Inactivated versus attenuated influenza vaccines trigger IgG versus IgA, in different locations.
Keywords: antibody forming cells, bone marrow, cold-adapted vaccine, upper and lower respiratory tract, inactivated vaccine, influenza virus
Abstract
Currently, there are two different types of licensed influenza virus vaccines available in the USA, the live attenuated cold-adapted vaccine and the inactivated vaccine. Children greater than 2 years of age and adults younger than 50 years (apart from those suffering from immunodeficiencies or lung disease) may choose between the two vaccines. Previous studies have shown that both vaccines elicit significant serum antibody responses. However, comprehensive analyses of antibody-forming cells (AFCs) in the upper respiratory tract (URT), the critical site of pathogen entry, have been lacking. We therefore compared influenza virus-specific antibody and AFC activities in systemic and mucosal tissues following immunizations of cotton rats with inactivated or live-attenuated vaccines, including vaccines from the 2009-10 and 2010-11 seasons. Results demonstrated that inactivated and live-attenuated vaccines induced virus-specific AFCs, but patterns of residence and function were highly disparate. The inactivated vaccine elicited AFCs predominantly in the spleen and bone marrow; IgG was the main isotype. In contrast, the live attenuated vaccine elicited acute and long-sustained AFC responses in the diffuse nasal-associated lymphoid tissue (d-NALT) and lung, with IgA being the predominant isotype. The appearance of these d-NALT URT responses was confirmed by a similar study of the 2009–10 live attenuated vaccine in ferrets. Data emphasize that the inactivated and live-attenuated vaccines that are each capable of protecting humans from influenza virus disease do so by very different modes of immune surveillance.
Introduction
Seasonal influenza virus constitutes a serious health threat responsible for tens of thousands of deaths per year in the USA alone (1, 2). The virus naturally undergoes sequence and antigenic changes (antigenic drift) and on rare occasions will jump species to yield dramatic changes in protein structure (antigenic shift) (3). Vaccines are produced at least once per year to represent and combat the fluctuating viral variants.
Young infants are susceptible to serious influenza virus disease and are often encouraged by public health authorities to receive an inactivated vaccine at 6 months of age (4). Older children greater than 2 years of age and adults younger than 50 years (apart from those suffering from immunodeficiencies or lung disease) may choose between two different vaccines, either inactivated or live-attenuated. The inactivated vaccine is prepared by isolation of the influenza virus membrane molecules and is given by the intramuscular (IM) route. The live attenuated vaccine is produced using reverse genetics technology to introduce desired membrane molecules into a standard influenza virus backbone. This replication-competent viral product is administered by the intranasal (IN) route and persists transiently in the upper respiratory tract (URT) (5). The antigens presented by either one of these vaccines are selected each year by the WHO to represent currently circulating influenza virus strains (6).
Recently, studies of a candidate parainfluenza virus vaccine (Sendai virus, SeV) in small animals demonstrated that IN administration of a replication-competent vaccine rapidly induced a population of antibody-forming cells (AFCs) in the diffuse nasal-associated lymphoid tissue (d-NALT) of the URT (7, 8). These cells were long sustained and well positioned to target incoming target virus at its point of entry. Might the same type of response be induced by licensed, influenza virus vaccines? To address this question, we tested the licensed inactivated and live-attenuated influenza virus vaccine products in cotton rats (9, 10) and ferrets. Experiments demonstrated that inactivated and live-attenuated vaccines elicited very different patterns of AFCs in terms of residence and isotype production. The live attenuated vaccine induced AFCs predominantly in the URT and lower respiratory tract (LRT) of cotton rats, most often expressing the IgA isotype (the presence of d-NALT resident AFCs was also observed when the vaccine was tested in ferrets). Measurable AFCs were sustained throughout a 4-month observation period. In contrast, the inactivated vaccine elicited predominantly IgG-expressing AFCs in the spleen and bone marrow of cotton rats. Results were similar when influenza virus vaccines from the 2009–10 and 2010–11 seasons were tested. These data emphasize that the inactivated and live-attenuated vaccine products, each capable of protecting humans from virus infection, can do so despite their induction of very different patterns of immune surveillance.
Materials and methods
Animals and immunizations
Cotton rats (Sigmodon hispidus ≥ 6 weeks of age) were purchased from Harlan Sprague Dawley. Ferrets (>3 months of age) were purchased from Triple F Farms. Animals were housed as specified by AALAC guidelines, and protocols were approved by the Institutional Animal Care and Use Committee (IACUC). Seasonal vaccines were Fluvirin® and FluMist® (2009–10 season) or Fluzone® and FluMist® (2010–11 season). Fluvirin® (Novartis Vaccine and Diagnostics Ltd) and Fluzone® (Sanofi Pasteur) were inactivated, purified surface antigen and split, respectively, influenza virus vaccines. Each 0.5ml dose of inactivated vaccine contained 15 µg haemagglutinin (HA) from each of three viruses, whereas each 0.2ml dose of FluMist® vaccine contained 106.5–7.5 FFU (fluorescent focus units) of each of three live-attenuated influenza viruses. All vaccines contained influenza virus antigens against A/Brisbane/59/2007 (H1N1), A/Brisbane/10/07 (H3N2) and B/Brisbane/60/08 viruses (2009–10 season vaccines), or ACalifornia/7/09 (H1N1), A/Perth/16/09 (H3N2) and B/Brisbane/60/08 viruses (2010–11 season vaccines).
The FluMist® vaccines were administered IN with a half human dose in cotton rats and a full human dose in ferrets. The Fluvirin® and Fluzone® vaccines were administered IM (in the gastrocnemius muscle, 0.2 ml per animal) in cotton rats. Cotton rats were studied in groups of 3 and ferrets were studied in groups of 2–3. All experiments were conducted twice with similar results.
Enzyme-linked immunosorbent assays (ELISAs)
Antibody responses toward influenza virus were determined by ELISA. Fluvirin® 2009–10 was plated as antigen to test all samples from animals that received Fluvirin® or FluMist® vaccines from the 2009–10 season. Fluzone® 2010–11 was plated as antigen to test all samples from animals that received Fluzone® or FluMist® vaccines from the 2010–11 season. Antigens were plated at 1 µg/ml on 96-well ELISA plates. After overnight incubation, plates were washed with PBS and blocked with PBS containing 1% BSA (Sigma, St Louis, MO, USA). Samples from vaccinated and control animals were diluted serially in PBS and 1% BSA and incubated on plates for 1h at 37°C. Plates were then washed 6× with PBS-Tween 20 (.05%). For cotton rat assays, plates were then incubated with alkaline phosphatase-conjugated goat anti-mouse IgM, IgG (gamma specific) or IgA (Southern Biotechnologies Assoc.) diluted 1:1000 in PBS and 1% BSA, for 1h at 37°C. For ferret assays, the reagent was goat anti-ferret IgG IgA IgM (H+L) alkaline phosphatase conjugated (Rockland). Plates were washed 6× with PBS-Tween and developed by addition of p-nitrophenyl phosphate substrate (1mg/ml) in diethanolamine buffer (pH 9.8). Assays were read at optical density (OD) 405nm after 30min. All experiments were conducted in replicate. Antibody titers were calculated using non-linear regression software, using a cut-off of 0.02 for the OD 405nm reading (one-site binding non-linear regression, GraphPad Prism, San Diego, CA, USA).
Tissue preparation for AFC analyses
To monitor AFCs, animals were sacrificed and bone marrow, d-NALT, lung, cervical lymph nodes (CLN) and spleen were sampled. To prepare d-NALT (11, 12), skin, lower jaws, soft palates (including the attached o-NALT), muscles, cheek bones and teeth were removed from the heads. Remaining snouts were cut into small pieces, after which cells were released by digestion with 4mg/ml collagenase in PBS at 37°C for 30min shaking. Cells were washed with PBS and then suspended in PBS and layered onto a 40/75% discontinuous percoll gradient. After centrifugation at 600g for 30min, cells were collected from the gradient interface for assay. The cells were washed 2× in PBS and suspended in RPMI1640 plus 10% heat-inactivated fetal bovine serum (R10). Lungs were suspended and similarly processed by collagenase digestion and purification on percoll gradients. Bone marrow, CLN and spleen were collected, red blood cells lysed (Sigma Red Blood Cell Lysing Buffer) and washed prior to assays.
Enzyme-linked immunospot (ELISPOT) assays
The ELISPOT plate was coated with the antigens as described above for ELISAs. The membrane was blocked for 1 hour with R10. Media were aspirated and 100 µl fresh R10 media were added to the wells. Then 1 × 105 cells in 100 µl R10 were added to each well. The plates were incubated at 37°C, 5% CO2 for 3h and washed 3× with PBS and 3× with PBS-Tween 20. Alkaline phosphatase-conjugated antibodies (specified above for ELISAs) in 100 µl buffer containing 1% BSA were added to each well. After overnight incubation, the antibodies were removed and plates were washed 6× with PBS and developed with 1mg/ml BCIP/NBT substrate (Sigma Aldrich). The plate was incubated at room temperature and monitored for spot appearance. The exposure was stopped by rinsing plates with water. Plates were dried and spots were counted using a Nikon dissecting scope. All experiments were conducted in replicate.
Haemagglutination inhibition assays
Sera were first diluted 1:4 in receptor-destroying enzyme (RDE ii, manufactured by Denka Seiken, purchased from Accurate Chemical) and incubated overnight at 37°C. RDE was then inactivated by incubating samples for 30min at 60°C. For the HAI assay, treated sera were diluted 1:10 and then serially diluted 1:2 in PBS in microtiter 96-well round-bottomed plates. Virus (4 agglutinating units) was added at an equal volume (25 µl serum and 25 µl virus) and incubated at room temperature for 30min. About 0.5% chick red blood cells in 50 µl were then mixed with each well and incubated for an additional 30min. HAI titers were read as the highest dilution of serum that blocked agglutination of chick red blood cells by virus. Viruses used in HAI assays were the licensed FluMist® vaccine [0.2ml contained 106.5–7.5 FFU of live attenuated influenza virus reassortants of three strains for the 2011–12 season: A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and B/Brisbane/60/2008] (MedImmune, LLC, Gaithersburg, MD, USA), or A/Puerto Rico/8/34 (A/PR8, stock kindly provided by the laboratory of Dr P. Thomas at St. Jude, Memphis TN for amplification).
Results
Differential IgG and IgA response patterns following immunizations with inactivated and live-attenuated influenza virus vaccines in cotton rats
To compare the systemic and mucosal immune responses elicited by two different forms of 2009–10 seasonal influenza virus vaccines, experiments were conducted in cotton rats. Animals were grouped to receive the inactivated vaccine by the IM route or the live attenuated vaccine by the IN route, after which responses were measured by ELISA at 1 week, 2 weeks, 1 month and 4 months post-vaccination.
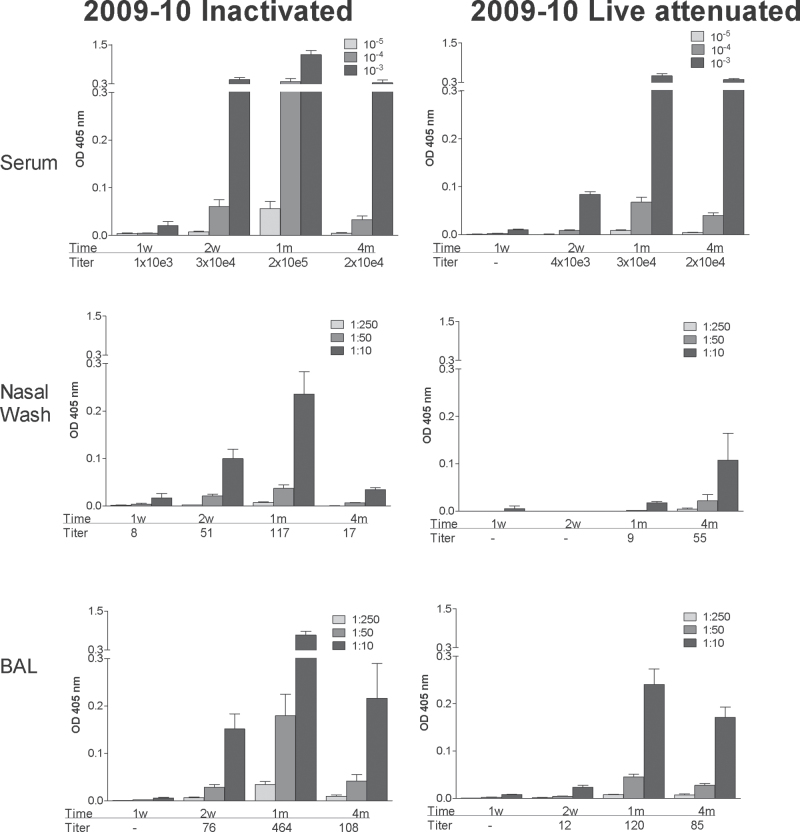
When responses were measured in serum, nasal wash and bronchoalveolar lavage (BAL) samples, virus-specific IgG was detected in all animals (Fig. 1). IgG responses were superior by orders of magnitude when serum samples were compared with respiratory wash samples, and serum samples from all vaccinated mice exhibited HAI activity against FluMist® and A/PR8 (Table 1). Antibody generally waned at the 4-month time point but was sustained at measureable levels in all vaccinated animals.
Fig. 1.
Serum and respiratory tract IgG responses elicited by inactivated and live attenuated 2009–10 influenza virus vaccines. Serum (top panels), nasal wash (middle panels) and BAL (bottom panels) samples were tested for virus-specific IgG responses by ELISA at various intervals (1 week, 2 weeks, 1 month, 4 months; x-axes) after vaccination with inactivated (left panels) or live attenuated vaccines (right panels) from the 2009–10 season. Animals were tested individually and results are shown as group means with standard error bars. Antibody titers were calculated using non-linear regression software and are shown below each bar set.
Table 1.
Serum antibodies inhibit virus haemagglutination
| Vaccine | Animal | HAI against FluMist® | HAI against A/PR8 |
|---|---|---|---|
| Inactivated 2009–10 | 1 | 160 | 160 |
| 2 | 160 | 80 | |
| 3 | 80 | 80 | |
| Live attenuated 2009–10 | 1 | 80 | 320 |
| 2 | 160 | 320 | |
| 3 | 160 | 320 | |
| None | 1 | <10 | <10 |
| 2 | <10 | <10 |
Animal sera were tested 1 month after animal vaccination for HAI activity against FluMist® (2011–12) or A/PR8. Serum titers are shown.
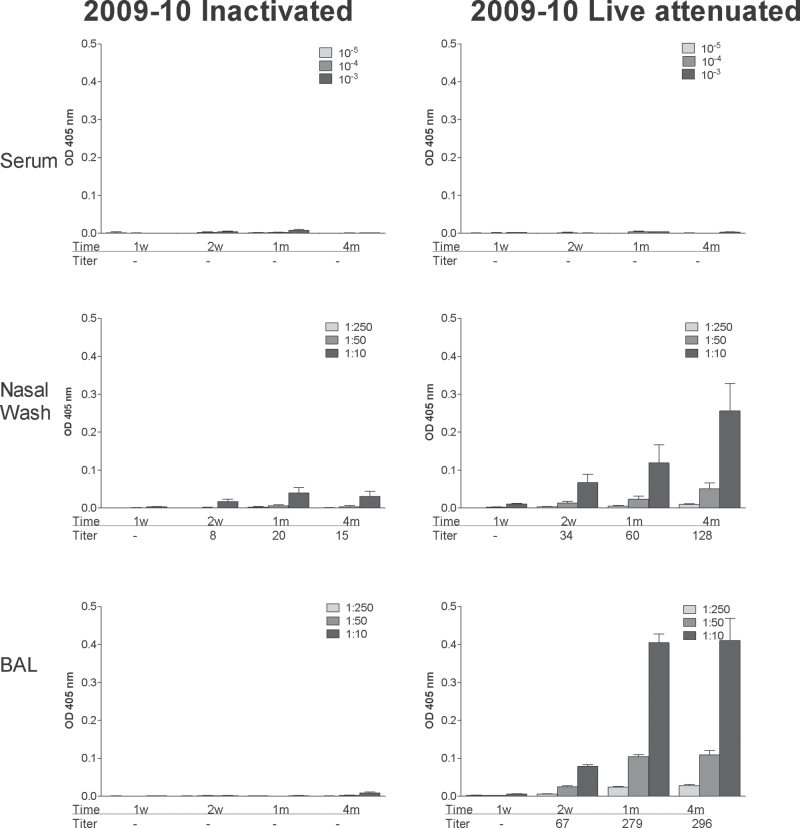
A dramatically different pattern was observed upon measurement of virus-specific IgA. As shown in Fig. 2, IgA appeared primarily in the nasal wash and BAL and primarily in animals that received the live attenuated vaccine rather than the inactivated vaccine (P < .05, unpaired t-test). At the 4-month time point, IgA had not waned.
Fig. 2.
IgA responses elicited by inactivated and live attenuated 2009–10 influenza virus vaccines. Serum (top panels), nasal wash (middle panels) and BAL (bottom panels) samples were tested for virus-specific IgA responses by ELISA at various intervals (1 week, 2 weeks, 1 month, 4 months; x-axes) after vaccination with inactivated (left panels) or live attenuated vaccines (right panels) from the 2009–10 season. Animals were tested individually and results are shown as group means with standard error bars. Antibody titers were calculated using non-linear regression software and are shown below each bar set.
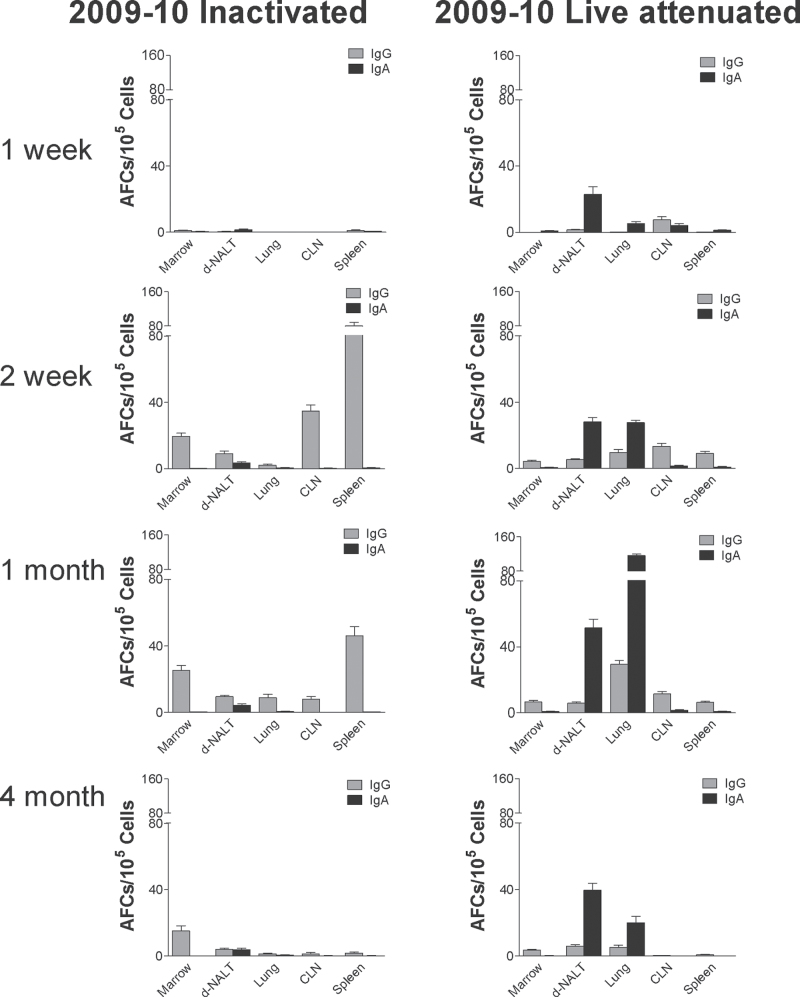
To explain the deposition of IgG and IgA isotypes in various tissues, we examined the patterns of virus-specific AFC residence. As shown in Fig. 3, these patterns were also dramatically different between groups of animals that received the two different forms of influenza virus vaccine. When animals received the inactivated product, AFCs were most frequent in the spleen at the 2-week time point. There was then a shift in AFC residence and AFCs were sustained primarily in the bone marrow at 4 months. In contrast, when animals received the live attenuated product, AFCs were most prevalent in the d-NALT and lung, with relatively little activity in the spleen and bone marrow. In these animals, IgA AFCs scored in higher numbers than IgG AFCs. Responses peaked at 1 month and were measureable above background at the 4-month time point.
Fig. 3.
IgG and IgA AFC elicited by inactivated and live attenuated 2009–10 influenza virus vaccines. IgG and IgA AFC were measured in various tissues (bone marrow, d-NALT, lung, CLN, spleen; x-axes) ELISPOT assay at various intervals (1 week-1st row, 2 weeks-2nd row, 1 month-3rd row, 4 months-4th row) after vaccination with inactivated (left panels) or live attenuated (right panels) vaccines from the 2009–10 season. Animals were tested individually and results are shown as group means with standard error bars.
Antibodies and AFCs induced in the ferret URT by the 2009–10 live attenuated vaccine
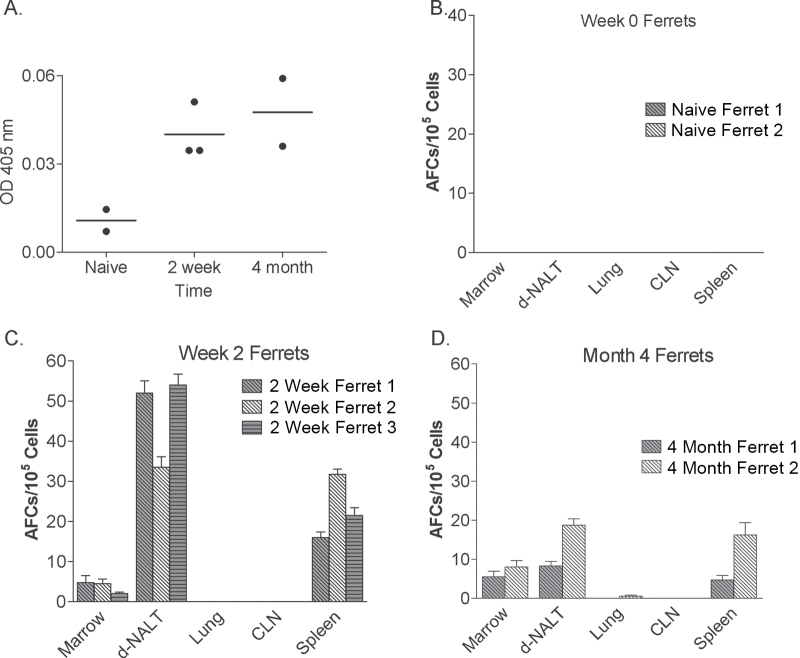
The cotton rat has been recommended as a new model for influenza virus vaccine analyses (10), but the ferret model is often preferred in the influenza virus field. We therefore asked if the FluMist® vaccine would be capable of inducing AFCs in the respiratory tract in ferrets, comparable to the situation in cotton rats. Ferrets were immunized with the FluMist® vaccine and monitored for antibodies and AFC responses after 2 weeks and 4 months. Results are shown in Fig. 4 (note that in this case, a reagent was used to measure all virus-specific antibodies rather than individual isotype subsets). In Fig. 4A are shown serum antibody responses from individual animals (each represented by a different circle). Data are shown only for sera diluted 1:103 as higher dilutions associated with no measurable antibody. Anti-virus responses were reproducibly induced by 2 weeks and were sustained for at least 4 months. Although the responses in ferrets appeared to be much weaker than those in cotton rats, this may have been a reflection of assay sensitivity rather than a difference between species.
Fig. 4.
Antibody and AFC responses elicited by inactivated and live attenuated 2009–10 influenza virus vaccines in ferrets. Panel A: Total immunoglobulin was tested in sera 0 weeks (naive), 2 weeks and 4 months after vaccination with the live attenuated vaccine of the 2009–10 season. Sera were diluted 1:1000 prior to testing (antibodies were below detection upon further dilution). Circles indicate results from individual animals. Panels B–D: Total AFC were measured in various tissues (bone marrow, d-NALT, lung, CLN, spleen; x-axes) by ELISPOT assays at week 0 (naive, panel B), week 2 (panel C) and month 4 (panel D) after vaccination with live attenuated vaccine from the 2009–10 season. For panels B–D, animals were tested individually and results are shown as means with standard error bars.
ELISPOT assays were also performed in ferrets on week 0 (naive animals), week 2 and month 4 (Fig. 4B, C and D, respectively). Of interest, the ferret results were qualitatively different from the results from cotton rats. The AFC responses in ferrets were observed only in the d-NALT and spleens, and not in the lungs or CLN. Apparently, vaccine antigen had not penetrated the LRT of ferrets and had thus induced durable AFC effectors in URT but not LRT tissues.
2009–10 and 2010–11 seasonal influenza virus vaccines are matched in patterns of antibody and AFC induction
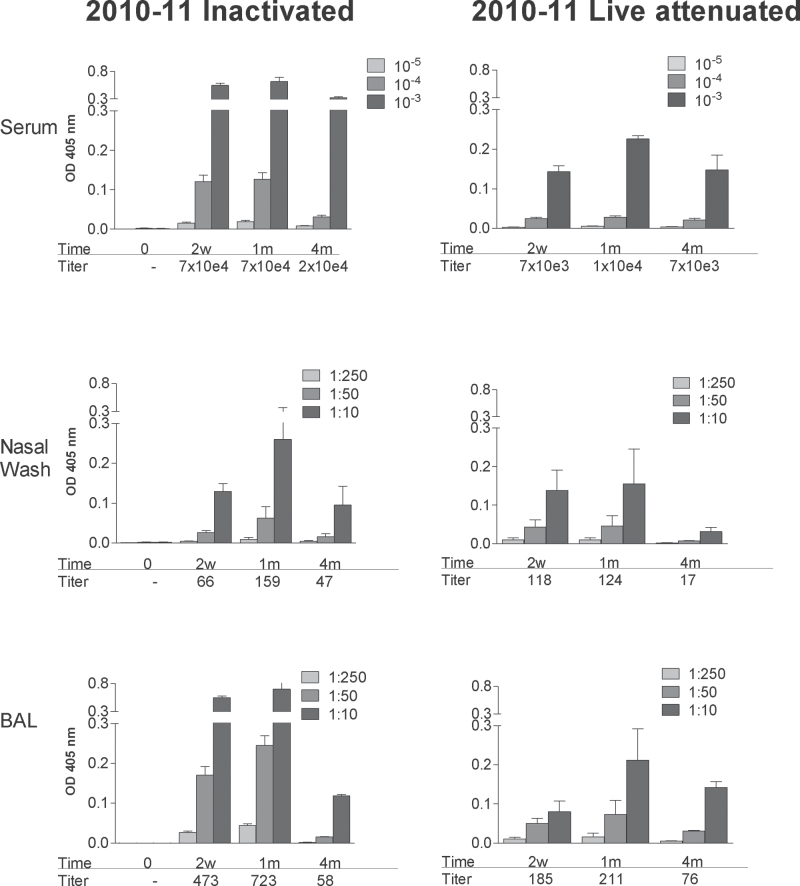
We next questioned whether the patterns of immune activity induced with the 2009–10 influenza virus vaccine might also be induced with other seasonal influenza virus vaccine products. To address this question, we repeated experiments the following year, when all three vaccine antigens were changed, with 2010–11 influenza virus vaccine products. As demonstrated in Fig. 5, IgG ELISA results again illustrated that the magnitude of antibodies was superior in serum samples compared with nasal wash samples in all animals. Also, as for the 2009–10 vaccine products, the 2010–11 vaccines induced IgG responses that peaked after 2 weeks or 1 month and waned at 4 months, but remained measureable throughout the observation period.
Fig. 5.
Serum and respiratory tract IgG responses elicited by inactivated and live attenuated 2010–11 influenza virus vaccines. Serum (top panels), nasal wash (middle panels) and BAL (bottom panels) samples were tested for virus-specific IgG responses by ELISA at various intervals (0 weeks, 2 weeks, 1 month, 4 months; x-axes) after vaccination with inactivated (left panels) or live attenuated vaccines (right panels) from the 2010–11 season. Animals were tested individually and results are shown as group means with standard error bars. Antibody titers were calculated using non-linear regression software and are shown below each bar set.
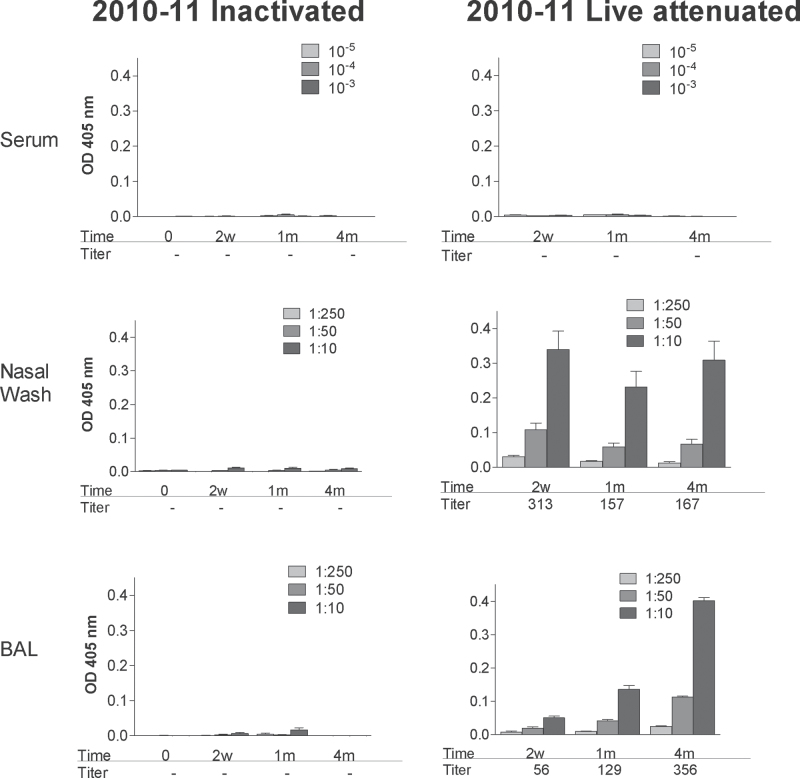
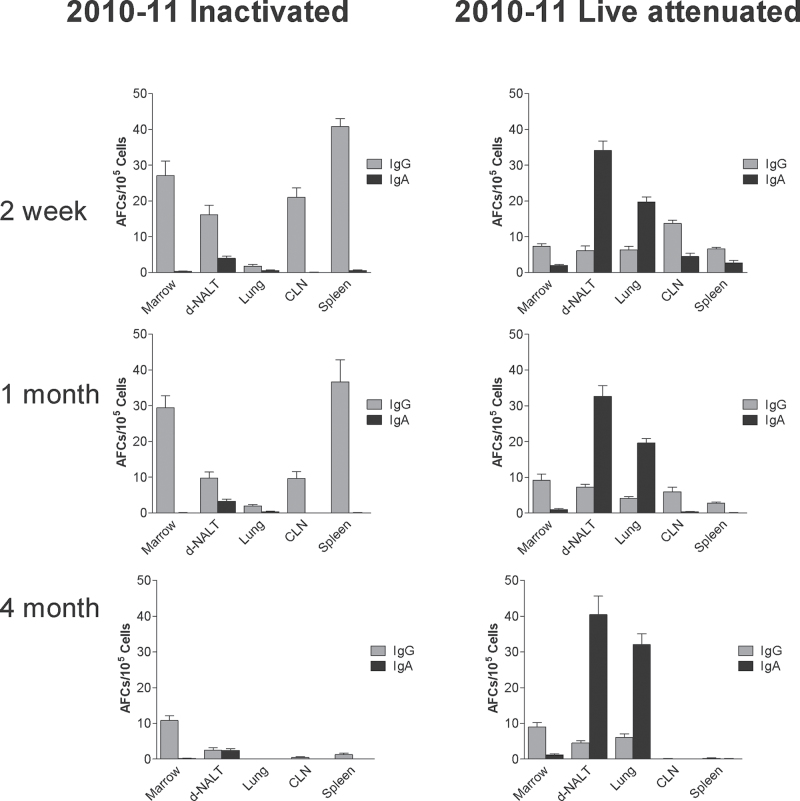
IgA responses were also comparable between the 2009–10 and 2010–11 vaccine products as shown in Fig. 6 (compare Figures 2 and 6) and ELLISPOT patterns were comparable among seasonal vaccines as shown in Fig. 7 despite the different sources of vaccines used for each season (compare Figures 3 and 7).
Fig. 6.
IgA responses elicited by inactivated and live attenuated 2010–11 influenza virus vaccines. Serum (top panels), nasal wash (middle panels) and BAL (bottom panels) samples were tested for virus-specific IgA responses by ELISA at various intervals (0 weeks, 2 weeks, 1 month, 4 months; x-axes) after vaccination with inactivated (left panels) or live attenuated vaccines (right panels) from the 2010–11 season. Animals were tested individually and results are shown as group means with standard error bars. Antibody titers were calculated using non-linear regression software and are shown below each bar set.
Fig. 7.
IgG and IgA AFCs elicited by inactivated and live attenuated 2010–11 influenza virus vaccines. IgG and IgA AFCs were measured in various tissues (bone marrow, d-NALT, lung, CLN, spleen; x-axes). ELISPOT assay at various intervals (2 week-1st row, 1 month-2nd row, 4 months-3rd row) after vaccination with inactivated (left panels) or live attenuated (right panels) vaccines from the 2010–11 season. Animals were tested individually and results are shown as group means with standard error bars.
Discussion
The study described in this report was performed to examine and compare the antibody and AFC activities induced by inactivated and live-attenuated seasonal influenza virus vaccines. Both of these vaccine formulations are licensed and have been demonstrated to provide protective efficacy against influenza virus infections in humans (13, 14). For example, in the year 2000, a 2-year multicenter, double-blind, placebo-controlled efficacy study of a live attenuated vaccine was reported; results showed the vaccine to be 92% efficacious in preventing culture-confirmed infections of influenza A/H3N2 and influenza B viruses in children (15, 16).
The two different vaccines each offer unique benefits. The inactivated influenza vaccine is associated with the better safety profile and is often administered to children greater than 6 months of age, adults and the elderly. When children are under 10 years of age, two doses are recommended to ensure the induction of a protective immune response (13). The live attenuated vaccine is not usually recommended for use in infants or the elderly but is effective after only one dose in children and adults (17).
The current study compared and contrasted the two licensed vaccines with particular attention paid to the URT because effector cells and molecules in this region are best positioned to block the influenza virus pathogen at its point of entry. Data in cotton rats demonstrated that inactivated and live-attenuated vaccines each generated a durable immune response but that the patterns of activity were dramatically different. The inactivated vaccine induced IgG as the predominant isotype and there was negligible production of IgA. Circulating IgG associated with the appearance of IgG-producing AFCs that were most frequent in the spleen in early weeks and most frequent in the bone marrow as time progressed. The live attenuated vaccines differed in that IgA was constitutively expressed in upper and lower airways, reflecting long-term residence of IgA-producing AFCs in d-NALT and lung. These cotton rat results were confirmed using vaccines from two different influenza virus seasons, 2009–10 and 2010–11, which included inactivated vaccines of two types (surface antigen and split).
To validate the discovery of influenza virus-specific AFCs in the d-NALT after FluMist® vaccination (Figures 3 and 7), we also vaccinated ferrets with the FluMist® vaccine (as described previously, a single dose of the inactivated vaccine does not elicit significant responses in ferrets) (18). The ferret shares sialic acid expression patterns and influenza virus-induced disease symptoms such as sneezing and wheezing with humans, and therefore serves as a popular model for influenza virus vaccination, infection and transmission studies (19). Results with the live attenuated influenza virus vaccine product in ferrets matched results in cotton rats, in that responses in the d-NALT were detected by week 2 and were sustained for at least 4 months. There were also qualitative differences between the two species, in that the ferrets did not generate detectable AFCs in the CLN or lung. This result suggests that the cotton rat, like the mouse (18), is more permissive than the ferret for virus and viral antigen penetration into the LRT, thus prompting recruitment of virus-specific AFCs to the lung.
The induction of durable AFCs ensures constitutive antibody production and is therefore an attractive feature of any vaccine. AFC persistence in the bone marrow of vaccinated animals was first described decades ago by Hyland and Coleclough (20), and then by Slifka et al. (21, 22). Cells in the bone marrow were associated with long-term serum antibody responses. The cause–effect relationship was confirmed when bone marrow cells were adoptively transferred from vaccinated mice to naive mice and supported durable antibody production in the hosts. Years later, the establishment of durable AFCs in the respiratory tract was recognized by a number of research investigators while testing live virus inoculations by the IN route. We have found, for example, that a single IN vaccination of cotton rats or mice with a replication-competent parainfluneza virus vaccine (SeV) rapidly and durably induces both CD8+ T cells and AFC-producing cells in the d-NALT (7, 8). The appearance of AFCs correlates with the detection of SeV-specific antibodies within 1 week and for many months after vaccination. AFC residence further correlates with acute and durable protection against challenge viruses (7, 8, 23).
The final residence of long-term AFCs, whether bone marrow or d-NALT, is likely a consequence of lymphocyte ‘imprinting’ at the time of first antigen exposure. Imprinting is initiated by complex interactions between lymphocytes and antigen-presenting cells, which determine the patterns of homing receptors expressed on lymphocyte surfaces. As lymphocytes traffic through blood, lymph and parenchymal tissues, their membrane receptors dictate the cell–cell interactions that will instruct cell residence. For example, the appearance of CCR9 and α4β7 membrane receptors on activated T lymphocyte surfaces promotes cell residence in the small intestine (24). Multiple factors including (i) the type of antigen to which a lymphocyte responds (e.g. internal versus external viral proteins) (25), (ii) the mode and location of antigen presentation and (iii) the cytokine milieu surrounding the activated lymphocyte will influence final outcome. Moreover, external factors such as dietary deficiencies can alter lymphocyte homing and residence (26).
The two vaccines tested in our recent study while inducing antibody responses in different locations of the animal were each able to promote AFC activity that persisted for several months. How is this achieved? There has been a long-standing debate as to the mechanism responsible for prolonged antibody responsiveness. Pertinent to this debate is the question of whether antigen persistence is required. Some researchers argue that antigens deposited by vaccination, particularly in the context of a replication-competent vaccine, can persist for weeks and months. However, antigen persistence does not fully explain the durability of an immune response as B cells that have been manipulated to lose antibody specificity for antigen are nonetheless capable of persisting for long periods (27). Experiments are now warranted, particularly in the URT, to define the degree of antigen persistence and its impact on AFC durability in the d-NALT and lung.
Although AFC residence in respiratory tissues typify responses to the replication-competent vaccine, they are clearly not necessary for the induction of protective immunity. Many vaccines for respiratory tract pathogens, including the inactivated influenza virus vaccine described in this report, are administered by the IM route yet provide protection at mucosal sites. IgG antibodies produced by spleen and bone marrow-resident AFCs clearly traffic to the respiratory tract. The migration of systemic antibody to URT and LRT locations has been previously demonstrated by the protective capacity of maternal antibodies in the newborn and by passive transfer studies in research animals with immunoglobulin products (28, 29). For example, the passive transfer of high-titered RSV-specific polyclonal or monoclonal IgG antibodies by a peripheral route into cotton rats has been shown to reduce RSV challenge virus significantly in the LRT. Of interest, a higher dose was necessary to confer a similar degree of protection in the URT (28), suggesting that peripheral IgG antibody penetrates the LRT more readily than the URT. This explains at least in part why IgG levels tended to be higher in the LRT compared with the URT in our experiments and was higher than might be predicted by numbers of resident IgG-producing AFCs (IgA, which is rare among serum antibodies, was more likely produced predominantly by local AFCs). Perhaps the relative resistance of the URT to protection by serum antibodies provides benefit to the host because a viral infection of the URT can ensue without severe morbidity and can enhance the endogenous immune response. Apart from the trafficking of antibodies from peripheral to mucosal sites, B cells primed in a peripheral location can also circulate through tissues and can quickly respond to antigen deposited in the URT or LRT mucosa upon challenge (30).
The contribution of various components of the vaccine-induced adaptive immune response to protection against respiratory tract pathogens is highly complex and has been evaluated extensively in previous literature. The passive transfer of virus-specific IgA or IgG directly into nasal passages is sufficient to protect mice against a virus challenge (31, 32). Polymeric and monomeric IgA can also be transferred by the intravenous (IV) route, in which case the polymeric form is more readily transported into nasal secretions than the monomeric form and can be protective (33). In contrast, as described above, when IgG is transferred by a peripheral route, it traffics more readily to the LRT than the URT, with limited or negligible protection of URT tissues (28, 33). IgA is well suited for protection of mucosal surfaces because it will transcytose across epithelial barriers and can be tethered to the surface of the airway lining via the poly Ig receptor and secretory components. However, IgG can also transcytose epithelial barriers using an FcRn escort (34). Multiple antibody isotypes can be produced by both B1-B cells and conventional B2 cells as a consequence of switch rearrangements and deletions at the immunoglobulin heavy chain locus (35–37). In some cases, a single B cell will produce IgM, then IgG and finally IgA, dependent on variables including antigen context, CD4+ T cells (antigen-specific or non-specific) (38), and interleukins in the B cell’s microenvironment (39–41). Successive switching may explain, at least in part, the differing kinetics of IgG and IgA in our research animals. Once mature, virus-specific plasma cells persist for months or years and can also be replenished from a long-standing memory B-cell pool (42, 43).
Should antibodies fail to eliminate virus completely, CD4+ and CD8+ T cells provide fail-safe functions (44). CD8+ T cells, best known for their capacity to kill virus-infected targets, are long lasting (7, 45) and can be transferred into naive mice to protect against virus infection (46). The CD4+ T-cell population will enhance the functions of other effector cells but can also confer protection against virus infections independent of B and CD8+ T-cell partners. In some cases, CD4+ T cells lyse infected targets with perforin-dependent mechanisms (47) and in other cases protection is independent of perforin and dependent on interferon production (48). Clearly, the immune system is multifaceted and redundant to ensure successful clearance of respiratory virus infections. The redundancies of function explain why two different vaccine formulations with two different immune outcomes can each confer protection.
In conclusion, the present report describes differential mechanisms by which two protective vaccines function. The inactivated vaccine induces AFCs that reside predominantly in non-mucosal tissue but that nonetheless bathe the respiratory tract with IgG. The live attenuated vaccine induces AFCs that are most often resident within the mucosa and that secrete IgA at this local site. These data are important for understanding the effectiveness of live, attenuated influenza vaccines in humans because protection correlates poorly with serum antibody titers (49). Vaccine efficacy in adults and children (50) is likely due to a complexity of influences including T-cell responses (51) and local mucosal IgA responses as described here. Development of assays to measure locally resident AFCs may allow a better definition of immune correlates in lieu of standard serum IgG measurements by the HAI assay (49). Our data further illustrate immune system flexibility and the diverse mechanisms driven by two different influenza virus vaccines to protect humans from virus infections.
Funding
National Cancer Institute at the National Institutes of Health (P30 CA21765); the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01 AI088729, HHSN266200700005C); American Lebanese Syrian Associated Charities (ALSAC).
Acknowledgements
We thank Dr. J. McCullers for reagents and useful discussions. We thank Dr. P. Thomas for contribution of A/PR8 virus for HAI studies.
References
- 1. Thompson W. W., Shay D. K., Weintraub E., et al. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 289: 179– [DOI] [PubMed] [Google Scholar]
- 2. Dushoff J., Plotkin J. B., Viboud C., Earn D. J., Simonsen L. 2006. Mortality due to influenza in the United States–an annualized regression approach using multiple-cause mortality data. Am. J. Epidemiol. 163: 181– [DOI] [PubMed] [Google Scholar]
- 3. Taubenberger J. K., Kash J. C. 2010. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host Microbe. 7: 440– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fraaij P. L., Bodewes R., Osterhaus A. D., Rimmelzwaan G. F. 2011. The ins and outs of universal childhood influenza vaccination. Future Microbiol. 6: 1171– [DOI] [PubMed] [Google Scholar]
- 5. Vesikari T., Karvonen A., Korhonen T., et al. 2006. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr. Infect. Dis. J. 25: 590– [DOI] [PubMed] [Google Scholar]
- 6. Ortiz J. R., Sotomayor V., Uez O. C., et al. 2009. Strategy to enhance influenza surveillance worldwide. Emerg. Infect. Dis. 15: 1271– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rudraraju R., Surman S., Jones B., Sealy R., Woodland D. L., Hurwitz J. L. 2011. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology. 410: 429– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sealy R., Jones B. G., Surman S. L., Hurwitz J. L. 2010. Robust IgA and IgG-producing antibody forming cells in the diffuse-NALT and lungs of Sendai virus-vaccinated cotton rats associate with rapid protection against human parainfluenza virus-type 1. Vaccine. 28: 6749– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ottolini M. G., Blanco J. C., Eichelberger M. C., et al. 2005. The cotton rat provides a useful small-animal model for the study of influenza virus pathogenesis. J. Gen. Virol. 86: 2823– [DOI] [PubMed] [Google Scholar]
- 10. Eichelberger M. C. 2007. The cotton rat as a model to study influenza pathogenesis and immunity. Viral Immunol. 20: 243– [DOI] [PubMed] [Google Scholar]
- 11. Davis S. S. 2001. Nasal vaccines. Adv. Drug Deliv. Rev. 51: 21– [DOI] [PubMed] [Google Scholar]
- 12. Asanuma H., Thompson A. H., Iwasaki T., et al. 1997. Isolation and characterization of mouse nasal-associated lymphoid tissue. J. Immunol. Methods. 202: 123– [DOI] [PubMed] [Google Scholar]
- 13. Frey S. E., Bernstein D. I., Gerber M. A., et al. 2012. Safety and immune responses in children after concurrent or sequential 2009 H1N1 and 2009–2010 seasonal trivalent influenza vaccinations. J. Infect. Dis. 206: 828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Madhun A. S., Akselsen P. E., Sjursen H., et al. 2010. An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine. 29: 266– [DOI] [PubMed] [Google Scholar]
- 15. Belshe R. B., Gruber W. C., Mendelman P. M., et al. 2000. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J. Infect. Dis. 181: 1133– [DOI] [PubMed] [Google Scholar]
- 16. Belshe R. B., Mendelman P. M., Treanor J., et al. 1998. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N. Engl. J. Med. 338: 1405– [DOI] [PubMed] [Google Scholar]
- 17. Glezen W. P. 2008. Universal influenza vaccination and live attenuated influenza vaccination of children. Pediatr. Infect. Dis. J. 27(10 Suppl):S104– [DOI] [PubMed] [Google Scholar]
- 18. Huber V. C., Kleimeyer L. H., McCullers J. A. 2008. Live, attenuated influenza virus (LAIV) vehicles are strong inducers of immunity toward influenza B virus. Vaccine. 26: 5381– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith H., Sweet C. 1988. Lessons for human influenza from pathogenicity studies with ferrets. Rev. Infect. Dis. 10: 56– [DOI] [PubMed] [Google Scholar]
- 20. Hyland L., Sangster M., Sealy R., Coleclough C. 1994. Respiratory virus infection of mice provokes a permanent humoral immune response. J. Virol. 68: 6083– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ahmed R. 2003. Understanding immunological memory to improve vaccination strategies. Keystone Meeting Presentation: HIV Vaccine Development: Immunological and Biological Challenges; Banff Canada: [Google Scholar]
- 22. Slifka M. K., Matloubian M., Ahmed R. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69: 1895– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones B., Zhan X., Mishin V., et al. 2009. Human PIV-2 recombinant Sendai virus (rSeV) elicits durable immunity and combines with two additional rSeVs to protect against hPIV-1, hPIV-2, hPIV-3, and RSV. Vaccine. 27: 1848– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mora J. R., Bono M. R., Manjunath N., et al. 2003. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 424: 88 [DOI] [PubMed] [Google Scholar]
- 25. Sealy R., Surman S., Hurwitz J. L., Coleclough C. 2003. Antibody response to influenza infection of mice: different patterns for glycoprotein and nucleocapsid antigens. Immunology. 108: 431– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cassani B., Villablanca E. J., De Calisto J., Wang S., Mora J. R. 2012. Vitamin A and immune regulation: role of retinoic acid in gut-associated dendritic cell education, immune protection and tolerance. Mol. Aspects Med. 33: 63– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gray D. 2002. A role for antigen in the maintenance of immunological memory. Nat. Rev. Immunol. 2: 60– [DOI] [PubMed] [Google Scholar]
- 28. Prince G. A., Horswood R. L., Chanock R. M. 1985. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J. Virol. 55: 517– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stensballe L. G., Ravn H., Kristensen K., Meakins T., Aaby P., Simoes E. A. 2009. Seasonal variation of maternally derived respiratory syncytial virus antibodies and association with infant hospitalizations for respiratory syncytial virus. J. Pediatr. 154: 296– [DOI] [PubMed] [Google Scholar]
- 30. Takamura S., Roberts A. D., Jelley-Gibbs D. M., Wittmer S. T., Kohlmeier J. E., Woodland D. L. 2010. The route of priming influences the ability of respiratory virus-specific memory CD8+ T cells to be activated by residual antigen. J. Exp. Med. 207: 1153– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tamura S., Funato H., Hirabayashi Y., et al. 1991. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur. J. Immunol. 21: 1337– [DOI] [PubMed] [Google Scholar]
- 32. Mazanec M. B., Lamm M. E., Lyn D., Portner A., Nedrud J. G. 1992. Comparison of IgA versus IgG monoclonal antibodies for passive immunization of the murine respiratory tract. Virus Res. 23: 1– [DOI] [PubMed] [Google Scholar]
- 33. Renegar K. B. Small P. A.Jr. 1991. Passive transfer of local immunity to influenza virus infection by IgA antibody. J. Immunol. 146: 1972 [PubMed] [Google Scholar]
- 34. Israel E. J., Taylor S., Wu Z., et al. 1997. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 92: 69– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amanna I. J., Slifka M. K., Crotty S. 2006. Immunity and immunological memory following smallpox vaccination. Immunol. Rev. 211: 320– [DOI] [PubMed] [Google Scholar]
- 36. Kataoka K., Fujihashi K., Terao Y., et al. 2011. Oral-nasopharyngeal dendritic cells mediate T cell-independent IgA class switching on B-1 B cells. PLoS. ONE. 6: e25396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaminski D. A., Stavnezer J. 2006. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. J. Immunol. 177: 6025– [DOI] [PubMed] [Google Scholar]
- 38. Sangster M. Y., Riberdy J. M., Gonzalez M., Topham D. J., Baumgarth N., Doherty P. C. 2003. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J. Exp. Med. 198: 1011– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gearhart P. J., Hurwitz J. L., Cebra J. J. 1980. Successive switching of antibody isotypes expressed within the lines of a B-cell clone. Proc. Natl. Acad. Sci. U.S.A. 77: 5424– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hurwitz J. L., Tagart V. B., Schweitzer P. A., Cebra J. J. 1982. Patterns of isotype expression by B cell clones responding to thymus-dependent and thymus-independent antigens in vitro. Eur. J. Immunol. 12: 342– [DOI] [PubMed] [Google Scholar]
- 41. Lutzker S., Rothman P., Pollock R., Coffman R., Alt F. W. 1988. Mitogen- and IL-4-regulated expression of germ-line Ig gamma 2b transcripts: evidence for directed heavy chain class switching. Cell. 53: 177– [DOI] [PubMed] [Google Scholar]
- 42. Amanna I. J., Carlson N. E., Slifka M. K. 2007. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 357: 1903– [DOI] [PubMed] [Google Scholar]
- 43. Slifka M. K., Antia R., Whitmire J. K., Ahmed R. 1998. Humoral immunity due to long-lived plasma cells. Immunity. 8: 363– [DOI] [PubMed] [Google Scholar]
- 44. Benton K. A., Misplon J. A., Lo C. Y., Brutkiewicz R. R., Prasad S. A., Epstein S. L. 2001. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J. Immunol. 166: 7437– [DOI] [PubMed] [Google Scholar]
- 45. Wiley J. A., Hogan R. J., Woodland D. L., Harmsen A. G. 2001. Antigen-specific CD8(+) T cells persist in the upper respiratory tract following influenza virus infection. J. Immunol. 167: 3293– [DOI] [PubMed] [Google Scholar]
- 46. Kast W. M., Roux L., Curren J., et al. 1991. Protection against lethal Sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc. Natl. Acad. Sci. U. S. A. 88: 2283– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brown D. M., Dilzer A. M., Meents D. L., Swain S. L. 2006. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J. Immunol. 177: 2888– [DOI] [PubMed] [Google Scholar]
- 48. Surman S. L., Brown S. A., Jones B. G., Woodland D. L., Hurwitz J. L. 2010. Clearance of HIV type 1 envelope recombinant sendai virus depends on CD4+ T cells and interferon-gamma but not B cells, CD8+ T cells, or perforin. Aids Res Hum Retroviruses. 26: 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCullers J. A., Huber V. C. 2012. Correlates of vaccine protection from influenza and its complications. Hum. Vaccin. Immunother. 8: 34– [DOI] [PubMed] [Google Scholar]
- 50. Belshe R. B., Edwards K. M., Vesikari T., et al. 2007. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J. Med. 356: 685– [DOI] [PubMed] [Google Scholar]
- 51. Hoft D. F., Babusis E., Worku S., et al. 2011. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J. Infect. Dis. 204: 845– [DOI] [PMC free article] [PubMed] [Google Scholar]