Abstract
Background
The CYP4503A5*1 genotype is associated with lower tacrolimus concentrations. Although its effect is important, it incompletely explains the variability in tacrolimus concentrations and has a relatively low minor allele frequency in Caucasians relative to African Americans (AA).
Methods
We studied clinical and recipient genetic correlates of dose-normalized tacrolimus troughs (n=12,277) in the first 6 months posttransplant using a customized single nucleotide polymorphism chip with 2,722 variants in a large, ethnically diverse (144 AA and 551 non-AA) adult kidney transplant population through a 7-center consortium.
Results
Over the 6 month study, AAs had consistently lower median (interquartile range) troughs than non-AAs, 6.2 (4.4–8.4) vs 8.3 (6.4–10.4) ng/mL (p<0.0001), in spite of 60% higher daily doses, 8 (5–10) vs 5 (4–7) mg (p<0.0001). The median tacrolimus trough concentration in week one posttransplant was particularly low in AAs [2.1 (1.2–3.5)] compared to non-AAs [5.0 (3.1–8.2) ng/mL](p<0.0001) despite similar initial doses. In single variant analysis, CYP3A5*3 (rs776746) was the top variant (p=2.4x10−33) associated with troughs. After adjustment for CYP3A5*3, clinical factors and race, thirty-nine additional variants were identified (p<0.01, not significant at FDR 20%). In the final multivariant, regression models beginning with these variants and clinical factors, 7 variants were identified in the non-AA and 7 variants in the AA group towards the first trough concentrations. Rs776746 (CYP3A5), rs2239393 (COMT) and diabetes were the only factors common in both populations.
Conclusion
We identified variants beyond CYP3A5*3 which may further explain pharmacokinetic variability of tacrolimus and demonstrated that important variants differ by race.
Keywords: tacrolimus, pharmacogenetics, cytochrome P450, pharmacokinetics
INTRODUCTION
Effective immunosuppression is essential for organ transplantation and recent improvements in outcomes have been due largely to advances in drug therapy. However, immunosuppression still fails in some while causing toxicity in others. Studies have reported that genetic variation is associated with immunosuppressant pharmacokinetics, toxicity and outcomes after transplantation.(1–6) Pharmacogenetic findings have not been adopted into clinical practice, in part due to the unclear impact on clinical outcome, conflicting findings and modest variant effects.
Tacrolimus is the most commonly used calcineurin inhibitor.(7) It is metabolized by CYP3A in the gut and liver, and transported in the gut by P-glycoprotein (P-gp), an efflux pump, which is encoded by the multidrug resistant protein (MDR1)/ABCB1 gene.(8–12) Tacrolimus has a narrow therapeutic index necessitating therapeutic monitoring of blood concentrations.(13–15) There is high interpatient variability in tacrolimus concentrations and the dose required to achieve the therapeutic range; therefore, there is intensive interest in using genetics to optimize dosing.
The influence of variants on tacrolimus metabolism has been extensively studied and recently reviewed.(16–17) One or more CYP3A5*1 alleles (rs776746) results in higher clearance, lower concentrations, higher dose requirements and delayed time to therapeutic concentrations.(18–30) Despite the clear differences between the CYP3A5*1 and *3 genotypes, they explain up to 45% of the variability in dose(31) and 30% of clearance variability(32) suggesting additional determinants of drug disposition. CYP3A4 variants have been studied with conflicting results.(18, 21, 23–24) Most studies have shown that the MDR1 variants have small or no effects on tacrolimus pharmacokinetics.(18, 21, 23–25, 29, 31, 33–36) To date these variants do not explain enough variation in metabolism thereby limiting the enthusiasm for clinical use. To directly address these issues we conducted an evaluation of 2,722 genetic variants towards tacrolimus pharmacokinetics in 695 kidney transplant recipients. Identification of the important variants will allow for the development of dosing equations to individualize therapy.
RESULTS
Transplant Recipients and Tacrolimus Trough Measurements
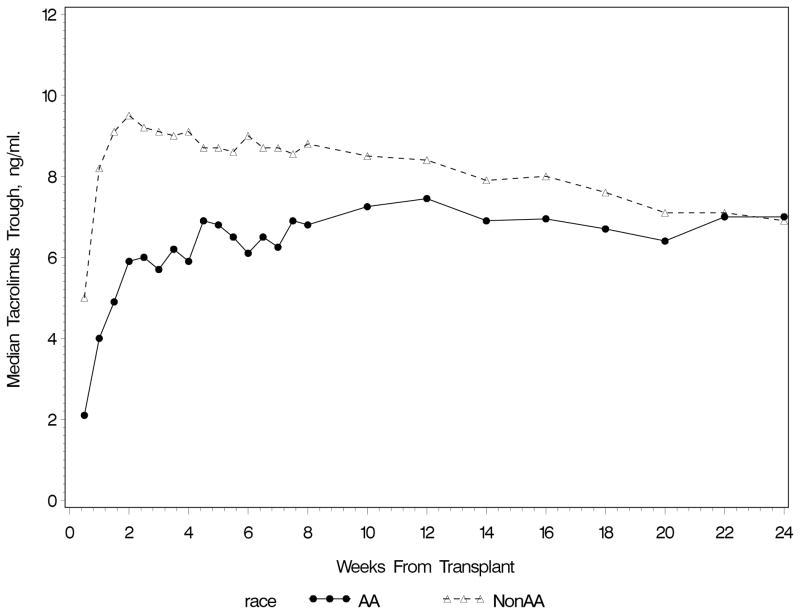
Recipient characteristics are shown in Table 1. A total of 12,277 tacrolimus trough concentrations were studied (Table 1) in 7 centers in an ethnically diverse population. Tacrolimus oral doses and troughs varied between centers. The median (interquartile range) daily dose (mg) over the 6 month study period in centers 1–7 were 8 (5–11), 5 (4–6), 4 (3–6), 6 (4–8), 6 (4–8), 6 (4–8) and 6 (4–8), respectively. The trough concentrations (ng/mL) in centers 1–7 were 9.5 (8.0–11.4), 10.5 (7.4–13.0), 7.9 (6.1–10.1), 9.7 (7.5–12.2), 8.9 (7.1–10.6), 9.1 (7.0–11.3) and 6.6 (4.7–8.7), respectively. Troughs increased over the first 2 weeks and then stabilized (Figure 1). The first trough concentration, measured within 4 days after transplantation, was <5 ng/mL in most subjects.
Table 1.
Characteristics, Tacrolimus Concentrations and Doses of Study Subjects
| Characteristics | All subjects (n=695) | nonAfrican American (n=551) | African American (n=144) |
|---|---|---|---|
| Male recipient, n (%) | 439 (63.2%) | 346 (62.8%) | 93 (64.6%) |
| Age at transplant (yrs)a | 50.4 (20.1–81.9) | 50.9 (201.-81.9) | 47.2 (20.3–72.9) |
| Weight at time of transplant (kg)a | 80.9 (37.7–151.7) | 80.7 (37.7–151.7) | 81.0 (42.3–133.0) |
| Living donor | 405 (58.3%) | 361 (65.5%) | 44 (30.6%) |
| Diabetes at time of transplant | 276 (39.7%) | 222 (40.3%) | 54 (37.5%) |
| Race/Ethnicityb (n) | |||
| Caucasian | 513 | 512 | 1 |
| African-American | 144 | 144 | |
| Asian | 22 | 22 | -- |
| Indian | 13 | 13 | -- |
| Hawaiian | 2 | 2 | -- |
| Hispanic Ethnicity | 9 | 8 | 1 |
| Primary cause of kidney disease (n) | |||
| Diabetes | 225 | 183 | 42 |
| Glomerular disease | 136 | 124 | 12 |
| Hypertensive nephrosclerosis | 100 | 37 | 63 |
| Polycystic kidney disease | 85 | 76 | 9 |
| Tubular and interstitial disease | 19 | 17 | 2 |
| Other | 130 | 114 | 16 |
| Acute rejections in first 6 months | |||
| No. of subjects | 130 | 116 | 14 |
| Time to first rejection (days)b | 22.5 (7–176) | 22.5 (7–176) | 21.5 (9–130) |
|
| |||
| Tacrolimus Trough Concentrations, Doses and Concomitant Medications and Events | |||
|
| |||
| No. trough concentrations | 12,277 | 9,881 | 2,396 |
| Total daily dose (mg)c | 5.5 (4–8) | 5 (4–7) | 8 (5–10)d |
| Trough concentration (ng/mL)c | 7.9 (5.9–10.1) | 8.3 (6.4–10.4) | 6.2 (4.4–8.4)d |
| Trough dose-normalized (ng/ml per mg/kg/day)c | 116.5 (70.7–186.3) | 131.4 (83.0–201.8) | 68.3 (46.1–103.6)d |
| Dosing intervale (n) | |||
| Twice daily | 12,150 | 9,767 | 2,383 |
| Once daily | 112 | 100 | 12 |
| Three times daily | 10 | 10 | 0 |
| % of trough concentrations with ACE inhibitorf | 10.1% | 9.5% | 12.3% |
| % of trough concentrations with calcium channel blockersf | 43.0% | 41.0% | 51.2% |
| % of trough concentrations with corticosteroidsf | 52.7% | 55.5% | 41.2% |
| % of trough concentrations around an acute rejection episodeg | 4.6% | 5.0% | 2.7% |
Data are median (range).
Race and ethnicity are self-identified. Totals do not always match number of subjects, because people could identify as no race or as more than one race. Patients identified as multiracial were classified as African American (AA) if one of the races was AA.
Data are median (interquartile range) over the first 6 months.
p-value is <0.0001 for comparison of AA and non-AAs.
Number of trough concentrations for each dosing interval. The numbers of dosing intervals do not match the number of trough concentrations since dosing interval was not known in all individuals.
Concomitant drug was in use at the visit nearest in time to the trough measurement.
% of subjects where acute rejection episode occurred ±14 days of the trough measurement.
Figure 1.
Median Tacrolimus Trough Concentrations In the First 6 Months Posttransplant by Race
Figure 1 shows the differences in troughs between African Americans (AA) and non-AAs in the first 6 months posttransplant. The first trough in the AA group was a median (interquartile range) of 2.1 (1.2–3.5) ng/mL with a daily tacrolimus dose of 4.0 (4.0–5.0) mg/day. In contrast, the non-AA group achieved a higher first trough (5.0 [3.1–8.2] ng/mL) despite a similar daily dose (4.0 [4.0–6.0] mg/day). Although the first trough was low in both groups, more AAs had troughs <8 ng/mL (96.6%) than non-AA (73.9%)(p<0.0001). Over the 6 month study period, AAs received a 60% higher median daily dose (p<0.0001) but yet achieved median troughs that were 2.1 ng/ml lower (p<0.0001) than non-AA recipients (Table 1).
Clinical Factors Associated With Tacrolimus Trough Concentrations
Clinical factors (AA vs non-AA, enrolling center, recipient gender, age, donor type, diabetes at transplant, time of trough posttransplant, calcium channel blocker (CCB), ACE inhibitor and corticosteroid use at time of trough, creatinine clearance (CrCl) nearest trough and acute rejection±14 days of trough) were explored for their association with log transformed dose-normalized tacrolimus trough concentrations. Race(p<0.0001), center(p<0.0001), gender(p<0.0001), age(p<0.0001), diabetes(p=0.003), CCB(p=0.04) use and time of trough measurement(p<0.0001) were associated with troughs and ACE inhibitor use neared significance (p=0.07). AA race and the first three troughs measurements were each associated with lower trough concentrations whereas male gender, diabetes, and CCB use were associated with higher troughs. AA race was associated with a reduction in log transformed dose-normalized trough of 0.04 relative to non-AAs. Male gender, diabetes and CCB use each were associated with an increase in log transformed dose-normalized troughs of 0.17, 0.12 and 0.03, respectively. The effect of age was quadratic, with higher troughs between 45 and 75 years with a maximum at 60 years.
Single Variant Analysis For Association with Tacrolimus Trough Concentrations
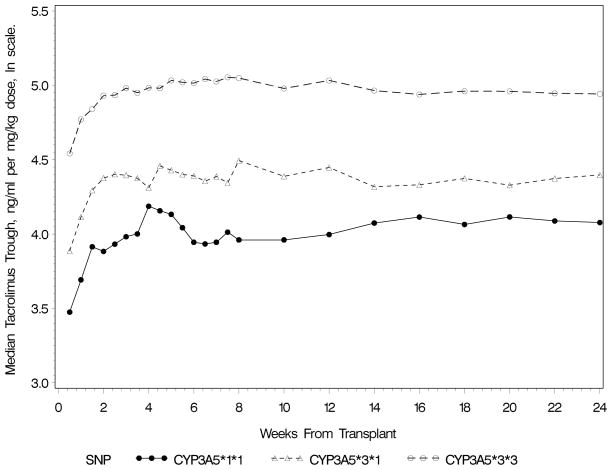
The CYP3A5 variant (rs776746, A=*1, G=*3) was the most important variant associated with troughs after adjustment for clinical factors. Dose requirements were higher and troughs lower in individuals with one or two A alleles. The presence of one A allele was associated with a 36% reduction in log transformed dose-normalized troughs (−0.44±0.03 [SE]) and a 59% reduction if two A alleles were present (p=2.4x10−33, Figure 2). Median (interquartile range) tacrolimus daily doses and troughs for the 3 genotype groups were as follows: 8(6–10) mg and 5.8(4.1–7.9) ng/mL for *1/*1, 7(5–10) mg and 7.1(5.1–9.3) ng/mL for *1/*3, and 4.5(3–6) mg and 8.4(6.5–10.5) ng/mL for the *3/*3 genotype. Dose-normalized troughs (ng/mL per mg/kg) were 54.3(37.9–80.3), 77.8(53.1–114.6) and 141.1(91.7–214.5) in those with CYP3A5*1/*1, *1/*3 and *3/*3 genotypes, respectively. The CYP3A5*1 allele was more frequent in AA (minor allele frequency (MAF)=0.65) than non-AA recipients (MAF=0.08). Fifty variants, besides CYP3A5*1, were significant towards dose-normalized troughs after controlling for the false discovery rate (FDR) at 20% and were primarily variants in CYP3A5, CYP3A7, CYP3A4 and CYP3A43 genes (variants not shown). These variants are in the same chromosomal region with linkage disequilibrium with rs776746; therefore, all further analyses were adjusted for rs776746.
Figure 2.
Median Log Transformed Dose-Normalized Tacrolimus Trough Concentrations by CYP3A5 Genotype Status (rs776746)
The top variants, after adjustment for rs776746 and the significant clinical factors, towards troughs are shown in Table A, Supplemental Digital Content 4, http://links.lww.com/TP/A325. No variants met significance after controlling the FDR at 20% although 39 variants achieved a p-value<0.01. The most frequently represented variants were from the CYP, COMT, MSH, ATF, XRCC5 and FMO genes. Variants within the CYP3A gene, CYP3A4 (rs7801671, rs12114000, rs2687117), CYP3A7 (rs2687080, rs776740) and CYP3A5 (rs10264272) were associated with 0.18 to 0.28 increase in log transformed dose-normalized troughs (20–32 % increase in dose-normalized trough). The CYP3A variants had higher MAF in the AA population (9–32%) than the non-AA (≤0.2%) and large effects on trough concentrations (0.18–0.28 for one allele). We previously described no association between variants and acute rejection, graft survival and survival.(37)
Multiple Variant Analysis For Association with Initial Tacrolimus Trough Concentrations
Multiple regression models for the first trough in week one were developed using the important clinical factors, CYP3A5 (rs776746) and the 39 variants from Table A (Supplemental Digital Content 4, http://links.lww.com/TP/A325). Final models were developed separately for all subjects, for non-AA and for AA (Table 2). The models explained 45.71%–52.54% of total variability. Center was important, explaining 19.19%–37.27% of variation. CYP3A5 was significant, accounting for 2–6% of variation. Six additional variants were significant in each of the AA and non-AA groups each explaining 2–6% of variation. CYP3A5 and COMT (rs2239393) variants overlapped between the groups. In AAs three variants (genes CYP3A4, CNAP1, FASTK) had large effect sizes (≥0.38). Diabetes was an important factor explaining ~2% of the variation. The were confirmed by bootstrapping and support their association with trough concentrations (see Appendix 1, Supplemental Digital Content 1, http://links.lww.com/TP/A322) although the AA model was relatively less stable compared to the full data.
Table 2.
Final Models for First Tacrolimus Trough Concentrationa
| Variableb | % Cumulative Contribution to Variation in Trough | Effect on Troughc (Estimate ± SE) | p-valuec |
|---|---|---|---|
| All subjectsd | |||
| Enrolling Center | 37.27 | <0.0001e | |
| rs776746 (CYP3A5) | 42.38 | −0.33 ± 0.05 | <0.0001 |
| Diabetes | 44.68 | 0.23 ± 0.05 | <0.0001 |
| Agef | 45.88 | 0.008 ± 0.002 | 0.0001 |
| rs2608555 (GAN) | 46.87 | 0.15 ± 0.04 | 0.0003 |
| rs2239393 (COMT) | 47.90 | −0.28 ± 0.08 | 0.0002 |
| rs2687117 (CYP3A4) | 48.86 | 0.26 ± 0.08 | 0.0019 |
| rs3734354 (SIM1) | 49.78 | 0.19 ± 0.06 | 0.0013 |
| rs3135506 (APOA5) | 50.62 | −0.23 ± 0.07 | 0.0009 |
| rs17567 (EPS15) | 51.01 | 0.10 ± 0.04 | 0.0141 |
| rs4646312 (COMT) | 51.42 | 0.22 ± 0.08 | 0.0075 |
| rs4926 (SERPING1) | 51.81 | −0.09 ± 0.04 | 0.0311 |
| rs2072374 (CNAP1) | 52.13 | −0.08 ± 0.04 | 0.0542 |
| rs3448 (GPX1) | 52.34 | 0.07 ± 0.04 | 0.0680 |
| rs13321 (TNC) | 52.54 | 0.06 ± 0.04 | 0.0823 |
| % total variation explained | 52.54 | ||
| Non-African American subjects only | |||
| Enrolling Center | 34.04 | <0.0001e | |
| Agef | 36.52 | 0.010 ± 0.002 | <0.0001 |
| rs776746 (CYP3A5) | 38.90 | −0.31 ± 0.07 | <0.0001 |
| Diabetes | 40.55 | 0.21 ± 0.06 | 0.0004 |
| rs2608555 (GAN) | 42.09 | 0.17 ± 0.04 | 0.0001 |
| rs3734354 (SIM1) | 43.74 | 0.20 ± 0.06 | 0.0008 |
| rs3135506 (APOA5) | 45.03 | −0.23 ± 0.08 | 0.0024 |
| rs4926 (SERPING1) | 45.96 | −0.12 ± 0.04 | 0.0053 |
| rs17567 (EPS15) | 46.78 | 0.12 ± 0.05 | 0.0122 |
| rs1800822 (FMO3) | 47.41 | 0.20 ± 0.09 | 0.0248 |
| rs2239393 (COMT) | 47.75 | −0.07 ± 0.04 | 0.0676 |
| % total variation explained | 47.75 | ||
| African American subjects only | |||
| Enrolling Center | 19.19 | <0.0001e | |
| rs776746 (CYP3A5) | 22.97 | −0.25 ± 0.08 | 0.0025 |
| rs2687117 (CYP3A4) | 29.41 | NAg | NAg |
| rs3136228 (MSH6) | 33.57 | −0.20 ± 0.08 | 0.0238 |
| rs2288648 (FASTK) | 35.62 | −0.41 ± 0.17 | 0.0210 |
| Diabetes | 37.95 | 0.45 ± 0.12 | 0.0003 |
| rs2072374 (CNAP1) | 40.09 | −0.45 ± 0.17 | 0.0110 |
| rs2239393 (COMT) | 41.74 | −0.19 ± 0.08 | 0.0246 |
| rs2280789 (CCL5) | 43.64 | 0.23 ± 0.10 | 0.0262 |
| rs12114000 (CYP3A4) | 45.63 | 0.38 ± 0.11 | 0.0008 |
| rs2687117 removed | 45.71 | NAg | NAg |
| % total variation explained | 45.71 | ||
Multiple linear regression models on the log transformed dose-normalized tacrolimus trough concentration at the first measurement posttransplant using stepwise variable selection.
Variables are listed in order of entry into the model.
Effect estimates on log transformed dose-normalized troughs, standard errors (SE) and p-values are for the final model.
Model is adjusted for race.
p-value for enrolling center is for the overall F-test.
Age is in years, mean-centered at 50.2 years.
variant rs2687117 was removed during variable selection, so it is not in the final model.
DISCUSSION
Tacrolimus is metabolized by CYP3A enzymes to form active and inactive metabolites.(11, 38–40) In vitro, the CYP3A5 enzyme has twice the intrinsic tacrolimus clearance of CYP3A4.(41) In hepatic enzymes from CYP3A5*1 allele carriers, 60% is metabolized through CYP3A5. Transplant recipients carrying CYP3A5*1 allele(s) have higher clearance, lower concentrations and delayed time to therapeutic concentrations.(18, 20, 22–23, 25–32) We confirmed CYP3A5*1 is an important variant and is associated with a reduction in troughs and explained only 2–6% of total variation in troughs. Other variants (including CYP3A4) explained equal or more of total variation (Table 2). This is consistent with previous data where variants each accounted for 3–9% of variability in tacrolimus clearance.(32) Variants in the COMT and CYP3A4 genes were important in our analysis. Catechol-O-methyltransferase (COMT) enzymes are responsible of O-methylation of dopamine, epinephrine and norepinephrine; and involvement in tacrolimus metabolism has not been described whereas variants in the CYP3A4 are relevant, particularly in AAs.(42) Additional variants (MSH, ATF, XRCC5 and FMO) are also potentially involved. Multigene involvement in may explain the findings of the prospective CYP3A5*1 guided tacrolimus dosing trial where genotype adapted dosing resulted in only 43.2% of subjects achieving the trough target (vs 29.1% control group, p=0.03)(43) Additional variants in the dosing model may improve genotype dosing. We found no association between the ABCB1 variants and troughs; consistent with published data.(28–29, 31)
The variants were different in the AA and non-AAs (Table 2) and were only identifiable when the races were analyzed separately. Several variants identified in the AAs had large effect sizes and may in part explain the differences in troughs between races. Warfarin pharmacogenetic studies have shown that clinical factors, effect sizes and pertinent genotypes differ by race.(44–45) We found that early troughs were low in both groups. The non-AA group quickly achieved troughs >8 ng/mL whereas the AAs median trough remained <8 ng/mL(Figure 1) despite 60% higher doses. Lower troughs and requirement for higher doses in AAs have been described.(38, 46–48) The low troughs may be due to high clearance due to CYP3A5*1 and/or other variants and inadequate starting dose; although, this does not sufficiently explain the persistently low troughs in AAs over the first 6 months. It is possible that the high degree of genetic diversity in the CYP3A or others, and the large effect size may result in longer periods of trial and error dosing. In a recent study, the intersubject pharmacokinetic variability in tacrolimus area under the curve was 41.1% higher in patients with CYP3A5*3/*3 compared to *1/*1 or *1/*3 suggesting that patients with variants are more difficult to manage.(49)
Center was an important factor towards troughs explaining 19–37% of the total variability. The effect of center is confounded by genotype (more AAs carry CYP3A5*1) and race (AAs were mostly from one center). Center effects are also possible given the heterogeneity in concomitant medications, timing of troughs, environment and diet. We found that diabetes at was important towards troughs with an effect as large as some variants. However, in a small study of 11 diabetics and 9 nondiabetics the rate of tacrolimus absorption was slowed in diabetics but the extent of absorption and troughs were unchanged.(50) Other factors influence tacrolimus pharmacokinetics including route of administration, hematocrit and hepatic function and should be evaluated in future studies.(8, 32, 51–52)
Drug interactions pose a dilemma in pharmacogenetic studies.(53–59) We found that the concomitant administration of CCBs, ACE inhibitors and corticosteroids were not significant towards troughs in the final models. Only class of concomitant medication was collected in our study. CCBs are not equipotent in their inhibitory activity towards tacrolimus and it is possible that the effect of CCBs with potent CYP3A inhibitory effects, such as diltiazem, are underestimated. The interaction between corticosteroids and tacrolimus is controversial.(53, 56, 58, 60–61) It is possible that high doses of corticosteroids may induce metabolism.(53) We did not collect corticosteroid dosing information and are unable test this possibility. We have no information on concomitant administration of antifungals which may be pertinent.(62–63)
To our knowledge, this is first pharmacogenetic study to explore a broad panel of variants (n=2722) and the largest group of AAs studied towards tacrolimus troughs. We confirmed that CYP3A5 is important and identified additional variants with effects as large or larger than CYP3A5. Trough concentrations are lower in AAs compared to non-AA subjects and therefore require higher tacrolimus doses. Variants important towards troughs differed between the AA and non-AA subjects. This study provides the data necessary to now develop dosing equations that use genotype and relevant clinical variables instead of weight to determine initial doses. These variants can now be validated in independent groups.
MATERIALS AND METHODS
Study Design
This is a 7-center, prospective, observational study in 695 subjects to identify novel variants associated with tacrolimus troughs using a customized SNP chip. Transplant recipients were recruited from the first 1000 subjects of the prospective arm of the Long-term Deterioration of Kidney Allograft Function (DeKAF) study, which is designed to characterize the causes of late allograft failure.(64–66) The study is registered at www.clinicaltrials.gov (NCT00270712). Transplant recipients were eligible if they were undergoing kidney or simultaneous kidney-pancreas transplantation, ≥18 years of age and to receive tacrolimus. Subjects were enrolled at time of transplant and signed informed consents approved by the Institutional Review Boards.
Tacrolimus trough concentrations, measured prior to an oral dose, were obtained as part of clinical care for the first 6 months posttransplant. Two measurements, if available, were obtained in each of weeks 1–8 and in each of months 3, 4, 5 and 6 for a maximum of 24 measurements per patient. Tacrolimus doses were adjusted to achieve individual institutional trough targets but in general were 8–12 ng/mL in months 0–3 and 6–10 ng/mL in months 4–6. Troughs were normalized for dose by taking the ratio of the trough concentration (ng/ml) by the total daily tacrolimus dose (mg/kg). Dose in mg/kg was used since this is the common clinical method for dose determination. Tacrolimus concentrations (n=12,277) were measured from whole blood by each institutions preferred analytical technique. Liquid chromatography-mass spectroscopy was used to measure 97.1% concentrations.
All participants received a tacrolimus based immunosuppressive regimen with mycophenolate, steroids, with and without antibody induction per transplant center preference. Donor and recipient characteristics, race, serum creatinine (SCr), and estimated CrCl(67), concomitant medications at time of each trough, acute rejection as diagnosed by the treating physician were obtained from the medical record.
Genotyping
Pretransplant recipient DNA was isolated from lymphocytes obtained from blood after RBC lysis. Genotyping was done using a customized Affymetrix GeneChip (Affymetrix, Santa Clara, CA).(68–69) Additional variants were genotyped using SNPlex (Applied Biosystems, Foster City, CA) and Sequenom (Sequenom, San Diego, CA). The variants were within genes in pathways associated with immunity, cell cycle, signaling, growth, proliferation, differentiation, movement, structure and death, inflammation, hematologic systems, and ~700 variants related to drug absorption, disposition, metabolism and excretion. Validated, functional polymorphisms including non-synonymous variants with a MAF >5%, and variants within conserved (in humans and mouse) transcriptional regulatory regions were chosen. In the absence of functional variants, intragenic tagging variants were used. Genotyping is described in (see Appendix 3, Supplemental Digital Content 3, http://links.lww.com/TP/A324).
Data quality was assessed by negative controls and duplicate samples (3% on Affymetrix, 7% SNPlex, and 1% Sequenom). On Affymetrix, duplicate samples from 31 individuals were genotyped with >99% concordance. Variants with concordance <90% and calls <60% were excluded. Twenty variants were run on multiple platforms and had a concordance rate of >97% and with calls >82%. The Hardy-Weinberg equilibrium assumption was tested by χ2 analysis and variants that deviated (p-value < 1x10−6) were removed from the analysis. Variants were excluded from analysis if the MAF was <5% in both the AA and non-AAs. The final analysis included 2,552 variants from Affymetrix, 165 from SNPlex and 7 from Sequenom (see Appendix 2, Supplemental Digital Content 2, http://links.lww.com/TP/A323).
Association Testing of Variants Towards Clinical Factors and Trough Concentrations
The importance of clinical factors towards log transformed dose-normalized troughs over the first 6 months posttransplant was evaluated, with no variants, using a general linear model with time trough obtained posttransplant (1 to 24) as a categorical variable and a covariance structure to account for correlation between repeated measurements on the same person. Factors explored were recipient race (AA vs. non-AA), age, gender, donor type (living or deceased), diabetes at transplant and center. We explored time varying covariates such as CCBs, ACE inhibitor and corticosteroid use at time of trough, acute rejection episode±14 days of the trough and CrCl nearest the trough. A number of model covariance structures (autoregressive, Toeplitz and compound symmetry) were considered. The structure which best fit the data, by the Akaike information criterion, in a model with all of the potential covariates listed above, was the Toeplitz covariance structure, which allows the correlation to decline slowly and non-uniformly over time. This structure was used for all models. Factors with F-test p<0.15 in backward selection were retained.
Each variant was first tested for association with the log transformed dose-normalized troughs obtained over the 6 months using a general linear model with time posttransplant (1 to 24) as a categorical variable and a Toeplitz covariance structure, using an additive genetic model. The analyses were adjusted for center and the clinical factors identified above. For variants where the MAF was >5% in non-AA and AAs, all subjects were used to estimate the variant effect. For variants where the MAF in one race was <5%, potentially resulting in unreliable estimates, the effect was estimated using the other race where the MAF was >5%. Since the subgroup analyses have somewhat less power than the analysis in all subjects, this procedure improves reliability at the cost of underestimating the significance of those variants. Rs776746 was the top variant and all further analyses were adjusted for it. This study aimed to identify novel variants and multiple testing was taken into account by controlling the FDR at 20% which is common for these type of studies. P-values for variant association were ordered in increasing order and denoted by P(1), …, P(m). They were considered significant if below a FDR of 20%, i.e., P(k)< 0.2k/m. We used an effective number of variants m= 2110, which was computed based on linkage disequilibrium between all variants.(70)
Pharmacogenetic testing is most useful at the beginning of therapy and the effect of variants and clinical factors on the first log transformed dose-normalized trough (within the first 4 days posttransplant) was evaluated through multiple variant analysis using linear regression models. The significant factors identified initially (center, rs776746 and variants in Table A, Supplemental Digital Content 4, http://links.lww.com/TP/A325) were considered for entry into the model. Stepwise selection was used with the criterion for entry set at p-value <0.15 and removal at >0.10. The reliability of the final regression models were confirmed by bootstrap resampling (see Appendix 1, Supplemental Digital Content 1, http://links.lww.com/TP/A322).
Troughs and daily doses were compared between AA and non-AAs using nonparametric Wilcoxon-Mann-Whitney. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
Supplementary Material
Apppendix 1. Detailed genotyping methods
Appendix 2. Single Nucleotide Polymorphisms Analyzed
Analyzed variants with reference sequence numbers and minor allele frequencies.
Appendix 3. Model Assessment
Table A. Top Variants in Single Variant Analysis Associated with Log Transformed Dose-Normalized Tacrolimus Trough Concentrations After Adjusting for CYP3A5*1 (rs776746) and Clinical Factors
Acknowledgments
We acknowledge the dedication and hard work of our coordinators: University of Alberta, Nicoleta Bobocea, Tina Wong, Adrian Geambasu and Alyssa Sader; University of Manitoba, Myrna Ross and Kathy Peters; University of Minnesota, Mandi DeGrote and Jill Nagorski; Hennepin County Medical Center, Lisa Berndt; Mayo Clinic, Tom DeLeeuw; University of Iowa, Wendy Wallace and Tammy Lowe; University of Alabama, Catherine Barker. We also acknowledge the dedicated work of our research scientists: Marcia Brott, Becky Willaert, Jennifer Vigliaturo and Winston Wildebush.
The project was supported by grant number (5U19-AI070119) from the National Institute of Allergy and Infectious Disease. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Disease or the National Institutes of Health.
Abbreviations
- FDR
false discovery rate – statistical method to correct for multiple comparisons. For a FDR of 20%, as used in this paper, we would expect no more than 20% false positives among the variants that are declared as significant
DeKAF Investigators
Arthur Matas, M.D.
Department of Surgery
University of Minnesota, Minneapolis, MN 55455
Email: matas001@umn.edu
J. Michael Cecka, M.D.
UCLA Immunogenetics Center, Los Angeles, CA 90095
Email: mcecka@ucla.edu
John Connett, Ph.D.
Division of Biostatistics. University of Minnesota, Minneapolis, MN 55455
Email: john-c@biostat.umn.edu
Fernando G. Cosio, M.D.
Division of Nephrology, Mayo Clinic, Rochester, MN 55905
Email: Cosio.Fernando@mayo.edu
Robert Gaston, M.D.
Division of Nephrology, University of Alabama, Division of Nephrology, Birmingham, AL 35294
Email: rgaston@uab.edu
Sita Gourishankar M.D.
Division of Nephrology and Immunology, University of Alberta, Edmonton, Alberta, Canada
Email: sitag@ualberta.ca
Joseph P. Grande, M.D., Ph.D.
Mayo Clinic College of Medicin, Rochester MN 55905
Email: Grande.Joseph@mayo.edu
Phillip Halloran, M.D.
Division of Nephrology and Immunology, University of Alberta, Edmonton, Alberta, Canada
Email: phil.halloran@ualberta.ca
Lawrence Hunsicker, M.D.
Nephrology Division, Iowa City, IA 52242-1082
Email: lawrence-hunsicker@uiowa.edu
Bertram Kasiske, M.D.
Division of Nephrology, Hennepin County Medical Center, Minneapolis, MN 55415
Email: kasis001@umn.edu
David Rush, M.D.
Health Sciences Center, Winnipeg MB, Canada
Email: drush@exchange.hsc.mb.ca
Participating Centers
Participating transplant centers were University of Alberta, Edmonton, Canada; University of Manitoba, Winnipeg, Canada; University of Minnesota, Minneapolis, MN; Hennepin County Medical Center, Minneapolis, MN; Mayo Clinic, Rochester, MN; University of Iowa, Iowa City, IA; and University of Alabama, Birmingham, AL.
Contributor Information
Pamala A. Jacobson, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN.
William S. Oetting, Department of Experimental and Clinical Pharmacology and Institute of Human Genetics, University of Minnesota, Minneapolis, MN.
Ann M Brearley, Clinical and Translational Science Institute, University of Minnesota, Minneapolis, MN.
Robert Leduc, Division of Biostatistics, University of Minnesota, Minneapolis, MN.
Weihau Guan, Division of Biostatistics, University of Minnesota, Minneapolis, MN.
David Schladt, Division of Biostatistics, University of Minnesota, Minneapolis, MN.
Arthur J. Matas, Department of Surgery, University of Minnesota, Minneapolis, MN.
Vishal Lamba, Department of Genetics, Cell Biology and Development, University of Minnesota, Minneapolis, MN.
Bruce A. Julian, Division of Nephrology, University of Alabama at Birmingham, Birmingham, AL.
Rosalyn B. Mannon, Division of Nephrology, University of Alabama at Birmingham, Birmingham, AL.
Ajay Israni, Department of Medicine, Hennepin County Medical Center, Minneapolis, MN.
References
- 1.Anglicheau D, Legendre C, Beaune P, Thervet E. Cytochrome P450 3A polymorphisms and immunosuppressive drugs: an update. Pharmacogenomics. 2007;8:835. doi: 10.2217/14622416.8.7.835. [DOI] [PubMed] [Google Scholar]
- 2.Coto E, Tavira B. Pharmacogenetics of calcineurin inhibitors in renal transplantation. Transplantation. 2009;88:S62. doi: 10.1097/TP.0b013e3181afe9e7. [DOI] [PubMed] [Google Scholar]
- 3.Hesselink DA, van Gelder T, van Schaik RH. The pharmacogenetics of calcineurin inhibitors: one step closer toward individualized immunosuppression? Pharmacogenomics. 2005;6:323. doi: 10.1517/14622416.6.4.323. [DOI] [PubMed] [Google Scholar]
- 4.Wavamunno MD, Chapman JR. Individualization of immunosuppression: concepts and rationale. Curr Opin Organ Transplant. 2008;13:604. doi: 10.1097/MOT.0b013e3283193bc5. [DOI] [PubMed] [Google Scholar]
- 5.Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006;112:184. doi: 10.1016/j.pharmthera.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Vicari-Christensen M, Repper S, Basile S, Young D. Tacrolimus: review of pharmacokinetics, pharmacodynamics, and pharmacogenetics to facilitate practitioners’ understanding and offer strategies for educating patients and promoting adherence. Prog Transplant. 2009;19:277. doi: 10.1177/152692480901900315. [DOI] [PubMed] [Google Scholar]
- 7.Bowman LJ, Brennan DC. The role of tacrolimus in renal transplantation. Expert Opin Pharmacother. 2008;9:635. doi: 10.1517/14656566.9.4.635. [DOI] [PubMed] [Google Scholar]
- 8.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43:623. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki K. Metabolism of tacrolimus (FK506) and recent topics in clinical pharmacokinetics. Drug Metab Pharmacokinet. 2007;22:328. doi: 10.2133/dmpk.22.328. [DOI] [PubMed] [Google Scholar]
- 10.Christians U, Strom T, Zhang YL, et al. Active drug transport of immunosuppressants: new insights for pharmacokinetics and pharmacodynamics. Ther Drug Monit. 2006;28:39. doi: 10.1097/01.ftd.0000183385.27394.e7. [DOI] [PubMed] [Google Scholar]
- 11.Shiraga T, Matsuda H, Nagase K, et al. Metabolism of FK506, a potent immunosuppressive agent, by cytochrome P450 3A enzymes in rat, dog and human liver microsomes. Biochem Pharmacol. 1994;47:727. doi: 10.1016/0006-2952(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 12.Saeki T, Ueda K, Tanigawara Y, Hori R, Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993;268:6077. [PubMed] [Google Scholar]
- 13.Undre NA, van Hooff J, Christiaans M, et al. Low systemic exposure to tacrolimus correlates with acute rejection. Transplant Proc. 1999;31:296. doi: 10.1016/s0041-1345(98)01633-9. [DOI] [PubMed] [Google Scholar]
- 14.Borobia AM, Romero I, Jimenez C, et al. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther Drug Monit. 2009;31:436. doi: 10.1097/FTD.0b013e3181a8f02a. [DOI] [PubMed] [Google Scholar]
- 15.Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;31:139. doi: 10.1097/FTD.0b013e318198d092. [DOI] [PubMed] [Google Scholar]
- 16.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clin Pharmacokinet. 2010;49:207. doi: 10.2165/11317550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. 2010;49:141. doi: 10.2165/11317350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Jun KR, Lee W, Jang MS, et al. Tacrolimus concentrations in relation to CYP3A and ABCB1 polymorphisms among solid organ transplant recipients in Korea. Transplantation. 2009;87:1225. doi: 10.1097/TP.0b013e31819f117e. [DOI] [PubMed] [Google Scholar]
- 19.Hesselink DA, van Schaik RH, van Agteren M, et al. CYP3A5 genotype is not associated with a higher risk of acute rejection in tacrolimus-treated renal transplant recipients. Pharmacogenet Genomics. 2008;18:339. doi: 10.1097/FPC.0b013e3282f75f88. [DOI] [PubMed] [Google Scholar]
- 20.Macphee IA, Fredericks S, Mohamed M, et al. Tacrolimus pharmacogenetics: the CYP3A5*1 allele predicts low dose-normalized tacrolimus blood concentrations in whites and South Asians. Transplantation. 2005;79:499. doi: 10.1097/01.tp.0000151766.73249.12. [DOI] [PubMed] [Google Scholar]
- 21.Hesselink DA, van Schaik RH, van der Heiden IP, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74:245. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 22.Renders L, Frisman M, Ufer M, et al. CYP3A5 genotype markedly influences the pharmacokinetics of tacrolimus and sirolimus in kidney transplant recipients. Clin Pharmacol Ther. 2007;81:228. doi: 10.1038/sj.clpt.6100039. [DOI] [PubMed] [Google Scholar]
- 23.Haufroid V, Wallemacq P, VanKerckhove V, et al. CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: guidelines from an experimental study. Am J Transplant. 2006;6:2706. doi: 10.1111/j.1600-6143.2006.01518.x. [DOI] [PubMed] [Google Scholar]
- 24.Op den Buijsch RA, Christiaans MH, Stolk LM, et al. Tacrolimus pharmacokinetics and pharmacogenetics: influence of adenosine triphosphate-binding cassette B1 (ABCB1) and cytochrome (CYP) 3A polymorphisms. Fundam Clin Pharmacol. 2007;21:427. doi: 10.1111/j.1472-8206.2007.00504.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya N, Satoh S, Tada H, et al. Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation. 2004;78:1182. doi: 10.1097/01.tp.0000137789.58694.b4. [DOI] [PubMed] [Google Scholar]
- 26.MacPhee IA, Fredericks S, Tai T, et al. The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. Am J Transplant. 2004;4:914. doi: 10.1111/j.1600-6143.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- 27.Thervet E, Anglicheau D, King B, et al. Impact of cytochrome p450 3A5 genetic polymorphism on tacrolimus doses and concentration-to-dose ratio in renal transplant recipients. Transplantation. 2003;76:1233. doi: 10.1097/01.TP.0000090753.99170.89. [DOI] [PubMed] [Google Scholar]
- 28.Kuypers DR, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin Pharmacol Ther. 2007;82:711. doi: 10.1038/sj.clpt.6100216. [DOI] [PubMed] [Google Scholar]
- 29.Bandur S, Petrasek J, Hribova P, Novotna E, Brabcova I, Viklicky O. Haplotypic structure of ABCB1/MDR1 gene modifies the risk of the acute allograft rejection in renal transplant recipients. Transplantation. 2008;86:1206. doi: 10.1097/TP.0b013e318187c4d1. [DOI] [PubMed] [Google Scholar]
- 30.Roy JN, Barama A, Poirier C, Vinet B, Roger M. Cyp3A4, Cyp3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genomics. 2006;16:659. doi: 10.1097/01.fpc.0000220571.20961.dd. [DOI] [PubMed] [Google Scholar]
- 31.Haufroid V, Mourad M, Van Kerckhove V, et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14:147. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Press RR, Ploeger BA, den Hartigh J, et al. Explaining variability in tacrolimus pharmacokinetics to optimize early exposure in adult kidney transplant recipients. Ther Drug Monit. 2009;31:187. doi: 10.1097/FTD.0b013e31819c3d6d. [DOI] [PubMed] [Google Scholar]
- 33.Anglicheau D, Verstuyft C, Laurent-Puig P, et al. Association of the multidrug resistance-1 gene single-nucleotide polymorphisms with the tacrolimus dose requirements in renal transplant recipients. J Am Soc Nephrol. 2003;14:1889. doi: 10.1097/01.asn.0000073901.94759.36. [DOI] [PubMed] [Google Scholar]
- 34.Macphee IA, Fredericks S, Tai T, et al. Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation. 2002;74:1486. doi: 10.1097/00007890-200212150-00002. [DOI] [PubMed] [Google Scholar]
- 35.Mourad M, Mourad G, Wallemacq P, et al. Sirolimus and tacrolimus trough concentrations and dose requirements after kidney transplantation in relation to CYP3A5 and MDR1 polymorphisms and steroids. Transplantation. 2005;80:977. doi: 10.1097/01.tp.0000174131.47469.d2. [DOI] [PubMed] [Google Scholar]
- 36.Fredericks S, Moreton M, Reboux S, et al. Multidrug resistance gene-1 (MDR-1) haplotypes have a minor influence on tacrolimus dose requirements. Transplantation. 2006;82:705. doi: 10.1097/01.tp.0000234942.78716.c0. [DOI] [PubMed] [Google Scholar]
- 37.Oetting W, Schladt D, Israni I, Jacobson P, Leduc R, Matas A. Association of Single Nucleotide Polymorphisms and Acute Rejection. Am J Transplant. 2010;10(Supl 4):389. [Google Scholar]
- 38.Mancinelli LM, Frassetto L, Floren LC, et al. The pharmacokinetics and metabolic disposition of tacrolimus: a comparison across ethnic groups. Clin Pharmacol Ther. 2001;69:24. doi: 10.1067/mcp.2001.113183. [DOI] [PubMed] [Google Scholar]
- 39.Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P-450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab Dispos. 1992;20:753. [PubMed] [Google Scholar]
- 40.Lecointre K, Furlan V, Taburet AM. In vitro effects of tacrolimus on human cytochrome P450. Fundam Clin Pharmacol. 2002;16:455. doi: 10.1046/j.1472-8206.2002.00114.x. [DOI] [PubMed] [Google Scholar]
- 41.Dai Y, Hebert MF, Isoherranen N, et al. Effect of CYP3A5 polymorphism on tacrolimus metabolic clearance in vitro. Drug Metab Dispos. 2006;34:836. doi: 10.1124/dmd.105.008680. [DOI] [PubMed] [Google Scholar]
- 42.Perera MA, Thirumaran RK, Cox NJ, et al. Prediction of CYP3A4 enzyme activity using haplotype tag SNPs in African Americans. Pharmacogenomics J. 2009;9:49. doi: 10.1038/tpj.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thervet E, Loriot MA, Barbier S, et al. Optimization of Initial Tacrolimus Dose Using Pharmacogenetic Testing. Clin Pharmacol Ther. 2010 doi: 10.1038/clpt.2010.17. [DOI] [PubMed] [Google Scholar]
- 44.Cavallari LH, Langaee TY, Momary KM, et al. Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin Pharmacol Ther. 2010;87:459. doi: 10.1038/clpt.2009.223. [DOI] [PubMed] [Google Scholar]
- 45.Voora D, Koboldt DC, King CR, et al. A polymorphism in the VKORC1 regulator calumenin predicts higher warfarin dose requirements in African Americans. Clin Pharmacol Ther. 2010;87:445. doi: 10.1038/clpt.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dirks NL, Huth B, Yates CR, Meibohm B. Pharmacokinetics of immunosuppressants: a perspective on ethnic differences. Int J Clin Pharmacol Ther. 2004;42:701. doi: 10.5414/cpp42701. [DOI] [PubMed] [Google Scholar]
- 47.Neylan JF. Racial differences in renal transplantation after immunosuppression with tacrolimus versus cyclosporine. FK506 Kidney Transplant Study Group. Transplantation. 1998;65:515. doi: 10.1097/00007890-199802270-00011. [DOI] [PubMed] [Google Scholar]
- 48.Neylan JF. Effect of race and immunosuppression in renal transplantation: three-year survival results from a US multicenter, randomized trial. FK506 Kidney Transplant Study Group. Transplant Proc. 1998;30:1355. doi: 10.1016/s0041-1345(98)00274-7. [DOI] [PubMed] [Google Scholar]
- 49.Yong Chung J, Jung Lee Y, Bok Jang S, Ahyoung Lim L, Soo Park M, Hwan Kim K. CYP3A5*3 genotype associated with intrasubject pharmacokinetic variation toward tacrolimus in bioequivalence study. Ther Drug Monit. 2010;32:67. doi: 10.1097/FTD.0b013e3181c49a4c. [DOI] [PubMed] [Google Scholar]
- 50.Mendonza AE, Zahir H, Gohh RY, Akhlaghi F. Tacrolimus in diabetic kidney transplant recipients: pharmacokinetics and application of a limited sampling strategy. Ther Drug Monit. 2007;29:391. doi: 10.1097/FTD.0b013e31811f319b. [DOI] [PubMed] [Google Scholar]
- 51.Kuypers DR, Vanrenterghem Y. Time to reach tacrolimus maximum blood concentration,mean residence time, and acute renal allograft rejection: an open-label, prospective, pharmacokinetic study in adult recipients. Clin Ther. 2004;26:1834. doi: 10.1016/j.clinthera.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Staatz CE, Willis C, Taylor PJ, Tett SE. Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin Pharmacol Ther. 2002;72:660. doi: 10.1067/mcp.2002.129304. [DOI] [PubMed] [Google Scholar]
- 53.Anglicheau D, Flamant M, Schlageter MH, et al. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18:2409. doi: 10.1093/ndt/gfg381. [DOI] [PubMed] [Google Scholar]
- 54.Park SI, Felipe CR, Pinheiro-Machado PG, et al. Tacrolimus pharmacokinetic drug interactions: effect of prednisone, mycophenolic acid or sirolimus. Fundam Clin Pharmacol. 2009;23:137. doi: 10.1111/j.1472-8206.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 55.Kuypers DR. Influence of interactions between immunosuppressive drugs on therapeutic drug monitoring. Ann Transplant. 2008;13:11. [PubMed] [Google Scholar]
- 56.Lam S, Partovi N, Ting LS, Ensom MH. Corticosteroid interactions with cyclosporine, tacrolimus, mycophenolate, and sirolimus: fact or fiction? Ann Pharmacother. 2008;42:1037. doi: 10.1345/aph.1k628. [DOI] [PubMed] [Google Scholar]
- 57.Jones TE, Morris RG. Pharmacokinetic interaction between tacrolimus and diltiazem: dose-response relationship in kidney and liver transplant recipients. Clin Pharmacokinet. 2002;41:381. doi: 10.2165/00003088-200241050-00005. [DOI] [PubMed] [Google Scholar]
- 58.Christians U, Jacobsen W, Benet LZ, Lampen A. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet. 2002;41:813. doi: 10.2165/00003088-200241110-00003. [DOI] [PubMed] [Google Scholar]
- 59.van Gelder T. Drug interactions with tacrolimus. Drug Saf. 2002;25:707. doi: 10.2165/00002018-200225100-00003. [DOI] [PubMed] [Google Scholar]
- 60.Kim JS, Aviles DH, Silverstein DM, Leblanc PL, Matti Vehaskari V. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatr Transplant. 2005;9:162. doi: 10.1111/j.1399-3046.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 61.Hesselink DA, Ngyuen H, Wabbijn M, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids. Br J Clin Pharmacol. 2003;56:327. doi: 10.1046/j.0306-5251.2003.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chandel N, Aggarwal PK, Minz M, Sakhuja V, Kohli KK, Jha V. CYP3A5*1/*3 genotype influences the blood concentration of tacrolimus in response to metabolic inhibition by ketoconazole. Pharmacogenet Genomics. 2009;19:458. doi: 10.1097/FPC.0b013e32832bd085. [DOI] [PubMed] [Google Scholar]
- 63.Kuypers DR, de Jonge H, Naesens M, Vanrenterghem Y. Effects of CYP3A5 and MDR1 single nucleotide polymorphisms on drug interactions between tacrolimus and fluconazole in renal allograft recipients. Pharmacogenet Genomics. 2008;18:861. doi: 10.1097/FPC.0b013e328307c26e. [DOI] [PubMed] [Google Scholar]
- 64.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for Antibody-Mediated Injury as a Major Determinant of Late Kidney Allograft Failure. Transplantation. 2010 doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 65.Gaston RS, Kasiske BL, Fieberg AM, et al. Use of cardioprotective medications in kidney transplant recipients. Am J Transplant. 2009;9:1811. doi: 10.1111/j.1600-6143.2009.02696.x. [DOI] [PubMed] [Google Scholar]
- 66.Gourishankar S, Leduc R, Connett J, et al. Pathological and clinical characterization of the ‘troubled transplant’: data from the DeKAF study. Am J Transplant. 2010;10:324. doi: 10.1111/j.1600-6143.2009.02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 68.Van Ness B, Ramos C, Haznadar M, et al. Genomic variation in myeloma: design, content, and initial application of the Bank On A Cure SNP Panel to detect associations with progression-free survival. BMC Med. 2008;6:26. doi: 10.1186/1741-7015-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hardenbol P, Baner J, Jain M, et al. Multiplexed genotyping with sequence-tagged molecular inversion probes. Nat Biotechnol. 2003;21:673. doi: 10.1038/nbt821. [DOI] [PubMed] [Google Scholar]
- 70.Galwey NW. A new measure of the effective number of tests, a practical tool for comparing families of non-independent significance tests. Genet Epidemiol. 2009;33:559. doi: 10.1002/gepi.20408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apppendix 1. Detailed genotyping methods
Appendix 2. Single Nucleotide Polymorphisms Analyzed
Analyzed variants with reference sequence numbers and minor allele frequencies.
Appendix 3. Model Assessment
Table A. Top Variants in Single Variant Analysis Associated with Log Transformed Dose-Normalized Tacrolimus Trough Concentrations After Adjusting for CYP3A5*1 (rs776746) and Clinical Factors