Abstract
The presence of epoxyeicosatrienoic acids (EETs) in tissues and their metabolism by soluble epoxide hydrolase (sEH) to 1,2-diols were first reported 30 years ago. However, appreciation of their importance in cell biology and physiology has greatly accelerated over the past decade with the discovery of metabolically stable inhibitors of sEH, the commercial availability of EETs, and the development of analytical methods for the quantification of EETs and their diols. Numerous roles of EETs in regulatory biology now are clear, and the value of sEH inhibition in various animal models of disease has been demonstrated. Here, we review these results and discuss how the pharmacological stabilization of EETs and other natural epoxy-fatty acids could lead to possible disease therapies.
Keywords: epoxy-fatty acid, hypertension, inflammation, pain, cancer
INTRODUCTION
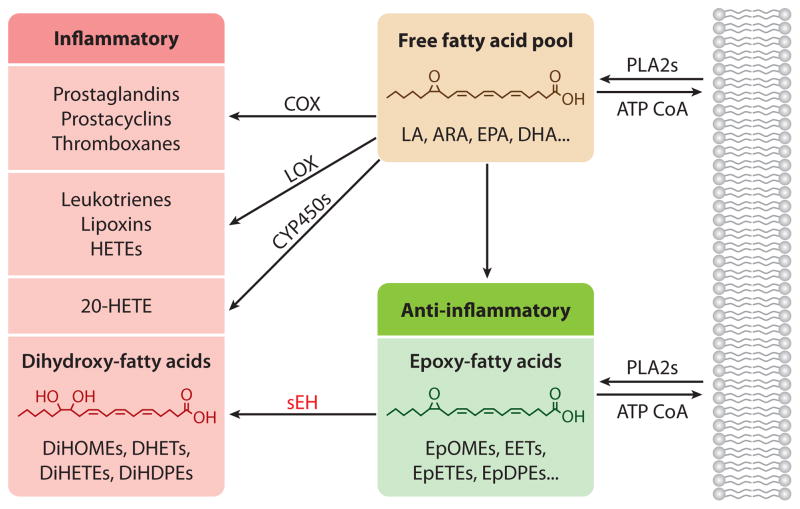
Arachidonic acid (ARA), an ω-6, 20-carbon polyunsaturated fatty acid, is at the center of a group of metabolic pathways, collectively termed the arachidonate cascade, that produces endogenous signaling molecules regulating multiple biological processes such as inflammation (Figure 1). Metabolic pathways for ω-3 fatty acid, such as eicosapentaenoic acid and docosahexaenoic acid, parallel and compete with the better described ARA cascade. A large proportion of the world’s pharmaceuticals target these lipid chemical mediators. Existing drugs target the cyclooxygenase (COX) and lipoxygenase (LOX) pathways of the ARA cascade (1, 2), which largely biosynthesize proinflammatory mediators (Figure 1), whereas the more recently discovered cytochrome P450 branch of the cascade has not been exploited as a pharmaceutical target. The P450 pathway produces both the proinflammatory, terminally hydroxylated metabolite 20-hydroxyeicosatetraenoic acid (20-HETE) (3) and the anti-inflammatory epoxy-fatty acids (EpFAs) such as epoxyeicosatrienoic acids (EETs) from ARA (4, 5). P450 epoxidation retains the cis geometry of most natural fatty acids; however, this geometry can isomerize, and peroxidase-dependent pathways can lead to bioactive trans epoxides (6). EETs and other EpFAs have numerous actions (4, 5). Generally, EETs are chemical mediators that move biological processes toward a homeostatic or status quo condition. For example, EETs seem to encourage the resolution of inflammation rather than prevent it, in a manner similar to that exhibited by mediators in the LOX pathway (7). EETs reduce inflammation but are also analgesic, antifibrotic, and antihypertensive, acting in both autocrine and paracrine manners (4, 5). Linoleic acid is our major dietary fat, and its metabolites—including its inflammatory diols or DiHOMEs—are known to be biologically active (Figure 1) (8). The high ω-6 composition of the American diet is of concern, but because ω-3 lipids are increasingly entering the diet as value-added products, the study of the metabolites of ω-3 lipids is also crucial. This review focuses largely on EETs and EpFAs, which encompass the isomers of EETs and the corresponding ω-3 lipids (9). The detailed biology of EET action was recently reviewed elsewhere (5).
Figure 1.
Overview of the arachidonic acid cascade emphasizing the conversion of largely anti-inflammatory epoxides to their corresponding 1,2-diols by the soluble epoxide hydrolase. Abbreviations: ARA, arachidonic acid; CoA, coenzyme A; COX, cyclooxygenase; CYP450, cytochrome P450; DHA, docosahexaenoic acid; DHET, dihydroxyeicosatrienoic acid; DiHDPE, dihydroxydocosapentaenoic acid; DiHETE, dihydroxyeicosatetraenoic acid; DiHOME, dihydroxyoctadecenoic acid; EET, epoxyeicosatrienoic acid; EPA, eicosapentaenoic acid; EpDPE, epoxydocosapentaenoic acid; EpETE, epoxyeicosatetraenoic acid; EpOME, epoxyoctadecenoic acid; HETE, hydroxyeicosatetraenoic acid; LA, linoleic acid; LOX, lipoxygenase; PLA2, phospholipase A2; sEH, soluble epoxide hydrolase.
There is likely an equilibrium between the P450-derived, largely anti-inflammatory EETs and the generally proinflammatory 20-HETE, and between anti- and proinflammatory pathways in general. For example, when EET levels are increased in soluble epoxide hydrolase (sEH) knockout mice, 20-HETE levels increase presumably to counterbalance the increase in EETs (10). However, many physiological and pathophysiological states, including diabetes, pregnancy, obesity, and possibly even aging, shift such equilibrium in favor of more inflammatory pathways (3). Blocking the production of 20-HETE is likely to be beneficial in many disease states, but it has not resulted in promising therapeutics. However, the inhibition of 20-HETE production in combination with the stabilization of EETs appears to be a promising approach (11).
Although EETs and other EpFAs are rapidly metabolized by numerous pathways including the sEH (discussed below), EETs have exceptionally high biological activity (5), making them attractive for some therapeutic applications such as ocular inflammation and organ baths. These beneficial biological effects of EETs illustrate the potential value of EET mimics (12). The optimization of EET mimics could be simplified by receptor-based screens, but the work to date has been based on mimicking the EET structure while blocking likely routes of metabolism, such as epoxide hydrolysis (13) and β-oxidation (14). Recently, an EET analog that also inhibits sEH was used as a biological probe (15). An advantage of this approach over sEH inhibition is that EET-like activity can be delivered to tissues where natural EET production is low.
EETs are metabolized by a variety of pathways (5). However, sEH, which adds water across the epoxide to give the corresponding 1,2-dihydroxy-fatty acids (DiHFAs or diols), is the dominant pathway in many tissues (4). Thus, inhibition of this pathway will likely increase the EET concentrations in plasma and tissues significantly, whereas the other routes of metabolism ensure that, even in the absence of sEH activity, EET levels can increase only to moderate levels. Thus, even massive doses of sEH inhibitors (sEHIs) cannot lead to exceptionally high EET levels and limit target-related side effects. Because sEHIs have advanced further toward clinical use than other approaches, this review focuses on sEH and its inhibitors as probes for understanding the ARA cascade and possibly as therapeutics.
MAMMALIAN EPOXIDE HYDROLASES
Epoxide hydrolases (EHs; EC 3.3.2.7–11) open epoxides to diols by the addition of water. Humans have several EHs (16, 17), and a systematic nomenclature has been proposed for the genes that code for EHs in the α/β-hydrolase fold family: EPHX1 corresponds to the microsomal epoxide hydrolase (mEH), EPHX2 corresponds to sEH, and additional sequential numbers are being assigned to new enzymes that have functional EH activity (17). Hepoxilin EH activity is largely due to sEH (18). The leukotriene A4 hydrolase opens LTA4 to the 5,12-LTB4 diol and is itself a drug target (1), and the cholesterol EH may be related to the tamoxifen-binding site (19). These proteins have a catalytic mechanism that differs from that of other EHs. Whereas the leukotriene A4 hydrolase and cholesterol EH work by a one-step mechanism, the sEH, mEH, and other EHs in the α/β-hydrolase fold family work by a two-step, base-catalyzed mechanism (16). Such EHs are common in evolution (17), but most work focuses on mEH (17, 20) and sEH (16, 21–24). Numerous reviews have addressed the biochemistry and roles of mEH and sEH (5, 16, 17, 22–24).
The mEH is best known as an enzyme involved in foreign compound metabolism, in which it degrades epoxides on cyclic systems such as arene oxides and aflatoxin epoxide, and it is the biochemical basis of the valpromide-chlorpromazine drug interaction (17, 22). The substrate selectivity of mEH is broader and quite different from that of sEH. The rapid reaction between most xenobiotic epoxides and glutathione and the turnover by glutathione S-transferase result in a minor role for mEH and no role for sEH (16, 17). Because some of the xenobiotic epoxides degraded by mEH are toxins, mutagens, and carcinogens and others are drug metabolites, inhibition of mEH is thought to be therapeutically undesirable. Thus, sEHIs are routinely counterscreened against mEH (25). Known inhibitors of mEH fail to inhibit sEH, and, reversibly, potent sEHIs do not inhibit mEH (25, 26).
Although mEH hydrates EpFAs (20), sEH is far more efficient at hydrolyzing such substrates (9, 27). In most tissues, such as the liver, the abundance and efficiency of sEH relative to mEH with lipid epoxides relegate mEH to a minor role (10, 16, 27–30). In the absence of sEH and the presence of high levels of mEH such as in some brain regions (27, 31), mEH may contribute significantly to the hydration of EpFAs. Potent inhibitors of mEH are now available, but their rapid metabolism makes them of marginal benefit in generating chemical knockouts in vivo (26).
EpFAs are the best substrates for sEH in terms of kcat/Km (9, 16); the sEH shows clear selectivity among regioisomers and enantiomers (9, 32). In general, the lowest rates of hydrolysis are observed for epoxides close to the carboxylic acid, and the highest rates are observed for regioisomeric epoxides that are 14–16 atoms away from the carboxylic acid. In addition, the ω-3 lipid epoxides are better substrates than the ω-6 lipid epoxides (9). Certainly, sEH is capable of degrading xenobiotic epoxides that match its substrate preference, but no potent toxic or mutagenic epoxides have been found to be good substrates. As expected, mice with the sEH gene knocked out are not more sensitive to the application of carcinogens that act through a reactive epoxide (33).
The sEH is expressed in multiple human tissues (17, 22). Although it has broad distribution in the liver, its expression in other tissues such as the kidney is localized (34). Similarly, in the brain, sEH is highly expressed in the smooth muscles of the arterioles, as well as in the neuronal cell bodies, oligodendrocytes, and astrocytes (31). Both peroxisome proliferator-activated receptor α (PPARα) and PPARγ agonists (e.g., fibrates and glitazones) induce sEH expression (22). Although PPARγ agonists upregulate sEH in adipose tissues, they have little effect in the liver (35), and they downregulate sEH in cardiomyocytes (36). The physiological role for the induction of sEH by PPAR agonists is not known. Interestingly, the 5′ flanking region of the sEH gene does not contain PPAR-binding sites, suggesting that the action of PPARs on sEH expression is indirect (37). The sEH is also induced by angiotensin II (38, 39), notably in adipocytes (35), and it is one of the major proteins that increase during adipocyte maturation (35). Multiple SP-1 transcription factor binding sites are present in the 5′ flanking region of the sEH gene (37), and methylation and binding of the transcription factor c-Jun to these sites seem to regulate angiotensin II effects on sEH expression (39, 40). A recent report shows that sEH induction is driven by homocysteine, which appears to depend on activating transcription factor 6 (41).
The sEH protein in mammals, a 125-kDa dimer composed of two identical 62-kDa monomers arranged in an antiparallel fashion, is found mostly in the cytosol but also in the peroxisomes of some organs (17, 22, 42). The N- and C-terminal regions of sEH are separated by a proline-rich linker (43). The C-terminal contains the EH activity, and the smaller N-terminal is a phosphatase (EC 3.1.3.76) (44) that apparently acts on lysophosphatidic acids (45). The few inhibitors developed for this phosphatase are not efficient in vivo, and its biological role is not known; the phosphatase and EH domains may be inhibited independently (46). The evolutionary pressure for sEH to remain as an EH-phosphatase fusion protein in higher animals remains mysterious (17). Several single-nucleotide polymorphisms of sEH have been identified (47–49), two of which have been associated with various cardiovascular diseases: K55R (17% of the population) and R287N (8–14% of the population) (recently reviewed in 5 and 17). These observations point to an essential role of sEH in human health.
SOLUBLE EPOXIDE HYDROLASE INHIBITORS
The catalytic mechanism and structure of sEH are detailed in other reviews (16, 17, 25, 50). The sEH adds water to its substrate in a two-step exothermic process that involves a covalent intermediate. The catalytic mechanism suggests that mimics of the reaction intermediates or transition states could be useful inhibitors, as observed for other α/β-hydrolase fold enzymes. Subsequently, the discovery of dicyclohexyl urea as a reversible nanomolar inhibitor of sEH paved the way for the generation of low-nanomolar to picomolar inhibitors with a variety of structures (Figure 2) (16, 25, 50, 51, 52). Similarly, amides and carbamates with appropriate substituents can also be powerful inhibitors of sEH (52). Crystallographic studies show that the urea binds to the sEH active site by mimicking the reaction intermediate (reviewed in 16, 17, 25, 50).
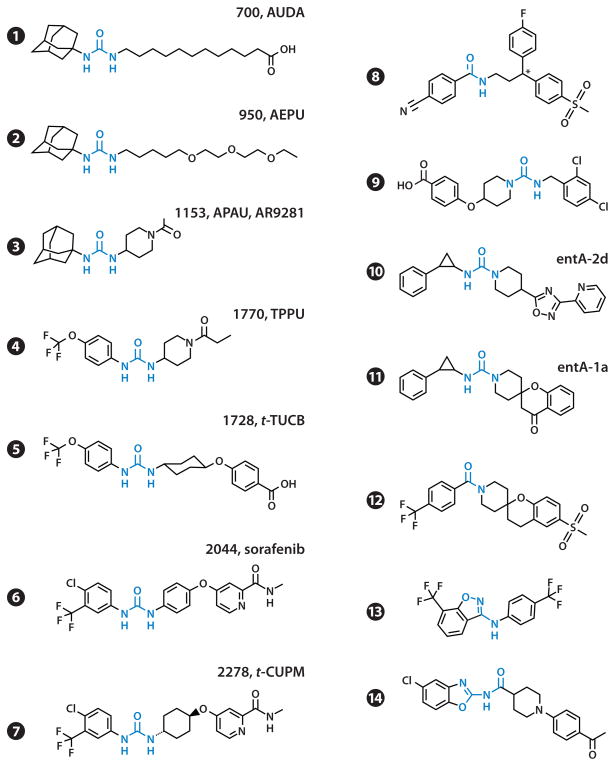
Figure 2.
Structure and commonly used acronyms of soluble epoxide hydrolase inhibitors used in biological assays, animal models of diseases, and clinical trials. References for compounds: 1 (53); 2 (55); 3–5, 7 (56); 6 (57); 8 (58); 9 (59); 10 (60); 11, 12 (61); 13 (62); and 14 (63). Abbreviations: AEPU, 1-adamantanyl-3-{5-[2-(2-ethoxyethoxy)ethoxy]pentyl]}urea; APAU, 1-(1-acetypiperidin-4-yl)-3-adamantanylurea; AUDA, 12-(3-adamantan-1-yl-ureido) dodecanoic acid; t-CUPM, trans-4-{4-[3-(4-chloro-3-trifluoromethyl-phenyl)-ureido]-cyclohexyloxy}-pyridine-2-carboxylic acid methylamide; TPPU, 1-trifluoromethoxyphenyl-3-(1-propionylpiperidin-4-yl) urea; t-TUCB, trans-4-{4-[3-(4-trifluoromethoxy-phenyl)-ureido]-cyclohexyloxy}-benzoic acid.
Structure-activity relationships show that the primary urea central pharmacophore should have a relatively small group such as a phenyl, hexyl, or cyclohexyl on one side but could accommodate a large group on the other side (53). Because EpFAs are endogenous substrates of sEH, the early compounds used fatty acid mimics, leading to AUDA (Figure 2, compound 1). With the adamantane sensitive to P450 oxidation and the fatty acid chain sensitive to β-oxidation, AUDA is rapidly metabolized in vivo. AUDA is a weak PPAR ligand and actually mimics EETs (54), but despite these limitations, AUDA has been used in many animal studies. A key in making sEHIs that have improved physical properties was the discovery that a polar group termed the secondary pharmacophore can be positioned approximately 7 Å from the carbonyl of the primary pharmacophore (55). The ether in AEPU (Figure 2, compound 2) is an example of the secondary pharmacophore, which in most modern sEHIs is an ether, a heterocycle, or an amide (55). Replacement of the ether chain by a conformationally restricted molecule—such as piperidine (Figure 2, compounds 3 and 4), cyclohexyl (Figure 2, compounds 5 and 7), or phenyl (Figure 2, compound 6)—has led to potent compounds that have favorable pharmacokinetics in a variety of species (56). Although APAU was taken through Phase IIA trials, the more metabolically stable and potent TPPU may represent a better clinical candidate (56). In general, compounds with cyclohexyl ether are active on a wider spectrum of mammalian species than are piperidine derivatives (56). Modern compounds show low-nanomolar potency, exhibit good oral bioavailability and pharmacokinetics (in rodents, canines, felines, and primates), and are easier to formulate. The compounds tested show little off-target activity on targets such as cytochrome P450s and hERG (human ether-à-go-go-related gene). Interestingly, the registered Raf kinase inhibitor sorafenib is a low-nanomolar sEHI that is similar to t-CUPM. Although most sEHIs are not kinase inhibitors, researchers have prepared a variety of compounds that are good sEHIs with interesting selectivity profiles on kinases (57).
The development of sEHIs, although led by academia, has also benefited from industry research. Using cell-based high-throughput screening, Boehringer Ingelheim found some potent amide and trisubstituted urea leads (e.g., Figure 2, compounds 8 and 9) that were exploited in several studies (58, 59). These compounds were optimized in part by solving a cocrystal with sEH (58). Merck scientists also developed promising compounds, including a phenylcyclopropyl derivative (Figure 2, compound 10) with the second nitrogen of the urea in a piperidine ring (60). This compound was used to generate spirocyclic trisubstituted ureas (e.g., Figure 2, compound 11), which were highly potent and showed good bioavailability (61). These developments led to other trisubstituted spirocyclic ureas, such as compound 12 in Figure 2, that show both high potency and good rat pharmacokinetics. However, these compounds failed to reduce blood pressure in a spontaneously hypertensive rat model (25). The Merck group also developed aminobenzisoxazoles, including compound 13 in Figure 2, which succeeded in replacing the urea or amide pharmacophore with a benzisoxazole (62). Pfizer chemists recently used structure-based virtual screening driven by new X-ray structures to design a combinatorial library that yielded nanomolar benzoxazole derivatives (Figure 2, compound 14) (63).
Numerous available structures are potent and selective enough to be used as orally available in vivo chemical probes and possibly even to be moved into the clinic. However, keeping the compounds—even most of the recent ones—in true solution to promote high biological activity is important. The evolution of sEHIs has been the subject of detailed reviews in the published and patent literature (25, 50, 51).
BIOLOGICAL EFFECTS OF EPOXY-FATTY ACIDS AND SOLUBLE EPOXIDE HYDROLASE INHIBITORS
EETs and other EpFAs have broad activities as both autocrine and paracrine chemical mediators (24). Increasingly, new activities are discovered through the use of biologically stable EET mimics and the use of sEHIs (5). EETs have numerous biochemical targets, but an EET receptor has not been identified. However, ample evidence shows that such receptors exist, and EETs have been proposed to act on vascular smooth muscle through a G protein (Gs)-dependent mechanism (5). EETs and their diol products (termed DHETs) are PPARα agonists (64). The regioisomers of EETs activate tissue plasminogen activator, PI3 kinase, protein kinase A, and the KATP and BK channels (65). They act on Trp channels; modulate MAP kinase activity (66); inhibit apoptosis (67); inhibit thromboxane A2 signaling, L-type Ca2+ channels, and cardiac sodium channels; and reduce nuclear factor κB (NF-κB) nuclear translocation (24, 68, 69). Many vascular effects of EETs are modulated by nitric oxide (NO) (70). EET concentration is regulated by both biosynthesis and release, as discussed above, and by their degradation, which is dominated by sEH in most situations (reviewed in 4). Thus, the discussion below about the effects of sEHIs reflects on the biological actions of both EETs and other EpFAs.
Effects of Soluble Epoxide Hydrolase Inhibitors on Inflammation
Inflammation is a complex biological response that helps an organism remove injurious stimuli and initiate the healing process. However, chronic inflammation can lead to a host of diseases, and most disease states are associated with a major inflammatory component. Inflammation was treated pharmacologically first using traditional medicines and then, starting 150 years ago, using synthetic chemicals such as aspirin. It was not until the 1970s that some oxylipins (prostaglandins and thromboxanes) were discovered to be at the center of the anti-inflammatory action of aspirin and other newer COX2-selective inhibitors (COXIBs) (2). At approximately the same time, some lipoxygenase products also were found to have proinflammatory roles (1). Discovered a decade later (71) the products of the P450 branch of the ARA cascade, especially the epoxides (EETs), also were thought to play a role in the inflammatory response. However, the lack of available EETs and their rapid metabolism by sEH (28) slowed progress. The first evidence of a role for EETs in inflammation was the observation that 11,12-EET prevents the tumor necrosis factor-α (TNF-α)-induced activation of NF-κB and the subsequent increase in VCAM-1 (vascular cell adhesion molecule-1) expression in mice (72, 73). 14,15-EET is also strongly anti-inflammatory (74). The role of EETs as potent anti-inflammatory lipid mediators was supported by the use of sEHIs (75, 76) and transgenic animals in various inflammatory models. The overexpression of CYP2J2, which produces EETs, is protective against ischemia-reperfusion injury (77), whereas the knockout of this P450 results in activation of the inflammatory response (78). The deletion of the sEH gene reduces the inflammatory response to endotoxin (79). The availability of ω-3 EpFAs has led to the recent observation that these natural oxiranes also have anti-inflammatory properties (80). The hydrolysis of EETs to DHETs by sEH was considered only a deactivation process (22); however, recent findings suggest that the DHETs are proinflammatory (81), as are the diols from the linoleate epoxides (8). Thus, sEH inhibition could be a powerful tool to reduce inflammation not only by stabilizing the anti-inflammatory EpFAs but also by reducing the production of proinflammatory diols.
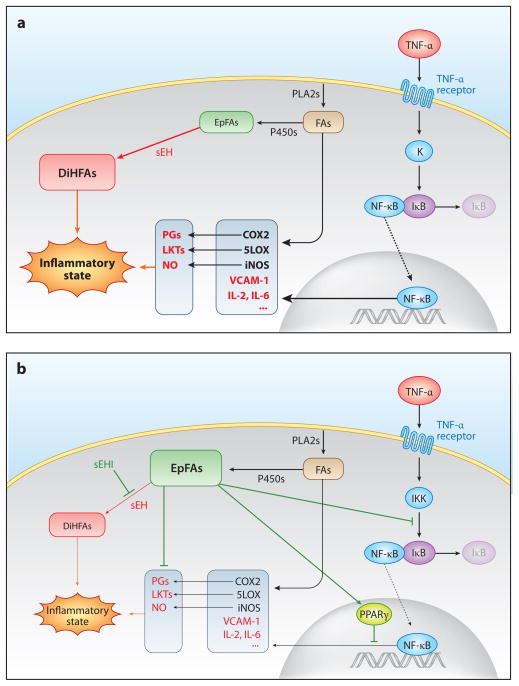
Reduced activation of NF-κB is a key event in the anti-inflammatory activity of EpFA (Figure 3). It results in transcriptional downregulation of numerous cytokines and enzymes induced during inflammation, such as iNOS, LOX5, and COX2; downregulation of COX2 results in lower production of proinflammatory prostaglandins (75, 76, 82). EETs seem to reduce the activation of NF-κB by three complementary cellular mechanisms (Figure 3b). First, EETs or epoxyeicosatetraenoic acids (EpETEs) reduce the TNF-α-induced activation of NF-κB by inhibiting IκB kinase (72, 80). Second, EETs and EpETEs activate PPARγ transcription activity (38, 80). The Kd values of EETs and EpETEs for the ligand-binding domain of PPARγ are in the micromolar range (38). PPARγ interferes with the activity of proinflammatory transcription factors such as NF-κB or AP-1, thus resulting in anti-inflammatory action (83). Third, EETs directly affect the inflammatory activity of PGE2 (84).
Figure 3.
(a) Role of epoxy-fatty acids (EpFAs) in inflammation. (b) Effects of soluble epoxide hydrolase inhibitors (sEHIs). Abbreviations: COX, cyclooxygenase; DiHFA, 1,2-dihydroxy-fatty acid; FA, fatty acid; IKK, IκB kinase; IL, interleukin; iNOS, inducible nitric oxide synthase; LKT, leukotriene; LOX, lipoxygenase; NF-κB, nuclear factor κB; NO, nitric oxide; PG, prostaglandin; PLA2, phospholipase A2; PPAR, peroxisome proliferator-activated receptor; sEH, soluble epoxide hydrolase; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1.
The stabilization of EpFAs by sEHIs represents a potentially novel way to treat numerous inflammatory diseases, such as atherosclerosis (85) and end organ damage (5). As discussed below, sEHIs synergize with classical anti-inflammatory drugs, including COX and LOX inhibitors (82, 86) as well as phosphodiesterase (PDE) inhibitors (84).
Regulation of Pain by Epoxy-Fatty Acids and Soluble Epoxide Hydrolase Inhibitors
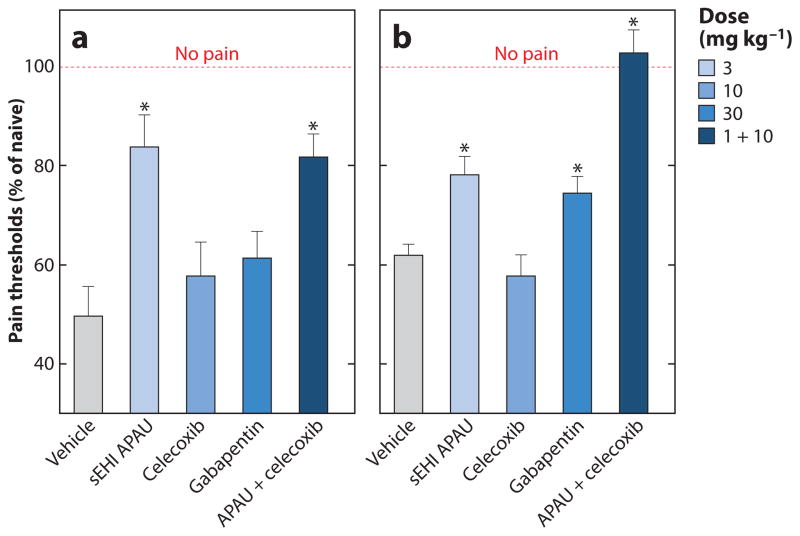
Like inflammation, pain is a component of many disease states. Because EpFAs and sEHIs dramatically reduce inflammation, they also were anticipated to reduce inflammatory pain (75). This effect was subsequently tested in multiple rodent models of inflammatory pain in which sEHIs and EpFAs reduce pain perception (Figure 4a) (87). The direct application of high-dose EETs to naive rodents increases pain-like behavior and is biphasic (88). In contrast, in an induced pain state, the same application of EETs is antihyperalgesic, as is sEHI treatment (88, 89). However, sEHIs do not alter pain perception in healthy animals. Intriguingly, sEH knockout mice show slow recovery from induced inflammatory pain, whereas exogenous EETs, with the exception of the 8,9-EET isomer, do not cause pain-like responses (90). In addition, regioisomers of docosahexaenoic acid epoxides show unequal effects on inflammatory pain (9). Thus, a more complex homeostatic balance has yet to be explored. Overall, however, increasing EpFA concentrations with sEHIs reduces heightened pain perception in inflammatory models.
Figure 4.
(a) Effect of a soluble epoxide hydrolase inhibitor (sEHI), APAU (Figure 2, compound 3), in a lipopolysaccharide (LPS)-induced inflammatory pain model (82, 88). (b) Effect of sEHIs in a streptozocin-induced type 1 diabetes neuropathic pain model (89). APAU is compared with celecoxib (which reduces inflammatory pain) and gabapentin (which reduces neuropathic pain). Nociceptive responses were measured with a von Frey apparatus and electronic aesthesiometer before the induction of diabetes or before the injection of LPS. Rats (n = 6 per group) were treated with the tested drugs 60 min prior to 10-μg intraplantar LPS injection in the inflammatory pain model; pretreatment was not possible for the neuropathic pain model. The measurements were taken at 2 h postinjection and are reported as a percent of the pretreatment naive baseline (no pain). All compounds were formulated in PEG400 vehicle and administered by subcutaneous injection. Asterisk indicates that compared to the vehicle control, the treatment significantly reduced the pain (p < 0.01). Abbreviation: APAU, 1-(1-acetypiperidin-4-yl)-3-adamantanylurea.
Chronic or neuropathic pain is notoriously resistant to treatment. Thus, it was surprising to find that sEHIs block pain-like behavior in streptozocin-induced type 1 diabetic neuropathy (Figure 4b) (89). sEHIs are more potent than the widely used drug gabapentin at reducing neuropathic pain, without inducing sedating effects. Neuropathic pain remains a poorly met clinical need, and the ability of sEHIs to reduce this type of pain in animal models suggests potential clinical applications (25).
The effectiveness of sEHIs in both inflammatory and neuropathic pain is associated with elevated levels of EpFAs (9, 75, 82, 84, 88, 89), suggesting a common mechanism of action (87). sEHIs are more efficient than the COXIB celecoxib at reducing inflammatory pain (Figure 4a), suggesting that sEHI/EpFA action on inflammatory pain goes beyond a simple decrease in COX2/PGE2-dependent inflammation (Figure 3). Furthermore, the analgesic effect of sEHIs may depend on the presence of cyclic adenosine monophosphate (cAMP), a common second messenger of pain perception (84). Both the mechanism of EETs and the action of sEHIs on neuropathic pain appear to involve neurosteroids (84). Furthermore, in the central nervous system, sEHIs alter the synthesis and release of endogenous analgesic peptides (91). An increase in the release of the opioid peptides β-endorphin and met-enkephalin is evident when EpFAs are injected directly into the ventrolateral periaqueductal grey matter, and EpFA regioisomers induce the release of somatostatin (92). Because of the mechanism of action, coadministering sEHIs with other synergizing drugs such as COX inhibitors and PDE inhibitors, particularly PDE isozymes 4 and 5 (82, 84), may offer therapeutic benefit. A dual COXIB/sEHI has been shown to be more effective at reducing pain than either of the inhibitors alone (93).
Soluble Epoxide Hydrolase Inhibition in Cardiovascular Diseases
Inhibition of sEH protects against a variety of cardiovascular diseases, including hypertension-induced end organ damage, atherosclerosis, inflammation, and cardiac hypertrophy (5). Possible clinical applications of sEHIs in these diseases are summarized in Table 1.
Table 1.
Clinical perspective for the usage of soluble epoxide hydrolase inhibitors in human health
| Disease state | Clinical perspective |
|---|---|
| Inflammation | In many models, sEHIs compare favorably with COXIBs in reducing inflammation. However, the best results are obtained with cotreatment of sEHIs with inhibitors of the COX or LOX pathway. sEHIs also reduce some side effects of COXIBs. |
| Inflammatory pain | sEHIs reduce inflammatory pain, but penetrating this market will be difficult because numerous drugs already exist in this area. Reducing pain in the companion animal area, however, is an unmet need. sEHIs appear to provide better safety profiles than do existing treatments for the treatment of chronic inflammatory pain. |
| Neuropathic pain | Because neuropathic pain is an unmet medical need, the analgesic effects of sEHIs suggest high potential for therapeutic benefit. In addition, sEHIs have the potential to be used in synergistic combinations with COX and PDE inhibitors. |
| Hypertension | sEHIs reduce hypertension in numerous but not all animal models. In addition, they often are less effective than existing drugs. One sEHI failed in a Phase IIA human clinical trial for hypertension. However, sEHIs can help reduce the hypertensive side effects of other drugs, including NSAIDs and antiangiogenesis compounds. |
| Cardiac hypertrophy/arrhythmia | In animal models, sEHIs reduce cardiac hypertrophy, arrhythmia, and electrical remodeling. These results provide a strong scientific basis for targeting heart failure and cardiac arrhythmia in humans. |
| Atherosclerosis/aneurysm | In rodent models, sEHIs effectively reduce atherosclerotic lesions and attenuate abdominal aortic aneurysm formation. |
| Renal failure | sEHIs display a protective effect on kidney damage induced by various conditions, including hypertension, inflammation, and chemotherapeutics. |
| Blood clotting | EETs reduce platelet aggregation. sEHI cotreatment with COX2 inhibitors does not result in an imbalance in prostacyclin and thromboxane or an increase in 20-HETE. Thus, sEHIs could increase the safety of COX2-selective drugs. |
| Stroke/heart attack | In rodent and dog models, sEHIs reduce infarct size and ischemia-reperfusion injury resulting from stroke and heart attack. |
| COPD | In a smoke-induced rat COPD model, sEHIs reduce lung inflammation and improve lung function. This benefit might extend to other disorders involving pulmonary inflammation. |
| Pulmonary hypertension | In rodent models, sEHIs show promise in treating pulmonary hypertension. However, some EpFAs increase pulmonary vascular remodeling, pulmonary artery constriction, and hypertension. The hypoxia-driven pulmonary vasoconstriction could be used to increase blood oxygenation. |
| Diabetes/metabolic syndrome | sEHIs tend to normalize dysregulated glycemic states in many but not all models of diabetes and metabolic syndrome. Unexpectedly, sEHIs and EETs are efficient at modulating insulin secretion in both type 1 and type 2 diabetes through a range of mechanisms. sEHIs currently are more attractive in treating the comorbidities associated with diabetes than in treating the underlying disease. |
Abbreviations: 20-HETE, 20-hydroxyeicosatetraenoic acid; COPD, chronic obstructive pulmonary disease; COX, cyclooxygenase; COXIB, COX2-selective inhibitor; EET, epoxyeicosatrienoic acid; EpFA, epoxy-fatty acid; LOX, lipoxygenase; NSAID, nonsteroidal anti-inflammatory drug; PDE, phosphodiesterase; sEH, soluble epoxide hydrolase; sEHI, soluble epoxide hydrolase inhibitor.
The first demonstration of the biological efficacy of sEHIs was in the reduction of blood pressure in spontaneously hypertensive rats (30). This observation was exciting because the sEHIs represented a new mechanism to treat hypertension, possibly providing another tool to reduce refractory hypertension with drug combinations. In the intervening years, sEH inhibition and the resulting increase in EETs have been shown to reduce hypertension in many models while not reducing blood pressure in normal animals, with the most dramatic effects found in angiotensin-driven and salt-sensitive hypertension (reviewed in 5, 23, 24, 66, 73). A caution with some hypertension models is that laboratory rats have two major haplotypes of the cis regulatory element of the EPHX2 gene that can alter sensitivity to sEHIs (25, 94).
The reduction in blood pressure by sEHIs is associated with elevated levels of EpFAs, especially EETs (23, 30, 66). EETs regulate blood pressure by two different mechanisms: action on the vascular tone in small resistance arteries, and action in the kidney through enhanced natriuresis (23, 95). In the wall of small arteries, EETs released from the endothelial cells activate large-conductance, calcium-activated potassium channels on the smooth muscle cells, causing vasodilatation. The endothelium-dependent mechanisms of EETs were recently reviewed (66). Supporting this endothelium-derived hyperpolarizing factor–like action, EETs also increase endothelial nitric oxide synthase expression and NO release in endothelial cells (70). Some but not all of the actions of sEHIs and EETs on blood pressure appear to be mediated by NO (96). In the kidney, EETs tend to promote sodium excretion and increase renal blood flow (95). In a 2-kidney, 1-clip model, sEHIs reduce blood pressure but fail to improve autoregulation of the glomerular filtration rate (97). In a transgenic rat model with overexpression of renin, sEHIs reduce blood pressure, improve the pressure-natriuresis relationship, and reduce development of hypertension and organ damage (98). These effects are greater when sEHIs are combined with inhibitors of 20-HETE biosynthesis (11). A caution is that sEHIs in mice with progressive renal disease cause an increase in albuminuria, possibly by altering LOX metabolites (99).
EPHX2 polymorphism in humans results in a significant association between forearm vasodilator response to vasoactive stimulants and sEH activity (100). AUDA (Figure 2, compound 1) given topically on the human forearm increases subcutaneous blood flow (101). Following oral administration, AR9281 (Figure 2, compound 3) showed a high level of safety in Phase I and IIA clinical trials (102) but failed to show efficacy in early-stage hypertension. This failure, coupled with effective existing drugs for angiotensin-driven hypertension, suggests that hypertension is not a good primary indication for sEHI clinical trials. However, each antihypertensive agent has its own efficacy and safety profiles, and agents with unique modes of action are greatly needed (73). In addition, the antihypertensive properties of sEHIs are attractive in dealing with hypertension as a comorbidity associated, for example, with the use of nonsteroidal anti-inflammatory drugs (NSAIDs) to treat inflammatory diseases (23, 73). sEHIs have the potential to lower blood pressure increased by antiangiogenesis drugs (66). Furthermore, sEHIs attenuate endothelial injury, which is a common underlying condition associated with hypertension, hyperhomocysteinemia, atherosclerosis, and coronary heart disease (5).
Low EET levels and high sEH levels have been associated with cardiac hypertrophy (103, 104). Treatment with sEHIs results in many cardioprotective and antihypertrophic effects—both therapeutic and prophylactic—including the lowering of fetal gene markers of hypertrophy, a reduction in heart size, and a reduction of fibrosis in numerous models (104–108). EETs appear to act in part by reducing inflammation in cardiomyocytes (104, 105). Enhanced susceptibility or tendency for cardiac arrhythmia is prevented by sEHIs in many hypertrophy models (103, 105, 108). These results provide a scientific basis for targeting heart failure as a possible clinical trial indication (108).
sEH inhibition protects not only against systemic inflammation (Figure 3) but also against vascular inflammation, which involves endothelial activation, leukocyte adhesion/infiltration into the arterial wall, and endothelial dysfunction. The anti-inflammatory actions of EETs and sEHIs in the vasculature include attenuation of cytokine-induced endothelial activation and leukocyte adhesion (72, 75, 78, 79), as well as prevention of endothelium-dependent vascular remodeling (109). Inhibition of sEH also attenuates homocysteine-induced endothelial injury (41) and improves vascular inflammation and endothelial dysfunction in cardiovascular diseases (96, 109). Vascular inflammation leads to atherosclerosis and aneurysm formation, both of which are reduced by sEHIs (110, 111). Therefore, sEHIs have potential utility to protect against vascular inflammation, atherosclerosis, and end organ damage (5).
EETs and sEHIs modulate blood clotting. Platelet aggregation plays a key role in thrombic events, and EETs, especially the 11,12- and 14,15-EETs, reduce platelet aggregation through membrane hyperpolarization and enhanced expression and activity of endothelial fibrolytic enzymes (112). Numerous hypotheses have been proposed to explain why rofecoxib (Vioxx® ) causes a higher incidence of strokes, perhaps by decreasing platelet stability, resulting in more rapid clotting (2). One hypothesis is that selective inhibition of COX2 causes an increase in 20-HETE (113); another is that it leads to a shift in relative prostacyclin and thromboxane levels, leading to platelet instability (reviewed in 2). sEHIs and the resulting increase in EET levels return this ratio to normal (82), resulting in more normal blood-clotting times. Thus, sEHIs could increase the safety of COXIBs.
EETs have a cardioprotective role in ischemia-reperfusion injury that results in vascular and myocardial dysfunction, leading to heart attacks and strokes (24, 79, 108, 114). Administration of sEHIs in multiple rodent and canine cardiac ischemia-reperfusion injury models prevented progressive cardiac remodeling and reduced infarct size in the heart (reviewed in 106, 115). Similar effects were observed in rodent stroke models (reviewed in 116), and the combination of sEHIs and EETs seems promising in this area in rodent and canine models (115, 117). The R287N single-nucleotide polymorphism, which results in a low-sEH-activity phenotype, is associated with improved tolerance against ischemia (106), suggesting that in humans sEHIs have the potential to improve recovery from stroke and heart attacks.
Soluble Epoxide Hydrolase and Pulmonary Diseases
Whereas LOX pathway inhibitors, steroids, and other drugs are widely used to reduce acute lung inflammation, medications are needed for chronic lung inflammation (118, 119). Because sEHIs can reduce systemic inflammation and hypertension in the periphery, investigators decided to examine the role of sEHIs and EpFAs in lung diseases. As a result, EpFAs were shown to regulate pulmonary vascular tone and human lung inflammation (80).
Chronic obstructive pulmonary disease (COPD) is poorly treatable. In a smoke-induced rat COPD model, sEHIs can attenuate the inflammation associated with acute exposure to tobacco smoke (76, 120). In addition, sEHI administration improved lung function by reducing tobacco smoke–induced total respiratory resistance and tissue damping, and attenuating the tobacco smoke–induced increase in alveolar airspace size (120). These data suggest that sEHIs have potential for treating COPD and possibly other pulmonary disorders that have an inflammatory component, such as asthma and cystic fibrosis.
Because of the potent effects of EpFAs on other vascular diseases (see above) and their in vitro effects on pulmonary vascular tone (80, 119), EpFAs were studied for their action on the lung vasculature. EETs, sEHIs, and sEH gene deletion reduced pulmonary artery pressure in rat models of pulmonary hypertension (reviewed in 121). Although sEHIs and EpFAs show promise in treating acute pulmonary hypertension and EETs dilate many peripheral blood vessels, they can cause hypoxia-driven pulmonary vessel constriction. This result raises the possibility that increasing EETs could worsen some forms of pulmonary hypertension.
Effects of Soluble Epoxide Hydrolase Inhibitors on Diabetes and Metabolic Syndrome
Although early work indicated that EETs are a component of the glycemic system (122), it took almost 25 years to reiterate the role of EETs in the pathophysiology of the endocrine system in relation to glucose homeostasis. EETs and sEHIs do not change blood glucose and insulin levels or insulin sensitivity of normal animals but tend to normalize dysregulated glycemic states in many but not all models of diabetes and metabolic syndrome (123, 124). These studies converge on the ability of EETs, sEH gene deletion, and sEHIs to increase insulin secretion in both type 1 and type 2 diabetes. Although the range of actions of sEHIs in diabetes is still unknown, current knowledge suggests that numerous mechanisms (including an increase in the size and output of pancreatic β-cells, the prevention of adipocyte dysregulation, and an effect on insulin tolerance) are at play (123, 124).
In rodent models of metabolic disorders, sEHIs are reported to lower blood pressure, lessen body weight gain, increase insulin sensitivity, reduce adipose tissue, and decrease inflammatory markers (125, 126). Expression of sEH is increased in developing adipose tissue of obese mice, suggesting a role of sEH and EETs in lipid regulation and adipogenesis (35). Moreover, in rats and mice fed different types of high-fat diets, sEHIs normalize the dysregulated metabolic parameters that are mirrored in human patients (124, 126). Taken as a whole, current knowledge suggests that inhibition of sEH has potential in addressing deregulated components of metabolic disorders.
Cautionary notes are that sEHIs are not effective in all rodent models of diabetes, that no clear mechanism of action for their positive effects is known, and that Arête Therapeutics failed to see benefit of an sEHI in a Phase IIA clinical trial. Thus, sEHIs have not shown clear promise as a monotherapy for metabolic disorders (27). However, in addition to possibly exerting direct effects on parameters related to glucose homeostasis, sEHI treatment may address major comorbidities of diabetes including inflammation, cardiac hypertrophy, renal failure, peripheral vascular disorders, fibrosis, and neuropathic pain.
Possible Beneficial Effects of Soluble Epoxide Hydrolase Inhibitors: Drug Combinations
PPARα agonists such as fibrates (which are used as hypolipidemic agents) and PPARγ agonists such as rosiglitazone (which are used as antidiabetic drugs) induce sEH expression in rodents (22, 35, 36). Because sEH appears to play roles in adipogenesis and in the development of diabetes (35, 124–126), the induction of sEH appears to be counterproductive to the goals of PPAR agonist administration. In addition, the overexpression of sEH could explain some of the adverse effects observed with PPAR agonists (127). Thus, cotreatment of PPARα or PPARγ ligands with sEHIs has the potential to improve their efficacy and reduce side effects.
The strong anti-inflammatory activities of sEHIs occur in part through the transcriptional downregulation of COX2, resulting in lower production of proinflammatory prostaglandins such as PGE2 (75, 76, 82). The data predict an additive or synergistic reduction in inflammation by combining sEHIs and COXIBs (82). This observation has since been extended to a beneficial interaction among sEHIs and aspirin, NSAIDs, COXIBs, and blockers of the LOX5 pathway (86). As discussed above, COXIBs increase cardiovascular risk, particularly in individuals with low levels of NO (2). In rodent models, both the prostacyclin/thromboxane ratio and the high levels of 20-HETE associated with COXIB treatment have been normalized with sEHIs, resulting in more normal bleeding times (82). Similarly, sEHIs are antihypertensive, whereas many NSAIDs and COXIBs are hypertensive. Thus, blood pressure reduction may represent another benefit of combining sEHIs with COX inhibitors. Of particular note in models of diabetic neuropathic pain, the combination of sEHIs with some NSAIDs causes a dramatic synergism in pain reduction (82, 87). Thus, combinations of sEHIs with other therapeutics that target the ARA cascade are attractive for enhancing efficacy while reducing dose and side effects.
sEHIs reduce the allodynia and hyperalgesia of the enhanced pain state but show little hypoalgesia and have little if any effect on pain perception in a normal animal (87). Coadministration of PDE inhibitors, particularly PDE4 inhibitors, enhances the action of sEHIs and EETs, supporting the hypothesis that they exert their analgesic effects in the presence of cyclic nucleotides such as cAMP (84). Because PDE inhibitors increase EET levels in the absence of sEHIs, PDE inhibitors and sEHIs should have additive or synergistic effects. This combination greatly improves the efficacy of sEHIs as anticonvulsants (84). The use of many PDE inhibitors has been limited because of side effects, but combining them with sEHIs may extend their utility by reducing the dose required for efficacy.
Potential Side Effects of Soluble Epoxide Hydrolase Inhibitors
No adverse reactions were observed in Phase I and IIA human clinical trials with AR9281 (Figure 2, compound 3), even when given at high doses (25, 102). However, this encouraging observation should be tempered with caution because sEHIs might cause side effects, which can be divided into mechanism-related side effects and compound-related ones. The high potency and good pharmacokinetic profile of the compounds developed recently suggests that there might be a good therapeutic index. Most sEHIs are not kinase inhibitors. However, the anticancer drug sorafenib represents an example whereby the activity on one target overlaps with that of another (128). A search for such off-target effects needs to be systemically addressed. To date, no off-target action by sEHIs of diverse structures has been reported (25, 50). One possible reason for the lack of mechanism-related side effects is that, even with total inhibition or knockout of sEH, it is difficult to obtain large increases in plasma levels of EpFAs because other metabolic pathways then dominate (4, 25). Nevertheless, the plethora of effects of EpFAs suggests caution.
Several eicosanoids have opposite effects in the peripheral versus pulmonary circulation. Whereas rodent studies suggest that sEHIs reduce some forms of pulmonary hypertension (121), EETs are tied to regulation of hypoxia-inducible transcription factors, pulmonary vascular remodeling, pulmonary artery constriction, and hypertension (121). Thus, their potential use in patients with pulmonary hypertension, hypoxia, or heart failure should be approached with caution (5, 24). Although sEHIs and presumably EETs improve cardiac function following ischemia and cardiac failure, survival was reduced in sEH knockout animals in a cardiac arrest resuscitation model (129). Whether this reduced survival rate resulted from an increase in EETs or from another strain-related change is not known. sEHIs stabilize blood clotting time, whether it is perturbed by agents such as rofecoxib, which decrease clotting time, or those such as aspirin, which increase clotting time (2). Because many people take aspirin to increase clotting time, overcoming this side effect could be seen as deleterious. EETs have long been known to be mildly angiogenic, particularly in the presence of vascular endothelial growth factor. A recent study (130) illustrates a common difficulty with such drugs. The very angiogenesis that is attractive in wound healing or the treatment of some developmental abnormalities can pose a danger of enhanced growth and metastasis of some tumors (5, 131). These data also suggest that the benefits and risks of EET mimics and sEHIs be balanced and that patient populations be selected carefully.
CONCLUSIONS AND FUTURE DIRECTIONS
Angiogenesis, which is the formation of new blood vessels from preexisting vessels, is vital for many physiologic processes including wound healing, tissue regeneration, and multiple pathological processes such as tumor progression and metastasis. Defining the conditions under which EETs and sEHIs can be used therapeutically to enhance or limit angiogenesis is critical (130–132). However, 17,18-EpETE-derived eicosapentaenoic acid inhibits endothelial proliferation (133); this apparent opposing effect requires further investigation into the roles played by EpFAs and sEH in angiogenesis and cancer biology.
Fibrosis, the formation of excess fibrous connective tissue in an organ or tissue, usually happens along with chronic inflammation in the remodeling process. An increasing number of studies show that EETs and sEHIs reduce undesirable fibrosis and, taken together, suggest that reduction of fibrosis could be a common mechanism across numerous diseases (107, 128, 134, 135). Of course, the availability of new tools to study the P450 branch of the ARA cascade should provide deeper insight into the biology it regulates.
The sEHIs have proven to be valuable tools for dissecting the biology of the ARA cascade by illustrating the numerous actions of EpFAs. Because many of these actions generate favorable biology, sEHIs and EpFA mimics can potentially be used as therapeutic agents—both as primary medicines and in combination with other agents (Table 1). The therapeutic index of sEHIs appears large, and both genetic and chemical knockout studies indicate a high degree of safety. However, future trials and risk-benefit evaluations will determine whether sEHIs have clinical utility.
Acknowledgments
This work was partially funded by National Institute of Environmental Health Sciences (NIEHS) grant ES02710, NIEHS Superfund Basic Research Program P42 ES04699. B.D.H. is a George and Judy Marcus Senior Fellow of the American Asthma Society. The authors thank Ms. Louisa Lo for her help in the preparation of this manuscript.
Glossary
- EpFA
epoxy-fatty acid
- EET
epoxyeicosatrienoic acid
- sEH
soluble epoxide hydrolase
- DiHFA
1,2-dihydroxy-fatty acid
- sEHI
soluble epoxide hydrolase inhibitor
- mEH
microsomal epoxide hydrolase
- PDE
phosphodiesterase
- COPD
chronic obstructive pulmonary disease
Footnotes
DISCLOSURE STATEMENT
The authors and the University of California hold several patents on sEHIs and their uses. Currently, none of these patents are licensed, and the authors do not hold equity in any company developing sEHIs as therapeutics.
LITERATURE CITED
- 1.Haeggström JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A. Advances in eicosanoid research, novel therapeutic implications. Biochem Biophys Res Commun. 2010;396:135–39. doi: 10.1016/j.bbrc.2010.03.140. [DOI] [PubMed] [Google Scholar]
- 2.Marnett LJ. The COXIB experience: a look in the rearview mirror. Annu Rev Pharmacol Toxicol. 2009;49:265–90. doi: 10.1146/annurev.pharmtox.011008.145638. [DOI] [PubMed] [Google Scholar]
- 3.Williams JM, Murphy S, Burke M, Roman RJ. 20-Hydroxyeicosatetraenoic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56:336–44. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector AA. Arachidonic acid cytochrome P450 epoxygenase pathway. J Lipid Res. 2009;50(Suppl):S52–56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–30. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang H, Quilley J, Doumad AB, Zhu AG, Falck JR, et al. Increases in plasma trans-EETs and blood pressure reduction in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;300:H1990–96. doi: 10.1152/ajpheart.01267.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel anti-inflammatory–pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–47. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswanathan S, Hammock BD, Newman JW, Meerarani P, Toborek M, Hennig B. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J Am Coll Nutr. 2003;22:502–10. doi: 10.1080/07315724.2003.10719328. [DOI] [PubMed] [Google Scholar]
- 9.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–90. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luria A, Weldon SM, Kabcenell AK, Ingraham RH, Matera D, et al. Compensatory mechanism for homeostatic blood pressure regulation in Ephx2 gene-disrupted mice. J Biol Chem. 2007;282:2891–98. doi: 10.1074/jbc.M608057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Čertíková Chábová V, Walkowska A, Kompanowska-Jezierska E, Sadowski J, Kujal P, et al. Combined inhibition of 20-hydroxyeicosatetraenoic acid formation and of epoxyeicosatrienoic acids degradation attenuates hypertension and hypertension-induced end-organ damage in Ren-2 transgenic rats. Clin Sci. 2010;118:617–32. doi: 10.1042/CS20090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gauthier KM, Falck JR, Reddy LM, Campbell WB. 14,15-EET analogs: characterization of structural requirements for agonist and antagonist activity in bovine coronary arteries. Pharmacol Res. 2004;49:515–24. doi: 10.1016/j.phrs.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Falck JR, Kodela R, Manne R, Atcha KR, Puli N, et al. 14,15-Epoxyeicosa-5,8,11-trienoic acid (14,15-EET) surrogates containing epoxide bioisosteres: influence upon vascular relaxation and soluble epoxide hydrolase inhibition. J Med Chem. 2009;52:5069–75. doi: 10.1021/jm900634w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W, Holmes BB, Gopal VR, Kishore RV, Sangras B, et al. Characterization of 14,15-epoxyeicosatrienoyl-sulfonamides as 14,15-epoxyeicosatrienoic acid agonists: use for studies of metabolism and ligand binding. J Pharmacol Exp Ther. 2007;321:1023–31. doi: 10.1124/jpet.107.119651. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Wang R, Li J, Rao J, Li W, et al. Stable EET urea agonist and soluble epoxide hydrolase inhibitor regulate rat pulmonary arteries through TRPCs. Hypertens Res. 2011;34:630–39. doi: 10.1038/hr.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–33. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 17.Decker M, Arand M, Cronin A. Mammalian epoxide hydrolases in xenobiotic metabolism and signaling. Arch Toxicol. 2009;83:297–318. doi: 10.1007/s00204-009-0416-0. [DOI] [PubMed] [Google Scholar]
- 18.Cronin A, Decker M, Arand M. Mammalian soluble epoxide hydrolase is identical to liver hepoxilin hydrolase. J Lipid Res. 2011;52:712–19. doi: 10.1194/jlr.M009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Medina P, Paillasse MR, Segala G, Poirot M, Silvente-Poirot S. Identification and pharmacological characterization of cholesterol-5,6-epoxide hydrolase as a target for tamoxifen and AEBS ligands. Proc Natl Acad Sci USA. 2010;107:13520–25. doi: 10.1073/pnas.1002922107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973;3:305–40. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- 21.Hammock BD, Gill SS, Mumby SM, Ota K. Comparison of epoxide hydrases in the soluble and microsomal fractions of mammalian liver. In: Bhatnagar RS, editor. Molecular Basis of Environmental Toxicity. Ann Arbor, MI: Ann Arbor Sci; 1980. pp. 229–72. [Google Scholar]
- 22.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Revermann M. Pharmacological inhibition of the soluble epoxide hydrolase—from mouse to man. Curr Opin Pharmacol. 2010;10:173–78. doi: 10.1016/j.coph.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Shen HC, Hammock BD. Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J Med Chem. 2012;55:1789–808. doi: 10.1021/jm201468j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morisseau C, Newman JW, Wheelock CE, Hill T, Morin D, et al. Development of metabolically stable inhibitors of mammalian microsomal epoxide hydrolase. Chem Res Toxicol. 2008;21:951–57. doi: 10.1021/tx700446u. [DOI] [PubMed] [Google Scholar]
- 27.Marowsky A, Burgener J, Falck JR, Fritschy JM, Arand M. Distribution of soluble and microsomal epoxide hydrolase in the mouse brain and its contribution to cerebral epoxyeicosatrienoic acid metabolism. Neuroscience. 2009;163:646–61. doi: 10.1016/j.neuroscience.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 28.Chacos N, Capdevila J, Falck JR, Manna S, Martin-Wixtrom C, et al. The reaction of arachidonic acid epoxides (epoxyeicosatrienoic acids) with a cytosolic epoxide hydrolase. Arch Biochem Biophys. 1983;223:639–48. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- 29.Borhan B, Mebrahtu T, Nazarian S, Kurth MJ, Hammock BD. Improved radiolabeled substrates for soluble epoxide hydrolase. Anal Biochem. 1995;231:188–200. doi: 10.1006/abio.1995.1520. [DOI] [PubMed] [Google Scholar]
- 30.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–98. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 31.Sura P, Sura R, Enayetallah AE, Grant DF. Distribution and expression of soluble epoxide hydrolase in human brain. J Histochem Cytochem. 2008;56:551–59. doi: 10.1369/jhc.2008.950659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeldin DC, Kobayashi J, Falck JR, Winder BS, Hammock BD, et al. Regio- and enantiofacial selectivity of epoxyeicosatrienoic acid hydration by cytosolic epoxide hydrolase. J Biol Chem. 1993;268:6402–7. [PubMed] [Google Scholar]
- 33.Sinal CJ, Miyata M, Tohkin M, Nagata K, Bend JR, Gonzalez FJ. Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J Biol Chem. 2000;275:40504–10. doi: 10.1074/jbc.M008106200. [DOI] [PubMed] [Google Scholar]
- 34.Yu Z, Davis BB, Morisseau C, Hammock BD, Olson JL, et al. Vascular localization of soluble epoxide hydrolase in the human kidney. Am J Physiol Renal Physiol. 2004;286:F720–26. doi: 10.1152/ajprenal.00165.2003. [DOI] [PubMed] [Google Scholar]
- 35.De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, et al. Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity. 2010;18:489–98. doi: 10.1038/oby.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang W, Li N, Ai D, Niu XL, Guan YF, Zhu Y. Activation of peroxisome proliferator-activated receptor-γ downregulates soluble epoxide hydrolase in cardiomyocytes. Clin Exp Pharmacol Physiol. 2011;38:358–64. doi: 10.1111/j.1440-1681.2011.05492.x. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka H, Kamita SG, Wolf NM, Harris TR, Wu Z, et al. Transcriptional regulation of the human soluble epoxide hydrolase gene EPHX2. Biochim Biophys Acta. 2008;1779:17–27. doi: 10.1016/j.bbagrm.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, et al. The antiinflammatory effect of laminar flow: the role of PPARγ, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2005;102:16747–52. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ai D, Fu Y, Guo D, Tanaka H, Wang N, et al. Angiotensin II up-regulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Proc Natl Acad Sci USA. 2007;104:9018–23. doi: 10.1073/pnas.0703229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D, Ai D, Tanaka H, Hammock BD, Zhu Y. DNA methylation of the promoter of soluble epoxide hydrolase silences its expression by an SP-1-dependent mechanism. Biochim Biophys Acta. 2010;1799:659–67. doi: 10.1016/j.bbagrm.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Zhang D, Xie X, Chen Y, Hammock BD, Kong W, Zhu Y. Homocysteine upregulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Circ Res. 2012;110:808–17. doi: 10.1161/CIRCRESAHA.111.259325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo B, Norris C, Bolstad ES, Knecht DA, Grant DF. Protein quaternary structure and expression levels contribute to peroxisomal-targeting-sequence-1-mediated peroxisomal import of human soluble epoxide hydrolase. J Mol Biol. 2008;380:31–41. doi: 10.1016/j.jmb.2008.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez GA, Morisseau C, Hammock BD, Christianson DW. Structure of human epoxide hydrolase reveals mechanistic inferences on bifunctional catalysis in epoxide and phosphate ester hydrolysis. Biochemistry. 2004;43:4716–23. doi: 10.1021/bi036189j. [DOI] [PubMed] [Google Scholar]
- 44.Newman JW, Morisseau C, Harris TR, Hammock BD. The soluble epoxide hydrolase encoded by EPXH2 is a bifunctional enzyme with novel lipid phosphate phosphatase activity. Proc Natl Acad Sci USA. 2003;100:1558–63. doi: 10.1073/pnas.0437724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morisseau C, Schebb NH, Dong H, Ulu A, Aronov PA, Hammock BD. Role of soluble epoxide hydrolase phosphatase activity in the metabolism of lysophosphatidic acids. Biochem Biophys Res Comm. 2012;419:796–800. doi: 10.1016/j.bbrc.2012.02.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tran KL, Aronov PA, Tanaka H, Newman JW, Hammock BD, Morisseau C. Lipid sulfates and sulfonates are allosteric competitive inhibitors of the N-terminal phosphatase activity of the mammalian soluble epoxide hydrolase. Biochemistry. 2005;44:12179–87. doi: 10.1021/bi050842g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enayetallah AE, Grant DF. Effects of human soluble epoxide hydrolase polymorphisms on iso-prenoid phosphate hydrolysis. Biochem Biophys Res Commun. 2006;341:254–60. doi: 10.1016/j.bbrc.2005.12.180. [DOI] [PubMed] [Google Scholar]
- 48.Sandberg M, Hassett C, Adman ET, Meijer J, Omiecinski CJ. Identification and functional characterization of human soluble epoxide hydrolase genetic polymorphisms. J Biol Chem. 2000;275:28873–81. doi: 10.1074/jbc.M001153200. [DOI] [PubMed] [Google Scholar]
- 49.Przybyla-Zawislak BD, Srivastava PK, Vazquez-Matias J, Mohrenweiser HW, Maxwell JE, et al. Polymorphisms in human soluble epoxide hydrolase. Mol Pharmacol. 2003;64:482–90. doi: 10.1124/mol.64.2.482. [DOI] [PubMed] [Google Scholar]
- 50.Ingraham RH, Gless RD, Lo HY. Soluble epoxide hydrolase inhibitors and their potential for treatment of multiple pathologic conditions. Curr Med Chem. 2011;18:587–603. doi: 10.2174/092986711794480212. [DOI] [PubMed] [Google Scholar]
- 51.Marino JP., Jr Soluble epoxide hydrolase, a target with multiple opportunities for cardiovascular drug discovery. Curr Top Med Chem. 2009;9:452–63. doi: 10.2174/156802609788340805. [DOI] [PubMed] [Google Scholar]
- 52.Morisseau C, Goodrow MH, Dowdy D, Zheng J, Greene JF, et al. Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc Natl Acad Sci USA. 1999;96:8849–54. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morisseau C, Goodrow MH, Newman JW, Wheelock CE, Dowdy DL, Hammock BD. Structural refinement of inhibitors of urea-based soluble epoxide hydrolases. Biochem Pharmacol. 2002;63:1599–608. doi: 10.1016/s0006-2952(02)00952-8. [DOI] [PubMed] [Google Scholar]
- 54.Olearczyk JJ, Field MB, Kim IH, Morisseau C, Hammock BD, Imig JD. Substituted adamantyl-urea inhibitors of the soluble epoxide hydrolase dilate mesenteric resistance vessels. J Pharmacol Exp Ther. 2006;318:1307–14. doi: 10.1124/jpet.106.103556. [DOI] [PubMed] [Google Scholar]
- 55.Kim IH, Morisseau C, Watanabe T, Hammock BD. Design, synthesis, and biological activity of 1,3-disubstituted ureas as potent inhibitors of the soluble epoxide hydrolase of increased water solubility. J Med Chem. 2004;47:2110–22. doi: 10.1021/jm030514j. [DOI] [PubMed] [Google Scholar]
- 56.Tsai HJ, Hwang SH, Morisseau C, Yang J, Jones PD, et al. Pharmacokinetic screening of soluble epoxide hydrolase inhibitors in dogs. Eur J Pharm Sci. 2010;40:222–38. doi: 10.1016/j.ejps.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu JY, Park SH, Morisseau C, Hwang SH, Hammock BD, Weiss RH. Sorafenib has soluble epoxide hydrolase inhibitory activity, which contributes to its effect profile in vivo. Mol Cancer Ther. 2009;8:2193–203. doi: 10.1158/1535-7163.MCT-09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eldrup AB, Soleymanzadeh F, Taylor SJ, Muegge I, Farrow NA, et al. Structure-based optimization of arylamides as inhibitors of soluble epoxide hydrolase. J Med Chem. 2009;52:5880–95. doi: 10.1021/jm9005302. [DOI] [PubMed] [Google Scholar]
- 59.Eldrup AB, Soleymanzadeh F, Farrow NA, Kukulka A, De Lombaert S. Optimization of piperidyl-ureas as inhibitors of soluble epoxide hydrolase. Bioorg Med Chem Lett. 2010;20:571–75. doi: 10.1016/j.bmcl.2009.11.091. [DOI] [PubMed] [Google Scholar]
- 60.Shen HC, Ding FX, Wang S, Deng Q, Zhang X, et al. Discovery of a highly potent, selective, and bioavailable soluble epoxide hydrolase inhibitor with excellent ex vivo target engagement. J Med Chem. 2009;52:5009–12. doi: 10.1021/jm900725r. [DOI] [PubMed] [Google Scholar]
- 61.Shen HC, Ding FX, Wang S, Xu S, Chen HS, et al. Discovery of spirocyclic secondary amine-derived tertiary ureas as highly potent, selective and bioavailable soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett. 2009;19:3398–404. doi: 10.1016/j.bmcl.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 62.Shen HC, Ding FX, Deng Q, Xu S, Tong X, et al. A strategy of employing aminoheterocycles as amide mimics to identify novel, potent and bioavailable soluble epoxide hydrolase inhibitors. Bioorg Med Chem Lett. 2009;19:5716–21. doi: 10.1016/j.bmcl.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Xing L, McDonald JJ, Kolodziej SA, Kurumbail RG, Williams JM, et al. Discovery of potent inhibitors of soluble epoxide hydrolase by combinatorial library design and structure-based virtual screening. J Med Chem. 2011;54:1211–22. doi: 10.1021/jm101382t. [DOI] [PubMed] [Google Scholar]
- 64.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor α. Drug Metab Dispos. 2007;35:1126–34. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 65.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 66.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflüg Arch. 2010;459:881–95. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, et al. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. Am J Physiol Heart Circ Physiol. 2006;291:H517–31. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 68.Lee HC, Lu T, Weintraub NL, VanRollins M, Spector AA, Shibata EF. Effects of epoxyeicosatrienoic acids on the cardiac sodium channels in isolated rat ventricular myocytes. J Physiol. 1999;519:153–68. doi: 10.1111/j.1469-7793.1999.0153o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther. 2009;328:231–39. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- 70.Hercule HC, Schunck WH, Gross V, Seringer J, Leung FP, et al. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- 71.Capdevila J, Chacos N, Werringloer J, Prough RA, Estabrook RW. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc Natl Acad Sci USA. 1981;78:5362–66. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Node K, Huo Y, Ruan X, Yang B, Spiecker M, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–79. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiamvimonvat N, Ho CM, Tsai HJ, Hammock BD. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50:225–37. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 74.Morin C, Sirois M, Echave V, Gomes MM, Rousseau E. 14,15-EET displays anti-inflammatory effects in TNFα-stimulated human bronchi: putative role of CPI-17. Am J Respir Cell Mol Biol. 2008;38:192–201. doi: 10.1165/rcmb.2007-0232OC. [DOI] [PubMed] [Google Scholar]
- 75.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci USA. 2005;102:9772–77. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, Hammock BD. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci USA. 2005;102:2186–91. doi: 10.1073/pnas.0409591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spiecker M, Liao J. Cytochrome P450 epoxygenase CYP2J2 and the risk of coronary artery disease. Trends Cardiovasc Med. 2006;16:204–8. doi: 10.1016/j.tcm.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, et al. Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice. FASEB J. 2011;25:703–13. doi: 10.1096/fj.10-171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Deng Y, Theken KN, Lee CR. Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation. J Mol Cell Cardiol. 2010;48:331–41. doi: 10.1016/j.yjmcc.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morin C, Sirois M, Echavé V, Albadine R, Rousseau E. 17,18-Epoxyeicosatetraenoic acid targets PPARγ and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. Am J Respir Cell Mol Biol. 2010;43:564–75. doi: 10.1165/rcmb.2009-0155OC. [DOI] [PubMed] [Google Scholar]
- 81.Norwood S, Liao J, Hammock BD, Yang GY. Epoxyeicosatrienoic acids and soluble epoxide hydrolase: potential therapeutic targets for inflammation and its induced carcinogenesis. Am J Transl Res. 2010;2:447–57. [PMC free article] [PubMed] [Google Scholar]
- 82.Schmelzer KR, Inceoglu B, Kubala L, Kim IH, Jinks SL, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:13646–51. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Becker J, Delayre-Orthez C, Frossard N, Pons F. Regulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases? Fundam Clin Pharmacol. 2006;20:429–47. doi: 10.1111/j.1472-8206.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 84.Inceoglu B, Wagner K, Schebb NH, Morisseau C, Jinks SL, et al. Analgesia mediated by soluble epoxide hydrolase inhibitors is dependent on cAMP. Proc Natl Acad Sci USA. 2011;108:5093–97. doi: 10.1073/pnas.1101073108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, et al. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in an apolipoprotein E-knockout mouse model. J Cardiovasc Pharmacol. 2008;52:314–23. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu JY, Yang J, Inceoglu B, Qiu H, Ulu A, et al. Inhibition of soluble epoxide hydrolase enhances the anti-inflammatory effects of aspirin and 5-lipoxygenase activation protein inhibitor in a murine model. Biochem Pharmacol. 2010;79:880–87. doi: 10.1016/j.bcp.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wagner K, Inceoglu B, Hammock BD. Soluble epoxide hydrolase inhibition, epoxygenated fatty acids and nociception. Prostaglandins Other Lipid Mediat. 2011;96:76–83. doi: 10.1016/j.prostaglandins.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79:2311–19. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci USA. 2008;105:18901–6. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brenneis C, Sisignano M, Coste O, Altenrath K, Fischer MJ, et al. Soluble epoxide hydrolase limits mechanical hyperalgesia during inflammation. Mol Pain. 2011;7:78. doi: 10.1186/1744-8069-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Terashvili M, Tseng LF, Wu HE, Narayanan J, Hart LM, et al. Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of β-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther. 2008;326:614–22. doi: 10.1124/jpet.108.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Capdevila J, Chacos N, Falck JR, Manna S, Negro-Vilar A, Ojeda SR. Novel hypothalamic arachidonate products stimulate somatostatin release from the median eminence. Endocrinology. 1983;113:421–23. doi: 10.1210/endo-113-1-421. [DOI] [PubMed] [Google Scholar]
- 93.Hwang SH, Wagner KM, Morisseau C, Liu JY, Dong H, et al. Synthesis and structure-activity relationship studies of urea-containing pyrazoles as dual inhibitors of cyclooxygenase-2 and soluble epoxide hydrolase. J Med Chem. 2011;54:3037–50. doi: 10.1021/jm2001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fornage M, Hinojos CA, Nurowska BW, Boerwinkle E, Hammock BD, et al. Polymorphism in soluble epoxide hydrolase and blood pressure in spontaneously hypertensive rats. Hypertension. 2002;40:485–90. doi: 10.1161/01.hyp.0000032278.75806.68. [DOI] [PubMed] [Google Scholar]
- 95.Imig JD. 20-HETE or EETs: which arachidonic acid metabolite regulates proximal tubule transporters and contributes to pressure natriuresis? Am J Physiol Regul Integr Comp Physiol. 2004;287:R3–5. doi: 10.1152/ajpregu.00151.2004. [DOI] [PubMed] [Google Scholar]
- 96.Bellien J, Iacob M, Remy-Jouet I, Lucas D, Monteil C, et al. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation. 2012;125:1266–75. doi: 10.1161/CIRCULATIONAHA.111.070680. [DOI] [PubMed] [Google Scholar]
- 97.Sporková A, Kopkan L, Varcabová S, Husková Z, Hwang SH, et al. Role of cytochrome P-450 metabolites in the regulation of renal function and blood pressure in 2-kidney 1-clip hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1468–75. doi: 10.1152/ajpregu.00215.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Honetschlägerová Z, Sporková A, Kopkan L, Husková Z, Hwang SH, et al. Inhibition of soluble epoxide hydrolase improves the impaired pressure-natriuresis relationship and attenuates the development of hypertension and hypertension-associated end-organ damage in Cyp1a1-Ren-2 transgenic rats. J Hypertens. 2011;29:1590–601. doi: 10.1097/HJH.0b013e328349062f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jung O, Jansen F, Mieth A, Barbosa-Sicard E, Pliquett RU, et al. Inhibition of the soluble epoxide hydrolase promotes albuminuria in mice with progressive renal disease. PLoS ONE. 2010;5:e11979. doi: 10.1371/journal.pone.0011979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee CR, Pretorius M, Schuck RN, Burch LH, Bartlett J, et al. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with forearm vasodilator responses in humans. Hypertension. 2011;57:116–22. doi: 10.1161/HYPERTENSIONAHA.110.161695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tran L, Kompa AR, Wang BH, Krum H. Evaluation of the effects of urotensin II and soluble epoxide hydrolase inhibitor on skin microvessel tone in healthy controls and heart failure patients. Cardiovasc Ther. 2012;30:295–300. doi: 10.1111/j.1755-5922.2011.00282.x. [DOI] [PubMed] [Google Scholar]
- 102.Chen D, Whitcomb R, Macintyre E, Tran V, Do ZN, et al. Pharmacokinetics and pharmaco-dynamics of AR9281, an inhibitor of soluble epoxide hydrolase, in single- and multiple-dose studies in healthy human subjects. J Clin Pharmacol. 2012;52:319–28. doi: 10.1177/0091270010397049. [DOI] [PubMed] [Google Scholar]
- 103.Monti J, Fischer J, Paskas S, Heinig M, Schulz H, et al. Soluble epoxide hydrolase is a susceptibility factor for heart failure in a rat model of human disease. Nat Genet. 2008;40:529–37. doi: 10.1038/ng.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ai D, Pang W, Li N, Xu M, Jones PD, et al. Soluble epoxide hydrolase plays an essential role in angiotensin II–induced cardiac hypertrophy. Proc Natl Acad Sci USA. 2009;106:564–69. doi: 10.1073/pnas.0811022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu D, Li N, He Y, Timofeyev V, Lu L, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci USA. 2006;103:18733–38. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao TT, Wasti B, Xu DY, Shen L, Du JQ, Zhao SP. Soluble epoxide hydrolase and ischemic cardiomyopathy. Int J Cardiol. 2012;155:181–87. doi: 10.1016/j.ijcard.2011.05.067. [DOI] [PubMed] [Google Scholar]
- 107.Kompa AR, Wang BH, Xu G, Zhang Y, Ho PY, et al. Soluble epoxide hydrolase inhibition exerts beneficial anti-remodeling actions post-myocardial infarction. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2011.12.062. In press. [DOI] [PubMed] [Google Scholar]
- 108.Qiu H, Li N, Liu JY, Harris TR, Hammock BD, Chiamvimonvat N. Soluble epoxide hydrolase inhibitors and heart failure. Cardiovasc Ther. 2010;29:99–111. doi: 10.1111/j.1755-5922.2010.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bellien J, Joannides R, Richard V, Thuillez C. Modulation of cytochrome-derived epoxyeicosatrienoic acids pathway: a promising pharmacological approach to prevent endothelial dysfunction in cardiovascular diseases? Pharmacol Ther. 2011;131:1–17. doi: 10.1016/j.pharmthera.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 110.Ulu A, Davis BB, Tsai HJ, Kim IH, Morisseau C, et al. Soluble epoxide hydrolase inhibitors reduce the development of atherosclerosis in an apolipoprotein E-knockout mouse model. J Cardiovasc Pharmacol. 2008;52:314–23. doi: 10.1097/FJC.0b013e318185fa3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang LN, Vincelette J, Cheng Y, Mehra U, Chen D, et al. Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia. Arterioscler Thromb Vasc Biol. 2009;29:1265–70. doi: 10.1161/ATVBAHA.109.186064. [DOI] [PubMed] [Google Scholar]
- 112.Sudhahar V, Shaw S, Imig JD. Epoxyeicosatrienoic acid analogs and vascular function. Curr Med Chem. 2010;17:1181–90. doi: 10.2174/092986710790827843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu JY, Li N, Yang J, Li N, Qiu H, et al. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc Natl Acad Sci USA. 2010;107:17017–22. doi: 10.1073/pnas.1011278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li N, Liu JY, Timofeyev V, Qiu H, Hwang SH, et al. Beneficial effects of soluble epoxide hydrolase inhibitors in myocardial infarction model: insight gained using metabolomic approaches. J Mol Cell Cardiol. 2009;47:835–45. doi: 10.1016/j.yjmcc.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nithipatikom K, Gross GJ. Epoxyeicosatrienoic acids: novel mediators of cardioprotection. J Cardiovasc Pharmacol Ther. 2010;15:112–19. doi: 10.1177/1074248409358408. [DOI] [PubMed] [Google Scholar]
- 116.Imig JD, Simpkins AN, Renic M, Harder DR. Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev Mol Med. 2011;13:e7. doi: 10.1017/S1462399411001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu XZ, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliver Rev. 2011;63:597–609. doi: 10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 118.Matera MG, Calzetta L, Segreti A, Cazzola M. Emerging drugs for chronic obstructive pulmonary disease. Expert Opin Emerg Drugs. 2012;17:61–82. doi: 10.1517/14728214.2012.660917. [DOI] [PubMed] [Google Scholar]
- 119.Lau EM, Corte TJ. Pulmonary hypertension in 2012: contemporary issues in diagnosis and management. Panminerva Med. 2012;54:11–28. [PubMed] [Google Scholar]
- 120.Wang L, Yang J, Guo L, Uyeminami D, Dong H, et al. Use of a soluble epoxide hydrolase inhibitor in smoke-induced chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2012;46:614–22. doi: 10.1165/rcmb.2011-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Loot AE, Fleming I. Cytochrome P450-derived epoxyeicosatrienoic acids and pulmonary hypertension: central role of transient receptor potential C6 channels. J Cardiovasc Pharmacol. 2011;57:140–47. doi: 10.1097/FJC.0b013e3181ed088d. [DOI] [PubMed] [Google Scholar]
- 122.Falck JR, Manna S, Moltz J, Chacos N, Capdevila J. Epoxyeicosatrienoic acids stimulate glucagon and insulin release from isolated rat pancreatic islets. Biochem Biophys Res Commun. 1983;114:743–49. doi: 10.1016/0006-291x(83)90843-4. [DOI] [PubMed] [Google Scholar]
- 123.Luo P, Chang HH, Zhou Y, Zhang S, Hwang SH, et al. Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J Pharmacol Exp Ther. 2010;334:430–38. doi: 10.1124/jpet.110.167544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, et al. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA. 2011;108:9038–43. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, et al. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–16. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, et al. Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats. Exp Diabetes Res. 2012;2012:758614. doi: 10.1155/2012/758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Friedland SN, Leong A, Filion KB, Genest J, Lega IC, et al. The cardiovascular effects of peroxisome proliferator-activated receptor agonists. Am J Med. 2012;125:126–33. doi: 10.1016/j.amjmed.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 128.Ionue H, Hwang SH, Wecksler AT, Hammock BD, Weiss RH. Sorafenib attenuates p21 in kidney cancer cells and augments cell death in combination with DNA-damaging chemotherapy. Cancer Biol Ther. 2011;12:827–36. doi: 10.4161/cbt.12.9.17680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hutchens MP, Nakano T, Dunlap J, Traystman RJ, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase gene deletion reduces survival after cardiac arrest and cardiopulmonary resuscitation. Resuscitation. 2008;76:89–94. doi: 10.1016/j.resuscitation.2007.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. J Clin Investig. 2012;122:178–91. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fleming I. The cytochrome P450 pathway in angiogenesis and endothelial cell biology. Cancer Metastasis Rev. 2011;30:541–55. doi: 10.1007/s10555-011-9302-3. [DOI] [PubMed] [Google Scholar]
- 132.Panigrahy D, Greene ER, Pozzi A, Wang DW, Zeldin DC. EET signaling in cancer. Cancer Metastasis Rev. 2011;30:525–40. doi: 10.1007/s10555-011-9315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cui PH, Petrovic N, Murray M. The ω-3 epoxide of eicosapentaenoic acid inhibits endothelial cell proliferation by p38 MAP kinase activation and cyclin D1/CDK4 down-regulation. Br J Pharmacol. 2011;162:1143–55. doi: 10.1111/j.1476-5381.2010.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Simpkins AN, Rudic RD, Schreihofer DA, Roy S, Manhiani M, et al. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174:2086–95. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao G, Tu L, Li X, Yang S, Chen C, et al. Delivery of AAV2-CYP2J2 protects remnant kidney in the 5/6-nephrectomized rat via inhibition of apoptosis and fibrosis. Hum Gene Ther. 2012;23:688–99. doi: 10.1089/hum.2011.135. [DOI] [PubMed] [Google Scholar]