Review on sepsis mediators, and roles in innate and adaptive immune systems, as well as implications for therapeutics.
Keywords: inflammation, innate and adaptive immunity, cytokines, sepsis
Abstract
Sepsis refers to severe systemic inflammation in response to invading pathogens. An overwhelming immune response, as mediated by the release of various inflammatory mediators, can lead to shock, multiple organ damage, and even death. Cytokines, proteases, lipid mediators, gaseous substances, vasoactive peptides, and cell stress markers play key roles in sepsis pathophysiology. Various adhesion molecules and chemokines sequester and activate neutrophils into the target organs, further augmenting inflammation and tissue damage. Although the anti-inflammatory substances counterbalance proinflammatory mediators, prolonged immune modulation may cause host susceptibility to concurrent infections, thus reflecting enormous challenge toward developing effective clinical therapy against sepsis. To understand the complex interplay between pro- and anti-inflammatory phenomenon in sepsis, there is still an unmet need to study newly characterized mediators. In addition, revealing the current trends of novel mediators will upgrade our understanding on their signal transduction, cross-talk, and synergistic and immunomodulating roles during sepsis. This review highlights the latest discoveries of the mediators in sepsis linking to innate and adaptive immune systems, which may lead to resolution of many unexplored queries.
Introduction
Sepsis, commonly referred to as SIRS, is associated with the abnormal host immune function in response to invading pathogens [1]. The term “severe sepsis” is accompanied by the mal-functioning of the vital organs, leading to multiple organ dysfunction syndrome in critically ill patients, whereas the “septic shock” occurs when sepsis is complicated by reduced blood pressure that does not respond to fluid resuscitation and vasopressors, in turn, leading to hypoxia in organ systems [1]. The current incidence of sepsis in United States is at least 240 patients/100,000 people, with the mortality rate from 25% to 30% for severe sepsis and up to 40–70% for septic shock [2].
Sepsis can initiate not only through the direct dissemination of pathogens into the bloodstream but also indirectly as a result of postsurgical complications, traumas, burn, hemorrhages, and gut IR-mediated bacterial translocations [1, 3–6]. Once invaded, the host response to pathogens is mediated through innate and adaptive immune systems. The innate-immune system constitutes the first line of defense, whereas the adaptive immune system comprises highly specialized and systemic cells to recognize specific pathogens and mount stronger attacks each time the pathogen is encountered [7]. After being triggered by an initial stimulus, the cells of the innate-immune system release plenteous amounts of cytokines, chemokines, complement-activation products, and intracellular alarmins during the early as well as late phase of sepsis [8–10]. Similarly, the adaptive immune response is induced upon interaction with the APCs that have ingested a pathogen. Upon antigen recognition, the cells of the adaptive immune system, such as naïve T cells, proliferate to generate effector cells, which in turn, liberate distinct cytokine profiles [11].
As the “cytokine storm” is thought to be responsible for triggering the inflammation in sepsis, the therapeutic benefits of anticytokine regimens have been demonstrated in animal models. Although neutralizing strategies against commonly encountered cytokines have been adopted in several clinical trials for sepsis, no such remarkable achievements are yet to be reported [12–14]. The better outcome of anticytokine therapies in septic animals as compared with humans is thought to be a result of a short therapeutic window period for reversing the events of lethal sepsis in animals [14]. Moreover, the ineffectiveness of anticytokine therapies in patients arises as a result of a prolonged immunosuppressive state at later time-points of sepsis development [15, 16]. Recently, the concept of combining more than one anticytokine agent has shown promising results in rescuing animals from sepsis [17, 18]. Hence, it is obvious that the identification of new mediators would increase the chances of understanding the complex pathophysiological events of sepsis and subsequent development of effective therapeutics.
With these fundamental views, the current review highlighting the latest mediators with their possible deleterious or beneficial roles in sepsis relies on the scope of delineating the unexplored notions of innate and adaptive immune systems, thereby imposing a better prognosis in sepsis.
SEPSIS PATHOPHYSIOLOGY: UNIVERSAL TRIGGERS AND MEDIATORS OF INNATE AND ADAPTIVE IMMUNE SYSTEM
Endotoxin/LPS, an outer-membrane component of all Gram-negative bacteria, has been widely recognized as the prime stimulating factor in sepsis [9]. The LPS-dependent TLR4 cascade has been well documented in several reviews, where the downstream signaling occurs through a series of interactions with several proteins, including LPS BP, CD14, and myeloid differentiation protein-2, leading to NF-κB- and MAPK-mediated up-regulation of pro- and anti-inflammatory cytokines [9, 19]. Prolonged activation of the LPS/TLR4 pathway leads to uncontrolled pathophysiology in sepsis [19].
Macrophages, after being triggered by endotoxin, can augment the release of a number of early proinflammatory mediators, such as TNF-α, IL-1β, IL-6, IFN-γ, IL-8, and MCP-1, as well as secondary mediators for tissue injury, e.g., NO and ROS [9, 19, 20]. In sepsis, although the activated neutrophils initially promote clearance of bacteria, they subsequently contribute to tissue injury through respiratory burst, cytotoxicity, degranulation, increased vascular permeability, and organ injury by releasing several proinflammatory mediators, MPO, and proteases [21]. In contrast, the anti-inflammatory cytokines, e.g., IL-10 and TGF-β, which are released in sepsis from macrophages and PMNs, play immune regulatory functions. However, excessive production of these anti-inflammatory mediators may later cause immune dysfunction against pathogens [22].
Upon interaction with APCs, the naïve Th cells proliferate and differentiate into Th1 cells that produce large amounts of TNF-α, IL-2, IL-12, IFN-γ, and leukotrienes. On the other hand, IL-4-driven Th2 cells give rise to immunomodulatory cytokines, such as IL-4, IL-5, IL-9, IL-10, and IL-13 [11, 23]. A majority of the Th1 cytokines are produced at the early phase of sepsis, followed by excessive production of Th2 cytokines. A shift in the balance from Th1 to Th2 cytokines can cause immune suppression, which is evident at the late stage of sepsis, rendering the host susceptibility to nosocomial infection [11, 23]. Likewise, the IL-17A is a proinflammatory cytokine that is mainly produced by Th17 cells [24]. IL-17A is involved in mediating proinflammatory responses by triggering the production of other cytokines (IL-1β, IL-6, and TNF-α) and provokes cross-talk between lymphocytes and phagocytes [24]. It has been shown recently that increased IL-17A levels have adverse effects in experimental sepsis; hence, neutralization of IL-17A markedly improves survival in animals [24]. As in some contexts, IL-17A is essential, blocking of IL-17A may not be beneficial in all cases. Therefore, the anti-IL-17A treatment of sepsis awaits further study.
A small subset of CD4+CD25+forkhead box P3+ T cells, commonly referred to as regulatory T cells, is being increased in sepsis, resulting in the release of IL-10 and TGF-β, thus regulating the Th1 phenotype via Th2 cell proliferation, activation, and differentiation [25]. Another subpopulation, the γδ T cells comprising 1–5% of the lymphocytes, produces IFN-γ, IL-4, IL-10, and TNF-α in response to pathogenic insults, hence demonstrating that γδ T cells to possess immune-activating and -regulatory phenotypes [25, 26]. NKT cells comprise 1–2% of lymphocytes in the spleen, LNs, and peripheral circulation and promote the production of IFN-γ, IL-4, IL-10, IL-13, and TGF-β [25, 27]. The potential of NKT cells to produce pro- and anti-inflammatory cytokines provides them with the capacity to promote or inhibit immune responses following severe sepsis. Recent studies using animal models of sepsis suggest that NKT cells appear to play a deleterious role following injury and might participate in the induction of immune dysfunctions by their polarization toward Th2 shift and IL-4 production [28]. In other cases, NKT cells might also play a role in organ injury as a result of their role in the recruitment of neutrophils [25, 29]. Detailed studies are necessary to confirm these findings and determine the extent to which they apply in the critically ill patients. Apart from the roles of B cells in antibody production and antigen presentation, recent findings demonstrated that the B cells could enhance early innate-immune responses during bacterial sepsis [30]. Rauch et al. [31] found an effector B cell population, which they termed as IRA B cells that protect against microbial sepsis. Specific deletion of IRA B cell activity impairs bacterial clearance, elicits a cytokine storm, and precipitates septic shock [31]. Based on the above features, here, we represent the sepsis pathophysiology in terms of universal mediators and their interaction with innate and adaptive immune systems (Fig. 1).
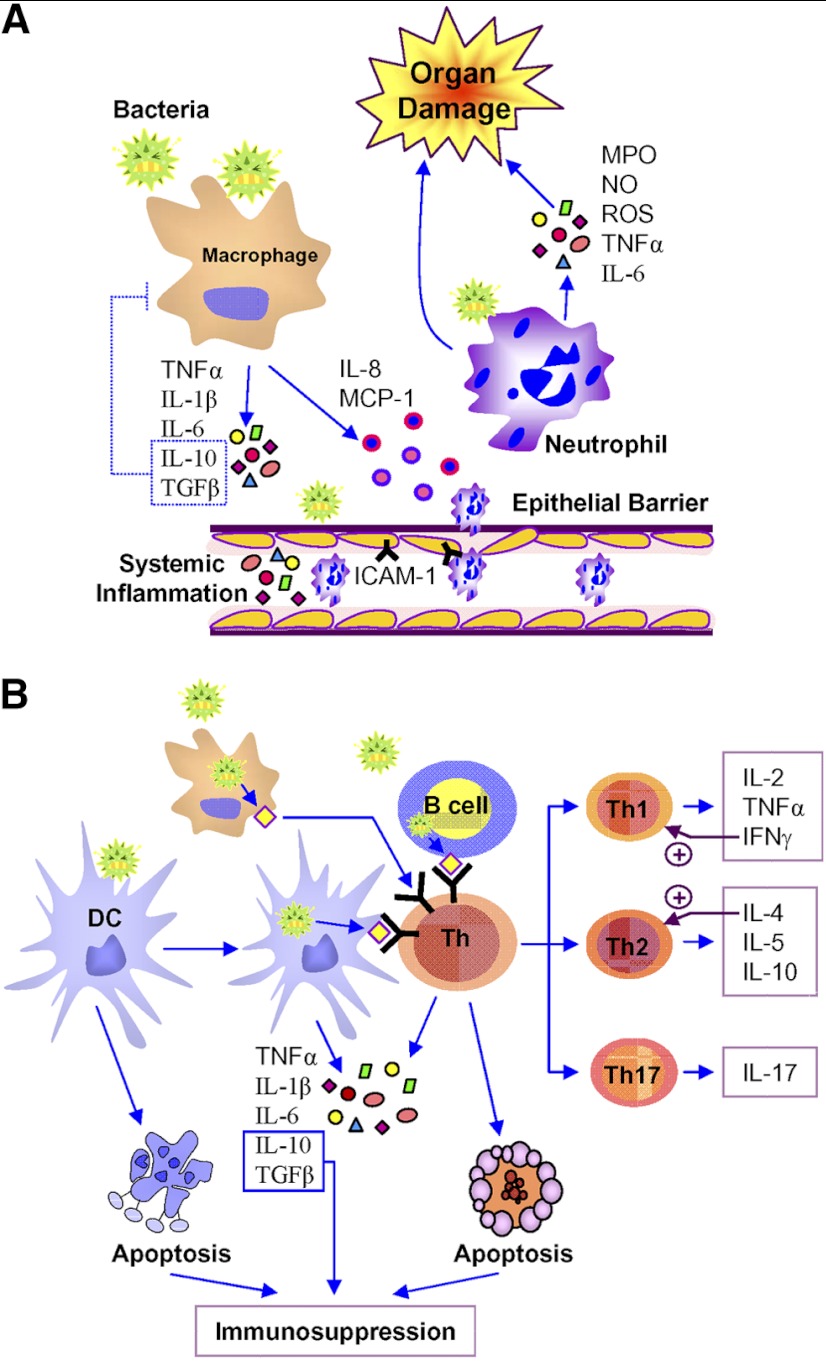
Figure 1. Roles of innate and adaptive immune system in sepsis pathophysiology.
(A) In response to invading pathogens, macrophages serve as the first line of defense, inducing the innate-immune response. Proinflammatory cytokines, e.g., TNF-α, IL-1β, and IL-6, as released from macrophages, further augment systemic inflammation and epithelial barrier dysfunction, whereas the anti-inflammatory cytokines, such as IL-10 and TGF-β, counterbalance excessive immune responses. Bacterial pathogens and systemic inflammatory mediators also promote epithelial barrier disruption to exaggerate inflammation and induce the expression of ICAM-1. The chemokines, such as IL-8 and MCP-1, released from macrophages, activate and promote neutrophil migration toward the site of inflammation, as well as to the remote organs. Excessive neutrophil infiltration exaggerates inflammation and severe organ injury by releasing several proinflammatory mediators, e.g., MPO, NO, ROS, TNF-α, and IL-6. (B) Immature DCs transform into its mature form upon interacting with the pathogen and serve as professional APCs to activate the adaptive immune system by promoting T cell functions through antigen presentation. Macrophages can also act as APCs to ingest, process, and present pathogens to T cells and promote their activation and differentiation. Activated T cells further differentiate into its lineages, generating distinct cytokine profiles, which include Th1: IL-2, TNF-α, and IFN-γ; Th2: IL-4, IL-5, and IL-10; and Th17: IL-17. DCs can also be activated by pathogens to trigger innate-immune functions by producing pro- and anti-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, IL-10, and TGF-β. In sepsis, immune cells undergo apoptosis, leading to an immunosuppressive environment.
SIRS AND CARS TRENDS IN SEPSIS
A finely tuned balance between pro- and anti-inflammatory events is a prerequisite for better prognosis in sepsis. The patients not only die during the initial, hyperinflammatory phase of sepsis but also at later time-points that are associated with a prolonged immunosuppressive state [15, 16]. We have described earlier that SIRS is an early acute hyperinflammatory state as a result of overwhelming immune response to infection that may help eliminating the pathogens, but in a prolonged course, they often cause injury to the host [1]. The occurrence of anti-inflammatory responses, which is coined as CARS, seeks to limit SIRS-mediated damage while not interfering with the pathogen elimination [32]. Thus, the CARS response can be unsafe when its effects are unchecked or miss-timed, leaving the host vulnerable to the next set of pathogens. However, the Hotchkiss and Karl model [33] best defines the complex immunologic response and therapeutic strategies of three hypothetical patients with sepsis. As the inflammatory mediators may have critical roles in mediating cell injury in sepsis, anti-inflammatory strategies applied early in patients with a hyperinflammatory immune response may be beneficial [33]. According to his schema, most deaths occur during the prolonged hypoimmune state, and reversal of this immune deficiency should generate a better outcome in sepsis. Alternatively, when patients are determined to be in a hypoimmune state, inflammatory strategies that enhance the function of the innate or adaptive immune system may be efficacious [33]. Based on these above concepts, it is reasonable to define the factors that are accountable for CARS initiation. Lymphocytes play a central role in modulating the sepsis response by their capacity to interact with the innate and adaptive immune responses [32]. The period of immunoparalysis has long been demonstrated in patients with sepsis as a result of lymphocyte apoptosis and anergy, a state that refers to diminished proliferation and inability to respond antigens [32]. In addition, decreased expression of antigen-presenting receptors on monocytes/macrophages and reduced amounts of TNF-α and IL-1β in response to bacterial challenges lead to facilitate CARS in sepsis patients [32]. Another commonly encountered event that may lead to CARS is through the overproduction of the anti-inflammatory cytokine IL-10, which suppresses TNF-α expression and thereby predicts an increased risk of secondary infection and poor prognosis [32]. Further work on current sepsis mediators in relation to SIRS versus CARS responses may reveal favorable outcomes in sepsis.
MEDIATORS OF SEPSIS: RECENT ADVANCES
Apart from the well-characterized functions of the above-mentioned cytokines, recent studies have unveiled several other mediators in sepsis. Herein, we randomly focus on newly identified cytokines, lipid mediators, alarmins, and vasoactive neuropeptides that exaggerate inflammation and tissue damage in sepsis. Whereas not all mediators were revealed to be harmful in sepsis, as some have immunomodulatory and protective roles, they are summarized altogether to improve our understanding of the core of sepsis pathophysiology (Table 1 and Fig. 2). These latest mediators are currently considered to be the hot topic in the sepsis field and predominantly implicate emerging roles in innate and adaptive immune systems.
Table 1. At a Glance: Current Trends in Sepsis Mediators.
| Mediators | Cellular sources | Roles in sepsis | References |
|---|---|---|---|
| Cytokines | |||
| IL-7 | Stromal cells | Promotes CD4 and CD8 T cell survival in sepsis | [34–36] |
| IL-17A | Th17 cells | Induces IL-1β, IL-6, and TNF-α release; neutralization of IL-17A improves survival in murine sepsis | [24] |
| IL-22 | DCs, T cells, NKT cells, mast cells | Increased IL-22 levels correlate with sepsis severity; its blocking leads to neutrophil accumulation and reduces bacterial load at the site of infection | [37–39] |
| IL-33 | T cells, mast cells, fibroblasts | Attenuates sepsis by mobilizing neutrophils to the site of infection and clears the pathogens | [40, 41] |
| Soluble receptor | |||
| sTREM-1 | Monocytes, neutrophils | Up-regulated in sepsis; induces inflammatory cytokine gene expression | [42–44] |
| Stress mediators | |||
| HMGB1 | Macrophages, NK cells, neurons, DCs, osteoclasts, epithelium | Induces TNF-α, IL-1β, and IL-8 production; Th1 polarization; tissue injury; barrier dysfunction; HMGB1 neutralization improves survival in sepsis | [10, 45, 46] |
| Histones | Eukaryotic cells | Cytotoxic; cause lethality in septic animals | [47, 48] |
| Glycoprotein | |||
| Osteopontin | Macrophages, T cells, DCs, epithelial cells, osteoblasts | Induces TNF-α, IL-1β, IFN-γ, and IL-6 release from macrophages; promotes Th1 differentiation; regulates iNOS expression; induces neutrophil migration | [49–51] |
| Lipid mediators | |||
| S1P | Endothelium, erythrocytes, platelets | Acts as a proinflammatory mediator; promotes immune cell trafficking; inhibition of Sphk1; protects against LPS-induced endotoxic shock in mice | [52–54] |
| RvD2 | Leukocytes, PMN | Reduces local and systemic bacterial burden, excessive cytokine production, and neutrophil recruitment; improves survival in sepsis | [55–57] |
| Resistin | Adipocyte, macrophages | Acts as a proinflammatory cytokine | [58–60] |
| Adipokines | |||
| Adiponectin | Adipose tissue, immune cells | Exhibits anti-inflammatory responses in macrophages and endothelial cells | [61, 62] |
| Visfatin | Adipose tissue, immune cells | Induces IL-1β, TNF-α, and IL-6 production in macrophages; inhibits neutrophil apoptosis | [63–66] |
| Vesoactive peptides | |||
| Ghrelin | Brain cells, stomach cells | Sympathoinhibition; suppresses inflammation; improves survival in sepsis. | [67, 68] |
| AM/AMBP-1 | Pheochromocytoma cells, immune cells | Attenuates inflammation; inhibits neutrophil activation and migration to inflamed sites | [69, 70] |
| ET-1 | Endothelial cells, fibroblasts | Causes vasospasm, vascular damage, cardiovascular remodeling, renal perfusion, and inflammation | [71–73] |
| Growth factor | |||
| MFG-E8 | Macrophages, DCs, epithelial cells | Phagocytosis of apoptotic cells; antiapoptotic; anti-inflammatory; improves survival in septic mice | [74–76] |
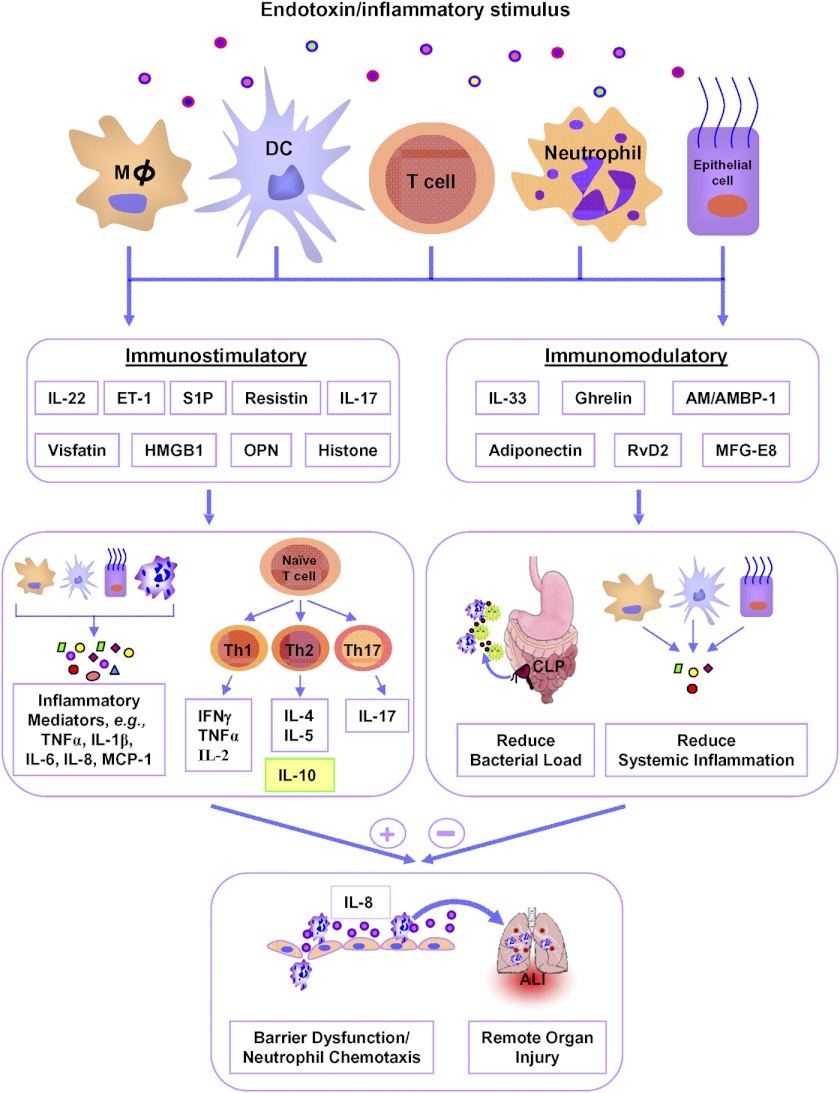
Figure 2. Latest trends in sepsis mediators.
In sepsis, the newly identified mediators are categorized into immunostimulatory, which includes IL-22, ET-1, S1P, resistin, IL-17, visfatin, HMGB1, OPN, and histone, and immunomodulatory, such as IL-33, ghrelin, AM/AMBP-1, adiponectin, RvD2, and MFG-E8, depending on their roles in exaggerating or regulating immune functions. Immunostimulatory mediators act on macrophages (Mϕ), DCs, neutrophils, lymphocytes, and epithelial cells to augment the production of proinflammatory cytokines, e.g., TNF-α, IL-1β, IL-6, IFN-γ, IL-2, IL-4, IL-5, IL-17 and chemokines, e.g., IL-8 and MCP-1, which exert profound effects on neutrophil activation and migration in various organs. On the other hand, IL-10 exhibits immune-regulatory roles to control excessive production of proinflammatory cytokines. As IL-10 has been considered a “negative” regulator in sepsis, it is shown in the yellow-filled box to be distinguished from other Th2 cytokines. Excessive neutrophil infiltration releases ROS and MPO, leading to multiple organ injuries. On the other hand, novel immunomodulatory substances attract neutrophils at the infectious area, promote bacterial clearance, attenuate systemic inflammation, reduce neutrophil infiltration at various organs, and improve survival in sepsis.
CYTOKINES, SOLUBLE MEMBRANE MARKER, STRESS MOLECULES, AND EXTRACELLULAR PHOSPHO-PROTEIN
IL-7
One of the characteristic features of sepsis is the profound loss of T cell numbers in various lymphoid organs [34]. Recently, IL-7, a hematopoietic growth factor, has been reported to possess antiapoptotic roles, which is essential for lymphocyte survival and expansion [35, 36]. IL-7 is produced by stromal cells in lymphoid tissues and transduces the T cell survival signal through the IL-7R, a heterodimer consisting of an IL-7Rα and common γ-chain receptor [77]. In addition to its antiapoptotic properties, IL-7 induces proliferation of naive CD4 and CD8 T cells [35, 36], which may in turn replenish the loss of naive T cells during sepsis. The therapeutic potential of IL-7 is best illustrated by the fact that it is currently being studied in multinational clinical trials to boost immune effector cell functions in immunodeficient patients [78, 79]. Studies by Unsinger et al. [80] showed increased circulating IL-7 levels, which can maintain the T cell viability in murine polymicrobial sepsis. Treatment of rhIL-7 in septic mice not only prevents the depletion in absolute counts of CD4 and CD8 T cells in spleen but also preserves their naive, central memory and effector functions in the lymphoid organs, ultimately leading to an improved survival benefit [80]. During sepsis, the antiapoptotic effects of IL-7 in T cells are mediated through the up-regulation of the intracellular antiapoptotic gene Bcl-2 and down-regulation of proapoptotic Bcl-2 homology domain 3 expression [80]. Moreover, they also revealed that rhIL-7 significantly increased the expression of the leukocyte adhesion molecules, which could improve leukocyte function and trafficking to the infectious focus [80]. Correspondingly, in another study, Kasten et al. [81] revealed that the treatment of rhIL-7 accelerated neutrophil recruitment and improved bacterial clearance in septic mice. Toward defining the mechanisms, they further proposed that the rhIL-7 treatment in septic mice increases local and systemic production of IL-17 from the recruited T cells, which can lead to neutrophil recruitment at the site of infection [81]. Altogether, these observations may underlie novel, potential therapeutic targets to improve the host immune response to sepsis.
IL-22
IL-22 is a new member of a group of cytokines called the IL-10 family or IL-10 superfamily (IL-19, IL-20, IL-24, and IL-26), which transduces intracellular signaling by binding to a cell-surface complex composed of IL-22R1 and IL-10R2 receptors [82]. IL-22 signaling is regulated by interactions with sIL-22BP, which shares sequence similarity with the extracellular region of IL-22R1 [82]. IL-22 is produced by activated DCs and T cells and initiates coordinated responses of adaptive and innate-immune systems against bacterial pathogens, particularly in epithelial cells, such as respiratory and intestinal epithelial cells [37]. Receptor interaction with IL-22 promotes dual functions in initiating and regulating immune responses in target cells. IL-22 can contribute to immune-related diseases through the stimulation of inflammatory responses. Animal studies suggest that IL-22 may play a crucial role in sepsis pathophysiology. However, little is known about IL-22 in sepsis patients. In a small group of single-center hospital settings, IL-22 levels were modestly elevated in serum of septic patients. IL-22 produced in the course of sepsis might contribute to host defense and stabilization of mucosal barrier functions under conditions of systemic infection [38]. However, adverse effects of IL-22 are also reported in a model of polymicrobial peritonitis, where the levels of IL-22 and its receptor in spleen and kidney are greatly elevated [39]. The biological activity of IL-22 is modulated by IL-22BP, which is considered a natural antagonist of IL-22. Treatment of mice with IL-22BP before sepsis induction led to enhanced accumulation of neutrophils and mononuclear phagocytes and a reduced bacterial load at the site of infection [39]. Thus, the beneficial effects of IL-22 are mediated by promoting tissue protection, whereas the deleterious effects are executed through delayed bacterial clearance and sustained inflammation. These dual immune functions of IL-22 implicate it to be a complex candidate in sepsis pathophysiology.
IL-33
IL-33 is the newest member belonging to the IL-1 cytokine family. This cytokine was previously named NF in high endothelial venules, as it was originally identified in these specialized cells. It is also expressed in structural and lining cells, including fibroblastic reticular cells of lymphoid tissues, and epithelial cells [40]. In the absence of inflammatory stimuli, IL-33 localizes to the nucleus, as mediated by the amino terminus of full-length IL-33. In macrophages, LPS can trigger the active release of IL-33 to the extracellular space after proteolytic cleavage of its precursor IL-33 [83, 84]. With respect to the mechanisms of release and activation/inactivation of IL-33, various studies reported conflicting findings. Initially, it was believed that IL-33 is activated through caspase-1-dependent cleavage of the IL-33 precursor into an active form. However, more recently, it has been demonstrated that the functional activity of IL-33 is independent of caspase-1 cleavage and that IL-33 may even be inactivated by caspase-1 [83, 84]. According to another study, cleavage of IL-33 into a less-active form is presumably mediated by the proapoptotic caspase-3 and -7, whereas caspase-1 cleavage only seems to play a minor role under physiological conditions [85]. IL-33 can induce Th cells, mast cells, eosinophils, and basophils to produce Th2 cytokines [86]. IL-33 mediates its biological effects by interacting with its receptors, ST2 and IL-1R accessory protein, which in particular, is expressed abundantly on the surface of Th2 cells and mast cells and finally leads to the activation of NF-κB and MAPK pathways that drive production of IL-5 and IL-13 from polarized Th2 cells [86]. IL-33 also functions as chemotactic factor in Th2 cell mobilization [87, 88]. On mast cells, IL-33 triggers the production and release of proinflammatory cytokines, e.g., TNF-α, IL-1β, and IL-6, promotes maturation, and induces degranulation [89]. Furthermore, IL-33 amplifies the polarization of alternatively activated macrophages and enhances TLR4-mediated cytokine production by macrophages [90]. ST2 exists in different splice variants, resulting in a localized form bound to the cellular membrane and a soluble form. The soluble variant, termed sST2, is generated by alternative splicing and does not induce signaling, hence acting as a decoy receptor for IL-33 [91]. A high level sST2 has been linked to the pathogenesis of sepsis and serves as a marker of poor prognosis [92]. In experimental sepsis, IL-33 exerts beneficial effects by enhancing the accumulation of neutrophils via up-regulation of CXCR2 through GRK2-dependent pathways at the site of infection and reduces systemic but not local proinflammatory responses, resulting in an improved outcome [41]. However, it remains to be determined whether administration of IL-33, in fact, represents a therapeutic strategy in the clinical treatment of patients with sepsis.
sTREM
Although the TREM-1, a member of the Ig superfamily, was originally described as a cell-surface receptor, selectively expressed on monocytes/macrophages and neutrophils, recent studies reported that the sTREM-1 is increased in the plasma of septic patients and in the BAL fluids of patients diagnosed with pneumonia [93]. Similarly, the high levels of sTREM-1 are also noted in the plasma of patients after surgical and IR stress [42]. As the direct LPS injection to healthy subjects results in an increase of plasma sTREM-1 levels, therefore, the circulating sTREM-1 seems to be useful in predicting bacteremia [43]. Moreover, the early serum levels of sTREM-1 in septic patients correlate with monocyte inflammatory gene expression. Hence, it may synergize with the TLR signaling pathway in amplifying the inflammatory response mediated by several microbial components [44]. Thus, blockade of sTREM-1 signaling protects animals from exaggerated inflammation and improves survival in a murine model of LPS-induced septic shock [94], as well as Pseudomonas aeruginosa-induced sepsis [95]. Collectively, these findings implicate sTREM-1 not only to be a useful diagnostic marker but also an effective therapeutic target in attenuating sepsis.
HMGB1
Although HMGB1 is considered to be a highly conserved DNA BP, necessary to maintain nucleosome structure and regulate gene transcription, it has been revealed as one of the potent proinflammatory mediators in sepsis [10]. HMGB1 is constitutively expressed in cells and localized into the nucleus as a result of its two structural, lysine-rich nuclear localization motifs [96]. It exerts its proinflammatory functions when it is liberated into the extracellular milieu. After treatment with endotoxin or various proinflammatory cytokines, such as TNF-α, IL-1β, and IFN-γ, the macrophages release HMGB1 starting at 8 h and reach a plateau in expression levels at 18–24 h poststimulation [10, 45, 97]. Studies show that HMGB1 is secreted, not only from activated macrophages but also from NK cells, DCs, endothelial cells, neurons, smooth-muscle cells, osteoclasts, and intestinal epithelial cells [45, 98]. HMGB1 can be released from cells through active or passive mechanisms. Intriguingly, HMGB1 lacks a classic leader peptide and does not travel through the ER and the Golgi apparatus, but large amounts of HMGB1 are released into the extracellular space by activated macrophages. Recent evidence suggests that the secretion of HMGB1 may require its translocation to the cytoplasm from nucleus, translocation into cytoplasmic organelles from cytosol, and exocytosis [45, 99]. Macrophages activated by endotoxin or various proinflammatory cytokines acetylate HMGB1 at lysine-rich nuclear localization sequences, leading to translocation of nuclear HMGB1 into cytoplasmic vesicles and subsequent release into the extracellular milieu [45, 100]. Other studies reveal that HMGB1 needs to be phosphorylated for secretion [101]. In LPS-stimulated macrophages, HMGB1 is phosphorylated by the classical PKC pathway and is secreted by a calcium-dependent mechanism. Apart from the active release, HMGB1 can also be passively secreted from cells undergoing necrosis. HMGB1 is bound loosely to the chromatin in interphase and mitotic cells and is leaked rapidly into the medium when membrane integrity is lost in necrotic cells [102]. In contrast, in apoptotic cells, HMGB1 is bound tightly to chromatin and not released into the extracellular milieu [100]. However, recent studies show that apoptotic cells can passively release HMGB1 at least in some cell types, which is likely to occur in late apoptosis [103].
HMGB1 initiates cellular responses by interacting with several different cell surface receptors, e.g., RAGE, TLR2, TLR4, and syndecan [98]. In macrophages and neutrophils, HMGB1 can dose-dependently up-regulate TNF-α, IL-1β, and IL-8 in a delayed and biphasic manner via phosphorylation of MAPKs and translocation of NF-κB through a RAGE-dependent pathway [104]. Hence, with the use of MAPK-blocking agents to inhibit the HMGB1-induced cytokine cascade and NO, production may offer novel therapeutic options [98, 104]. Moreover, when added to endothelial cells, HMGB1 can induce the release of chemokines and cytokines and up-regulate the expression of adhesion molecules in activated endothelial cells, which promote the adhesion and migration of leukocytes and aggravate an inflammatory response [45, 98]. Furthermore, HMGB1 induces the maturation of DCs and Th1 polarization and also induces secretion of IL-12 from DCs, as well as IL-2 and IFN-γ from T cells [46]. Exposure of epithelial cell monolayers to HMGB1 increased their permeability in a time- and dose-dependent manner, implicating HMGB1 as a mediator of epithelial barrier dysfunction [105].
In addition to specific neutralizing antibodies, HMGB1 antagonists exhibit protective roles against lethal sepsis. Nicotine and acetylcholine can inhibit LPS- or TNF-α-induced HMGB1 release by inhibiting the NF-κB pathway [106]. Recent evidence shows that ethyl pyruvate significantly decreases serum HMGB1 levels by inhibiting the activation of p38 MAPK and NF-κB and confers significant improvement in survival of septic mice [107]. Beside these, oleanolic acid and edaravone have been found to be effective in reducing HMGB1 release through scavenging the free radicals, thereby prolonging survival in septic mice [108, 109]. Consistently, i.p. administration of the green tea major component, epigallocatechin-3-gallate, rescues mice from lethal sepsis by reducing HMGB1 release [110]. These results suggest that the HMGB1 antagonist could be the strong therapeutic candidate for ameliorating sepsis.
Histones
Histones are found in the nuclei of eukaryotic cells, which act as spools around which DNA winds, and play a role in gene regulation [47]. Recently, Xu et al. [48] revealed that extracellular histones released in response to inflammatory challenge contributed to endothelial dysfunction, organ failure, and death during sepsis. Extracellular histones are cytotoxic toward endothelium and exhibit lethality in mice. In vivo histone administration results in neutrophil margination, vacuolated endothelium, intra-alveolar hemorrhage, and macro- and microvascular thrombosis [48]. Histones can be targeted pharmacologically by neutralizing strategy or by activated protein C, which cleaves histones and reduces their cytotoxicity and finally, improves survival in experimental sepsis [48]. These findings suggest that the extracellular histones are potent inflammatory mediators in sepsis and can be a therapeutic target to prevent this inflammatory disorder.
OPN
OPN is an integrin-binding ECM glycophosphoprotein that is produced by the cells of the immune system, osteoblasts, and tumor cells as well as secreted in bodily fluids, including milk, blood, and urine [49]. OPN exists as a component of the ECM and as a soluble cytokine [49]. An iOPN has been reported to be expressed in DCs and macrophages. Recent studies suggest that iOPN is generated as a result of translation initiation downstream of the usual start site in bone marrow-derived DCs [111]. Use of this downstream start site generates the truncated iOPN that lacks the N-terminal signal sequence and consequently, localizes to the cytoplasm, where it associates with TLR9 and the MyD88 adaptor. The interaction of iOPN with MyD88 appears to activate the transcription factor, IFN regulatory factor 7, and to induce expression of IFN-γ, ultimately leading to Th1 cell-mediated immunity and proinflammatory responses [111]. Proteolytic processing of OPN is necessary for its functional bioactivity. OPN is a substrate for thrombin and matrix metalloproteinases. The highly conserved N-terminal RGD and SVVYGLR sites are generated by thrombin cleavage [112, 113]. The RGD motif of OPN is capable of binding to the αvβ3-integrin, whereas the cryptic SVVYGLR-binding domain can recognize α9β1 and α4β1 integrins [113]. On the other hand, the C-terminal fragment is suggested to contain a CD44-binding domain [114].
OPN is known to be induced in macrophages by several inflammatory cytokines, including TNF-α, IL-1β, IFN-γ, and IL-6 [49]. Functionally, OPN plays a key role in macrophage biology by regulating migration, survival, phagocytosis, and proinflammatory cytokine production [115]. OPN stimulates the production of IL-12, while inhibiting the production of IL-10, thereby promoting Th1 cell-mediated responses. Interestingly, OPN-dependent IL-12 production is mediated via an N-terminal fragment interaction with αvβ3 integrin, whereas IL-10 production is inhibited via its C-terminal fragment interacting with CD44R [114, 116]. Beside its roles in macrophages, OPN is important for the recruitment of neutrophils at the site of inflammation, as neutrophils from OPN-null mice display reduced chemotaxis toward chemokines [117]. In response to TCR ligation, OPN is strongly up-regulated, where they act on T cells by initiating their migration, adhesion, and proliferation [118]. Recent results reveal that OPN gene expression in T cells is controlled by T-bet, a transcription factor that promotes Th1 lineage commitment. Further, T-bet-dependent expression of OPN in T cells is essential for efficient skewing of CD4 and CD8 T cells toward the Th1 and type 1 CD8 T cell pathway, respectively [118]. A recent report reveals that the number of peripheral NKT cells is significantly reduced in OPN-deficient mice compared with WT mice, thus implicating OPN roles in maintaining intrathymic NKT cell maturation and subsequent proliferation [119].
The roles of OPN in sepsis pathophysiology have not been clearly elucidated. In a case-control study, serum OPN levels were strikingly higher in septic patients than in controls and then decreased during the resolution phase [50]. However, in another study using CLP-induced sepsis mice, OPN is induced dramatically in liver and bone marrow macrophages at 12 h, and the expression was even higher at a 24-h time-point, indicating it to be a late sepsis mediator [51]. Although OPN is considered to activate innate and adaptive immune systems by releasing elevated levels of proinflammatory mediators, recently, it has been demonstrated to play protective roles in tissue injury by modulating the expression of iNOS [51]. Collectively, these findings implicate OPN to be a novel mediator in sepsis, which needs further studies to clearify its pro- and anti-inflammatory functions.
LIPID MEDIATORS
S1P
S1P is a potent bioactive sphingolipid metabolite that regulates diverse cellular processes, including cell growth, survival, differentiation, lymphocyte trafficking, vascular integrity, and proinflammatory cytokine production [52]. Sphingosine can be released from ceramides, a process catalyzed by the enzyme ceramidase. S1P is formed by phosphorylation of sphingosine, a backbone component of all sphingolipids, in a reaction catalyzed by two isoforms of sphingosine kinase, Sphk1 and Sphk2, which have distinct and overlapping functions [52]. Sphk1 is activated by numerous stimuli, including LPS and proinflammatory cytokines, and promotes the formation of S1P [52]. Recently, Puneet et al. [53] presented striking findings that suggest that Sphk1 in neutrophils and macrophages is activated by various inflammatory signals and participates in the TLR2 and TLR4 signaling pathways and the development of sepsis. It was demonstrated that deletion or inhibition of Sphk1 prevents the mouse from CLP-induced sepsis. S1P generation by Sphk1 mediates the activation of PKCδ, which phosphorylates an unknown target to promote NF-κB activation [53]. It is also possible that S1P enhances the autoubiquitylation of TRAF6, which then recruits and activates TGF-β-activated kinase 1, which phosphorylates the IKK complex, leading to the activation of NF-κB and MAPK cascades [52]. Recent studies also suggest that DC activation induced by coagulation in the lymphatics, which promotes systemic inflammation and lethality during severe sepsis, depends on inside-out signaling by S1P via activation of its receptor, S1PR3 [54]. However, Puneet et al. [53] proposed a completely different mechanism for the involvement of Sphk1 and intracellular S1P in sepsis, focusing on the functions of macrophages. They showed that Sphk1 expression is up-regulated in peritoneal macrophages isolated from patients with severe sepsis and that administration of a Sphk1 inhibitor to mice suppresses LPS-induced production of inflammatory cytokines. Inhibiting S1P by the drug fingolimod (FTY720), which acts through the S1P receptor, prevents autoimmune lymphocytes from moving from the lymphoid organs into the CNS. FTY720 has also been shown to have anti-inflammatory properties and prevents monocyte-endothelial interactions in aorta, possibly through the S1P receptor [120, 121]. Inhibition of Sphk1 with 5c (selective inhibitor of Sphk1) exhibits protective roles against lethality in a murine polymicrobial sepsis by enhancing bacterial clearance [53]. Strikingly, it can be seen that the administration of 5c in septic animals, together with the broad spectrum of antibiotics, greatly improved the efficacy of antibiotics in protecting infection [53]. Thus, the above features suggest that S1P, as mediated by Sphk1, serves as a novel, inflammatory mediator in sepsis, implicating it as a promising therapeutic target against sepsis.
Resolvin
Resolvins are endogenous lipid mediators generated during the resolution phase of acute inflammation from ω-3 polyunsaturated fatty acids and display potent proresolving and anti-inflammatory actions [55]. RvD1 and RvE1 are derived from DHA and eicosapentaenoic acid, respectively. Recent studies have shown that peripheral and central administration of RvD1 and RvE1 could effectively reduce inflammatory and postoperative pain [56]. Although the anti-inflammatory roles of RvD1 and RvE1 are established in various inflammatory diseases, there remains inadequate information regarding sepsis. RvD2, derived from DHA, is a novel family member of resolvins, which acts as a potent regulator of leukocytes and controls microbial sepsis [55, 57]. RvD2 decreases leukocyte-endothelial interactions in vivo by endothelial-dependent NO production and by direct modulation of leukocyte adhesion receptor expression [57]. In mice with polymicrobial sepsis initiated by CLP, RvD2 sharply decreases local and systemic bacterial burden, excessive cytokine production, and neutrophil recruitment, while increasing peritoneal mononuclear cells and macrophage phagocytosis. These multilevel, proresolving actions of RvD2 translate to increased survival from experimental sepsis [57]. Together, these results identify RvD2 as a potent, endogenous regulator of excessive inflammatory responses that acts via multiple cellular targets to stimulate resolution of inflammation and preserve immune vigilance.
An analogous class, LXs are short-lived endogenously produced eicosanoids that are derived enzymatically from arachidonic acid, an ω-6 fatty acid. At present, two LXs have been identified: LXA4 and LXB4. LXA4 acts through its receptor, LXA4R, to inhibit chemotaxis, transmigration, superoxide generation, and NF-κB activation [122]. Based on the inflammatory resolution properties of LXA4, Walker et al. [123] showed that LXA4 treatment in sepsis animals reduced systemic inflammation and NF-κB activation without compromising host defense. Moreover, they also revealed that LXA4 could reduce blood bacterial load by enhancing macrophage recruitment. As a result of these striking features of reducing systemic inflammation, as well as bacterial spread, LXA4 improves survival in septic animals, thus implicating it to be an extraordinary therapeutic potential in sepsis.
Resistin
Although resistin, an adipose tissue-derived, cysteine-rich secretory protein, was initially thought to play pivotal roles in insulin resistance, further research suggests its functions as a proinflammatory cytokine [58]. In mammals, resistin is also secreted from macrophages and epithelial cells [58]. In a hospital-based study, Sundén-Cullberg et al. [59] reported a profound elevation of resistin in patients' sera in severe sepsis and septic shock, which was positively correlated with their serum levels of TNF-α, IL-6, IL-8, L-10, and procalcitonin and was associated with an unfavorable outcome in overall survival. In accordance with this finding, others also revealed significant up-regulation of resistin in the serum of sepsis patients as compared with healthy controls [60]. In vitro, resistin can be released from monocytes after stimulation with LPS or HMGB1 protein [59]. On the other hand, recombinant resistin itself can up-regulate ICAM-1 on monocytes and promotes their trafficking [59]. Considering resistin as one of the potent mediators in sepsis, elaborative studies are necessary to elucidate its mechanism of actions so that it can be established as a novel, therapeutic target in resisting sepsis.
ADIPOCYTOKINES
Adipose tissue has long been considered to be an organ that stores and supplies excess energy when food is lacking to favor survival. However, recent studies have revealed that adipose tissue secretes a variety of bioactive substances, namely adipocytokines, which exert protective roles in different organs [124]. Herein, the adipocytokines that have recently been revealed to play pivotal roles in sepsis are reviewed.
Adiponectin
A novel adipocytokine, named adiponectin, has a strong ability to exert several anti-inflammatory responses in macrophages and endothelial cells [124]. Treatment of cultured macrophages with adiponectin significantly inhibits LPS-induced production of TNF-α [61]. In murine polymicrobial sepsis, exogenous administration of adiponectin reduces the mortality rate and plays an anti-inflammatory role through inhibiting HMGB1 [62]. Adiponectin suppresses TNF-α-induced NF-κB activation without affecting other TNF-α-mediated signals, including MAPK and Akt kinase [125]. This inhibitory effect of adiponectin is accompanied by cAMP accumulation and is blocked by an adenylate cyclase inhibitor or a PKA inhibitor. These observations suggest that adiponectin, which is naturally present in the bloodstream, modulates the inflammatory response of macrophages and endothelial cells through a cross-talk between cAMP-PKA and NF-κB signaling pathways [124]. Recent studies have demonstrated that adiponectin also induces various anti-inflammatory cytokines, such as IL-10R and IL-1R antagonists [126]. As a result of these anti-inflammatory properties of endogenous adiponectin, it can be considered a potential therapeutic substance in sepsis.
Visfatin
Visfatin, also known as a pre-B cell colony-enhancing factor, has recently been identified as a new adipocytokine, affecting the innate-immune system. Recent studies showed that recombinant visfatin activates human leukocytes to release IL-1β, TNF-α, and IL-6, as well as increases the surface expression of costimulatory molecules CD54, CD40, and CD80 via MAPK pathways [63, 64]. Visfatin expression is up-regulated in sepsis, where it plays a key role in the persistence of inflammation through its capacity to inhibit neutrophil apoptosis [65]. Higher levels of visfatin in neonatal sepsis can be a useful, diagnostic marker [66] and incriminate further studies to reveal any potential roles in sepsis pathobiology.
VASOACTIVE PEPTIDES
Ghrelin
Ghrelin, a stomach-derived vasoactive peptide, plays essential roles in stimulating growth-hormone release from the anterior pituitary gland and energy homeostasis [127]. Recent studies demonstrated that serum ghrelin levels were reduced during sepsis, and administration of exogenous ghrelin improved survival in sepsis [67]. It has been demonstrated recently that ghrelin suppresses inflammation in sepsis by modulating the peripheral, as well as central sympathetic nerve activity through its action on receptors, known as the GHSR in the brain [68]. To act on its receptors located in the brain, ghrelin must be able to cross the blood-brain barrier. However, in sepsis, ghrelin is likely to enter the brain easily, as a result of the disruption of the blood-brain barrier during systemic inflammation [68]. Apart from ghrelin's roles in sympathoinhibition by recognizing its cognate receptor GHSR-1a in the hypothalamus, several studies have demonstrated its functions to be mediated by the vagus nerve stimulation [67, 68]. In another study, ghrelin administration in sepsis was shown to down-regulate the MAPK pathway by up-regulating MKP-1 [128]. Ghrelin, through its mechanisms of sympathoinhibition and suppression of inflammation, was shown to rescue rodents from sepsis-associated, multiple-organ failure by attenuating ALI and intestinal barrier dysfunction [67, 68]. Sepsis may also be aggravated in the presence of other kinds of tissue injury that alter the immune function. Recent studies demonstrated that human ghrelin decreased proinflammatory cytokine levels and improved survival in an animal model of radiation combined with sepsis [129, 130].
AM and AMBP-1
AM, a member of the calcitonin gene-related peptide family of proteins, is an evolutionarily conserved neuropeptide, first isolated from human pheochromocytoma cells and later found to be widely distributed throughout the mammalian tissues [131]. Elevated plasma AM levels were reported in patients with sepsis [132] and following major surgery [133]. The effects of AM are mediated by the functional receptor combination of calcitonin receptor-like receptor with receptor activity-modifying protein-2 or -3 [134]. Our group recently revealed that the small intestine is a major source of AM during CLP-induced polymicrobial sepsis in rats, which may account for the increased portal blood flow observed in the early stage of sepsis [69].
Recently, the understanding of the regulation of AM activity in sepsis has been greatly expanded after the discovery of AMBP-1 in mammalian blood [69, 70]. Inflammatory cytokines, particularly IFN-γ, have been recognized as positive regulators of AMBP-1 expression, whereas oxidative stress and NF-κB-sensitive microRNA-146a have been shown to down-regulate AMBP-1 [135, 136]. Administration of AM/AMBP-1 may provide a novel approach to the treatment of sepsis [137]. Our previous studies have shown that AM or AMBP-1 alone only moderately reduces LPS-induced TNF-α production in Kupffer cells, whereas AM and AMBP-1, in combination, dramatically down-regulate TNF-α production [138]. The direct anti-inflammatory effects of AM/AMBP-1 are mediated through the cAMP-dependent pathway and proline-rich tyrosine kinase-2 ERK1/2-dependent induction of peroxisome proliferator-activated receptor γ [139]. Moreover, AM has been shown to down-regulate chemokine levels in in vivo and in vitro conditions [140]. AM also has the potential to inhibit neutrophil activation and migration to inflammatory sites by suppressing up-regulation of the adhesion molecule CD11 [141]. Thus, the multifunctional, beneficial effects of AM/AMBP-1 lead to a potent therapeutic regimen for sepsis treatment.
ET-1
ET-1, another vasoactive peptide ubiquitously expressed in many cell types, plays critical roles in maintaining the vascular wall character. ET-1 is formed from its biological precursor, a full-length, 38-aa-long peptide, and after its synthesis in the cytoplasm, it is cleaved by an ET conversion enzyme to yield active ET-1 (aa 1–21) and a C-terminal fragment (aa 22–38) [142]. Under normal condition, vasculature, endothelial cells, cardiac myocytes, and fibroblasts synthesize ET-1, while its expression is elevated in a diseased condition [143]. An excessive level of ET-1 has been associated with vasospasm, vascular damage, cardiovascular remodeling, renal perfusion, and inflammation [143, 144]. In patients with severe sepsis, plasma levels of ET-1 are markedly increased and correlate significantly with renal dysfunction [145]. Clinical manifestations of sepsis in newborns are accompanied by increased concentrations of ET-1, which serves as one of the key mediators to cause multiple organ dysfunctions [146]. In septic patients, the ET-1 level correlates with the degree of sepsis severity, which in particular, reflects cardiac dysfunction [147]. In recent studies, it has been demonstrated that during the early phase of sepsis, the initial up-regulation of ET-1 seems to be beneficial in terms of maintaining blood pressure and organ perfusion [71]. However, the excessive rise in the plasma level of ET for longer periods evokes profound vasoconstriction and tissue hypoperfusion in the vascular beds, which is harmful [72]. Hence, the therapeutic strategies were attained by using the ETR antagonist to abrogate the effects of excessive ET-1, which protects from multiple organ injuries, splanchnic hypoperfusion, and bacterial translocation in septic shock [73].
GROWTH FACTOR
Several growth factors and hormones are up-regulated to play crucial roles in sepsis by promoting immunomodulatory, antiapoptotic, and neoangiogenesis effects to the immune-reactive cells of inflamed and ischemic tissues [148]. We have recently identified such an immunomodulatory factor, MFG-E8, which exerts beneficial roles in sepsis [149].
MFG-E8, a secretory glycoprotein plays pivotal roles in engulfment of apoptotic cells by the professional phagocytes [150]. MFG-E8 is ubiquitously expressed in mononuclear immune-reactive cells, as well as others [150]. The detailed immunomodulatory, antiapoptotic, and tissue-protective functions of MFG-E8 in sepsis have been clearly demonstrated in recent reviews [74]. During CLP-induced sepsis, endogenous MFG-E8 is reduced dramatically, which leads to accumulation of apoptotic cells in the spleen and thymus and causes exaggerated inflammation accompanied by elevated levels of TNF-α, IL-6, IL-8, and MPO. However, exogenous rMFG-E8 administration greatly restores immune homeostasis by reducing proinflammatory mediators and accelerating tissue regeneration [74, 149]. An intriguing feature of MFG-E8 in regulating the deleterious effects of neutrophils in lungs and other organs has been reported recently in gut I/R and endotoxin-mediated ALI [75, 76]. We revealed that the inhibition of neutrophil migration in lungs after endotoxin administration was mediated through MFG-E8 binding to its receptor, αvβ3-integrin, followed by down-regulation of surface exposure of chemokine receptor CXCR2 via GRK2-dependent pathways [75]. Moreover, the anti-inflammatory roles of MFG-E8 are mediated directly through down-regulating NF-κB or indirectly, by enhancing phagocytosis of apoptotic cells [149, 151]. In experimental sepsis, MFG-E8 also inhibits the activation of caspase-3 and MPO, which protects tissues from apoptosis and injuries [74–76]. Although the protective roles of MFG-E8 are being mediated through the modulation of hyperactive innate-immune functions, its roles in the adaptive immune system need to be defined.
POTENTIAL PITFALLS OF ANIMAL MODELS OF SEPSIS
The current review highlighting the latest mediators has been generated from the findings of animal models of sepsis. Therefore, the widely used sepsis models with their possible variability factors related to the clinical settings are worth mentioning. Mitchell Fink [152] concisely reviewed the animal models of sepsis and their possible complications, where the most extensively studied animal models of sepsis are generated by injecting LPS into mice or rats, which initiates a systemic inflammatory response, resembling the patients with severe sepsis. However, there are substantial differences in the kinetics and magnitude of cytokinemia between rodent LPS models and clinical sepsis. For example, in mice challenged with a lethal dose of LPS, circulating TNF-α levels spear rapidly (within 60–90 min), followed by a sharp decline to an undetectable level within short periods [152]. In contrast, TNF-α levels in septic patients are not as high as murine endotoxemia, but they persist for several days, even among patients with fatal illness [152]. A controversial result between LPS model and clinical trial has been noted in anti-IL-1R antibody treatment, where the murine model shows better therapeutic efficacy than patients [153, 154]. Direct bacterial inoculations can also generate sepsis, while they are limited to certain variability factors, such as bacterial load, strain, and route of administration [155]. Moreover, considering the anti-TNF-αR antagonist treatment, bacteremic animals exhibit better responses than clinical trials [156, 157]. However, several host-barrier disruption models, which mimic clinical situations, are now adopted. Colon ascendens stent peritonitis and CLP are the most commonly used sepsis models in animals, as these are close to several critical factors, such as needle size used for perforation, number of perforations, amount of necrosis induced, amount of stool diffusing into peritoneum, as well as sex, age, and strain [158]. Apart from these, several clinically relevant models that mimic systemic inflammation [74, 76], e.g., intestinal I/R, are now generated. Extensive studies are needed to ascertain how much less deviation and controversies these models have as compared with clinical conditions. Despite potential pitfalls in generating suitable animal models that mimic human sepsis, they are still in use for investigating pathophysiological mechanisms or testing the efficacy of new therapeutic agents. Further studies are needed to upgrade animal models that can adequately replicate the clinically relevant conditions of sepsis.
CONCLUSION
This current review highlights new advances on sepsis mediators that have profound effects on innate and adaptive immune systems in exaggerating or modulating inflammation, organ damage, and mortality. Based on literature reviews, there has been substantial progress over the decades in understanding the roles of inflammatory mediators in the pathogenesis of sepsis. Despite extensive research, none of the promising therapeutic approaches for sepsis that target the inflammatory cascades have been successfully translated to the clinical setting, and rates of sepsis mortality have not decreased notably. From this review, it is clear that the influence of a single key mediator causing sepsis and neutralization of such a single factor curing all sepsis patients may not be true. Instead, we understand that sepsis is a complex, dynamic syndrome with great heterogeneity to pro- and anti-inflammatory mediators. The current review deals with the novel mediators that are being up-regulated or down-regulated during the early and late stages of sepsis, hence exacerbating or modulating the immune responses. Based on these viewpoints, we suggest that replenishing the protective mediators that are down-regulated in sepsis, while withdrawing the elevated deleterious factors, may lead to the discovery of new therapies for improving survival in septic patients, a goal that has been elusive for decades. We hope that clinical trials targeting these novel mediators, as well as anti-inflammatory pharmacological strategies, will reduce the morbidity and mortality of this deadly clinical condition.
ACKNOWLEDGMENTS
This study was supported by the U.S. National Institutes of Health grants R01 GM057468, R01 GM053008, and R33 AI 080536 (P.W.).
We acknowledge Cletus Cheyuo and Jose M. Prince for their valuable discussion.
Footnotes
- ALI
- acute lung injury
- AM
- adrenomedullin
- AMBP-1
- adrenomedullin-binding protein 1
- BP
- binding protein
- CARS
- compensatory anti-inflammatory response syndrome
- CLP
- cecal ligation and puncture
- DHA
- docosahexaenoic acid
- ET-1
- endothelin-1
- GHSR
- growth hormone secretagogue receptor
- GRK2
- GPCR kinase 2
- h
- human
- HMGB1
- high mobility group box 1
- iOPN
- intracellular osteopontin
- IR
- ischemia-reperfusion
- IRA
- innate response activator
- LX
- lipoxins
- MFG-E8
- milk fat globule-EGF factor 8
- OPN
- osteopontin
- RAGE
- receptor for advanced glycation end-products
- RGD
- arginine-glycine-aspartate
- RvD1/2 and RvE1
- resolvin D1/2 and E1, respectively
- s
- soluble
- S1P
- sphingosine-1 phosphate
- SIRS
- systemic inflammatory response syndrome
- Sphk1
- sphingosine-1 phosphate kinase 1
- TREM-1
- triggering receptor expressed on myeloid cells 1
AUTHORSHIP
M.A. composed, revised, and edited the manuscript. A.J. worked on critical reviewing and editing of the manuscript. W-L.Y. and A.M. reviewed the manuscript. P.W. generated overall concepts and reviewed the manuscript.
REFERENCES
- 1. Lever A., Mackenzie I. (2007) Sepsis: definition, epidemiology, and diagnosis. BMJ 335, 879–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont. G., Carcillo J., Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 3. Mokart D., Leone M., Sannini A., Brun J. P., Tison A., Delpero J. R., Houvenaeghel G., Blache J. L., Martin C. (2005) Predictive perioperative factors for developing severe sepsis after major surgery. Br. J. Anaesth. 95, 776–781 [DOI] [PubMed] [Google Scholar]
- 4. Bognar Z., Foldi V., Rezman B., Bogar L., Csontos C. (2010) Extravascular lung water index as a sign of developing sepsis in burns. Burns 36, 1263–1270 [DOI] [PubMed] [Google Scholar]
- 5. Cai B., Deitch E. A., Ulloa L. (2010) Novel insights for systemic inflammation in sepsis and hemorrhage. Mediators Inflamm. 2010, 642462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haglund U. (1994) Gut ischaemia. Gut 1 (Suppl.), S73–S76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oberholzer A., Oberholzer C., Moldawer L. L. (2001) Sepsis syndromes: understanding the role of innate and acquired immunity. Shock 16, 83–96 [DOI] [PubMed] [Google Scholar]
- 8. Ayala A., Chaudry I. H. (1996) Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock 6 (Suppl. 1), S27–S38 [PubMed] [Google Scholar]
- 9. Opal S. M., Huber C. E. (2002) Bench-to-bedside review: Toll-like receptors and their role in septic shock. Crit. Care 6, 125–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang H., Yang H., Czura C. J., Sama A. E., Tracey K. J. (2001) HMGB1 as a late mediator of lethal systemic inflammation. Am. J. Respir. Crit. Care Med. 164, 1768–1773 [DOI] [PubMed] [Google Scholar]
- 11. Rittirsch D., Flierl M. A., Ward P. A. (2008) Harmful molecular mechanisms in sepsis. Nat. Rev. Immunol. 8, 776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dinarello C. A. (2001) Anti-cytokine therapies in response to systemic infection. J. Investig. Dermatol. Symp. Proc. 6, 244–250 [DOI] [PubMed] [Google Scholar]
- 13. Minnich D. J., Moldawer L. L. (2004) Anti-cytokine and anti-inflammatory therapies for the treatment of severe sepsis: progress and pitfalls. Proc. Nutr. Soc. 63, 437–441 [DOI] [PubMed] [Google Scholar]
- 14. Dinarello C. A., Abraham E. (2002) Does blocking cytokines in sepsis work? Am. J. Respir. Crit. Care Med. 166, 1156–1157 [DOI] [PubMed] [Google Scholar]
- 15. Vincent J. L., Abraham E., Annane D., Bernard G., Rivers E., Van den Berghe G. (2002) Reducing mortality in sepsis: new directions. Crit. Care 6, S1–S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Brien J. M., Jr., Abraham E. (2003) New approaches to the treatment of sepsis. Clin. Chest Med. 24, 521–548 [DOI] [PubMed] [Google Scholar]
- 17. Vincent J. L. (2006) Management of sepsis in the critically ill patient: key aspects. Expert Opin. Pharmacother. 7, 2037–2045 [DOI] [PubMed] [Google Scholar]
- 18. Opal S. M. (2010) New perspectives on immunomodulatory therapy for bacteraemia and sepsis. Int. J. Antimicrob. Agents 36, S70–S73 [DOI] [PubMed] [Google Scholar]
- 19. Lakhani S. A., Bogue C. W. (2003) Toll-like receptor signaling in sepsis. Curr. Opin. Pediatr. 15, 278–282 [DOI] [PubMed] [Google Scholar]
- 20. Ramnath R. V., Weing S., He M., Sun J., Zhang H., Bawa M. S., Bhatia M. (2006) Inflammatory mediators in sepsis: cytokines, chemokines, adhesion molecules and gases. J. Organ Dysfunction 2, 80–92 [Google Scholar]
- 21. Hoesel L. M., Neff T. A., Neff S. B., Younger J. G., Olle E. W., Gao H., Pianko M. J., Bernacki K. D., Sarma J. V., Ward P. A. (2005) Harmful and protective roles of neutrophils in sepsis. Shock 24, 40–47 [DOI] [PubMed] [Google Scholar]
- 22. Opal S. M., DePalo V. A. (2000) Anti-inflammatory cytokines. Chest 117, 1162–1172 [DOI] [PubMed] [Google Scholar]
- 23. Perl M., Chung C. S., Garber M., Huang X., Ayala A. (2006) Contribution of anti-inflammatory/immune suppressive processes to the pathology of sepsis. Front. Biosci. 11, 272–299 [DOI] [PubMed] [Google Scholar]
- 24. Weaver C. T., Hatton R. D., Mangan P. R., Harrington L. E. (2007) IL-17 family cytokines and the expanding diversity of effector T-cell lineages. Annu. Rev. Immunol. 25, 821–852 [DOI] [PubMed] [Google Scholar]
- 25. Venet F., Chung C. S., Monneret G., Huang X., Horner B., Garber M., Ayala A. (2008) Regulatory T cell populations in sepsis and trauma. J. Leukoc. Biol. 83, 523–535 [DOI] [PubMed] [Google Scholar]
- 26. Carding S. R., Egan P. J. (2002) γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2, 336–345 [DOI] [PubMed] [Google Scholar]
- 27. Jiang H., Chess L. (2004) An integrated view of suppressor T cell subsets in immunoregulation. J. Clin. Invest. 114, 1198–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rhee R. J., Carlton S., Chung C. S., Lomas J. L., Cioffi W. G., Ayala A. (2003) Inhibition of CD1d activation suppresses septic mortality: a role for NK-T cells in septic immune dysfunction. J. Surg. Res. 115, 74–81 [DOI] [PubMed] [Google Scholar]
- 29. Li L., Huang L., Sung S. S., Lobo P. I., Brown M. G., Gregg R. K., Engelhard V. H., Okusa M. D. (2007) NKT cell activation mediates neutrophil IFN-γ production and renal ischemia-reperfusion injury. J. Immunol. 178, 5899–5911 [DOI] [PubMed] [Google Scholar]
- 30. Kelly-Scumpia K. M., Scumpia P. O., Weinstein J. S., Delano M. J., Cuenca A. G., Nacionales D. C., Wynn J. L., Lee P. Y., Kumagai Y., Efron P. A., Akira S., Wasserfall C., Atkinson M. A., Moldawer L. L. (2011) B cells enhance early innate immune responses during bacterial sepsis. J. Exp. Med. 208, 1673–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rauch P. J., Chudnovskiy A., Robbins C. S., Weber G. F., Etzrodt M., Hilgendorf I., Tiglao E., Figueiredo J. L., Iwamoto Y., Theurl I., Gorbatov R., Waring M. T., Chicoine A. T., Mouded M., Pittet M. J., Nahrendorf M., Weissleder R., Swirski F. K. (2012) Innate response activator B cells protect against microbial sepsis. Science 335, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ward N. S., Casserly B., Ayala A. (2008) The compensatory anti-inflammatory response syndrome (CARS) in critically ill patients. Clin. Chest Med. 29, 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hotchkiss R. S., Karl I. E. (2003) The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348, 138–150 [DOI] [PubMed] [Google Scholar]
- 34. Hotchkiss R. S., Osmon S. B., Chang K. C., Wagner T. H., Coopersmith C. M., Karl I. E. (2005) Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J. Immunol. 174, 5110–5118 [DOI] [PubMed] [Google Scholar]
- 35. Fry T. J., Mackall C. L. (2005) The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 174, 6571–6576 [DOI] [PubMed] [Google Scholar]
- 36. Geiselhart L. A., Humphries C. A., Gregorio T. A., Mou S., Subleski J., Komschlies K. L. (2001) IL-7 administration alters the CD4: CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J. Immunol. 166, 3019–3027 [DOI] [PubMed] [Google Scholar]
- 37. Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. (2001) Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 [DOI] [PubMed] [Google Scholar]
- 38. Bingold T. M., Ziesché E., Scheller B., Sadik C. D., Franck K., Just L., Sartorius S., Wahrmann M., Wissing H., Zwissler B., Pfeilschifter J., Mühl H. (2010) Interleukin-22 detected in patients with abdominal sepsis. Shock 34, 337–340 [DOI] [PubMed] [Google Scholar]
- 39. Weber G. F., Schlautkötter S., Kaiser-Moore S., Altmayr F., Holzmann B., Weighardt H. (2007) Inhibition of interleukin-22 attenuates bacterial load and organ failure during acute polymicrobial sepsis. Infect. Immun. 75, 1690–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Baekkevold E. S., Roussigné M., Yamanaka T., Johansen F. E., Jahnsen F. L., Amalric F., Brandtzaeg P., Erard M., Haraldsen G., Girard J. P. (2003) Molecular characterization of NF-HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am. J. Pathol. 163, 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alves-Filho J. C., Sônego F., Souto F. O., Freitas A., Verri W. A., Jr., Auxiliadora-Martins M., Basile-Filho A., McKenzie A. N., Xu D., Cunha F. Q., Liew F. Y. (2010) Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat. Med. 16, 708–712 [DOI] [PubMed] [Google Scholar]
- 42. Gibot S., Massin F., Alauzet C., Montemont C., Lozniewski A., Bollaert P. E., Levy B. (2008) Effects of the TREM-1 pathway modulation during mesenteric ischemia-reperfusion in rats. Crit. Care Med. 36, 504–510 [DOI] [PubMed] [Google Scholar]
- 43. Knapp S., Gibot S., de Vos A., Versteeg H. H., Colonna M., van der Poll T. (2004) Cutting edge: expression patterns of surface and soluble triggering receptor expressed on myeloid cells-1 in human endotoxemia. J. Immunol. 173, 7131–7134 [DOI] [PubMed] [Google Scholar]
- 44. Dimopoulou I., Pelekanou A., Mavrou I., Savva A., Tzanela M., Kotsaki A., Kardara M., Orfanos S. E., Kotanidou A., Giamarellos-Bourboulis E. J. (2012) Early serum levels of soluble triggering receptor expressed on myeloid cells-1 in septic patients: correlation with monocyte gene expression. J. Crit. Care 27, 294–300 [DOI] [PubMed] [Google Scholar]
- 45. Huang W., Tang Y., Li L. (2010) HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 51, 119–126 [DOI] [PubMed] [Google Scholar]
- 46. Messmer D., Yang H., Telusma G., Knoll F., Li J., Messmer B., Tracey K. J., Chiorazzi N. (2004) High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J. Immunol. 173, 307–313 [DOI] [PubMed] [Google Scholar]
- 47. Grunstein M. (1990) Histone function in transcription. Annu. Rev. Cell Biol. 6, 643–678 [DOI] [PubMed] [Google Scholar]
- 48. Xu J., Zhang X., Pelayo R., Monestier M., Ammollo C. T., Semeraro F., Taylor F. B., Esmon N. L., Lupu F., Esmon C. T. (2009) Extracellular histones are major mediators of death in sepsis. Nat. Med. 15, 1318–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lund S. A., Giachelli C. M., Scatena M. (2009) The role of osteopontin in inflammatory processes. J. Cell Commun. Signal. 3, 311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vaschetto R., Nicola S., Olivieri C., Boggio E., Piccolella F., Mesturini R., Damnotti F., Colombo D., Navalesi P., Della Corte F., Dianzani U., Chiocchetti A. (2008) Serum levels of osteopontin are increased in SIRS and sepsis. Intensive Care Med. 34, 2176–2184 [DOI] [PubMed] [Google Scholar]
- 51. Guo H., Wai P. Y., Mi Z., Gao C., Zhang J., Kuo P. C. (2008) Osteopontin mediates Stat1 degradation to inhibit iNOS transcription in a cecal ligation and puncture model of sepsis. Surgery 144, 182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spiegel S., Milstien S. (2001) The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Puneet P., Yap C. T., Wong L., Yulin L., Koh D. R., Moochhala S., Pfeilschifter J., Huwiler A., Melendez A. J. (2010) SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science 328, 1290–1294 [DOI] [PubMed] [Google Scholar]
- 54. Niessen F., Schaffner F., Furlan-Freguia C., Pawlinski R., Bhattacharjee G., Chun J., Derian C. K., Andrade-Gordon P., Rosen H., Ruf W. (2008) Dendritic cell PAR1-S1P3 signalling couples coagulation and inflammation. Nature 452, 654–658 [DOI] [PubMed] [Google Scholar]
- 55. Serhan C. N., Chiang N., Van Dyke T. E. (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park C. K., Xu Z. Z., Liu T., Lu N., Serhan C. N., Ji R. R. (2011) Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J. Neurosci. 31, 18433–18438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Spite M., Norling L. V., Summers L., Yang R., Cooper D., Petasis N. A., Flower R. J., Perretti M., Serhan C. N. (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pang S. S., Le Y. Y. (2006) Role of resistin in inflammation and inflammation-related diseases. Cell. Mol. Immunol. 3, 29–34 [PubMed] [Google Scholar]
- 59. Sundén-Cullberg J., Nyström T., Lee M. L., Mullins G. E., Tokics L., Andersson J., Norrby-Teglund A., Treutiger C. J. (2007) Pronounced elevation of resistin correlates with severity of disease in severe sepsis and septic shock. Crit. Care Med. 35, 1536–1542 [DOI] [PubMed] [Google Scholar]
- 60. Koch A., Gressner O. A., Sanson E., Tacke F., Trautwein C. (2009) Serum resistin levels in critically ill patients are associated with inflammation, organ dysfunction and metabolism and may predict survival of non-septic patients. Crit. Care 13, R95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yokota T., Oritani K., Takahashi I., Ishikawa J., Matsuyama A., Ouchi N., Kihara S., Funahashi T., Tenner A. J., Tomiyama Y., Matsuzawa Y. (2000) Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96, 1723–1732 [PubMed] [Google Scholar]
- 62. Li S., Bao H. G., Han L., Liu L., Wang X. (2012) Effects of adiponectin on mortality and its mechanism in a sepsis mouse model. J. Invest. Surg. 25, 214–219 [DOI] [PubMed] [Google Scholar]
- 63. Luk T., Malam Z., Marshall J. C. (2008) Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J. Leukoc. Biol. 83, 804–816 [DOI] [PubMed] [Google Scholar]
- 64. Moschen A. R., Kaser A., Enrich B., Mosheimer B., Theurl M., Niederegger H., Tilg H. (2007) Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J. Immunol. 178, 1748–1758 [DOI] [PubMed] [Google Scholar]
- 65. Jia S. H., Li Y., Parodo J., Kapus A., Fan L., Rotstein O. D., Marshall J. C. (2004) Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J. Clin. Invest. 113, 1318–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cekmez F., Canpolat F. E., Cetinkaya M., Aydinöz S., Aydemir G., Karademir F., Ipcioglu O. M., Sarici S. Ü. (2011) Diagnostic value of resistin and visfatin, in comparison with C-reactive protein, procalcitonin and interleukin-6 in neonatal sepsis. Eur. Cytokine Netw. 22, 113–117 [DOI] [PubMed] [Google Scholar]
- 67. Wu R., Dong W., Zhou M., Zhang F., Marini C. P., Ravikumar T. S., Wang P. (2007) Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am. J. Respir. Crit. Care Med. 176, 805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cheyuo C., Jacob A., Wang P. (2012) Ghrelin-mediated sympathoinhibition and suppression of inflammation in sepsis. Am. J. Physiol. Endocrinol. Metab. 302, E265–E272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang J., Wu R., Zhou M., Wang P. (2010) Human adrenomedullin and its binding protein ameliorate sepsis-induced organ injury and mortality in jaundiced rats. Peptides 31, 872–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fowler D. E., Yang S., Zhou M., Chaudry I. H., Simms H. H., Wang P. (2003) Adrenomedullin and adrenomedullin binding protein-1: their role in the septic response. J. Surg. Res. 109, 175–181 [DOI] [PubMed] [Google Scholar]
- 71. Vemulapalli S., Chiu P. J., Rivelli M., Foster C. J., Sybertz E. J. (1991) Modulation of circulating endothelin levels in hypertension and endotoxemia in rats. J. Cardiovasc. Pharmacol. 8, 895–903 [DOI] [PubMed] [Google Scholar]
- 72. Ruetten H., Thiemermann C. (1996) Effect of selective blockade of endothelin ETB receptors on the liver dysfunction and injury caused by endotoxaemia in the rat. Br. J. Pharmacol. 119, 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Iskit A. B., Sungur A., Gedikoglu G., Guc M. O. (1999) The effects of bosentan, aminoguanidine and L-canavanine on mesenteric blood flow, spleen and liver in endotoxaemic mice. Eur. J. Pharmacol. 379, 73–80 [DOI] [PubMed] [Google Scholar]
- 74. Matsuda A., Jacob A., Wu R., Zhou M., Nicastro J. M., Coppa G. F., Wang P. (2011) Milk fat globule-EGF factor VIII in sepsis and ischemia-reperfusion injury. Mol. Med. 17, 126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Aziz M., Matsuda A., Yang WL., Jacob A., Wang P. (2012) Milk fat globule-epidermal growth factor-factor 8 attenuates neutrophil infiltration in acute lung injury via modulation of CXCR2. J. Immunol. 189, 393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cui T., Miksa M., Wu R., Komura H., Zhou M., Dong W., Wang Z., Higuchi S., Chaung W., Blau S. A., Marini C. P., Ravikumar T. S., Wang P. (2010) Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am. J. Respir. Crit. Care Med. 181, 238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mazzucchelli R., Durum S. K. (2007) Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 7, 144–154 [DOI] [PubMed] [Google Scholar]
- 78. Sportès C., Hakim F. T., Memon S. A., Zhang H., Chua K. S., Brown M. R., Fleisher T. A., Krumlauf M. C., Babb R. R., Chow C. K., Fry T. J., Engels J., Buffet R., Morre M., Amato R. J., Venzon D. J., Korngold R., Pecora A., Gress R. E., Mackall C. L. (2008) Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J. Exp. Med. 205, 1701–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sereti I., Dunham R. M., Spritzler J., Aga E., Proschan M. A., Medvik K., Battaglia C. A., Landay A. L., Pahwa S., Fischl M. A., Asmuth D. M., Tenorio A. R., Altman J. D., Fox L., Moir S., Malaspina A., Morre M., Buffet R., Silvestri G., Lederman M. M.; ACTG 5214 Study Team. (2009) IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113, 6304–6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Unsinger J., McGlynn M., Kasten K. R., Hoekzema A. S., Watanabe E., Muenzer J. T., McDonough J. S., Tschoep J., Ferguson T. A., McDunn J. E., Morre M., Hildeman D. A., Caldwell C. C., Hotchkiss R. S. (2010) IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol. 184, 3768–3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kasten K. R., Prakash P. S., Unsinger J., Goetzman H. S., England L. G., Cave C. M., Seitz A. P., Mazuski C. N., Zhou T. T., Morre M., Hotchkiss R. S., Hildeman D. A., Caldwell C. C. (2010) Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through γ δ T-cell IL-17 production in a murine model of sepsis. Infect. Immun. 78, 4714–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xie M. H., Aggarwal S., Ho W. H., Foster J., Zhang Z., Stinson J., Wood W. I., Goddard A. D., Gurney A. L. (2000) Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 275, 31335–31339 [DOI] [PubMed] [Google Scholar]
- 83. Schmitz J., Owyang A., Oldham E., Song Y., Murphy E., McClanahan T. K., Zurawski G., Moshrefi M., Qin J., Li X., Gorman D. M., Bazan J. F., Kastelein R. A. (2005) IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 [DOI] [PubMed] [Google Scholar]
- 84. Carriere V., Roussel L., Ortega N., Lacorre D. A., Americh L., Aguilar L., Bouche G., Girard J. P. (2007) IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl. Acad. Sci. USA 104, 282–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ohno T., Oboki K., Kajiwara N., Morii E., Aozasa K., Flavell R. A., Okumura K., Saito H., Nakae S. (2009) Caspase-1, caspase-8, and calpain are dispensable for IL-33 release by macrophages. J. Immunol. 183, 7890–7897 [DOI] [PubMed] [Google Scholar]
- 86. Chackerian A. A., Oldham E. R., Murphy E. E., Schmitz J., Pflanz S., Kastelein R. A. (2007) IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J. Immunol. 179, 2551–2555 [DOI] [PubMed] [Google Scholar]
- 87. Kurowska-Stolarska M., Kewin P., Murphy G., Russo R. C., Stolarski B., Garcia C. C., Komai-Koma M., Pitman N., Li Y., Niedbala W., McKenzie A. N., Teixeira M. M., Liew F. Y., Xu D. (2008) IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J. Immunol. 181, 4780–4790 [DOI] [PubMed] [Google Scholar]
- 88. Komai-Koma M., Xu D., Li Y., McKenzie A. N. J., McInnes I. B., Liew F. Y. (2007) IL-33 is a chemoattractant for human Th2 cells. Euro. J. Immunol. 37, 2779–2786 [DOI] [PubMed] [Google Scholar]
- 89. Ali S., Huber M., Kollewe C., Bischoff S. C., Falk W., Martin M. U. (2007) IL-1 receptor accessory protein is essential for IL-33-induced activation of T lymphocytes and mast cells. Proc. Natl. Acad. Sci. USA 104, 18660–18665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kurowska-Stolarska M., Stolarski B., Kewin P., Murphy G., Corrigan C. J., Ying S., Pitman N., Mirchandani A., Rana B., van Rooijen N., Shepherd M., McSharry C., McInnes I. B., Xu D., Liew F. Y. (2009) IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 183, 6469–6477 [DOI] [PubMed] [Google Scholar]
- 91. Iwahana H., Yanagisawa K., Ito-Kosaka A., Kuroiwa K., Tago K., Komatsu N., Katashima R., Itakura M., Tominaga S. (1999) Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur. J. Biochem. 264, 397–406 [DOI] [PubMed] [Google Scholar]
- 92. Hoogerwerf J. J., Tanck M. W. T., Van Zoelen M. A. D., Wittebole X., Laterre P. F., Van Der Poll T. (2010) Soluble ST2 plasma concentrations predict mortality in severe sepsis. Inten. Care Med. 36, 630–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gibot S. (2006) Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia and severe sepsis. Semin. Respir. Crit. Care Med. 27, 29–33 [DOI] [PubMed] [Google Scholar]
- 94. Bouchon A., Facchetti F., Weigand M. A., Colonna M. (2001) TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410, 1103–1107 [DOI] [PubMed] [Google Scholar]
- 95. Wang F., Liu S., Wu S., Zhu Q., Ou G., Liu C., Wang Y., Liao Y., Sun Z. (2012) Blocking TREM-1 signaling prolongs survival of mice with Pseudomonas aeruginosa induced sepsis. Cell. Immunol. 272, 251–258 [DOI] [PubMed] [Google Scholar]
- 96. Andersson U., Erlandsson-Harris H., Yang H., Tracey K. J. (2002) HMGB1 as a DNA-binding cytokine. J. Leukoc. Biol. 72, 1084–1091 [PubMed] [Google Scholar]
- 97. Wang H., Vishnubhakat J. M., Bloom O., Zhang M., Ombrellino M., Sama A., Tracey K. J. (1999) Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery 126, 389–392 [PubMed] [Google Scholar]
- 98. Yang H., Tracey K. J. (2010) Targeting HMGB1 in inflammation. Biochim. Biophys. Acta 1799, 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gardella S., Andrei C., Ferrera D., Lotti L. V., Torrisi M. R., Bianchi M. E., Rubartelli A. (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 3, 995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bonaldi T., Talamo F., Scaffidi P., Ferrera D., Porto A., Bachi A., Rubartelli A., Agresti A., Bianchi M. E. (2003) Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 22, 5551–5560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Oh Y. J., Youn J. H., Ji Y., Lee S. E., Lim K. J., Choi J. E., Shin J. S. (2009) HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J. Immunol. 182, 5800–5809 [DOI] [PubMed] [Google Scholar]
- 102. Scaffidi P., Misteli T., Bianchi M. E. (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 [DOI] [PubMed] [Google Scholar]
- 103. Bell C. W., Jiang W., Reich C. F., III, Pisetsky D. S. (2006) The extracellular release of HMGB1 during apoptotic cell death. Am. J. Physiol. Cell Physiol. 291, C1318–C1325 [DOI] [PubMed] [Google Scholar]
- 104. Wang H., Bloom O., Zhang M., Vishnubhakat J. M., Ombrellino M., Che J., Frazier A., Yang H., Ivanova S., Borovikova L., Manogue K. R., Faist E., Abraham E., Andersson J., Andersson U., Molina P. E., Abumrad N. N., Sama A., Tracey K. J. (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 [DOI] [PubMed] [Google Scholar]
- 105. Sappington P. L., Yang R., Yang H., Tracey K. J., Delude R. L., Fink M. P. (2002) HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology 123, 790–802 [DOI] [PubMed] [Google Scholar]
- 106. Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., Al-Abed Y., Wang H., Metz C., Miller E. J., Tracey K. J., Ulloa L. (2004) Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10, 1216–1221 [DOI] [PubMed] [Google Scholar]
- 107. Ulloa L., Ochani M., Yang H., Tanovic M., Halperin D., Yang R., Czura C. J., Fink M. P., Tracey K. J. (2002) Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl. Acad. Sci. USA 99, 12351–12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Kawahara K., Hashiguchi T., Masuda K., Saniabadi A. R., Kikuchi K., Tancharoen S., Ito T., Miura N., Morimoto Y., Biswas K. K., Nawa Y., Meng X., Oyama Y., Takenouchi K., Shrestha B., Sameshima H., Shimizu T., Adachi T., Adachi M., Maruyama I. (2009) Mechanism of HMGB1 release inhibition from RAW264.7 cells by oleanolic acid in Prunus mume Sieb. et Zucc. Int. J. Mol. Med. 23, 615–620 [DOI] [PubMed] [Google Scholar]
- 109. Kato S., Hussein M. H., Kakita H., Goto T., Daoud G. A., Kato T., Sugiura T., Nobata M., Nakajima Y., Endo T., Mizuno K., Ito T., Kato I., Suzuki S., Togari H. (2009) Edaravone, a novel free radical scavenger, reduces high-mobility group box 1 and prolongs survival in a neonatal sepsis model. Shock 32, 586–592 [DOI] [PubMed] [Google Scholar]
- 110. Li W., Ashok M., Li J., Yang H., Sama A. E., Wang H. (2007) A major ingredient of green tea rescues mice from lethal sepsis partly by inhibiting HMGB1. PLoS One 2, e1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Shinohara M. L., Kim H. J., Kim J. H., Garcia V. A., Cantor H. (2008. a) Alternative translation of osteopontin generates intracellular and secreted isoforms that mediate distinct biological activities in dendritic cells. Proc. Natl. Acad. Sci. USA 105, 7235–7239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Smith L. L., Giachelli C. M. (1998) Structural requirements for α 9 β 1-mediated adhesion and migration to thrombin-cleaved osteopontin. Exp. Cell. Res. 242, 351–360 [DOI] [PubMed] [Google Scholar]
- 113. Bayless K. J., Davis G. E. (2001) Identification of dual α4 β1 integrin binding sites within a 38 amino acid domain in the N-terminal thrombin fragment of human osteopontin. J. Biol. Chem. 276, 13483–13489 [DOI] [PubMed] [Google Scholar]
- 114. Weber G. F., Zawaideh S., Hikita S., Kumar V. A., Cantor H., Ashkar S. (2002) Phosphorylation-dependent interaction of osteopontin with its receptors regulates macrophage migration and activation. J. Leukoc. Biol. 72, 752–761 [PubMed] [Google Scholar]
- 115. Nystrom T., Duner P., Hultgardh-Nilsson A. (2007) A constitutive endogenous osteopontin production is important for macrophage function and differentiation. Exp. Cell. Res. 313, 1149–1160 [DOI] [PubMed] [Google Scholar]
- 116. Ashkar S., Weber G. F., Panoutsakopoulou V., Sanchirico M. E., Jansson M., Zawaideh S., Rittling S. R., Denhardt D. T., Glimcher M. J., Cantor H. (2000) Eta-1 (osteopontin): an early component of type-1 (cellmediated) immunity. Science 287, 860–864 [DOI] [PubMed] [Google Scholar]
- 117. Koh A., da Silva A. P., Bansal A. K., Bansal M., Sun C., Lee H., Glogauer M., Sodek J., Zohar R. (2007) Role of osteopontin in neutrophil function. Immunology 122, 466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shinohara M. L., Jansson M., Hwang E. S., Werneck M. B., Glimcher L. H., Cantor H. (2005) T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc. Natl. Acad. Sci. USA 102, 17101–17106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Diao H., Kon S., Iwabuchi K., Kimura C., Morimoto J., Ito D., Segawa T., Maeda M., Hamuro J., Nakayama T., Taniguchi M., Yagita H., Van Kaer L., Onoe K., Denhardt D., Rittling S., Uede T. (2004) Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity 21, 539–550 [DOI] [PubMed] [Google Scholar]
- 120. Brinkmann V., Billich A., Baumruker T., Heining P., Schmouder R., Francis G., Aradhye S., Burtin P. (2010) Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 9, 883–897 [DOI] [PubMed] [Google Scholar]
- 121. Mullershausen F., Zecri F., Cetin C., Billich A., Guerini D., Seuwen K. (2009) Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat. Chem. Biol. 5, 428–434 [DOI] [PubMed] [Google Scholar]
- 122. Chiang N., Arita M., Serhan C. N. (2005) Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot. Essent. Fatty Acids 73, 163–177 [DOI] [PubMed] [Google Scholar]
- 123. Walker J., Dichter E., Lacorte G., Kerner D., Spur B., Rodriguez A., Yin K. (2011) Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock 36, 410–416 [DOI] [PubMed] [Google Scholar]
- 124. Lago F., Dieguez C., Gómez-Reino J., Gualillo O. (2007) Adipokines as emerging mediators of immune response and inflammation. Nat. Clin. Pract. Rheumatol. 3, 716–724 [DOI] [PubMed] [Google Scholar]
- 125. Ouchi N., Kihara S., Arita Y., Okamoto Y., Maeda K., Kuriyama H., Hotta K., Nishida M., Takahashi M., Muraguchi M., Ohmoto Y., Nakamura T., Yamashita S., Funahashi T., Matsuzawa Y. (2000) Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 102, 1296–1301 [DOI] [PubMed] [Google Scholar]
- 126. Tilg H., Wolf A. M. (2005) Adiponectin: a key fat-derived molecule regulating inflammation. Expert Opin. Ther. Targets 9, 245–251 [DOI] [PubMed] [Google Scholar]
- 127. Inui A., Asakawa A., Bowers C. Y., Mantovani G., Laviano A., Meguid M. M., Fujimiya M. (2004) Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 18, 439–456 [DOI] [PubMed] [Google Scholar]
- 128. Jacob A., Rajan D., Pathickal B., Balouch I., Hartman A., Wu R., Zhou M., Wang P. (2010) The inhibitory effect of ghrelin on sepsis-induced inflammation is mediated by the MAPK phosphatase-1. Int. J. Mol. Med. 25, 159–164 [PMC free article] [PubMed] [Google Scholar]
- 129. Shah K. G., Wu R., Jacob A., Blau S. A., Ji Y., Dong W., Marini C. P., Ravikumar T. S., Coppa G. F., Wang P. (2009) Human ghrelin ameliorates organ injury and improves survival after radiation injury combined with severe sepsis. Mol. Med. 15, 407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Jacob A., Shah K. G., Wu R., Wang P. (2010) Ghrelin as a novel therapy for radiation combined injury. Mol. Med. 16, 137–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kitamura K., Kangawa K., Kawamoto M., Ichiki Y., Nakamura S., Matsuo H., Eto. T. (1993) Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 192, 553–560 [DOI] [PubMed] [Google Scholar]
- 132. Nishio K., Akai Y., Murao Y., Doi N., Ueda S., Tabuse H., Miyamoto S., Dohi K., Minamino N., Shoji H., Kitamura K., Kangawa K., Matsuo H. (1997) Increased plasma concentrations of adrenomedullin correlate with relaxation of vascular tone in patients with septic shock. Crit. Care Med. 25, 953–957 [DOI] [PubMed] [Google Scholar]
- 133. Fujioka S. (2001) Increased plasma concentration of adrenomedullin during and after major surgery. Surg. Today 31, 575–579 [DOI] [PubMed] [Google Scholar]
- 134. McLatchie L. M., Fraser N. J., Main M. J., Wise A., Brown J., Thompson N., Solari R., Lee M. G., Foord S. M. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339 [DOI] [PubMed] [Google Scholar]
- 135. Wu Z., Lauer T. W., Sick A., Hackett S. F., Campochiaro P. A. (2007) Oxidative stress modulates complement factor H expression in retinal pigmented epithelial cells by acetylation of FOXO3. J. Biol. Chem. 282, 22414–22425 [DOI] [PubMed] [Google Scholar]
- 136. Lukiw W. J., Zhao Y., Cui J. G. (2008) An NF-κ B-sensitive micro RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J. Biol. Chem. 283, 31315–31322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Yang S., Zhou M., Chaudry I. H., Wang P. (2002) Novel approach to prevent the transition from the hyperdynamic phase to the hypodynamic phase of sepsis: role of adrenomedullin and adrenomedullin binding protein-1. Ann. Surg. 236, 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wu R., Zhou M., Wang P. (2003) Adrenomedullin and adrenomedullin binding protein-1 downregulate TNF-α in macrophage cell line and rat Kupffer cells. Regul. Pept. 112, 19–26 [DOI] [PubMed] [Google Scholar]
- 139. Miksa M., Wu R., Cui X., Dong W., Das P., Simms H. H., Ravikumar T. S., Wang P. (2007) Vasoactive hormone adrenomedullin and its binding protein: anti-inflammatory effects by up-regulating peroxisome proliferator-activated receptor-γ. J. Immunol. 179, 6263–6272 [DOI] [PubMed] [Google Scholar]
- 140. lez-Rey E., Chorny A., Varela N., Robledo G., Delgado M. (2006) Urocortin and adrenomedullin prevent lethal endotoxemia by down-regulating the inflammatory response. Am. J. Pathol. 168, 1921–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Saito Y., Nakagawa C., Uchida H., Sasaki F., Sakakibara H. (2001) Adrenomedullin suppresses fMLP-induced upregulation of CD11b of human neutrophils. Inflammation 25, 197–201 [DOI] [PubMed] [Google Scholar]
- 142. Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332, 411–415 [DOI] [PubMed] [Google Scholar]
- 143. Kedzierski R. M., Yanagisawa M. (2001) Endothelin system: the double-edged sword in health and disease. Annu. Rev. Pharmacol. Toxicol. 41, 851–876 [DOI] [PubMed] [Google Scholar]
- 144. Guarda E., Myers P. R., Brilla C. G., Tyagi S. C., Weber K. T. (1993) Effects of endothelin on collagen turnover in cardiac fibroblasts. Cardiovasc. Res. 27, 1004–1008 [DOI] [PubMed] [Google Scholar]
- 145. Tschaikowsky K., Sagner S., Lehnert N., Kaul M., Ritter J. (2000) Endothelin in septic patients: effects on cardiovascular and renal function and its relationship to proinflammatory cytokines. Crit. Care Med. 28, 1854–1860 [DOI] [PubMed] [Google Scholar]
- 146. Figueras-Aloy J., Gomez-Lopez L., Rodriguez-Miguelez J. M., Jordán-García Y., Salvia-Roiges M. D., Jiménez W., Carbonell-Estrany X. (2004) Plasma endothelin-1 and clinical manifestations of neonatal sepsis. J. Perinat. Med. 32, 522–526 [DOI] [PubMed] [Google Scholar]
- 147. Piechota M., Banach M., Irzmanski R., Barylski M., Piechota-Urbanska M., Kowalski J., Pawlicki L. (2007) Plasma endothelin-1 levels in septic patients. J. Intensive Care Med. 22, 232–239 [DOI] [PubMed] [Google Scholar]
- 148. Pierrakos C., Vincent J. L. (2010) Sepsis biomarkers: a review. Crit. Care 14, R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Miksa M., Wu R., Dong W., Das P., Yang D., Wang P. (2006) Dendritic cell-derived exosomes containing milk fat globule epidermal growth factor-factor VIII attenuate proinflammatory responses in sepsis. Shock 25, 586–593 [DOI] [PubMed] [Google Scholar]
- 150. Aziz M., Jacob A., Matsuda A., Wang P. (2011) Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis 16, 1077–1086 [DOI] [PubMed] [Google Scholar]
- 151. Aziz M., Jacob A., Wu R., Matsuda A., Zhou M., Dong W., Wang P. (2011) MFG-E8 attenuates LPS-induced TNF-α production in macrophages via STAT3-mediated SOCS3 activation. PLoS One 6, e27685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Fink M. P. (2008) Animal models of sepsis and its complications. Kidney Int. 74, 991–993 [DOI] [PubMed] [Google Scholar]
- 153. McNamara M. J., Norton J. A., Nauta R. J., Alexander H. R. (1993) Interleukin-1 receptor antibody (IL-1rab) protection and treatment against lethal endotoxemia in mice. J. Surg. Res. 54, 316–321 [DOI] [PubMed] [Google Scholar]
- 154. Fisher C. J., Jr., Dhainaut J. F., Opal S. M., Pribble J. P., Balk R. A., Slotman G. J., Iberti T. J., Rackow E. C., Shapiro M. J., Greenman R. L.., et al. (1994) Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271, 1836–1843 [PubMed] [Google Scholar]
- 155. Doi K., Leelahavanichkul A., Yuen P. S., Star R. A. (2009) Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Invest. 119, 2868–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. (1987) Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330, 662–664 [DOI] [PubMed] [Google Scholar]
- 157. Fisher C. J., Jr., Agosti J. M., Opal S. M., Lowry S. F., Balk R. A., Sadoff J. C., Abraham E., Schein R. M., Benjamin E. (1996) Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N. Engl. J. Med. 334, 1697–1702 [DOI] [PubMed] [Google Scholar]
- 158. Marshall J. C. (2003) Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat. Rev. Drug Discov. 2, 391–405 [DOI] [PubMed] [Google Scholar]