Abstract
Plasmacytoid dendritic cells (pDC) are rare cells found in peripheral blood and lymphoid tissues. pDC are considered to be “professional” type I interferon (IFN) producing cells and produce 10–100-fold more IFN-α than other cell types in response to enveloped viruses or synthetic TLR-7 and -9 agonists. In this study, purified pDC were found to express high levels of IFN-λ receptor mRNA as well as cell-surface IFN-λ receptor. We have developed intracellular flow cytometry assays using antibodies to IFN-λ1/3 or -λ2 to assess the expression of IFN-λ proteins by pDC. We observed that a subset of human pDC expresses only intracellular IFN-α while another subset produces both IFN-α and IFN-λ after stimulation with virus or the TLR9 agonist, CpGA; the cells that co-expressed IFN-α and IFN-λ were the cells with the highest levels of IFN-α expression. Antibody cross-linking of CD4 or BDCA-2 molecules on pDC inhibited both HSV-induced IFN-λ and IFN-α production. Like the production of IFN-α, the HSV-induced IFN-λ production in pDC was mediated through TLR9 and independent of virus replication. Exogenous IFN-λ treatment of pDC resulted in increased virus-induced expression of both IFN-α and IFN-λ. In addition, both exogenous IFN-λ and –α inhibited dexamethasone-induced apoptosis of pDC. We conclude that pDC are major producers of IFN-λ1 and –λ2 in response to viral stimulation and also express functional receptors for this cytokine. Thus, IFN-λ can serve as an autocrine signal to strengthen the antiviral response of pDC by increasing IFN-α and IFN-λ production, resulting in prolonged pDC survival.
Keywords: Human, dendritic cells, cytokines, cytokine receptors, viral infection
Introduction
The interferons (IFNs) are classified into three types : Type I, Type II and Type III. Type I IFN comprises IFN-α, β, κ, δ, ε, π, ω, and ξ. A common receptor, the IFN-α/β receptor, mediates cell interactions of all Type I IFNs. The IFN-α/β receptor comprises two subunits, IFNAR-1 and IFNAR-2, which form a heterodimer upon type I IFN stimulation (1, 2) leading to the expression of IFN response genes (3). Type I IFNs play a critical role in cellular antiviral and antiproliferative responses (4, 5). Type I IFNs are also important in regulating innate as well as adaptive immunity (6). In contrast to the multiple Type I IFNs, Type II interferon comprises a single cytokine, IFN-γ, which is essential to cell-mediated immunity by activating macrophages and promoting the development of CD4+ Th1 and cytotoxic CD8+ T cells (7). IFN-γ has a specific receptor, a heterodimer of IFNGR-1 and IFNGR-2 subunits (8). A limited number of cell types can produce IFN-γ, including CD4+ Th1 cells, cytotoxic CD8+ T cells, and natural killer cells.
A third interferon class, type III, has been recently described and includes three members: λ1, λ2, and λ3, also known as IL-29, IL-28A, IL-28B, respectively (9, 10). Genes for all three type III IFNs are clustered on human chromosome 19. The type III IFNs bind to a receptor complex that includes a unique ligand-binding subunit, IFN-λR1 (also known as IL-28RA) and an accessory chain, IL-10R2, that is also utilized in signaling of several IL-10-related cytokines (9, 10). Although the type III IFNs are genetically and structurally most closely related to the cytokines in the IL-10 family, functionally they behave more like members of the type I IFN family than like members of the IL-10 family. Like IFN-α, IFN-λ has both antiviral and anti-proliferative activity (11–13). These functional similarities result from a common signaling pathway that is activated by both the type I and type III IFNs: signaling through either the IFN-α or IFN-γ receptor results in the formation of the ISGF3 complex (comprising STAT-1/STAT-2 and IRF9) to activate what appears to be a common set of genes (10, 12–18). Although the signaling pathways for the type I and type III IFNs are similar, the expression of the IFN-λ receptors is reported to be much more limited than the ubiquitously expressed IFN-α receptors. Sommereyns et al. demonstrated that epithelial cells are the primary target of IFN-λ in vivo, whereas a much broader range of cell types are targets of IFN-α activation (19). In a murine system, Paludan and colleagues reported that in additional to epithelial cells, IFN-λ can also target plasmacytoid dendritic cells (pDC) and that these cell types contribute to TLR-induced antiviral activity (20). In other studies, macrophages, MDDC and intrahepatic NK cells have been reported to respond to IFN-λ activation (21–23).
A number of different cell types have been reported to produce IFN-λ in response to viral infection and/or maturation stimuli including monocytes, monocyte-derived dendritic cells (MDDC) (24–26) and plasmacytoid dendritic cells (pDC) (26). Sommereyns et al. recently reported that the expression of IFN-λ is more restricted than that of IFN-α on a tissue basis, with the brain readily expressing IFN-α but not IFN-λ following infection with RNA viruses (19). Although many cell types can produce IFN-α in response to viral infection, plasmacytoid dendritic cells (pDC) are considered to be “professional” type I interferon-producing cells that can produce 1–2 IU (3–10 pg)/cell of IFN-α in response to enveloped virus (27), which is 10 to 100-fold more than that produced by monocytes (28). The pDC are rare cells in peripheral blood, accounting for only about 0.2–0.5% of peripheral blood mononuclear cells (PBMC) (29). In addition to their function as IFN-α producing cells, pDC have been recognized for their roles in innate and adaptive immunity such as production of chemokines to regulate cell trafficking (30), facilitation of B cell antibody production (31), providing help for NK cells (32) and regulation of Th1 and Th2 responses (6, 33, 34). Given the importance of pDC in the immune response, the current study was undertaken to further understand the potential role of type III IFNs as both products of and modulators of human pDC. In these studies, we found that a subset of pDC co-express IFN-λ along with IFN-α after stimulation with a broad range of DNA and RNA viruses including herpes simplex virus (HSV), influenza virus (Flu), Sendai virus (SeV) and HIV-1. We also found that IFN-λ can enhance function and improve survival of pDC.
Materials and Methods
Viruses and cell lines
Herpes simplex virus-1 strain 2931 (HSV) was originally obtained from Dr. C. Lopez, then at the Sloan-Kettering Institute (New York, NY). The HSV stocks were expanded in VERO cells (American Type Culture Collection, Manassas, VA) and titered by a plaque assay on VERO cells. For some experiments, sucrose-gradient enriched HSV-1 was used (35). Titers of standard and sucrose gradient purified HSV were 2 × 107 and 4 × 108 PFU/ml, respectively. Influenza virus A strain PR8 (Flu) was kindly provided by Dr. T. Moran of the Mount Sinai Medical Center, New York, NY. Sendai virus strain Sendai/Cantell (SeV) was purchased from Charles River Laboratory (SPAFAS, N. Franklin, CT). AT-2 inactivated human immunodeficiency virus-1 (HIV-1), strains MN and ADA, were kindly provided by Dr. Jeff Lifson, (SAIC-Frederick, MD). The viruses were stored at −70°C until use. UV inactivated HSV (UV-HSV) was prepared by irradiating 100 µl virus in a 40×10 mm plate with a UV Stratalinker 1800 (Stratagene, La Jolla, CA) with a total of 7.8 × 105 µJ. Mycoplasma-free cell lines and virus preparations were routinely confirmed using a kit from Stratagene.
Reagents
PE-anti-CD123 (6H6), Biotin-anti-CD3 (HIT3a), PE-anti-CD4 (RPA-T4), APC-anti-CD8 (HIT8a), PE-anti-CD56 (HCD56), PerCP/Cy5.5-anti-CD83 (HB15e), APC-Cy7-anti-CD40 (5C3) and FITC-anti-HLA-ABC antibodies (clone W6/32) were purchased from BioLegend (San Diego, CA); FITC-lineage cocktail (lin1), Alexa Fluor® 488-anti-Stat1(pY701, clone 4a), FITC- rabbit anti-active caspase-3 monoclonal antibody (clone C92-605), PE-anti-CD16 (clone 3G8) and FITC-anti-CD83 (clone HB15e) antibodies were purchased from BD Pharmingen (San Diego, CA); APC-anti-BDCA-2 (clone AC114), PE-anti-BDCA-3 (AD514H12) and PE-anti-CD14 (clone TUK4) were purchased from Miltenyi Biotec (Auburn, CA). Unconjugated mouse-anti-human IFN-α monoclonal antibody clone MMHA-2 was purchased from PBL InterferonSource (Piscataway, NJ) and mouse-anti-human IFN-λ1/3 (clone # 247801, which has a 20% cross-reactivity with IFN-λ2) and IFN-λ2 (clone # 248526, and which does not react with IFN-λ1) monoclonal antibodies were purchased from R&D Systems, Minneapolis, MN. The anti-IFN-α and –λ1/3 were biotinylated with biotin N-hydroxysuccinimide ester according to the manufacturer’s protocol (Sigma). The anti-IFN-λ2 antibody, and for some experiments, the anti-IFN-λ1/3 antibody were labeled using an Alexa-fluor 488-anti-mouse Zenon labeling kit (Invitrogen, San Diego, CA). For some experiments, PE- or FITC-labeled anti-IFN-α (Miltenyi Biotec) was used. Mouse neutralizing monoclonal antibody against human IFN-α/β receptor chain 2 (clone MMHAR-2) was purchased from PBL InterferonSource. The anti-mouse-FITC secondary antibody was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA).
Recombinant IFN-α2b and IFN-λ1 were obtained from Schering-Plough (Kenilworth, NJ) and Peprotech Inc. (Rocky Hill, NJ), respectively. The TLR 9 agonist CpG A (ODN 2663) (sequence: 5’ G*G*GGACGACGTCGTGG*G*G*G*G*G 3’) was purchased from Coley Pharmaceutical Group Inc (Wellesley, MA). The TLR 9 agonist CpG B (ODN 2006) (sequence: 5’ T*C*G*T*C*G*T*T*T*T*G*T*C*G*T*T*T*T*G*T*C*G*T*T 3’) and the TLR 9 inhibitory (ODN 2008) (sequence: 5’T*C*C*T*G*G*C*G*G*G*G*A*A*G*T 3’) were synthesized at the Molecular Resource Facility, UMDNJ-New Jersey Medical School, Newark, NJ. The asterisks in the above three sequences indicate a phosphorothioate bond. The TLR7 agonist, imiquimod, was obtained from 3M (Minneapolis, MN). Dexamethasone was purchased from Sigma.
Preparation of the anti-IFN-λR1 mAb
Monoclonal antibodies against the extracellular domain of human IL-28RA were generated at ZymoGenetics by repeatedly immunizing BALB/c mice (Charles River Laboratories, Wilmington, MA) with IFN-λR1-Fc (ZymoGenetics, Seattle,) in combination with the Ribi Adjuvant System (Sigma-Aldrich, Saint Louis, MI). Animal sera was assessed for the presence of anti- IFN-λR1 antibodies after three to four immunizations by whole cell ELISA assay with the use of parental HEK293 cells and HEK293 cells overexpressing IFN-λR1. The ability of antibody to function in flow cytometry assays was determined using the above-mentioned HEK293 cell lines as well as SupB15, U266, HepG2 cell lines endogenously expressing IFN-λR1. The sera were also screened by ELISA for the lack of cross-reactivity against human IL-20RA, IL -22RA1 and IL-31RA (ZymoGenetics, Seattle, WA). Spenocytes from the mouse with the highest titer of IL-28RA antibodies were used to generate hybridoma clones producing high titers of antibodies specifically recognizing human IL-28RA as determined by the above-mentioned assays. In this study, mAb from clonal hybridoma E10891 cell line was used and its specificity was confirmed in flow cytometry with the use of CHO-derived cells overexpressing modified human IFN-λ (IL-28/29) receptor complex (10) vs. non-transfected CHO cells (shown in Fig 6B).
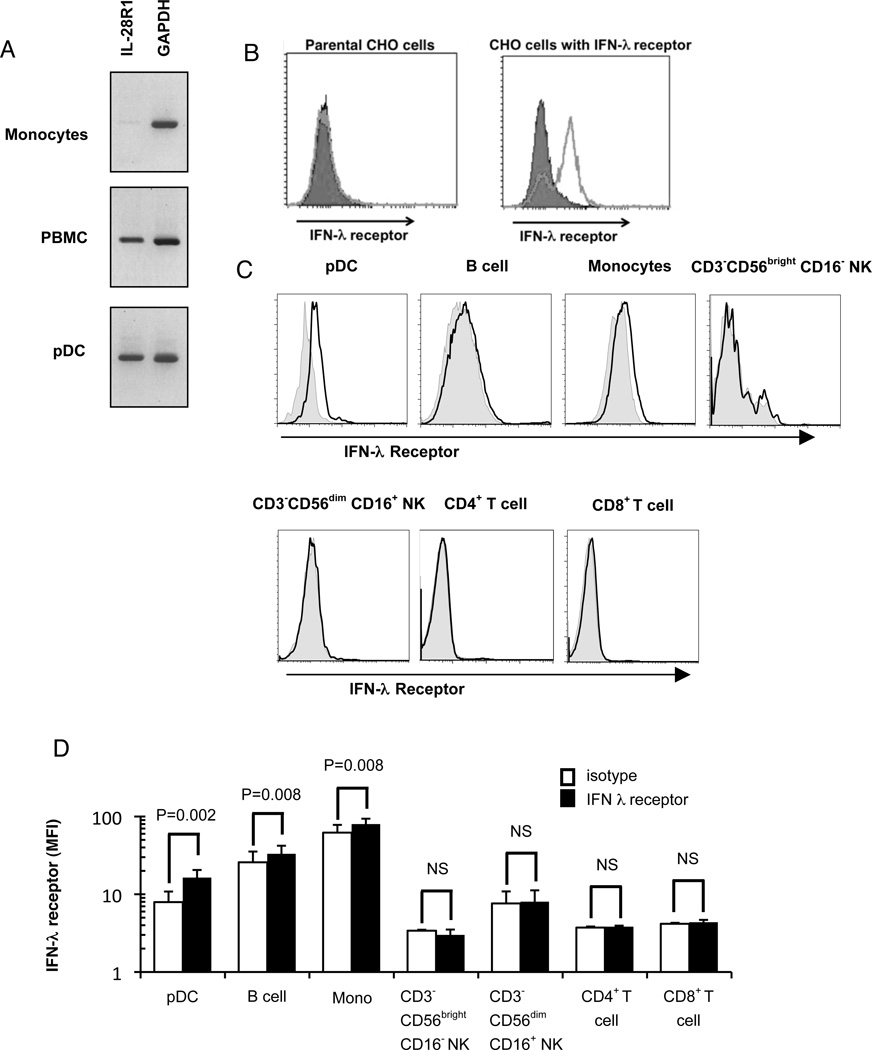
Figure 6. PDC express the IFN-λ receptor.
A) Total RNA was extracted from monocytes, PBMC, and purified pDC. An RT-PCR kit was used to determine the expression of IL-28R1 (IFN-λR1), with GAPDH as control gene. B) Parental CHO cells and CHO cells transfected with the IFN-λ receptor were examined for the expression of IFN-λR1. Closed symbols: isotype; open symbols IFN-λR1; C) PDC, B cells, monocytes, CD3−CD56brightCD16− NK cells, CD3−CD56dimCD16+ NK cells, CD4+ T cells, and CD8+ T cells in PBMC were labeled with specific markers (pDC: BDCA-2+/CD123+, monocytes: CD14+,;B cells: CD19+, CD3−CD56brightCD16− NK cells: CD3−/CD56bright/CD16−; CD3−CD56dimCD16+ NK cells: CD3−/CD56dim/CD16+; CD4+ T cells: CD3+/CD4+; CD8+ T cells: CD3+/CD8+). The expression of IFN-λR1 was examined by flow cytometry. Representative histograms are shown for isotype controls (filled histograms and IFN-λR1 (open histograms). D) The mean fluorescence intensity (MFI) of IFN-λR1 of pDC, monocytes, CD3−CD56brightCD16− NK cells, CD3−CD56dimCD16+ NK cells, CD4+ T cells, and CD8+ T cells was compared with that of isotype. The results represent 4 experiments for pDC and B cells and 3 experiments for monocytes, CD3−CD56brightCD16− NK cells, CD3−CD56dimCD16+ NK cells, CD4+ T cells, and CD8+ T cells. (NS indicates not significant.)
Preparation of Peripheral Blood Mononuclear Cells (PBMC)
PBMC were isolated by Ficoll-Hypaque (Mediatech, Inc, Herndon, VA) density centrifugation from fresh, heparinized blood obtained from healthy adult volunteers. The Institutional Review Board of the New Jersey Medical School approved the human studies protocol and informed consent documentation. The PBMC were resuspended in RPMI 1640 medium (Mediatech Inc, Herndon, VA) containing 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (Gemini Bio-Products Inc., West Sacramento, CA) and 25 mM HEPES, and counted with a Coulter Particle Counter Z1 (Beckman Coulter).
Preparation of enriched pDC and monocytes
“Untouched” pDC were purified from prepared PBMC with the Human Plasmacytoid Dendritic Cell Isolation Kit (Miltenyi Biotec Inc., Auburn, CA) according to the manufacturer’s instructions. The purity of enriched pDC was determined by flow cytometry (CD123 and BDCA-2 double positive), and was routinely 92–99%; enrichment preparations below 92% were not used. The viability of enriched pDC was always >95% by trypan blue exclusion. Monocytes were selected by positive selection of CD14+ cells using the Miltenyi monocyte isolation kit; purities of ≥95% were routinely obtained.
RT-PCR evaluation of mRNA level of IFN-λ and IFN-λ receptor
Total RNA was extracted from highly-enriched pDC and PBMC separately with RNeasy Mini kits (QIAGEN, Valencia, CA). The purity and concentration of total RNA were measured with NanoDrop ND-1000 UV-Vis spectrophotometer and ND-1000 V3.3.1 software (Thermo Scientific, Wilmington, DE). The nucleotide sequences of the primers used to measure expression of the human IFN-λ1 (IL29) and IFN-λ2 (IL28A) genes were as follows:
IFN-λ1 (IL29) sense: 5’-CGCCTTGGAAGAGTCACTCA-3’
IFN-λ1 (IL29) antisense: 5’-GAAGCCTCAGGTCCCAATTC-3’
IFN-λ2 (IL28A) sense: 5’-AGTTCCGGGCCTGTATCCAG-3’
IFN-λ2 (IL28A) antisense: 5’-GAGCCGGTACAGCCAATGGT-3’
The mRNA levels of IFN-λ1 and -λ2 were determined by quantitative real-time PCR with SYBR green I (Qiagen) using the designed primer sets (12); data were normalized to β-actin. IFN-λ receptor gene expression (IFN-λR1, IL28RA) was measured by standard RT-PCR and agarose gel electrophoresis. The nucleotide sequences of the primers used to measure expression of the human IFN-λR1 (IL28RA) gene were as follows:
IFN-λR1 (IL28RA) sense: 5’-TTTCAGCGGGCAAAGATG-3’
IFN-λR1 (IL28RA) antisense: 5’-GGGACAGAGGAACAAGTCATTC-3’
The RT-PCR products were resolved by electrophoresis on 2% agarose gels, stained with ethidium bromide and visualized with UV light.
Stimulation of PBMC and pDC with viruses for induction of IFNs
Cells suspensions at 2×106 /ml, were incubated with viruses at a multiplicity of infection (MOI) of 1 for HSV and flu, with 500 hemagglutination units (HAU)/ml Sendai virus or 500 ng/ml p24 equivalents of AT-2 inactivated HIV-1 (strain MN or ADA) in 1ml (for PBMC) or 100 µl volumes (for pDC). Incubation of the cells continued for 7h at 37°C in a 5% CO2 atmosphere. To prevent protein secretion, at 4h post-stimulation, Brefeldin A (Sigma-Aldrich Corp., St. Louis, MO) was added to a final concentration 5µg/ml. At 7h post-stimulation, cells were collected and washed with 0.1% BSA (Sigma-Aldrich Corp., St. Louis, MO) in PBS, labeled with anti-CD123-PE and anti-BDCA-2-APC (BD) and fixed overnight with 1% paraformaldehyde in PBS. The fixed cells were washed with 2% FCS in PBS, permeabilized with 0.5% Saponin (Sigma-Aldrich Corp., St. Louis, MO) in 2% FCS-PBS (permeabilization buffer) and labeled for 30 minutes with mAbs to anti-IFN-λ1/3, anti-IFN-λ2, or anti-IFN-α. The cells were washed with the permeabilization buffer and incubated for 30 minutes with streptavidin-cy7. The cells were washed three times and fixed again with 1% paraformaldehyde. Data were acquired using a BD FACSCalibur or LSRII, and analyzed using Cellquest (BD) or FloJo (Treestar) software.
ELISAs for IFN-λ1 and IFN–α
The supernatants from enriched pDC were collected after treatment with viruses or TLR9 (CpG A and CpG B) or TLR7 (3M003) agonists. The concentrations of secreted IFN-λ1/3 and IFN-α were measured by ELISA in accordance with the manufacturer’s protocols (R&D Systems Inc., Minneapolis, MN for IFN- λ1/3 (DuoSet DY1598B), and Bender MedSystems, Burlingame, CA for IFN-α). The IFN-λ 1/3 ELISA assay is reported by the manufacturer to have no cross-reactivity with IFN-α or –β and 36% cross-reactivity with IFN-λ2; in our hands, we obtained a 26% cross-reactivity of the ELISA with IFN-λ2.
Antibody cross-linking of BDCA-2 or CD4 on pDC
Cross-linking of BDCA-2 and CD4 on pDC was carried out as previously described (34). Briefly, PBMC (2×106 cells/sample) were washed with MACS buffer (PBS with 0.5% BSA and 2 mM EDTA), incubated for 20 mins at 4°C with mouse anti-human BDCA-2 Ab (Miltenyi, clone AC114, 1µg/sample) or CD4 MicroBeads (Miltenyi, M-T466, 20 µl/sample) and washed again with MACS buffer. For BDCA-2 cross-linking, the washed cells were supplemented with rat anti-mouse IgG1 MicroBeads (Miltenyi, 1µg/sample), incubated for 15 min at 4°C and washed again with MACS buffer.
Treatment of cells with dexamethasone
Partially-enriched pDC (30% purity, 1 × 106 cell/ml) were pretreated with or without dexamethasone (DEX) (1uM) for 1 hour, and then treated with HSV (MOI: 1), AT-2 inactivated HIV-1 (500 ng/ml), or highly-purified HSV-GFP (MOI: 1) for another 18 hours. The amount of IFN-λ in supernatants was measured by ELISA as described above. For measurement of apoptosis in DEX-treated pDC, PBMC were treated with DEX in the presence or absence of recombinant IFN-α (10,000 IU/ml) or IFN-λ (10ng/ml) for 6 hr. Cells were stained for pDC markers and intracellularly for active caspase-3 expression. In separate experiments, annexin V binding of DEX-treated pDC in the presence or absence of IFN-α or IFN-λ was determined using an Annexin V binding kit from BD Pharmingen as described by the manufacturer.
Statistical analysis
The statistical significance of differences between samples was determined by ANOVA with Tukey’s post-hoc test. P values <0.05 were considered significant.
Results
pDC produce IFN-λ in response to viral stimulation
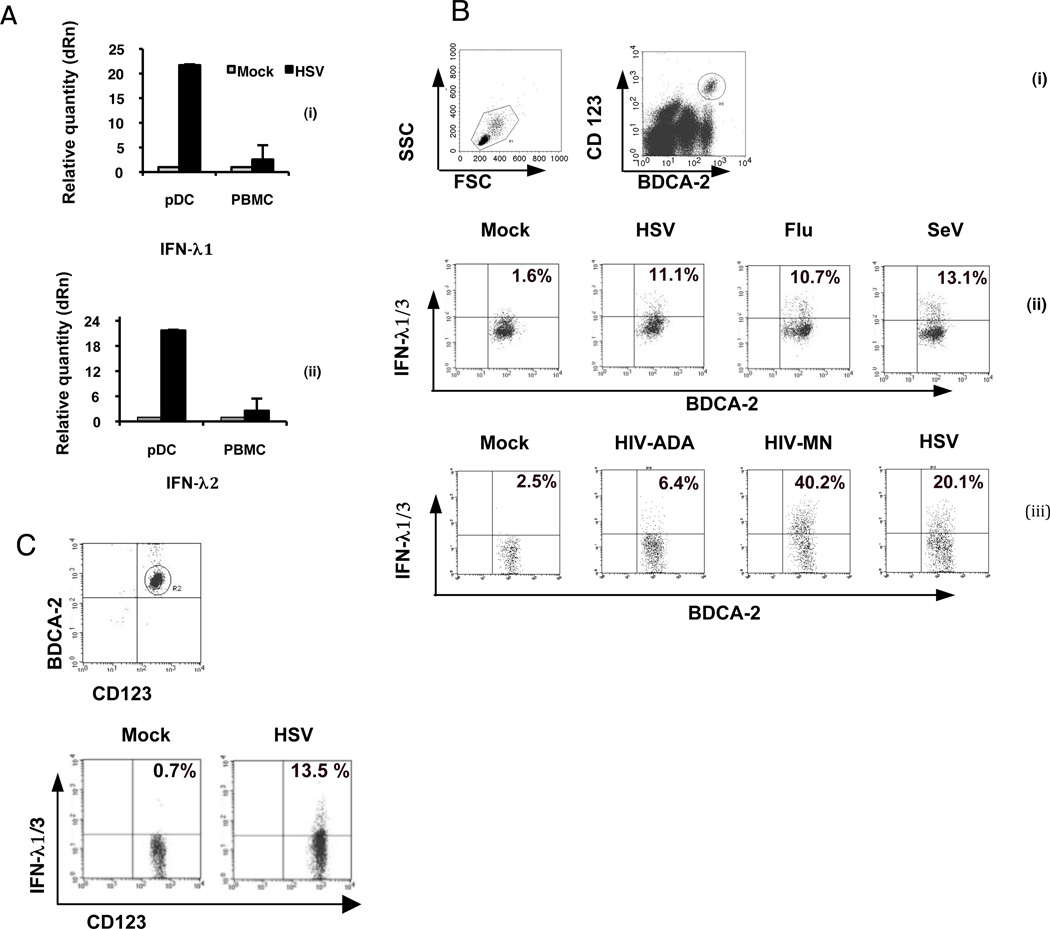
In order to determine whether pDC express IFN-λ in response to stimulation with HSV-1, which is a strong inducer of IFN-α in pDC, PBMC and purified pDC were cultured for 7 hr with HSV-1 or were unstimulated and IFN-λ transcript levels in stimulated and unstimulated pDC were determined using real time RT-PCR. HSV induced a 10–20-fold increase in both IFN-λ1 and IFN-λ2 transcripts in pDC, compared with the unstimulated controls (Figure 1A). In contrast, PBMC demonstrated only very modest upregulation of IFN-λ1 and IFN-λ2 mRNA in response to HSV stimulation, strongly implicating pDC as the source of IFN-λ. A flow cytometric assay was developed to assess the expression of IFN-λ protein by pDC in PBMC, by using cell surface staining of pDC and antibody to IFN-λ1/3 (the available monoclonal antibody detects both IFN-λ1 and –λ3) or -λ2. The expression of IFN-λ1/3 protein by pDC in PBMC was induced by a number of DNA and RNA viruses, including HSV, Flu, SeV and AT-2-inactivated HIV-1MN and HIVADA (Fig. 1B). To determine whether the induction of IFN-λ in pDC is directly due to HSV stimulation and not indirect effects of other cells present in PBMC and/or cytokines produced by these cells, enriched pDC were isolated by negative selection and then stimulated with HSV. Purified pDC produced IFN-λ1/3 in response to HSV stimulation (Figure 1C), thus demonstrating a direct stimulation of IFN-λ production by HSV-1 in pDC. It has been reported that human BDCA-3+ mDC2 produce IFN-λ in response to polyI:polyC (36). Therefore, to address the contribution of these cells to the HSV-induced IFN-λ1/3, we examined the ability of lineage negative, BDCA3+/BDCA2− (mDC2) vs. lineage negative, BDCA3−/BDCA2+ (pDC), within the same population of DC, to produce IFN-λ1/3 in response to 8-hr stimulation with HSV. By intracellular flow for IFN-λ1/3, we found that 14.2 ± 2.8 (mean ± SEM for three separate donors) BDCA2+/BDCA3− cells expressed IFN-λ, while for BDCA2−/BDCA3+ cells in the same populations, only 2.6 ± 0.9 were IFN-λ (p<0.001); mock-stimulated cultures (without virus) for these two populations were 1.8 ± 1.3 and 0.95 ± 0.4 % positive, respectively; thus, in response to HSV-1, the BDCA2+ pDC were the major producers of IFN-λ.
Figure 1. pDC produce IFN-λ in response to viruses and CpGA.
A) Induction of IFN- λ1 and IFN- λ2 mRNA in PBMC and purified pDC. Cells were treated with HSV (MOI=1) for 7 hr. The mRNA levels of IFN-λ1 (i) and IFN-λ2 (ii) were determined by real-time RT-PCR. Data represent mean ± 1 SD (n=3 separate experiments). B) Virus-induced production of IFN-λ1 by pDC in PBMC suspensions. pDC were distinguished from other PBMC cell types by simultaneous detection of BDCA-2 and CD123 markers (i) gating for pDC; PBMC were stimulated with (ii) HSV, Flu, or SeV or, (iii) AT-2 inactivated HIVADA or HIVMN for 7 hr, then stained for intracellular IFN-λ and intracellular expression of IFN-λ1/3 was detected by flow cytometry in gated pDC populations. Data are representative of at least 3 separate experiments. C) HSV-induced IFN-λl production by purified pDC. (i) pDC were negatively enriched using magnetic bead sorting and (ii) expression of intracellular IFN-λ1 in the purified pDC was detected by flow cytometry following 7 hr stimulation with HSV-1. Data are representative of 3 experiments.
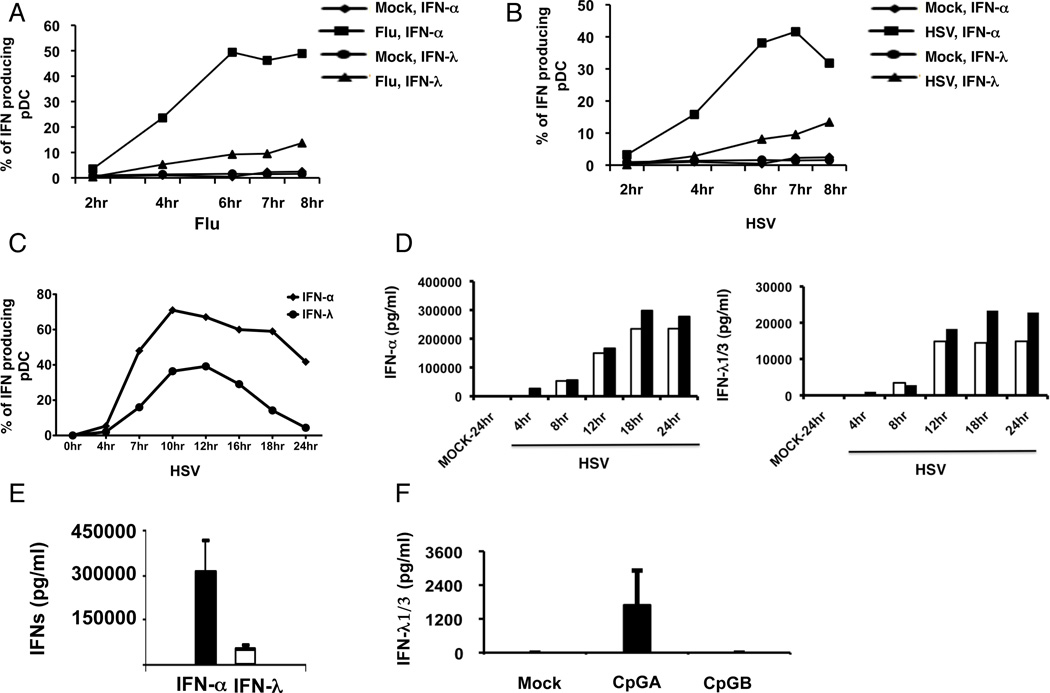
To determine the kinetics of IFN-λ1/3 protein production in pDC, we compared the intracellular expression of IFN-α and IFN-λ1/3 in pDC after stimulation with HSV and Flu for different time periods. The expression of intracellular IFN-α in pDC was first detected at 2hr and reached its peak by 6hr while IFN-λ appeared slightly delayed (Fig. 2A,B). In an additional two experiments, we addressed intracellular IFN-λ1/3 in HSV-stimulated pDC over a longer time course, with similar results (a representative figure is shown in Fig. 2C). In order to determine the quantity of IFN-λ1/3 secreted by purified pDC after stimulation with HSV, the supernatants from purified pDC:virus co-cultures collected at different time points were assayed by IFN-λ and IFN-α ELISA. In addition to detecting IFN-λ1 and -3, in agreement with the manufacturer, we found that the antibodies in the ELISA kit used also partially cross-reacted with recombinant IFN-λ2 (average cross-reactivity was 26%). Purified pDC routinely produced much more IFN-α than -λ in response to HSV-1, but with similar kinetics (Figure 2D); total IFN-λ production measured at 18 hr was approximately 10-fold lower than that of IFN-α. Similar to what is reported for induction of IFN-α (37), CpGA but not CpGB induced the expression of IFN-λ in pDC, albeit to a much lower extent than that induced by HSV-1 (Figure 2F).
Figure 2. Kinetics of IFN-α and –λ expression in purified pDC.
PBMCs were stimulated with Influenza virus (panel A) or HSV (panel B) for different time periods, and then stained for pDC markers and intracellular expression of IFN-α and IFN-λ expression. Data are representative of 2 separate experiments. C) PBMC were stimulated with HSV-1 for varying times, with BFA added two hours before each time point. Data are representative of two separate donors. D) Kinetics of expression of IFN-α (left panel) and IFN-λ (right panel). Enriched pDC were stimulated with HSV-1 (without the addition at BFA) and supernatants were collected at the indicated time points and tested by ELISA for IFN-α and IFN-λ. Data for two separate donors are shown. E) Summary of IFN-α and –λ in supernatants of purified pDC stimulated with HSV for 18 hr. Data are mean ± 1 SD for 4 separate donors. F) ELISA-determined concentration of IFN-λ in the supernatants of purified pDC after treatment with CpGA or CpGB for 18 hr. Data are mean ± 1 SD for 4 separate donors.
A subset of pDC that produces IFN-α also produces IFN-λ
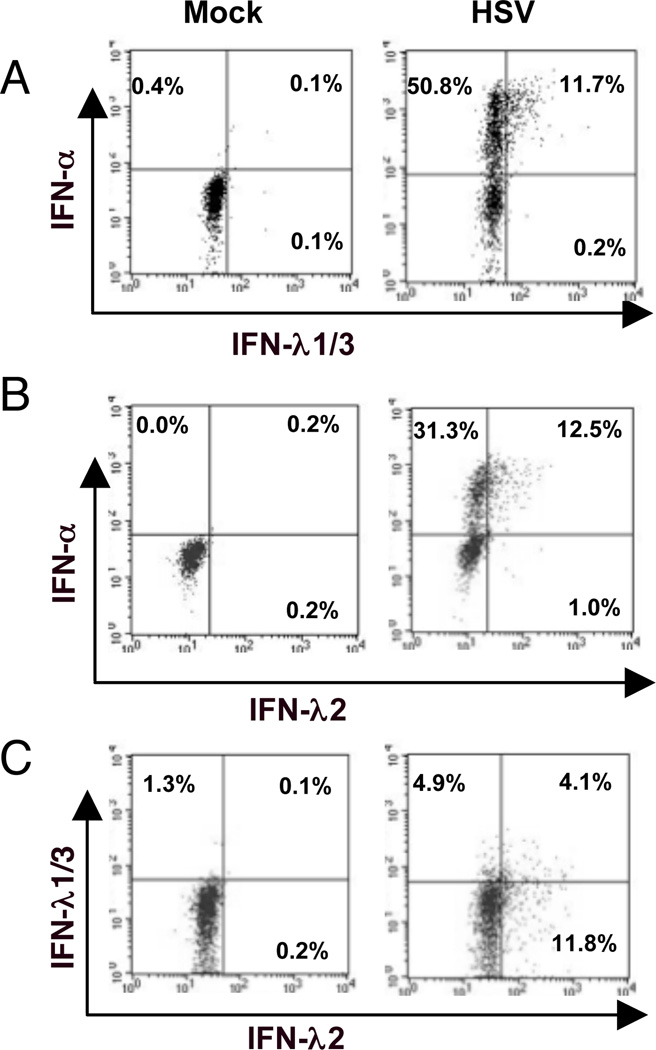
We next compared the expression of IFN-α, IFN-λ1/3 and IFN-λ2 in pDC preparations and characterized the IFN-λ producing pDC. An intracellular flow cytometry assay was adapted to stain for IFN-α in addition to either IFN-λ1/3 or IFN-λ2 in HSV-stimulated pDC. In response to HSV stimulation, only pDC that produced high levels of IFN-α also produced IFN-λ1/3 or IFN-λ2 (Figure 3 A,B). Some pDC produced only IFN-λ1/3, some produced only IFN-λ2, while others produced both IFN-λ1/3 and IFN-λ2 (Figure 3 C). Both IFN-λ/IFN-α double-positive and IFN-α single-positive pDC upregulated activation and maturation markers PDL-1, CD83 and CD40 to a similar extent in response to stimulation with HSV-1 (Supplemental Figure 1.)
Figure 3. A subset of IFN-α+ pDC co-expresses IFN-λ.
A, B) Co-expression of IFN-α and –λ: After stimulation of PBMC with HSV for 7hr, pDC were stained and the cells were permeabilized and stained for the co-expression of IFN-α and IFN-λ1/3 (Panel A), and for IFN-α and IFN-λ2 (panel B) and for IFN-λ1/3 and IFN-λ2 (panel C). Data are representative of three separate experiments for each panel.
IFN-λ1/3 expression by pDC results directly from activation with virus and is independent of IFN-α
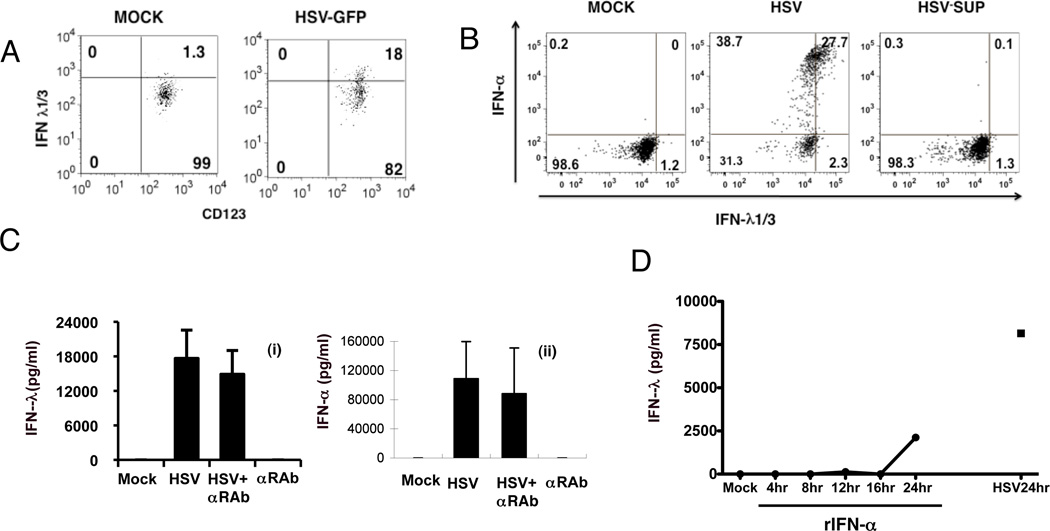
Ank et al. reported that IFN-λ message is expressed in HepG2 cells in response to both IFN-α and IFN-λ (12). Moreover, VERO cells, where we produce our HSV-1 preparations, can produce IFN-λ but not IFN-α in response to virus stimulation (38). Indeed, our VERO sonicate HSV-1 preparation was found to have 560 pg/ml IFN-λ, which means that we were carrying over a final concentration of 16 pg/ml of IFN-λ1/3 along with the HSV into our PBMC and pDC stimulations. To address the possibility of carried-over IFN-λ being responsible for induction of IFN-λ in our cultures, we utilized a sucrose-gradient purified HSV (which also expressed GFP, which was not relevant to the experiment) to stimulate pDC; this virus, which yielded <0.5 pg/IFN-λ/ml added to the cultures, efficiently induced IFN-λ production (Figure 4A). Moreover, supernatants of our standard virus subjected to ultrafugation did not stimulate IFN-λ while the pelleted virus did (Figure 4B).
Figure 4. Production of IFN-λ by pDC is not dependent upon IFN-α or –λ.
A) PBMC were stimulated with purified HSV-GFP with final stimulation conditions containing <5 pg/ml of IFN-λ, then stained for IFN-λ expression in pDC as described in Figure 2. Data re representative of two similar experiments. B) HSV-1 was pelleted in an ultracentrifuge then PBMC were stimulated with pelleted virus (MOI of 1) or supernatant from the centrifuged virus for 7 hr and intracellular expression of IFN-α and IFN-λ1/3 were determined as described in Figure 3. C) Purified pDC were incubated with HSV for 14 hr with or without a 30 min pre-treatment with IFN-α receptor neutralizating antibody (αRAb, 12.5µg/ml). The amount of IFN-λ (i) and IFN-α (ii) in supernatants was measured by ELISA, with mock and IFN-α receptor neutralization antibody (12.5µg/ml) treatment alone as controls. Data are mean ± 1 SD (n=3). D) Purified pDC (98–99% pure) were treated with 10,000 IU rIFN-α for the times shown; as a control, pDC were stimulated for 24 with HSV-1 or without HSV (MOCK). Supernatants were collected and the levels of IFN-λ were determined by ELISA. Data are representative of two similar experiments.
In some cell types, IFN-α treatment is known to enhance or “prime” IFN-α via a positive feedback pathway through the IFN-α/β receptor (39). Therefore, we asked whether virus induced IFN-λ production by pDC also depends on endogenous IFN-α that is secreted by pDC in response to virus stimulation. To address the potential role for IFN-α produced in response to HSV-1 leading to IFN-λ production in a feed-back loop, we used blocking receptors to the IFN-α/β receptor; there was no decrease in IFN-λ or –α producing cells under these conditions (Figure 4C). Finally, although our results clearly indicate that intracellular IFN-λ observed at 7 hr of stimulation was clearly independent of endogenous and exogenous IFNs, we were interested in knowing whether exogenous IFN-α by itself is sufficient to induce IFN-λ in pDC, as we have previously shown for IFN-α induction of CXCL10 by pDC (30). Purified pDC (98.2% and 99% pure for two separate donors) were stimulated with 10,000 IU of recombinant IFN-α2 that mimics the maximum levels produced by virus-stimulated pDC in PBMC and supernatants were collected and IFN-λ levels determined by ELISA. IFN-λ was undetectable in the supernatants at all time points except for the 24 hr time point, when low levels were detected relative to the levels induced by HSV (Figure 4D). Likewise, we did not detect intracellular IFN-λ in pDC treated for 7 hr with rIFN-α at concentrations ranging from 100 to 2 × 104 IU/ml (data not shown), a time at which IFN-λ is distinctly expressed by virus-stimulated pDC. Together, these results suggest that the virus induced IFN-λ production of pDC results from the direct stimulation of virus instead of an indirect action of IFN-λ.
HSV-induced IFN-λ expression in pDC is inhibited by CD4 and CD303 (BDCA-2) cross-linking, is TLR9-mediated, and independent of virus replication
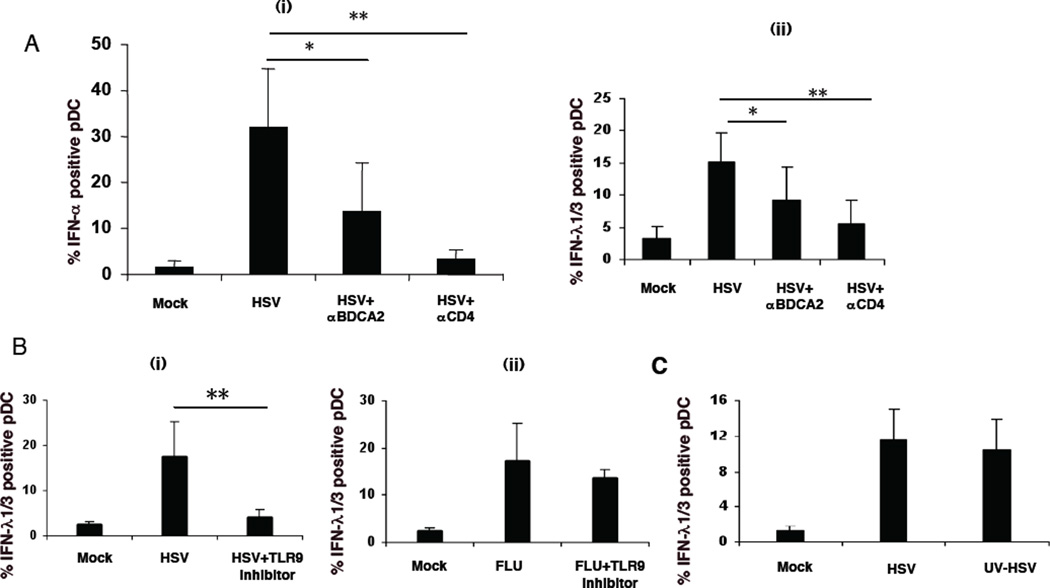
In agreement with previous findings (34, 40), cross-linking of BDCA-2 or CD4 molecules on pDC inhibits an IFN-α response to HSV stimulation (Figure 5A i). Similarly, cross-linking of either BDCA-2 or CD4 also inhibited IFN-λ production by pDC (Figure 5A ii).
Figure 5. Modulation of production of IFN-λ of pDC.
A) Cross-linking of CD4 or BDCA-2 on pDC inhibits IFN-λ and IFN-α production of PDC: CD4 or BDCA-2 on pDC within PBMC was cross-linked with anti-CD4 or anti-BDCA-2 microbeads, and then PBMC were simulated for 7 hr with HSV. The expression of IFN-α (i) or IFN-λ1 (ii) by pDC was measured by flow cytometry as described above. Data represent mean ± 1 SD for 6 separate experiments. * P<0.05 vs. no crosslinking, ** P<0.01 vs. no crosslinking as determined by ANOVA. B) A TLR9 inhibitor inhibits HSV- but not Flu-induced IFN-λ production of pDC. IFN-λ1 production by pDC in PBMC was measured by flow cytometry after stimulation with HSV (i) and Flu (ii), with or without a TLR9 inhibitory ODN (5.42 µg/ml). Data represent mean ± 1 SD for 6 separate donors. ** P<0.01 vs. sample without CpG inhibitor as determined by ANOVA. C) HSV replication is not required for the induction of IFN-λ in pDC. PBMC were simulated with HSV or UV-HSV. The expression of IFN-λ1 of PDC in PBMC was measured by flow cytometry as described above. Data represent mean ± 1 SD for 3 separate donors.
HSV-induced IFN-α production by pDC is mediated through the endosomal receptor, TLR9 (41, 42). A TLR9 inhibitory ODN was found to inhibit HSV-induced IFN-λ1/3 expression by pDC (Figure 5B i). In contrast, the TLR9 inhibitor did not affect the IFN-λ1/3 production by pDC in response to influenza virus, which signals through TLR7 (Figure 5B ii) (43). UV-inactivated HSV is able to induce pDC expression of IFN-α (44). Similarly, UV-inactivated HSV induced pDC to produce IFN-λ (Figure 5C), as did AT-2 inactivated strains of HIV-1 (Figure 1B ?), indicating that virus replication in pDC was not required for induction of IFN-λ. In contrast, live but not UV-inactivated HSV was reported to induce IFN-λ expression in either monocyte-derived macrophages or monocyte-derived dendritic cells (23).
pDC express functional IFN-λ receptors
Given the ability of pDC to produce IFN-λ, we next investigated whether pDC are able to respond to IFN-λ. RT-PCR was used to determine the expression of IFN-λ receptor on cells. We found that freshly-isolated PBMC expressed IFN-λR1; while purified monocytes did not express large amounts of this receptor as evidenced by a very weak band, highly-enriched pDC expressed transcripts for IFN-λR1 (Figure 6A). We also investigated the levels of the IFN-λ receptor expression on pDC and other cell types in peripheral blood by flow cytometry using an antibody to the IFN-λR1. This antibody, which was developed by Zymogenetics as described in the Materials and Methods, recognized CHO cells transfected with the IFN-λ receptor (10) but not the parental CHO cells (Figure 6B). Using this antibody, peripheral blood pDC were found to express IFN-λR1 compared to low but significant levels in B cells and monocytes and no expression in NK cell and T cell subsets (Figure 6C, and D).
IFN-λ enhances pDC function and partially protects from dexamethasone-induced apoptosis
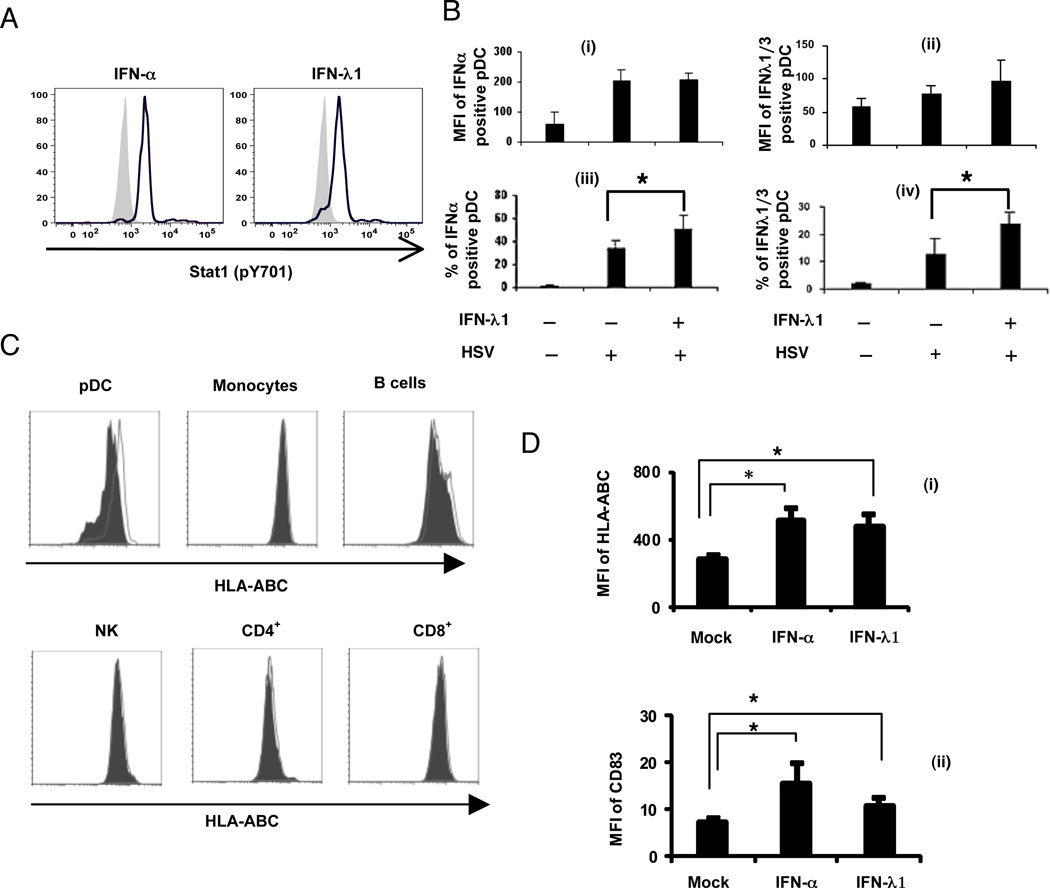
Signaling through both the IFN-α and IFN-λ receptors leads to the phosphorylation of STAT-1 (10, 45). As expected, treatment of pDC with either rIFN-α2 or IFN-λ1 resulted in phosphorylation of STAT-1 (Figure 7A). Pretreatment of pDC and other cell types with IFN-α is known to enhance virus-induced IFN-α production, in part through the upregulation of IRF-7 (39, 46). Similarly, when pDC were pretreated with IFN-λ1 and then stimulated with HSV, the subsequent expression of IFN-λ1/3 and IFN-α occurred in a larger percentage of the pDC population compared to cells only stimulated with HSV (Figure 7B iii and iv). However, there was no significant difference of mean fluorescence intensity (MFI) of the IFN-λ1 or IFN-α expression in individual pDC (Figure 7B i and ii). Further evidence for functional activity of the IFN-λR on pDC but not on most other PBMC subpopulations was demonstrated by the upregulation of MHC Class I (HLA-ABC) on pDC and on B cells, but not monocytes, NK cells or CD4+ or CD8+ T cells (Figure 7C). IFN-α and –λ upregulated both MHC Class I and CD83 on pDC to a similar extent (Figure 7D).
Figure 7. Functional activation of pDC by IFN-λ1.
A) PBMC were treated with either rIFN-α (1000 IU/ml) or IFN-λ1 (10 ng/ml) for 40 minutes; the cells were collected and immediately surface stained with CD123-PE and BDCA2-APC to identify the pDC population, then permeabilized for intracellular staining for the detection of phosphorylated Stat1. Histogram overlays comparing mock (filled) and stimulated pDC (lines). Data are representative of two independent experiments. B) Functional activation of pDC by IFN-λ1 priming: PBMC were primed with IFN-λ1 (10 ng/ml) for 3hr, and then cells were stimulated by HSV for another 4 hr. The percentage and mean fluorescence intensity (MFI) of IFN-α positive (left panels) and IFN-λ positive (right panels) pDC were detected by flow cytometry. Data are mean ± 1 SD for 5 separate donors. C) PBMC were treated with IFN-λ1 (25ng/ml) for 6 hr, then PDC, monocytes, B cells, NK cells, CD4+ T cells, and CD8+ T cells were labeled with specific markers. The expression of HLA-ABC in these subpopulations was measured by flow cytometry with anti-HLA-ABC antibody. (Filled: isotype control; Open: anti-HLA-ABC antibody.) Data are representative of 3 different donors. D) PBMC were treated with IFN-α (1000 U/ml) or IFN-λ1 (25 ng/ml) for 7 hr, and stained with anti-CD83-FITC or anti-HLA-ABC-FITC vs. isotype controls. The mean fluorescence intensities (MFI) of HLA-ABC (i) and CD83 (ii) of PDC after IFN-α or IFN-λ treatment was compared with that of mock for n=3 donors. *p<0.05.
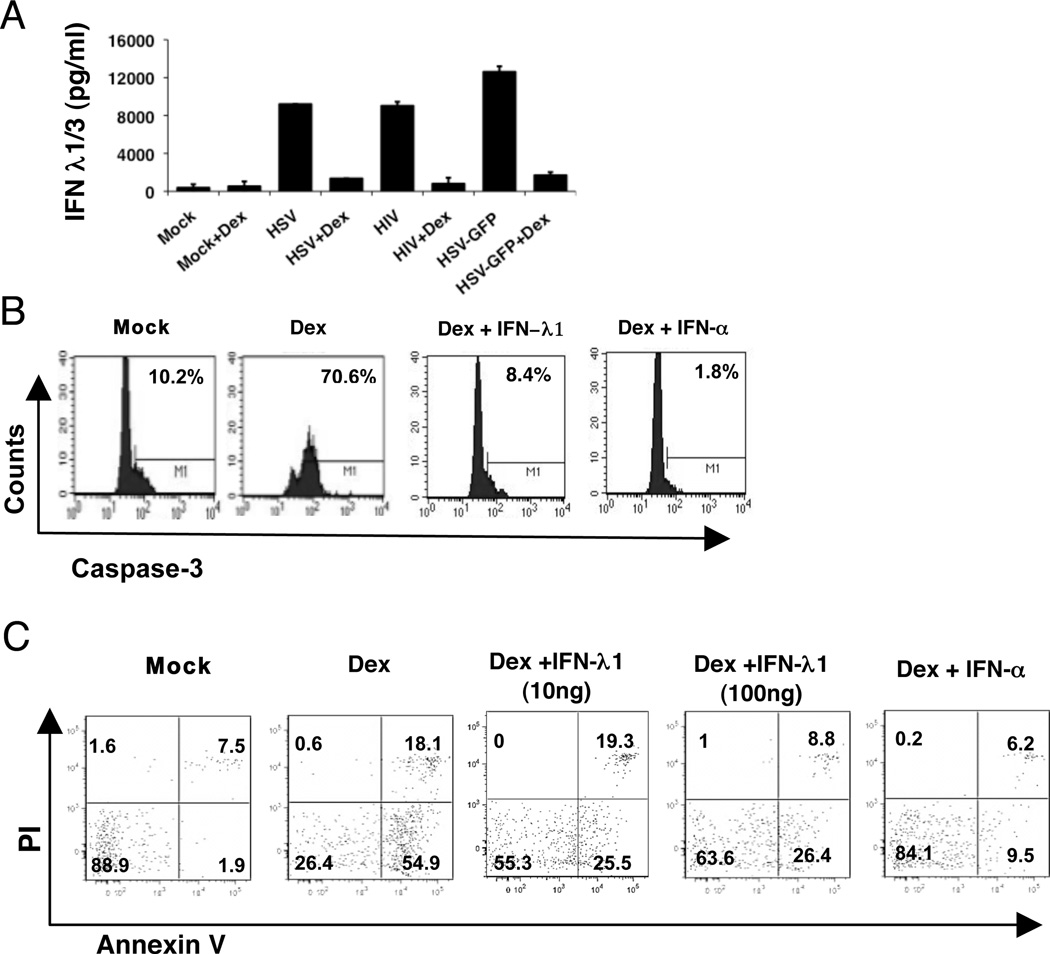
pDC have been reported to be very sensitive in vivo to glucocorticoid treatment (47). We have previously observed that dexamethasone (DEX) inhibits IFN-α production in pDC (Feng et al., in preparation). Likewise, IFN-λ production was strongly inhibited by pretreatment with DEX (Figure 8A.) In addition, we have observed that pDC are very sensitive to DEX treatment and rapidly undergo apoptosis upon in vitro treatment with the drug; furthermore, addition of exogenous IFN-α protected the pDC from dexamethasone-induced cell death (Feng et al., in preparation and Figure 8B, C). Similarly, IFN-λ1 also protected the pDC from dexamethasone-induced apoptosis as measured by expression of active caspase-3 (Figure 8B), and, to a lesser extent, by Annexin-V binding (Figure 8C); while there was some decrease in Dex-induced Annexin V binding with 10 ng/ml of IFN-λ, 100 ng/ml of IFN-λ was more effective at inhibiting apoptosis (Figure 8C).
Figure 8. IFN-λ production by pDC is sensitive to treatment with dexamethasone (Dex) and exogenous IFN-λ partially protects from Dex-mediated apoptosis.
A) Enriched pDC were treated with 1 µM dexamethasone (Dex) for 1 hr prior to stimulation with HSV-1, HIV-1 or highly purified GFP-HSV for another 18 hr. IFN-λ in supernatants was determined by ELISA. Data represent mean and range for two separate donors. B) Exogenous IFN-λ inhibits apoptosis of pDC. PBMC were treated with Dex, with or without IFN-α (10,000 U/ml) or IFN-λ1 (10ng/ml) for 6 hr. Active caspase-3 in pDC was stained intracellularly in pDC. Data are representative of 3 different donors. C) Annexin V/PI staining was carried out for pDC within PBMC treated with 1 µM Dex in the presence or absence of rIFN-α (100 ng/ml) or –λ1 (10 or 100 ng/ml). Data are representative of three experiments.
Discussion
Since the first description of the type III IFNs and their receptor (9, 10), there has been much interest in its cellular origins and unique functions. Among cells of hematopoietic origin, macrophages and MDDC have been shown to express IFN-λ1 (IL-29) and IFN-λ2 and IFN-λ3 (IL-28A and IL-28B, respectively) in response to virus infection, as reflected by increased mRNA levels (23, 25). Likewise, pDC have been reported by several groups to express IFN-λ mRNA or protein. To study the production of IFN-λ and its effects on pDC in more detail, we developed a flow cytometric assay to assess intracellular levels of IFN-λ protein and found that a subset of pDC produce IFN-λ1/3 in response to stimulation by viruses including HSV, Flu, SeV and HIV-1, or in response to the synthetic TLR9 agonist, CpGA. Interestingly, the viruses were better inducers of IFN-λ than CpGA. As is also seen with IFN-α, CpGB did not induce appreciable levels of IFN-λ in pDC.
Given their presence in blood and secondary lymphoid tissue, the expression and secretion of IFN-λ by pDC may be important in host defenses against viral infection by way of signaling other cells in the immune system. Ank et al. have shown that live but not UV-inactivated HSV can induce non-lymphoid cells to produce IFN-λ (12), suggesting that active viral infection of these cells is required for induction of IFN-λ. In contrast, as we previously demonstrated for the induction of IFN-α (44, 48), we found that either infectious or UV-inactivated HSV-1 or inactivated HIV-1 can induce pDC to express IFN-λ. Thus, HSV and HIV-stimulated expression of IFN-λ in pDC is independent of viral replication, thus making these cells uniquely poised to respond to viruses that the pDC sense through non-infectious routes, e.g. through endocytosis of infectious or non-infectious viral particles. Our results further indicate that HSV induces both IFN-α and IFN-λ through TLR9, which signals in endosomal compartments (42, 49, 50). In contrast, IFN-λ production in response to Flu was independent of TLR9 signaling; in recent studies, we have found that inhibition of TLR7 signaling inhibits both Flu-induced IFN-λ and -α (Natalia et al., in preparation).
While the regulation of IFN-α expression has been widely studied, less is known about the corresponding mechanisms of virus-stimulated induction of IFN-λ. We found that purified pDC produce IFN-λ1 at levels about 10-fold lower than the production of IFN-α. This may in part by accounted for by the multiple subtypes of IFN-α that are produced by pDC (51) and which are detected by the IFN-α ELISA kit we utilize. For IFN-λ detection, the ELISA kit we used recognizes IFN-λ1 and –λ3 and has partial cross-reactivity with IFN-λ2, making it unlikely that we are missing massive amounts of IFN-λ production. Differential expression of the IFN subsets in pDC subpopulations likely contributes to the lower overall amount of IFN-λ than IFN-α production by pDC; in support of this, we observed that, typically, a larger percentage of pDC produce IFN-α than -λ in response to virus stimulation, with the IFN-λ1/3 expression restricted to the pDC expressing the highest mean fluorescence intensity for IFN-α production; this was observed at all time points tested (Supplementary Figure 2). Our data also suggest that pDC that produce IFN-λ1/3 vs -λ2 represent partially non-overlapping subsets. Similarly, we have previously shown that, in response to viral stimulation, while all IFN-α positive cells also express TNF-α, not all of the TNF-α positive cells produce IFN-α. These results, as well as our results with IFN-α vs. IFN-λ expression, suggest that at least partially non-overlapping mechanisms are involved in the induction of type I and III IFNs and type I IFNs and TNF-α. Iversen et al. (52) recently reported that vaginal expression of type III IFN is more dependent on NF-κB signaling than is IFN-α. Possible differences in intracellular signaling pathways leading to Type I vs. Type III induction are currently under investigation in our laboratory. For example, it is possible that different endosomal compartments and/or different downstream events following engagement of TLR9 by HSV leads to differential expression of IFN-α and –λ. Moreover, it is possible that there are phenotypic differences in the cells that produce both IFN-α and –λ and those that produce only IFN-α; however, our initial data suggests that while cells that produced neither IFN-α nor –λ barely upregulated the expression of CD40, CD83 or PD-L1 during the 7 hr co-culture, both the IFN-α single positive cells and IFN-α/IFN-λ double positive cells did upregulate these markers (shown in Supplementary Figure 1).
Another difference observed between type I and type III IFN produced by pDC was the relatively delayed kinetics in the numbers of pDC positive for intracellular IFN-λ vs. IFN-α. However, 24 hr kinetics of either the intracellular IFNs or secreted IFNs did not show substantial differences in the rate of production of these cytokines, but with a much lower magnitude of IFN-λ released for each time point. At all time points, the cells brightest for the expression of IFN-α were the ones that expressed IFN-λ (Supplementary Figure 2). The differences in the total amount of IFN-λ produced vs. IFN-α can be accounted for, in part, by the lower portion of pDC that produce IFN-λ vs. –α and also by the fact that the IFN-λ ELISA assay does not detect all of the secreted IFN-λ.
The expression of IFN-λ by pDC could not be accounted for by an indirect effect of virus-induced IFN-α being released and then inducing the expression of IFN-λ: in contrast to what was reported in cell lines (12), IFN-α was not able to induce IFN-λ expression in pDC by 7 hr independent of virus stimulation, a time at which we routinely measure intracellular IFN-λ, and blocking of IFN-α signaling failed to inhibit virus-induced IFN-λ expression. We were, however, able to detect low levels of IFN-λ in supernatants of purified pDC stimulated with 10,000 IU/ml of IFN-α for 24 hr, but at lower levels than that induced by HSV stimulation. Together, these results indicate that IFN-λ production by pDC can be induced directly by virus, but that eventually, high levels of IFN-α production might feed-back and enhance IFN-λ production. Other reports have also indicated that IFN-λ1 can be induced in an IFN-α-independent manner in different model systems (52–54).
In addition to the ability of a similar range of viruses to induce type I and type III IFN in pDC, there appear to be some similarities in regulation of the production of these IFNs: first, we observed that antibody cross-linking of CD4 or BDCA-2 (CD303) molecules on pDC inhibits HSV-induced IFN-λ as has previously been demonstrated for IFN-α (34, 40). Similar to induction of IFN-α (42, 55, 56), we demonstrated that HSV-induced production of IFN-λ in pDC is also mediated by TLR9, and, as also seen for IFN-α (44), the induction of IFN-λ in pDC by HSV or HIV-1 was independent of virus infection and proliferation. Likewise, AT2-inactivated HIV-1 strains, which are replication incompetent, also induced strong IFN-λ responses in pDC. Others have shown that key regulators in the induction of type I interferons such as RIG-I, IPS-1, TBK1, and IRF-3, in cell types other than pDC, are also involved in the regulation of type III IFN expression in these cells (14), but these are all dependent on the presence of virus replication intermediates and are subject to viral immune evasion strategies. Therefore, the ability to respond to viruses in the absence of virus infection of the pDC makes these cells uniquely positioned to detect a broad range of viruses, many of which, like HSV-1 cannot infect the pDC (30). The ability of pDC to detect viruses in the absence of viral replication limits the ability of the viruses to escape from these innate sentinels by actively interfering with the induction of IFNs.
Functionally, IFN-λ has both antiviral and anti-proliferative activity, although with lower potency than IFN-α (13). An important distinction between the functions of IFN-α and –λ is the ubiquitous nature of the type I vs. the more limited expression of the type III IFN receptors. While it was recently reported that pDC express IFN-λR1 mRNA and can respond to IFN-λ treatment (57), our results show for the first time the cell surface expression of the IFN-λ receptor on human pDC, which was greater than the expression in other PBMC sub-populations. This dual expression of type I and type III IFN receptors by pDC as well as their production of both of these families of IFNs in response to a wide range of viral and TLR 7 and -9 stimuli makes them unique among cells of the immune system. The type III IFN receptors on pDC are functional: pDC responded with phosphorylation of STAT-1 in response to both IFN-α and –λ. pDC also responded to IFN-λ stimulation with upregulation of MHC Class I and CD83 and IFN-λ pretreatment lead to enhanced production of both type I and III IFNs in response to virus in these cells. This latter finding is in contrast to the observations of Paluden and colleagues who failed to find positive feedback by IFN-λ in an in vivo system, perhaps because they were not directly looking at pDC (20). Virus-induced IFN-α production (Feng et al., in preparation) and IFN-λ production (shown herein) are very sensitive to treatment with dexamethasone (DEX). In addition, we have observed that IFN-λ, like IFN–α, can have an immunoprotective role in pDC, being able to protect these cells from DEX-induced apoptosis as measured by Annexin V binding and expression of active caspase-3. The inhibition of active caspase-3 by IFN-λ was more pronounced than that seen with Annexin V binding; moreover, the survival effect for IFN-λ was not as potent as that seen with IFN-α and was most evident in 6 hr cultures, with IFN-λ less ability to protect at 18 hr (data not shown). In vivo, under conditions of stress such as occurs in virus infections, glucosteroids are synthesized and function, in part to prevent overzealous immune responses. We hypothesize that production of type I and type III IFNs can exert positive feedback to keep the pDC alive at least over the short-run. We speculate that this protective effect of type I and III IFNs for pDC is important in that in the case of viral infection, the pDC need to survive and go on to present antigen to virus-specific T cells. To put our dexamethasone-induced studies into a physiological perspective, further studies will need to be conducted to determine whether IFN-α and/or –λ produced in the course of a viral infection extends pDC life by preventing apoptosis. From our in vitro priming studies, we extrapolate that in vivo, IFN-λ can enhance the function of pDC by increasing the amount of IFN-α and IFN-λ production in either an autocrine or paracrine manner, thus amplifying the total IFN response of these pivotal cells.
What is still puzzling, however, is the benefit of pDC expressing both type I and type III IFN receptors, given the largely overlapping functions of these IFNs on pDC. It is possible that the IFN-λ signaling response in pDC is more prolonged in addition to being slightly delayed compared to the IFN-α response, as has been reported in the HaCaT cell line (58), and would thus potentially facilitate pDC survival during crucial maturation and antigen presentation stages; studies are currently underway to assess this possibility.
Together, our results indicate that pDC, well-known for being “professional” type I IFN producing cells, are also unique among PBMC in their ability to both produce and respond to IFN-λ. It has recently been proposed based on elegant studies in murine models that IFN-λ primarily exerts host protection at epithelial surfaces (59); however, given the unique properties of pDC, which are found in circulation and within secondary lymphoid tissues as well at some infected tissue sites, we propose that IFN-λ production and response by these cells has the potential to influence global anti-viral host resistance mechanisms.
Supplementary Material
Acknowledgments
We gratefully acknowledge the provision of influenza virus by Dr. Thomas Moran, Mt. Sinai Medical Center and antibody to the IFN-λ receptor from ZymoGenetics, Seattle, WA. These experiments also utilized HIV-1 provided by the AIDS and Cancer Virus Program, SAIC Frederick, Inc./National Cancer Institute, Frederick, supported with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
Abbreviations
- Flu
influenza virus
- HAU
hemagglutination units
- HIV-1
human immunodeficiency virus
- HSV
herpes simplex virus
- IFN
interferon
- MFI
mean fluorescence intensity
- MOI
multiplicity of infection
- NK
natural killer cells
- PBMC
peripheral blood mononuclear cells
- pDC
plasmacytoid dendritic cells
- SeV
Sendai virus
- TLR
toll-like receptor
- UV-HSV
UV-inactivated HSV
Footnotes
References
- 1.Novick D, Cohen B, Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994;77:391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 2.Uze G, Lutfalla G, Gresser I. Genetic transfer of a functional human interferon alpha receptor into mouse cells: cloning and expression of its cDNA. Cell. 1990;60:225–234. doi: 10.1016/0092-8674(90)90738-z. [DOI] [PubMed] [Google Scholar]
- 3.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 4.Taniguchi T, Takaoka A. The interferon-alpha/beta system in antiviral responses: a multimodal machinery of gene regulation by the IRF family of transcription factors. Curr Opin Immunol. 2002;14:111–116. doi: 10.1016/s0952-7915(01)00305-3. [DOI] [PubMed] [Google Scholar]
- 5.Biron CA. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin Immunol. 1998;10:383–390. doi: 10.1006/smim.1998.0138. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki N, Liu YJ. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum Immunol. 2002;63:1126–1132. doi: 10.1016/s0198-8859(02)00751-6. [DOI] [PubMed] [Google Scholar]
- 7.Gattoni A, Parlato A, Vangieri B, Bresciani M, Derna R. Interferon-gamma: biologic functions and HCV therapy (type I/II) (1 of 2 parts) Clin Ter. 2006;157:377–386. [PubMed] [Google Scholar]
- 8.Pestka S, Kotenko SV, Muthukumaran G, Izotova LS, Cook JR, Garotta G. The interferon gamma (IFN-gamma) receptor: a paradigm for the multichain cytokine receptor. Cytokine Growth Factor Rev. 1997;8:189–206. doi: 10.1016/s1359-6101(97)00009-9. [DOI] [PubMed] [Google Scholar]
- 9.Sheppard P, Kindsvogel W, Xu W, Henderson K, Schlutsmeyer S, Whitmore TE, Kuestner R, Garrigues U, Birks C, Roraback J, Ostrander C, Dong D, Shin J, Presnell S, Fox B, Haldeman B, Cooper E, Taft D, Gilbert T, Grant FJ, Tackett M, Krivan W, McKnight G, Clegg C, Foster D, Klucher KM. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–68. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 10.Kotenko SV, Gallagher G, Baurin VV, Lewis-Antes A, Shen M, Shah NK, Langer JA, Sheikh F, Dickensheets H, Donnelly RP. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 11.Zitzmann K, Brand S, Baehs S, Goke B, Meinecke J, Spottl G, Meyer H, Auernhammer CJ. Novel interferon-lambdas induce antiproliferative effects in neuroendocrine tumor cells. Biochem Biophys Res Commun. 2006;344:1334–1341. doi: 10.1016/j.bbrc.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 12.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–4509. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. Biological activity of interleukins-28 and -29: comparison with type I interferons. Cytokine. 2005;31:109–118. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Onoguchi K, Yoneyama M, Takemura A, Akira S, Taniguchi T, Namiki H, Fujita T. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- 15.Bartlett NW, Buttigieg K, Kotenko SV, Smith GL. Murine interferon lambdas (type III interferons) exhibit potent antiviral activity in vivo in a poxvirus infection model. J Gen Virol. 2005;86:1589–1596. doi: 10.1099/vir.0.80904-0. [DOI] [PubMed] [Google Scholar]
- 16.Doyle SE, Schreckhise H, Khuu-Duong K, Henderson K, Rosler R, Storey H, Yao L, Liu H, Barahmand-pour F, Sivakumar P, Chan C, Birks C, Foster D, Clegg CH, Wietzke-Braun P, Mihm S, Klucher KM. Interleukin-29 uses a type 1 interferon-like program to promote antiviral responses in human hepatocytes. Hepatology. 2006;44:896–906. doi: 10.1002/hep.21312. [DOI] [PubMed] [Google Scholar]
- 17.Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Z, Hamming OJ, Ank N, Paludan SR, Nielsen AL, Hartmann R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. Journal of virology. 2007;81:7749–7758. doi: 10.1128/JVI.02438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommereyns C, Paul S, Staeheli P, Michiels T. IFN-lambda (IFN-lambda) is expressed in a tissue-dependent fashion and primarily acts on epithelial cells in vivo. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000017. e1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol. 2008;180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 21.Mennechet FJ, Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of FOXP3-expressing suppressor T cells. Blood. 2006;107:4417–4423. doi: 10.1182/blood-2005-10-4129. [DOI] [PubMed] [Google Scholar]
- 22.Jordan WJ, Eskdale J, Srinivas S, Pekarek V, Kelner D, Rodia M, Gallagher G. Human interferon lambda-1 (IFN-lambda1/IL-29) modulates the Th1/Th2 response. Genes Immun. 2007;8:254–261. doi: 10.1038/sj.gene.6364382. [DOI] [PubMed] [Google Scholar]
- 23.Melchjorsen J, Siren J, Julkunen I, Paludan SR, Matikainen S. Induction of cytokine expression by herpes simplex virus in human monocyte-derived macrophages and dendritic cells is dependent on virus replication and is counteracted by ICP27 targeting NF-kappaB and IRF-3. J Gen Virol. 2006;87:1099–1108. doi: 10.1099/vir.0.81541-0. [DOI] [PubMed] [Google Scholar]
- 24.Wolk K, Witte K, Witte E, Proesch S, Schulze-Tanzil G, Nasilowska K, Thilo J, Asadullah K, Sterry W, Volk HD, Sabat R. Maturing dendritic cells are an important source of IL-29 and IL-20 that may cooperatively increase the innate immunity of keratinocytes. J Leukoc Biol. 2008;83:1181–1193. doi: 10.1189/jlb.0807525. [DOI] [PubMed] [Google Scholar]
- 25.Osterlund P, Veckman V, Siren J, Klucher KM, Hiscott J, Matikainen S, Julkunen I. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol. 2005;79:9608–9617. doi: 10.1128/JVI.79.15.9608-9617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coccia EM, Severa M, Giacomini E, Monneron D, Remoli ME, Julkunen I, Cella M, Lande R, Uze G. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur J Immunol. 2004;34:796–805. doi: 10.1002/eji.200324610. [DOI] [PubMed] [Google Scholar]
- 27.Feldman SB, Ferraro M, Zheng HM, Patel N, Gould-Fogerite S, Fitzgerald-Bocarsly P. Viral induction of low frequency interferon-alpha producing cells. Virology. 1994;204:1–7. doi: 10.1006/viro.1994.1504. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald-Bocarsly P. Natural interferon-alpha producing cells: the plasmacytoid dendritic cells. Biotechniques. 2002;(Suppl):16–20. 22, 24–19. [PubMed] [Google Scholar]
- 29.Cederblad B, Alm GV. Infrequent but efficient interferon-alpha-producing human mononuclear leukocytes induced by herpes simplex virus in vitro studied by immuno-plaque and limiting dilution assays. J Interferon Res. 1990;10:65–73. doi: 10.1089/jir.1990.10.65. [DOI] [PubMed] [Google Scholar]
- 30.Megjugorac NJ, Young HA, Amrute SB, Olshalsky SL, Fitzgerald-Bocarsly P. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J Leukoc Biol. 2004;75:504–514. doi: 10.1189/jlb.0603291. [DOI] [PubMed] [Google Scholar]
- 31.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 32.Zitvogel L, Terme M, Borg C, Trinchieri G. Dendritic cell-NK cell cross-talk: regulation and physiopathology. Curr Top Microbiol Immunol. 2006;298:157–174. doi: 10.1007/3-540-27743-9_8. [DOI] [PubMed] [Google Scholar]
- 33.Ito T, Amakawa R, Inaba M, Hori T, Ota M, Nakamura K, Takebayashi M, Miyaji M, Yoshimura T, Inaba K, Fukuhara S. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J Immunol. 2004;172:4253–4259. doi: 10.4049/jimmunol.172.7.4253. [DOI] [PubMed] [Google Scholar]
- 34.Fanning SL, George TC, Feng D, Feldman SB, Megjugorac NJ, Izaguirre AG, Fitzgerald-Bocarsly P. Receptor cross-linking on human plasmacytoid dendritic cells leads to the regulation of IFN-alpha production. J Immunol. 2006;177:5829–5839. doi: 10.4049/jimmunol.177.9.5829. [DOI] [PubMed] [Google Scholar]
- 35.Paladino P, Collins SE, Mossman KL. Cellular localization of the herpes simplex virus ICP0 protein dictates its ability to block IRF3-mediated innate immune responses. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010428. e10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A, Wu L, Shortman K, Chaplin P, Suter M, O'Keeffe M, Hochrein H. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. The Journal of experimental medicine. 2010;207:2703–2717. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 38.Prescott J, Hall P, Acuna-Retamar M, Ye C, Wathelet MG, Ebihara H, Feldmann H, Hjelle B. New World hantaviruses activate IFNlambda production in type I IFN-deficient vero E6 cells. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011159. e11159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- 40.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison LA. The Toll of herpes simplex virus infection. Trends Microbiol. 2004;12:353–356. doi: 10.1016/j.tim.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- 44.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 45.Maher SG, Sheikh F, Scarzello AJ, Romero-Weaver AL, Baker DP, Donnelly RP, Gamero AM. IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer biology & therapy. 2008;7:1109–1115. doi: 10.4161/cbt.7.7.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai J, Megjugorac NJ, Amrute SB, Fitzgerald-Bocarsly P. Regulation of IFN regulatory factor-7 and IFN-alpha production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J Immunol. 2004;173:1535–1548. doi: 10.4049/jimmunol.173.3.1535. [DOI] [PubMed] [Google Scholar]
- 47.Shodell M, Shah K, Siegal FP. Circulating human plasmacytoid dendritic cells are highly sensitive to corticosteroid administration. Lupus. 2003;12:222–230. doi: 10.1191/0961203303lu362xx. [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald-Bocarsly P, Feldman M, Mendelsohn M, Curl S, Lopez C. Human mononuclear cells which produce interferon-alpha during NK (HSV-FS) assays are HLA-DR positive cells distinct from cytolytic natural killer effectors. Journal of Leukocyte Biology. 1988;43:323–334. doi: 10.1002/jlb.43.4.323. [DOI] [PubMed] [Google Scholar]
- 49.Guiducci C, Ott G, Chan JH, Damon E, Calacsan C, Matray T, Lee KD, Coffman RL, Barrat FJ. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, Taya C, Taniguchi T. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 51.Izaguirre A, Barnes BJ, Amrute S, Yeow WS, Megjugorac N, Dai J, Feng D, Chung E, Pitha PM, Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 52.Iversen MB, Ank N, Melchjorsen J, Paludan SR. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-kappaB than type I IFNs. J Virol. 84:4579–4586. doi: 10.1128/JVI.02591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stoltz M, Klingstrom J. Alpha/beta interferon (IFN-alpha/beta)-independent induction of IFN-lambda1 (interleukin-29) in response to Hantaan virus infection. Journal of virology. 2010;84:9140–9148. doi: 10.1128/JVI.00717-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou P, Cowled C, Todd S, Crameri G, Virtue ER, Marsh GA, Klein R, Shi Z, Wang LF, Baker ML. Type III IFNs in pteropid bats: differential expression patterns provide evidence for distinct roles in antiviral immunity. Journal of immunology. 2011;186:3138–3147. doi: 10.4049/jimmunol.1003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hochrein H, Schlatter B, O'Keeffe M, Wagner C, Schmitz F, Schiemann M, Bauer S, Suter M, Wagner H. Herpes simplex virus type-1 induces IFN-alpha production via Toll-like receptor 9-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2004;101:11416–11421. doi: 10.1073/pnas.0403555101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004;103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 57.Megjugorac NJ, Gallagher GE, Gallagher G. Modulation of human plasmacytoid DC function by IFN-lambda1 (IL-29) J Leukoc Biol. 2009;86:1359–1363. doi: 10.1189/jlb.0509347. [DOI] [PubMed] [Google Scholar]
- 58.Maher SG, Sheikh F, Scarzello AJ, Romero-Weaver AL, Baker DP, Donnelly RP, Gamero AM. IFNalpha and IFNlambda differ in their antiproliferative effects and duration of JAK/STAT signaling activity. Cancer Biol Ther. 2008;7:1109–1115. doi: 10.4161/cbt.7.7.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ank N, Paludan SR. Type III IFNs: new layers of complexity in innate antiviral immunity. Biofactors. 2009;35:82–87. doi: 10.1002/biof.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.