doi: 10.1111/j.1532-5415.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 53.Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65(10):1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2007;62(8):844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 55.Christensen H, Korten AE, Mackinnon AJ, et al. Are changes in sensory disability, reaction time, and grip strength associated with changes in memory and crystallized intelligence? Gerontology. 2000;46(5):276–292. doi: 10.1159/000022172. [DOI] [PubMed] [Google Scholar]
- 56.Angevaren M, Aufdemkampe G, Verhaar H, et al. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;16(3) doi: 10.1002/14651858.CD005381.pub3. doi:10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 57.Liu-Ambrose T, Nagamatsu LS, Graf P, et al. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170(2):1–8. doi: 10.1001/archinternmed.2009.494. doi:10.1186/1471-2318-1110-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piccinin AM, Muñiz G, Matthews FE, et al. Terminal decline from within- and between-person perspectives, accounting for incident dementia. J Gerontol B Psychol Sci Soc Sci. 2011;66(4):391–401. doi: 10.1093/geronb/gbr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sliwinski M, Hoffman L, Hofer SM. Evaluating convergence of within-person change and between-person age differences in age-heterogeneous longitudinal studies. Res Hum Dev. 2010;7(1):45–60. doi: 10.1080/15427600903578169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andersen-Ranberg K, Petersen I, Frederiksen H, et al. Cross-national differences in grip strength among 50+ year-old Europeans: results from the SHARE study. Eur J Ageing. 2009;6(3):227–236. doi: 10.1007/s10433-009-0128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Werle S, Goldhahn J, Drerup S, et al. Age- and gender-specific normative data of grip and pinch strength in a healthy adult Swiss population. J Hand Surg Eur Vol. 2009;34(1):76–84. doi: 10.1177/1753193408096763. [DOI] [PubMed] [Google Scholar]
- 62.Currie J. Healthy, wealthy, and wise: socioeconomic status, poor health in childhood, and human capital development. J Econ Lit. 2009;47(1):87–122. [Google Scholar]
- 63.Richards M, Sacker A. Lifetime antecedents of cognitive reserve. J Clin Exp Neuropsychol. 2003;25(5):614–624. doi: 10.1076/jcen.25.5.614.14581. [DOI] [PubMed] [Google Scholar]
- 64.Hegewald MJ, Crapo RO. Socioeconomic status and lung function. Chest. 2007;132(5):1608–1614. doi: 10.1378/chest.07-1405. [DOI] [PubMed] [Google Scholar]
- 65.Brunner E, Shipley M, Spencer V, et al. Social inequality in walking speed in early old age in the Whitehall II study. J Gerontol A Biol Sci Med Sci. 2009;64(10):1082–1089. doi: 10.1093/gerona/glp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steffens D, Otey E, Alexopoulos G, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch Gen Psychiatry. 2006;63(2):130–138. doi: 10.1001/archpsyc.63.2.130. [DOI] [PubMed] [Google Scholar]
- 67.Buchner DM, Cress ME, Esselman PC, et al. Factors associated with changes in gait speed in older adults. J Gerontol A Biol Sci Med Sci. 1996;51(6):M297–M302. doi: 10.1093/gerona/51a.6.m297. [DOI] [PubMed] [Google Scholar]
- 68.Atkinson HH, Cesari M, Kritchevsky SB, et al. Predictors of combined cognitive and physical decline. J Am Geriatr Soc. 2005;53(7):1197–1202. doi: 10.1111/j.1532-5415.2005.53362.x. [DOI] [PubMed] [Google Scholar]
- 69.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85(11):867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurland BF, Johnson LL, Egleston BL, et al. Longitudinal data with follow-up truncated by death: match the analysis method to research aims. Stat Sci. 2009;24(2):211–222. doi: 10.1214/09-STS293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the Health Aging and Body Composition Study. Neuroepidemiology. 2007;29(3-4):156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacDonald SWS, DeCarlo CA, Dixon RA. Linking biological and cognitive aging: toward improving characterizations of developmental time. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i59–i70. doi: 10.1093/geronb/gbr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59(4):601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 74.Taekema DG, Gussekloo J, Maier AB, et al. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39(3):331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]