Abstract
Background
Ecstasy use is commonly linked with memory deficits in abstinent ecstasy users. Similar impairments are being found during ecstasy intoxication after single doses of ± 3,4 metylenedioxymethamphetamine (MDMA). The concordance of memory impairments during intoxication and abstinence suggests a similar neuropharmacological mechanism underlying acute and chronic memory impairments. The mechanism underlying this impairment is to date not known. We hypothesized that cortisol might play an important role in this mechanism as cortisol, implicated in the regulation of memory performance, can be brought out of balance by stressors like MDMA.
Methods
In the present study, we aimed to block the MDMA-induced acute memory defect by giving participants a cortisol synthesis inhibitor (metyrapone) together with a single dose of MDMA. Seventeen polydrug MDMA users entered this placebo-controlled within subject study with four treatment conditions. The treatments consisted of MDMA (75 mg) and metyrapone (750 mg), alone and in combination, and double placebo. Pre-treatment with metyrapone or Placebo occurred 1 h prior to MDMA or Placebo administration. Memory performance was tested at peak drug concentrations by means of several memory tests. Cortisol levels were determined in blood and oral fluid; this served as a control measure to see whether manipulations were effective.
Results
Main findings indicated that whereas treatment with metyrapone blocked the expected MDMA-induced increase in cortisol levels in blood, it did not prevent the MDMA-induced memory deficit from happening.
Conclusion
We therefore conclude that MDMA-induced increments in cortisol concentrations are not related to MDMA-induced memory impairments.
Keywords: MDMA, cortisol, memory, metyrapone
Introduction
Ecstasy (MDMA) use is commonly linked with impairment of short-term and long-term memory in heavy ecstasy users (Parrott, 2001; Cole and Sumnall, 2003). Chronic memory deficits in abstinent, heavy ecstasy users resemble the acute memory deficits observed after a single dose of MDMA. These acute effects, first shown in an experimental, placebo-controlled, within-subject study in 2005 (Kuypers and Ramaekers, 2005) and later replicated in other studies (e.g. van Wel et al., 2011), revealed an MDMA-induced impairment of immediate recall and delayed recall for verbal and spatial information, 1.5–2 h after administration (Kuypers and Ramaekers, 2005, Kuypers and Ramaekers, 2007a). This apparent concordance between acute and long-term MDMA effects may indicate that the chronic deficits in heavy ecstasy users and acute MDMA effects may share a common neuropharmacological mechanism.
Cortisol is implicated in the regulation of neurotransmission, cognitive function and a number of psychobiological functions such as psychiatric well-being (Erickson et al., 2003; Scholey et al., 2011). The relation between cortisol levels and memory performance is represented by an inverted U-shaped curve: that is, extreme (high/low) levels of cortisol negatively affect memory performance, whereas medium levels are required for optimal performance(Riedel et al., 2002; Erickson et al., 2003). Cortisol levels can be brought out of balance by stressors, such as MDMA (Parrott, 2009). Several studies have shown that single doses of ecstasy (75–125 mg) cause significant elevations in cortisol concentrations in saliva and blood, with peak concentrations at 120 min post-MDMA administration (Mas et al., 1999; Harris et al., 2002; Lamers, 2003). These peak cortisol concentrations remarkably fall together with the MDMA-induced memory impairment (Kuypers and Ramaekers, 2005). Therefore, it is postulated that there might be a causal relation between MDMA-induced rises in cortisol and memory impairment.
Previous research has shown that at high doses, glucocorticoids can impair memory performance, although not permanently (McEwen, 1998; Andreano and Cahill, 2006; Brunner et al., 2006). The fact that memory impairment is not permanent would coincide with the observation that MDMA-induced memory impairment (single dose) is transient in light MDMA users (Kuypers and Ramaekers, 2005). Repetitive high levels of cortisol can also cause structural changes in certain brain regions implicated in memory functions (i.e. hippocampus, frontal areas and amygdala) (McEwen, 2005). Heavy MDMA users expose themselves frequently to multiple stressors (e.g. MDMA), sleep deprivation, prolonged physical exercise (dancing), elevated environmental temperatures, which supposedly lead to repetitive elevations of cortisol and possibly structural and functional impairment (Parrott, 2009). Previous research has shown that neuroendocrine reactions in heavy ecstasy users differ from reactions in drug-naïve controls (Price et al., 1989; Gerra et al., 2000; Verkes et al., 2001). Cortisol responses to a 5-HT challenge are blunted in the heavy ecstasy users, but not in drug-naïve subjects. Consequently, the possibility exists that the cortisol response to MDMA challenges may contribute to the memory impairment during acute intoxication as well after prolonged heavy use.
The present study was designed to investigate the role of cortisol in MDMA-induced memory deficits. Subjects participated in four drug conditions on separate test days and received a pre-treatment (placebo or metyrapone) and treatment (MDMA or placebo), before conducting a series of memory tests. Cortisol levels were assessed at baseline, and before and after cognitive testing, in blood and oral fluid. It was hypothesized that pre-treatment with metyrapone, a cortisol synthesis inhibitor, would prevent MDMA effects on memory from occurring.
Methods
Design and treatments
The study design was double-blind, placebo-controlled, four-way cross-over with a pre-treatment (750 mg metyrapone or placebo) which preceded treatment (75 mg MDMA or placebo) by 1 h.
Metyrapone, a cortisol synthesis inhibitor, reaches peak plasma concentrations around 1 h post administration. Its effects last for approximately 4 h (Young and Ribeiro, 2006). Previously, it has been shown that a 750 mg dose effectively suppresses the synthesis of cortisol within 2 h post dose (Sawin et al., 1971; Winhusen et al., 2005).
Metyrapone treatment consisted of three 250 mg capsules; MDMA was administered as a 25 mL solution in bitter orange peel syrup, mixed subsequently with 200 mL orange juice. Placebo capsules and syrup were administered along with the active treatments to insure blinding for both participant and researcher. Treatments were randomized by the pharmacy of the Academic Hospital Maastricht. A permit for obtaining, storing and administering MDMA was obtained from the Dutch drug enforcement administration.
Participants
Participant recruitment occurred by means of advertisements in local newspapers and by word of mouth. In total, 19 polydrug MDMA users were medically screened and assigned a subject number, they underwent an ECG measurement, and blood and urine samples were taken for examination (for in- and exclusion criteria, see van Wel et al., 2011). Two participants dropped out prematurely, both because of personal reasons. The remaining 17 participants completed all conditions. Participants' lifetime use varied from light to heavy (15 participants, <20 times; two participants, 85–120 times). Besides ecstasy or MDMA, subjects used other illicit substances; an overview of types of substances and mean use of the sample are represented in Table 1. Their age ranged from 19 to 24 years, and their verbal IQ 105–117 (See Table 1).
Table 1.
Demographic variables
| Min | Max | Mean (SD) | N | |
|---|---|---|---|---|
| Age | 19 | 24 | 21 (1.23) | 17 |
| Verbal IQ | 105 | 117 | 110.29 (4.04) | 17 |
| Males | Females | ||
|---|---|---|---|
| Sex | 11 | 6 | 17 |
| Drug use (number of times used in lifetime) | Min | Max | Mean (SD) | N |
|---|---|---|---|---|
| Ecstasy/MDMA | 1 | 120 | 18 (33) | 17 |
| Amphetamine | 0 | 25 | 2 (6) | 17 |
| Cannabis | 0 | 300 | 55 (92) | 16* |
| Cocaine | 0 | 40 | 5 (12) | 17 |
| Mushrooms | 0 | 8 | 3 (2) | 17 |
| LSD | 0 | 0 | 0 (0) | 17 |
| Ketamine | 0 | 5† | 0 (1) | 17 |
One subject answered: ‘sometimes’;
Only one subject used ketamine.
Procedures
Participants were requested to abstain from any drug use 1 week before the medical examination until 14 days after the last test-day. They were asked not to use any caffeinated or alcoholic beverages 24 h before testing and to get a normal night's sleep. Prior to experimental sessions at 9:00 a.m., they were screened for drugs of abuse consumption in urine (THC/opiates/cocaine/amphetamines/methamphetamines) and had to pass a breathalyser ethanol test. Women were given a pregnancy test. When tests were negative, subjects had breakfast and filled out two questionnaires (to assess sleep complaints and their mood state). At 9:30 a.m., a saliva sample was taken, and participants received the pre-treatment. An hour later, a blood sample was taken subsequently followed by the treatment. One and a half hours later, a second blood sample was taken. From 9:30 to 12:00, subjects were seated in a waiting room equipped with a TV. Between 12:00 and 13:00, at (pre-)treatment peak plasma concentrations, they were administered a test battery of cognitive tests and a mood questionnaire (see Table 2 ).
Table 2.
Schematic representation of the timeline of the test day. Sample: S1, saliva sample; B1–B2, blood samples 1–2. Checks: Urine screen for recent drug use (THC/opiates/cocaine/amphetamines/methamphetamines) and pregnancy (women); breath test for recent alcohol use. Activity: GSS; POMS1–2; (P)T, (Pre)Treatment; CTB, Cognitive Test Battery.
| Time | 9:00 h | 9:30–9:35 h | 10:00–10:30 h | 10:30–10:35 h | 10:35–12:00 h | 12:00 h | 13:00 h |
|---|---|---|---|---|---|---|---|
| Sample | – | S1 | – | B1 | – | B2 | – |
| Treatments | – | PT | – | T | – | – | – |
| Checks | Drugs/pregnancy, alcohol | – | – | – | – | – | – |
| Activity | GSS, POMS1 | – | Break | – | Break | POMS2, CTB | TQ |
Test days were minimally separated by 7 days. Before test days, participants were familiarized with the tests on a training day. Participants signed an informed consent and were paid upon completion of the testing periods for their participation.
The study was performed in accordance with the Helsinki Declaration of 1975 (Latest revision, Seoul 2008) and was approved by the Medical Ethics Committee of the Academic Hospital of Maastricht and the University of Maastricht.
Cognitive measures
National adult reading test (Dutch version)
This test is used to estimate the verbal intelligence of participants (Schmand et al., 1991; Crawford et al., 2001; Bright et al., 2002).
Word learning task (WLT)
Thirty Dutch monosyllabic meaningful nouns and adjectives were consecutively presented for 1 s on a computer screen. Participants' task was to repeat every word aloud upon appearance and recall as many words as possible at the end of the trial. This procedure was repeated three times. After a 30 min delay, participants were asked again to recall as many words as possible from the previously shown list, which resulted in a delayed recall score(Klaassen et al., 2002). Hereafter, subjects were given a delayed recognition task containing 15 new words and 15 words of the original list. They had to categorize the words as ‘old’ or ‘new’ (Rey, 1958; Wingen et al., 2007).
Continuous recognition memory task
A series of 400 black-and-white pictures were presented on a computer screen with a duration of 500 ms and an inter-stimulus interval of 2500 ms. Ten pictures were presented ten times, and this occurred randomly in the series, either 1, 3 or 10 pictures after the first occurrence of the picture. The other pictures were only repeated once in the series(Curran et al., 1998; Van Strien et al., 2007). Participants' task was to categorize pictures as ‘new’ or ‘old’, according to whether they were presented for the first or as a repetition. The task assesses recognition memory and can be seen as a model of time-dependent forgetting.
Spatial memory task
Ten black-and-white pictures were presented subsequently in 10 different locations. After presentation, each picture was presented alone with two possible locations where it appeared. Participants' task was to choose the correct location; this was the immediate recall phase. This procedure was repeated six times with different stimuli and locations. After a 30 min delay, the recall phase was repeated; this test served as a delayed recall measure (adjusted version of Kessels et al., 1999).
Prospective memory task
Participants saw three components on a computer screen: a letter in the centre (A or B), a counter in the left upper corner, to keep track of the trial number and a memory set in the right upper corner. Their task was to respond to successive presentations of the letters by pressing the corresponding response button (= simple task) while remembering prospective trial numbers (memory set) on which they had to withhold their response. This prospective memory signal occurred randomly for 1 s in 30 trials during the test. The memory set never exceeded three items at a time (Ramaekers et al., 2009; van Wel et al., 2011). In total, 240 letters were displayed for 3 s with an inter-stimulus interval of 0.5 s.
Sternberg memory task
Participants were briefly shown a set of unrelated consonants they had to memorize. This ‘memory load’ changed and expanded over three trial blocks from one to two, to four letters. After presentation of a memory set, a series of 90 letters was displayed on the computer screen. Participants had to respond to each letter as rapidly as possible by pressing either a ‘yes’ or ‘no’ button to indicate whether or not the letter belonged to the memory set. Half of the presented letters was part of the memory set (Sternberg, 1966).
Questionnaires
Groninger sleep scale (GSS)
This questionnaire consists of 15 dichotomous questions about sleep complaints and an open question concerning the duration/h of sleep in order to assess respectively sleep quality and quantity (Mulder-Hajonides van der Meulen et al., 1980).
Profile of mood states (POMS)
In this self-assessment mood questionnaire, participants indicate in how far the 72 five-point Likert scale items represent his/her mood. Ten scales, representing mood states, were derived: that is, anxiety, depression, anger, vigour, fatigue, confusion, friendliness and elation, arousal [(Anxiety + Vigour) – (Fatigue + Confusion)] and positive mood (Elation – Depression) (de Wit et al., 2002).
Pharmacokinetic assessments
Blood samples, centrifuged immediately at 4000× g for 10 min, served to determine cortisol concentrations and peak drug concentrations in blood plasma. One saliva sample was collected at the beginning of the test day and served to determine baseline cortisol concentrations.
Cortisol concentrations
Blood plasma samples were not stored but centrifuged immediately and sent away for analysis after each test day with the Cobas assay (Roche Diagnostics Limited, West Sussex, UK). The quantification limit was 0.5 nmol·L−1.
Oral fluid samples were collected in clean tubes and frozen immediately at minus 20°C until analysis for cortisol concentrations. A freezing step facilitates the breakdown of mucous before centrifugation (Chiu and Collier, 2003). After thawing at room temperature, samples were vortex-mixed for 30 s and centrifuged at 2880 g for 10 min. Samples were analysed with the AxSYM® Cortisol Assay (Abbott Diagnostics, Abbott Park, IL) that utilizes fluorescence polarization immunoassay (FPIA) (Nejtek, 2002). The LOD was 0.64 μg·dL−1, and intra- and inter-assay variability were below 6% and 11% respectively.
Peak drug concentrations
Blood plasma samples were frozen at –20°C until analysis for drug concentrations. MDMA, MDA, HMMA and HMA were determined using a method previously described by Pizarro et al. (2002). Metyrapone plasma concentrations were determined following a modified method of Chiarotto and Wainer (1995). Briefly, daily standard curves were fortified with metyrapone at 0, 25, 50, 100, 250 and 500 ng·mL−1 using 1 mL of blank plasma. After liquid extraction and evaporation step, each sample was reconstituted with 50 μL of the mobile phase, MilliQ water–Acetonitrile (75:25, v/v). Separation of metyrapone and its internal standard (diphenylamine) was carried out using a 4.6 × 150 mm Waters Atlantis® (Waters Corporation, Milford, MA) dC18 5 um column. The linear gradient elution system was as follows: 75% A for the initial time, rising to 50% A at the first 6 min, holding 4 min and then reached to 30% A and standing during 12 to 15 min. The gradient returned to 75% A in 2 min, standing at 75% A for others 3 min as post time. The flow rate was 1 mL·min−1. Identification of compounds was carried out by comparing retention times and UV spectra of the unknown peaks with those of the standards at 264 nm. LOD and LOQ were 3.6 and 10.9 ng·mL−1, respectively. Intra- and inter-assay variability was below 15%.
Statistical analyses
Sample size
A power calculation for detecting a significant effect on the primary parameter of this study, total number of recalled words (verbal word learning task) was conducted by means of G-Power (version 3.0.10), a computer programme for power analyses. The significance level (α) was set at 0.05, and the effect size at 0.30 (small to moderate). Based on these numbers, it has been shown that 17 subjects are sufficient to detect a significant difference between treatment conditions with 81% power and an α of 0.05.
Statistical analyses were conducted by means of SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Data entered a general linear model, repeated measures procedure, with metyrapone (two levels: metyrapone: Yes/No) and MDMA (two levels: MDMA: Yes/No) as main within-subject factors. Extra within-subject factors were included in following tasks: word learning task (trial: three levels), prospective memory task (NoGo delay: three levels), Sternberg memory test (memory load: three levels). The α criterion level of significance was set at P = 0.05.
Results
The main effects of the statistical analyses are displayed in Tables 3 and 4.
Table 3.
In this table, a summary of means (SE) and F- and P-values accompanying main and interaction effects in cognitive tasks are shown (Overall df = 3, 48)
| Test/DV | Treatments | ANOVA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metyrapone | MDMA | Metyrapone * MDMA interaction | |||||||||
| MDMA | Metyrapone | Metyrapone + MDMA | Placebo | F | P | F | P | F | P | ||
| WLT | |||||||||||
| IRT1 | 8.82 (0.54) | 10.94 (0.84) | 8.47 (0.56) | 10.00 (0.66) | 0.234 | 0.635 | 28.122 | <0.001 | 0.106 | 0.749 | |
| IRT2 | 12.18 (0.79) | 14.47 (0.89) | 11.53 (0.86) | 14.29 (0.94) | |||||||
| IRT3 | 14.53 (0.82) | 17.00 (1.38) | 14.00 (1.05) | 18.18 (1.26) | |||||||
| IRTOT | 35.53 (1.89) | 42.41 (2.88) | 34.00 (2.29) | 42.47 (2.59) | 0.234 | 0.635 | 28.122 | <0.001 | 0.106 | 0.749 | |
| DR | 10 (1.14) | 14.12 (1.32) | 10.88 (1.06) | 13.94 (1.34) | 0.581 | 0.457 | 28.216 | <0.001 | 0.137 | 0.716 | |
| RECCOR | 13.06 (0.38) | 13.29 (0.43) | 12.23 (0.60) | 12.59 (0.77) | 0.010 | 0.922 | 0.324 | 0.577 | 4.072 | 0.061 | |
| RECRT | 760 (32.50) | 741 (20.67) | 753 (26.54) | 759 (34.34) | 0.263 | 0.615 | 0.126 | 0.727 | 0.033 | 0.858 | |
| CRMT | |||||||||||
| New | 57.53 (0.57) | 57.94 (0.41) | 56.18 (0.69) | 58.35 (0.35) | 1.456 | 0.245 | 16.192 | 0.001 | 0.591 | 0.453 | |
| Old | 56.06 (0.94) | 57.76 (0.56) | 55.88 (0.52) | 57.53 (0.46) | |||||||
| RTNEW | 755 (26.24) | 770 (37.16) | 742 (31.94) | 774 (35.40) | 0.691 | 0.418 | 0.081 | 0.780 | 0.061 | 0.808 | |
| RTOLD | 731 (27.93) | 711 (27.58) | 741 (33.29) | 732 (29.48) | |||||||
| SMT | |||||||||||
| IRCOR | 48.47 (1.86) | 53.29 (1.25) | 47.70 (1.65) | 52.18 (1.42) | 0.018 | 0.895 | 15.82 | 0.001 | 1.227 | 0.284 | |
| IRRT | 821 (49.85) | 833 (54.61) | 770 (54.34) | 781 (55.15) | 0.001 | 0.982 | 0.119 | 0.735 | 0.829 | 0.376 | |
| DRCOR | 43.65 (1.85) | 45.41 (1.88) | 42.12 (1.98) | 45.12 (1.62) | 0.380 | 0.546 | 3.387 | 0.084 | 0.683 | 0.421 | |
| DRRT | 745 (45.23) | 756 (70.19) | 728 (49.32) | 739 (50.41) | 0.000 | 0.997 | 0.148 | 0.706 | 0.153 | 0.701 | |
| PMT | |||||||||||
| GoCOR | 203.76 (1.37) | 204.23 (1.74) | 203.82 (0.84) | 205.53 (0.67) | 1.498 | 0.239 | 1.969 | 0.180 | 0.251 | 0.623 | |
| GoRT | 713 (49.18) | 715 (54.82) | 718 (41.60) | 653 (43.97) | 0.345 | 0.565 | 2.199 | 0.158 | 0.000 | 0.985 | |
| NoGo1 | 8.82 (0.26) | 8.65 (0.31) | 8.47 (0.32) | 8.88 (0.27) | 1.045 | 0.322 | 5.538 | 0.032 | 0.471 | 0.502 | |
| NoGo2 | 8.82 (0.39) | 9.35 (0.28) | 8.35 (0.44) | 8.76 (0.23) | |||||||
| NoGo3 | 7.76 (0.54) | 8.29 (0.32) | 7.53 (0.45) | 8.82 (0.23) | |||||||
| NoGoTOT | 25 (1.04) | 26 (0.80) | 24 (0.93) | 26 (0.53) | 1.045 | 0.322 | 5.538 | 0.032 | 0.471 | 0.502 | |
| Sternberg | |||||||||||
| Mem1COR | 87.59 (0.44) | 87.70 (0.50) | 87.88 (0.38) | 86.53 (1.74) | 2.005 | 0.176 | 0.258 | 0.618 | 0.157 | 0.697 | |
| Mem2COR | 86.41 (0.65) | 87.47 (0.38) | 87.47 (0.47) | 85.41 (1.78) | |||||||
| Mem4COR | 85.70 (0.97) | 86.88 (0.54) | 87.18 (0.52) | 85.18 (1.90) | |||||||
| Mem1RT | 483 (29.48) | 460 (21.09) | 459 (23.13) | 462 (18.08) | 1.003 | 0.331 | 2.013 | 0.175 | 0.010 | 0.921 | |
| Mem2RT | 504 (25.71) | 488 (19.06) | 503 (22.02) | 505 (20.84) | |||||||
| Mem4RT | 566 (22.99) | 542 (21.28) | 557 (22.46) | 549 (23.51) | |||||||
Bold figures indicate statistically significant values. CRMT, continuous recognition memory task; DR, delayed recall; DV, dependent variables; IR, immediate recall; Mem1–4, memory load in the Sternberg task (1–4 letters); RECN, number of correct recognised words; RECRT, reaction time of correct recognized words; RT, reaction time (in ms).
Table 4.
In this table, a summary of means (SE) and F- and P-values accompanying main and interaction effects in 10 scales of the POMS are shown (Overall Df = 3, 48)
| Mood scales | M | Treatments | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Metyrapone | MDMA | Metyrapone * MDMA interaction | |||||||||
| MDMA | Metyrapone | Metyrapone + MDMA | Placebo | F | P | F | P | F | P | ||
| Anxiety | 1 | 4 (0.55) | 3.70 (0.57) | 4.59 (0.70) | 3.70 (0.72) | 0.511 | 0.485 | 3.178 | 0.094 | 0.250 | 0.624 |
| 2 | 6.18 (0.76) | 3.18 (0.48) | 6.82 (1.25) | 2.94 (0.66) | 0.841 | 0.373 | 25.175 | <0.001 | 0.068 | 0.798 | |
| Depression | 1 | 0.29 (0.19) | 0.53 (0.28) | 0.59 (0.41) | 0.88 (0.55) | 0.030 | 0.864 | 3.429 | 0.083 | 0.838 | 0.374 |
| 2 | 0.53 (0.24) | 0.59 (0.44) | 1.12 (0.51) | 0.29 (0.29) | 3.543 | 0.078 | 1.178 | 0.294 | 0.595 | 0.452 | |
| Anger | 1 | 1.76 (0.48) | 1.76 (0.52) | 2.65 (0.89) | 1.82 (0.71) | 2.237 | 0.154 | 1.953 | 0.181 | 0.664 | 0.427 |
| 2 | 0.82 (0.32) | 2.00 (0.53) | 1.18 (0.71) | 1.23 (0.61) | 3.339 | 0.086 | 14.283 | 0.002 | 0.206 | 0.656 | |
| Vigour | 1 | 13.06 (1.05) | 14.41 (1.09) | 13.59 (1.41) | 11.23 (1.37) | 5.647 | 0.030 | 0.220 | 0.645 | 2.419 | 0.139 |
| 2 | 15.23 (1.51) | 11.65 (1.11) | 13.59 (1.71) | 11.82 (1.22) | 0.956 | 0.343 | 3.874 | 0.067 | 0.635 | 0.437 | |
| Fatigue | 1 | 1.76 (0.46) | 2.06 (0.61) | 2.06 (0.52) | 2.06 (0.71) | 0.102 | 0.754 | 0.089 | 0.769 | 0.082 | 0.778 |
| 2 | 2.59 (1.08) | 4.00 (1.04) | 2.70 (0.79) | 1.70 (0.43) | 1.939 | 0.183 | 0.054 | 0.819 | 3.441 | 0.082 | |
| Confusion | 1 | 4.29 (0.79) | 4.00 (0.78) | 3.53 (0.54) | 3.82 (0.56) | 1.146 | 0.300 | 0.000 | 1.000 | 2.977 | 0.104 |
| 2 | 8.41 (0.78) | 4.47 (0.62) | 8.94 (0.94) | 5.23 (0.60) | 0.060 | 0.810 | 28.308 | <0.001 | 3.811 | 0.069 | |
| Friendliness | 1 | 18.35 (1.32) | 18.70 (1.08) | 17.06 (1.45) | 16.23 (1.49) | 0.462 | 0.506 | 0.053 | 0.820 | 3.914 | 0.065 |
| 2 | 21.35 (1.30) | 16.12 (1.26) | 20.70 (1.62) | 16.12 (1.24) | 0.165 | 0.690 | 13.944 | 0.002 | 0.100 | 0.756 | |
| Elation | 1 | 12.00 (0.82) | 11.88 (0.65) | 11.06 (0.80) | 10.18 (1.08) | 0.391 | 0.541 | 0.335 | 0.571 | 3.680 | 0.073 |
| 2 | 15.18 (1.18) | 10.29 (0.76) | 13.76 (1.10) | 10.70 (0.86) | 1.049 | 0.321 | 16.384 | 0.001 | 0.334 | 0.571 | |
| Arousal | 1 | 11.00 (1.93) | 12.06 (1.80) | 12.59 (1.84) | 9.06 (2.16) | 3.521 | 0.079 | 0.908 | 0.355 | 0.463 | 0.506 |
| 2 | 10.41 (2.45) | 6.35 (2.15) | 8.76 (2.68) | 7.82 (1.74) | 0.741 | 0.402 | 1.052 | 0.320 | 0.003 | 0.960 | |
| Positive mood | 1 | 11.70 (0.83) | 11.35 (0.64) | 10.47 (0.86) | 9.29 (1.20) | 0.458 | 0.508 | 0.844 | 0.372 | 4.630 | 0.047 |
| 2 | 14.65 (1.32) | 9.70 (0.92) | 12.65 (1.25) | 10.41 (0.84) | 1.815 | 0.197 | 10.222 | 0.006 | 0.529 | 0.478 | |
M = Measurement (1 = 9:00 am; 2 = 11:45 am).
Pharmacokinetic assessments
Cortisol concentrations
Two blood samples and one saliva sample were taken in order to determine cortisol concentrations in blood and saliva respectively. Statistical analysis of the baseline saliva samples revealed no significant differences between baseline cortisol levels on test days [mean (SD): 38.23 nmol·L−1 (20.01)]. Therefore, we felt it was justified to use the placebo condition as reference for the plasma cortisol concentrations as we did not collect blood samples at baseline.
Blood
Analysis of blood sample 2 (Tmax MDMA) revealed significant main effects of metyrapone (P < 0.001), MDMA (P < 0.001) and a metyrapone × MDMA interaction effect (P < 0.001). Cortisol concentrations doubled after MDMA treatment and were halved after metyrapone treatment, relative to placebo. Pre-treatment with metyrapone prevented the MDMA-induced increase in cortisol concentrations (Figure 1).
Figure 1.
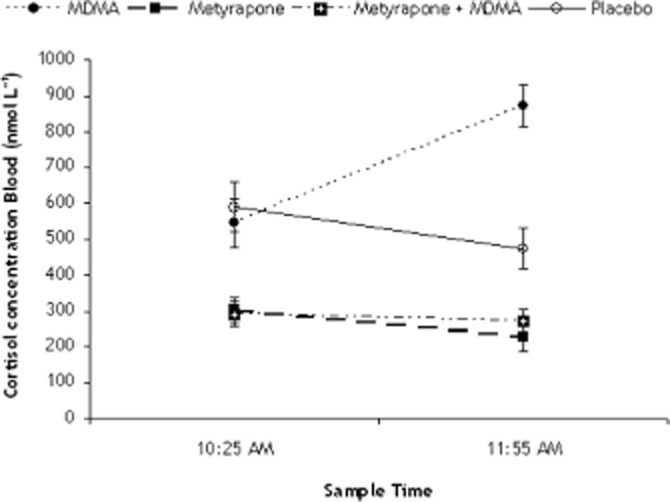
Cortisol levels in blood, respectively, 1 h after treatment with placebo or metyrapone and 2.5 h after treatment with placebo and metyrapone or 1.5 h after treatment with Placebo or MDMA.
Peak drug concentrations
Blood plasma concentrations of MDMA were on average (SD) 135.7 ng·mL−1 (34.6) and 138.5 ng·mL−1 (38.4) 1.5 h post dosing, respectively, after MDMA alone and MDMA combined with metyrapone (Table 5). MDMA or metyrapone concentrations did not significantly differ when given alone or in combination.
Table 5.
Mean (SD) MDMA, MDA, HMMA, HMA and metyrapone concentrations in the different treatment conditions (ng mL−1)
| 1 h post Pre-treatment | 2.5 h post Pre-treatment = 1.5 h post Treatment | |||||
|---|---|---|---|---|---|---|
| Metyrapone | Metyrapone | MDMA | MDA | HMMA | HMA | |
| MDMA alone | – | – | 135.7 (34.6) | 4.7 (2.4) | 184.1 (115.7) | 2.0 (1.5) |
| Metyrapone alone | 1060.01 (1100.47) | 265.20 (189.49) | – | – | – | – |
| Metyrapone + MDMA | 883.78 (1322.11) | 201.84 (182.49) | 138.5 (38.4) | 4.3 (1.9) | 207.1 (123.9) | 1.2 (0.9) |
Cognitive measures
There was no difference in performance between the two ‘heavy’ users and the rest of the sample; therefore, they were not treated differently in terms of statistical analysis (i.e. 17 subjects entered the GLM).
Word learning task
Analysis revealed significant main effects of MDMA (P < 0.001) and trial (P < 0.001) on immediate recall scores. There was no main or interaction effect of metyrapone or metyrapone × MDMA on immediate recall scores.
The trial effect reflects the overall increase in the number of words recalled over three subsequent learning trials. The MDMA effect exemplifies that subjects learned less words in the MDMA conditions compared with placebo. The mean (SE) difference from placebo summed over three trials was 6.9 (2.7) and 8.5 (1.7) words for both MDMA conditions. The absence of a metyrapone × MDMA interaction effect shows that even after metyrapone, the MDMA impairing effect on memory was still present.
Delayed recall scores revealed a significant MDMA effect (P < 0.001). Delayed recall decreased significantly after treatment with MDMA compared with placebo. While under influence of MDMA, participants recalled approximately 3.9 (SE 1.2) and 3.1 (.8) words less during delayed recall, compared with placebo. There was no main or interaction effect of metyrapone or metyrapone × MDMA on delayed recall scores. The latter reflects the presence of the MDMA-induced memory impairment even after pre-treatment with metyrapone.
Recognition scores (number correct and reaction time) were not affected by metyrapone, MDMA nor their interaction.
Continuous recognition memory test
Analyses revealed a main effect of MDMA (P = 0.001) on number of correct recognized items, independent of category (i.e. New/Old). Under the influence of MDMA, subjects recognized on average 1.5 items less compared with placebo.
There was a main effect of category on mean reaction time (of correct recognized items) (P = 0.003). Subjects responded on average 31 ms faster on old items compared with new items (RT = 760 ms). There was also a MDMA by category interaction effect on mean reaction time (P = 0.031). When under influence of MDMA, subjects reacted faster on new items [RT (New) = 748] compared with placebo [RT (New) = 772] and slower on old items [RT(Old) = 736] compared with placebo [RT (Old) = 721].
There were no main or interaction effects of metyrapone or MDMA on reaction time of number of correct recognized items; there was no main effect of metyrapone, nor an interaction effect, on number of correct recognized items.
Spatial memory test (SMT)
Analyses revealed a main effect of MDMA on number of correct immediately recalled locations (P = 0.001). Subjects recalled 4.6 (1.2) locations less under influence of MDMA compared with placebo.
There were no main effects of metyrapone or MDMA, nor interaction effects on other variables of the SMT, that is, reaction time on immediate and delayed recalled locations and number of correct delayed recalled locations although the latter (treatment number correct DR) approached statistical significance (P = 0.084). Under the influence of MDMA, subjects recalled 2.4 locations less compared with placebo.
Prospective memory test
Analysis revealed a main effect of MDMA on total number of correct inhibitions (NoGos) (P = 0.032). Under the influence of MDMA, subjects inhibited less on 1.5 of the NoGo trials. There were no main effects of metyrapone, MDMA or interaction effects on other variables (number correct Go, RT Go trials).
Sternberg memory test
There were no main or interaction effects of metyrapone or MDMA on number correct or RT (correct). There was a main effect of memory load on both dependent variables. An increase in memory load caused a decrease in accuracy and an increase in reaction speed.
Behavioural data and cortisol concentrations: a correlational analysis
In order to investigate the relation between memory performance and cortisol concentrations, immediate recall scores were correlated (Pearson correlation) with blood cortisol concentration. Analysis revealed an absence of a significant correlation (r = −0.134; P = ns) between total number of correct recalled words of the WLT and cortisol concentrations in blood (at the moment of WLT) (Figure 2).
Figure 2.
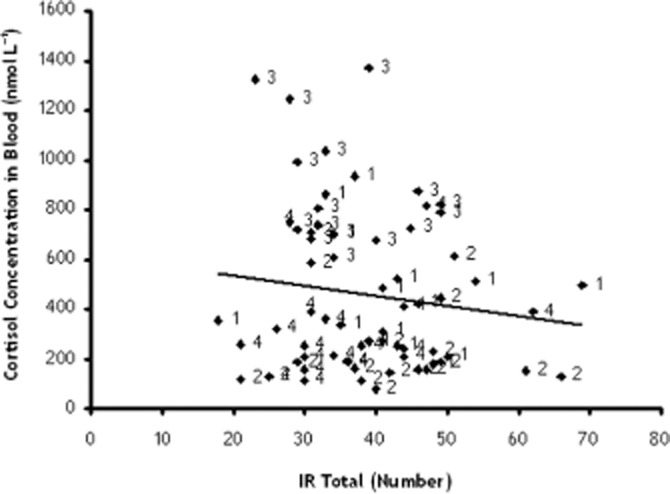
Correlation between total number of recalled words during the immediate recall phase of the word learning task and the cortisol concentrations in blood at drug peak (= before cognitive tests). 1: MDMA = Placebo (9.30AM) + MDMA (75 mg) (10.30AM); 2: metyrapone = metyrapone (750 mg) + Placebo; 3: metyrapone (750 mg) + MDMA (75 mg); 4: Placebo = Placebo MDMA + Placebo metyrapone.
Questionnaires
GSS
There was no difference in quality and quantity of sleep over the four test sessions. Subjects slept on average 7.3 h (SD: 0.8) and had an average score of 2.15 (SD: 2.99) on the GSS.
POMS
Analyses revealed main effects of MDMA on 6 out of 10 scales of the POMS, that is, anxiety (P < 0.001), anger (P = 0.002), confusion (P < 0.001), friendliness (P < 0.002), elation (P = 0.001) and positive mood (P = 0.006). Subjects under influence of MDMA were more anxious, more confused, friendlier, more elated and felt an increase in positive mood compared with placebo. They felt less anger under influence of MDMA compared with placebo (Table 4).
There were no main effects of metyrapone or interaction effects of metyrapone × MDMA.
Discussion
The present study was designed to investigate the role of cortisol in the MDMA-induced memory impairment. It was hypothesized that the suppression of the MDMA-induced increase in cortisol levels would prevent the acute impairing effect of MDMA on memory performance. It was shown that MDMA impaired verbal, spatial and working memory performance irrespective of the fact whether it was administered alone or in combination with metyrapone, a cortisol synthesis inhibitor. Pre-treatment with metyrapone prevented the expected MDMA increase in cortisol plasma concentrations. Together, these findings show that successfully blocking the cortisol response is not an effective measure to block the MDMA-induced memory impairment. This suggests that cortisol does not play a pivotal role in the MDMA-induced memory impairment at drug peak concentration.
Our findings with regard to the MDMA-induced memory deficit are in line with previous findings (Kuypers and Ramaekers, 2005, 2007b), showing that MDMA caused impairments in spatial, verbal and working memory performance. Although the blockade of the cortisol response did not lead to a reverse in the MDMA-induced memory deficits, a potential role for cortisol in these effects cannot be totally excluded, as there are more types of memory than tested here. It is known that cortisol plays a role in emotional memory, and it has been shown that increasing or decreasing those levels results in memory impairment (Van Honk et al., 2003; Marin et al., 2011). In line with the pro-social effects MDMA has, and the potential to use it in the therapeutic setting, this could be another interesting topic to study. A recent study revealed more about the mechanism underlying the MDMA-induced memory deficit. This study, aiming to block the MDMA-induced memory impairment by means of either a 5-HT1a or a 5-HT2a receptor blocker, showed that only pre-treatment with the latter was effective. However, this only holds true for the verbal memory deficit, as spatial and prospective memory performance were still impaired by MDMA, whether or not it was combined with a pre-treatment (van Wel et al., 2011). This finding is interesting as it suggests that perhaps different mechanisms could be involved in the effects MDMA has on different forms of memory. This could result from the fact that MDMA induces a massive serotonin release, and that a large number of serotonin receptors play a role in learning and memory (Barnes and Sharp, 1999; White et al., 1996).
With respect to mood, MDMA caused an increase in positive emotions (like friendliness and elation) and a reduction in anger levels. Besides these positive mood effects, it was also shown that MDMA caused an increase in feelings of confusion and anxiety. These are typical side effects observed in clubbers on ecstasy (Britt and McCance Katz, 2005) and also consistent with previous laboratory findings (Parrott et al., 2011; van Wel et al., 2012). MDMA effects on mood were not affected by pretreatment with metyrapone. This finding appears in line with a study of Harris et al. (2003) that showed that increasing or decreasing cortisol levels did not alter the pleasurable effects of an acute dose of methamphetamine. A recent study has shown that positive mood effects of MDMA may be mediated by the 5-HT2a receptor (van Wel et al., 2012). There is also some evidence for a role of the 5-HT1a receptor in negative moods such as anxiety, but this association could not be demonstrated for MDMA (Raymond et al., 2001; van Wel et al., 2012).
There was no difference in performance or cortisol levels between the two heavy users and the light-to-moderate users. When re-examining the categorization of ‘type of user’ we applied, it might be suggested that we were too strict in terms of labelling. Other studies that did find differences in cortisol levels between moderate and heavy users reported much higher numbers of lifetime use, for example, a mean of 73 number of occasions of ecstasy use (lifelong) for the moderate users and a mean of 230 number of occasions of ecstasy use (lifelong) for the heavy users (Verkes et al., 2001). In that sense, all the subjects in our sample could be classified as light-to-moderate users.
MDMA alone caused a 200% increase in cortisol concentrations in blood compared with placebo. This is in line with previous studies, showing an increase of 150% in sedentary humans (Harris et al., 2002; Dumont and Verkes, 2006; Parrott, 2009). Parrott (2009) interestingly showed that this increase was even (four times) higher in ecstasy users at a party. He suggested this could be due to the combined effect of different stressors (e.g. the drug, the environmental temperature, dancing) (Parrott et al., 2008; Parrott, 2009). Metyrapone caused a reduction of 50% in cortisol concentrations compared with placebo, irrespective of whether it was combined with MDMA or not. These data together show that the pharmacological manipulations produced the expected hormonal changes.
An interesting discussion point related to cortisol is whether the time point of treatments and testing would matter as cortisol levels follow a circadian rhythm. These levels peak between 8:00 and 9:00 a.m., where after they start decreasing until their lowest levels between 12:00 p.m. and 1:00 a.m. (Debono et al., 2009). All the subjects in our study received the treatments between 9:30 a.m. (metyrapone/placebo) and 10:30 a.m. (MDMA/placebo), when cortisol was at its return to low levels. However, cortisol levels in the placebo condition show that there is a mild, but non-significant decrease in levels over the specific time period. Metyrapone and MDMA caused a 50% decrease and increase, relatively, compared with placebo levels. This shows that the change in levels due to the treatments was proportionally larger than the normal changes in circadian cortisol levels. Therefore, it is reasonable to assume that the time period in which we tested was good and did not bias our findings.
Another point potentially related to cortisol levels is the activity subjects undertook during their breaks. Between the start of the test day and the start of the cognitive test battery, there were breaks in which subjects just had to sit in our waiting room to allow pre/treatments reach their peak blood concentrations. They could self-choose the activity they wanted to do with the only requirement that they had to stay in that room (i.e. either watch TV/DVD, read a book or listen to music). Form previous research, it is known that, for example, listening to music can reduce stress levels and cortisol levels (Suda et al., 2008). It can therefore be expected that cortisol levels were possible reduced during the breaks. However, the cortisol concentrations found in the current study were in line with our expectations; for example, these started high at baseline in the morning, when subjects arrived at 9:00 a.m. and slowly lowered during the day, in the placebo condition, according to the circadian rhythm of cortisol, apparently not influenced by the activities subjects undertook during the breaks.
A possible limitation of this study is that we did not collect baseline blood samples to determine cortisol concentrations. However, we did collect saliva samples, and results showed that there was no difference between baseline cortisol concentrations over the four drug conditions. Previous studies have shown that blood and saliva samples are comparable; therefore, it is assumed that it was justified to compare blood cortisol concentrations over the different drug conditions (Ljubijankic et al., 2008). Furthermore, the fact that both saliva samples and blood samples were collected at drug peak concentration is a strength as this enabled us to determine whether cortisol concentrations were influenced by the blood drawing. This was not the case as cortisol concentrations in blood and saliva were comparable (Ljubijankic et al., 2008). Another possible shortcoming of the study is that we did not test women during the same period in their menstrual cycle over the four drug conditions. This could possible act as a confounder with respect to the endocrine (i.e. cortisol) levels. However, comparison of the baseline cortisol concentrations of male and female subjects learned that there were no differences between both groups. The lack of gender effects at baseline supports the notion that menstrual cycle in female subjects in the present study did not act as a confounder.
In sum, main findings indicated that whereas treatment with metyrapone blocked the expected MDMA-induced increase in cortisol levels in blood, it did not prevent the MDMA-induced memory deficit from happening. We therefore conclude that MDMA-induced increments in cortisol concentrations are not related to memory impairments during MDMA intoxication.
Acknowledgments
The authors would like to thank J Schwechheimer, I Verstegen, AMC van Oers and E Theuniszen for their help with the data collection. This research was funded by the Netherlands Organization for Scientific Research (NWO), Grant number: 400-07-213, awarded to JR and KK. The ‘open access’ fee was paid by NWO.
Glossary
- DR
delayed recall
- GSS
Groninger Sleep Scale
- IQ
intelligence quotient
- MDMA
± 3,4 metylenedioxymethamphetamine, ecstasy
- PMT
prospective memory task
- POMS
Profile of Mood States
- SMT
spatial memory task
- THC
tetrahydrocannabinol, cannabis
- TQ
treatment questionnaire
- WLT
word learning task
Conflict of interest
RdlT, MP and MF declare no conflict of interest.
References
- Andreano JM, Cahill L. Glucocorticoid release and memory consolidation in men and women. Psychol Sci. 2006;17:466–470. doi: 10.1111/j.1467-9280.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bright P, Jaldow E, Kopelman MD. The national adult reading test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. J Int Neuropsychol Soc. 2002;8:847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- Britt GC, McCance Katz EF. A brief overview of the clinical pharmacology of ‘club drugs’. Subst Use Misuse. 2005;40:1189–1201. doi: 10.1081/JA-200066730. [DOI] [PubMed] [Google Scholar]
- Brunner R, Schaefer D, Hess K, Parzer P, Resch F, Schwab S. Effect of high-dose cortisol on memory functions. Ann N Y Acad Sci. 2006;1071:434–437. doi: 10.1196/annals.1364.037. [DOI] [PubMed] [Google Scholar]
- Chiarotto JA, Wainer IW. Determination of metyrapone and the enantiomers of its chiral metabolite metyrapol in human plasma and urine using coupled achiral-chiral liquid chromatography. J Chromatogr B Biomed Sci Appl. 1995;665:147–154. doi: 10.1016/0378-4347(94)00500-5. [DOI] [PubMed] [Google Scholar]
- Chiu SK, Collier CP. Salivary cortisol on ROCHE Elecsys immunoassay system: pilot biological variation studies. Clin Biochem. 2003;36:211–214. doi: 10.1016/s0009-9120(02)00471-x. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. Altered states: the clinical effects of Ecstasy. Pharmacol Ther. 2003;98:35–58. doi: 10.1016/s0163-7258(03)00003-2. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Deary IJ, Starr J, Whalley LJ. The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66-year interval. Psychol Med. 2001;31:451–458. doi: 10.1017/s0033291701003634. [DOI] [PubMed] [Google Scholar]
- Curran HV, Pooviboonsuk P, Dalton JA, Lader MH. Differentiating the effects of centrally acting drugs on arousal and memory: an event-related potential study of scopolamine, lorazapam and diphenhydramine. Psychopharmacology (Berl) 1998;135:27–36. doi: 10.1007/s002130050482. [DOI] [PubMed] [Google Scholar]
- Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab. 2009;94:1548–1554. doi: 10.1210/jc.2008-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont GJ, Verkes RJ. A review of acute effects of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Psychopharmacol. 2006;20:176–187. doi: 10.1177/0269881106063271. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets W, Schulkin J. Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neurosci Biobehav Rev. 2003;27:233–246. doi: 10.1016/s0149-7634(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Ferri M, Zambelli U, Timpano M, Neri E, et al. Long-lasting effects of (+/-)3,4-methylenedioxymethamphetamine (ecstasy) on serotonin system function in humans. Biol Psychiatry. 2000;47:127–136. doi: 10.1016/s0006-3223(99)00180-8. [DOI] [PubMed] [Google Scholar]
- Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology (Berl) 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- Harris DS, Reus VI, Wolkowitz OM, Mendelson JE, Jones RT. Altering cortisol level does not change the pleasurable effects of methamphetamine in humans. Neuropsychopharmacology. 2003;28:1677–1684. doi: 10.1038/sj.npp.1300223. [DOI] [PubMed] [Google Scholar]
- Kessels RP, Postma A, de Haan EH. Object Relocation: a program for setting up, running, and analyzing experiments on memory for object locations. Behav Res Methods Instrum Comput. 1999;31:423–428. doi: 10.3758/bf03200721. [DOI] [PubMed] [Google Scholar]
- Klaassen T, Riedel WJ, Deutz NE, Van Praag HM. Mood congruent memory bias induced by tryptophan depletion. Psychol Med. 2002;32:167–172. doi: 10.1017/s003329170100438x. [DOI] [PubMed] [Google Scholar]
- Kuypers KP, Ramaekers JG. Acute dose of MDMA (75 mg) impairs spatial memory for location but leaves contextual processing of visuospatial information unaffected. Psychopharmacology (Berl) 2007a;189:557–563. doi: 10.1007/s00213-006-0321-7. [DOI] [PubMed] [Google Scholar]
- Kuypers KPC, Ramaekers JG. Transient memory impairment after acute dose of 75 mg 3.4-Methylenedioxymethamphetamine. J Psychopharmacol. 2005;19:633–639. doi: 10.1177/0269881105056670. [DOI] [PubMed] [Google Scholar]
- Kuypers KPC, Ramaekers JG. Acute dose of MDMA (75 mg) impairs spatial memory for location but leaves contextual processing of visuospatial information unaffected. Psychopharmacology (Berl) 2007b;189:557–563. doi: 10.1007/s00213-006-0321-7. [DOI] [PubMed] [Google Scholar]
- Lamers CTJ. Influence of Marijuana and Ecstasy (MDMA) on Cognitive Function and Driving Performance. Maastricht: University of Maastricht; 2003. [Google Scholar]
- Ljubijankic N, Popovic-Javoric R, Sceta S, Sapcanin A, Tahirovic I, Sofic E. Daily fluctuation of cortisol in the saliva and serum of healthy persons. Bosn J Basic Med Sci. 2008;8:110–115. doi: 10.17305/bjbms.2008.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54(5 Suppl. 1):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Marin M-F, Hupbach A, Maheu FS, Nader K, Lupien SJ. Metyrapone administration reduces the strength of an emotional memory trace in a long-lasting manner. J Clin Endocrinol Metab. 2011;96:1–7. doi: 10.1210/jc.2011-0226. [DOI] [PubMed] [Google Scholar]
- Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, et al. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- Mulder-Hajonides van der Meulen WREH, Wijnberg JR, Hollanders JJ, DeDiana I, Hoofdakker R. Measurement of subjective sleep quality. Amsterdam: Fifth European Congress on Sleep Research; 1980. [Google Scholar]
- Nejtek VA. High and low emotion events influence emotional stress perceptions and are associated with salivary cortisol response changes in a consecutive stress paradigm. Psychoneuroendocrinology. 2002;27:337–352. doi: 10.1016/s0306-4530(01)00055-5. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Human psychopharmacology of Ecstasy (MDMA): a review of 15 years of empirical research. Hum Psychopharmacol. 2001;16:557–577. doi: 10.1002/hup.351. [DOI] [PubMed] [Google Scholar]
- Parrott AC. Cortisol and 3,4-methylenedioxymethamphetamine: neurohormonal aspects of bioenergetic stress in ecstasy users. Neuropsychobiology. 2009;60:148–158. doi: 10.1159/000253551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Lock J, Conner AC, Kissling C, Thome J. Dance clubbing on MDMA and during abstinence from ecstasy/MDMA: prospective neuroendocrine and psychobiological changes. Neuropsychobiology. 2008;57:165–180. doi: 10.1159/000147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC, Gibbs A, Scholey AB, King R, Owens K, Swann P, et al. MDMA and methamphetamine: some paradoxical negative and positive mood changes in an acute dose laboratory study. Psychopharmacology (Berl) 2011;215:527–536. doi: 10.1007/s00213-011-2184-9. [DOI] [PubMed] [Google Scholar]
- Pizarro N, Ortuño J, Farré M, Hernández-López C, Pujadas M, Llebaria A, et al. Determination of MDMAand its metabolitesin blood and urine by gas chromatography-mass spectrometry and analysis of enantiomersby capillary electrophoresis. J Anal Toxicol. 2002;26:157–165. doi: 10.1093/jat/26.3.157. [DOI] [PubMed] [Google Scholar]
- Price LH, Ricaurte GA, Krystal JH, Heninger GR. Neuroendocrine and mood responses to intravenous L-tryptophan in 3,4-methylenedioxymethamphetamine (MDMA) users. Preliminary observations. Arch Gen Psychiatry. 1989;46:20–22. doi: 10.1001/archpsyc.1989.01810010022003. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kuypers KPC, Wingen M, Heinecke A, Formisano E. Involvement of inferior parietal lobules in prospective memory impairment during acute MDMA (Ecstasy) intoxication: an event-related fMRI study. Neuropsychopharmacology. 2009;34:1641–1648. doi: 10.1038/npp.2008.219. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, et al. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Rey A. The Clinical Examination in Psychology/L'examen Clinique En Psychologie. Oxford: Presses Universitaires De France; 1958. [Google Scholar]
- Riedel WJ, Sobczak S, Nicolson N, Honig A. Stress, cortisol and memory as markers of serotonergic vulnerability. Acta Neuropsychiatrica. 2002;14:186–191. doi: 10.1034/j.1601-5215.2002.140405.x. [DOI] [PubMed] [Google Scholar]
- Sawin CT, Spark RF, Mitchell ML. Lack of response of serum growth hormone to metyrapone. J Clin Endocrinol Metab. 1971;32:854–856. doi: 10.1210/jcem-32-6-854. [DOI] [PubMed] [Google Scholar]
- Schmand B, Bakker D, Saan R, Louman J. De Nederlandse Leestest voor Volwassenen: een maat voor het premorbide intelligentieniveau. Tijdschr Gerontol Geriatr. 1991;22:15–19. [PubMed] [Google Scholar]
- Scholey AB, Owen L, Gates J, Rodgers J, Buchanan T, Ling J, et al. Hair MDMA samples are consistent with reported ecstasy use: findings from a study investigating the effects of ecstasy on mood and memory. Neuropsychobiology. 2011;63:15–21. doi: 10.1159/000321833. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Suda M, Morimoto K, Obata A, Koizumi H, Maki A. Emotional responses to music: towards scientific perspectives on music therapy. Neuroreport. 2008;19:75–78. doi: 10.1097/WNR.0b013e3282f3476f. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Kessels RPC, Putman P, Jager G, Koppeschaar HPF, Postma A. Attentionally modulated effects of cortisol and mood on memory for emotional faces in healthy young males. Psychoneuroendocrinology. 2003;28:941–948. doi: 10.1016/s0306-4530(02)00116-6. [DOI] [PubMed] [Google Scholar]
- Van Strien JW, Verkoeijen PP, Van der Meer N, Franken IH. Electrophysiological correlates of word repetition spacing: ERP and induced band power old/new effects with massed and spaced repetitions. Int J Psychophysiol. 2007;66:205–214. doi: 10.1016/j.ijpsycho.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Verkes RJ, Gijsman HJ, Pieters MS, Schoemaker RC, de Visser S, Kuijpers M, et al. Cognitive performance and serotonergic function in users of ecstasy. Psychopharmacology (Berl) 2001;153:196–202. doi: 10.1007/s002130000563. [DOI] [PubMed] [Google Scholar]
- van Wel JHP, Kuypers KPC, Theunissen EL, Bosker WM, Bakker K, Ramaekers JG. Blockade of 5-HT2 receptor selectively prevents MDMA induced verbal memory impairment. Neuropsychopharmacology. 2011;36:1932–1939. doi: 10.1038/npp.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wel JHP, Kuypers KPC, Theunissen EL, Bosker WM, Bakker K, Ramaekers JG. Effects of acute MDMA intoxication on mood and impulsivity: role of the 5-HT2 and 5-HT1 receptors. PLoS ONE. 2012;7:e40187. doi: 10.1371/journal.pone.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Obradovic T, Imel KM, Wheaton MJ. The effects of methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) on monoaminergic neurotransmission in the central nervous system. Prog Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- Wingen M, Kuypers KPC, Ramaekers JG. Selective verbal and spatial memory impairment after 5-HT1a and 5-HT2a receptor blockade in healthy volunteers pre-treated with an SSRI. J Psychopharmacol. 2007;21:477–485. doi: 10.1177/0269881106072506. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Somoza E, Harrer JM, Moore E, Ussery T, Kropp F, et al. Metyrapone and cocaine: a double-blind, placebo-controlled drug interaction study. Pharmacol Biochem Behav. 2005;80:631–638. doi: 10.1016/j.pbb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Young EA, Ribeiro SC. Sex differences in the ACTH response to 24H metyrapone in depression. Brain Res. 2006;1126:148–155. doi: 10.1016/j.brainres.2006.05.053. [DOI] [PubMed] [Google Scholar]


