Abstract
Although mitochondria are usually considered as supporters of life, they are also involved in cellular death. Mitochondrial outer membrane permeabilization (MOMP) is a crucial event during apoptosis because it causes the release of proapoptotic factors from the mitochondrial intermembrane space to the cytosol. MOMP is mainly controlled by the Bcl-2 family of proteins, which consists of both proapoptotic and antiapoptotic members. We discuss the current understanding of how activating and inhibitory interactions within this family lead to the activation and oligomerization of MOMP effectors Bax and Bak, which result in membrane permeabilization. The order of events leading to MOMP is then highlighted step by step, emphasizing recent discoveries regarding the formation of Bax/Bak pores on the outer mitochondrial membrane. Besides the Bcl-2 proteins, the mitochondrial organelle contributes to and possibly regulates MOMP, because mitochondrial resident proteins and membrane lipids are prominently involved in the process.
During apoptosis, mitochondrial outer membrane permeabilization (MOMP) causes the activation of proapoptotic effectors (Bax and Bak). It is controlled by Bcl-2 family members and possibly mitochondrial factors.
Mitochondria are essential for the life of the cell. They produce most of the ATP via oxidative phosphorylation thanks to the respiratory chain that is embedded in the inner mitochondrial membrane. Consequently, mitochondrial dysfunction is implicated in the development of many human diseases, in particular, neurodegenerative disorders (Lin and Beal 2006). Mitochondria are also prominently involved in cell death, because they play a crucial role in many apoptotic responses. Apoptosis is a self-destruction program that is essential during the development of multicellular organisms. Its dysregulation has also been recognized as a main feature of many pathological conditions, especially cancer (Llambi and Green 2011).
The executioners of apoptosis are a family of cysteine proteases termed caspases that cleave a variety of cellular targets, resulting in morphological changes, degradation of genomic DNA, and, ultimately, phagocytic removal of the apoptotic cell (Taylor et al. 2008). Caspases are synthesized as inactive zymogens that become activated after regulated limited proteolysis. Two different pathways of apoptotic signaling that result in the activation of executioner caspases 3 and 7 can be distinguished. In the extrinsic pathway, binding of ligands such as FasL or TNFα to a death receptor on the plasma membrane leads to the activation of initiator caspase 8. Active caspase 8 propagates the signal by directly cleaving and thereby activating caspases 3 and 7, which continue a proteolytic cascade ultimately leading to the removal of the cell.
The intrinsic pathway, on the other hand, is initiated upon exposure to a number of stress situations, including DNA damage. A subclass of the Bcl-2 protein family termed BH3-only proteins (see below) becomes activated after an internal stress stimulus and translocates to the outer mitochondrial membrane (OMM), where they orchestrate a process called mitochondrial outer membrane permeabilization (MOMP). As an outcome of this process, pores are formed in the OMM, membrane integrity is lost, and contents of the intermembrane space gain access to the cytosol. One of the molecules that is rapidly released to the cytosol is cytochrome c, which is normally a soluble electron carrier between respiratory complexes III and IV. Together with the proapoptotic cytosolic factor APAF1, cytochrome c assembles into a caspase-activating complex termed the “apoptosome.” This complex subsequently activates caspase 9, which is able to cleave caspases 3 and 7, proceeding with the same downstream cascade as in the extrinsic pathway. Other intermembrane space proteins also contribute to cell death after being released into the cytosol (e.g., SMAC/Diablo, which blocks the caspase inhibitor protein XIAP).
Remarkably, the two pathways are not completely independent. Cross talk between the extrinsic and intrinsic pathways exists because of caspase 8-dependent cleavage of the BH3-only protein Bid. Upon cleavage, Bid becomes activated, and the truncated version, tBid, translocates to the surface of mitochondria to induce MOMP. In so-called type II cells, this mitochondrial feedback loop is needed to induce apoptosis through the extrinsic pathway, because of the requirement of XIAP antagonism by SMAC.
The loss of OMM integrity caused by MOMP is usually considered the point of no return in the whole process, because cells are committed to die once MOMP is initiated. Therefore, this process represents a major checkpoint of apoptosis and must be tightly controlled to ensure that it is initiated at the right time and place. The main molecular players of MOMP belong to the Bcl-2 protein family. Integration of proapoptotic and antiapoptotic signals by the network of Bcl-2 proteins determines whether or not the OMM is permeabilized. In the following sections, we describe in detail the stimulatory and inhibitory protein–protein interactions within this family, discussing various models of how the MOMP effectors, Bax and Bak, become activated. Furthermore, we focus on the actual event of membrane permeabilization, summarizing the current understanding of how pores are formed in the OMM by Bax and Bak oligomers.
MITOCHONDRIA AS A PLATFORM FOR INTEGRATION OF APOPTOTIC SIGNALS
Models for Interaction between Bcl-2 Proteins
The family of Bcl-2 proteins can be subdivided both on the basis of the number of Bcl-2 homology (BH) domains they possess and on their proapoptotic or antiapoptotic function (Youle and Strasser 2008; Chipuk et al. 2010). Both the antiapoptotic Bcl-2-like proteins (Bcl-2, Bcl-XL, MCL-1, Bcl-w, A1) and proapoptotic members Bax and Bak share four short homologous regions of high conservation, termed BH1 to 4. A variety of other Bcl-2 proteins like Bid, Bim, Bad, Puma, Noxa, and others, belong to a third subgroup called the BH3-only proteins. As the name indicates, they contain the conserved BH3 region only and are, apart from that, very heterogeneous. BH3-only proteins, generally speaking, serve as sensors of intrinsic stimuli because in response to DNA damage or ER stress, specific members become activated by transcriptional induction (Puma and Noxa), posttranslational modification (Bad), or limited proteolysis (Bid). BH3-only proteins are thus proapoptotic and transmit the signal by translocating to the OMM, where they either activate Bax and Bak or inhibit the antiapoptotic family members. The subsequent step is the homo-oligomerization of activated Bax and Bak at the OMM. Because this oligomerization is essential for membrane permeabilization, Bax and Bak are often termed the “effectors” of MOMP. The essential role of Bax and Bak at the downstream end of the signaling cascade was shown in cells lacking both proteins, because those cells are resistant to MOMP following diverse apoptotic stimuli (Wei et al. 2001).
Proapoptotic effectors Bax and Bak and the antiapoptotic Bcl-2-like proteins share a similar structure, being small globular proteins that display a characteristic arrangement of seven amphipathic α-helices surrounding the central hydrophobic helix 5 (Muchmore et al. 1996; Suzuki et al. 2000; Moldoveanu et al. 2006). Interactions between individual proteins commonly involve the BH3 region located in helix 2. This region is also present in the BH3-only proteins, which, except for Bid, are believed to be intrinsically unstructured in solution and do not share any common features with other Bcl-2 family proteins except for the BH3 region. In nonapoptotic cells, Bax is primarily a cytosolic protein, whereas Bak and most of its antiapoptotic relatives are already attached to the OMM via a carboxy-terminal tail anchor (Lindsay et al. 2011). The carboxy-terminal α-helix 9 of Bax also serves as a tail anchor that is able to direct the protein to the OMM. However, the helix is sequestered in a hydrophobic groove on the surface of the same molecule, composed of the BH1 and 3 regions. The tail anchor is displaced from the groove upon activation, leading to a cytosol-to-mitochondria redistribution of the protein (Hsu et al. 1997; Eskes et al. 2000). Further structural rearrangements then cause oligomerization of Bax or Bak so that MOMP can proceed.
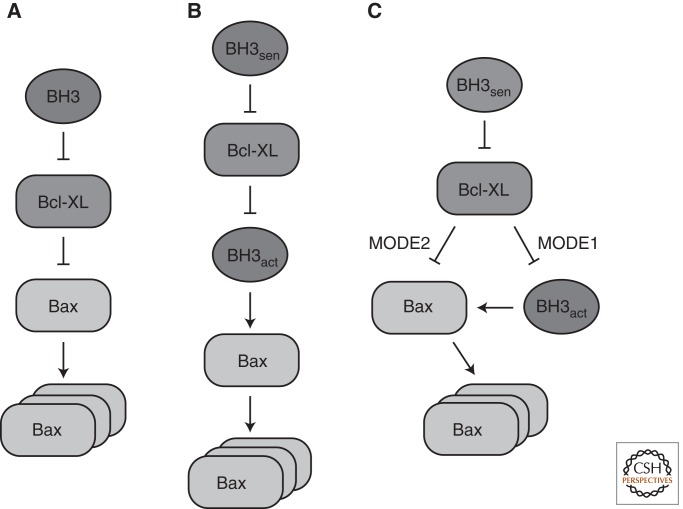
Several models have been discussed for the activation of MOMP effectors that involve protein–protein interaction between the subgroups of Bcl-2 proteins (Fig. 1). In the derepressor model, Bax and Bak are considered active by default but are kept in check by Bcl-XL or another antiapoptotic protein (Chen et al. 2005; Willis et al. 2005, 2007). Activation of the effectors, according to this model, is achieved if a BH3-only protein replaces Bax or Bak at the binding site on Bcl-XL so that the active effector becomes liberated. The direct activator model, however, proposes that Bax and Bak must be activated by direct contact with a BH3-only protein (Desagher et al. 1999; Kuwana et al. 2002; Letai et al. 2002; Terradillos et al. 2002; Kim et al. 2006). This model postulates two classes of BH3-only proteins. “Direct activators” such as tBid and Bim are able to activate MOMP effectors unless they are sequestered by antiapoptotic Bcl-2 proteins. To relieve this inhibition, “sensitizer” BH3-only proteins such as Bad or Noxa replace the direct activators by binding to Bcl-XL, so that Bax or Bak activation can occur. Indeed, by reconstituting membrane permeabilization in liposomes, direct activation of Bax by the BH3-only protein tBid could be shown (Kuwana et al. 2002; Lucken-Ardjomande et al. 2008b). Besides tBid, Bim and Puma have also been described as direct activators of Bax (Kuwana et al. 2005; Kim et al. 2006; Gallenne et al. 2009). Although the mode of action of Puma is more controversial and some investigators classify it as a sensitizer (Chipuk et al. 2008), the fact that a Bid/Bim/Puma triple knockout displays the same phenotype as a Bax/Bak double knockout strongly supports the notion of Puma being a direct activator (Ren et al. 2010).
Figure 1.
Models for activation of MOMP effectors. (A) Derepressor model. (B) Direct activator model. (C) Current model, which is a refinement of the two former models. Antiapoptotic Bcl-2-like Bcl-XL proteins inhibit both direct activator BH3-proteins (MODE1) and effectors Bax and Bak (MODE2). Inhibition through Bcl-XL is relieved by sensitizer BH3-only proteins.
Another model that has been proposed is termed “embedded together” because it emphasizes the role of the lipid bilayer in the interactions between proapoptotic and antiapoptotic Bcl-2 proteins (Leber et al. 2007, 2010). The model can be regarded as a refinement of the conflicting two former models because it integrates concepts from both (Fig. 1). In particular, antiapoptotic proteins are not seen as either inhibitors of activator BH3-only proteins (as in the “direct activator” model) or inhibitors of Bax and Bak (as in the “derepressor” model), but engage both. Like the “direct activator” model, the “embedded together” model differentiates between activator and sensitizer BH3 proteins. An elegant study using fluorescently labeled proteins in a reconstituted system in liposomes was able to establish a clear order of events during Bax activation (Lovell et al. 2008). Using FRET measurements between a variety of protein-attached fluorophores, different protein–protein interactions as well as membrane integration of proteins could be followed simultaneously and in real time. In this experimental setting, the first step is the targeting of tBid, the caspase 8-cleaved active form of Bid, to the membrane. Bax is subsequently recruited by tBid and integrates into the membrane, which can be inhibited by the addition of Bcl-XL. When Bax becomes activated at the membrane, the rate-limiting step is its oligomerization, which involves the recruitment and activation of more Bax molecules (Tan et al. 2006; Lovell et al. 2008). While Bax oligomerizes, liposome contents are released because of membrane permeabilization at the same time. By examining several Bax mutants, it could be confirmed that activation of Bax proceeds in a stepwise manner, and membrane targeting and oligomerization are separate events (Lucken-Ardjomande et al. 2008a; Kim et al. 2009). Interestingly, Bad could displace tBid from Bcl-XL, again confirming its role as a sensitizer (Lovell et al. 2008). Thus, both the initial recruitment of tBid and the activation of Bax require the presence of a membrane. We discuss the role of different membrane lipids in Bax activation below, including the controversial issue of whether or not cardiolipin is involved in this process.
The “embedded together” model not only proposes an important role for the mitochondrial outer membrane in the activation of Bax by direct activators Bid and Bim, but also for the recruitment of antiapoptotic Bcl-2 proteins. Just like Bax, its antiapoptotic opponent Bcl-2 undergoes extensive structural rearrangements when it becomes active at the OMM. These structural changes, which are required for inhibiting Bax oligomerization, appear to be initialized upon binding to activator BH3 proteins (Dlugosz et al. 2006). Furthermore, both Bax and Bcl-XL were shown to be recruited to the membrane by tBid in a comparable way in another study (Billen et al. 2008). Based on these observations, it was proposed that Bcl-XL acts as a dominant-negative version of Bax, providing an explanation for why two structurally similar proteins have opposing activities (Billen et al. 2008). Whereas Bax becomes activated by tBid, Bcl-XL remains associated with this activator, thus inhibiting its function. Likewise, Bax could interact with Bcl-XL as it would with another Bax molecule during oligomerization. However, in this case, Bax oligomerization would be prevented because no membrane-perforating higher-order structure can be formed by Bax/Bcl-XL heterodimers.
Further refinement to the “embedded together” model was made by a recent study that used an elegant domain-shuffling approach between homologous proteins to dissect the antiapoptotic proteins’ binding to activator versus effector proteins (Llambi et al. 2011). Because tBid is inhibited by Bcl-XL, Bcl-2, and MCL-1 equally well, the investigators constructed chimeric proteins in which the BH3 domain of tBid was replaced by the BH3 domain of Bax or Bak. This yielded direct activator protein versions that could still activate both Bax and Bak, but which were inhibited only by a subset of the three antiapoptotic proteins. This allowed the investigators to distinguish inhibition of direct activators (termed MODE1) from inhibition of effectors (termed MODE2), showing that the latter mode of inhibition is more efficient. A scenario was proposed in which direct activators are under MODE1 inhibition at low stress levels, whereas MODE2 inhibition prevents the initiation of MOMP when the amount of direct activator rises. Under MODE2 inhibition, Bax was shown to be in an active conformation and tightly bound to mitochondria. This must be distinguished from the weaker association with mitochondria of unactivated Bax, as described in another recent study (Edlich et al. 2011). Cytosolic Bax is in a constant equilibrium with the loosely mitochondria-associated form in the presence of Bcl-XL because Bcl-XL promotes its retrotranslocation back to the cytosol by a yet-undiscovered mechanism. However, even if a mutation in Bcl-XL prevents its interaction with Bax and Bax localizes to the OMM because of blocked retrotranslocation, MOMP is not readily caused (Edlich et al. 2011), presumably because of “MODE1” sequestration of direct activators (Llambi et al. 2011). Interestingly, in cancer cells, levels of BH3-only proteins are high, but inhibition by antiapoptotic Bcl-2 proteins still causes cell survival (Certo et al. 2006). This condition has been termed “primed to death,” because BH3-only levels in these cancer cells are constantly close to the threshold that would initiate apoptosis. Therefore, BH3-only mimetics like the drug ABT-737 provide an attractive tool to push “primed” cells over the limit in favor of apoptosis (Certo et al. 2006). Indeed, a recent study showed that the responsiveness of cancer cells to chemotherapeutic treatment correlates with “priming” by BH3-only levels (Ni Chonghaile et al. 2011).
Structural Reorganization of Bax/Bak during Activation
The discussed models of MOMP initiation agree that BH3-only proteins provide the death signal for tipping the balance between proapoptotic and antiapoptotic Bcl-2 family members. Reconstitution of the whole process in liposomes, using fluorescently labeled recombinant proteins, has established the order of events leading to the activation of effectors and, ultimately, membrane permeabilization, as described above. But what is the structural basis for the interactions taking place in vivo on the mitochondrial surface? Which structural rearrangements lead to the transition of inactive cytosolic Bax to the active killer in the OMM, and what triggers them?
As observed for complexes of antiapoptotic Bcl-2 proteins with BH3 peptides derived from either activators or effectors, interactions involve the binding of a BH3 domain in the hydrophobic groove built from BH1 and BH3 (Sattler et al. 1997; Petros et al. 2000). The sequestration of a BH3 domain of a proapoptotic protein in the hydrophobic groove of an antiapoptotic inhibitor thus seems to be a common feature. However, it might also be assumed that all interactions between Bcl-2 proteins involve the BH3/groove contact, because the proapoptotic multidomain proteins also contain the groove structure on their surface. Interestingly, the hydrophobic groove of Bax in its cytosolic state is occupied by its own transmembrane helix, thereby preventing translocation to mitochondria. Therefore, in the case of Bax, the BH3 domain of an activator BH3-only protein would have to replace the transmembrane helix in the groove. However, it was shown that the stepwise activation of Bax by tBid leads to structural rearrangements at the amino terminus first (Kim et al. 2009). In the same study, it was proposed, based on the analysis of Bax mutants, that the displacement of helix 1 is required to release the tail-anchor helix 9. Hence, it is very likely that the first contact between an activating BH3 domain and Bax occurs at its amino-terminal region (Kim et al. 2009). This view is supported by the discovery of a binding region at helices 1 and 6, using an α-helical Bim-BH3-derived peptide stabilized by hydrocarbon stapling (Walensky et al. 2006; Gavathiotis et al. 2008). This novel interaction site is often termed the “rear pocket,” because it is located on the opposite surface of Bax compared with the canonical BH1/BH3 groove. Binding of the stapled Bim BH3 helix leads to the displacement of the loop between helices 1 and 2 (Gavathiotis et al. 2008). This rearrangement at the amino terminus of Bax has been recognized for a long time as a structural feature accompanying Bax activation (Hsu and Youle 1997). Subsequently, the amino-terminal rearrangements allow the tail-anchor helix 9 to pop out of the groove and to integrate into the OMM (Fig. 2) (Gavathiotis et al. 2008; Kim et al. 2009).
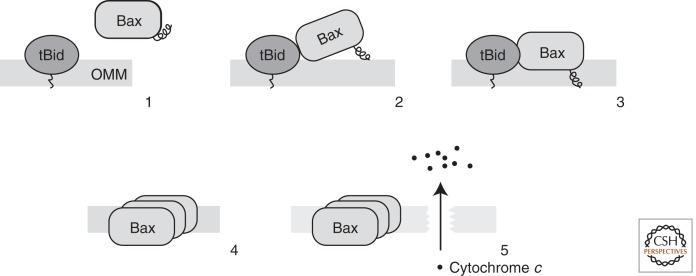
Figure 2.
The order of events leading to MOMP. At first, an activator BH3 protein like tBid is targeted to the membrane (Step 1), which binds and recruits Bax to the membrane (Step 2). Upon activation, Bax exposes its carboxy-terminal membrane-anchor helix and becomes membrane integrated (Step 3). Activated Bax homo-oligomerizes (Step 4), which causes the formation of pores so that proapoptotic contents of the mitochondrial intermembrane space like cytochrome c can be released (Step 5).
The BH3 domain of Bax is exposed as a consequence of the amino- and carboxy-terminal rearrangements triggered by BH3 binding to the rear pocket (Gavathiotis et al. 2010). On the basis of similarity in primary structures of the Bim and Bax BH3 domains, it was proposed that the exposure of the Bax BH3 domain could provide yet another device that is able to recruit and activate further Bax molecules. In that case, the exposed Bax BH3 domain would act just like the BH3 domain of a direct activator BH3-only protein. This would be consistent with the observation that a small number of BH3-only proteins can recruit a molar excess of Bax to the membrane through a Bax-autoactivating mechanism (Tan et al. 2006). Indeed, experimental evidence obtained with a stapled Bax BH3 peptide similar to the Bim peptide described above suggests self-propagating autoactivation of Bax (Gavathiotis et al. 2010). These results provide a very attractive model of Bax oligomerization. After initial activation of Bax through interaction with a BH3 domain at the rear pocket, not only is the membrane-anchor helix 9 released, but also the BH3 domain becomes exposed. By engaging another Bax molecule at its rear pocket, the activation could be propagated from molecule to molecule, leading to a higher-order structure of Bax molecules in a homo-oligomer at the OMM (Fig. 3A). Interestingly, if the release of helix 9 is blocked by mutation, the subsequent BH3 exposure does not lead to oligomerization (Gavathiotis et al. 2010), emphasizing that Bax must be targeted to the membrane in order to oligomerize.
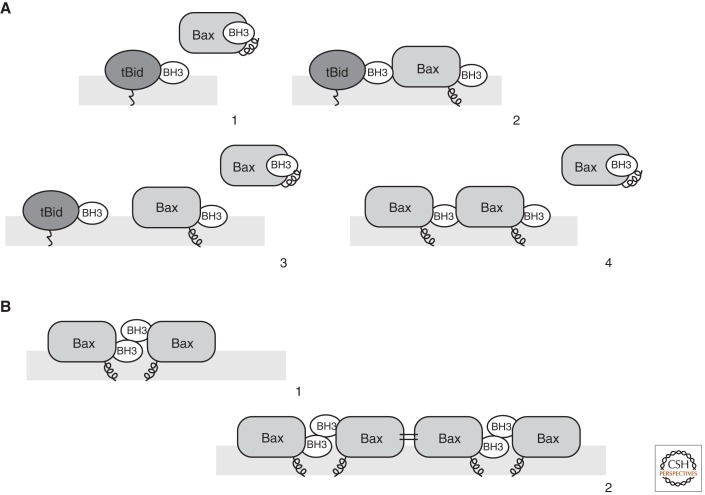
Figure 3.
Bax activation and oligomerization. (A) Asymmetric autoactivation of Bax. Membrane-embedded tBid interacts through its BH3 domain with Bax at its rear pocket (Step 1). This results not only in the exposure of the Bax carboxy-terminal helix, but also in the exposure of the BH3 domain of Bax (Step 2). According to the asymmetric autoactivation model, the exposed BH3 domain of Bax can recruit further Bax molecules to the OMM (Step 3) and activate them (Step 4). (B) Symmetric dimer formation causes Bax oligomerization. Activated Bax, which exposes its BH3 domain, forms symmetric dimers by a reciprocal interaction of one molecule’s BH3 domain with the hydrophobic groove of another, and vice versa (Step 1). Bax dimers can then form higher-order oligomers through another symmetric interaction involving the α-helices 6 at the surface opposite from the BH3–groove interaction (Step 2).
In contrast to this asymmetric Bax oligomeric assembly, the formation of symmetric dimers has been suggested for Bak (Fig. 3B) (Dewson et al. 2008). The search for mutations in Bak that inhibit oligomer formation revealed that the BH1 and BH3 regions are prominently involved in this process. Moreover, cysteine residues were engineered in Bak, which were designed to match in a proposed reciprocal interaction of the BH3 domain of one monomer with the groove region of another. Because disulfide cross-linking stabilized Bak dimers, this is a strong indication of symmetric dimer formation. Finally, reciprocal mutations that allow noncovalent interactions between Bak monomers by the symmetric BH3/groove contact could complement the loss-of-function mutations in oligomerization, pointing to a functional significance of this interaction (Dewson et al. 2008). This evidence is supported by another study aimed at determining the minimal requirements for Bax oligomerization, which found helices 2, 4, and 5 to be sufficient for this process (George et al. 2007). This region overlaps with the BH1 and BH3 domains found to be essential in symmetric dimer formation. Furthermore, several biochemical studies confirmed symmetric dimers involving the BH3/groove interaction for both Bax and Bak (Bleicken et al. 2010; Oh et al. 2010; Zhang et al. 2010). This potential interaction, however, would be reciprocal, resulting in both the BH3 domains and hydrophobic grooves of Bax being occupied. Therefore, to form higher-order oligomers, as observed during MOMP, dimers would need to be connected by yet another intermolecular contact. This additional contact is provided by another symmetric contact, because helix 6 of one Bax molecule was shown to interact with helix 6 of another (Dewson et al. 2009). Therefore, dimers formed by the BH3/groove contact could oligomerize by helix 6/helix 6 interactions (Fig. 3B). A previously reported oligomerization mechanism via the coordination of zinc ions (Moldoveanu et al. 2006), on the other hand, could be ruled out (Dewson et al. 2009).
Thus, two conflicting views exist regarding Bax or Bak oligomer formation, based on the interaction of monomers in an either symmetric or asymmetric fashion. However, it is likely that neither model describes the mode of oligomerization exclusively. The symmetric model raises the question whether the BH3 exposure of one monomer, provoked by a direct activator, is sufficient to also activate the other monomer, or if this second monomer also has to be activated by a BH3-only direct activator binding to its rear pocket. The observation that BH3 ligands leave the rear pocket in a hit-and-run fashion and then seem to engage the effector at another site, possibly the BH1/BH3 region (Kim et al. 2009), suggests that the initial interactions are highly dynamic. Thus, it is possible to envision a scenario in which the exposed BH3 domain of activated Bax can either activate other Bax molecules via the asymmetric rear-pocket activation site or interact with an already activated Bax molecule via the symmetric BH3/groove interface to initialize oligomerization.
Pore Formation by Bax/Bak Oligomers
The activation and oligomerization of proapoptotic Bcl-2 family effector proteins Bax and Bak are the prerequisites for MOMP. Although over the last few years models and concepts have emerged regarding the interactions between different Bcl-2 family members, as well as the structural rearrangements of Bax and Bak causing their oligomerization, the molecular connection between Bax or Bak oligomers and the actual event of membrane permeabilization is less evident (Fig. 1). Experiments with fluorescent dextrans of different sizes encapsulated by liposomes have shown that pores formed by activated Bax allow the release of molecules with a broad range of molecular mass from 10 kDa to 2 MDa (Kuwana et al. 2002). Although another study suggests that the maximum size of intermembrane space contents released in vivo is lower (Rehm et al. 2003), it is generally agreed that Bax pores accommodate the release of all proapoptotic intermembrane space (IMS) molecules like cytochrome c or SMAC/Diablo.
So what is the mode of pore formation by oligomerized Bax? Bax alone is able to form pores in artificial planar lipid membranes (Antonsson et al. 1997; Schlesinger et al. 1997), although the experimental setup used in these experiments is differing substantially from the in vivo situation. The structural similarities of multidomain Bcl-2 proteins like Bax or Bcl-XL to bacterial pore-forming toxins like colicins, in particular, the arrangement of amphipathic helices surrounding a central hydrophobic helix, suggest that Bax itself is able to porate membranes (Muchmore et al. 1996). Indeed, following activation, helices 5 and 6 of Bax were shown to insert into the membrane (Annis et al. 2005), emphasizing that these central helices might play an important role in membrane permea-bilization. Moreover, replacement of helix 5 of Bcl-XL by the respective Bax helix enabled pore formation by the chimeric protein, thereby turning Bcl-XL into a killer molecule (George et al. 2007). This supports the notion that Bax oligomers are forming a proteinaceous channel in the OMM. Patch-clamp measurements of pores formed by Bak have led to the description of a channel structure termed the mitochondrial apoptosis-induced channel (MAC) by Martinez-Caballero et al. (2009). This channel shows an increase in conductance over time, being consistent with the stepwise incorporation of activated Bax homodimers into an oligomeric Bax pore. Alternatively, Bax oligomers could provoke the opening of a preexisting mitochondrial pore structure like the permeability-transition pore. However, this was effectively ruled out by experiments in cells lacking the central components of this pore structure (Baines et al. 2005; Nakagawa et al. 2005). Likewise, an involvement of the voltage-dependent anion channel (VDAC) was ruled out (Baines et al. 2007), although Bak was shown to interact with VDAC at the OMM, VDAC having an inhibitory function on Bak (Cheng et al. 2003).
Apart from observations in favor of a proteinaceous channel formed directly by a complex of activated Bax or Bak subunits, evidence also exists that a lipidic pore is formed. In a scenario like this, Bax or Bak could act like another bacterial toxin called melittin, which induces the formation of inverted micelle structures in membranes, resulting in lipidic pores. This view is supported by observations that pore formation is facilitated by the incorporation of lipids into the membrane that induce positive membrane curvature (Basanez et al. 2002). Remarkably, the pores formed by isolated Bax helix 5 were shown to be lipidic in nature (Garcia-Saez et al. 2007; Qian et al. 2008), although, of course, a single helix might have different effects from full-length Bax. The analysis of pores formed by activated Bax in liposomes by cryoelectron microscopy, on the other hand, revealed that the pore edges are devoid of proteinaceous particles, thus strongly supporting the lipidic pore model (Schafer et al. 2009). Furthermore, a recent structural study using small-angle neutron scattering revealed that activated Bax in liposomes adopts a shape reminiscent of bacterial toxins known to form lipidic pores (Satsoura et al. 2012).
Yet another intriguing possibility for the mode of action of Bax oligomers was recently proposed by Montessuit et al. (2010). The researchers observed that the mitochondrial fission factor Drp-1 facilitates pore formation by Bax in liposomes. Interestingly, a direct interaction of Bax and Drp-1 was not responsible for this effect, but the shape of the membrane played a key role. In particular, the existence of hemifusion intermediates promotes Bax oligomerization in this study (Montessuit et al. 2010). This type of intermediate is formed transiently during both the mitochondrial fusion or fission process and is characterized by an interconnected stalk between the outer leaflets of the two lipid bilayers, whereas the inner leaflets remain intact (Chernomordik and Kozlov 2008). Complete separation of the membranes or the formation of a fusion pore then completes membrane fission or fusion, respectively. Although Drp1 has so far only been described as a fission factor, it seems that it can also support vesicle fusion to the intermediate stage under the experimental conditions used (Montessuit et al. 2010). Interestingly, intermediate structures common to membrane fusion or fission have been described to be transiently leaky. Moreover, any perturbations in the fusion process in general can lead to permanent membrane leakage and thus the loss of membrane integrity (Engel and Walter 2008). It is thus conceivable that transient leakage also occurs during mitochondrial fission. Because mitochondria commonly fragment during apoptosis, Bax oligomers might take advantage of membrane vulnerability, insert into fragile sections at or near hemifission intermediates, and cause further perturbation during the fission process, leading to a loss of mitochondrial integrity (Martinou and Youle 2011). Consistent with this model, Bax has formerly been shown to insert at constriction sites, where it colocalizes with components of both the fusion and fission machineries (Karbowski et al. 2002).
The important role of membrane properties, especially membrane shape, not only in the recruitment of Bcl-2 proteins to the OMM but also in pore formation itself, raises the question as to whether specific membrane lipids influence the course or the outcome of MOMP. The role of cardiolipin, a lipid restricted to mitochondrial membranes, has been controversially discussed in the literature. Several studies show that cardiolipin is required for tBid/Bax-induced permeabilization of liposomes (Kuwana et al. 2002; Terrones et al. 2004; Lucken-Ardjomande et al. 2008b). The targeting of tBid, in particular, seems to require an interaction with this membrane lipid (Lutter et al. 2000). It remains questionable, however, if cardiolipin is required for MOMP in vivo, because it is primarily found in the inner mitochondrial membrane and its concentration in the outer membrane is low. One possible explanation for this obvious discrepancy is that cardiolipin is enriched in contact sites between the outer and inner membrane (Lutter et al. 2001), in such a way that local concentrations could be high enough to promote Bax activation. Other investigators have suggested that in vivo outer membrane proteins can take over the role that cardiolipin has in liposomes (Schafer et al. 2009). Candidate proteins include components of the TOM receptor complex, components of the fusion or fission machineries, or the mitochondrial carrier homolog MCTH2/MIMP (Ott et al. 2009; Zaltsman et al. 2010).
An additional study introduced yet another possibility for the requirement of membrane lipids in MOMP. Chipuk et al. (2012) discovered that contaminants originating from the endoplasmic reticulum, which are usually present in the mitochondrial preparations used for in vitro experiments, contain an MOMP-promoting factor. By stepwise purification of this factor from the contaminating microsomal fraction, a neutral sphingomyelinase was identified that promoted membrane permeabilization by tBid and Bak or Bax. Further investigation of the sphingolipid metabolism then revealed that Bak and Bax interact in the membrane with the sphingolipid metabolites sphigosin-1-phosphate and hexadecenal, respectively (Chipuk et al. 2012). Interestingly, this interaction resulted in the exposure of the 6A7 epitope in Bax, indicating activating structural rearrangements (see above). One might thus speculate that interaction of MOMP effectors with specific lipid components cooperates with direct activator BH3-only proteins by lowering the activation energy for the required structural remodeling. Furthermore, the researchers showed that cardiolipin is not needed for Bax activation in liposomes if the respective sphingolipid metabolite, hexadecenal, is present (Chipuk et al. 2012). All of these observations give rise to a potentially important role of the contact between mitochondria and the endoplasmic reticulum during apoptosis.
CONCLUDING REMARKS
Besides hosting numerous essential metabolic pathways, mitochondria are also prominently involved in cell death. In vertebrate cells, death signals arising from internal stress-sensing pathways converge on the outer mitochondrial membrane, where the effector Bcl-2 proteins Bax and Bak become activated, integrate into the membrane, homo-oligomerize, and thereby cause the loss of membrane integrity. The participation in the execution of apoptosis is just one example of how mitochondria are part of an interconnected cellular network that not only maintains life but also determines a cell’s fate in a variety of situations. In this respect, it is not surprising that, besides dysregulation of cellular proliferation, the inhibition of cell death through apoptosis is also a common feature in the development of cancer (for review, see Llambi and Green 2011). Still, because regulation involves the interplay of proapoptotic and antiapoptotic Bcl-2 family proteins, apoptosis is not completely abolished in cancer cells. On the contrary, cancer cells have been described as “primed” for death, because a high level of BH3-only proteins is counteracted by up-regulated antiapoptotic Bcl-2-like proteins. BH3-only mimetics like ABT-737 represent tools for tipping the balance in favor of death, thereby providing a promising possibility for therapeutic intervention (Vogler et al. 2009).
However, although our knowledge of the early steps of MOMP (i.e., Bax targeting, activation, and oligomerization) has expanded substantially over the past few years, the actual event of membrane permeabilization by Bax or Bak oligomers remains, in part, mysterious. It has become evident that the outer membrane of mitochondria itself is not only providing the grounds for MOMP, but actively participates in this process. Membrane lipids and also resident mitochondrial proteins seem to be involved in all of the steps leading to MOMP, from the recruitment of tBid or other direct activators to the pore formation itself. Like cytochrome c, which normally serves as an electron carrier between respiratory complexes, other mitochondrial proteins are likely to have dual functions both in mitochondrial biology and apoptosis. On the other hand, there is growing evidence that Bcl-2 proteins also participate in other cellular processes besides apoptosis, like the regulation of mitochondrial fusion and fission (Martinou and Youle 2011). Therefore, it is most likely that we are awaiting many more exciting discoveries regarding the role of mitochondria in cell death in the near future.
ACKNOWLEDGMENTS
We thank Dr. C. Rodley for critical reading of the manuscript and all members of the laboratory for their support. Work in our laboratory is supported by subsidy 31003A-141068/1 of the Swiss National Foundation. T.B. receives a fellowship from the Deutsche Forschungsgemeinschaft (BE 5103/1-1).
Footnotes
Editors: Douglas C. Wallace and Richard J. Youle
Additional Perspectives on Mitochondria available at www.cshperspectives.org
REFERENCES
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW 2005. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J 24: 2096–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, et al. 1997. Inhibition of Bax channel-forming activity by Bcl-2. Science 277: 370–372 [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, et al. 2005. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662 [DOI] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD 2007. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9: 550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanez G, Sharpe JC, Galanis J, Brandt TB, Hardwick JM, Zimmerberg J 2002. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem 277: 49360–49365 [DOI] [PubMed] [Google Scholar]
- Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW 2008. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol 6: e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Classen M, Padmavathi PV, Ishikawa T, Zeth K, Steinhoff HJ, Bordignon E 2010. Molecular details of Bax activation, oligomerization, and membrane insertion. J Biol Chem 285: 6636–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A 2006. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 9: 351–365 [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC 2005. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403 [DOI] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ 2003. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301: 513–517 [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM 2008. Mechanics of membrane fusion. Nat Struct Mol Biol 15: 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Fisher JC, Dillon CP, Kriwacki RW, Kuwana T, Green DR 2008. Mechanism of apoptosis induction by inhibition of the anti-apoptotic BCL-2 proteins. Proc Natl Acad Sci 105: 20327–20332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR 2010. The BCL-2 family reunion. Mol Cell 37: 299–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, Obeid LM, Green DR 2012. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell 148: 988–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou JC 1999. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol 144: 891–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM 2008. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3: groove interactions. Mol Cell 30: 369–380 [DOI] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Czabotar P, Day CL, Adams JM, Kluck RM 2009. Bak activation for apoptosis involves oligomerization of dimers via their α6 helices. Mol Cell 36: 696–703 [DOI] [PubMed] [Google Scholar]
- Dlugosz PJ, Billen LP, Annis MG, Zhu W, Zhang Z, Lin J, Leber B, Andrews DW 2006. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J 25: 2287–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle RJ 2011. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell 145: 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A, Walter P 2008. Membrane lysis during biological membrane fusion: Collateral damage by misregulated fusion machines. J Cell Biol 183: 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC 2000. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 20: 929–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, Geneste O, Cartron PF, Vallette FM, Manon S, et al. 2009. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol 185: 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Chiantia S, Salgado J, Schwille P 2007. Pore formation by a Bax-derived peptide: Effect on the line tension of the membrane probed by AFM. Biophys J 93: 103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, et al. 2008. BAX activation is initiated at a novel interaction site. Nature 455: 1076–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD 2010. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell 40: 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Evans JJ, Luo X 2007. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev 21: 1937–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem 272: 13829–13834 [DOI] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ 1997. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci 94: 3668–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ 2002. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol 159: 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH 2006. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 8: 1348–1358 [DOI] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH 2009. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 36: 487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111: 331–342 [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD 2005. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell 17: 525–535 [DOI] [PubMed] [Google Scholar]
- Leber B, Lin J, Andrews DW 2007. Embedded together: The life and death consequences of interaction of the Bcl-2 family with membranes. Apoptosis 12: 897–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber B, Lin J, Andrews DW 2010. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene 29: 5221–5230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2: 183–192 [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF 2006. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443: 787–795 [DOI] [PubMed] [Google Scholar]
- Lindsay J, Esposti MD, Gilmore AP 2011. Bcl-2 proteins and mitochondria—Specificity in membrane targeting for death. Biochim Biophys Acta 1813: 532–529 [DOI] [PubMed] [Google Scholar]
- Llambi F, Green DR 2011. Apoptosis and oncogenesis: Give and take in the BCL-2 family. Curr Opin Genet Dev 21: 12–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, Dillon CP, Green DR 2011. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell 44: 517–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW 2008. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135: 1074–1084 [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S, Montessuit S, Martinou JC 2008a. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell Death Differ 15: 484–493 [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S, Montessuit S, Martinou JC 2008b. Contributions to Bax insertion and oligomerization of lipids of the mitochondrial outer membrane. Cell Death Differ 15: 929–937 [DOI] [PubMed] [Google Scholar]
- Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X 2000. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat Cell Biol 2: 754–761 [DOI] [PubMed] [Google Scholar]
- Lutter M, Perkins GA, Wang X 2001. The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol 2: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Caballero S, Dejean LM, Kinnally MS, Oh KJ, Mannella CA, Kinnally KW 2009. Assembly of the mitochondrial apoptosis-induced channel, MAC. J Biol Chem 284: 12235–12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ 2011. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 21: 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu T, Liu Q, Tocilj A, Watson M, Shore G, Gehring K 2006. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell 24: 677–688 [DOI] [PubMed] [Google Scholar]
- Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, et al. 2010. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell 142: 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, et al. 1996. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381: 335–341 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y 2005. Cyclophilin D–dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658 [DOI] [PubMed] [Google Scholar]
- Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, et al. 2011. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science 334: 1129–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Singh P, Lee K, Foss K, Lee S, Park M, Aluvila S, Kim RS, Symersky J, Walters DE 2010. Conformational changes in BAK, a pore-forming proapoptotic Bcl-2 family member, upon membrane insertion and direct evidence for the existence of BH3–BH3 contact interface in BAK homo-oligomers. J Biol Chem 285: 28924–28937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Norberg E, Zhivotovsky B, Orrenius S 2009. Mitochondrial targeting of tBid/Bax: A role for the TOM complex? Cell Death Differ 16: 1075–1082 [DOI] [PubMed] [Google Scholar]
- Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB, et al. 2000. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci 9: 2528–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S, Wang W, Yang L, Huang HW 2008. Structure of transmembrane pore induced by Bax-derived peptide: Evidence for lipidic pores. Proc Natl Acad Sci 105: 17379–17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm M, Dussmann H, Prehn JH 2003. Real-time single cell analysis of Smac/DIABLO release during apoptosis. J Cell Biol 162: 1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH 2010. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science 330: 1390–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsoura D, Kucerka N, Shivakumar S, Pencer J, Griffiths C, Leber B, Andrews DW, Katsaras J, Fradin C 2012. Interaction of the full-length Bax protein with biomimetic mitochondrial liposomes: A small-angle neutron scattering and fluorescence study. Biochim Biophys Acta 1818: 384–401 [DOI] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, et al. 1997. Structure of Bcl-xL–Bak peptide complex: Recognition between regulators of apoptosis. Science 275: 983–986 [DOI] [PubMed] [Google Scholar]
- Schafer B, Quispe J, Choudhary V, Chipuk JE, Ajero TG, Du H, Schneiter R, Kuwana T 2009. Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. Mol Biol Cell 20: 2276–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ 1997. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci 94: 11357–11362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N 2000. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell 103: 645–654 [DOI] [PubMed] [Google Scholar]
- Tan C, Dlugosz PJ, Peng J, Zhang Z, Lapolla SM, Plafker SM, Andrews DW, Lin J 2006. Auto-activation of the apoptosis protein Bax increases mitochondrial membrane permeability and is inhibited by Bcl-2. J Biol Chem 281: 14764–14775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ 2008. Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9: 231–241 [DOI] [PubMed] [Google Scholar]
- Terradillos O, Montessuit S, Huang DC, Martinou JC 2002. Direct addition of BimL to mitochondria does not lead to cytochrome c release. FEBS Lett 522: 29–34 [DOI] [PubMed] [Google Scholar]
- Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu J, Lee RM, Herrmann A, Basanez G 2004. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J Biol Chem 279: 30081–30091 [DOI] [PubMed] [Google Scholar]
- Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJS, Cohen GM 2009. Different forms of cell death induced by putative BCL-2 inhibitors. Cell Death Differ 16: 1030–1039 [DOI] [PubMed] [Google Scholar]
- Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, Smith E, Verdine GL, Korsmeyer SJ 2006. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell 24: 199–210 [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ 2001. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC 2005. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19: 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. 2007. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315: 856–859 [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A 2008. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59 [DOI] [PubMed] [Google Scholar]
- Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, Vaz FM, De Leonardis F, Fiermonte G, Palmieri F, et al. 2010. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat Cell Biol 12: 553–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhu W, Lapolla SM, Miao Y, Shao Y, Falcone M, Boreham D, McFarlane N, Ding J, Johnson AE, et al. 2010. Bax forms an oligomer via separate, yet interdependent, surfaces. J Biol Chem 285: 17614–17627 [DOI] [PMC free article] [PubMed] [Google Scholar]