Abstract
OBJECTIVE
The aim of this study was to evaluate the association of urinary cystatin C, a tubular damage marker, with the progression of type 2 diabetic nephropathy.
RESERCH DESIGN AND METHODS
The baseline values of serum and urinary cystatin C were measured as primary parameters and those of urinary nonalbumin protein (NAP) were measured as secondary parameters. In this prospective observational study, a total of 237 type 2 diabetic patients were followed up for 29 months (13–44 months).
RESULTS
Both the urinary cystatin C-to-creatinine ratio (CCR) and NAP-to-creatinine ratio (NAPCR) were significantly different according to the degree of albuminuria. Both markers had strongly positive correlations at baseline. After adjusting for several clinical factors, both urinary CCR and NAPCR had significant associations with the decline of the estimated glomerular filtration rate (eGFR) (r = 0.160, P = 0.021; r = 0.412, P < 0.001, respectively). Urinary CCR had positive correlations with the decline of eGFR in the subpopulation of patients with eGFR ≥60 mL/min/1.73 m2. In patients with eGFR ≥60 mL/min/1.73 m2 and normoalbuminuria, only urinary NAPCR showed a significant association with the decline of eGFR; urinary CCR did not. In multivariate regression analysis, the number of patients who progressed to chronic kidney disease stage 3 or greater was higher in those in the upper tertiles of both the urinary levels of cystatin C and NAP than in those in the lower tertiles.
CONCLUSIONS
The results of this study suggest that urinary cystatin C and NAP may be predictors of the progression of type 2 diabetic nephropathy.
Diabetic nephropathy is a complication with high morbidity and mortality as well as a major cause of end-stage renal disease. Although glomerular dysfunction is thought to be a major factor for the development and progression of diabetic nephropathy, tubulointerstitial damage may also play an important role in the pathogenesis of diabetic nephropathy (1–3). Recently, several studies have shown that some tubular damage markers have clinical implications as biomarkers for the development and progression of diabetic nephropathy (4–10).
Cystatin C is a 13-kDa cysteine proteinase inhibitor and is produced by all nucleated cells at a constant rate (11). In healthy subjects, cystatin C is almost freely filtered by the renal glomeruli and almost entirely reabsorbed in the proximal tubule like other low molecular weight proteins; there is no tubular secretion of cystatin C (12–14). Similar to the serum cystatin C, the urinary cystatin C level is not affected by age or muscle mass in healthy subjects or in proteinuric patients without renal tubular damage (15). On the other hand, increased urinary cystatin C has been recognized as a marker of renal tubular dysfunction (16,17). In addition, urinary leakage of proteins other than albumin (nonalbumin protein [NAP]) can also indicate tubular damage rather than glomerular damage (18).
The aims of this study were to evaluate the impact of urinary cystatin C on the progression of type 2 diabetic nephropathy and to determine whether urinary cystatin C has an association with the decline of the glomerular filtration rate (GFR) in type 2 diabetic patients. In addition, we also evaluated whether urinary NAP has any correlation with urinary cystatin C or has any effect on the decline in GFR.
RESEARCH DESIGN AND METHODS
Patients
This was a prospective observational study of patients attending the Department of Endocrinology at Pusan National University Hospital. The study was conducted with the approval of the Institutional Review Board of Pusan National University Hospital. A total of 264 Korean type 2 diabetic patients were consecutively enrolled at the outpatient clinics between May 2008 and December 2009. All patients fulfilled the following inclusion criteria: age ≥18 years and estimated GFR (eGFR) ≥30 mL/min/1.73 m2. We excluded patients with thyroid disorders or who had been medicated within 6 months prior to the study because thyroid function could affect the cystatin C level (19). Additional exclusion criteria were 1) active urinary tract infection, 2) renal disease other than diabetic nephropathy, 3) neoplastic disorders, 4) severe liver dysfunction, 5) active or chronic infection or inflammatory disorders, 6) pregnancy, and 7) a recent (within 6 months) history of acute myocardial infarction, stroke, or occlusive peripheral vascular disease.
A random spot urine sample and a blood sample were obtained from each patient at the clinic visit. Medical histories and anthropometric measurements were also recorded the same day. The eGFR level was calculated using the Modification of Diet in Renal Disease (MDRD) Study formula for the Korean population: MDRD = 107.904 × (serum creatinine [mg/dL])−1.009 × age−0.02 (20). A correction factor of 0.667 was used for women. The serum and urinary cystatin C levels were measured by the latex agglutination test (Modular P800; Roche Diagnostics, Mannhein, Germany). The interassay and intra-assay coefficients of variations of cystatin C in our laboratory were as follows: <4.2 and <3.4%, respectively, for serum and <7.9 and <10.1%, respectively, for urine. Urine specimens with cystatin C levels <0.01 mg/L were assumed to have a concentration of 0.01 mg/L. The data on urinary cystatin C was also expressed as ratios of urinary cystatin C to urinary creatinine in order to assess different hydration states and renal functions of the patients. The ratio between urine mass concentrations of cystatin C and creatinine in micrograms per millimole was calculated and designated as the urinary cystatin C-to-creatinine ratio (CCR). Since we obtained total proteinuria and albuminuria values from each patient at baseline, we were able to estimate the amount of nonalbumin proteinuria through the following calculation: NAP-to-creatinine ratio (NAPCR) = protein-to-creatinine ratio (PCR) – albumin-to-creatinine ratio (ACR). The lowest detectable level and the coefficient of variation in our laboratory were as follows: for total proteinuria, 0.7 mg/dL and <4.8%, respectively; for albuminuria, 0.2 mg/dL and <7.4%, respectively.
The patients were followed up at our clinic until March 2012. They were managed to give priority to the best treatment according to standard guidelines at each outpatient clinic of two endocrinologists (I.J.K. and S.S.K.). Thirteen patients were excluded during follow-up as follows: four patients died of other causes, six patients were hospitalized for acute myocardial infarction and active infections, such as pneumonia, and three patients were diagnosed with additional malignancies during the follow-up period. Finally, a total of 237 patients with type 2 diabetes were enrolled for this study. The two tubular damage markers were measured at intervals of 12 ± 1 (mean ± SD) months at the outpatient clinic during the follow-up period. Serum creatinine was routinely measured for the estimation of GFR at intervals of 6 ± 1 (mean ± SD) months during the follow-up period using the same methods. The latest eGFR calculations were used in the assessment of the annual decline in eGFR. Chronic kidney disease (CKD) stage 3 or greater was defined as having eGFR <60 mL/min/1.73 m2 in two consecutive measurements from the last follow-up visit.
Statistical analysis
All statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL). The data are presented as mean ± SD for normally distributed variables and the medians (interquartile range) for nonnormally distributed variables. The distribution of continuous variables was examined for skewness and kurtosis, and logarithm-transformed values of nonnormally distributed variables were used for analysis. Differences between the groups were analyzed by ANOVA, followed by Bonferroni test. Categorical variables are reported as frequencies and proportions. Pearson χ2 test was used to analyze the categorical data as appropriate. Pearson correlation coefficient was used to test the correlations between individual variables. We conducted multivariate regression analyses with the annual rates of decline in eGFR as dependent variables and urinary CCR and NAPCR as independent variables, respectively. Several models were gradually built to adjust for confounding factors. Multivariate logistic analysis, using a backward procedure on the basis of likelihood ratios, was conducted to determine whether both tubular markers would be predictive factors for CKD stage 3 or greater. The odds ratios (ORs) of urinary levels of cystatin C and NAP were calculated with reference to the lowest tertile of each variable. A P value of <0.05 derived from the two-tailed Student t test was considered statistically significant.
RESULTS
Baseline patient characteristics
The baseline characteristics of the patients are shown in Table 1. The mean age of the patients was 58.5 ± 11.1 years (range, 18–80 years), and there were 115 males and 122 females. The patients were categorized into three groups according to ACR: those with ACR <30 mg/g creatinine (normoalbuminuria group, n = 149), those with ACR 30–299 mg/g creatinine (microalbuminuria group, n = 58), and those with ACR ≥300 mg/g creatinine (macroalbuminuria group, n = 30). Age, duration of diabetes, systolic blood pressure (SBP), HbA1c, HDL cholesterol, and triglycerides were significantly different between the groups. Estimated GFR tended to decrease with increasing degrees of albuminuria (P value for trend <0.001). More antihypertensive and lipid-lowering agents were administered in the macroalbuminuria group than in the normo- and microalbuminuria groups.
Table 1.
Baseline characteristics of metabolic and laboratory parameters in patients with type 2 diabetes
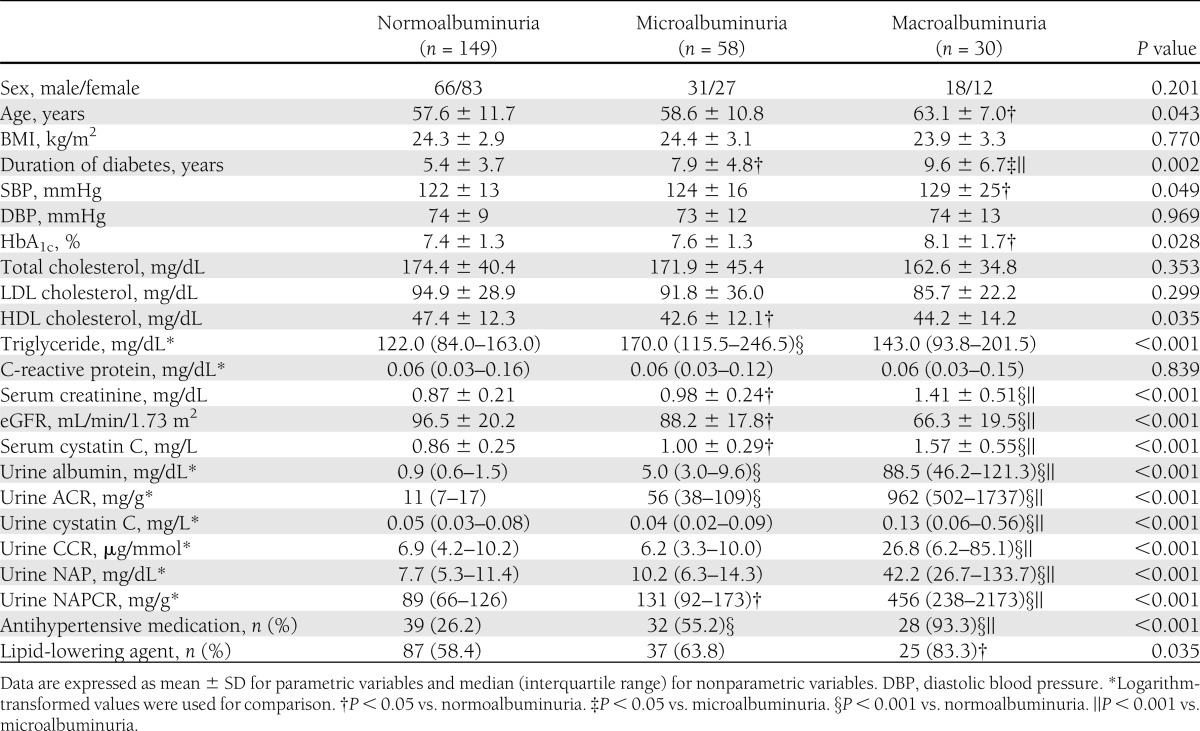
The urinary cystatin C level and CCR were significantly higher in the macroalbuminuria group than in the normoalbuminuria and microalbuminuria groups (both P < 0.001), whereas they were not significantly different between the normoalbuminuria and microalbuminuria groups (Table 1). Urinary NAPCR was significantly higher in the macroalbuminuria group than in the normo- and microalbuminuria groups (both P < 0.001), and they were also significantly different between the normo- and microalbuminuria groups (P < 0.001). Urinary ACR positively correlated with urinary CCR (r = 0.450, P < 0.001) and urinary NAPCR (r = 0.699, P < 0.001). In addition, at baseline, urinary CCR and NAPCR, both markers of tubular damage, positively correlated with each other (r = 0.597, P < 0.001).
Associations of the annual eGFR decline with urinary CCR and NAPCR
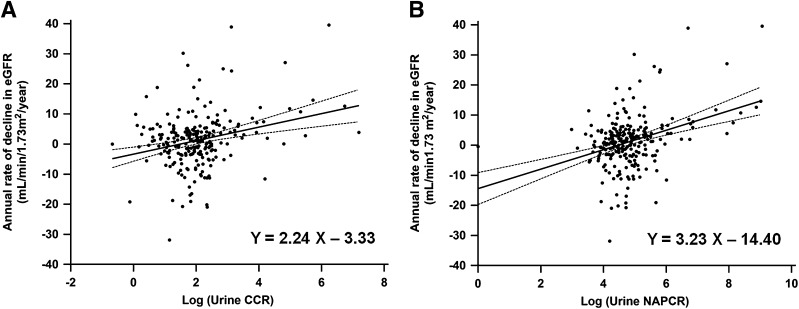
The median follow-up period was 29.0 months (range, 13.0–44.0 months). There was a median annual decline in eGFR of 1.31 mL/min/1.73 m2/year during the follow-up period. The values of annual eGFR decline significantly correlated with both baseline urinary CCR (r = 0.272, P < 0.001) (Fig. 1A) and NAPCR (r = 0.361, P < 0.001) (Fig. 1B) in univariate regression analysis (Supplementary Table 2). After adjusting for age and significant clinical factors affecting the decline of eGFR, both urinary CCR and NAPCR remained significantly associated with a decline in eGFR (Table 2). After additionally adjusting for baseline eGFR and serum cystatin C (only analysis for urinary CCR), both urinary CCR and NAPCR remained significantly associated with the annual decline in eGFR in the final model (r = 0.160, P = 0.021; r = 0.412, P < 0.001, respectively).
Figure 1.
Single regression analysis of the annual rate of the decline in eGFR by using urinary CCR (A) and NAPCR (B). Logarithm-transformed values of urinary CCR and NAPCR were used for analysis.
Table 2.
Multiple regression analysis of the annual rate of decline in eGFR as a dependent variable
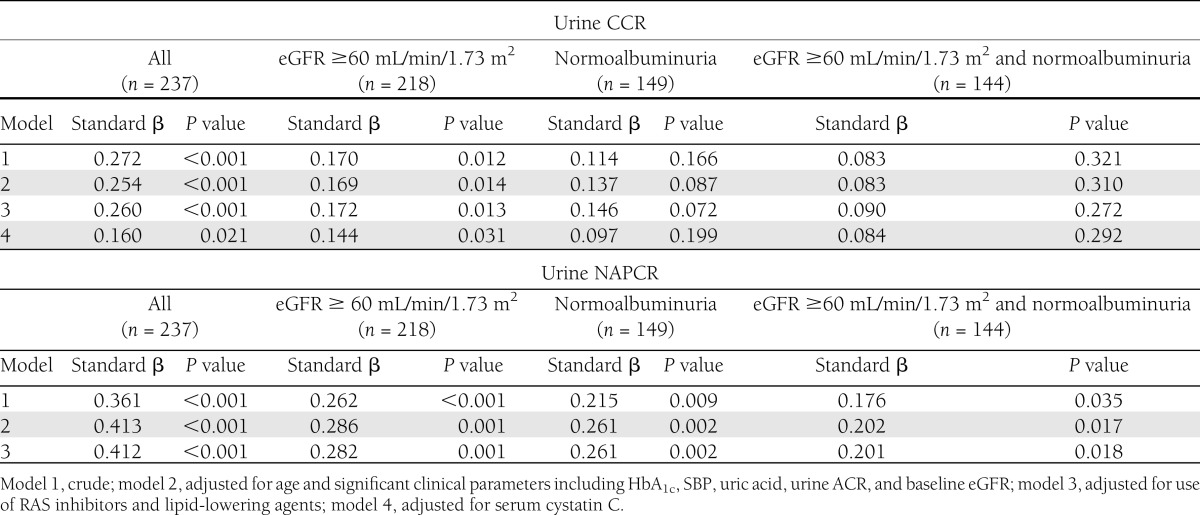
To test whether urinary CCR and NAPCR might have prognostic values in early diabetic nephropathy, we analyzed patients with baseline eGFR ≥60 mL/min/1.73 m2 or with normoalbuminuria. In patients with eGFR ≥60 mL/min/1.73 m2, both urinary CCR and NAPCR showed positive correlations with a decline in eGFR in the final model after adjusting for several clinical parameters (r = 0.144, P = 0.031; r = 0.282, P = 0.001, respectively). In the normoalbuminuria group, the urinary NAPCR remained significantly associated with a decline in eGFR (r = 0.261, P = 0.002) in the final model, whereas urinary CCR did not (r = 0.097, P = 0.199). In addition, urinary NAPCR showed a significant association with a decline in eGFR (r = 0.201, P = 0.018) in patients with both eGFR ≥60 mL/min/1.73 m2 and normoalbuminuria.
Urinary CCR and NAPCR as predictive factors for the development of CKD stage 3 or greater
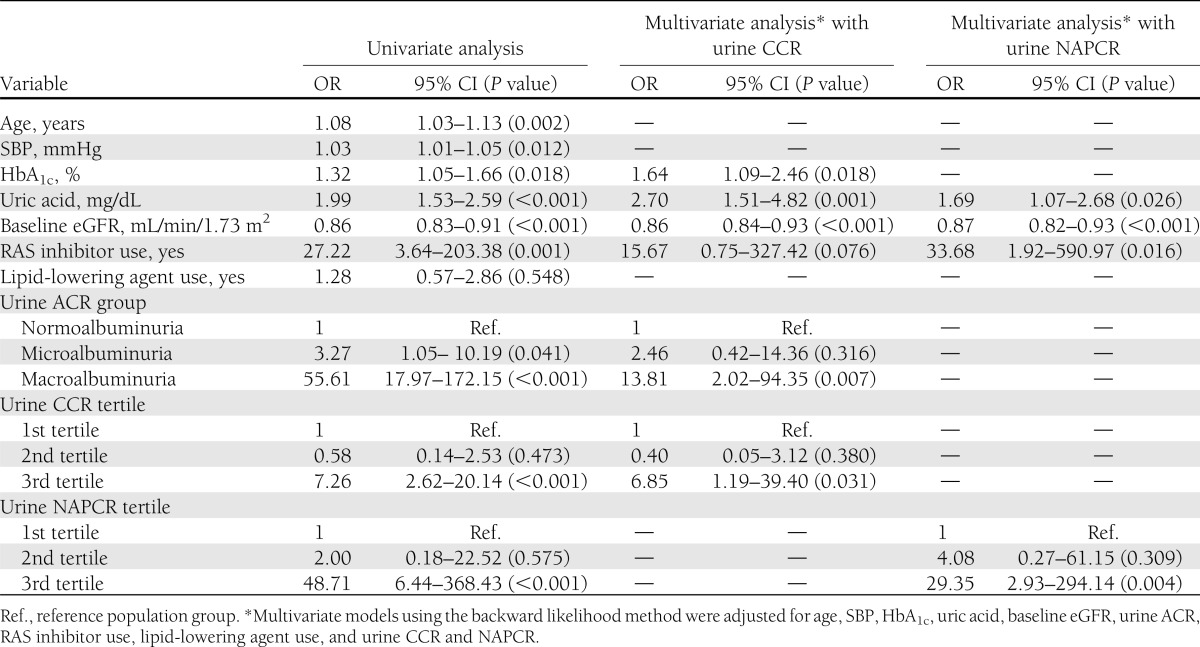
In univariate logistic regression analysis, the number of patients who progressed to CKD stage 3 or greater (eGFR <60 mL/min/1.73 m2) was higher in those in the upper tertiles of both the urinary levels of cystatin C and NAP than in those in the lower tertiles (OR 7.26 for urinary cystatin C; 48.71 for NAPCR; P < 0.001 in both) (Table 3). The increased levels of both urinary CCR and NAPCR remained significantly associated with progression to CKD stage 3 or greater after adjusting for several clinical factors in each multivariate model.
Table 3.
Logistic regression of development of CKD stage 3 or greater (eGFR <60 mL/min/1.73 m2) at last follow-up
CONCLUSIONS
In this study, urinary cystatin C and NAP, both clinical tubular damage markers, positively correlated with each other at baseline. Both markers were significantly associated with the annual decline in eGFR in type 2 diabetic nephropathy. In particular, both tubular damage makers affected a decline in eGFR at the early stage of nephropathy in type 2 diabetic patients (eGFR ≥60 mL/min/1.73 m2). Urinary NAP affected eGFR decline in patients with both eGFR ≥60 mL/min/1.73 m2 and normoalbuminuria, although urinary cystatin C did not reach statistical significance. In addition, the increased levels of the two markers were also associated with the progression of CKD stage 3 or greater at the last follow-up.
In general, unlike healthy subjects, diabetic patients are continuously exposed to the various metabolic and hemodynamic risks associated with this disease (21). Recent studies have mainly focused on tubular damage, which is known to correlate with acute kidney injury in patients with diabetic nephropathy (4–10). Some cross-sectional studies have reported that several tubular markers increase more in diabetic patients than in healthy controls, and this correlated with the severity of albuminuria (4–8). In our study, NAP correlated with the severity of baseline albuminuria, which is consistent with the results of previous studies. However, urinary cystatin C mainly increased in the macroalbuminuria group and was not significantly different between the microalbuminuria and normoalbuminuria groups.
Microalbuminuria has generally been considered the earliest marker for the development of diabetic nephropathy in clinical settings (22). Microalbuminuria is diagnosed when significant glomerular damage has occurred (23) and does not necessarily lead to renal impairment because nephropathy sometimes occurs in the normoalbuminuric patients (24,25). In our study, although urinary cystatin C was not associated with a decline in eGFR in normoalbuminuric patients, urinary NAP was significantly associated with a decline in eGFR independent of baseline albuminuria and eGFR. It is necessary to assess tubular damage independent of albuminuria in patients with early development and progression of diabetic nephropathy because tubular damage may play a significant role in the normoalbuminuric renal insufficiency.
Serum cystatin C is the most valid marker to estimate the GFR, rather than serum creatinine, and to predict progression of renal dysfunction (26). An increase in urinary cystatin C, independent of serum cystatin C, is suggestive of renal tubular damage (13). In our study, urinary cystatin C was a predictor of renal impairment independent of serum cystatin C. Thus, urinary cystatin C also plays some role in predicting renal decline independent of serum cystatin C, although serum cystatin C itself, an indicator for the estimation of GFR, is very important for predicting renal decline.
NAPs include α-1 microglobulin, β-2 macroglobulin, IgG, cystatin C, transferrin nephrin, matrix metalloproteinase-9, and tissue inhibitor of metalloproteinases-1 (27). It is well known that each NAP is related to renal damage in various chronic renal diseases, including diabetic nephropathy (28). Because there is little evidence to support the role of NAP in diabetic nephropathy, it is worthwhile to investigate this issue. Interestingly, in our type 2 diabetic patients, NAP has been shown to be a stronger predictor for renal impairment than urinary cystatin C. At normal levels of protein loss, albumin is a minor component of total urinary protein, although albumin becomes the most significant single protein present as protein loss increases (29). This supports our result that indicates the clinical value of NAP in the prediction of renal impairment in normoalbuminuric patients.
A recent study on tubular damage markers in acute kidney injury has demonstrated that unnormalized values are also useful for predicting ongoing injury (30). Thus, we analyzed unnormalized values of the two aforementioned markers. The unnormalized values had similar patterns as the normalized values in baseline characteristics and univariate analysis results (Table 1 and Supplementary Table 2). The statistical significance of unnormalized values was lost in multivariate analysis, whereas that of normalized values was not (data not shown).
Interestingly, serum uric acid was found to be associated with renal decline in the most analyzed model in this study and has recently been shown to be related to diabetic nephropathy (31,32). Uric acid might induce renal microvascular disease independent of blood pressure through stimulation of the renin-angiotensin system (RAS) and inhibition of endothelial nitric oxide (33). In addition, lowering therapy in a diabetic animal model significantly reduced albuminuria and ameliorated tubulointerstitial inflammation, suggesting a role for uric acid in diabetic nephropathy (34).
The results of this study are subject to some limitations. First, the follow-up period of this study was relatively short, even though the development and progression of diabetic nephropathy require a longer time frame. However, it is considered that the occurrence of tubular damage, as expressed by biomarkers, could be a significant predictor for relatively rapid decline in renal function during a short period of time. Second, we measured the urinary levels of cystatin C, albumin, and protein with single random spot urine samples, although urine samples were collected at the outpatient clinic from patients without illness or renal diseases other than diabetic nephropathy. Despite these limitations, it is noteworthy that the two aforementioned tubular damage markers can easily be checked and used to assess the development and progression of diabetic nephropathy in clinical settings. Third, although there have been a few studies reporting that urinary cystatin C and NAP mainly increase due to tubular rather than glomerular damage, it is unclear whether urinary cystatin C and NAP originate from tubular damage alone in clinical conditions involving both tubular and glomerular damage, such as diabetic nephropathy. Further studies are needed to investigate whether both urinary cystatin C and NAP are biomarkers of tubular damage in clinical conditions with a massive glomerular protein load, such as diabetic nephropathy.
In conclusion, it is suggested that urinary cystatin C and NAP, along with albuminuria, may be sensitive and specific markers for predicting renal impairment in type 2 diabetic patients and may help to further elucidate the role of tubular damage in the pathophysiological mechanisms of the development and progression of type 2 diabetic nephropathy.
Supplementary Material
Acknowledgments
This study was supported by a grant from Pusan National University.
No potential conflicts of interest relevant to this article were reported.
S.S.K. and S.H.S. researched data, contributed to discussion, and wrote and edited the manuscript. I.J.K. researched data, contributed to discussion, and reviewed the manuscript. Y.K.J., B.H.K., I.S.K., and Y.K.K. contributed to discussion and reviewed the manuscript. E.K.L. contributed to discussion and edited the manuscript. I.J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0849/-/DC1.
References
- 1.Bangstad HJ, Seljeflot I, Berg TJ, Hanssen KF. Renal tubulointerstitial expansion is associated with endothelial dysfunction and inflammation in type 1 diabetes. Scand J Clin Lab Invest 2009;69:138–144 [DOI] [PubMed] [Google Scholar]
- 2.Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol 2002;17:247–252 [DOI] [PubMed] [Google Scholar]
- 3.Wolkow PP, Niewczas MA, Perkins B, et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol 2008;19:789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen SE, Schjoedt KJ, Astrup AS, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM1) in patients with diabetic nephropathy: a cross-sectional study and the effects of lisinopril. Diabet Med 2010;27:1144–1150 [DOI] [PubMed] [Google Scholar]
- 5.Nauta FL, Boertien WE, Bakker SJ, et al. Glomerular and tubular damage markers are elevated in patients with diabetes. Diabetes Care 2011;34:975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon YK, Kim MR, Huh JE, et al. Cystatin C as an early biomarker of nephropathy in patients with type 2 diabetes. J Korean Med Sci 2011;26:258–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SS, Song SH, Kim IJ, et al. Clinical implication of urinary tubular markers in the early stage of nephropathy with type 2 diabetic patients. Diabetes Res Clin Pract 2012;97:251–257 [DOI] [PubMed] [Google Scholar]
- 8.Fu WJ, Xiong SL, Fang YG, et al. Urinary tubular biomarkers in short-term type 2 diabetes mellitus patients: a cross-sectional study. Endocrine 2012;41:82–88 [DOI] [PubMed] [Google Scholar]
- 9.Vaidya VS, Niewczas MA, Ficociello LH, et al. Regression of microalbuminuria in type 1 diabetes is associated with lower levels of urinary tubular injury biomarkers, kidney injury molecule-1, and N-acetyl-β-D-glucosaminidase. Kidney Int 2011;79:464–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen SE, Sugaya T, Hovind P, Baba T, Parving HH, Rossing P. Urinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care 2010;33:1320–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abrahamson M, Olafsson I, Palsdottir A, et al. Structure and expression of the human cystatin C gene. Biochem J 1990;268:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest 1996;56:409–414 [DOI] [PubMed] [Google Scholar]
- 13.Herget-Rosenthal S, Feldkamp T, Volbracht L, Kribben A. Measurement of urinary cystatin C by particle-enhanced nephelometric immunoassay: precision, interferences, stability and reference range. Ann Clin Biochem 2004;41:111–118 [DOI] [PubMed] [Google Scholar]
- 14.Tian S, Kusano E, Ohara T, et al. Cystatin C measurement and its practical use in patients with various renal diseases. Clin Nephrol 1997;48:104–108 [PubMed] [Google Scholar]
- 15.Uchida K, Gotoh A. Measurement of cystatin-C and creatinine in urine. Clin Chim Acta 2002;323:121–128 [DOI] [PubMed] [Google Scholar]
- 16.Conti M, Moutereau S, Zater M, et al. Urinary cystatin C as a specific marker of tubular dysfunction. Clin Chem Lab Med 2006;44:288–291 [DOI] [PubMed] [Google Scholar]
- 17.Herget-Rosenthal S, van Wijk J A, Bröcker-Preuss M, Bökenkamp A. Increased urinary cystatin C reflects structural and functional renal tubular impairment independent of glomerular filtration rate. Clin Biochem 2007;40:946–951 [DOI] [PubMed] [Google Scholar]
- 18.Sakatsume M, Kubota R, Ogawa A, et al. Rapid and sensitive electrophoresis of urinary protein clearly reveals the pathophysiological feature of renal diseases. Nephrology (Carlton) 2007;12:191–196 [DOI] [PubMed] [Google Scholar]
- 19.Wiesli P, Schwegler B, Spinas GA, Schmid C. Serum cystatin C is sensitive to small changes in thyroid function. Clin Chim Acta 2003;338:87–90 [DOI] [PubMed] [Google Scholar]
- 20.Lee CS, Cha RH, Lim YH, et al. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease Study equations in the Korean population. J Korean Med Sci 2010;25:1616–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis 2005;12:177–186 [DOI] [PubMed] [Google Scholar]
- 22.Narita T, Hosoba M, Kakei M, Ito S. Increased urinary excretions of immunoglobulin g, ceruloplasmin, and transferrin predict development of microalbuminuria in patients with type 2 diabetes. Diabetes Care 2006;29:142–144 [DOI] [PubMed] [Google Scholar]
- 23.Barratt J, Topham P. Urine proteomics: the present and future of measuring urinary protein components in disease. CMAJ 2007;177:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen MP, Lautenslager GT, Shearman CW. Increased collagen IV excretion in diabetes. A marker of compromised filtration function. Diabetes Care 2001;24:914–918 [DOI] [PubMed] [Google Scholar]
- 25.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR, UKPDS Study Group Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006;55:1832–1839 [DOI] [PubMed] [Google Scholar]
- 26.Macisaac RJ, Premaratne E, Jerums G. Estimating glomerular filtration rate in diabetes using serum cystatin C. Clin Biochem Rev 2011;32:61–67 [PMC free article] [PubMed] [Google Scholar]
- 27.Halimi JM, Matthias B, Al-Najjar A, et al. Respective predictive role of urinary albumin excretion and nonalbumin proteinuria on graft loss and death in renal transplant recipients. Am J Transplant 2007;7:2775–2781 [DOI] [PubMed] [Google Scholar]
- 28.Matheson A, Willcox MD, Flanagan J, Walsh BJ. Urinary biomarkers involved in type 2 diabetes: a review. Diabetes Metab Res Rev 2010;26:150–171 [DOI] [PubMed] [Google Scholar]
- 29.Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem 2009;46:205–217 [DOI] [PubMed] [Google Scholar]
- 30.Ralib AM, Pickering JW, Shaw GM, et al. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol 2012;23:322–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ficociello LH, Rosolowsky ET, Niewczas MA, et al. High-normal serum uric acid increases risk of early progressive renal function loss in type 1 diabetes: results of a 6-year follow-up. Diabetes Care 2010;33:1337–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study. Diabetes 2009;58:1668–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menè P, Punzo G. Uric acid: bystander or culprit in hypertension and progressive renal disease? J Hypertens 2008;26:2085–2092 [DOI] [PubMed] [Google Scholar]
- 34.Kosugi T, Nakayama T, Heinig M, et al. Effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol 2009;297:F481–F488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.