Abstract
Many motivated and addiction-related behaviors are sustained by activity of both dopamine D1- and D2-type receptors (D1Rs and D2Rs) as well as CB1 receptors (CB1Rs) in the nucleus accumbens (NAc). Here, we use in vitro whole-cell patch-clamp electrophysiology to describe an endocannabinoid (eCB)–dopamine receptor interaction in adult rat NAc core neurons. D1R and D2R agonists in combination enhanced firing, with no effect of a D1R or D2R agonist alone. This D1R+D2R-mediated firing increase required CB1Rs, since it was prevented by the CB1R antagonists AM251 and Rimonabant. The D1R+D2R firing increase also required phospholipase C (PLC), the major synthesis pathway for the eCB 2-arachidonoylglycerol (2-AG) and one of several pathways for anandamide. Further, inhibition of 2-AG hydrolysis with the monoglyceride lipase (MGL) inhibitor JZL184 allowed subthreshold levels of D1R+D2R receptor agonists to enhance firing, while inhibition of anandamide hydrolysis with the fatty acid amide hydrolase (FAAH) inhibitors URB597 or AM3506 did not. Filling the postsynaptic neuron with 2-AG enabled subthreshold D1R+D2R agonists to increase firing, and the 2AG+D1R+D2R increase in firing was prevented by a CB1R antagonist. Also, the metabotropic glutamate receptor 5 (mGluR5) blocker MPEP prevented the ability of JZL184 to promote subthreshold D1R+D2R enhancement of firing, while the 2-AG+D1R+D2R increase in firing was not prevented by the mGluR5 blocker, suggesting that mGluR5s acted upstream of 2-AG production. Thus, our results taken together are consistent with the hypothesis that NAc core eCBs mediate dopamine receptor (DAR) enhancement of firing, perhaps providing a cellular mechanism underlying the central role of NAc core D1Rs, D2Rs, CB1Rs, and mGluR5s during many drug-seeking behaviors.
Keywords: endocannabinoid, cannabinoid receptors, nucleus accumbens, dopamine receptors, drug addiction
Dopamine (DA) receptor (DAR) signaling in VTA target regions such as the nucleus accumbens (NAc) is known to regulate a number of motivated and addiction-related behaviors (Di Chiara, 2002; Nicola, 2007; Le Foll et al., 2009). DARs occur in two general types, D1-type receptors (D1 or D5, termed here D1R) and D2-type receptors (D2, D3, or D4, termed here D2R) (Le Foll et al., 2009). Interestingly, many behavioral studies suggest that NAc D1Rs and D2Rs can act in a similar manner to promote a number of motivated and addiction-related behaviors, including cue control over reward seeking, drug self-administration, and reinstatement of drug seeking (Hiroi and White, 1991; Hodge et al., 1997; Nowend et al., 2001; Bachtell et al., 2005; Bari and Pierce, 2005; Ferretti et al., 2005; Schmidt and Pierce, 2006; Nicola, 2007; Suto et al., 2009), and we previously showed that co-activation of D1Rs and D2Rs in vitro is required for dopamine enhancement of firing in the NAc shell (Hopf et al., 2003).
CB1 receptors (CB1Rs) are abundantly expressed in the NAc (Herkenham, 1992; Pickel et al., 2006), and NAc CB1Rs also mediate many addiction-related behaviors (Hiranita et al., 2008; Azizi et al., 2009; Morra et al., 2010), including forms of drug self-administration and reinstatement that also require NAc DARs (Xi et al., 2006; Caillé et al., 2007; Alvarez-Jaimes et al., 2008; Malinen and Hyytiä, 2008; Orio et al., 2009). However, despite the potential behavioral importance of NAc DAR/CB1R interactions, very little is known about the molecular mechanisms that could mediate these comparable effects of DARs and CB1Rs on behaviors. At a cellular level, NAc/striatal endocannabinoids (eCBs) are known to act as a retrograde modulator which presynaptically suppresses release of glutamate and GABA (Hoffman and Lupica, 2001; Robbe et al., 2001; Adermark and Lovinger, 2007; Centonze et al., 2007), in addition to CB1Rs present postsynaptically on striatal neurons (Köfalvi et al., 2005; Kearn et al., 2005; Pickel et al., 2006; Ferré et al., 2009). Also, some behavioral studies have found antagonistic interactions between CB1R and DARs (Giuffrida et al., 1999; Martín et al., 2008), and cellular studies have suggested antagonistic CB1R/DAR interactions in the striatum, including CB1R inhibition of PKA stimulation by D1R (Meschler and Howlett, 2001) and direct CB1R inhibition of D2Rs (Ferré et al., 2009).
Thus, much uncertainty remains about the cellular mechanisms through which CB1Rs and DARs can positively interact to promote behavior. CB1Rs are activated by eCBs, and the best studied members of the eCB family are 2-arachidonoylglycerol (2-AG) and anandamide (AEA) (Placzek et al., 2008; Wang and Ueda, 2008). Here, we examined whether DARs and CB1Rs might interact to enhance action potential firing of adult rat NAc core neurons. We focused on the core region of NAc, since it is critical for expression of many types of drug relapse and other addiction-related behaviors (Kalivas and McFarland, 2003; Everitt and Robbins, 2005; Nicola, 2007; Di Ciano et al., 2008).
EXPERIMENTAL PROCEDURES
Animals
All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted by the NIH, and with approval of an Institutional Animal Care and Use Committee. A total of 157 adult, male Wistar rats (~300–400 g) were individually housed. Animals were maintained on a 12-h light/dark cycle with ad libitum access to food and water.
Slice preparation
Rats were deeply anesthetized with 100 mg/kg pentobarbital administered i.p. and perfused transcardially with ~30 ml of nearly frozen (~0 °C) modified artificial cerebrospinal fluid (aCSF) at a rate of ~10 ml/min. The modified aCSF for perfusion contained (in mM): 225 sucrose, 119 NaCl, 2.5 KCl, 1.0 NaH2PO4, 4.9 MgCl2, 0.1 CaCl2, 26.2 NaHCO3, 1.25 glucose, 3 kynurenic acid, and 1 ascorbate acid. After decapitation with a guillotine, the brain was removed rapidly, and brain slices were cut in this same modified aCSF. Coronal slices (275–300 µm) containing the NAc core were prepared using a Leica VT1200S vibratome (Bannockburn, IL, USA). Slices recovered at 32 °C in carbogen-bubbled aCSF (126 mM NaCl, 2.5 mM KCl, 1.2 mM NaH2PO4, 1.2 mM MgCl2, 2.4 mM CaCl2, 18 mM NaHCO3, 11 mM glucose, with pH 7.2–7.4 and mOsm 302–305) containing 1 mM ascorbic acid for 30 min to 5 h. During experiments, slices were submerged and continuously perfused (~2 ml/min) with carbogen-bubbled aCSF warmed to 31–32 °C, and supplemented with CNQX (10 µM, to block AMPA-type glutamate receptors) and picrotoxin (50 µM, to block GABA-A receptors); CNQX and picrotoxin isolate the cell from several major sources of neurotransmitter input whose release is known to be inhibited by DA (Nicola and Malenka, 1997). For most experiments, drugs were bath applied by adding them to the aCSF.
Electrophysiology
All experiments were performed using whole-cell recording using visualized infrared-DIC with 2.5–3.5 MΩ electrodes. Whole-cell patch-clamp experiments were performed with a potassium methanesulfonate- based internal solution containing (in mM): 130 KOH, 105 methanesulfonic acid, 17 HCl, 20 HEPES, 0.2 EGTA, 2.8 NaCl, with 2.5 mg/ml Mg-ATP, 0.25 mg/ml GTP, pH 7.2–7.4, and 278–287 mOsm. Current pulses were applied and electrical signals were recorded using Clampex 10 and an Axon 700A patch amplifier (Axon Instruments, Foster City, CA, USA) in current clamp mode. On breaking into neurons, the resting membrane potential was between −90 and −80 mV. The membrane potential for each neuron was set to ~−80 mV during the experiment by DC injection via patch amplifier (D’Ascenzo et al., 2009).
To measure firing, current pulses were applied using a patch amplifier in current-clamp mode. A series of seven to eight depolarizing current pulses (300 ms duration, 20 pA apart) were applied every 30 s, where the minimum current amplitude was set for each cell so that the first pulse was just subthreshold for spike firing. Depolarizing pulses were alternated with a 100 pA hyperpolarizing pulse to examine the input resistance. Changes in firing after 8–10 min of exposure to a given compound were determined as the percent change in number of action potentials (APs) generated relative to baseline, determined at the current step at baseline with three APs, or four APs if no current steps at baseline had three APs.
Our experiments were restricted to GABAergic medium spiny neurons, which represent more than 90% of the neurons within the NAc and dorsal striatum, and exhibit specific firing characteristics, such as slow, repetitive spike firing (Mahon et al., 2000). Other cell types can be easily distinguished by a large soma (cholinergic neurons) or by very high rates of firing, a larger fast afterhyperpolarization, and a more depolarized resting membrane potential (fast-spiking interneuron; Bracci et al., 2002), and are not studied further.
Analysis of spike firing
Firing data for all neurons was analyzed with Clampfit (Axon Instruments, Foster City, CA, USA) using the same criteria. To calculate percent change in spiking for a given cell, a current pulse was selected that exhibited three to four spikes at baseline. The same current pulse was then used for all time points for that cell. Spike firing rates during the 4 min before addition of the reagent were averaged, and this value was normalized to 100%. We then determined the percent change in firing versus baseline during the average of 7–9 min after starting exposure to a given reagent. Thus, even if it is unclear whether a DAR/CB1R-related change in firing a given cell has reached plateau, we applied consistent criteria for analysis in every cell; in general, however, 7–9 min application was sufficient to observe a plateau level for changes in firing.
Reagents
Drugs were obtained from Sigma (St. Louis, MO, USA), except cannabinoid reagents which were purchased from Tocris Bioscience (Ellisville, MO, USA). Compounds were dissolved in Tocrisolve (all cannabinoid reagents) or DMSO, except DNQX and picrotoxin which were dissolved in water. Compounds were made in aliquots and stored at 4 °C for reagents in Tocrisolve and −20 °C for others.
Statistics
Statistical significance of changes in spike rate for a particular experimental condition was determined in comparison with an appropriate control condition. Data were analyzed with ANOVAs using StatView (SAS Institute Inc., Cary, NC, USA), with Sheffe post hoc tests, unless otherwise indicated. We should also note that cannabinoid reagents in general did not by themselves enhance action potential firing, consistent with other studies showing minimal post-synaptic effect of CB1R activation alone (Hoffman and Lupica, 2001), and sometimes produced a small decrease in firing (see Fig. 3B, C). Thus, we only present the statistical significance of the interactions of cannabinoid reagents with DAR agonists. All data shown are the means±SEM.
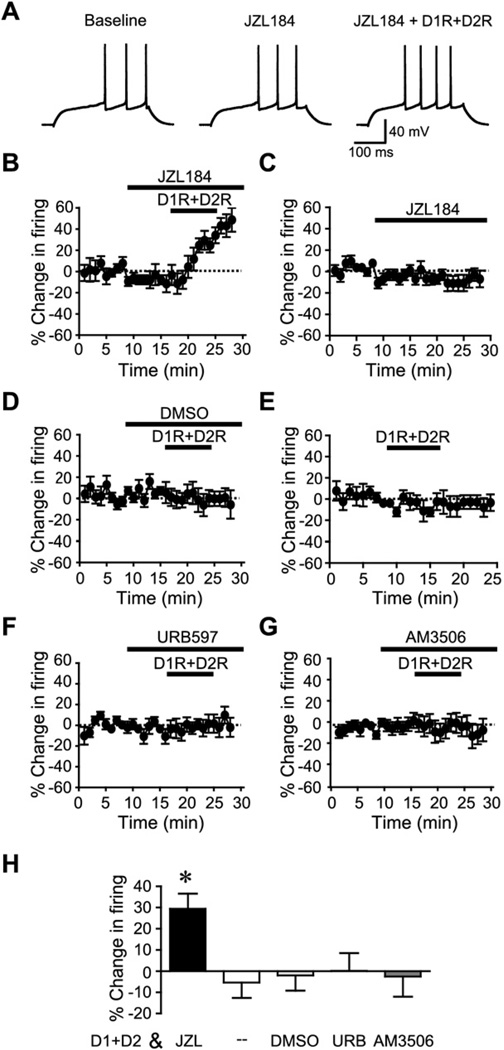
Fig. 3.
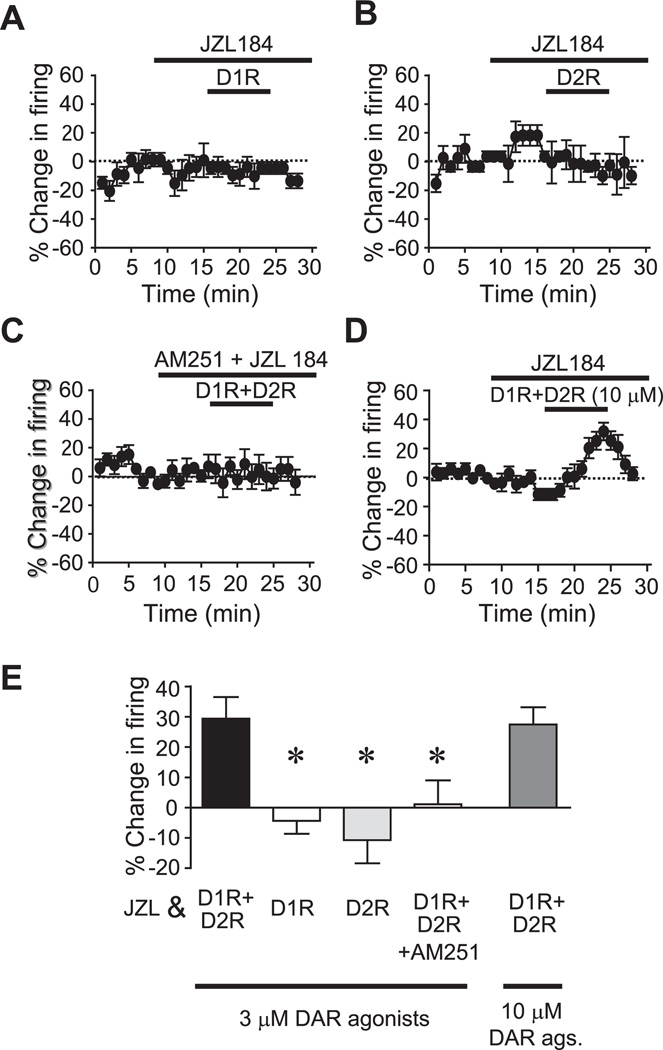
Subthreshold concentrations of D1R+D2R agonists (3 µM each) enhance firing in the presence of a 2-AG hydrolysis inhibitor. (A, B) Example traces (A) and time course experiments (B) showing that pre-exposure to the MGL inhibitor JZL184 allowed subthreshold levels of D1R+D2R agonists (3 µM each of SKF82597 and quinpirole) to enhance firing. (C) Prolonged exposure to JZL had no effect on firing. (D) Pre-exposure to the vehicle DMSO (0.1%) did not allow a DAR-mediated increase in firing. (E) 3 µM each of D1R and D2R agonists in combination were subthreshold for firing enhancement. (F, G) FAAH inhibitors URB597 (F) and AM3506 (G) did not promote the DAR-mediated firing enhancement. (H) Grouped data. JZL, JZL184; URB, URB597. * P<0.05 JZL vs. other groups.
RESULTS
Both D1Rs and D2Rs are required to enhance NAc core spike firing
We examined action potential firing of adult rat NAc core medium spiny neurons during depolarizing current steps, where a series of 300 ms depolarizing and hyperpolarizing current pulses was applied every 30 s. We previously showed that DA enhances firing in NAc shell neurons through D1R+D2R co-activation, which may be mediated by G-protein βγ-subunits released from the Gi/o-linked D2Rs acting in combination with Gαs-like subunits from D1Rs (Hopf et al., 2003). Here, we observed that co-activation of D1Rs and D2Rs was required to increase firing in NAc core neurons. Bath application of 10 µM each of SKF81297 (D1R agonist) and quinpirole (D2R agonist) significantly enhanced firing of NAc core neurons (Fig. 1A–C; n=21; P<0.01, paired t-test; 23.9±3.9% change in firing). However, spike firing was not altered by the D1R agonist alone (10 µM; Fig. 1C; n=7; 1.4±4.4% change in firing) or the D2R agonist alone (10 µM; Fig. 1C; n=9; 2.8±6.4% change in firing) (both P<0.01 vs. D1R+D2R). In all experiments, neurons were held at ~−80 mV by passage of DC current with the patch amplifier (D’Ascenzo et al., 2009), and thus changes in firing were not a consequence of depolarization (see also Hopf et al., 2003). Thus, as seen in the NAc shell of younger rats (Hopf et al., 2003), D1R and D2R agonists are required in combination to enhance action potential firing in NAc core neurons from adult rats.
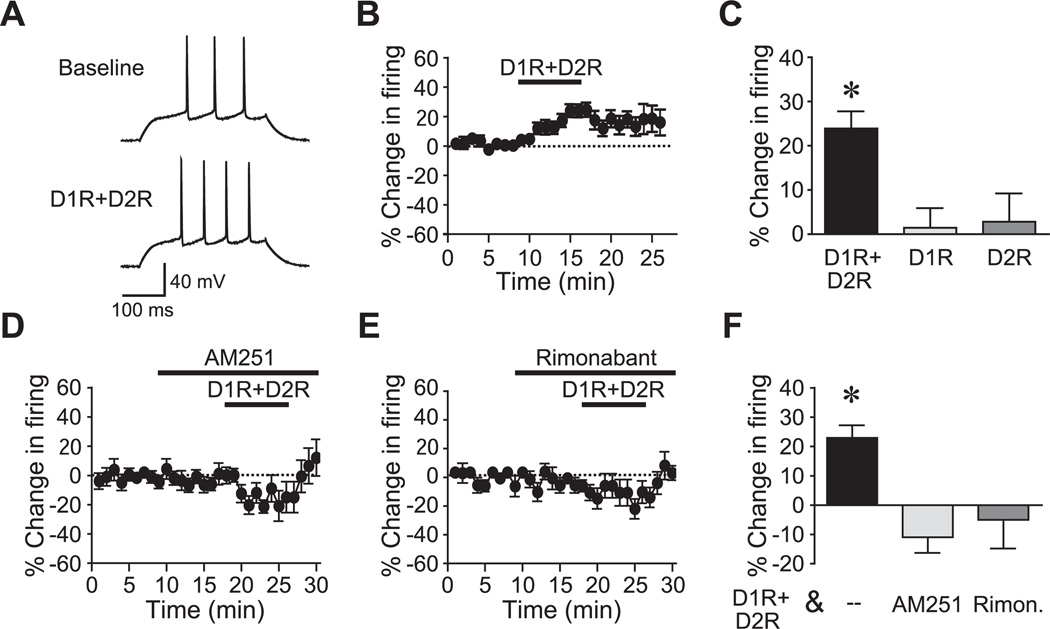
Fig. 1.
D1R+D2R agonists in combination enhance firing of NAc core neurons through CB1Rs. (A, B) Representative traces (A) and time course data (B) showing that exposure to D1R+D2R agonists (10 µM each of SKF-81297, D1R agonist, and quinpirole, D2R agonist) in combination enhanced firing of NAc core neurons. (C) D1R agonist alone or D2R agonist alone did not increase firing. (D–F) Time course and grouped data showing that the CB1R antagonists AM251 (D, F) and Rimonabant (E, F) prevented the D1R+D2R firing increase. D1+D2 data in (F) are the same as in (C). Rimon, Rimonabant. * P<0.05 vs. D1R or D2R alone.
CB1R antagonists prevent the D1R+D2R enhancement of firing
To examine whether the eCB system and CB1R activation were important for the D1R+D2R enhancement of firing, CB1Rs were blocked with two different antagonists, AM251 (2 µM) or Rimonabant (3 µM). Thus, after 8 min stable baseline, AM251 was bath applied for 8 min, followed by addition of D1R+D2R agonists (SKF81297 and quinpirole, 10 µM each). Pretreatment of NAc core neurons with AM251 prevented the D1R+D2R-mediated enhancement of firing (Fig. 1D, F; n=11; P<0.01; −14.1±5.9% change in firing). AM251 inhibition of the D1R+D2R enhancement of firing suggests a role for the CB1Rs, and thus we determined whether the D1R+D2R enhancement of firing was also inhibited by a structurally-unrelated CB1R antagonist, Rimonabant (also called SR 141716A). As with AM251, bath application of Rimonabant prevented the D1R+D2R enhancement of firing (Fig. 1E, F; n=7; P<0.05; −6.9±9.8% change in firing). We should also note that D1R+D2R actually inhibited firing in the presence of CB1R antagonists, which was significant for AM251 (P<0.05). Although our results below (Fig. 8) suggest that DARs enhance firing through action on the slow A-type potassium current, it has also been recognized that DARs in NAc neurons can act on multiple targets which have divergent effects on firing (see Greengard et al., 1999; Nicola et al., 2000; and Discussion). Nonetheless, our results suggest that CB1Rs contribute to DAR enhancement of NAc core firing.
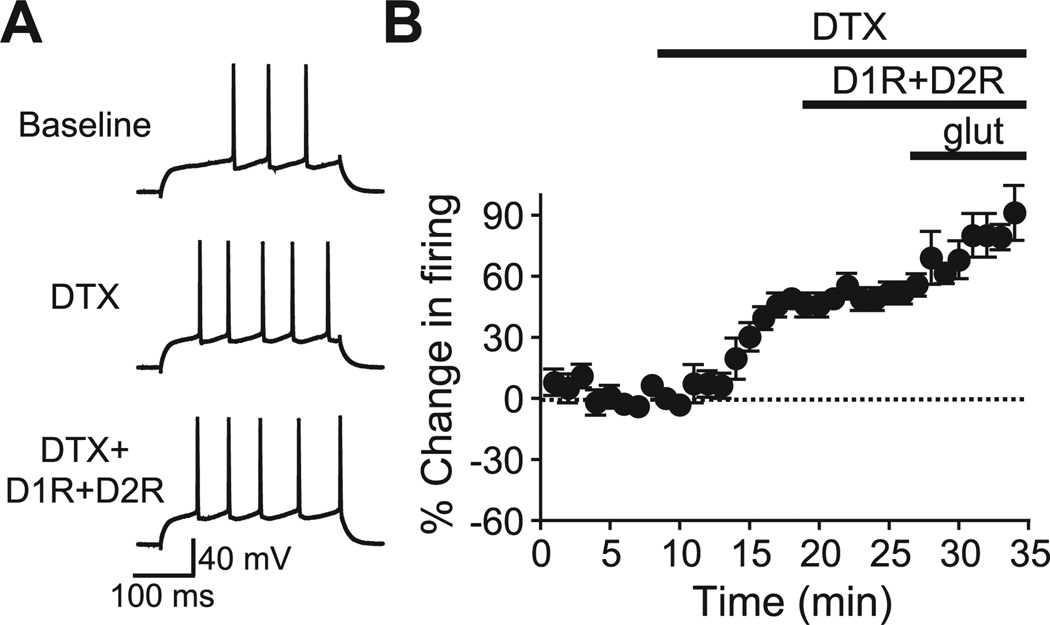
Fig. 8.
(A) Examples and (B) grouped data showing that inhibition of the slow A-type potassium current with α-dendrotoxin (DTX) significantly occluded the D1R+D2R enhancement of NAc core firing. DTX (0.5 µM) pre-exposure occluded the ability of 10 µM D1R+D2R agonists to enhance firing, as previously observed in the NAc shell (Hopf et al., 2003). (B) This was not due to a ceiling effect, as glutamate exposure (200 µM in the presence of DNQX which blocks AMPA receptors) was able to enhance firing in the presence of DTX (Hopf et al., 2003).
PLC-dependent pathways contribute to D1R+D2R enhancement of firing
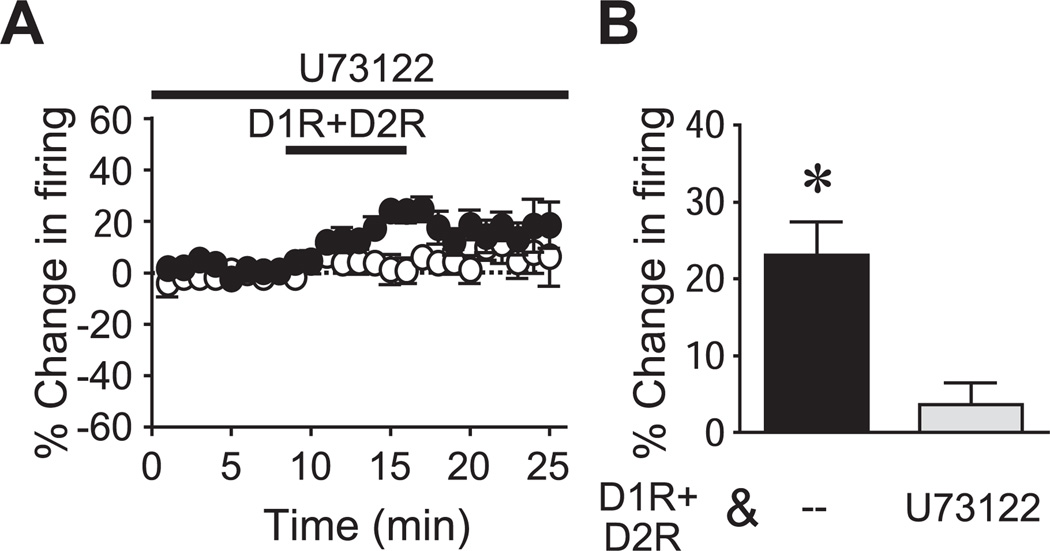
To investigate the intracellular pathways through which CB1R and DAR activation could facilitate NAc core firing, and to gain insight into which eCB mediates the D1R+D2R enhancement of firing, we first examined the involvement of phospholipase C (PLC). PLC mediates several eCB synthesis pathways, including the major biosynthetic pathway for 2-AG and one of the several biosynthetic pathways for AEA (Freund et al., 2003; Placzek et al., 2008; Wang and Ueda, 2008). We found that the PLC inhibitor U73122 (10 µM, with 30 min pre-exposure and exposure during the recording, Hopf et al., 2003) significantly prevented the D1R+D2R enhancement of firing in NAc core neurons (Fig. 2; n=11; P<0.01; 2.7±4.1% change in firing). This suggests that PLC-dependent pathways were required for the D1R+D2R enhancement of NAc core firing, which could constrain the possible biosynthetic pathways contributing to eCB production.
Fig. 2.
PLC-dependent pathways are needed for D1R+D2R enhancement of firing. (A, B) Time course (A) and grouped data (B) showing that the PLC antagonist U73122 prevented the D1R+D2R (10 µM of each) mediated increase in firing. D1R+D2R data same as in Fig. 1. * P<0.05 vs. D1R+D2R.
Inhibition of 2-AG hydrolysis facilitates the DAR enhancement of NAc core firing
Since eCBs and CB1Rs seem to mediate the NAc core D1R+D2R enhancement of firing, we examined whether inhibition of eCB hydrolysis might facilitate the DAR enhancement of firing. We hypothesized that inhibition of eCB hydrolysis could promote the ability of subthreshold concentrations of DAR agonists to enhance firing. In particular, by using agents that more selectively reduce 2-AG versus AEA hydrolysis, we could perhaps determine whether 2-AG or AEA contribute to the D1R+D2R enhancement of NAc core firing. AEA is hydrolyzed by fatty acid amide hydrolase (FAAH), while 2-AG is primarily hydrolyzed by a monoglyceride lipase (MGL) but also sometimes by FAAH (Deutsch and Chin, 1993; Dinh et al., 2002; Cravatt and Lichtman, 2003; Long et al., 2009).
Pre-exposure to the selective MGL inhibitor, JZL184 (10 µM; Long et al., 2009) allowed subthreshold concentrations of DAR agonists (3 µM each of SKF81297 and quinpirole) to significantly enhance NAc core firing (Fig. 3A, B, H; n=7; P<0.05; 29.4±7.1% change in firing), while subthreshold DAR agonists did not increase firing in neurons pretreated with the DMSO control (Fig. 3D, H; n=8; −2.0±7.2% change in firing) or in neurons not pre-treated with DMSO (Fig. 3E, H; n=6; −5.4±7.2% change in firing). Thus 3 µM of DAR agonists have no effect on firing in the NAc core, although they can increase firing in the NAc shell (Hopf et al., 2003). Also, no increases in firing were seen with longer treatment with JZL184 (JZL) alone (Fig. 3C; n=7; P<0.05 vs. JZL+D1R+D2R; −11.0±6.6% change in firing), suggesting that the JZL+D1R+D2R increase did not reflect slowly-developing JZL-mediated changes in firing. Importantly, in contrast to JZL, subthreshold DAR agonists did not increase firing in neurons pretreated with the FAAH inhibitors URB597 (1 µM; Adermark and Lovinger, 2007; Fig. 3F, H; n=7; 0.0±8.4% change in firing) or a novel FAAH inhibitor AM3506 (2 µM; Godlewski et al., 2010; Fig. 3G, H; n=8; −3.0±9.5% change in firing). Together, these results suggest that 2-AG rather than AEA contributes to the DAR enhancement of firing. Also, JZL pre-exposure did not enable firing when only a D1R agonist (10 µM; Fig. 4A, E; n=5; P<0.01 vs. JZL+D1R+D2R; −4.0±4.2% change in firing) or a D2R agonist (10 µM; Fig. 4B, E; n=5; P<0.01 vs. JZL+D1R+D2R; −10.7±7.7% change in firing) was applied, indicating that both D1Rs and D2Rs were required for JZL to enhance firing. In addition, pretreatment with AM251 blocked the JZL+D1R+D2R enhancement of NAc core firing (Fig. 4C, E; n=10; P<0.05; 1.1±7.9% change in firing), suggesting that the ability of JZL to promote DAR enhancement of firing required activation of CB1Rs. Finally, higher concentrations of D1R and D2R agonists (10 µM of each) also increased firing in the presence of JZL (Fig. 4D, E; n=10; −27.0±5.6% change in firing), but this firing increase was not greater than observed with JZL+3 µM DAR agonists or 10 µM DAR agonists without JZL (P>0.5).
Fig. 4.
JZL promotion of the DAR firing increase requires D1Rs, D2Rs, and CB1Rs. (A, B) Time course experiments showing that JZL did not allow a D1R agonist alone (A, 10 µM) or a D2R agonist alone (B, 10 µM) to promote firing. (C) JZL facilitation of subthreshold D1R+D2R enhancement of firing (3 µM each, see Fig. 3B) required CB1Rs. (D) DAR agonist (10 µM each) enhancement of firing in the presence of JZL. (E) Grouped data. JZL+subthreshold D1R+D2R agonists data same as in Fig. 3. Ags, agonists; JZL, JZL184. * P<0.05 vs. JZL+subthreshold DAR agonists.
We also examined the change in firing frequency in neurons exposed to JZL+D1R+D2R, since the firing increase was most consistent in this group. NAcb MSNs show little accommodation during firing (Hopf et al., 2010), and thus we examined the instantaneous firing frequency of the first pair of action potentials in the spike train at three different depolarizing current steps: the current step at which percent change in firing was measured, and the current step below and above that step. At the lowest current step, firing frequency changed from 13.6±0.7 Hz at baseline to 17.5±0.8 Hz, a 29.6±4.4% increase. At the intermediate current step (at which percent change in action potential firing was measured), firing frequency changed from 17.9±0.8 Hz at baseline to 22.3±1.2 Hz, a 23.9±2.4% increase. At the higher current step, firing frequency changed from 22.8±1.3 Hz at baseline to 26.8±1.9 Hz, a 16.9±2.2% increase. Thus, JZL+D1R+ D2R significantly enhanced firing frequency (P<0.01 by post-hoc at each current step after two-way repeated measures ANOVA) in addition to the number of action potentials fired.
2-AG-mediated enhancement of firing requires co-activation of D1 and D2 receptors
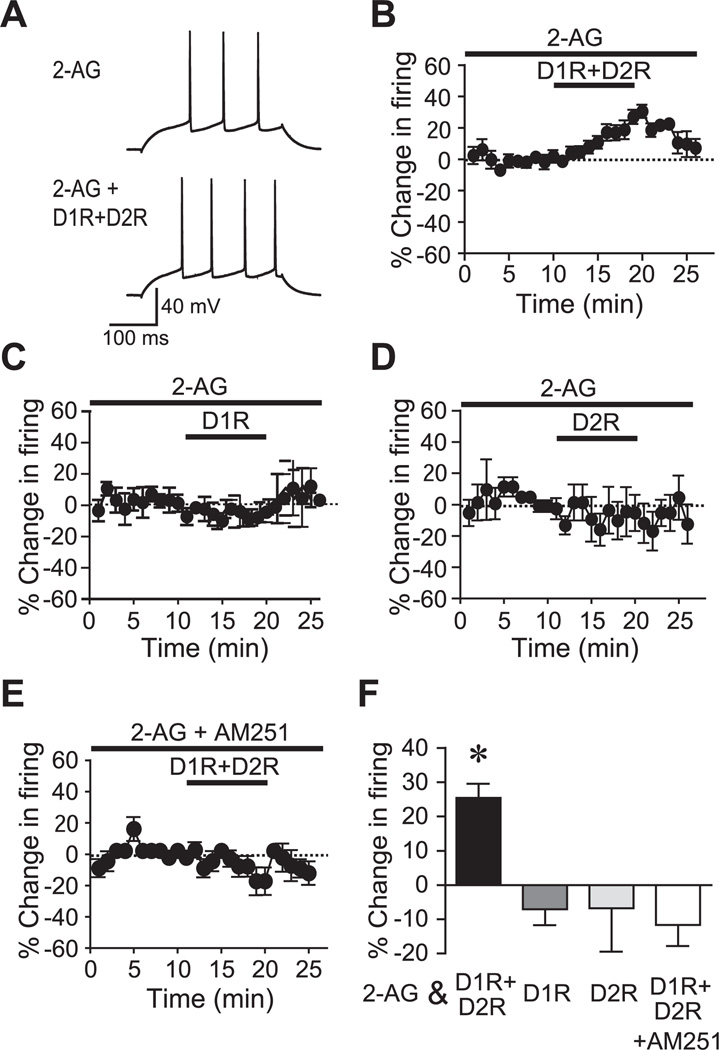
JZL enhancement of firing when combined with subthreshold DAR agonists suggests that 2-AG but not AEA may be required for the CB1R/DAR-mediated enhancement of NAc core firing. Thus, we next examined whether filling the postsynaptic neuron with 2-AG could act as a CB1R agonist. Following the methods of Adermark and Lovinger (2007), we filled NAc core neurons with 2-AG (75 µM) and determined whether this could promote a DAR-mediated increase in firing. Postsynaptic loading with 2-AG enabled subthreshold concentration (3 µM) of D1R+D2R agonists to significantly enhance firing (Fig. 5A, B, F; n=10; P<0.01; 25.4±4.1% change in firing), whereas application of a D1R or a D2R agonist alone in the presence of 2-AG did not increase firing (Fig. 5C, D, F; n=7 for 2-AG+D1R and n=5 for 2-AG+D2R; P<0.05, 2AG with D1R+D2R vs. 2AG with D1R or D2R; −7.0±4.6% or −6.7%±12.6% change in firing for 2-AG with D1R or D2R). Also, the 2-AG+DAR enhancement of firing was prevented by the CB1R blocker AM251 (Fig. 5E, F; n=5; −11.0±6.1% change in firing). In combination with our JZL results, these data support the hypothesis that D1R+D2R enhancement of NAc core firing requires the eCB 2-AG and CB1R activation.
Fig. 5.
Postsynaptic 2-AG promotes D1R+D2R enhancement of firing through CB1Rs. (A, B) Example traces (A) and time course experiments (B) showing that filling a post synaptic neuron with 2-AG allowed subthreshold doses of D1R+D2R agonists to increase firing. (C, D) Filling the postsynaptic neuron with 2-AG did not allow a D1R agonist (C, 10 µM) or D2R agonist (D, 10 µM) alone to enhance firing. (E) The 2-AG+D1R+D2R increase in firing required CB1Rs. (F) Grouped data. * P<0.05 2-AG+D1R+D2R vs. other groups.
mGluR5 activation provides the 2-AG required for the D1R+D2R firing enhancement
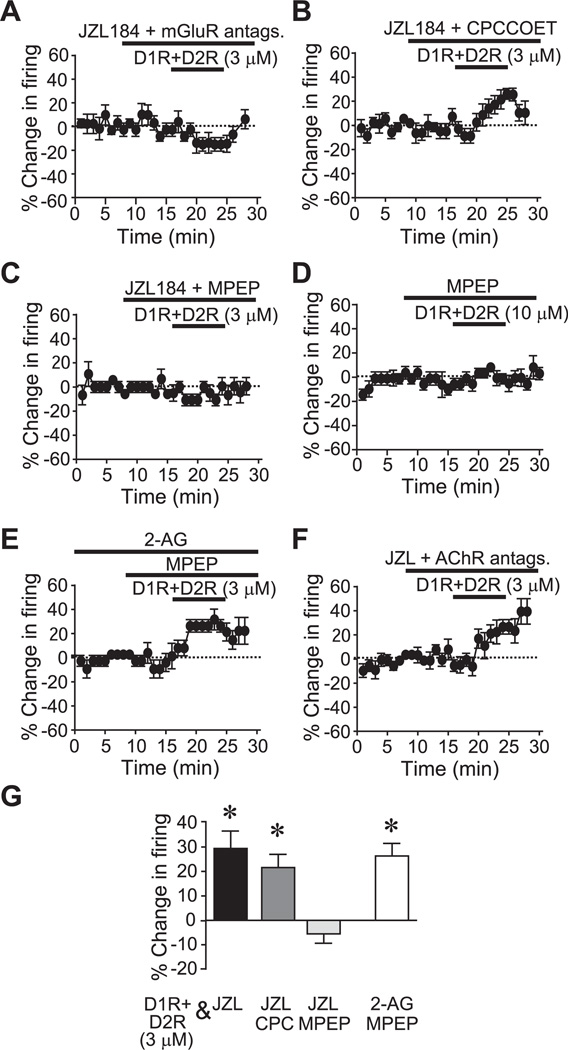
2-AG synthesis through PLC has been linked to the activation of group I metabotropic glutamate receptors (mGluR) (Jung et al., 2005; Adermark and Lovinger, 2007; Centonze et al., 2007; Uchigashima et al., 2007). Thus, we found that pre-exposure to mGluR group I blockers for mGluR5 (MPEP, 10 µM) and mGluR1 (CPCCOEt, 50 µM) in combination blocked the firing enhancement by JZL+D1R+D2R (Fig. 6A, G; n=5; P<0.01; −15.0±3.9% change in firing). In addition, the mGluR5 antagonist MPEP (10 µM) prevented the JZL+D1R+D2R enhancement of firing (Fig. 6C, G; n=5; P<0.05; −5.6±3.8% change in firing), while the mGluR1 antagonist, CPCCOEt (50 µM) did not prevent the enhancement in firing (Fig. 6B, G; n=8; P<0.05; 22.0±5.5% change in firing). MPEP also prevented the increase in firing normally elicited by 10 µM of D1R and D2R agonists in combination (Fig. 6D; n=6; P<0.05 vs. no MPEP; −2.5±3.6% change in firing). These results suggest a primary role for mGluR5s in the eCB/DAR enhancement of NAc core firing.
Fig. 6.
Group I mGluR activation is required for the DAR-mediated firing increase. (A) The JZL+D1R+D2R (3 µM each) firing increase was prevented by a cocktail of the mGluR1 antagonist CPCCOET and the mGluR5 antagonist MPEP. (B, C) The mGluR1 antagonist did not prevent the DAR (3 µM each) firing increase (B), while the mGluR5 antagonist did prevent the firing increase (C). (D) MPEP prevented the enhancement of firing by 10 µM each of the D1R and D2R agonists. (E) The mGluR5 antagonist did not prevent the 2-AG+D1R+D2R (3 µM each of DAR agonist) enhancement of firing, suggesting that mGluR5s are upstream of 2-AG. (F) The JZL+DAR-mediated enhancement of firing (3 µM of each DAR agonist) was not prevented by a cocktail containing antagonists for both nicotinic (methyllycaconitine; dihydro-β-erythroidine) and muscurinic ACh receptors (scopolamine). (G) Grouped data. JZL+D1R+D2R data same as in Fig. 3. JZL, JZL184; CPC, CPCCOET. * P<0.05 change in firing vs. appropriate control groups.
We next examined whether mGluR5s are required for the enhancement of firing when a neuron is loaded with 2-AG before DAR agonist application. If mGluR5s only contribute to DAR enhancement of firing through 2-AG synthesis, then mGluR5 blockers should not prevent the firing enhancement when cells are directly filled with 2-AG before DAR agonist application. In fact, MPEP did not block the increase in firing with 2-AG and subthreshold D1R+D2R agonists (Fig. 6E, G; n=5; P<0.05 vs. 2-AG with D1R or D2R agonist alone; 26.3±5.2% change in firing), suggesting that mGluR5 activation is upstream of 2-AG and thus is not required when 2-AG is already present.
The neurotransmitter acetylcholine (ACh) and cholinergic receptors (AChR) can regulate firing activity of striatal neurons (Howe and Surmeier, 1995; Lin et al., 2004; Musella et al., 2010), and eCBs can modulate the activity of striatal cholinergic neurons (Narushima et al., 2007; Bonsi et al., 2008). Therefore, we investigated the potential involvement of AChRs in the eCB/DAR enhancement of NAc core neuron firing. A cocktail containing nicotinic and muscarinic AChR antagonists (scopolamine: 3 µM; methylly-caconine: 100 nM; dihydro-β-erythroidine: 1 µM) did not block the enhancement of firing by JZL and subthreshold D1R+D2R agonists (Fig. 6F; n=6; P<0.05; 25.1±7.2% change in firing). Thus, AChRs do not contribute to the eCB/DAR-mediated enhancement of firing.
Since mGluR5s are thought to be localized post-synaptically in NAc core neurons (Shigemoto et al., 1993; Romano et al., 1995), and mGluR5s have been linked to 2-AG production through PLC (Jung et al., 2005; Adermark and Lovinger, 2007; Centonze et al., 2007; Uchigashima et al., 2007; current study), we examined whether eCBs from the post-synaptic neurons under patch could contribute to the CB1R/DAR-mediated firing enhancement. Evidence suggests that eCB release from neurons involves an eCB transporter protein (Di Marzo et al., 1994; Gerdeman et al., 2002; Adermark and Lovinger, 2007). Thus, we followed the methodology described in Gerdeman et al. (2002), where a transporter inhibitor was loaded postsynaptically into a neuron by inclusion of the drug in the recording pipette solution; this study showed that eCBs released from the cell under patch-clamp were required for LTD induction. The authors interpreted their results to indicate that, under normal conditions, only eCBs released by a given neuron are able to influence the development of plasticity in that neuron and that, in some cases, eCBs can be considered to act in an autocrine-like manner.
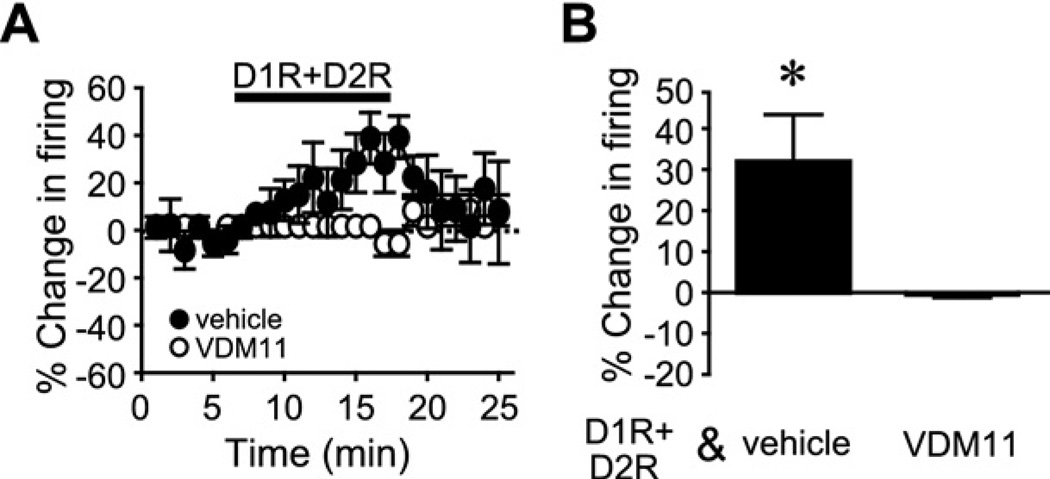
Loading a neuron with an eCB transporter inhibitor VDM11 (1 µM) (Gerdeman et al., 2002) by including the inhibitor in the recording pipette blocked the D1R+D2R enhancement of firing (10 µM each SKF81297 and quin-pirole), while filling neurons with the vehicle Tocrisolve (1 µM) did not prevent the D1R+D2R increase in firing (Fig. 7; n=5 VDM11, n=7 vehicle; P<0.05, t-test; −0.6±0.6% or 31.9%±11.5% change in firing for VDM11 or vehicle). These results suggest that eCBs that might be generated by nearby neurons were unable to influence firing in the cell under study.
Fig. 7.
The eCBs needed for the D1R+D2R enhancement of NAc core firing are synthesized within the cell under patch-clamp. (A, B) Time course (A) and grouped data (B) showing that postsynaptic loading of a neuron with the eCB transporter inhibitor VDM11 prevented the D1R+D2R enhancement of firing, while filling the neuron with the Tocrisolve vehicle had no effect. * P<0.05 vehicle vs. VDM11.
Ionic mechanism of D1R+D2R firing enhancement
To address the ionic mechanism through which DARs enhance NAc core firing, we examined the effect of α-dendrotoxin (DTX), a slow A-type potassium channel inhibitor which mediates dopamine enhancement of NAc shell firing (Hopf et al., 2003). As in the NAc shell, pre-exposure to 0.5 µM DTX enhanced firing alone (44.8±4.8% change in firing) and occluded the ability of DARs to increase firing (Fig. 8; n=9; 9.0±4.5% change in firing 10 µM D1R+D2R after DTX; P<0.05 vs. no DTX), suggesting that DARs enhance NAc core firing through inhibition of A-type potassium currents. In addition, as seen in the NAc shell, application of glutamate (200 µM) was able to increase firing in the presence of DTX (Fig. 8; tested in five cells), suggesting that DTX pre-exposure did not prevent DAR firing enhancement through a ceiling effect on the ability to increase firing.
DISCUSSION
Here, we provide evidence that eCBs mediate the ability of DA receptors to enhance action potential firing in adult rat NAc core neurons in vitro. D1R and D2R agonists in combination increased action potential firing, while neither agonist alone modified firing. This enhancement of NAc core firing required CB1Rs, suggesting a role for eCBs, and also was mediated by PLC, which mediates several eCB synthesis pathways including the major pathway for 2-AG. Further, inhibition of 2-AG hydrolysis but not AEA hydrolysis allowed subthreshold concentrations of D1R and D2R agonists in combination to enhance firing in a CB1R-dependent manner, suggesting a 2-AG role in the DAR firing increase. Loading the post-synaptic neuron with 2-AG also allowed subthreshold D1R+D2R to enhance firing. DAR enhancement of NAc core firing required mGluR5 but not mGluR1 or cholinergic receptors. Interestingly, the ability of 2-AG to promote the D1R+D2R-mediated firing increase required CB1Rs but not mGluR5s, suggesting that mGluR5 acted upstream of 2-AG production. Also, mGluR5 is thought to be post-synaptic in the NAc, and the D1R+D2R enhancement of firing was blocked by preventing eCB release from the cell under patch-clamp. Finally, DTX occlusion of the DAR enhancement of firing suggests that the latter occurred through the A-slow potassium current, as seen in the NAc shell (Hopf et al., 2003). Taken together, our results suggest that the eCB 2-AG and mGluR5 may play a prominent role in the D1R+D2R-mediated firing increase in NAc core neurons.
The striatum/NAc has one of the highest concentrations of CB1Rs (Herkenham, 1992; Pickel et al., 2006). CB1Rs are activated by eCBs, the best studied of which are 2-AG and AEA. These eCBS generally have different synthesis and degradation pathways, with AEA hydrolyzed primarily by FAAH, and 2-AG hydrolyzed primarily by MGL (Placzek et al., 2008; Wang and Ueda, 2008). Here, we found that subthreshold concentrations of DAR agonists in combination did not increase firing in cells pretreated with the DMSO control or the FAAH inhibitors URB597 or AM3506. In contrast, subthreshold D1R+D2R activation enhanced firing after pretreatment with JZL, an MGL inhibitor that would be predicted to enhance 2-AG levels (Long et al., 2009). Further, 2-AG infusion into a neuron allowed an increase in firing with D1R+D2R agonists in combination, but not with a D1R agonist or D2R agonist alone, similar to what was observed after pre-exposure to JZL. eCB hydrolysis and CB1R blockers and 2-AG had no affect on firing alone. Taken together, these results suggest that 2-AG mediated activation of CB1Rs, and that CB1R in combination with both D1R and D2R activation was needed for the DAR enhancement of firing.
We should note that DAR agonists actually inhibited firing in the presence of CB1R blockers but not other signaling blockers. Although our results suggest that DARs enhanced firing through a DTX-sensitive potassium current, DARs in striatal neurons are known to be able to act on multiple targets which could have divergent effects on firing (see Greengard et al., 1999; Nicola et al., 2000); we speculate that the increase in firing is usually greater than any DAR-mediated inhibition, but when the signaling pathway that enhances firing is inhibited, a DAR-dependent but CB1R-independent decrease in firing becomes apparent. Why DAR-mediated inhibition is not seen with other blockers (e.g. PLC) is unclear, although there is some indication of DAR agonist inhibition with mGluR antagonists (Fig. 6A, C); thus, our results could indicate that some aspects of the DAR modulation of firing require, for example, PLC for both excitation and inhibition. In addition, we should note that washout of DAR/CB1R enhancement of firing is somewhat variable across neurons, which is consistent with our previous NAc shell studies showing more variable washout under whole-cell patch-clamp conditions relative to more robust washout using perforated-patch, when the intracellular mileau is intact. Finally, we found that the increase in firing was similar when applying JZL plus subthreshold concentrations of DAR agonists (3 µM) versus effective concentrations (10 µM) of DAR agonists. This perhaps suggests a ceiling effect in terms of the ability of DAR/CB1R-activated signaling to enhance firing, which would be consistent with what we previously observed in the NAc shell where a similar increase in firing was seen with 10 µM D1/D2 agonists and with forskolin, a very strong activator of PKA (Hopf et al., 2003). In addition, in the present study, the increase in firing with higher concentrations of DAR agonists was similar with or without JZL, making experiments with subthreshold concentrations of DAR agonists necessary to determine the impact of JZL on firing.
We also found a role for mGluR5 in the D1R+D2R and DAR/CB1R enhancement of NAc core firing. Group I mGluRs, primarily mGluR5 but also mGluR1, have been linked to PLC and eCB production in the striatum (Jung et al., 2005; Kreitzer and Malenka, 2005; Adermark and Lovinger, 2007; Centonze et al., 2007; Uchigashima et al., 2007). Importantly, mGluR5s are postsynaptic in NAc/striatum medium spiny neurons (Shigemoto et al., 1993; Romano et al., 1995), and striatal mGluR5 activation has been linked to synthesis of 2-AG but not AEA (Maccarrone et al., 2008), consistent with our data suggesting a role for 2-AG. Also, 2-AG can be generated through PLC and subsequent action of DAG lipase-α (Bisogno et al., 2003), and group I mGluRs, PLC, and DAG lipase-α may be enriched together around spines in the striatum (Nakamura et al., 2004; Mitrano and Smith, 2007; Uchigashima et al., 2007), and mGluRs and PLC involved in eCB synthesis are not required at a stage where eCBs have been already produced (Piomelli, 2003). Also, we found no role for mGluR1, even though it is present postsynaptically in the NAc (Mitrano and Smith, 2007). Although the mechanism leading to mGluR5 activation remains unclear, mGluR5 and mGluR1 can exist in a constitutively active state even in the absence of an agonist (Ango et al., 2001); alternately, mGluRs could be activated by tonically released glutamate, perhaps from glia (Reissner and Kalivas, 2010). In this regard, eCBs are considered to be synthesized and released extracellularly on demand (Placzek et al., 2008; Wang and Ueda, 2008), but tonic CB1R activation both in vitro and in vivo has been reported (Gifford and Ashby, 1996; Zhou and Shearman, 2004; but see Sperlágh et al., 2009). It would be interesting in future experiments to examine the impact of modulators of glial metabolism and glutamate transporter activity in order to address the possibility of tonic glutamate levels activating mGluR5s. Finally, we should note that our 2-AG+D1R+D2R effects on firing are more modest than those seen with JZL+D1R+D2R. Nonetheless, these 2-AG/DAR results exhibited a similar pharmacological pattern as JZL/DARs (blocked by CB1R antagonists, requiring D1R and D2R agonists in combination) and thus our 2-AG experiments support the hypothesis that 2-AG mediates the DAR/CB1R enhancement of NAc core firing.
Many studies of eCB function have examined the ability of striatal eCBs to act as a retrograde modulator which presynaptically suppresses release of glutamate and GABA (Hoffman and Lupica, 2001; Robbe et al., 2001; Adermark and Lovinger, 2007; Centonze et al., 2007). For example, D2Rs and group I mGluRs acting through eCBs can work in concert to depress striatal glutamatergic transmission (Kreitzer and Malenka, 2005; Yin and Lovinger, 2006). It has also been suggested that CB1Rs can enhance striatal DA release, although mixed results have been reported (Gardner, 2005; Sidló et al., 2008; Sperlágh et al., 2009). Here, we have added picrotoxin to block GABA-A receptors and CNQX to block AMPA receptors. Also, mGluR5 seems to contribute upstream of eCB production. Thus, eCB effects on presynaptic release of GABA or glutamate could have little role. Also, our previous studies in the NAcb shell found that DAR excitation was not inhibited by preventing neurotransmitter release with calcium channel blockers or tetrodotoxin (TTX) (Hopf et al., 2003). Instead, results from several studies suggest that eCBs could also act on post-synaptic CB1Rs that have been observed in striatal neurons (Köfalvi et al., 2005; Pickel et al., 2006). For example, CB1Rs can form postsynaptic heteromers with D2Rs (Ferré et al., 2009), and, interestingly, this association allows these receptors to activate the cAMP system (Glass and Felder, 1997; Kearn et al., 2005); our previous work has shown a role for PKA in the D1R+D2R-mediated enhancement of NAc shell firing (Hopf et al., 2003). We should also note that some cellular studies find antagonistic CB1R/DAR interactions in the striatum, including CB1R inhibition of PKA stimulation by D1R (Meschler and Howlett, 2001) and direct CB1R inhibition of D2Rs (Ferré et al., 2009), similar to behavioral studies showing antagonistic CB1R/DAR interactions (Giuffrida et al., 1999; Martín et al., 2008). Although our results do not conclusively demonstrate the localization of the CB1R required for the DAR effects, they do suggest that post-synaptic 2-AG from the neuron under patch-clamp, perhaps a product of mGluR5 activity, was required for the CB1R/DAR enhancement of firing.
Our studies also do not directly address whether the D1R+D2R enhancement of NAc core firing involves D1Rs and D2Rs on the same or different neurons. It is widely considered that D1Rs and D2Rs are segregated into direct- and indirect-pathway striatal neurons, respectively, with little co-expression (Gerfen, 2004; Surmeier et al., 2007), and thus D1R/D2R interactions would occur between neurons (e.g. Jones et al., 2001). Recent BAC-transgenic mouse studies elegantly demonstrate that direct- and indirect-pathway neurons have mutually exclusive afferent projections, as well as different physiological properties (Kreitzer and Malenka, 2007; Surmeier et al., 2007; Cepeda et al., 2008). However, rat (Kawaguchi et al., 1990; Tripathi et al., 2010) and primate (Lévesque and Parent, 2005; Nadjar et al., 2006) direct-pathway neurons also project to pallidal areas typically thought to be targets only for indirect-pathway neurons, perhaps suggesting some species differences. For example, several studies have used acutely dissociated rat striatal neurons, where inter-cellular interactions are minimal, to show that isolated neurons can respond electrophysiologically to both D1R and D2R agonists (Surmeier et al., 1995, 1996; Waszczak et al., 1998; Zhang et al., 1998; Flores-Hernández et al., 2002; Hu et al., 2005). Some rat antibody and in situ studies have also suggested co-localization of D1Rs and D2Rs (Ariano et al., 1992; Surmeier et al., 1995) or peptides associated with direct- and indirect-pathway neurons (Furuta et al., 2002; cf. Lévesque and Parent, 2005), with greater co-localization in the NAc compared with dorsal striatum (Furuta et al., 2002; Perreault et al., 2010). Accumbens D1R/D2R heteromers acting via PLC have also been reported (Perreault et al., 2010). Also, mouse indirect pathway dorsal striatal neurons have greater basal firing than direct pathway neurons (e.g. Kreitzer and Malenka, 2007), while rat dorsal striatal neurons do not show evidence of two populations of basal firing (Hopf et al., 2010). Although this remains an area of considerable controversy, we speculate that there may be species differences in the amount of D1R/D2R overlap, where rat striatal neurons may contain a preponderance of one type of DAR, but possess some functional receptors of both types. For example, segregation of these rarer receptors into microdomains (Ferré et al., 2009; Perreault et al., 2010) could allow physiological action of these receptors. Although it is very difficult to definitively address whether the D1R+D2R enhancement of firing occurs through D1Rs and D2Rs on the same or different neurons (or even glia) in the intact slice, our results identify a novel interaction whereby the mGluR5, 2-AG, and CB1Rs mediate DAR enhancement of NAc core firing.
The neurotransmitter ACh plays a prominent role in regulating the activity of striatal projection neurons. For example, postsynaptic M1 mAChR activation in striatal neurons reduces GABA release via 2-AG (Narushima et al., 2007; Uchigashima et al., 2007). Our data, however, suggest that AChRs are not involved with the eCB/DAR-mediated enhancement of firing, since increased firing was observed even after pre-exposure to nicotinic and muscarinic AChR antagonists. In this regard, mGluR5, PLC, and DAG lipase are localized in striatal spines, while M1 mAChRs are excluded from this association (Uchigashima et al., 2007). In addition, studies suggest an opposite role for CB1Rs and nAChRs in the NAc core during methamphetamine reinstatement (Hiranita et al., 2008).
We found that DTX occluded the ability of D1R/D2R agonists to enhance firing, suggesting that, as seen in the NAc shell (Hopf et al., 2003), the slow A-type potassium current mediates the dopaminergic enhancement of NAc core firing. In addition, the CB1R/DAR increase in firing was not accompanied by a change in input resistance (Table 1), suggesting that DAR activation did not enhance firing by modulating ion channels active at the resting membrane potential. Also, although we found no DAR/CB1R-associated change in input resistance (Table 1), other studies have shown a dopamine receptor-mediated depolarization in the NAc (Podda et al., 2010) and NAc shell (Hopf et al., 2003) which was associated with a change in input resistance in Podda et al. (2010) although not in Hopf et al. (2003). Here, we applied DC current to maintain the resting potential near ~−80 mV throughout the experiment, which would obviate any effects of depolarization on firing. In addition, our experiments here were performed in adult rats, while the studies of Podda et al. (2010) were performed in very young (p13–p17) mice; the complement of ion channels as well as other aspects of electrophysiology and morphology can be quite different between juvenile and adult animals (Tepper et al., 1998; Butler et al., 1998; Colwell et al., 1998; Partridge et al., 2000). We should also note that activation of mGluR5s can enhance NAc spike firing in very young mice through increasing persistent sodium currents (D’Ascenzo et al., 2009), and low concentrations of the sodium channel blocker TTX prevent the mGluR5 enhancement of firing (D’Ascenzo et al., 2009). However, we find that low TTX concentrations produce a small but significant depolarization of the action potential threshold and a decrease in the action potential amplitude in striatal neurons (Hopf et al., 2010), as predicted for changes in sodium channel function (Zhang et al., 1998), but the CB1R/DAR enhancement of firing occurred without any change in action potential threshold or amplitude (Table 1). Finally, a number of electrophysiology studies have examined the impact of D1R and D2R agonists on NAc/striatal firing in vitro, with mixed results (Nicola et al., 2000). However, nearly all such studies have been performed in adolescent (p30–p50) animals (Zhang et al., 1998; Hu et al., 2005; Perez et al., 2006; but see Uchimura and North, 1990), in contrast to the adult animals used here.
Table 1.
Increased firing with JZL/D1/D2 was not accompanied by a change in the action potential threshold or amplitude (suggesting no changes in sodium channel function) or input resistance (suggesting no changes in ion channels such as inwardly rectifying potassium channel and potassium leak channels that are active at the resting potential). Data are taken 8 min after addition of JZL, or 8 min after addition of D1R and D2R agonists in the presence of JZL
| Baseline | Post-JZL | JZL+D1R+D2R | |
|---|---|---|---|
| Action potential threshold (mV) | −45.54±1.53 | −45.64±1.58 | −46.35±1.87 |
| Action potential amplitude (mV) | 79.43±2.13 | 79.04±2.61 | 77.85±2.94 |
| Input resistance (MΩ) | 63.17±6.21 | 62.87±5.53 | 63.83±6.45 |
JZL, JZL184.
All F<1 and P>0.1.
Our in vitro results suggest that the D1R+D2R enhancement of NAc core firing was mediated through mGluR5, eCBs, and CB1Rs, and we are particularly interested in the possible behavioral importance of the mGluR5/eCB/DAR interaction. The only behavior we are aware of where D1Rs, D2Rs, CB1Rs, and mGluR5 in the NAc core have all been tested is ethanol self-administration, and all these receptors support ethanol intake (Hodge et al., 1997; Caillé et al., 2007; Malinen and Hyytiä, 2008; Besheer et al., 2010). In addition, alcohol self-administration is not altered by systemic inhibition of AEA hydrolysis with URB597 (Cippitelli et al., 2008), in concordance with our in vitro results that 2-AG rather than AEA contributes to the DAR/CB1R enhancement of NAc core firing. Further, D1R+D2R enhancement of firing in NAc shell neurons in vitro involves Gβγ subunits (Hopf et al., 2003), and increased NAc core levels of AGS3, a promoter of Gβγ-dependent signaling, facilitates motivation for ethanol (Bowers et al., 2008), while NAc Gβγ subunit inhibition reduces ethanol self-administration (Yao et al., 2002). Other addiction-related behaviors are also regulated by NAc CB1Rs and DARs. For example, reinstatement for heroin is promoted by NAc core CB1Rs (Caillé et al., 2007; Alvarez-Jaimes et al., 2008) and D1Rs (Bossert et al., 2007), cocaine-primed reinstatement for cocaine is regulated by NAc D1Rs and D2Rs (Schmidt et al., 2006) and mGluR5s (Kumaresan et al., 2009), and NAc CB1Rs mediate both cocaine reinstatement (Xi et al., 2006) and self-administration under long access (Orio et al., 2009). Although these results suggest that CB1R/DAR interactions through the mechanisms described here could contribute to addiction-related behaviors, there are also NAc-dependent behaviors where D1Rs and D2Rs can have different or no contribution, including some aspects of drug seeking and impulsivity (McFarland and Kalivas, 2001; Goto and Grace, 2005; Pezze et al., 2007; Haluk and Floresco, 2009).
ECBs modulate many diverse biological targets in the central nervous system, and therefore play a critical role in addiction as well as the pathophysiology of severe neurological disorders such Parkinson’s disease (Gubellini et al., 2002), Huntington’s disease (Centonze et al., 2005), dystonia (Richter and Löscher, 2002). Pharmacological intervention in this system may allow us to identify new therapeutic routes for several neurological disorders while eliminating undesirable side effects associated with cannabis. The result from this study may be useful in identifying novel signaling interactions that could represent translational targets for treating addiction, drug-related behavior or other diseases.
Acknowledgments
This work was supported by the RO1AA015358 (F.W.H.), NIDA F32DA028065 (T.S.), and funds provided by the State of California for medical research for alcohol and substance abuse through the University of California, San Francisco (A.B.).
Abbreviations
- 2-AG
2-arachidonoylglycerol
- Ach
acetylcholine
- AChR
cholinergic receptor
- aCSF
artificial cerebrospinal fluid
- AEA
anandamide
- AP
action potential
- CB1R
CB1 receptor
- DA
dopamine
- DAR
dopamine receptor
- DTX
α-dendrotoxin
- D1R
D1-type receptor (D1 or D5 receptor)
- D2R
D2-type receptor (D2, D3, or D4 receptor)
- eCB
endocannabinoid
- FAAH
fatty acid amide hydrolase
- JZL
JZL184
- MGL
monoglyceride lipase
- mGluR
metabotropic glutamate receptor;
- NAc
nucleus accumbens
- PLC
phospholipase C
- TTX
tetrodotoxin
REFERENCES
- Adermark L, Lovinger DM. Retrograde endocannabinoid signaling at striatal synapses requires a regulated postsynaptic release step. Proc Natl Acad Sci U S A. 2007;104:20564–20569. doi: 10.1073/pnas.0706873104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–2493. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Stromski CJ, Smyk-Randall EM, Sibley DR. D2 dopamine receptor localization on striatonigral neurons. Neurosci Lett. 1992;144:215–220. doi: 10.1016/0304-3940(92)90753-t. [DOI] [PubMed] [Google Scholar]
- Azizi P, Haghparast A, Hassanpour-Ezatti M. Effects of CB1 receptor antagonist within the nucleus accumbens on the acquisition and expression of morphine-induced conditioned place preference in morphine-sensitized rats. Behav Brain Res. 2009;197:119–124. doi: 10.1016/j.bbr.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intra-nucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharmacology (Berl) 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–822. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Platania P, Martella G, Madeo G, Vita D, Tassone A, Bernardi G, Pisani A. Distinct roles of group I mGlu receptors in striatal function. Neuropharmacology. 2008;55:392–395. doi: 10.1016/j.neuropharm.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, Hopf FW, Chou JK, Guillory AM, Chang S-J, Janak PH, Bonci A, Diamond IF. Nucleus accumbens AGS3 expression drives ethanol seeking through Gβγ. Proc Natl Acad Sci U S A. 2008;105:12533–12538. doi: 10.1073/pnas.0706999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci E, Centonze D, Bernardi G, Calabresi P. Dopamine excites fast-spiking interneurons in the striatum. J Neurophysiol. 2002;87:2190–2194. doi: 10.1152/jn.00754.2001. [DOI] [PubMed] [Google Scholar]
- Butler AK, Uryu K, Chesselet MF. A role for N-methyl-d-aspartate receptors in the regulation of synaptogenesis and expression of the polysialylated form of the neural cell adhesion molecule in the developing striatum. Dev Neurosci. 1998;20:253–262. doi: 10.1159/000017319. [DOI] [PubMed] [Google Scholar]
- Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Prosperetti C, Gasperi V, De Chiara V, Bari M, Tscherter A, Febbraro F, Bernardi G, Maccarrone M. Endocannabinoids limit metabotropic glutamate 5 receptor-mediated synaptic inhibition of striatal principal neurons. Mol Cell Neurosci. 2007;35:302–310. doi: 10.1016/j.mcn.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Prosperetti C, Tscherter A, Bernardi G, Maccarrone M, Calabresi P. Abnormal sensitivity to cannabinoid receptor stimulation might contribute to altered gamma-aminobutyric acid transmission in the striatum of R6/2 Huntington’s disease mice. Biol Psychiatry. 2005;57:1583–1589. doi: 10.1016/j.biopsych.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cepeda C, André VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, Defonseca FR, Piomelli D, Ciccocioppo R. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology (Berl) 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Cepeda C, Crawford C, Levine MS. Postnatal development of glutamate receptor-mediated responses in the neostriatum. Dev Neurosci. 1998;20:154–163. doi: 10.1159/000017310. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. Fatty acid amide hydrolase: an emerging therapeutic target in the endocannabinoid system. Curr Opin Chem Biol. 2003;7:469–475. doi: 10.1016/s1367-5931(03)00079-6. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo M, Podda MV, Fellin T, Azzena GB, Haydon P, Grassi C. Activation of mGluR5 induces spike afterdepolarization and enhanced excitability in medium spiny neurons of the nucleus accumbens by modulating persistent Na+ currents. J Physiol. 2009;587:3233–3250. doi: 10.1113/jphysiol.2009.172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, Chin SA. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Ferré S, Goldberg SR, Lluis C, Franco R. Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology. 2009;56(Suppl 1):226–234. doi: 10.1016/j.neuropharm.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti V, Florian C, Costantini VJ, Roullet P, Rinaldi A, De Leonibus E, Oliverio A, Mele A. Co-activation of glutamate and dopamine receptors within the nucleus accumbens is required for spatial memory consolidation in mice. Psychopharmacology (Berl) 2005;179:108–116. doi: 10.1007/s00213-005-2144-3. [DOI] [PubMed] [Google Scholar]
- Flores-Hernández J, Cepeda C, Hernández-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, Greengard P, Levine MS. Dopamine enhancement of NMDA currents in dissociated medium-sized striatal neurons: role of D1 receptors and DARPP-32. J Neurophysiol. 2002;88:3010–3020. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Furuta T, Zhou L, Kaneko T. Preprodynorphin-, preproenkephalin-, preprotachykinin A- and preprotachykinin B-immunoreactive neurons in the accumbens nucleus and olfactory tubercle: double-immunofluorescence analysis. Neuroscience. 2002;114:611–627. doi: 10.1016/s0306-4522(02)00312-3. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal ganglia. In: Paxinos G, editor. The rat nervous system. 3rd ed. San Diego, CA: Elsevier Academic Press; 2004. pp. 455–508. [Google Scholar]
- Gifford AN, Ashby CR., Jr Electrically evoked acetylcholine release from hippocampal slices is inhibited by the cannabinoid receptor agonist, WIN 55212-2, and is potentiated by the cannabinoid antagonist, SR 141716A. J Pharmacol Exp Ther. 1996;277:1431–1436. [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci. 1997;17:5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski G, Alapafuja SO, Bátkai S, Nikas SP, Cinar R, Offertáler L, Osei-Hyiaman D, Liu J, Mukhopadhyay B, Harvey-White J, Tam J, Pacak K, Blankman JL, Cravatt BF, Makriyannis A, Kunos G. Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol. 2010;17:1256–1266. doi: 10.1016/j.chembiol.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Picconi B, Bari M, Battista N, Calabresi P, Centonze D, Bernardi G, Finazzi-Agrò A, Maccarrone M. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22:6900–6907. doi: 10.1523/JNEUROSCI.22-16-06900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluk DM, Floresco SB. Ventral striatal dopamine modulation of different forms of behavioral flexibility. Neuropsychopharmacology. 2009;34:2041–2052. doi: 10.1038/npp.2009.21. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann N Y Acad Sci. 1992;654:19–32. doi: 10.1111/j.1749-6632.1992.tb25953.x. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Yamamoto T. Methamphetamine-seeking behavior is due to inhibition of nicotinic cholinergic transmission by activation of cannabinoid CB1 receptors. Neuropharmacology. 2008;55:1300–1306. doi: 10.1016/j.neuropharm.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Hiroi N, White NM. The amphetamine conditioned place preference: differential involvement of dopamine receptor subtypes and two dopaminergic terminal areas. Brain Res. 1991;552:141–152. doi: 10.1016/0006-8993(91)90672-i. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein betagamma subunits. J Neurosci. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Seif T, Mohamedi ML, Chen BT, Bonci A. The small-conductance calcium-activated potassium channel is a key modulator of firing and long-term depression in the dorsal striatum. Eur J Neurosci. 2010;31:1946–1959. doi: 10.1111/j.1460-9568.2010.07231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe AR, Surmeier DJ. Muscarinic receptors modulate N-, P-, and L-type Ca2+ currents in rat striatal neurons through parallel pathways. J Neurosci. 1995;15:458–469. doi: 10.1523/JNEUROSCI.15-01-00458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, Dong Y, Zhang XF, White FJ. Dopamine D2 receptor-activated Ca2+ signaling modulates voltage-sensitive sodium currents in rat nucleus accumbens neurons. J Neurophysiol. 2005;93:1406–1417. doi: 10.1152/jn.00771.2004. [DOI] [PubMed] [Google Scholar]
- Jones EA, Wang JQ, McGinty JF. Intrastriatal GABA(A) receptor blockade does not alter dopamine D(1)/D(2) receptor interactions in the intact rat striatum. Neuroscience. 2001;102:381–389. doi: 10.1016/s0306-4522(00)00451-6. [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–1202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Emson PC. Projection subtypes of rat neostriatal matrix cells revealed by intracellular injection of biocytin. J Neurosci. 1990;10:3421–3438. doi: 10.1523/JNEUROSCI.10-10-03421.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol. 2005;67:1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Köfalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlágh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25:10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Parent A. The striatofugal fiber system in primates: a reevaluation of its organization based on single-axon tracing studies. Proc Natl Acad Sci U S A. 2005;102:11888–11893. doi: 10.1073/pnas.0502710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Chung KK, de Castro D, Funk GD, Lipski J. Effects of muscarinic acetylcholine receptor activation on membrane currents and intracellular messengers in medium spiny neurones of the rat striatum. Eur J Neurosci. 2004;20:1219–1230. doi: 10.1111/j.1460-9568.2004.03576.x. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agrò A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- Mahon S, Delord B, Deniau JM, Charpier S. Intrinsic properties of rat striatal output neurones and time-dependent facilitation of cortical inputs in vivo. J Physiol. 2000;527(Pt 2):345–354. doi: 10.1111/j.1469-7793.2000.t01-1-00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinen H, Hyytiä P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2008;32:1976–1983. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- Martín AB, Fernandez-Espejo E, Ferrer B, Gorriti MA, Bilbao A, Navarro M, Rodriguez de Fonseca F, Moratalla R. Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology. 2008;33:1667–1679. doi: 10.1038/sj.npp.1301558. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschler JP, Howlett AC. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology. 2001;40:918–926. doi: 10.1016/s0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Morra JT, Glick SD, Cheer JF. Neural encoding of psychomotor activation in the nucleus accumbens core, but not the shell, requires cannabinoid receptor signaling. J Neurosci. 2010;30:5102–5107. doi: 10.1523/JNEUROSCI.5335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musella A, De Chiara V, Rossi S, Cavasinni F, Castelli M, Cantarella C, Mataluni G, Bernardi G, Centonze D. Transient receptor potential vanilloid 1 channels control acetylcholine/2-arachidonoylglicerol coupling in the striatum. Neuroscience. 2010;167:864–871. doi: 10.1016/j.neuroscience.2010.02.058. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Brotchie JM, Guigoni C, Li Q, Zhou SB, Wang GJ, Raven-scroft P, Georges F, Crossman AR, Bezard E. Phenotype of striatofugal medium spiny neurons in parkinsonian and dyskinetic nonhuman primates: a call for a reappraisal of the functional organization of the basal ganglia. J Neurosci. 2006;26:8653–8661. doi: 10.1523/JNEUROSCI.2582-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sato K, Fukaya M, Araishi K, Aiba A, Kano M, Watanabe M. Signaling complex formation of phospholipase Cbeta4 with metabotropic glutamate receptor type 1alpha and 1,4,5-trisphosphate receptor at the perisynapse and endoplasmic reticulum in the mouse brain. Eur J Neurosci. 2004;20:2929–2944. doi: 10.1111/j.1460-9568.2004.03768.x. [DOI] [PubMed] [Google Scholar]
- Narushima M, Uchigashima M, Fukaya M, Matsui M, Manabe T, Hashimoto K, Watanabe M, Kano M. Tonic enhancement of endocannabinoid-mediated retrograde suppression of inhibition by cholinergic interneuron activity in the striatum. J Neurosci. 2007;27:496–506. doi: 10.1523/JNEUROSCI.4644-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC. Dopamine depresses excitatory and inhibitory synaptic transmission by distinct mechanisms in the nucleus accumbens. J Neurosci. 1997;17:5697–5710. doi: 10.1523/JNEUROSCI.17-15-05697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29:4846–4857. doi: 10.1523/JNEUROSCI.0563-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Tang KC, Lovinger DM. Regional and postnatal heterogeneity of activity-dependent long-term changes in synaptic efficacy in the dorsal striatum. J Neurophysiol. 2000;84:1422–1429. doi: 10.1152/jn.2000.84.3.1422. [DOI] [PubMed] [Google Scholar]
- Perez MF, White FJ, Hu XT. Dopamine D(2) receptor modulation of K(+) channel activity regulates excitability of nucleus accumbens neurons at different membrane potentials. J Neurophysiol. 2006;96:2217–2228. doi: 10.1152/jn.00254.2006. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O Dowd BF, George SR. The dopamine D1–D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem. 2010;285:36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology. 2007;32:273–283. doi: 10.1038/sj.npp.1301073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Placzek EA, Okamoto Y, Ueda N, Barker EL. Membrane microdomains and metabolic pathways that define anandamide and 2-arachidonyl glycerol biosynthesis and breakdown. Neuropharmacology. 2008;55:1095–1104. doi: 10.1016/j.neuropharm.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda MV, Riccardi E, D’Ascenzo M, Azzena GB, Grassi C. Dopamine D1-like receptor activation depolarizes medium spiny neurons of the mouse nucleus accumbens by inhibiting inwardly rectifying K+ currents through a cAMP-dependent protein kinase A-independent mechanism. Neuroscience. 2010;167:678–690. doi: 10.1016/j.neuroscience.2010.02.075. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol. 2010;21:514–522. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A, Löscher W. Effects of pharmacological manipulations of cannabinoid receptors on severity of dystonia in a genetic model of paroxysmal dyskinesia. Eur J Pharmacol. 2002;454:145–151. doi: 10.1016/s0014-2999(02)02477-9. [DOI] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC. Cooperative activation of D1-like and D2-like dopamine receptors in the nucleus accumbens shell is required for the reinstatement of cocaine-seeking behavior in the rat. Neuroscience. 2006;142:451–461. doi: 10.1016/j.neuroscience.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Sidló Z, Reggio PH, Rice ME. Inhibition of striatal dopamine release by CB1 receptor activation requires nonsynaptic communication involving GABA, H2O2, and KATP channels. Neurochem Int. 2008;52:80–88. doi: 10.1016/j.neuint.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperlágh B, Windisch K, Andó RD, Sylvester Vizi E. Neurochemical evidence that stimulation of CB1 cannabinoid receptors on GABAergic nerve terminals activates the dopaminergic reward system by increasing dopamine release in the rat nucleus accumbens. Neurochem Int. 2009;54:452–457. doi: 10.1016/j.neuint.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Ecke LE, Wise RA. Control of within-binge cocaine-seeking by dopamine and glutamate in the core of nucleus accumbens. Psychopharmacology (Berl) 2009;205:431–439. doi: 10.1007/s00213-009-1553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Sharpe NA, Koós TZ, Trent F. Postnatal development of the rat neostriatum: electrophysiological, light- and electron-microscopic studies. Dev Neurosci. 1998;20:125–145. doi: 10.1159/000017308. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Prensa L, Cebrián C, Mengual E. Axonal branching patterns of nucleus accumbens neurons in the rat. J Comp Neurol. 2010;518:4649–4673. doi: 10.1002/cne.22484. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura N, North RA. Actions of cocaine on rat nucleus accumbens neurones in vitro. Br J Pharmacol. 1990;99:736–740. doi: 10.1111/j.1476-5381.1990.tb12999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ueda N. Role of the endocannabinoid system in metabolic control. Curr Opin Nephrol Hypertens. 2008;17:1–10. doi: 10.1097/MNH.0b013e3282f29071. [DOI] [PubMed] [Google Scholar]
- Waszczak BL, Martin LP, Greif GJ, Freedman JE. Expression of a dopamine D2 receptor-activated K+ channel on identified striatopallidal and striatonigral neurons. Proc Natl Acad Sci U S A. 1998;95:11440–11444. doi: 10.1073/pnas.95.19.11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–8536. doi: 10.1523/JNEUROSCI.0726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Arolfo MP, Dohrman DP, Jiang Z, Fan P, Fuchs S, Janak PH, Gordon AS, Diamond I. Betagamma dimers mediate synergy of dopamine D2 and adenosine A2 receptor-stimulated PKA signaling and regulate ethanol consumption. Cell. 2002;109:733–743. doi: 10.1016/s0092-8674(02)00763-8. [DOI] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci U S A. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ. Whole-cell plasticity in cocaine withdrawal: reduced sodium currents in nucleus accumbens neurons. J Neurosci. 1998;18:488–498. doi: 10.1523/JNEUROSCI.18-01-00488.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Shearman LP. Voluntary exercise augments acute effects of CB1-receptor inverse agonist on body weight loss in obese and lean mice. Pharmacol Biochem Behav. 2004;77:117–125. doi: 10.1016/j.pbb.2003.10.015. [DOI] [PubMed] [Google Scholar]