Abstract
Cardiovascular disease (CVD) and type 2 diabetes mellitus have their roots in childhood, particularly in obese children and adolescents, raising important opportunities for early lifestyle intervention in at-risk individuals. However, not all obese individuals are at the same risk for disease progression. Accurate screening of obese adolescents may identify those in greatest need for intensive intervention to prevent or delay future disease. One potential screening target is obesity-related inflammation, which contributes to insulin resistance, metabolic syndrome, and CVD. In adults, the inflammatory marker high-sensitivity C-reactive protein (hsCRP) has utility for risk stratification and treatment initiation in individuals of intermediate CVD risk. In adolescents, hsCRP shares many of the associations of hsCRP in adults regarding the degree of insulin resistance, metabolic syndrome, and carotid artery media thickness. However, long-term data linking increased hsCRP levels—and increased insulin or decreased adiponectin—in childhood to adult disease outcomes are lacking at this time. Future efforts continue to be needed to identify childhood clinical and laboratory characteristics that could be used as screening tests to predict adult disease progression. Such tests may have utility in motivating physicians and patients' families toward lifestyle changes, ultimately improving prevention efforts.
Keywords: Obesity, Metabolic syndrome, Insulin resistance, Risk, Adolescents, Inflammation, High-sensitivity C-reactive protein, Adiponectin
Introduction
The increasing incidence of cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) worldwide has raised interest in means of early identification of risk and preventative treatment in those with multiple risk factors [1]. A major factor in the increase in these adult chronic diseases has been the obesity epidemic: compared with those of normal weight, adults who are obese have a 50% to 75% increased risk of CVD over 3- to 14-y periods [2], whereas obesity starting in childhood carries a higher risk of adult CVD and T2DM [3]. These risks make childhood obesity a logical target for intervention. Nevertheless, not all obese individuals carry the same risk for future disease, which is encapsulated in the concept of the “healthy obese,” a subset of obese children and adults who do not exhibit increases in CVD risk factors [4,5]. In addition, outside of intensive lifestyle interventions as part of research protocols, attempts at weight loss for children and adolescents in clinical settings have been modest [6–8]. This raises a question about the need for tools to identify risk for future disease—tools that could be used to trigger more intensive intervention in a subset of children at higher risk.
Currently, there are few evidence-based tools available for use in risk stratification in pediatrics. Such tools could end up being factors related to the underlying processes that connect obesity to future disease. One set of such processes is the metabolic syndrome (MetS), a cluster of CV factors, including central obesity (as assessed by increases in waist circumference), hypertension, increased fasting blood glucose, high levels of triacylglycerols, and low high-density lipoprotein cholesterol [9–11]. MetS itself represents a potential screening tool for risk in adolescents [12] but is not widely used clinically, likely because of its complexity of use, requiring that patients have increases above specific cutoff values in at least three of the five components of MetS [13]. In this sense—if possible—a single blood test would likely represent an easier clinical tool. A further drawback is that MetS exhibits racial/ethnic differences in its accuracy [14].
Another important target for risk-related screening is obesity-related inflammation. One inflammation-associated screening tool that is used for risk stratification in adults is high-sensitivity C-reactive protein (hsCRP). Higher levels of hsCRP have been linked to risk for CVD and T2DM in adults [15–21], although no such long-term data are available in adolescents. In this review, I will consider the processes underlying the connections among obesity, inflammation, and adult disease; the data for hsCRP as a screening tool for future CVD and T2DM in adults; and data regarding obesity-related inflammation in children. Using this as background, I will consider multiple potential screening tools that in the future may be used to identify obese adolescents at highest risk for future CVD and T2DM.
Obesity and systemic inflammation
The concept of obesity as an inflammatory state has continued to develop over the past 15 y, and as our understanding of the process has progressed, multiple factors have been identified that may serve as reasonable screening tools. The model (Fig. 1) that has emerged from multiple studies features adipocytes—and in particular visceral adipocytes—as playing important endocrine roles in the process of inflammation and worsening insulin sensitivity [22]. In response to excess lipid stores, visceral adipocytes secrete increasing amounts of inflammatory cytokines such as interleukin-6 and tumor necrosis factor-α and chemokines such as monocyte chemo-attractant protein-1 [23]. These chemokines in turn promote the migration of macrophages to the adipose tissue, greatly increasing cytokine release. Another factor in obesity-associated inflammation appears to be the adipokine adiponectin, which is secreted by adipocytes in inverse proportion to the amount of stored lipid and appears to confer insulin sensitivity in animal models of obesity [24,25]. These low levels of adiponectin and increasing insulin resistance are also associated with the clinical features of MetS [9,11,26]. Notably, low levels of adiponectin are associated with higher levels of inflammatory cytokines, whereas infusions of adiponectin in animal models result in a decrease in systemic inflammation from unclear mechanisms [24]. Another factor released from adipocytes is retinol-binding protein-4, which appears to suppress the peripheral expression of glucose transporter-4 (GLUT4)—a key glucose transporter in skeletal muscle—and is associated with an increased expression of monocyte chemoattractant protein-1 adipocytes [27–30].
Fig. 1.
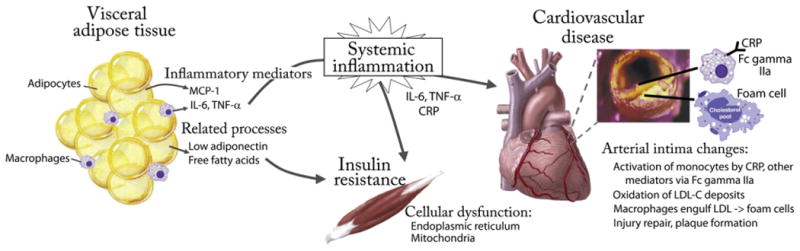
A simplified model of the role of obesity-related inflammation in disease development. Hypertrophied visceral adipocytes secrete MCP-1, recruiting macrophages that secrete inflammatory cytokines including IL-6 and TNF-α. These enter the systemic circulation and stimulate the production of CRP. These inflammatory molecules contribute to peripheral cellular dysfunction of the endoplasmic reticulum and mitochondria, resulting in a worsening of insulin resistance that is further exacerbated by low levels of adiponectin and high levels of free fatty acids. In the intima of arteries, inflammatory molecules including CRP activate monocytes, contributing to reactive oxygen species and the oxidation of LDL-C, which is then taken up by macrophages to form lipid-laden foam cells. Further injury remodeling and fibroblast migration contribute to a growing atherosclerotic plaque. CRP, C-reactive protein; IL-6, interleukin-6; LCL-C, low-density lipoprotein cholesterol; MCP-1, monocyte chemoattractant protein-1; TNF-α, tumor necrosis factor-α.
These proinflammatory conditions conferred by excess visceral adipose tissue combine to produce a tonic degree of systemic inflammation that worsens with increasing central obesity. As will become clear, these processes appear to play a central role in the development of insulin resistance and future disease, making these processes a logical target for screening and risk assessment.
C-reactive protein is arguably the most commonly used marker to assess systemic inflammation. CRP is produced by the liver, peripheral leukocytes, and even the adipose tissue in response to multiple cues, particularly increases in interleukin-6 and other systemic inflammatory cytokines [22,31]. In the periphery, CRP has specific roles including the activation of phagocytic cells—at least in part by binding to the Fc-γ-RIIa receptor (Fig. 1) [32]. Although CRP has long been used as a marker of high degrees of inflammation such as in acute infections and chronic inflammatory diseases (with levels typically >0.6 or >6 mg/L), it has been convincingly demonstrated using highly sensitive assays that report hsCRP levels that even mild increases in CRP (range 2-6 mg/L) are indicative of low-grade inflammation that carry relevance in other clinical situations, which are discussed further below [33]. Given the proinflammatory state associated with visceral adipose tissue, it is not surprising that hsCRP is increased in the setting of central obesity. The degree of increase in hsCRP associated with visceral obesity varies by individual [34]; thus, given the known correlations between hsCRP and future CVD, assessing levels of hsCRP may be a way to differentiate “healthy obese” individuals from those at higher risk [4]. Indeed, higher levels of CRP appear to be not only a marker of risk but also have an active role in cardiovascular disease. This has been demonstrated using animal models of atherosclerosis in which human CRP is increased experimentally, resulting in worsening of arterial thrombosis and endothelial injury repair [35]. Besides obesity, other lifestyle factors contribute toward higher hsCRP such as smoking and gender, with women exhibiting stronger associations between waist circumference and hsCRP than men [34]. Higher levels of hsCRP are also associated with incident myocardial infarctions, emphasizing further the pervasive relation between inflammation and heart disease [36].
Inflammation and insulin resistance
Another important consideration in the potential use of obesity-related inflammation for risk determination is its link to insulin resistance. Insulin resistance refers to the inability of physiologic levels of insulin to produce the movement of glucose into the cells. Although the underlying molecular processes involved in insulin resistance continue to be described, it is clear that multiple factors contribute to systemic insulin resistance, including genetics, visceral obesity, low levels of adiponectin, and high levels of free fatty acids [37]. Systemic inflammation in the setting of obesity is also independently associated with insulin resistance, as supported by basic science and clinical information. Animal models of obesity have demonstrated that when obese mice are deficient in their abilities to make or respond to tumor necrosis factor-α and other factors involved in inflammatory signaling, this results in less insulin resistance than seen in equally obese wild-type mice with intact inflammatory signaling [23,38–40].
Human data linking inflammation to insulin resistance began with association studies that consistently demonstrated inter-relations between systemic markers of inflammation (including hsCRP) and the presence of insulin resistance—links that have been noted in adults [41–43] and adolescents [44–46]. Supportive of these connections are historical data that suppression of inflammation with high-dose salicylate treatment was noted to improve blood sugars in patients with T2DM [47]. Multiple prospective cohort studies in adults have demonstrated a relation between inflammation and future development of T2DM (Table 1) [15,19]. These studies have revealed that in subjects as young as 45 y followed for 3 to 10 y, those with hsCRP levels in the highest quartile (starting at 2.86–6.1 mg/L, depending on the study) had adjusted odds ratios for developing T2DM of 2.03 to 4.2 compared with those in the lowest quartile (Table 1) [15,18,19]. The mechanism connecting inflammation to insulin resistance is likely to be complex but appears to involve increases in oxidative stress in target tissues, with effects on the endoplasmic reticulum and mitochondrial function in affected cells [22]. Notably, levels of inflammation and insulin resistance decrease in concert in the setting of lifestyle modification-induced weight loss in adults [48] and children [49,50], further highlighting the potential for these processes to be targets not just for screening but for following treatment progress.
Table 1.
Studies in adults linking hsCRP levels to increased risk of T2DM and CVD
| Publication | Study population, follow-up | hsCRP levels used in RR | RR of disease event |
|---|---|---|---|
| Diabetes risk | |||
| Freeman et al., [18], 2002 | 5245 men, mean age 55.4 y, followed 5 y | quintile 5 (>4.18 mg/L) versus 1 (<0.66 mg/L) | T2DM RR 3.1 for quintile 5 versus 1 |
| Pradhan et al., [19], 2001 | 27 628 women, mean age 54.7 y, followed 4 y | quartile 4 (>6.1 mg/L) versus 1 (<1 mg/L) | T2DM RR 4.2 for quartile 4 versus 1 |
| Barzilay et al., [15], 2001 | 5888 men and women ≥65 y old, followed 3-4 y | quartile 4 (>2.8 mg/L) versus 1 (<0.82 mg/L) | T2DM RR 2.0 for quartile 4 versus 1 |
| CVD event risk | |||
| Ridker etal., [21], 2002 | 27 939 women ≥45 y old, followed mean 8 y | quintile 5 (>4.19 mg/L) versus 1 (<0.49 mg/L) | CVD event RR 2.3 for quintile 5 versus 1 |
| Danesh et al., [17], 2000 | 1531 men 40-59 y old, followed mean 9.5 y | tertile 3 (>2.4 mg/L mg/L) versus 1 (<0.9 mg/L) | CVD event OR 2.13 for tertile 3 versus 1 |
| Danesh et al., [16], 2004 | 6428 men and women, mean age 55.8 y, followed mean 18 y | tertile 3 (>2 mg/L) versus 1 (<0.78 mg/L) | CVD event OR 1.45 for tertile 3 versus 1 |
| Ridker et al., [20], 2000 | 28 263 ≥45 y old, followed mean 3 y | quartile 4 (median 0.06 mg/L) versus 1 (median 0.85 mg/L) | CVD event RR 1.5 for quartile 4 versus 1 |
CVD, cardiovascular disease; hsCRP, high-sensitivity C-reactive protein; OR, odds ratio; RR, relative risk; T2DM, type 2 diabetes mellitus All associations listed are adjusted for common confounders (e.g. socioeconomic status, smoking) and are significant at P < 0.05
Inflammation and CVD
In addition to its relation to insulin resistance and T2DM risk, systemic inflammation has been even more notable as a marker of CVD risk in adults. Indeed, it has been pointed out that 1) many of the best-known CVD risk factors (smoking, hypertension, and hyperglycemia) promote noxious stimuli that lead to the production of leukocyte adhesion molecules and 2) this promotes an inflammatory response that is part of the mechanism of atherosclerotic plaque formation and growth [51]. Monocytes that are recruited to the arterial intima differentiate into macrophages, engulf oxidized lipoproteins, and become lipid-laden foam cells [52]. Continued systemic inflammation—with CRP as an important marker—contributes to this process, with neutrophils that infiltrate the plaque and release reactive oxygen species, further oxidizing lipoproteins and ultimately contributing to the growing atheroma (Fig. 1) [52].
Additional markers related to the atherosclerotic pathway or subsequent worsening cardiac function are used to identify individuals with prevalent disease. These include troponin and B-type natriuretic peptide, which are used in the evaluation for related processes such as acute coronary syndrome, myocardial infarction, and heart failure [53]. In addition, C-X-C motif chemokine ligand 12 (CXCL12) (also called stromal derived factor-1) is a survival factor that is involved in myocardial protection and appears to be decreased in its release in the setting of CVD, including in stable angina and after myocardial infarction, potentially serving as a marker of progressive CVD [54,55]. However, fewer markers are available for long-term risk assessment, as would be needed for screening tools in adolescents.
Although the development of atherosclerosis rarely progresses to a point of clinical significance before mid-adulthood, the process clearly begins in childhood, with the development of fatty streaks and thickening of the arterial media. Fibrous plaque lesions have been noted to have a surprisingly high prevalence in childhood at autopsy examination, occurring in coronary arteries in 33% of 16- to 20-y-old adolescents who died of accidental causes (Fig. 2) [56]. Although the presence of fibrous plaques was particularly associated with levels of low-density lipoprotein (LDL) cholesterol, the prevalence of plaque precursors at such young ages suggests a potential utility of hsCRP as a marker for future disease risk even in adolescence.
Fig. 2.
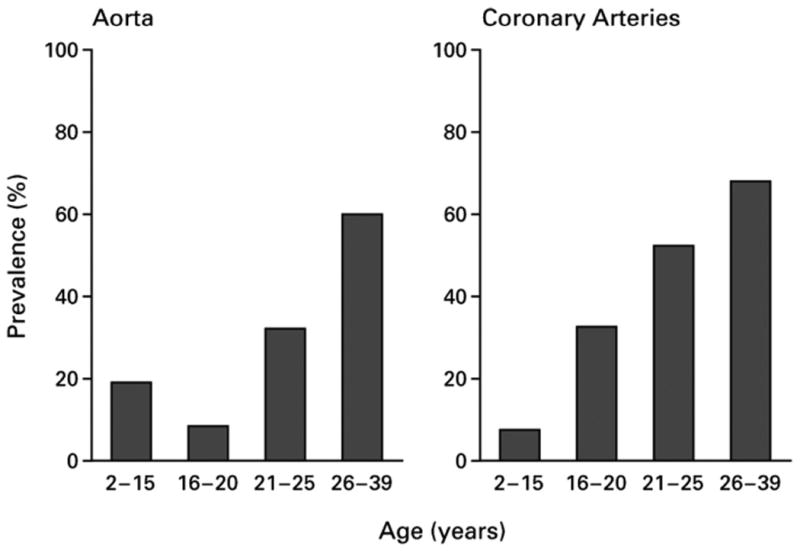
Prevalence of fibrous plaques at autopsy examination in individuals by age group. As part of the Bogalusa Heart Study, individuals who died of all causes in the region of Bogalusa, Louisiana underwent autopsy examination, including the determination of fibrous plaques in the aorta and coronary arteries. Results shown are from 204 children and adults, separated by age. Reprinted by permission from Berenson et al. [56].
hsCRP as a risk assessment tool in adults
Thus far, the data linking hsCRP and disease risk come entirely from adult studies. Multiple cohort studies including adults as young as 40 y old over follow-up time frames of 3 to 18 y have demonstrated consistent relations between increased hsCRP and risk for future CVD (Table 1) [33]. When subjects were divided into quartiles or quintiles of hsCRP, those with in the highest category of hsCRP had adjusted relative risks of 2.3 to 4.8 for CVD events compared with the lowest category, even after an adjustment for other CVD risk factors such as obesity, socioeconomic status, age, and smoking [21]. When subjects were divided into tertiles of hsCRP, those in the highest tertile (with hsCRP values as low as 2.0 mg/dL) exhibited relative risks of CVD events of 1.4 to 1.7 compared with the lowest tertile of hsCRP (up to 1.0 mg/dL; Table 1) [16,33,57]. It is important to note that these levels of hsCRP associated with increased risk in these studies were almost entirely within the “normal” range.
These strong associations of hsCRP and future risk in adults led to testing for isolated mild increases in hsCRP as a determinant of risk in otherwise intermediate-risk adults in a trial of rosuvastatin use [58]. This trial enrolled individuals without CVD, with LDL cholesterol levels lower than 120 mg/dL but with hsCRP levels of at least 2.0 mg/L Subjects who were randomized to receive therapy with rosuvastatin (versus placebo) had a decrease in CVD events during treatment, coinciding with decreases in hsCRP (mean 4.2 mg/L to mean 2.2 mg/dL after 2 y), and decreased LDL cholesterol (108 mg/dL to mean 54 mg/dL after 2 y). Based on these results, in February 2010 the Food and Drug Administration approved the use of rosuvastatin for decreasing the risk of CVD events in men at least 50 y old and women at least 60 y old without LDL cholesterol increases but with increased hsCRP (≥2.0 mg/L) and at least one other CVD risk factor such as hypertension, low high-density lipoprotein cholesterol, smoking, or a family history of premature coronary heart disease. This was the first approved CVD treatment based on increases in hsCRP and underscores the potential importance of low-grade inflammation as a clinically useful marker of risk.
Consideration of hsCRP as a risk assessment tool in adolescents
Although longitudinal data regarding the predictive importance of hsCRP in adolescents are lacking, inferential data support the potential for such a relation. As in adults, hsCRP levels in childhood are increased in the settings of obesity and are strongly associated with waist circumference, body mass index, and adiposity, as summarized in Table 2 [59–63]. With respect to insulin resistance, levels of hsCRP levels are strongly associated with measurements of insulin resistance (such as fasting insulin and the homeostasis model of insulin resistance) and are higher in overweight adolescents with insulin resistance versus without insulin resistance as assessed by insulin clamp (Table 2) [60–62,64]. Regarding MetS, hsCRP levels are higher in adolescents with MetS than in those without MetS (Table 2) [60, 61,65]. Perhaps most importantly, in a cohort of 2195 individuals who had CRP levels measured at 3 to 18 y of age, CRP levels predicted the presence of MetS as adults 21 y later [66]. In addition, hsCRP is independently associated with carotid artery media thickness in adolescence, an early finding related to atherosclerotic plaque formation [67]—although this has not been demonstrated in all studies on the topic [68].
Table 2.
Relations between hsCRP and obesity, insulin resistance, and MetS in adolescents
| Publication | Study population | hsCRP and obesity | hsCRP and insulin resistance | hsCRP and MetS |
|---|---|---|---|---|
| Sinaiko et al., [59], 2005 | selected study population 295 adolescents, mean age 15 y divided by weight and insulin sensitivity | hsCRP: overweight/obese insulin sensitive 1.21; lean insulin sensitive 0.78* | hsCRP: overweight/obese insulin resistant 1.34; overweight/obese insulin sensitive 1.21§ | |
| Ford et al., [65], 2005 | US representative sample (NHANES 1999–2000): 1366 subjects 12–17 y old | hsCRP: geometric mean 1.4 mg/L with MetS versus 0.4 mg/L without MetS†; hsCRP ≥3 mg/L in adolescents with MetS 38.4%, without MetS 10.3%† | ||
| Retnakaran et al., [62], 2006 | Native Canadian cohort 228 subjects 10–19 y old | hsCRP associated with WC, BMI, adiposity‡ | hsCRP associated with fasting insulin, HOMA-IR‡ | |
| Oliveira et al., [61], 2008 | Brazilian school cohort 407 subjects 4–18 y old | hsCRP associated with WC using linear regression‡ | hsCRP associated with HOMA-IR using linear regression‡ | hsCRP: geometric mean 1.4 mg/L with MetS versus 1.1 mg/L without MetS‡ |
| DeBoer et al., [60], 2011 | USA representative sample (NHANES 1999–2008): 3559 subjects 12–19 y old | hsCRP: highly correlated with BMI and WC in all gender/ethnicity groups evaluated (r = 0.48–0.58)‡ | mean hsCRP: adolescents with MetS 1.0–2.4 mg/L (depending on sex/ethnicity), without MetS 0.32–0.56 mg/L† |
BMI, body mass index; HOMA-IR, homeostasis model of insulin resistance; hsCRP, high-sensitivity C-reactive protein; MetS, metabolic syndrome; NHANES, National Health and Nutrition Survey; WC, waist circumference
P < 0.05.
P < 0.01.
P < 0.001.
P > 0.05, not significant.
The tight associations of hsCRP with 1) MetS in children, 2) future CVD risk in adults, and 3) incident carotid artery media thickness and future MetS raise the potential to use hsCRP as a way to identify higher-risk adolescents in need of an increased weight-loss intervention as a means of preventing future adult disease. Unfortunately, as mentioned earlier, long-term data linking increased hsCRP levels in childhood to adult disease outcomes are lacking. Given a paucity of data for most potential screening tools in pediatrics, I will nevertheless consider hsCRP as a prototype for screening. In considering this, it is notable that there could be some benefits to such a screen but also that critical information is lacking that would be needed before applying hsCRP in adolescents on a clinical basis.
Pros for use of hsCRP screening in adolescents
As mentioned earlier, a seductive motive for using hsCRP in adolescents is the ease of use. As a single test that does not rely on a fasting sample, the use of hsCRP levels to determine risk would avoid fasting blood draws and integrate several laboratory studies that are used to diagnose MetS. The hsCRP levels appear to be stable in individuals over the course of several years in adulthood—or at least as stable as measurements of blood pressure and cholesterol [33]. Moreover, hsCRP appears to identify higher metabolic risk in individuals of multiple ethnicities [69], although current criteria used to categorize MetS appear to have an inherent bias against diagnosing MetS in African Americans even in the setting of high hsCRP and other markers of future risk [60,70–72]. Thus, the benefits of hsCRP are that it would provide an easy-to-use tool without obvious racial bias that may discriminate the “healthy” obese from those at higher risk for future CVD.
Cons against use of hsCRP screening in adolescents
Arguments against the use of hsCRP in adolescents at this point outweigh any potential benefits. Foremost among the drawbacks of using hsCRP as a screening tool are the lack of prospective data linking increased hsCRP levels in adolescents to any clinical outcome. The adult studies linking hsCRP to future risk were performed over relatively short intervals because of their high or intermediate risk of developing disease. Given the low overall risk for adolescents and young adults for developing CVD and T2DM over a long interval, it is unlikely that any study with childhood hsCRP data will provide such a link in the near future. Without such data, providers would lack specifics regarding what cutoff level of hsCRP to use to identify higher risk in adolescents. In addition, the data regarding the stability of hsCRP over time come predominantly from adult studies. A potentially larger amount of intercurrent illnesses in childhood and adolescence may affect the specificity of a single increased hsCRP, necessitating at minimum an assessment of the symptoms of illness before testing.
Moreover, there are no long-term data regarding how clinical interventions targeting lifestyle change might modify any long-term risk identified by increased hsCRP levels. Indeed, attempts at lifestyle intervention in pediatric obesity clinics frequently result in only modest changes in weight status [7,8]—although improvements in measurements of insulin resistance (such as decrease in fasting insulin or increase in adiponectin) can be seen with physical-activity changes in the absence of weight loss [7,8,49]. Conceivably the identification of a higher degree of risk might be used to trigger more intensive techniques of lifestyle modification. Large-scale research studies have been more effective than standard care, in one case demonstrating a mean weight loss of 2.8 kg over 2 y [6]. These intensive tools targeting lifestyle change nevertheless have an unclear durability of effect regarding long-term CVD and T2DM risk decreases, even if such short-term changes are clearly desirable for any adolescents at increased disease risk.
Consideration of other potential screening tools in adolescents
Although hsCRP may not yet be the correct tool to use in identifying adolescents at higher risk for adult disease, such tools may prove beneficial, particularly as a motivator for obese adolescents and their treating physicians. In the National Weight Control Registry of individuals who have lost at least 13.6 kg of weight and kept it off for at least 1 y, 83% reported a trigger that started their lifestyle intervention in earnest and for 23% this trigger was a medical reason, such as a physician's recommendation [73]. Chang et al. [74] reported a strong correlation between a physician's advice regarding healthy lifestyle behaviors such as increases in exercise or improved diet and a patient's adoption of these practices. It is not known if adolescents respond as well as adults do to physician recommendations. Nevertheless, if physicians know that the patient in front of them is at higher risk for future disease, they may be more likely to keep after the family regarding recommendations and may look more closely to screen for other CVD risk factors such as hypertension and smoking. Ultimately, this effect of screening may be just as valuable as motivating the patients toward change.
In addition to hsCRP, other potential tools associated with obesity-related inflammation may have the potential to identify obese adolescents at higher risk for CVD and T2DM. Similar to hsCRP, most of these tools have a logical basis for use as risk markers but lack long-term data demonstrating their utility.
Fasting insulin
Fasting insulin levels can provide a rough estimate of an individual's degree of insulin resistance [75], which gives some indication of the presence of underlying processes such as obesity-related inflammation [37]. On a population level, these higher levels of insulin are associated with an increase in risk of future T2DM in prediabetic adults [76]. However, although widely used by practitioners, the assessment of insulin levels are complicated by a high degree of variation between assays, which makes it difficult to compare an individual patient's results to any published insulin level associated with risk. An additional problem is that a single insulin level may not distinguish well between individuals who have limitations in β-cell function (thus progressing to T2DM) and those who are able to continue an increased level of insulin production without progression [37]. These limitations make it impossible currently to assign a cutoff value to increased fasting insulin levels as a marker of future disease risk.
Adiponectin
Adiponectin appears to be in the causative pathway of producing insulin sensitivity and seems to distinguish between the “healthy obese” children and those at higher metabolic risk [5], and as such may in the future prove an effective marker of long-term risk [25]. Lower levels of adiponectin have been shown to predict future risk of MetS over a 6-y period [77]. However, similar to insulin, adiponectin testing currently lacks the widespread availability of standardized assays. In addition, as for hsCRP, testing for adiponectin in adolescents lacks proved cutoff levels demarcating an increase in long-term risk. Overall, with improvements in assay uniformity and with the addition of long-term data, adiponectin may offer significant benefits as a screening tool in the future.
Retinol-binding protein-4
Retinol-binding protein-4 represents another untested potential marker for long-term risk [78]. Although the data are mixed, some reports have noted increased levels of retinol-binding protein-4 in obese adolescents, corresponding to the amount of fat mass [79] and changes in insulin resistance [80]. Issues regarding the appropriate assay and the consistency of the findings would need to be resolved, in addition to data on the long-term prediction for the development of T2DM or CVD [78].
MetS diagnosis
A diagnosis of MetS serves as an example of the potential for predictive tools in that a longitudinal study demonstrated an odds ratio of 11.5 for future diabetes risk by 25 to 30 y later in individuals diagnosed with MetS during childhood [12]. Such an impressive increase in risk could be used to motivate children, parents, and medical professionals to encourage weight loss in at-risk children. However, the current criteria to diagnose MetS appear to have a decreased ability to detect other markers of obesity-related inflammation in African Americans. For example, an Adult Treatment Panel II-based criterion for diagnosing MetS in adolescents has a poor sensitivity for detecting increases in hsCRP, insulin, and uric acid in African Americans compared with other ethnicities [60,70,81]. Furthermore, as mentioned earlier, the use of cutoffs for multiple different laboratory studies makes the current MetS criteria difficult to use from a clinical perspective. Potential variations on a MetS diagnosis include the use of a continuous set of criteria [82,83] or a risk score that is specific for each ethnicity [84]. As with any of these potential risk markers, linking risk scores to childhood data with long-term adult outcomes would be critical to accurately characterize future risk in adolescents.
Ultimately, just as MetS is a composite of multiple factors, a combination of factors such as hsCRP and adiponectin may be needed to identify increased risk. Until long-term data are available demonstrating the utility of hsCRP screening—and other approaches—in adolescents, traditional predictors such as family history may be the best screening tools with long-term data available at this time. Compared with those without a parental history of T2DM, adolescents with a parental history of T2DM have a odds ratio of 5.2 for developing diabetes over only a 9-y follow-up period [85]. This high degree of risk serves to underscore that there may be a greater sense of urgency to promote intensive lifestyle changes in some certain groups of adolescents. This type of urgency (when backed by data) holds potential for motivating physicians and patients to modify the future risk for disease and to postpone or prevent disease for those at highest risk.
Conclusion
Many of the processes leading to CVD and T2DM are present already in childhood, although the likelihood of developing disease appears higher for some adolescents than others. Screening adolescents for MetS-related risk for CVD and T2DM may help in identifying individuals at particularly great need for lifestyle modification. hsCRP is used as such a screening tool in adults of intermediate risk for CVD to trigger more immediate intervention. However, although data regarding hsCRP and other factors related to inflammation are similarly correlated to insulin resistance and arterial changes in childhood, long-term data are lacking in adolescents linking levels of these factors to true disease risk. Such tools for identifying future risk are needed to better motivate patients and their physicians toward lifestyle changes—and toward effective decreases in risk.
Acknowledgments
This work was supported by grants 5K08HD060739-03 and 1R21DK085363 from the National Institutes of Health.
References
- 1.Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: summary report. Pediatrics. 2011;128(suppl 5):S1–44. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162:1867–72. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 3.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365:1876–85. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- 4.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–24. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 5.Morrison JA, Glueck CJ, Daniels S, Wang P, Stroop D. Paradoxically high adiponectin in obese 16-year-old girls protects against appearance of the metabolic syndrome and its components seven years later. J Pediatr. 2011;158:208–14. doi: 10.1016/j.jpeds.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savoye M, Nowicka P, Shaw M, Yu S, Dziura J, Chavent G, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics. 2011;127:402–10. doi: 10.1542/peds.2010-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickham EP, Stern M, Evans RK, Bryan DL, Moskowitz WB, Clore JN, et al. Prevalence of the metabolic syndrome among obese adolescents enrolled in a multidisciplinary weight management program: clinical correlates and response to treatment. Metab Syndr Relat Disord. 2009;7:179–86. doi: 10.1089/met.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker SE, Smolkin ME, O'Leary LL, Cluett SB, Norwood VF, DeBoer MD, et al. Predictors of retention and BMI loss or stabilization in obese youth enrolled in a weight loss intervention. Obes Res Clin Pract. 2011 doi: 10.1016/j.orcp.2011.08.157. Available at: http://www.sciencedirect.com/science/journal/1871403X. [DOI] [PubMed]
- 9.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–32. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 11.Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents. Lancet. 2007;369:2059–61. doi: 10.1016/S0140-6736(07)60958-1. [DOI] [PubMed] [Google Scholar]
- 12.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–6. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 13.DeBoer MD, Gurka MJ. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with type 2 diabetes and cardiovascular disease: data from the National Health and Nutrition Examination Survey 1999–2006. Metab Syndr Relat Disord. 2010;8:343–53. doi: 10.1089/met.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBoer MD. Ethnicity, obesity and the metabolic syndrome: implications on assessing risk and targeting intervention. Expert Rev Endocrinol Metab. 2011;6:279–89. doi: 10.1586/eem.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, et al. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384–9. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- 16.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 17.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 19.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 22.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–55. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 23.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–31. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fatderived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care. 2006;29:51–6. doi: 10.2337/diacare.29.1.51. [DOI] [PubMed] [Google Scholar]
- 26.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 27.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–33. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 28.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 29.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–63. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 30.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–7. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calabro P, Chang DW, Willerson JT, Yeh ET. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112–3. doi: 10.1016/j.jacc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 32.Stein MP, Edberg JC, Kimberly RP, Mangan EK, Bharadwaj D, Mold C, et al. C-reactive protein binding to FcgammaRIIa on human monocytes and neutrophils is allele-specific. J Clin Invest. 2000;105:369–76. doi: 10.1172/JCI7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 34.Wee CC, Mukamal KJ, Huang A, Davis RB, McCarthy EP, Mittleman MA. Obesity and C-reactive protein levels among white, black, and Hispanic US adults. Obesity (Silver Spring) 2008;16:875–80. doi: 10.1038/oby.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danenberg HD, Szalai AJ, Swaminathan RV, Peng L, Chen Z, Seifert P, et al. Increased thrombosis after arterial injury in human C-reactive protein–transgenic mice. Circulation. 2003;108:512–5. doi: 10.1161/01.CIR.0000085568.13915.1E. [DOI] [PubMed] [Google Scholar]
- 36.Datta S, Iqbal Z, Prasad KR. Comparison between serum hsCRP and LDL cholesterol for search of a better predictor for ischemic heart disease. Indian J Clin Biochem. 2011;26:210–3. doi: 10.1007/s12291-010-0100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–4. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 39.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–7. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 40.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 41.Festa A, D'Agostino R, Jr, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 42.Wu T, Dorn JP, Donahue RP, Sempos CT, Trevisan M. Associations of serum C-reactive protein with fasting insulin, glucose, and glycosylated hemoglobin: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2002;155:65–71. doi: 10.1093/aje/155.1.65. [DOI] [PubMed] [Google Scholar]
- 43.Forouhi NG, Sattar N, McKeigue PM. Relation of C-reactive protein to body fat distribution and features of the metabolic syndrome in Europeans and South Asians. Int J Obes Relat Metab Disord. 2001;25:1327–31. doi: 10.1038/sj.ijo.0801723. [DOI] [PubMed] [Google Scholar]
- 44.Herder C, Schneitler S, Rathmann W, Haastert B, Schneitler H, Winkler H, et al. Low-grade inflammation, obesity, and insulin resistance in adolescents. J Clin Endocrinol Metab. 2007;92:4569–74. doi: 10.1210/jc.2007-0955. [DOI] [PubMed] [Google Scholar]
- 45.Moran A, Steffen LM, Jacobs DR, Jr, Steinberger J, Pankow JS, Hong CP, et al. Relation of C-reactive protein to insulin resistance and cardiovascular risk factors in youth. Diabetes Care. 2005;28:1763–8. doi: 10.2337/diacare.28.7.1763. [DOI] [PubMed] [Google Scholar]
- 46.Shea S, Aymong E, Zybert P, Shamoon H, Tracy RP, Deckelbaum RJ, et al. Obesity, fasting plasma insulin, and C-reactive protein levels in healthy children. Obes Res. 2003;11:95–103. doi: 10.1038/oby.2003.15. [DOI] [PubMed] [Google Scholar]
- 47.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566–72. doi: 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balagopal P, George D, Patton N, Yarandi H, Roberts WL, Bayne E, et al. Lifestyle-only intervention attenuates the inflammatory state associated with obesity: a randomized controlled study in adolescents. J Pediatr. 2005;146:342–8. doi: 10.1016/j.jpeds.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 50.Reinehr T, Stoffel-Wagner B, Roth CL, Andler W. High-sensitive C-reactive protein, tumor necrosis factor alpha, and cardiovascular risk factors before and after weight loss in obese children. Metabolism. 2005;54:115, 5–61. doi: 10.1016/j.metabol.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 52.Mizuno Y, Jacob RF, Mason RP. Inflammation and the development of atherosclerosis. J Atheroscler Thromb. 2011;18:351–8. doi: 10.5551/jat.7591. [DOI] [PubMed] [Google Scholar]
- 53.Halim SA, Newby LK, Ohman EM. Biomarkers in cardiovascular clinical trials: past, present, future. Clin Chem. 2012;58:45–53. doi: 10.1373/clinchem.2011.165787. [DOI] [PubMed] [Google Scholar]
- 54.Boyle AJ, Yeghiazarians Y, Shih H, Hwang J, Ye J, Sievers R, et al. Myocardial production and release of MCP-1 and SDF-1 following myocardial infarction: differences between mice and man. J Transl Med. 2010;9:150. doi: 10.1186/1479-5876-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farouk SS, Rader DJ, Reilly MP, Mehta NN. CXCL12: a new player in coronary disease identified through human genetics. Trends Cardiovasc Med. 2010;20:204–9. doi: 10.1016/j.tcm.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study N Engl J Med. 1998;338:1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 57.de Maat MP, Trion A. C-reactive protein as a risk factor versus risk marker. Curr Opin Lipidol. 2004;15:651–7. doi: 10.1097/00041433-200412000-00005. [DOI] [PubMed] [Google Scholar]
- 58.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 59.Sinaiko AR, Steinberger J, Moran A, Prineas RJ, Vessby B, Basu S, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111:1985–91. doi: 10.1161/01.CIR.0000161837.23846.57. [DOI] [PubMed] [Google Scholar]
- 60.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the metabolic syndrome is associated with disproportionately high levels of high-sensitivity C-reactive protein in non-Hispanic black adolescents: an analysis of NHANES 1999–2008. Diabetes Care. 2011;34:734–40. doi: 10.2337/dc10-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliveira AC, Oliveira AM, Adan LF, Oliveira NF, Silva AM, Ladeia AM. C-reactive protein and metabolic syndrome in youth: a strong relationship? Obesity (Silver Spring) 2008;16:1094–8. doi: 10.1038/oby.2008.43. [DOI] [PubMed] [Google Scholar]
- 62.Retnakaran R, Hanley AJ, Connelly PW, Harris SB, Zinman B. Elevated C-reactive protein in Native Canadian children: an ominous early complication of childhood obesity. Diabetes Obes Metab. 2006;8:483–91. doi: 10.1111/j.1463-1326.2005.00533.x. [DOI] [PubMed] [Google Scholar]
- 63.Siervo M, Ruggiero D, Sorice R, Nutile T, Aversano M, Iafusco M, et al. Body mass index is directly associated with biomarkers of angiogenesis and inflammation in children and adolescents. Nutrition. 2012;28:262–6. doi: 10.1016/j.nut.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Lambert M, Delvin EE, Paradis G, O'Loughlin J, Hanley JA, Levy E. C-reactive protein and features of the metabolic syndrome in a population-based sample of children and adolescents. Clin Chem. 2004;50:1762–8. doi: 10.1373/clinchem.2004.036418. [DOI] [PubMed] [Google Scholar]
- 65.Ford ES, Ajani UA, Mokdad AH. The metabolic syndrome and concentrations of C-reactive protein among U.S. youth Diabetes Care. 2005;28:878–81. doi: 10.2337/diacare.28.4.878. [DOI] [PubMed] [Google Scholar]
- 66.Mattsson N, Ronnemaa T, Juonala M, Viikari JS, Raitakari OT. Childhood predictors of the metabolic syndrome in adulthood. The Cardiovascular Risk in Young Finns Study Ann Med. 2008;40:542–52. doi: 10.1080/07853890802307709. [DOI] [PubMed] [Google Scholar]
- 67.Jarvisalo MJ, Harmoinen A, Hakanen M, Paakkunainen U, Viikari J, Hartiala J, et al. Elevated serum C-reactive protein levels and early arterial changes in healthy children. Arterioscler Thromb Vasc Biol. 2002;22:1323–8. doi: 10.1161/01.atv.0000024222.06463.21. [DOI] [PubMed] [Google Scholar]
- 68.Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima–media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117:1560–7. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 69.Albert MA, Ridker PM. C-reactive protein as a risk predictor: do race/ethnicity and gender make a difference? Circulation. 2006;114:e67–74. doi: 10.1161/CIRCULATIONAHA.106.613570. [DOI] [PubMed] [Google Scholar]
- 70.DeBoer MD, Dong L, Gurka MJ. Racial/Ethnic and sex differences in the ability of metabolic syndrome criteria to predict elevations in fasting insulin levels in adolescents. J Pediatr. 2011;159:975–81. doi: 10.1016/j.jpeds.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sumner AE. Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J Pediatr. 2009;155:S7. doi: 10.1016/j.jpeds.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Renzaho AM, Halliday JA, Nowson C. Vitamin D, Obesity, and obesity-related chronic disease among ethnic minorities: a systematic review. Nutrition. 2011;27:868–79. doi: 10.1016/j.nut.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 73.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82(1 suppl):222S–5S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 74.Chang MH, Valdez R, Ned RM, Liu T, Yang Q, Yesupriya A, et al. Influence of familial risk on diabetes risk-reducing behaviors among U.S. adults without diabetes Diabetes Care. 2011;34:2393–9. doi: 10.2337/dc11-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz B, Jacobs DR, Jr, Moran A, Steinberger J, Hong CP, Sinaiko AR. Measurement of insulin sensitivity in children: comparison between the euglycemic–hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31:783–8. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 76.Osei K, Rhinesmith S, Gaillard T, Schuster D. Impaired insulin sensitivity, insulin secretion, and glucose effectiveness predict future development of impaired glucose tolerance and type 2 diabetes in pre-diabetic African Americans: implications for primary diabetes prevention. Diabetes Care. 2004;27:1439–46. doi: 10.2337/diacare.27.6.1439. [DOI] [PubMed] [Google Scholar]
- 77.Kynde I, Heitmann BL, Bygbjerg IC, Andersen LB, Helge JW. Hypo-adiponectinemia in overweight children contributes to a negative metabolic risk profile 6 years later. Metabolism. 2009;58:1817–24. doi: 10.1016/j.metabol.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 78.McTernan PG, Kumar S. Editorial: retinol binding protein 4 and pathogenesis of diabetes. J Clin Endocrinol Metab. 2007;92:2430–2. doi: 10.1210/jc.2007-1054. [DOI] [PubMed] [Google Scholar]
- 79.Friebe D, Neef M, Erbs S, Dittrich K, Kratzsch J, Kovacs P, et al. Retinol binding protein 4 (RBP4) is primarily associated with adipose tissue mass in children. Int J Pediatr Obes. 2011;6:e345–52. doi: 10.3109/17477166.2010.491228. [DOI] [PubMed] [Google Scholar]
- 80.Goodman E, Graham TE, Dolan LM, Daniels SR, Goodman ER, Kahn BB. The relationship of retinol binding protein 4 to changes in insulin resistance and cardiometabolic risk in overweight black adolescents. J Pediatr. 2009;154:67–73. doi: 10.1016/j.jpeds.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DeBoer MD, Gurka MJ. Low sensitivity for the metabolic syndrome to detect uric acid elevations in females and non–Hispanic-black male adolescents: an analysis of NHANES 1999–2006. Atherosclerosis. 2012;220:575–80. doi: 10.1016/j.atherosclerosis.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Eisenmann JC. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc Diabetol. 2008;7:17. doi: 10.1186/1475-2840-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 84.DeBoer MD. Underdiagnosis of the metabolic syndrome in non-Hispanic black adolescents: a call for ethnic-specific criteria. Curr Cardiovasc Risk Rep. 2010;4:302–10. doi: 10.1007/s12170-010-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morrison JA, Glueck CJ, Horn PS, Wang P. Childhood predictors of adult type 2 diabetes at 9- and 26-year follow-ups. Arch Pediatr Adolesc Med. 2010;164:53–60. doi: 10.1001/archpediatrics.2009.228. [DOI] [PubMed] [Google Scholar]


