Abstract
The tumor microenvironment can polarize innate immune cells to a proangiogenic phenotype. Decidual natural killer (dNK) cells show an angiogenic phenotype, yet the role for NK innate lymphoid cells in tumor angiogenesis remains to be defined. We investigated NK cells from patients with surgically resected non-small cell lung cancer (NSCLC) and controls using flow cytometric and functional analyses. The CD56+CD16- NK subset in NSCLC patients, which represents the predominant NK subset in tumors and a minor subset in adjacent lung and peripheral blood, was associated with vascular endothelial growth factor (VEGF), placental growth factor (PIGF), and interleukin-8 (IL-8)/CXCL8 production. Peripheral blood CD56+CD16- NK cells from patients with the squamous cell carcinoma (SCC) subtype showed higher VEGF and PlGF production compared to those from patients with adenocarcinoma (AdC) and controls. Higher IL-8 production was found for both SCC and AdC compared to controls. Supernatants derived from NSCLC CD56+CD16- NK cells induced endothelial cell chemotaxis and formation of capillary-like structures in vitro, particularly evident in SCC patients and absent from controls. Finally, exposure to transforming growth factor-β1 (TGFβ1), a cytokine associated with dNK polarization, upregulated VEGF and PlGF in peripheral blood CD56+CD16- NK cells from healthy subjects. Our data suggest that NK cells in NSCLC act as proangiogenic cells, particularly evident for SCC and in part mediated by TGFβ1.
Introduction
Lung cancer is the predominant cause of cancer-related mortality in the developed world and is the leading cause of death from cancer in man. Approximately 80% of all lung cancers are non-small cell lung cancer (NSCLC) that can be divided phenotypically into two principal subtypes, squamous cell carcinoma (SCC) and adenocarcinoma (AdC). Although often treated similarly in therapy, they differ not only pathologically and functionally but also in their response to targeted therapeutic agents.
Experimental and expanding clinical evidence indicates that while immune cells can contribute to tumor rejection [1], they often play a key role in initiating and promoting cancer [2,3]. These opposing functions are in large part due to polarization of immune cells within tumors as well as to immune cell editing, modulation of tumor micro-environment, and immunosuppression [1–3]. This concept of polarization resulting in attenuation of antitumor activity and enhancement of processes favoring tumor growth, including angiogenesis, is well established for macrophages [4,5]. Similar phenomena have been observed for most immune cells depending on the model system, including neutrophils and T and B cells [2,3]. However, relatively little is known concerning the effects of tumor and tumor microenvironment-derived factors on natural killer (NK) cell polarization.
NK cells are the major subset of innate lymphoid cells endowed with complex regulatory roles [6] originally identified as innate immune cells able to recognize and kill cells lacking—or with very low expression of—class I major histocompatibility complex molecules [7]. Additional analyses have identified multiple subsets of NK cells and the complexity of NK cells is increasingly appreciated [7,8]. There are two main NK cell subsets. The major subset, approximately 95% of circulating NK cells in peripheral blood, is characterized as being CD56dimCD16+, have strong production of granzyme and perforin, and are generally associated with cytotoxicity. The remaining approximately 5% of circulating NK cells are CD56brightCD16- and show lower cytotoxicity and higher levels of cytokine production [7,8]. Remarkably, within the developing decidua, there is another very different NK cell subset [decidual NK (dNK) cells] that displays a cytokine-secreting, highly angiogenic phenotype [9–11] in humans and mice. Human dNK cells are CD56superbrightCD16-, produce vascular endothelial growth factor (VEGF), placental growth factor (PIGF), and interleukin-8 (IL-8), and can significantly enhance growth of transplanted tumors by their angiogenic activity [9].
NK cells play an important role in tumor immune control and in the modulation of adaptive antitumor T cell immunity, as well as in the cooperation with dendritic cells, for example, by secretion of interferon-γ (IFN-γ) and tumor necrosis factor α (TNFα) [12,13]. The majority of studies on human NK cell biology have been performed on peripheral blood NK cells rather than on those infiltrating tissues. Lung tissues are particularly rich in NK cells [14,15] possibly because of spontaneous IL-15 production by bronchial epithelial cells [16]. Human NSCLCs are infiltrated by the predominant subset of CD56brightCD16- NK cells [17,18] that appear to be selectively recruited into tumors, showing substantial cytokine production, expressing killer inhibitory receptors [17,18] yet have a reduced killing capacity [17].
Given the strong proangiogenic activity of dNK cells and the consistent activation of angiogenic programs in immune cells by tumors, here we show angiogenic cytokine production and angiogenesis-associated activities by NK cells from NSCLC cancer, particularly evident in NK cells from patients with SCC.
Transforming growth factor-β (TGFβ), an immunosuppressive cytokine [19] with a pleiotropic role in tumor biology [20], is a cytokine frequently overexpressed in many cancers [20] including NSCLC [21]. TGFβ also has a role in the tumor microenvironment immune cell polarization [19], including macrophages, neutrophils, and NK cells, associated with tumor immune evasion [19]. High expression of TGFβ is characteristic of NSCLC and predictive of poor survival for patients with the SCC subtype [22]. The development and differentiation of human NK cell subsets in vitro is known to be effected by TGFβ and has been previously suggested to induce a polarization of peripheral blood NK cells toward a dNK-like CD56superbrightCD16- phenotype [23,24]. In vitro exposure of peripheral blood NK cells from healthy donors to TGFβ1 upregulated production of angiogenic cytokines, suggesting a role for this cytokine in inducing a proangiogenic NK phenotype.
Patients, Materials, and Methods
Patient Selection and Samples
Samples (tumor tissue and macroscopically normal adjacent tissues) from 31 patients with NSCLC were obtained during surgical resections after obtaining informed consent in an institutional ethics committee-approved study. The patient population characteristics are shown in Table W1. Tissue samples were placed in phosphate-buffered saline (PBS; LONZA, Basel, Switzerland) with 1% Pen/Strep (Sigma- Aldrich, St Louis, MO) at 4°C for no more than 18 hours before processing. Peripheral blood samples were drawn from the same patients before surgical intervention into blood collection heparinized tubes, stored at 4°C, and processed within 18 hours. Patients with diabetes, human immunodeficiency virus (HIV)/hepatitis C virus (HCV)/hepatitis B virus (HBV) infection, overt chronic inflammatory conditions, previously treated with chemotherapy or radiotherapy, or those iatrogenically immunosuppressed or having undergone myeloablative therapies were excluded. As controls, adjacent normal lung samples were obtained from patients who underwent minimal lung resection for bullectomy to treat pneumothorax following informed consent and processed as above (Table W1). Peripheral blood samples were obtained from healthy donors.
Patient Characteristics
NK cells were isolated from blood, lung tumor, and adjacent healthy tissues from 31 NSCLC patients having undergone tumor resection (median age, 71; range, 44–79), as well as blood and macroscopically normal lung tissue from 10 patients having undergone minimal lung resection for bullectomy (median age, 27; range, 16–69), whose characteristics are shown in Tables 1 and W1. Consistent with the population at risk, the majority of the cancer patients were males (90%) and either former or current smokers (90%). The most frequent subtype was AdC (17; 55%), followed by SCC (9; 29%) and tumors of other subtypes. Lung tissue controls were predominantly male (90%) and current or former smokers (70%; Table W1).
Table 1.
Characteristics of All Patients with Resected NSCLCs Analyzed.
| Patient No. | Gender | Age (Years) | Histology | T | N | M | Stage* | Smoking Status | Tumor Infiltrating NK | Lung Tissue Infiltrating NK | Peripheral Blood NK | |||
| Percentage of Total CD45+,† | Percentage of CD16- NK‡ | Percentage of Total CD45+,† | Percentage of CD16- NK‡ | Percentage of Total CD45+,† | Percentage of CD16- NK‡ | |||||||||
| 1 | M | 77 | AdC | 4 | 0 | 0 | IIIA | Former | 1.4 | 77.7 | 0.7 | 1 | 9.9 | 2.8 |
| 2 | M | 70 | AdC | 1a | 0 | 0 | IA | Former | 0.4 | 83 | 0.1 | 40.5 | 15 | 2.3 |
| 3 | M | 77 | AdC | 2a | 0 | 0 | IB | Former | 1.6 | 69.3 | 0.8 | 13.2 | 35.9 | 3.9 |
| 4 | M | 54 | AdC | 2a | 0 | 0 | IB | Former | 1.2 | 74.8 | 0.7 | 16.1 | 20.9 | 2 |
| 5 | M | 60 | NLC | 1b | 2 | 0 | IIIA | Current | 0.2 | 59.1 | 2 | 18.4 | 12.9 | 10.3 |
| 6 | M | 75 | AdC | 3 | 1 | 0 | IIIA | Former | 23.3 | 83.3 | 0.2 | 32.1 | 21.3 | 3.2 |
| 7 | M | 67 | AdC | 2b | 0 | 0 | IIA | Former | 1.7 | 64.9 | 7.5 | 13.9 | 0.5 | 28.4 |
| 8 | M | 71 | St | 3 | 1 | 0 | IIIA | Former | 7.5 | 72.4 | 0.7 | 21 | 36.8 | 1.1 |
| 9 | M | 59 | SCC | 1a | 0 | 0 | IA | Current | 0.2 | 52.1 | 0.9 | 19.8 | 3.3 | 10.2 |
| 10 | M | 73 | AdC | 2a | 0 | 0 | IB | Current | 1.9 | 87.3 | 0.3 | 21.4 | 0.6 | 13.5 |
| 11 | M | 56 | AdC | 2a | 0 | 0 | IB | Current | 0.3 | 55.9 | 0.2 | 38.5 | 30.7 | 1.6 |
| 12 | F | 72 | LCC | 3 | 0 | 0 | IIB | Current | 0.8 | 75 | 6.9 | 27.4 | 0.2 | 43.3 |
| 13 | M | 78 | SCC | 1a | 0 | 0 | IA | Former | 0.6 | 64 | 8.8 | 18 | 1.4 | 22.6 |
| 14 | M | 70 | AdC | 1a | 0 | 0 | IA | Current | 5.5 | 55.5 | 0.8 | 19.7 | 0.6 | 9.7 |
| 15 | M | 67 | AdC | 2a | 0 | 0 | IB | Current | 2.4 | 56.9 | 0.2 | 47.3 | 2 | 33.3 |
| 16 | M | 73 | AdC | 2a | 0 | 0 | IB | Former | 0.2 | 88.5 | 1.1 | 14.3 | 5.2 | 33.3 |
| 17 | M | 56 | LCC | 2a | 1 | 0 | IIA | Current | 0.1 | 87.5 | 1.7 | 14.4 | 0.1 | 22.7 |
| 18 | M | 67 | SCC | 2b | 0 | 0 | IIA | Current | 0.9 | 98.3 | 3.1 | 21.1 | 7.9 | 7.6 |
| 19 | M | 79 | AdC | 2a | 0 | 0 | IB | Former | 0.6 | 89.9 | 0.7 | 0.3 | 0.1 | 43.3 |
| 20 | M | 52 | AdC | 1b | 1 | 0 | IIA | Never | 3.3 | 96.1 | 0.9 | 4.1 | 0.7 | 1.4 |
| 21 | M | 73 | SCC | 2a | 0 | 0 | IB | Former | 1.6 | 72.7 | 0.1 | 25 | 0.8 | 25 |
| 22 | M | 66 | SCC | 1b | 0 | 0 | IA | Former | 0.1 | 92.4 | 0.6 | 6.3 | 2 | 17.4 |
| 23 | M | 73 | SCC | 1b | 2 | 0 | IIIA | Former | 2.9 | 73 | 2 | 8.3 | 0.3 | 23.7 |
| 24 | M | 66 | SCC | 1b | 0 | 0 | IA | Former | 1.3 | 89.9 | 0.8 | 20.8 | 0.8 | 19 |
| 25 | M | 72 | SCC | 2 | 2 | 0 | IIIA | Current | ND | ND | ND | ND | 12.3 | ND |
| 26 | M | 74 | SCC | 3 | 0 | 0 | IIB | Former | ND | ND | ND | ND | 0.1 | ND |
| 27 | M | 71 | AdC | 2 | 1 | 0 | IIB | Current | ND | ND | ND | ND | 0.1 | ND |
| 28 | M | 63 | AdC | 1b | 1 | 0 | IA | Current | ND | ND | 0.4 | ND | 1.4 | ND |
| 29 | F | 74 | AdC | 1b | 0 | 0 | IA | Never | 1 | ND | 0.3 | ND | 0.1 | ND |
| 30 | M | 73 | AdC | 2a | 0 | 0 | IB | Former | 0.2 | ND | 2.3 | ND | 0.3 | ND |
| 31 | F | 72 | SCC | 1 | 0 | 0 | IA | Never | 0.4 | ND | 0.3 | ND | 13 | ND |
AdC indicates lung adenocarcinoma; SCC, squamous cell lung carcinoma; NLC, neuroendocrine large cell lung carcinoma; LCC, large cell lung carcinoma; St, sarcomatoid lung cancer; ND, not determined.
Isolation of Peripheral Blood Mononuclear Cells
To obtain peripheral blood mononuclear cells, a density gradient was performed on heparinized peripheral blood by diluting the sample 1:1 with RPMI 1640 (LONZA). This suspension was then carefully stratified on Lymphocyte Separation Medium (LONZA) and centrifuged at 500g for 30 minutes at room temperature with no brake. The lymphocyte-containing ring at the interface was collected in a new tube and washed twice in PBS by centrifugation.
Solid Tissue Enzymatic Digestion
The solid tissues obtained (tumor, adjacent normal, and non-oncologic lung tissues) were extensively washed in PBS to remove cell debris and eventual red blood cell aggregates and mechanically minced by scissors to obtain small fragments that were enzymatically digested with a cocktail containing DNAse (100 µg/ml; Roche, Mannhein, Germany) and Collagenase (1 mg/ml; Sigma-Aldrich) in RPMI 1640 supplemented with Pen/Strep for 1 hour at 37°C. The suspension was then filtered on cell strainers [Becton Dickinson (BD), San Jose, CA], while the remaining tissue fragments were processed in a tissue dissociator (gentleMACS; Miltenyi Biotec, Auburn, CA) and subsequently filtered as above. The total single cell suspension was washed by centrifugation in PBS to remove residual enzymes.
Phenotypic Characterization of Tumor Infiltrating NK Cells
Cells from blood, tumor, and normal adjacent tissue (3 x 105 cells per sample) were stained with the following monoclonal antibodies (mAbs) in a direct immunofluorescence assay and assessed by flow cytometry (FACS Canto I; BD): Leucogate [BD; fluorescein isothiocyanate (FITC)-conjugated anti-human CD45 and phycoerythrin-conjugated (PE) anti-human CD14] was used to gate on lymphocytes. FITC-conjugated anti-human CD16 (BD, clone 3G8), peridinin-chlorophyll-protein complex (PerCP)-conjugated anti-human CD3 (Miltenyi Biotec, clone BW264/56), and allophycocyanin (APC)-conjugated anti-human CD56 (Miltenyi Biotec, clone AF12-7H3) were used to detect NK cells. Negative controls included directly labeled FITC-conjugated, PerCP-conjugated, and APC-conjugated isotype-matched irrelevant mAbs (BD). Briefly, after physical parameter setting (Forward and Side Scatter), lymphocyte populations were identified by gating on CD45-positive cells, and then the NK cell subpopulations were distinguished by gating on CD3-negative cells/CD56-positive cells using the isotypic controls. The CD3-CD56+ NK population was evaluated for CD16 expression.
Cytokine and Angiogenic Growth Factor Expression by NK Cells
The total cell suspensions were incubated overnight in RPMI 1640 supplemented with heat-inactivated FBS (Euroclone, Milan, Italy), Pen/Strep, and IL-2 (100 U/ml; R&D Systems, Minneapolis, MN) at 37°C and 5% CO2. Cells (3 x 105/ml) were then stimulated for 6 hours with 10 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich, St Louis, MO), 500 ng/ml ionomycin (Sigma-Aldrich), and protein transport inhibitor Brefeldin-A (Golgi Plug, BD) and then treated with Cytofix/Cytoperm fixation and permeabilization kit (BD). The expression of specific cytokines and angiogenic growth factors by NK subpopulations were evaluated by flow cytometric analyses after staining with anti-human Leucogate and phenotype detection markers (CD16-FITC/CD3-PerCP/CD56-APC) combined with PE-conjugated anti-human VEGF (R&D Systems; clone 23410), PE-conjugated anti-human IL-8 (R&D Systems; clone 6217), and PE-conjugated anti-human IFN-γ (BD; clone 25723.11). Negative controls included directly labeled isotype-matched irrelevant mAbs (BD). For PlGF staining, an unconjugated mAb was used (R&D Systems; clone 37203.111) coupled with a secondary PE-conjugated mAb goat anti-mouse IgG1 (BioLegend, San Diego, CA); the secondary antibody alone was used as a negative control.
Immunohistochemistry of Tumor Samples
A portion of each tumor sample was retained fixed in formalin and embedded in paraffin for routine histopathology. Sections were stained for CD31 (Dako, Glostrup, Denmark; clone JC/70A) as a marker for vessels, CD57 (clone HNK-1; R&D Systems) as a marker for activated NK cells, and CD56 (Dako; clone T199) and CD3 (Ventana, Tucson, AZ; clone 2GV6) as markers for NK cells. Serial sections showing cells staining with CD56 but not CD3 and with appropriate morphology were considered to be NK cells.
NK Cell Enrichment
Total NK cells derived from blood or tissue samples were enriched with an immunomagnetic negative NK cell selection kit (MagCellect Human Kit; R&D Systems) from the cell suspensions obtained as above from blood, tumor, and adjacent lung tissue. Briefly, cells were resuspended into MagCellect buffer, and negative selection was performed by incubating for 15 minutes with MagCellect NK cell biotinylated antibody cocktail and for 15 minutes with MagCellect streptavidin ferrofluid reagent and then placed in a magnetic field that retains the unwanted fraction. NK enrichment greater than 85% was confirmed using flow cytometry assays.
Chemotaxis and Morphogenesis of Human Umbilical Vein Endothelial Cells
NK cells purified from blood and tissues were incubated overnight as previously described. Cells were incubated in culture medium and stimulated for 6 hours with PMA (10 ng/ml) and ionomycin (500 ng/ml) or left untreated. Supernatants were collected; residual cells and debris were discarded by centrifugation and concentrated with Concentricon devices (Millipore, Temecula, CA) with a 5-kDa membrane pore cutoff. A sequential step of PBS dilution and concentration was performed to remove residual PMA and ionomycin that we found to be toxic for endothelial cells (data not shown).
We evaluated the ability of NK-secreted factors to induce chemotaxis of endothelial cells in Boyden chamber assays [25,26] using 5 x 104 human umbilical vein endothelial cells (HUVECs; Promocell, Heidelberg, Germany), 12-µm pore size polycarbonate filters pre-coated with collagen (50 µg/ml), and NK supernatants derived from mitogen-stimulated or unstimulated purified NK cells. After 6 hours of incubation, the filters were recovered, and the cells migrated to the lower filter surface stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) and counted in a double-blind manner in eight consecutive fields per filter with a fluorescence microscope (Zeiss, Oberkochen, Germany).
The capacity to induce formation of capillary-like networks by endothelial cells seeded on matrigel (BD) was performed as previously described [27]. HUVECs (5 x 104 cells/well) were resuspended in 1 ml of tumor infiltrating stimulated or unstimulated NK cell supernatants obtained as above and transferred to the matrigel-coated wells for 6- or 24-hour incubation on matrigel, and the morphologic organization was documented with an inverted microscope (Zeiss).
TGFβ1 Polarization and Angiogenic Cytokine Production by NK Cells
We evaluated cytokine production (VEGF, PlGF, IL-8, and IFN-γ) after TGFβ1 stimulation on peripheral blood NK cells isolated from buffy coats of healthy donors by negative selection NK cell enrichment as above. Total NK cells were cultured in RPMI 1640 (1 x 106/ml) supplemented with FBS, IL-2 (100 U/ml), Stem Cell Factor (20 ng/ml; Miltenyi Biotec), and TGFβ1 at 2, 5, or 10 ng/ml (Miltenyi Biotec), while the same medium without TGFβ1 was used as control. The growth factors were added every third day, and after 3 weeks, the cultures were processed for assessment of surface markers and intracellular cytokine production as described above.
Flow Cytometric and Statistical Analyses
Flow cytometric analyses were performed using BD FACSDiva (v6.1.2) and FlowJo (v7.2.5) software. Statistical analyses were performed using the GraphPad Prism statistics and graphing program (GraphPad Software, San Diego, CA). Two-tailed t tests were used for comparison of paired data sets and quartile localization for population distribution.
Results
NK Cell Subsets Associated with Angiogenic Cytokine Production
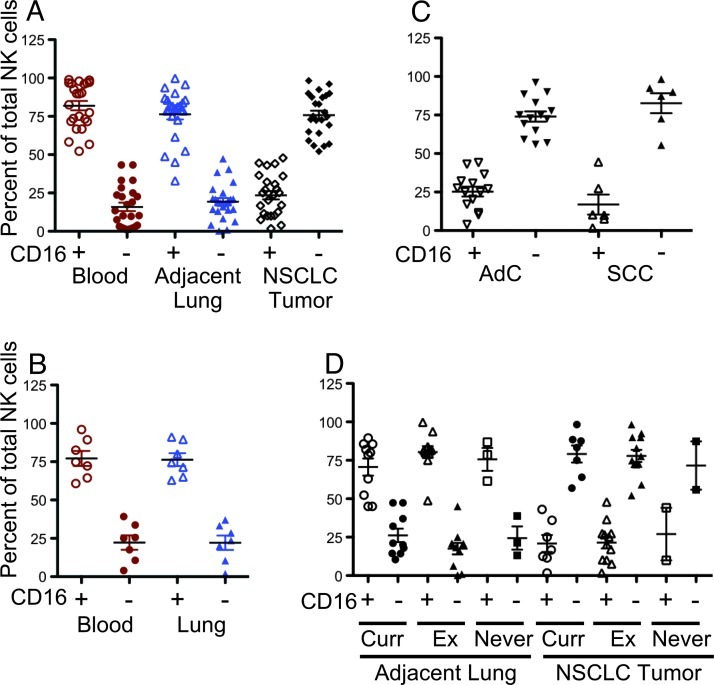
Previous studies have shown that tumor samples from patients with NSCLC are enriched in the CD56+CD16- NK subset, which have been extensively characterized for the expression of specific NK markers displaying a distinct surface molecular pattern [17,18]. Further, the CD56+CD16- NSCLC infiltrating cells have a limited capacity to degranulate and kill tumor cells through an IFN-γ and TNFα-mediated mechanism [17,18]. As previously observed [17], NK cells represent 2% to 3% of the whole CD45+ leukocytes population within the tumors, 1.6% on average in adjacent lung tissues, and 7.7% on average in the peripheral blood (Table 1). The CD56+CD16- NK cell subset is the major subset in lung tumor samples (Figure 1A), significantly higher (P < .001) than that in the adjacent lung tissue and peripheral blood samples and showing higher levels of CD56 expression (Figure W1). The normal lung tissues obtained from patients that did not have oncologic disease showed a CD56dimCD16+ profile comparable to that of the adjacent tissues resected along with the NSCLCs (Figure 1B). We did not observe any significant differences regarding the prevalence of the CD56+CD16- NK subset between NSCLC subtypes: the CD56+CD16- NK subset predominated both in AdC and SCC (Figure 1C), as well as in occasional mixed adenosquamous or large cell carcinomas (data not shown). Further, we did not observe any difference in distribution of NK cell phenotype on the basis of smoking status (Figure 1D). No correlations with tumor grade, stage, or tumor lymph node metastasis (TNM) statuses were found (data not shown). Smoking status also did not alter the distribution of the CD56+CD16- NK subset in control patients as well (data not shown), indicating that a chronic inflammatory status induced by smoking did not affect the NK cell phenotype distribution.
Figure 1.
Phenotypic distribution of the CD3-CD56+CD16+ and CD3-CD56+CD16- NK cell subsets as determined by flow cytometry. (A) NK cell distribution in samples derived from peripheral blood, normal adjacent lung tissues, and tumor tissues of patients with NSCLC. (B) A similar distribution of NK cell subsets is found in peripheral blood and lung tissues from non-oncologic patients. (C) The tumor infiltrating NK cells are primarily of the CD3-CD56+CD16- subset in both AdC and SCC. (D) Smoking status [current, former (Ex), and non-smokers] did not influence the NK cell subset distribution in tumor or adjacent lung tissues. Significant differences between the CD16+ and CD16- NK subsets for each group were all P < .0001 except for former smokers in adjacent tissue (P = .0085) and tumors of non-smokers (insufficient data).
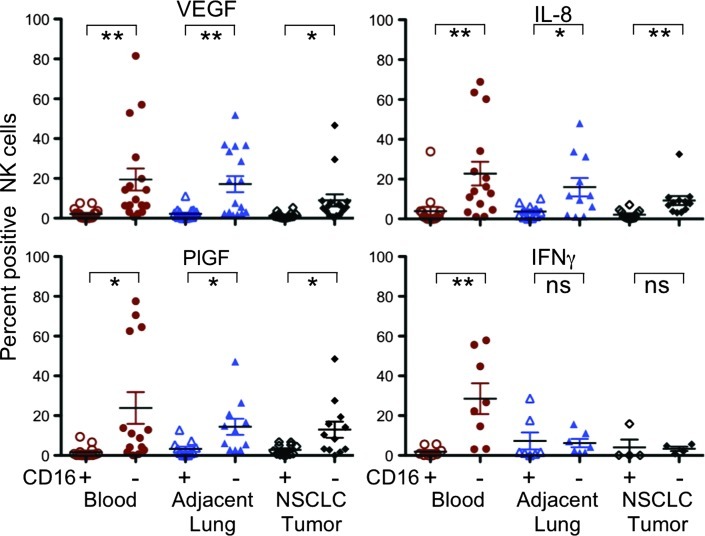
The CD56+CD16- subset has been described as a potent producer of several cytokines following appropriate stimulation [28]. Since CD56+CD16- dNK cells have been reported to produce angiogenic factors [9], we evaluated the capacity of the CD56+CD16- subset, predominantly infiltrating NSCLC, to produce several angiogenic factors, in particular VEGF, PlGF, and IL-8. Production of IFN-γ, a key immunomodulatory cytokine endowed with antiangiogenic potential [29], was also investigated. Following standard PMA and ionomycin stimulation, the CD56+CD16- NK cell subset was clearly associated with significantly higher production of the proangiogenic factors VEGF, PlGF, and IL-8 (Figure 2), in all the compartments examined. The significantly higher expression of angiogenic cytokines in the peripheral blood suggests that other innate lymphoid cell subsets, which can express CD56 but are essentially not found in peripheral blood, are probably not a confounding factor. Production of IFN-γ after stimulation was lower in adjacent lung tissue and tumor infiltrating NK cells and higher in the peripheral blood CD56+CD16- NK cells (Figure 2).
Figure 2.
Characterization of tumor infiltrating NK cells by intracellular staining for angiogenic and antiangiogenic cytokines. The CD3-CD56+CD16- NK cell subset is clearly associated with angiogenic cytokine production, including VEGF, PlGF, and IL-8 in the tumor and adjacent normal tissues as well as in peripheral blood, where this subset was also associated with enhanced production of IFN-γ. **P < .01, *P < .05; ns, not significant.
Differences in NK Cell Angiogenic Factor Production in NSCLC Subtypes
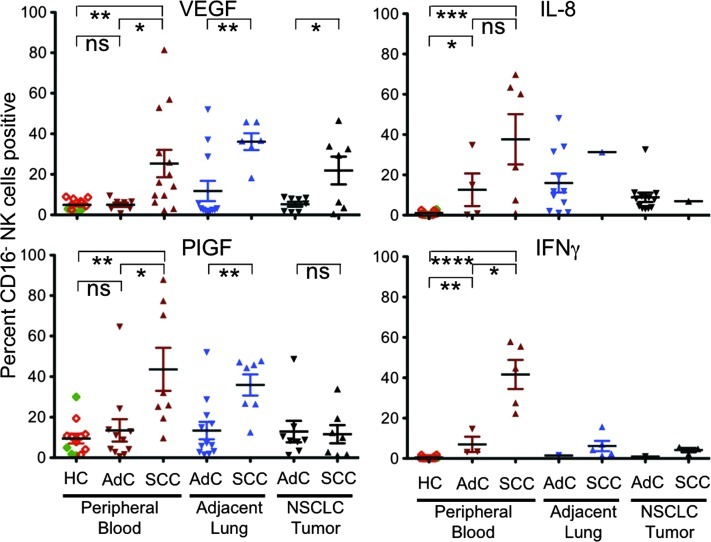
We then examined the distribution of cytokines and angiogenic factors production as a function of tumor subtype and patients' clinical parameters. We noted that the VEGF production by CD56+CD16- NK cells in patients with SCC was significantly higher than in those with AdC in tumor, adjacent lung tissues, and in particular in peripheral blood (Figure 3). CD56+CD16- NK cells from SCC patients also produced significantly higher levels of PlGF compared to AdC in the adjacent lung tissues and in peripheral blood compartments, although there was no difference between the AdC and SCC tumor infiltrating NK cells (Figure 3).
Figure 3.
Differences in angiogenic factor production based on NSCLC subtype. CD3-CD56+CD16- NK cells from patients with SCC produce significantly more VEGF than those from patients with AdC. This was evident both locally in the tumor and in the adjacent tissues. This effect appeared to be systemic, as CD3-CD56+CD16- NK cells from patients with SCC showed significantly higher production of VEGF when compared to patients with AdC or to healthy donor controls [HC, eight donors with a median age of 54; range, 44 to 68; 63% male and 25% smokers (open diamonds, ⋄) and four anonymous donors (filled diamonds, ♦)]. In addition, CD3-CD56+CD16- NK cells from peripheral blood and adjacent tissue from patients with SCC produced significantly more PlGF. In the peripheral blood, patients with both SCC and AdC showed significantly higher production of IL-8 and IFN-γ compared to healthy controls. While there was no difference in IL-8 production between NK cells from patients with AdC and SCC, those with SCC showed significantly higher production of IFN-γ compared to AdC. ****P < .0001, ***P < .001, **P < .01, *P < .05; ns, not significant.
Given the systemic effect on peripheral blood NK cells in patients with SCC, we then compared cytokine production with that of peripheral blood from 12 healthy donors. The expression of VEGF and PlGF in the CD56+CD16- NK cells from the peripheral blood of healthy controls was similar to that of patients with AdC and significantly less than the CD56+CD16- NK cells from patients with SCC (Figure 3). Interestingly, we noted that peripheral blood CD56+CD16- NK cells from both AdC and SCC produced high levels of IL-8 that was significantly different from that of healthy controls (Figure 3). Expression of IFN-γ was slightly (albeit significantly) higher in peripheral blood CD56+CD16- NK cells from AdC compared to healthy controls and even higher in SCC with significant differences between AdC and healthy controls (Figure 3). In some SCC patients, more than 50% of the CD56+CD16- NK cells expressed VEGF, and in the same samples, more than 50% also expressed IFN-γ. These data suggest that at least some NK cells from patients with NSCLC can express multiple cytokines with proangiogenic and antiangiogenic functions. Sample material was insufficient for comparisons within the tissue compartments for IL-8 and IFN-γ expression.
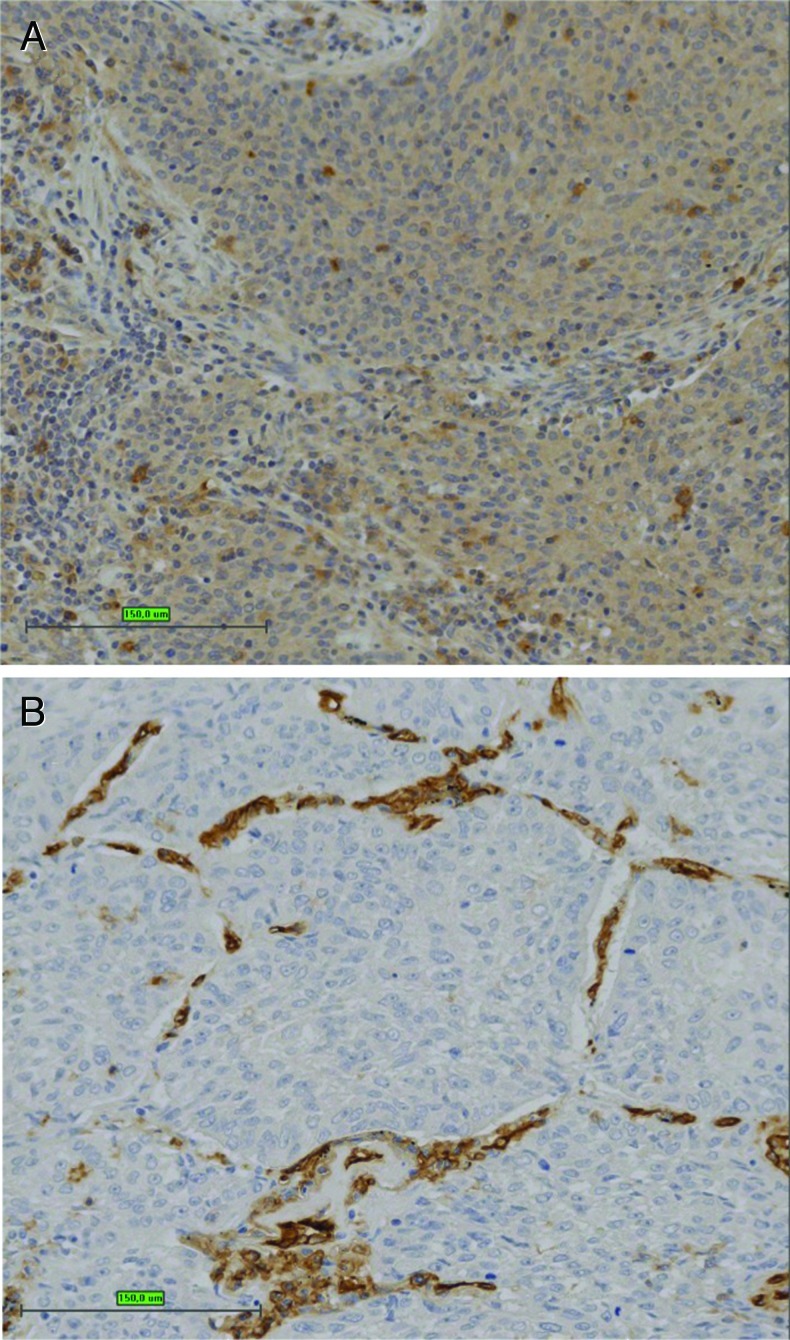
The correlation between angiogenic cytokine production and histologic and clinical parameters in SCC patients was also examined. In most of the NSCLC samples, expression of CD56+ was limited to few cells showing NK characteristics (Figure 4A), with occasional staining of the tumor epithelial compartment. Most of the CD56+ cells with an NK phenotype were CD3-, and the distribution of these cells correlated with flow cytometric data (Table 1 and Figure W1). CD31 staining showed that all the tumor samples had a highly vascularized microenvironment (Figure 4B) characteristic of most of the NSCLC samples. CD57 is not expressed on CD56bright NK cells and is a marker for a mature, activated phenotype [30]. Interestingly, the SCC patients showing high angiogenic cytokine production by NK cells were essentially negative for CD57 staining. A retrospective immunohistochemical study examining CD57+ NK cells found a positive correlation with survival in resected SCC NSCLC [31]. These data further highlight the role of NK polarization in SCC NSCLC.
Figure 4.
Immunohistochemistry of SCC. (A) CD56 staining shows occasional positive cells scattered throughout the tumor. (B) CD31 staining shows extensive tumor vascularization. Bar, 150 µm.
Tumor Infiltrating CD56+CD16- NK Cells Functionally Promote Angiogenesis-Associated Effects on Endothelial Cells
Given the range of factors produced by NSCLC NK cells, to further determine their angiogenic potential, we examined in vitro the effects of NK cell products on biologic correlates of angiogenesis, in particular endothelial cell recruitment and morphogenesis.
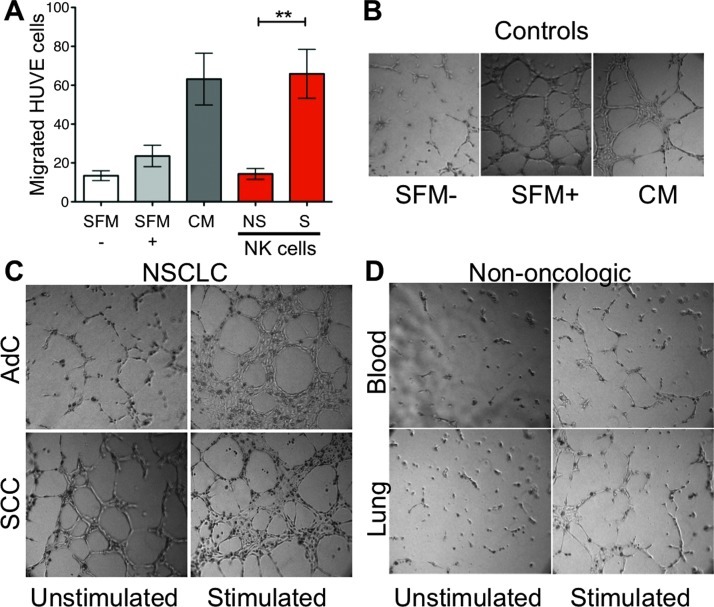
Supernatants from stimulated NSCLC tumor infiltrating NK cells were able to induce a significant level HUVEC chemotaxis (Figure 5A) similar to that of positive controls. Supernatants from unstimulated cultures showed little chemotactic activity, identical to serum-free controls. Supernatants from both AdC and SCC tumor-infiltrating NK cells showed a comparable level of chemotactic activity.
Figure 5.
Proangiogenic activity potential of NK cells. (A) Analysis of the capacity of supernatants from NSCLC-derived NK cells to induce endothelial cell chemotaxis. SFM-, serum-free medium as a negative control, containing RPMI 1640 alone. SFM+, serum-free RPMI 1640 with 1% l-glutamine, fibroblast growth factors (10 ng of acidic fibroblast growth factor plus 10 ng of basic fibroblast growth factor/ml), epidermal growth factor (10 ng/ml), heparin (0.1 mg/ml), and hydrocortisone (010 µg/ml). CM, complete medium containing SFM+ medium supplemented with 10% FBS as a positive control. Supernatants were isolated from AdC and SCC NK cells cultured for 6 hours either unstimulated (NS) or stimulated (S) with PMA and ionomycin. Supernatants from stimulated NK cells showed enhanced induction of chemotaxis compared to supernatants from unstimulated NK cells (NS). Similar data were obtained from AdC and SCC supernatants individually. Media containing only PMA and ionomycin had little chemotactic activity (data not shown). **P < .01. (B–D) NK cells from patients with NSCLC promote endothelial cell capillary-like morphogenesis, (B) positive (SFM+; CM) and negative (SFM-) controls as above. (C) Supernatants from NK cells isolated from AdCs that were stimulated for 6 hours with PMA and ionomycin (stimulated) showed induction of endothelial cell morphogenesis compared to supernatants from unstimulated NK cells. Supernatants from NK cells isolated from SCC showed induction of morphogenesis even when unstimulated, which was further enhanced upon stimulation, indicating that these cells harbor a strong angiogenic activity. (D) NK cells isolated from the peripheral blood (blood) or lung tissues (lung) of non-oncologic patients did not show significant enhancement of morphogenesis in the presence or absence of stimulation.
We also examined the ability of NK cell supernatants to promote capillary-like organization of HUVECs seeded onto matrigel three-dimensional support (Figure 5, B–D). Supernatants from AdC and SCC NSCLC infiltrating NK cells, stimulated by PMA and ionomycin, induced endothelial cell morphogenesis in vitro following stimulation (Figure 5C). Interestingly, in this assay, the unstimulated NK cells derived from SCC showed a baseline angiogenic activity that was enhanced following stimulation. Network formation in the presence of NK cell supernatants from lung tissues and peripheral blood of control patients without oncologic disease was very limited (Figure 5D). Taken together, these data suggest that NSCLC infiltrating NK cells display an enhanced angiogenic potential compared to non-tumor tissues infiltrating NK cells.
NK Cell Polarization and Cytokine Production after TGFβ1 Stimulation
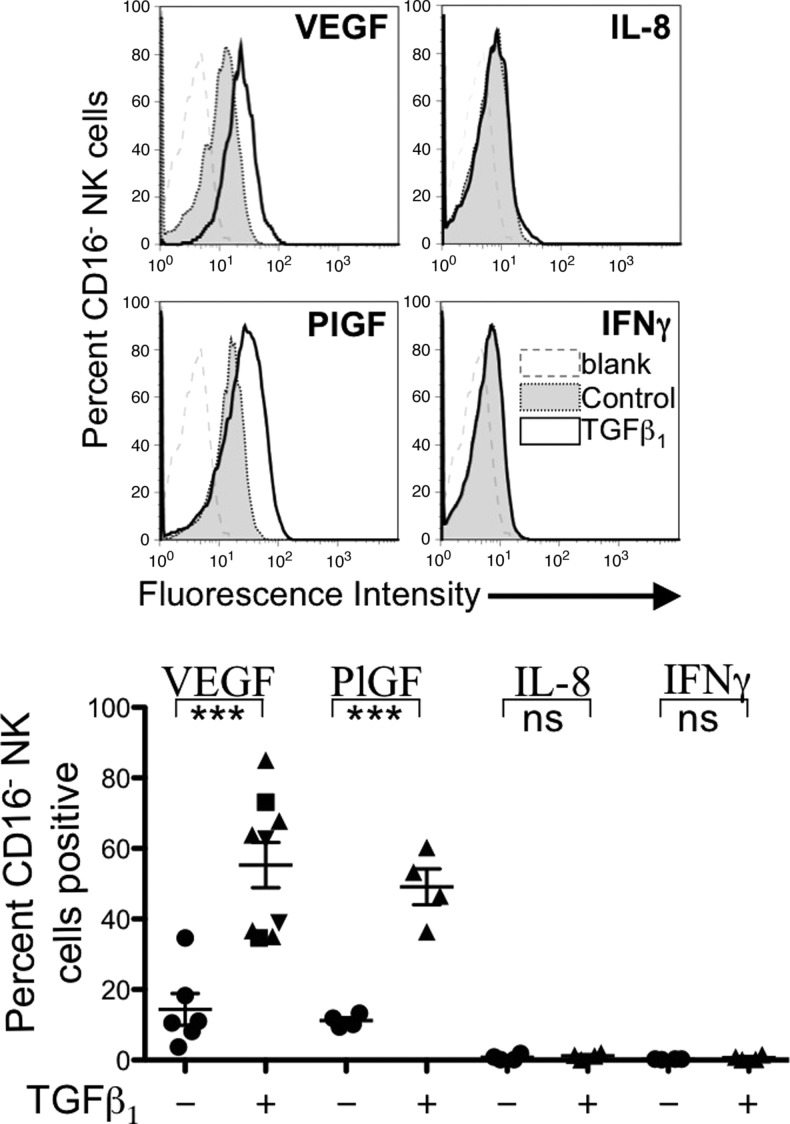
TGFβ1 has been shown, at least in vitro, to affect development and differentiation of human NK cell subsets. TGFβ1 has been reported to convert a fraction of peripheral blood CD56dimCD16+ and CD56brightCD16+ NK cells into CD56brightCD16- cells that express killer inhibitory receptors, CD9, and CD103 [23,24], all features of dNK cells [11]. To our knowledge, however, the capability of in vitro TGFβ-polarized peripheral blood NK cells to produce proangiogenic cytokines has not been evaluated. In keeping with previous observations [23,24], after 7 days of TGFβ1 exposure of healthy donor-derived NK cells, a significant increase of the CD56brightCD16- subset (approximately 70% of all NK cells) compared to untreated controls (approximately 30% of NK cells) was observed (data not shown). More importantly, exposure of NK cells to TGFβ1 significantly upregulated the expression of VEGF and PlGF within the CD56+CD16- subset (Figure 6). The percentages of cells expressing IL-8 or IFN-γ were quite low and not significantly affected by the TGFβ1 treatment.
Figure 6.
Angiogenic phenotype switch induced by TGFβ1 on healthy donor buffy coat-derived NK cells. After 3 weeks of culture in 10% FBS, IL-2 (100 U/ml), and SCF (20 ng/ml) either alone (control; circles, ●) or with TGFβ1 at 2 ng/ml (squares, ■), 5 ng/ml (triangles, ▴), or 10 ng/ml (inverted triangles, ▾), angiogenic cytokine expression was measured by flow cytometry. As previously reported [24], after exposure to TGFβ1, the CD56+CD16- subset was significantly enhanced with respect to the CD56+CD16+ subset compared to controls (data not shown). Exposure to TGFβ1 significantly induced the expression of the proangiogenic cytokines VEGF and PlGF. ***P < .001; ns, not significant.
Discussion
NK cells are lymphocytes of the innate immune system that can recognize tumor cells as targets and play a key role in antitumor immunity. Our data demonstrate that, like many other leukocytes, tumors can polarize these cells to a proangiogenic and protumorigenic environment, possibly linked to tumor progression. At the moment, there is very little literature on the capability of NK cells to induce tumor-sustaining angiogenesis. Given the fact that lung cancer is one of major causes of cancer-related deaths and that the lung parenchyma plays a role in tumor progression, we investigated NK cells in NSCLC.
Here, we show that NSCLC tumors induce both local and systemic polarization of NK cells toward phenotype associated with angiogenesis and that TGFβ may be one of the mechanisms involved in this process. The significantly higher expression of VEGF and PlGF by CD56+CD16- NK cells in SCC and IL-8 in both SCC and AdC correlated with the angiogenic activities found for these cells in functional assays. NK cells in the peripheral blood of NSCLC patients also expressed IFN-γ, an antiangiogenic cytokine expressed at high levels by human, porcine, and murine dNK cells. Studies in mice have indicated that IFN-γ secretion by dNK cells is involved in positively regulating decidual vascular lumen size and the spiral artery alterations (see [32] for review). These data suggest that tumor-polarized NK cells producing both proangiogenic cytokines and IFN-γ may induce structural alterations in vessels that might have clinical relevance [33]. The angiogenic activity of NSCLC “educated” NK cells that also produce IFN-γ is similar to that of dNK cells and clearly different from the CD56+CD16- NK cells found in healthy individuals. Further studies are needed to fully address whether NK cells from NSCLC patients have other functions and markers of “bona fide” dNK cells or if they represent a novel NK cell subset.
The presence of an NSCLC, even of modest dimensions, appears to have a potent systemic effect on the phenotype of the CD56+CD16- NK cells that is more pronounced in SCC. This is in keeping with transcriptome profiling studies indicating that NSCLC has a distinct effect on peripheral blood mononuclear cells [34,35], in particular for SCC. Interestingly, many of the genes making up a prognostic signature were NK associated [34,35].
VEGF staining has been reported to be an independent prognostic indicator of poor survival for resectable lung cancer patients [36,37], particularly for the SCC histotype [38], although this is controversial [39]. TGFβ has been associated with NK cell dysfunction in melanoma [40], esophageal SCC [41], and ovarian carcinoma [42]. Changes in NK receptor profiles in patients with NSCLC [17,18] have been linked in part to TGFβ [18]. Further, high TGFβ expression by tumor cells and infiltrating lymphocytes is characteristic of NSCLC and was found to have a prognostic value for SCC, but not for AdC, for both compartments [22]. TGFβ-positive tumor-infiltrating lymphocytes represented the only independent immunologic parameter with prognostic significance after multivariate analysis [22]. TGFβ1 significantly enhanced expression of VEGF and PlGF from healthy donors, yet did not enhance expression of IL-8 or IFN-γ, suggesting that other factors produced by NSCLC tumors or the tumor microenvironment also influence the NK cell angiogenic phenotype. A potential factor may be HLA-G, an immunoregulatory class I major histocompatibility complex molecule expressed in the decidua and in many tumors capable of modulating NK cell function [43,44]. Interestingly, both tumor and circulating soluble HLA-G have been found to be particularly high in SCC patients and predictive of poor survival of this subtype [45,46].
Previous studies have shown that surgically resected samples of SCC have a significantly shorter (>25% less) tumor doubling times compared to those of surgically resected AdC origin [47], a prognostic factor [47] that is even more evident using computed tomography scans that identifies earlier stage tumors [48,49]. However, to date, on the basis of the TNM system, globally there is no clear-cut difference in the aggressiveness of these two tumors when size, lymph node infiltration, and metastases are considered [50]. A lower overall survival of SCC compared to AdC NSCLC patients has been reported [51] possibly attributable to more advanced and invasive cancer status on resection as well as smoking or age-related comorbidities. The systemic effect on NK cells shown here and peripheral blood lymphocytes in general [34,35] may promote comorbidities.
While additional therapeutic options are available for patients with non-squamous cell subtypes, such as bevacizumab and pemetrexed, fewer options are available for SCC patients [33], yet are urgently needed. In addition to suggesting that aberrant activation of NK cells in NSCLC may be a therapeutic target, the angiogenic profile of NK cells might provide a marker for potential responses to, or complications of, targeted therapies that are currently lacking for SCC of the lung.
Supplementary Material
Acknowledgments
We thank Paolo Carrega (IRCCS San Martino IST, Genova, Italy) and Lorenzo Dominioni (Department of Surgical and Morphological Sciences, University of Insubria, Varese, Italy) for helpful discussion. We are grateful to Marta Pinter (University of Insubria) for establishing initial conditions, Paola Corradino for bibliographic searches, and Alessandra Panvini Rosati for assistance.
Abbreviations
- NSCLC
non-small cell lung cancer
- SCC
squamous cell carcinoma
- AdC
adenocarcinoma
- NK
natural killer
Footnotes
This work was supported by grants from the Associazione Italiana per la Ricerca sul Cancro (No. IG5968 to D.M.N., IG10228 to A.A., and IG11650 to G.F.) and the Ministero Italiano della Salute, Programma Strategico Ricerca Oncologica to G.F. and A.A., funds of the University of Insubria (Contributo Straordinario del Rettore and Fondo Ateneo della Ricerca (FAR) to D.M.N. and L.M.), and MultiMedica Onlus (to A.A.). C.F. was a Ministero dell'Istruzione, dell'Universita e della Ricerca (MIUR) “Grande Progetto Strategico” fellow; A.B. was supported by the University of Insubria Cellular and Molecular Biology Doctoral program. A.B., A.P., and A.R.C. were participants in the Cellular and Molecular Biology Doctoral program of the University of Insubria. A.B. and A.P. are recipients of Fondazione Italiana per la Ricerca sul Cancro (FIRC) fellowships. The authors declare no conflict of interest.
This article refers to supplementary materials, which are designated by Table W1 and Figure W1 and are available online at www.neoplasia.com.
References
- 1.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 2.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 3.Noonan DM, De Lerma Barbaro A, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 7.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moretta A, Locatelli F, Moretta L. Human NK cells: from HLA class I-specific killer Ig-like receptors to the therapy of acute leukemias. Immunol Rev. 2008;224:58–69. doi: 10.1111/j.1600-065X.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 9.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 10.Santoni A, Carlino C, Stabile H, Gismondi A. Mechanisms underlying recruitment and accumulation of decidual NK cells in uterus during pregnancy. Am J Reprod Immunol. 2008;59:417–424. doi: 10.1111/j.1600-0897.2008.00598.x. [DOI] [PubMed] [Google Scholar]
- 11.Vacca P, Moretta L, Moretta A, Mingari MC. Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol. 2011;32:517–523. doi: 10.1016/j.it.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Ferlazzo G, Munz C. Dendritic cell interactions with NK cells from different tissues. J Clin Immunol. 2009;29:265–273. doi: 10.1007/s10875-009-9283-y. [DOI] [PubMed] [Google Scholar]
- 13.Morandi B, Mortara L, Chiossone L, Accolla RS, Mingari MC, Moretta L, Moretta A, Ferlazzo G. Dendritic cell editing by activated natural killer cells results in a more protective cancer-specific immune response. PLoS One. 2012;7:e39170. doi: 10.1371/journal.pone.0039170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128:151–163. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–182. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge N, Nishioka Y, Nakamura Y, Okano Y, Yoneda K, Ogawa H, Sugita A, Yanagawa H, Sone S. Synthesis and secretion of interleukin-15 by freshly isolated human bronchial epithelial cells. Int Arch Allergy Immunol. 2004;135:235–242. doi: 10.1159/000081309. [DOI] [PubMed] [Google Scholar]
- 17.Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, Ratto GB, Mingari MC, Moretta L, Ferlazzo G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–875. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 18.Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, Alifano M, Regnard JF, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 19.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFβ. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikushima H, Miyazono K. TGFβ signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 21.Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-β1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004;172:7335–7340. doi: 10.4049/jimmunol.172.12.7335. [DOI] [PubMed] [Google Scholar]
- 22.Sterlacci W, Wolf D, Savic S, Hilbe W, Schmid T, Jamnig H, Fiegl M, Tzankov A. High transforming growth factor β expression represents an important prognostic parameter for surgically resected non-small cell lung cancer. Hum Pathol. 2011;43:339–349. doi: 10.1016/j.humpath.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Allan DS, Rybalov B, Awong G, Zuniga-Pflucker JC, Kopcow HD, Carlyle JR, Strominger JL. TGF-β affects development and differentiation of human natural killer cell subsets. Eur J Immunol. 2010;40:2289–2295. doi: 10.1002/eji.200939910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL. TGFβ promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA. 2007;104:3378–3383. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albini A, Noonan DM. The ‘chemoinvasion’ assay, 25 years and still going strong: the use of reconstituted basement membranes to study cell invasion and angiogenesis. Curr Opin Cell Biol. 2010;22:677–689. doi: 10.1016/j.ceb.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Albini A, Benelli R. The chemoinvasion assay: a method to assess tumor and endothelial cell invasion and its modulation. Nat Protoc. 2007;2:504–511. doi: 10.1038/nprot.2006.466. [DOI] [PubMed] [Google Scholar]
- 27.Cantelmo AR, Cammarota R, Noonan DM, Focaccetti C, Comoglio PM, Prat M, Albini A. Cell delivery of Met docking site peptides inhibit angiogenesis and vascular tumor growth. Oncogene. 2010;29:5286–5298. doi: 10.1038/onc.2010.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 29.Indraccolo S, Pfeffer U, Minuzzo S, Esposito G, Roni V, Mandruzzato S, Ferrari N, Anfosso L, Dell'Eva R, Noonan DM, et al. Identification of genes selectively regulated by IFNs in endothelial cells. J Immunol. 2007;178:1122–1135. doi: 10.4049/jimmunol.178.2.1122. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Verges S, Milush JM, Pandey S, York VA, Arakawa-Hoyt J, Pircher H, Norris PJ, Nixon DF, Lanier LL. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK-cell subset. Blood. 2010;116:3865–3874. doi: 10.1182/blood-2010-04-282301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 32.Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA. Interferon gamma in successful pregnancies. Biol Reprod. 2009;80:848–859. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for non-squamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 34.Kossenkov AV, Vachani A, Chang C, Nichols C, Billouin S, Horng W, Rom WN, Albelda SM, Showe MK, Showe LC. Resection of non-small cell lung cancers reverses tumor-induced gene expression changes in the peripheral immune system. Clin Cancer Res. 2011;17:5867–5877. doi: 10.1158/1078-0432.CCR-11-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Showe MK, Vachani A, Kossenkov AV, Yousef M, Nichols C, Nikonova EV, Chang C, Kucharczuk J, Tran B, Wakeam E, et al. Gene expression profiles in peripheral blood mononuclear cells can distinguish patients with non-small cell lung cancer from patients with nonmalignant lung disease. Cancer Res. 2009;69:9202–9210. doi: 10.1158/0008-5472.CAN-09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donnem T, Al-Shibli K, Andersen S, Al-Saad S, Busund LT, Bremnes RM. Combination of low vascular endothelial growth factor A (VEGF-A)/VEGF receptor 2 expression and high lymphocyte infiltration is a strong and independent favorable prognostic factor in patients with nonsmall cell lung cancer. Cancer. 2010;116:4318–4325. doi: 10.1002/cncr.25333. [DOI] [PubMed] [Google Scholar]
- 37.Seto T, Higashiyama M, Funai H, Imamura F, Uematsu K, Seki N, Eguchi K, Yamanaka T, Ichinose Y. Prognostic value of expression of vascular endothelial growth factor and its flt-1 and KDR receptors in stage I non-small-cell lung cancer. Lung Cancer. 2006;53:91–96. doi: 10.1016/j.lungcan.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Imoto H, Osaki T, Taga S, Ohgami A, Ichiyoshi Y, Yasumoto K. Vascular endothelial growth factor expression in non-small-cell lung cancer: prognostic significance in squamous cell carcinoma. J Thorac Cardiovasc Surg. 1998;115:1007–1014. doi: 10.1016/S0022-5223(98)70398-8. [DOI] [PubMed] [Google Scholar]
- 39.Regina S, Rollin J, Blechet C, Iochmann S, Reverdiau P, Gruel Y. Tissue factor expression in non-small cell lung cancer: relationship with vascular endothelial growth factor expression, microvascular density, and K-ras mutation. J Thorac Oncol. 2008;3:689–697. doi: 10.1097/JTO.0b013e31817c1b21. [DOI] [PubMed] [Google Scholar]
- 40.Holtan SG, Creedon DJ, Thompson MA, Nevala WK, Markovic SN. Expansion of CD16-negative natural killer cells in the peripheral blood of patients with metastatic melanoma. Clin Dev Immunol. 2011;2011:316314. doi: 10.1155/2011/316314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe M, Kono K, Kawaguchi Y, Mizukami Y, Mimura K, Maruyama T, Izawa S, Fujii H. NK cell dysfunction with down-regulated CD16 and up-regulated CD56 molecules in patients with esophageal squamous cell carcinoma. Dis Esophagus. 2010);23:675–681. doi: 10.1111/j.1442-2050.2010.01073.x. [DOI] [PubMed] [Google Scholar]
- 42.Wilson EB, El-Jawhari JJ, Neilson AL, Hall GD, Melcher AA, Meade JL, Cook GP. Human tumour immune evasion via TGF-β blocks NK cell activation but not survival allowing therapeutic restoration of anti-tumour activity. PLoS One. 2011;6:e22842. doi: 10.1371/journal.pone.0022842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carosella ED. The tolerogenic molecule HLA-G. Immunol Lett. 2011;138:22–24. doi: 10.1016/j.imlet.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Yan WH. Human leukocyte antigen-G in cancer: are they clinically relevant? Cancer Lett. 2011;311:123–130. doi: 10.1016/j.canlet.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 45.Schutt P, Schutt B, Switala M, Bauer S, Stamatis G, Opalka B, Eberhardt W, Schuler M, Horn PA, Rebmann V. Prognostic relevance of soluble human leukocyte antigen-G and total human leukocyte antigen class I molecules in lung cancer patients. Hum Immunol. 2010;71:489–495. doi: 10.1016/j.humimm.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 46.Yie SM, Yang H, Ye SR, Li K, Dong DD, Lin XM. Expression of human leucocyte antigen G (HLA-G) is associated with prognosis in non-small cell lung cancer. Lung Cancer. 2007;58:267–274. doi: 10.1016/j.lungcan.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Arai T, Kuroishi T, Saito Y, Kurita Y, Naruke T, Kaneko M. Tumor doubling time and prognosis in lung cancer patients: evaluation from chest films and clinical follow-up study. Japanese Lung Cancer Screening Research Group. Jpn J Clin Oncol. 1994;24:199–204. [PubMed] [Google Scholar]
- 48.Aoki T, Nakata H, Watanabe H, Nakamura K, Kasai T, Hashimoto H, Yasumoto K, Kido M. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am J Roentgenol. 2000;174:763–768. doi: 10.2214/ajr.174.3.1740763. [DOI] [PubMed] [Google Scholar]
- 49.Hasegawa M, Sone S, Takashima S, Li F, Yang ZG, Maruyama Y, Watanabe T. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73:1252–1259. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 50.Rami-Porta R, Ball D, Crowley J, Giroux DJ, Jett J, Travis WD, Tsuboi M, Vallieres E, Goldstraw P. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 51.Kawase A, Yoshida J, Ishii G, Nakao M, Aokage K, Hishida T, Nishimura M, Nagai K. Differences between squamous cell carcinoma and adenocarcinoma of the lung: are adenocarcinoma and squamous cell carcinoma prognostically equal? Jpn J Clin Oncol. 2012;42:189–195. doi: 10.1093/jjco/hyr188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.