This study aims to determine how the lymph node ratio may be used to predict the likelihood of recurrence for patients with papillary thyroid cancer.
Keywords: Thyroid cancer, Recurrence, Lymph node metastases, Quality measure, Lymph node ratio
Learning Objectives
Explain how lymph node ratio can be used to predict disease recurrence for papillary thyroid cancer.
Cite the threshold total and central lymph node ratios that best predict recurrence.
Describe ways in which lymph node ratio can be useful in guiding postoperative follow-up.
Abstract
Background.
Lymph node metastasis occurs in 20%–50% of patients presenting for initial treatment of papillary thyroid cancer (PTC). The significance of lymph node metastases remains controversial, and the aim of this study is to determine how the lymph node ratio (LNR) may predict the likelihood of disease recurrence.
Methods.
We conducted a retrospective review of patients undergoing total thyroidectomy for PTC at our institution from 2005 to 2010. A total LNR (positive nodes to total nodes) and central lymph node ratio (cLNR) was calculated. Regression was used to determine a threshold LNR that best predicted recurrence. Multivariate logistic regression then determined the influence of LNR on recurrence while accounting for other known predictors of recurrence. Kaplan-Meier analysis and the log-rank test were used to compare differences in disease-free survival.
Results.
Of the 217 patients undergoing total thyroidectomy for PTC, 69 patients had concomitant neck dissections. Sixteen (23.2%) patients developed disease recurrence. When disease-free survival functions were compared, we found that patients with a total LNR ≥0.7 (p < .01) or a cLNR ≥0.86 (p = .04) had significantly worse disease-free survival rates than patients with ratios below these threshold values. Considering other known predictors of recurrence, we found that LNR was significantly associated with recurrence (odds ratio: 19.5, 95% confidence interval: 4.1–22.9; p < .01).
Conclusions.
Elevated total LNR and cLNR are strongly associated with recurrence of PTC after initial operation. LNR in PTC is a tool that can be used to determine the likelihood of the patient developing recurrent disease and inform postoperative follow-up.
Implications for Practice:
Patients with PTC and lymph node metastases are currently staged according to presence or absence of lymph node metastases in anatomic compartments. The extent of disease in the lymph nodes is not considered in current staging systems. The LNR can be used to further risk-stratify patients with PTC for their risk of recurrence. In the postoperative period, the LNR is helpful in deciding frequency of follow-up, the need for radioactive iodine, or to provide a more informed discussion with the patient regarding the likelihood of recurrence.
Introduction
Lymph node metastases occur in 20%–50% of patients presenting for initial treatment of papillary thyroid cancer (PTC) [1, 2]. This percentage reaches 90% when considering micrometastatic disease [3]. Presence of lymph node metastases is an independent risk factor for recurrence [4, 5], and recurrence can add significant treatment morbidity. Population-based studies have demonstrated that lymph node metastases do carry a small but significant negative effect on disease-specific survival rates [6]. The strength of this association, however, has varied widely in other studies [7, 8].
When recurrence does occur, it often leads to a second operation that carries a higher complication rate, especially if the recurrence is in the central neck [9, 10]. Treating PTC with total thyroidectomy then permits radioactive iodine (RAI) ablation of micrometastatic disease in the lymph nodes and elsewhere. Recently, the long-term safety of radioactive iodine, especially when liberally applied, has been called into question [11, 12]. More sensitive ultrasonography and serum thyroglobulin measurements are capable of detecting subcentimeter recurrences in patients treated with thyroidectomy and RAI [13, 14], leading to surgical referral or repeated RAI treatment.
These issues have led some clinicians to advocate for routine prophylactic lymph node dissection for PTC [15–17], but the optimal approach to the central compartment lymph nodes remains quite controversial and practice patterns vary widely [18]. Moreover, performance measures to assess the adequacy or completeness of lymph node dissection do not exist for PTC as they do in other cancers for which lymphadenectomy is performed [19, 20]. As the ability to detect lymph node metastases improves and the indications for lymph node dissection are debated, there is a need to understand how the extent of lymph node metastases affects oncologic outcomes. The recent literature contains a variety of methods to further risk-stratify patients with PTC and lymph node metastases, but the optimal method has yet to be determined [21–24]. Because the disease-specific mortality from PTC is extremely low, the most relevant oncologic outcome is disease recurrence. The purpose of this study was to determine how the lymph node ratio (LNR) may be used to predict the likelihood of disease recurrence.
Methods
We conducted a retrospective review of our prospectively collected thyroid database. We selected patients with PTC undergoing total thyroidectomy with lymph node dissection between 2005 and 2010. At our institution, patients are offered therapeutic central or lateral compartment lymph node dissection at the time of their thyroidectomy based on pathologically proven lymph node metastases or suspicious lymph nodes seen intraoperatively or on preoperative imaging.
Data on demographics, number of positive lymph nodes, lymph node yield, histologic tumor features, and recurrence were extracted from our database. LNR was calculated by dividing the total number of metastatic lymph nodes by the total number of lymph nodes removed for both the central compartment alone (cLNR) and the total number of nodes removed. Recurrence was defined as any pathologically or cytologically confirmed evidence of disease that was not present at the time of initial surgery. Cases in which there was gross disease left behind or staged neck dissections were not considered recurrent disease. Logistic regression was used to calculate the predicted probability function of recurrence for each LNR, and a threshold total and cLNR was selected that corresponded to a greater than 50% observed probability of recurrence.
Kaplan-Meier analysis was used to plot disease-free survival curves. Differences in the Kaplan-Meier estimates using our threshold total and cLNRs were compared by the log-rank test. Finally, both univariate and multivariate regression with the Cox proportional hazards model was used to determine the influence of LNR on disease recurrence while accounting for other known clinical and pathologic predictors of outcome. Binary comparisons were made using the Student's t test, Wilcoxon rank sum test, or χ2 test where appropriate. A p value <.05 was considered significant.
Results
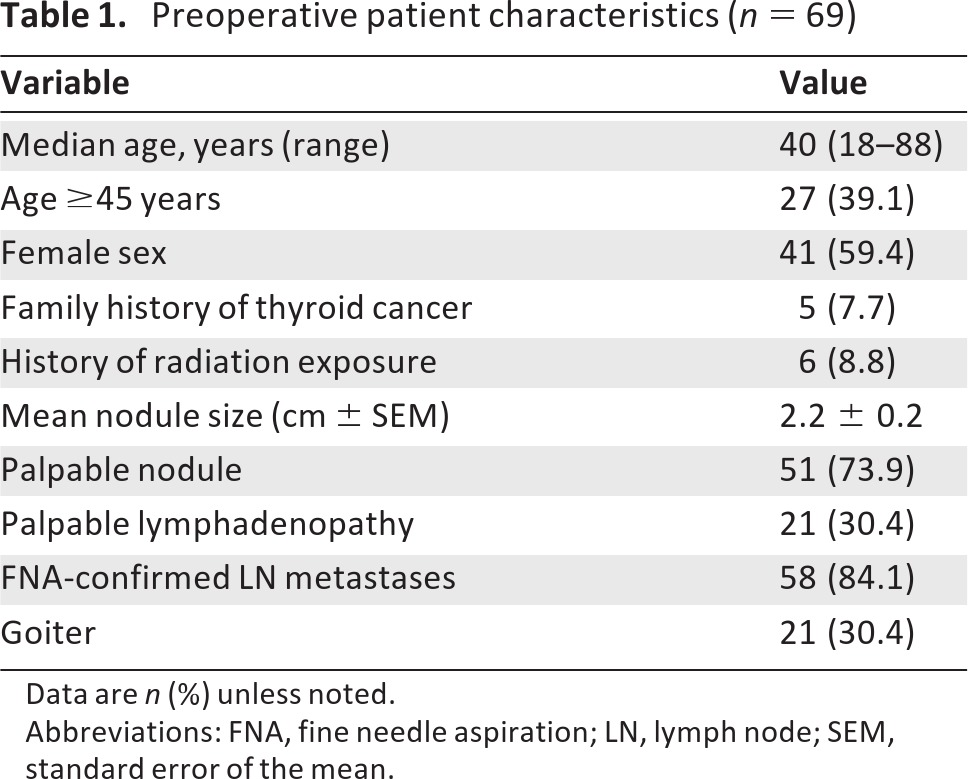
Of the 217 patients undergoing total thyroidectomy for PTC at our institution between 2005 and 2010, a total of 69 patients had concomitant neck dissections, and this subset was selected for further analysis. The median age was 40 years (range 18–88) and 39.1% of patients were 45 or older (Table 1). In all, 59.4% of patients were female. Six (8.8%) patients had a history of head and neck radiation exposure, and five (7.7%) had a family history of thyroid cancer (Table 1). In all but six patients (8.8%), PTC was confirmed by fine needle aspiration biopsy (FNAB) preoperatively; these six patients had FNABs read as suspicious for PTC or hypercellular. The mean nodule size was 2.2 ± 0.2 cm, and 73.9% had a palpable nodule (Table 1).
Table 1.
Preoperative patient characteristics (n = 69)
Data are n (%) unless noted.
Abbreviations: FNA, fine needle aspiration; LN, lymph node; SEM, standard error of the mean.
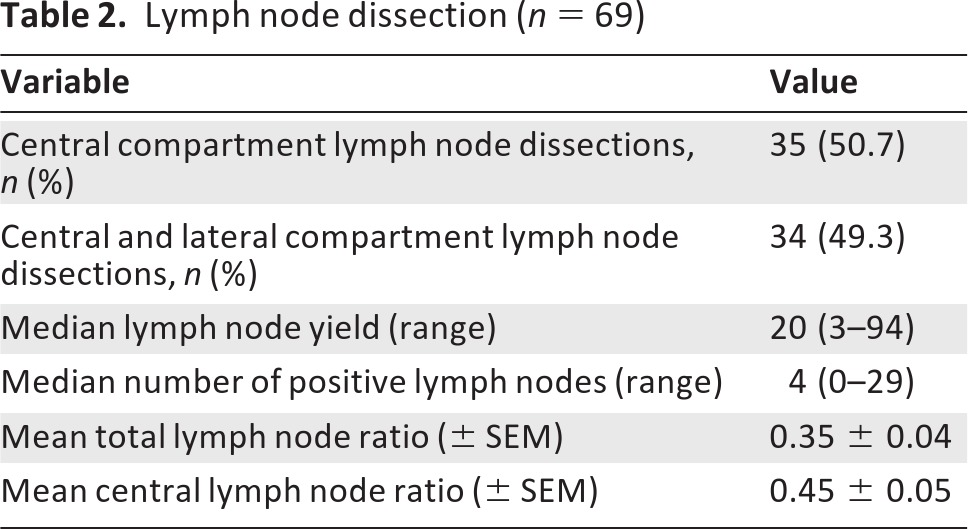
Lymphadenopathy was preoperatively palpable by the surgeon in 21 patients (30.4%), and FNAB confirmed lymph node metastases in 58 patients (84.1%) preoperatively (Table 1). In eight patients (11.6%), one or more lymph nodes were evaluated by intraoperative frozen section. A total of 35 patients (50.7%) underwent central compartment lymph node dissection, whereas 34 patients (49.3%) had both central and lateral compartments dissected (Table 2).
Table 2.
Lymph node dissection (n = 69)
Threshold LNR
Final pathology reports were reviewed and lymph node ratios were calculated for both the total number of lymph nodes and the central compartment lymph nodes retrieved. The mean total LNR was 0.35 ± 0.04, whereas the mean cLNR was 0.45 ± 0.05 (Table 2).
Regression was used to calculate the predicted probability of recurrence for each patient (Fig. 1). The threshold LNR was calculated by selecting the LNR associated with greater than 50% observed probability of recurrence. For the total lymph nodes retrieved, this LNR was 0.70 (Fig. 1), whereas the threshold cLNR was 0.86.
Figure 1.
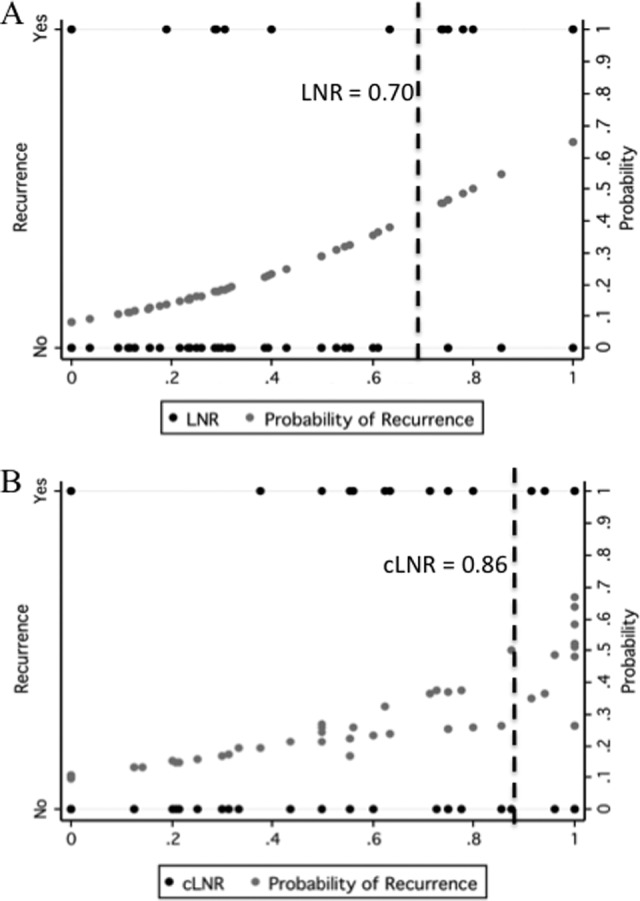
Probability of recurrence by lymph node ratio (LNR). The predicted probability of recurrence for each LNR is also displayed (black dots). The dashed line indicates the calculated threshold LNR for the total number of lymph nodes based on 50% observed probability of recurrence.
Abbreviations: cLNR, central lymph node ratio; LNR, lymph node ratio.
Disease-Free Survival and LNR
We then evaluated these threshold LNRs by their ability to distinguish disease-free survival curves. In all, 16 of the 69 (23.2%) patients analyzed developed pathologically proven recurrence, and 14 underwent subsequent operations. The median time to recurrence was 11.8 months (range 4.3–59.5 months).
Kaplan-Meier estimates for disease-free survival were plotted for both cLNR and total LNR (Fig. 2). Patients with a total LNR greater than or equal to the threshold of 0.70 had significantly worse disease-free survival compared to those with a total LNR less than 0.70 (p < .01, Fig. 2A). Similarly, patients with a cLNR greater than or equal to the threshold of 0.86 had worse disease-free survival compared to those with a cLNR less than 0.86 (p = .04, Fig. 2B).
Figure 2.
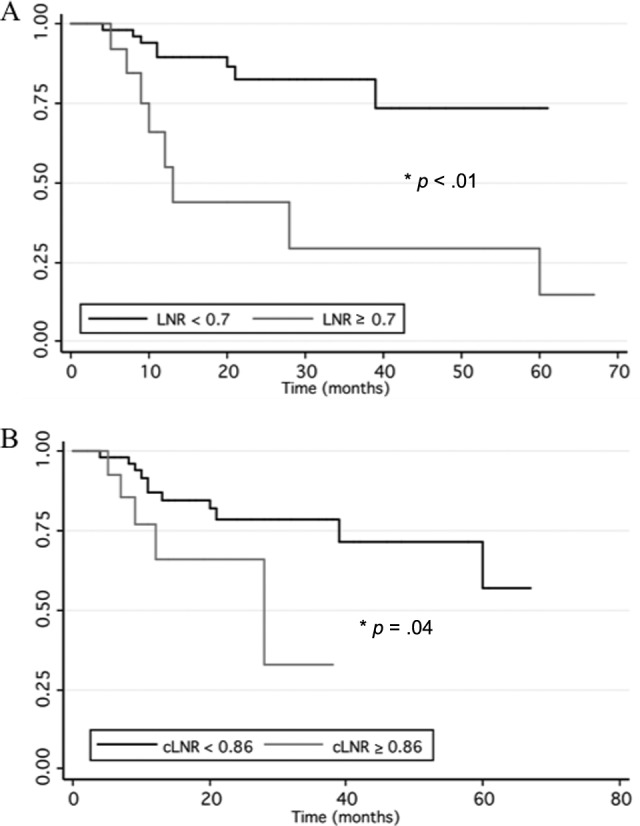
Disease-free survival. Kaplan-Meier estimates of disease free recurrence are plotted by grouping the patients according to the threshold total (A) and central (B) lymph node ratio.
Abbreviations: cLNR, central lymph node ratio; LNR, lymph node ratio.
Only two patients recurred at distant sites outside of the neck. Of those that recurred in the neck, 50% were in the previously operated field and the other 50% were in the contralateral side of the neck.
Multivariate Analysis
Univariate analysis revealed that male sex (hazard ratio [HR]: 1.3, 95% confidence interval [CI]: 1.1–2.1; p = .04) and LNR (HR: 9.6, 95% CI: 1.9–28.8; p < .01) were significant predictors of disease recurrence, whereas age, tumor size, extrathyroidal extension, multifocality, number of metastatic lymph nodes, total lymph node yield, or radioactive iodine treatment were not significantly associated with recurrence (data not shown).
To determine how strongly LNR and cLNR were associated with recurrence relative to other known predictors of recurrence in PTC, we performed multivariate analysis. Instead of limiting the multivariate analysis to the significant terms from the univariate analysis, we chose to include all the variables due to the low sample size and the fact that these factors have been previously shown to be important in predicting disease recurrence and mortality in differentiated thyroid cancer. Age, sex, tumor size, multifocality, extrathyroidal extension, lymphovascular invasion, extranodal extension, treatment with radioactive iodine, and total LNR were included in this analysis (Table 3). We also included total number of lymph nodes dissected in the model to control for the variability in lymph node yield that would offset LNRs to zero or one due to low yield. We found that total LNR was significantly associated with disease recurrence (odds ratio [OR]: 20.7, 95% CI: 1.9–26.8, p = .01; Table 3). This analysis was repeated using cLNR instead of total LNR, and cLNR was also strongly associated with recurrence (OR: 18.1, 95% CI: 1.5–22.2, p = .02). Importantly, LNR and cLNR were the only significant variables in this model (Table 3).
Table 3.
Multivariate logistic regression for recurrence
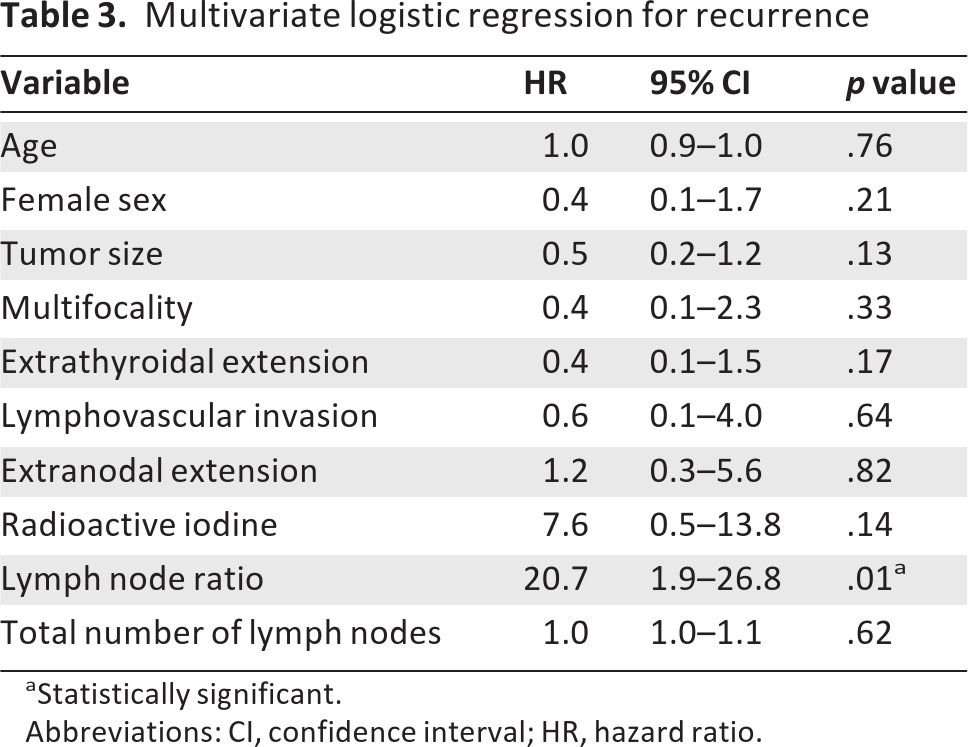
aStatistically significant.
Abbreviations: CI, confidence interval; HR, hazard ratio.
Discussion
In this study, we describe how LNR in PTC affects the relevant oncologic outcome of disease recurrence. LNR is an important determinant of recurrence relative to other previously described clinicopathologic features associated with disease recurrence in PTC. Furthermore, we provide threshold LNRs (0.70 for total LNR and 0.86 for cLNR) that indicate increased probability of recurrence. We believe this information can assist clinicians who care for patients with PTC and lymph node metastases in the postoperative period.
Although LNR and optimal node yield have been well described for other cancers [19, 20, 25], few studies exist for LNR in PTC [26]. Unlike other analyses of LNR, we provide a calculated threshold LNR rather than dividing LNRs into arbitrary divisions [26]; these thresholds are useful for the clinician who must decide who is at greater risk for recurrence by using more of the information gleaned from the final pathology report.
Numerous studies have examined the issue of lymph node metastases in PTC, but these studies simply examine lymph node metastases as a binary factor (presence vs. absence), and controversy has surrounded the impact of lymph node metastases on recurrence and survival [4, 27–28]. Our results support the notion of lymph node metastases as a risk factor for recurrence given that a higher LNR was the only significant factor associated with recurrence when combined with other known determinants of recurrence in a multivariate analysis (Table 3). Moreover, LNR provides a quantitative assessment of the extent of lymph node disease rather than considering lymph node metastases as a binary entity or by compartments as in the current American Joint Committee on Cancer staging system. That is, LNR permits further risk stratification of patients with lymph node metastases rather than grouping a patient with 1 of 10 lymph nodes positive in the same category as a patient with 9 of 10 lymph nodes positive.
Several prognostic schemes place importance on factors such as tumor size and extrathyroidal extension in addition to age and sex [29–34], although these factors were not significant in our multivariate analysis (Table 3). The results presented here, however, cannot be directly compared to these studies because we only evaluated a subset of patients with known lymph node metastases. All the prognostic schemes cited incorporate all comers, including patients without lymph node metastases. Many patients with lymph node metastases will also have other poor prognostic features such as large tumors or extrathyroidal extension, so when considering only patients with lymph node metastases, factors such as extrathyroidal extension may become less important or are collinear with other factors as found (Table 3). The other caveat is that these staging systems incorporate both papillary and follicular histology, whereas this study only includes PTC.
Several authors have examined the number of positive nodes alone to assess risk of recurrence. Although the number of positive nodes correlated with recurrence, the exact number of nodes and indications for lymph node dissection has varied widely [21, 23–24, 35]. When we examined the number of positive lymph nodes, the association with disease recurrence was not nearly as strong as with LNR (OR: 2.08 vs. 20.7). This study demonstrated the patient-to-patient variability in nodal yield, especially in the central compartment (Table 2). Therefore, LNR assesses the proportion of positive nodes in each compartment and provides a quality assessment of the completeness of lymph node dissection within the compartment. As an example, assume that a central neck compartment contains 10 lymph nodes. If a surgeon only removes five and all five are positive, then the lymph node ratio is 100%. A different (more complete) surgeon may remove all 10 nodes, but only 5 of these are positive. In this case, the LNR is only 50%. Obviously, the patient with an incomplete dissection is more likely to recur, and the higher LNR reflects that difference.
Not only does intraoperative technique influence LNR, but preoperative lymph node evaluation can also assist the surgeon in deciding the appropriate extent of dissection. Preoperative imaging identifies the highest pathologic nodes. Then, the surgeon should carry his or her dissection one level beyond these pathologic appearing nodes to capture nodes with micrometastatic disease and a “margin” of negative nodes. This practice obviously lowers the LNR and decreases the chance of recurrence [36–38].
Further stratifying patients with lymph node metastases has several advantages. First, it allows for a more informed discussion with the patient regarding his or her chances of recurrence. In the case of an LNR of 0.1, this might enable the clinician to put the patient at ease, whereas while a patient with a LNR of 0.9 can be given more realistic expectations regarding the potential need for future treatment(s). Although this study did not evaluate various interventions based on LNR, it may prompt clinicians to search for distant metastases and appropriately dose radioactive iodine for patients with a higher LNR. Similarly, a patient with a higher LNR may warrant more frequent follow-up ultrasounds or thyroglobulin levels after the initial treatment phase.
This study is limited by its retrospective nature. Even in the context of a single institution, great variability exists in the conduct, extent, and yield of lymph node dissection among surgeons, and this is especially evident in our data on central compartment lymph node dissection. Lymph node yield depends on how extensively lymph nodes are sought and counted in the specimen, introducing another source of variability that may reduce the LNR denominator. Hence, surgeon, patient, and pathologist factors can influence the ultimate LNR calculation. The single-institution nature of this study should limit this bias. Nonetheless, this variability combined with a low sample size may have biased our calculation of a threshold lymph node ratio to a higher number and may limit widespread applicability of this data.
Future studies using population-based data or multiple institutions will increase the sample size, dampening the effect of such variability. Our group is currently pursuing these studies to provide more accurate data on LNR. A low sample size also limited our ability to perform subset analyses and to exclude patients with very low lymph node yields as these patients will inevitably have lymph node ratios of zero or one. Because we do not practice routine prophylactic central neck dissection and this was a retrospective analysis, this study cannot evaluate this practice, nor does it evaluate the utility of LNR in patients with primarily microscopic disease. All 69 cases included in this study were therapeutic neck dissections where lymph nodes were evaluated with cytology or frozen section in addition to preoperative imaging. Although prophylactic neck dissection has been debated in the literature, the present study only evaluates the utility of LNR in a therapeutic setting.
Despite these limitations, this study demonstrated a very strong association between LNR and disease recurrence in PTC. We feel that LNR can be a useful tool for clinicians involved in the postoperative treatment, surveillance, and counseling of patients with PTC and lymph node metastases.
This article is available for continuing medical education credit at CME.TheOncologist.com.
Author Contributions
Conception and design: David F. Schneider, Herbert Chen, Rebecca S. Sippel
Provision of study material or patients: David F. Schneider, Herbert Chen, Rebecca S. Sippel
Collection and/or assembly of data: David F. Schneider, Herbert Chen, Rebecca S. Sippel
Data analysis and interpretation: David F. Schneider, Haggi Mazeh, Herbert Chen, Rebecca S. Sippel
Manuscript writing: David F. Schneider, Haggi Mazeh, Herbert Chen, Rebecca S. Sippel
Final approval of manuscript: David F. Schneider, Haggi Mazeh, Herbert Chen, Rebecca S. Sippel
Disclosures
The authors indicated no financial relationships.
Section Editor: Stan Sidhu: None
Reviewer “A”: None
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Grubbs EG, Evans DB. Role of lymph node dissection in primary surgery for thyroid cancer. J Natl Compr Canc Netw. 2007;5:623–630. doi: 10.6004/jnccn.2007.0053. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi S, Noguchi A, Murakami N. Papillary carcinoma of the thyroid: Developing pattern of metastasis. Cancer. 1970;26:1053–1060. doi: 10.1002/1097-0142(197011)26:5<1053::aid-cncr2820260513>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Qubain SW, Nakano S, Baba M, et al. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery. 2002;131:249–256. doi: 10.1067/msy.2002.120657. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferri EL, Young RL. Papillary thyroid carcinoma: A 10-year follow-up report of the impact of therapy in 576 patients. Am J Med. 1981;70:511–518. doi: 10.1016/0002-9343(81)90573-8. [DOI] [PubMed] [Google Scholar]
- 5.Wada N, Suganuma N, Nakayama H, et al. Microscopic regional lymph node status in papillary thyroid carcinoma with and without lymphadenopathy and its relation to outcomes. Arch Surg. 2007;392:417–422. doi: 10.1007/s00423-007-0159-4. [DOI] [PubMed] [Google Scholar]
- 6.Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery. 2008;144:1070–1078. doi: 10.1016/j.surg.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 7.Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: A population-based, nested case-control study. Cancer. 2006;106:524–531. doi: 10.1002/cncr.21653. [DOI] [PubMed] [Google Scholar]
- 8.Scheumann GF, Gimm O, Wegener G, et al. Prognostic significance and surgical management of locoregional lymph node mestastases in papillary thyroid cancer. World J Surg. 1994;18:559–567. doi: 10.1007/BF00353765. [DOI] [PubMed] [Google Scholar]
- 9.Schuff KG, Weber SM, Givi B, et al. Efficacy of nodal dissection for treatment of persistent/recurrent papillary thyroid cancer. Laryngoscope. 2008;118:768–775. doi: 10.1097/MLG.0b013e318162cae9. [DOI] [PubMed] [Google Scholar]
- 10.Sippel RS, Chen H. Controversies in the surgical management of newly diagnosed and recurrent/residual thyroid cancer. Thyroid. 2009;19:1373–1380. doi: 10.1089/thy.2009.1606. [DOI] [PubMed] [Google Scholar]
- 11.Sacks W, Fung CH, Chang JT, et al. The effectiveness of radioactive iodine for treatment of low-risk thyroid cancer: A systematic analysis of the peer-reviewed literature from 1966 to April 2008. Thyroid. 2010;20:1235–1245. doi: 10.1089/thy.2009.0455. [DOI] [PubMed] [Google Scholar]
- 12.Iyer NG, Morris LG, Tuttle RM, et al. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–4446. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn JE, Lee JH, Yi JS, et al. Diagnostic accuracy of CT and ultrasonography for evaluating metastatic cervical lymph nodes in patients with thyroid cancer. World J Surg. 2008;32:1552–1558. doi: 10.1007/s00268-008-9588-7. [DOI] [PubMed] [Google Scholar]
- 14.Kloos RT. Thyroid cancer recurrence in patients clinically free of disease with undetectable or very low serum thyroglobulin values. J Clin Endocrinol Metab. 2010;95:5241–5248. doi: 10.1210/jc.2010-1500. [DOI] [PubMed] [Google Scholar]
- 15.Sywak M, Cornford L, Roach P, et al. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery. 2006;140:1000–1005. doi: 10.1016/j.surg.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Hughes DT, White ML, Miller BB, et al. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148:1100–1106. doi: 10.1016/j.surg.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Tisell LE, Nilsson B, Molne J, et al. Improved survival of patients with papillary thyroid cancer after surgical microdissection. World J Surg. 1996;20:854–859. doi: 10.1007/s002689900130. [DOI] [PubMed] [Google Scholar]
- 18.Mazzaferri EL, Doherty GM, Steward DL. The pros and cons of prophylactic central compartment lymph node dissection for papillary thyroid carcinoma. Thyroid. 2009;19:683–689. doi: 10.1089/thy.2009.1578. [DOI] [PubMed] [Google Scholar]
- 19.Engstrom PF, Benson AB, Chen YJ, et al. Colon cancer clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3:468–491. doi: 10.6004/jnccn.2005.0024. [DOI] [PubMed] [Google Scholar]
- 20.Chagpar AB, Scoggins CR, Martin RC, et al. Factors determining adequacy of axillary node dissection in breast cancer patients. Breast J. 2007;13:233–237. doi: 10.1111/j.1524-4741.2007.00415.x. [DOI] [PubMed] [Google Scholar]
- 21.Leboulleux S, Rubino C, Baudin E, et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90:5723–5729. doi: 10.1210/jc.2005-0285. [DOI] [PubMed] [Google Scholar]
- 22.Bardet S, Malville E, Rame JP, et al. Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol. 2008;158:551–560. doi: 10.1530/EJE-07-0603. [DOI] [PubMed] [Google Scholar]
- 23.Sugitani I, Kasai N, Fujimoto Y, et al. A novel classification system for patients with PTC: Addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135:139–148. doi: 10.1016/s0039-6060(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 24.Ricarte-Filho J, Ganly I, Rivera M, et al. Papillary thyroid carcinomas with cervical lymph node metastases can be stratified into clinically relevant prognostic categories using oncogenic BRAF, the number of nodal metastases, and extra-nodal extension. Thyroid. 2012;22:575–584. doi: 10.1089/thy.2011.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mocellin S, Pasquali S, Rossi CR, et al. Validation of the prognostic value of lymph node ratio in patients with cutaneous melanoma: A population-based study of 8,177 cases. Surgery. 2011;150:83–90. doi: 10.1016/j.surg.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 26.Beal SH, Chen SL, Schneider PD, et al. An evaluation of lymph node yield and lymph node ratio in well-differentiated thyroid carcinoma. Am Surg. 2010;76:28–32. [PubMed] [Google Scholar]
- 27.Hughes CJ, Shaha AR, Shah JP, et al. Impact of lymph node metastasis in differentiated carcinoma of the thyroid: A matched-pair analysis. Head Neck. 1996;18:127–132. doi: 10.1002/(SICI)1097-0347(199603/04)18:2<127::AID-HED3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Podnos YD, Smith D, Wagman LD, et al. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71:731–734. doi: 10.1177/000313480507100907. [DOI] [PubMed] [Google Scholar]
- 29.Byar DP, Green SB, Dor P, et al. A prognostic index for thyroid carcinoma: A study of the EORTC Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15:1033–1041. doi: 10.1016/0014-2964(79)90291-3. [DOI] [PubMed] [Google Scholar]
- 30.Hay ID, Grant CS, Taylor WF, et al. Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: A retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102:1088–1095. [PubMed] [Google Scholar]
- 31.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–953. [PubMed] [Google Scholar]
- 32.Hay ID, Bergstralh EJ, Goellner JR, et al. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–1057. [PubMed] [Google Scholar]
- 33.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 34.Sherman SI, Brierley JD, Sperling M, et al. Prospective multicenter study of thyroid carcinoma treatment: Initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer. 1998;83:1012–1021. doi: 10.1002/(sici)1097-0142(19980901)83:5<1012::aid-cncr28>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Ito Y, Fukushima M, Tomoda C, et al. Prognosis of patients with papillary thyroid carcinoma having clinically apparent metastasis to the lateral compartment. Endocr J. 2009;56:759–766. doi: 10.1507/endocrj.k09e-025. [DOI] [PubMed] [Google Scholar]
- 36.Poehls JL, Chen H, Sippel RS. Preoperative ultrasonography findings predict the need for repeated surgery in papillary thyroid cancer. Endocr Pract. 2012;18:403–409. doi: 10.4158/EP11221.OR. [DOI] [PubMed] [Google Scholar]
- 37.Porterfield JR, Factor DA, Grant CS. Operative technique for modified radical neck dissection in papillary thyroid carcinoma. Arch Surg. 2009;144:567–574. doi: 10.1001/archsurg.2009.89. [DOI] [PubMed] [Google Scholar]
- 38.Ito Y, Tomoda C, Uruno T, Takamura Y, et al. Preoperative ultrasonographic examination for lymph node metastasis: Usefulness when designing lymph node dissection for papillary microcarcinoma of the thyroid. World J Surg. 2004;28:498–501. doi: 10.1007/s00268-004-7192-z. [DOI] [PubMed] [Google Scholar]


