Abstract
In the present study, archived U.S biosolids from the 2001 Environmental Protection Agency (EPA) National Sewage Sludge Survey were analyzed with an expanded U.S EPA Method 1694, to determine the occurrence of 26 previously unmonitored pharmaceuticals and personal care products (PPCPs) among a total of 120 analytes. The study further served to examine the reproducibility of a mega-composite approach for creating chemical mass inventories in biosolids based on pooled samples from wastewater treatment plants (WWTPs) nationwide. Five mega-composites reflecting 94 WWTPs in 32 states and the District of Columbia were constructed from archived biosolids and analyzed by LC/ESI-MS/MS using a newly introduced analytical method expanding upon U.S EPA Method 1694. In addition, soil-biosolids mixtures from a mesocosm setup were analyzed to experimentally determine the half-lives of biosolids-borne compounds applied on U.S land. Among 59 analytes detected, 33 had been reported previously, whereas 26 are reported in biosolids for the first time, at levels ranging from 1.65 to 673 μg kg−1 dry weight. Newly recognized biosolids constituents were identified as Ca2+ channel blockers, antidepressants, diuretics, β-blockers and analgesics. Using a mass balance approach, the total loading of these 26 pharmaceuticals to U.S soils from biosolids land application was estimated at 5–15 tons year−1. Past and present datasets for 30 pharmaceuticals and personal care products (PPCPs) were determined to be statistically indistinguishable (paired t-test; p = 0.01). This study expands the list of PPCPs reported in U.S biosolids, provides the first estimates of nationwide release rates to and environmental half-lives in U.S agricultural soils, and confirms the utility of using mega-composite sampling for economical tracking of chemical inventories in biosolids on a national scale.
Keywords: Biosolids, Pharmaceuticals, Risk assessment, Persistence, Half-life, PPCPs
1. Introduction
Growth of the U.S population necessitates the disposal of steadily increasing volumes of treated municipal sewage sludge (biosolids). Similarly, between 1988 and 2004, the rate of biosolids disposal on land has increased from 36% to approximately 55% of the total volume produced, which in 2007 was estimated at 7.2 million dry tons annually for the U.S. (USEPA, 1992; NEBRA, 2007). Whereas land-applied biosolids offer a source of inexpensive nutrients, they also are a recognized sink for domestic and industrial chemicals that become sequestered in solids during the wastewater treatment processes (Chu and Metcalfe, 2007; Wu et al., 2010). High aqueous-phase removal rates (>95%) reported for wastewater treatment plants (WWTPs) are not necessarily indicative of the extent of transformation of chemicals within a given plant, and many compounds that are effectively removed from the liquid phase persist and become sequestered and highly enriched in biosolids during treatment (Heidler and Halden, 2007). Land-applied biosolids can release pollutants into groundwater and waterways through leaching and runoff (Kinney et al., 2006; Gottschall et al., 2012).
With U.S. per-capita consumption of medicines growing annually at a rate of about 0.6% (IMS Health, 2009), a proportionally higher amount of pharmaceuticals is being discharged into sewers and ultimately into the environment. Drugs can enter the environment as parent compounds, as metabolites excreted by patients (Halling-Sorensen et al., 1998; Williams and Cook, 2007), and as a waste stream from manufacturing plants, hospitals and domestic discharges (Bound and Voulvoulis, 2005) as well as from leachates originating from terrestrial depositions (Langdon et al., 2010). Some conjugated metabolites that are excreted by humans have the capacity to be transformed back to the parental compounds due to bacterial action in the environment (Halling-Sorensen et al., 1998; Richards and Cole, 2006) or within the sewage treatment process (Gobel et al., 2005; Schultz et al., 2006).
The occurrence of pharmaceuticals and their metabolites in WWTPs and in surface waters has been well documented (Ternes, 1998; Kolpin et al., 2002). Analytical challenges concerning solid matrices are the physical characteristics of the sample matrix and the extensive cleanup processes required prior to analysis. Biosolids of all forms (pelletized, composted or semi-solid) consist of particles with large surface areas, negative surface charges and interstitial spaces that all promote sorption and sequestration of compounds deep within the matrix. Adding to the complexity are the variety of chemicals such as ferric chloride, lime and polymers used during the conditioning process that need to be neutralized during sample cleanup (Jones-Lepp and Stevens, 2007). However, with the release of the EPA Method 1694 and developing analytical technologies, there has been a marked increase in the number of studies reporting PPCPs in biosolids. A recent study employed EPA Method 1694 to establish trends in PPCP occurrences in WWTPs from across the U.S. by utilizing archived biosolids contained in the U.S National Biosolids Repository maintained at Arizona State University (McClellan and Halden, 2010). This latter study also pioneered the use of mega composite samples to establish national release inventories for biosolids-borne chemicals.
The purpose of the present study was to examine the occurrence of previously unmonitored pharmaceuticals in archived biosolids samples, predict their behavior in the environment and determine the risks posed by matching their porewater and soil-biosolids concentrations with reported toxicity values from literature and to critically evaluate the mega composite sampling approach that – for matters of convenience, speed and cost-effectiveness – relies on a very limited number of measurements to create estimates of national inventories of chemicals in biosolids, but whose reproducibility from an experimental perspective is as of yet unknown. Building on the list of 72 previously reported compounds (McClellan and Halden, 2010), an additional 48 compounds were monitored in this work using a newly introduced analytical method (AXYS Method MLA-075) that extends the analyte range of U.S. EPA Method 1694 without changing any of the attributes inherent to the originally reported protocol. Study results reveal the identities of 26 newly reported PPCPs in biosolids and their importance from an ecotoxicological perspective, yield U.S mass inventories for the latter, and provide evidence for the reproducible preparation and analysis of large biosolids composite samples constructed from 94 WWTPs across the U.S.
2. Materials and methods
2.1. Sampling procedure
This study utilized 113 biosolids samples obtained by the U.S. Environmental Protection Agency (EPA) for the 2001 National Sewage Sludge Survey (NSSS). These samples make up a small fraction of the U.S National Biosolids Repository, maintained at the Biodesign Institute at Arizona State University in the laboratory of Dr. Halden. During the 10-year period between sample acquisition and analysis, samples were stored at −20 °C. Along with the NSSS samples, soil-biosolids mixtures from an outdoor mesocosm study conducted in Baltimore, Maryland were also analyzed as part of this study to experimentally determine the half-lives of the extended list of PPCPs. Details about the design of the mesocosm studies have been provided previously (Walters et al., 2010).
2.2. Composite sample preparation
Of the 113 NSSS samples three were excluded from analysis as the containers were broken or compromised (McClellan and Halden, 2010). Five groups were created with the remaining 110 samples, by weighing out approximately one g of dry weight from each sample and pooling it to obtain five composite samples each containing solids from between 21 and 24 individual WWTPs. A split sample of composite 1 was prepared to serve as a blind duplicate. All procedural steps were identical to those described previously for the initial mega composite study (McClellan and Halden, 2010; see SM for details).
2.3. Sample analysis
Samples were analyzed by AXYS Analytical Services (2045 Mills Road West, Sydney, British Columbia, V8L 358) according to AXYS Method MLA – 075, a modification of the USEPA Method 1694. All analytes were separated by liquid chromatography and detected by tandem mass spectrometry. Analytes were quantified using isotope dilution technique or internal standard quantification with linear regression calibration. More detailed information on the analysis method is available in supplementary material (SM).
2.4. Quality assurance
Before sample analyses were performed, several tests were carried out to ensure system and laboratory performance. A verification of calibration accuracy was performed using calibration standard solution with native and labeled analytes. The retention times of both the native and labeled compounds were required to be within ±15 s of the respective retention times determined during initial calibration. Throughout the analysis precision and recovery were ensured. Lab blanks were analyzed prior to each sample analysis. Analysis of duplicate samples was performed by the lab for each batch consisting of seven to 20 samples. In addition to these, a blind duplicate was included in the sample set to evaluate analysis precision according to the following formula.
| (1) |
2.5. Reproducibility of results
To gauge the integrity of the samples and efficiency of the analytical method, a statistical comparison was carried out between the present and previous datasets obtained for composites created from the same archived individual biosolids samples. Data from the study by McClellan and Halden (2010; see SM for details) and from the present study were compared statistically using a paired t-test approach and scatter-plot correlation analysis.
2.6. Modeling of porewater and equilibrium soil concentration
In order to inform environmental risk assessments for the compounds newly detected in biosolids, the soil concentrations following land application were calculated following a previously established approach (McClellan and Halden, 2010). Calculations took into account a soil-biosolids mixing ratio of 25:1. Bulk densities of soil and biosolids used in these calculations were assumed to be 1.3 g cm−3 and 1.6 g cm−3 respectively, and an average soil moisture content of 22% (v/v) was assumed as reported earlier by others (De Lannoy et al., 2006). The organic carbon fraction of dried biosolids was assumed to equal 0.4 (USEPA, 2007). Calculations involved the two equations below:
| (2) |
| (3) |
where m is the dry mass in kg m−3 of the solids, C is the concentration in μg kg−1, ρ is the density in kg m−3 and fporewater and fOC are the dimensionless fractions, respectively, of the pore-water and organic carbon in the soil/biosolids mixture, respectively.
2.7. Drug usage and ecotoxicity data
Information on drug sales and uses were obtained from Internet sources (http://www.rxlist.com) and from the IMS Health database (2009). Ecotoxicity and half-life data were predicted using the PBT Profiler software provided online by the U.S EPA as described previously (McClellan and Halden, 2010).
2.8. Modeling of annual loading to agricultural soil
Annual loading of PPCPs to agricultural soils was calculated for a biosolids production rate of 7.2 million dry tons per year, of which 55% is land applied (NEBRA, 2007) using a previously established approach (McClellan and Halden, 2010; see SM for details).
2.9. Experimental calculation of half-life
As a part of this study, archived mesocosm samples that contained soil:biosolids mixtures were analyzed using the same analytical procedure described previously (Walters et al., 2010) in order to calculate the half-lives of the sequestered compounds.
3. Results
3.1. Data quality assurance
No detections above the method detection limit were observed in the lab blanks for any of the analytes; hence, measured concentrations of all analytes were accepted. An On-going Precision and Recovery (OPR) procedure was carried out for each target analyte as part of the Quality Assurance/Quality Control (QA/QC) protocol. This approach included the fortification of performance samples to establish the recovery rates for analytes of interest.
The average recovery for a subset of 26 compounds that were quantified using internal standards was 103% with a range of recoveries from 47 to 357%. Individual recoveries for all these compounds were noted to be within the method’s lower and upper control limits with the notable exception of desmethyldiltiazem (357%) whose recovery exceeded the method’s upper control limit range of 350%. The values reported for this compound thus may represent an overestimation (Table 1). Recovery rates for two compounds were close to the method’s lower control limit, amlodipine (47 vs. 45–130%) and alprazolam (73 vs. 70–130%). A significant analytical cross-interference was seen between hydrocodone and codeine compounds. An algebraic correction was performed for both compounds that enabled detection and correction of false positive occurrences. Values for hydrocodone represent approximate concentrations with the interferences taken into account.
Table 1.
Results for 26 PPCPs monitored in five composite samples representing 94 U.S. wastewater treatment plants. Compounds observed inconsistently are marked with superscript letter (a) and the number of positive detections (n <5) is shown in parentheses.
| # | Compound | MDL μg kg−1 | Mean ± Std. Dev. μg kg−1 | Matrix-spike recovery % | Use |
|---|---|---|---|---|---|
| 1 | 10-Hydroxy-amitriptyline | 0.4 | 14.4 ± 5.7 | 97.4 | Metabolite |
| 2 | Amitriptyline | 0.6 | 275.4 ± 92.8 | 78.8 | Antidepressant |
| 3 | Amlodipinea | 4.2 | ND (n = 3) | 47 | Ca2+ Channel blocker |
| 4 | Alprazolama | 0.7 | ND (n = 1) | 72.9 | Anxiolytic |
| 5 | Atenolola | 1.7 | ND (n = 1) | 94.9 | â blocker |
| 6 | Atorvastatina | 1.4 | ND (n = 4) | 79.7 | Antilipidemic |
| 7 | Benzoylecgoninea | 0.3 | ND (n = 2) | 92.1 | Metabolite |
| 8 | Benztropine | 0.5 | 2.9 ± 0.1 | 101 | Anticholinergic |
| 9 | Cocaine | 0.1 | 3.6 ± 3 | 97.6 | Illicit drug |
| 10 | DEET | 0.6 | 7.4 ± 2.8 | 112 | Insect repellant |
| 11 | Desmethyl-Diltiazem | 0.3 | 7.4 ± 6.1 | 357 | Metabolite |
| 12 | Furosemidea | 104 | ND (n = 2) | 92.6 | Diuretic |
| 13 | Glyburidea | 15.6 | ND (n = 2) | 104 | Antidiabetic |
| 14 | Hydrocodonea | 4.5 | ND (n = 4) | 83.8 | Narcotic analgesic |
| 15 | Metoprolol | 4.5 | 24.5 ± 10.1 | 93.4 | â blocker |
| 16 | Norfluoxetine | 2 | 42 ± 25.1 | 100 | Metabolite |
| 17 | Norverapamil | 0.3 | 458 ± 169.4 | 82.7 | Metabolite |
| 18 | Oxycodonea | 1.2 | ND (n = 3) | 108 | Narcotic analgesic |
| 19 | Paroxetine | 7.1 | 61.6 ± 21.7 | 105 | Anti-depressant |
| 20 | Promethazine | 0.6 | 22 ± 6.2 | 108 | Antihistamine |
| 21 | Propoxyphene | 1.2 | 50 ± 23.2 | 88.7 | Narcotic analgesic |
| 22 | Propranolol | 2.5 | 107.4 ± 36 | 91.2 | â-blocker |
| 23 | Sertraline | 0.5 | 458 ± 168.3 | 74 | Anti-depressant |
| 24 | Triamterene | 0.5 | 430.4 ± 139.9 | 81.2 | Diuretic |
| 25 | Valsartana | 9.2 | ND (n = 4) | 107 | Antihypertensive |
| 26 | Verapamil | 0.3 | 551.4 ± 226.2 | 92.2 | Ca2+ channel blocker |
More comprehensive information on each compound is available in SM.
Duplicate analyses revealed a 20% relative percent difference (RPD) for all compounds detected and 10% for the subset of 26 compounds consistently detected in each sample and its corresponding duplicate.
3.2. Study representativeness and sample integrity
The results of this study aim to provide mean estimates of the analytes concentration in biosolids from the perspective of storage time, as prolonged storage of samples may have allowed for degradation of labile compounds and sample pooling may have diluted the concentrations of some analytes to levels below the detection limit. Additional pertinent information is provided in Section 3.4.
3.3. Occurrence of PPCPs in biosolids
Of the 120 pharmaceuticals tested for, 59 compounds were detected in at least one composite sample (Refer to Figure S1). The mean concentration of the sum of all PPCPs detected in the five composite samples was approximately 58.7 ± 19.8 mg/kg. Four compounds previously reported to occur in the ppm range in these samples were detected again at similar levels; these included triclocarban, triclosan, ciprofloxacin and ofloxacin. Combined, these four analytes contributed about 85% of the total mass of all PPCPs detected.
Overall, 26 unreported pharmaceuticals were detected in at least one of the composite samples (Fig. 1). In the following section these compounds have been grouped as major and minor contaminants based on the detected concentrations and frequency of detection in the samples. The majority of these compounds have not been reported previously in the NSSS nor were they detected in U.S biosolids samples, although several were detected previously in sludges from other parts of the world (Ternes et al., 2007).
Fig. 1.
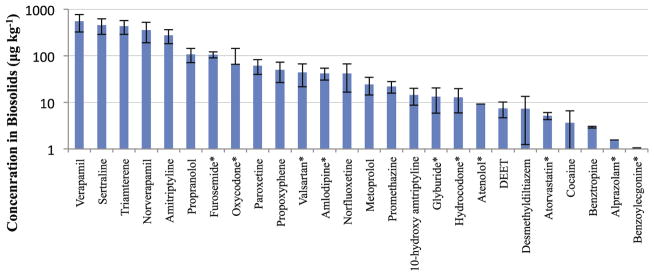
Rank order of mean concentrations of 26 previously unmonitored PPCPs that were detected for the first time in composites of 110 U.S biosolids samples from the 2001 NSSS. Error bars depict ± one standard deviation (n = 5), and asterisks (*) indicate compounds that were detected inconsistently.
3.4. Reproducibility of results
When comparing the present list of detected analytes to those reported previously by McClellan and Halden (2010), 30 analytes were found to be common to both studies, whereas eight compounds that had been detected previously were uniformly not detected in any of the samples during the present study. The eight compounds themselves with the exception of nor-floxacin were reported as inconsistent detects only in the McClellan and Halden (2010) study; lack of detection in this work may be due to a number of factors, including potential degradation, irreversible sorption, and dilution to levels below the method detection limit. A comparison of mean concentrations of compounds detected in this study and those reported in 2010 (Fig. 2) shows good agreement between the two. A paired t-test conducted on both the log-transformed and original datasets showed the results to be statistically indistinguishable at the 99% confidence level. Mean concentrations of compounds were within a factor of about 1.3 between previously obtained and current data.
Fig. 2.
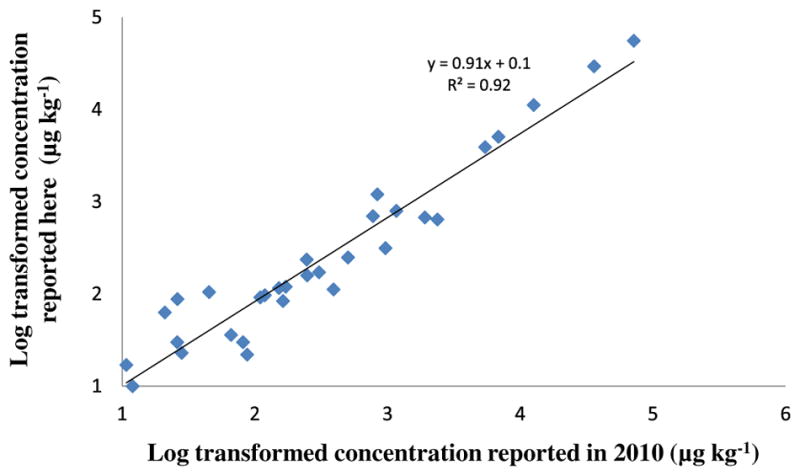
Log–log scatterplot comparing mean concentrations from the present study to those reported previously for 30 compounds commonly detected. Both datasets represent results obtained from analysis of composites created two years apart from the same group of archived samples.
3.5. Experimental half-life determination
Analytical results from the mesocosm samples revealed consistent first-order loss rates for four compounds, namely amitriptyline, paroxetine, propranolol and sertraline (Fig. 3). Compound-specific half-lives in biosolids-amended soils were calculated by fitting the experimental data to first-order kinetics, yielding values ranging from 533 to 866 days (Table 2).
Fig. 3.

Decreasing concentrations of compounds plotted as natural logarithms vs. time (common x-axis). Compound structures were obtained from the database of the Royal Society of Chemistry.
Table 2.
Half-lives determined experimentally in mesocosm experiments versus, shown in parentheses, the corresponding values estimated using the PBT Profiler of the U.S. EPA.
| Compound | Calculated half-life, days−1 | Predicted loading to U.S. soils, kg yr−1 |
|---|---|---|
| Amitriptyline | 533 (120) | 570–1402 |
| Paroxetine | 770 (NA) | 122–356 |
| Propranolol | 866 (30) | 231–578 |
| Sertraline | 630 (NA) | 883–2519 |
4. Discussion
4.1. Study limitations
For the purpose of this study, 110 archived biosolids samples were pooled together to create five mega composite samples. This approach is economically attractive but does not allow for extrapolating obtained results to individual treatment plants and operating conditions. Due to sample pooling, the detection method’s accuracy around the mean concentration is high. In contrast, the corresponding maximum and minimum concentrations reported likely are lower and higher, respectively, than those that would be found if all 110 samples were analyzed individually. Yet, the pooling technique effectively reduced the number of samples required in order to estimate the mean concentrations of drug residues in U.S. sludges nationwide. Compounds not detected in this study may still occur at detectable concentrations in some of the individual samples from specific plants because the pooling of samples can dilute out low-level analytes that occur infrequently. In addition, the mass loading estimates to soils nationwide should be interpreted as rough estimates only. Some states represented in this study may not dispose of any biosolids on land, whereas others may do so extensively. Such state-to-state variability in biosolids use as well as differences in concentrations between individual treatment plants influence the reliability of the data presented here. Nevertheless, this technique was found to be suitable for identifying contaminants and their average concentrations in a large sample set in an economical and efficient fashion.
It is important to recall that separate batches of composite samples were prepared and analyzed for both studies. Since labile compounds are eliminated during the wastewater treatment process, more refractory compounds tend to persist and accumulate in sludge. Such compounds are fairly resilient to environmental stress and tend to have extended half-lives in the sequestering matrix. The closeness of results between the two analysis campaigns (Fig. 2) indicates that the sample integrity had been preserved over the years during storage and that good reproducibility can be achieved with the composite sample approach.
4.2. Newly reported PPCPs in biosolids
4.2.1. Major contaminants
4.2.1.1. Calcium channel blockers and metabolites
Two parent compounds (amlodipine, verapamil) and two metabolites (norverapamil, desmethyldiltiazem) were detected in the biosolids samples. Amlodipine was detected at a maximum concentration of 51.7 μg kg−1 and verapamil was detected in all samples at a mean concentration of 551.4 ± 226.2 μg kg−1. The metabolites norverapamil and desmethyldiltiazem were uniformly detected in all samples at mean concentrations of 360.2 ± 169.4 μg kg−1 and 7.4 ± 6.1 μg kg−1, respectively. Calcium channel blockers have been reported previously in the μg L−1 to ng L−1 range in wastewater effluents and in surface waters (Batt et al., 2008; Nagarnaik et al., 2010). All four compounds lacked data for establishing a comparative analysis, as they have not been reported in any major study.
4.2.1.2. Antidepressants and metabolites
Three antidepressants (paroxetine, sertraline, amitriptyline) and two metabolites (10-hydroxy-amitriptyline, norfluoxetine) were detected as well. Paroxetine, sertraline and norfluoxetine were uniformly detected in all samples at mean concentrations of, respectively, 61.6 ± 21.7, 458 ± 168.3 and 41.6 ± 25.1 μg kg−1. Paroxetine, sertraline, norverapamil and 10-hydroxy-amitriptyline have been reported to occur in surface waters across the U.S in the ng L−1 range (Schultz and Furlong, 2008; Wu et al., 2009; Batt et al., 2008) and interestingly, in the low to high μg L−1 range in fish tissues (Chu and Metcalfe, 2007; Ramirez et al., 2009). Radjenović et al. (2009) reported paroxetine in biosolids at a concentration of 40.7 ± 13.0 μg kg−1, similar to the mean concentration reported here. While in this study the highest detected concentration of sertraline was 636 μg kg−1, Barron (2009) reported levels as high as 22 mg kg−1 in biosolids samples from Sweden and Norway; they also reported values for amitriptyline and its metabolite similar to those presented here (275.4 ± 92.8 and 14.4 ± 5.7 μg kg−1, respectively). The latter two compounds have been detected in aquatic matrices (Kasprzyk-Hordern et al., 2008; Batt et al., 2008).
4.2.1.3. Diuretics
Triamterene was uniformly detected in all samples at a mean concentration of 430.4 ± 139.9 μg kg−1, whereas furosemide was found at a maximum level of 122 μg kg−1. Furosemide has been reported to have a highly variable removal range (8–54%) during wastewater treatment including nearly zero removal in winter (Castiglioni et al., 2006). The most recent study conducted on the behavior of pharmaceuticals during conventional wastewater treatment reported a maximum detected value of 75 μg kg−1 in biosolids and calculated a removal range of 30–80% for furosemide (Jelic et al., 2011). There were no reports on the occurrence of triamterene in biosolids.
4.2.1.4. β-blockers
Metoprolol and propranolol were uniformly detected in all samples at mean concentrations of 24.5 ± 10.1 and 107.4 ± 36 μg kg−1, respectively, whereas atenolol was detected at a maximum concentration of 9.25 μg kg−1. Metoprolol has been observed previously in biosolids at 35 ± 7 μg kg−1 (Barron, 2009). Apart from this, there have been several published studies conducted on metoprolol and its presence in aquatic matrices. These studies indicated a range of removal efficiencies exhibited by WWTPs, from 0 to 80% (Jelic et al., 2011). Propranolol also has been detected in aquatic and solid matrices alike. Levels in the low ppb range were reported in WWTP process streams and in surface waters (Bendz et al., 2005; Scheurer et al., 2010). Concentrations found in Norwegian biosolids (101 ± 3 μg kg−1) mirrored the range reported here, whereas Radjenović et al., 2009 observed Propranolol at lower concentrations of 26.2 ± 10.7 μg kg−1 in sludge samples from Spain. Atenolol has been detected previously in sludge at levels similar to those reported here (Barron, 2009; Jelic et al., 2011); it is one of the most frequently tested for pharmaceuticals in leachates from biosolids (Lapen et al., 2008; Topp et al., 2008).
4.2.1.5. (Narcotic) analgesics
Propoxyphene was detected in all samples at mean concentration of 49.9 ± 23.2 μg kg−1, whereas hydrocodone and oxycodone were detected at maximum concentrations of 21.7 μg kg−1 and 157 μg kg−1, respectively. In addition to their various legitimate uses, all three drugs in recent times have been associated with abuse, and are frequently detected by U.S forensics labs (Daughton, 2011). Both hydrocodone and oxycodone have been detected previously in surface waters and wastewater streams, most frequently in the low to high ppb range in surface waters of the U.S. and other nations (Hummel et al., 2006; Batt et al., 2008; Chiaia et al., 2008; Phillips et al., 2010) although no published studies were found with regards to analyses of solid matrices.
4.2.2. Minor contaminants
4.2.2.1. Ungrouped compounds
DEET (N,N-diethyl-meta-tol-uamide) has been reported in aquatic matrices including surface waters (Kolpin et al., 2002), landfill leachates (Eggen et al., 2010), WWTP streams (Trenholm et al., 2006; Bartelt-Hunt et al., 2009) and in U.S groundwater (Barnes et al., 2004). In the present study, DEET was uniformly detected in all biosolids samples at a mean concentration of 7.4 ± 2.8 μg kg−1.
Howard and Muir (2010) listed promethazine and benztropine as high production volume (HPV) pharmaceuticals that have not been detected in the environment. In the present study both pharmaceuticals were uniformly detected in all samples at mean concentration of 21.9 ± 6.2 μg kg−1 and 2.9 ± 0.1 μg kg−1.
The maximum detected concentrations of valsartan, glyburide, alprazolam, and atorvastatin were 64.1, 20.6, 1.56, and 6.2 μg kg−1. Atorvastatin has previously been detected in WWTP process streams and in biosolids at concentrations of 20–46 ppb (Miao and Metcalfe, 2003; Batt et al., 2008; Jelic et al., 2011).
4.2.2.2. Cocaine and metabolite
Testing of environmental matrices (most commonly aquatic) for the presence of illicit drugs has recently gained impetus. The presence of cocaine and benzoylecgonine (metabolite) has been reported in wastewater streams and surface waters across Europe (Castiglioni et al., 2006; Huerta-Fontela et al., 2007; Kasprzyk-Hordern et al., 2008; Postigo et al., 2010; Gonzáles-Mariño et al., 2010) and in U.S WWTP influent (Chiaia et al., 2008). Here, we report uniform detection of cocaine in all biosolids composites analyzed. To our knowledge, this is the first study to report the presence of cocaine in U.S biosolids; however, the mean concentration found was low at 3.6 ± 3 μg kg−1. Benzoylecgonine was detected at even lower levels, never exceeding 1.05 μg kg−1
These results provide some of the first documented occurrences of select compounds in biosolids. For a more detailed analysis of worldwide occurrences of these and other seldom monitored compounds, readers should refer to Table S2.
4.3. Soil/porewater equilibria and half-life calculation
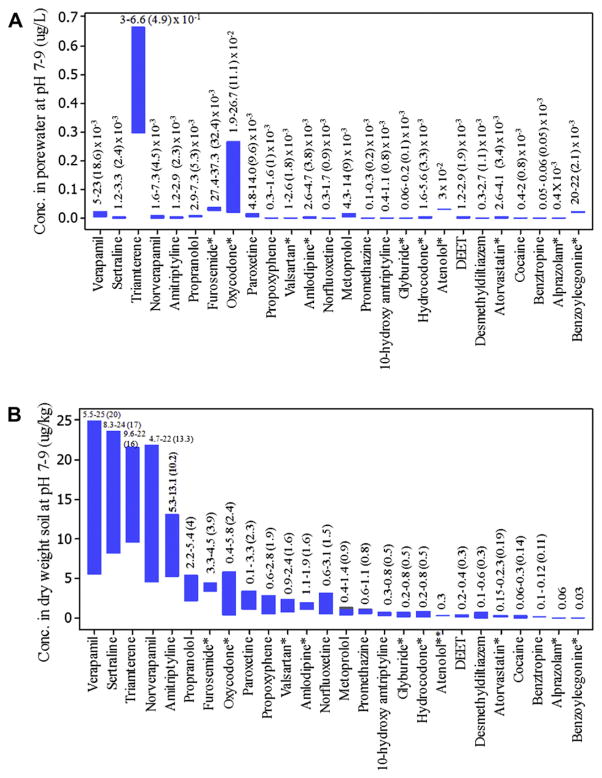
In order to determine if the compounds detected in biosolids potentially pose a threat to aquatic organisms, toxicity values were estimated using the PBT Profiler of the U.S. EPA. From a comparison of the modeled porewater concentrations for these compounds with predicted toxicity data (Fig. 4A vs. Fig. 5), it was concluded that the presence of these compounds in biosolids at the detected concentrations posed no threat to aquatic organisms in nearby surface waters. The EC50 values were at least one order of magnitude higher than the pore-water concentrations calculated for these compounds. Since we followed EPA-recommended soil-biosolids mixing ratio of 25:1 when predicting analyte levels in surface water impacted by porewater leaching, there is a possibility that these compounds may be toxic to organisms in situations where mixing ratios may differ (e.g. applications in forests draining into adjacent surface waters).
Fig. 4.
Predicted porewater concentration range (A) and equilibrium concentration range in soils-biosolids mixtures (B) for 26 PPCPs at environmentally relevant pH range 7–9. Concentration range for each compound has been printed with corresponding mean values in parenthesis. Compounds marked with (*) were inconsistently detected.
Fig. 5.
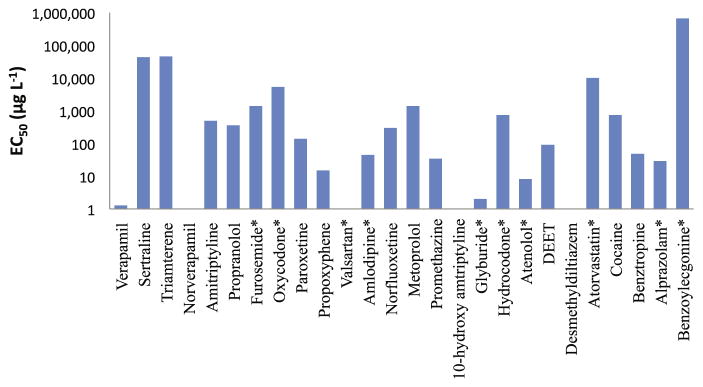
Predicted EC50 values for 22 compounds. Values for some compounds could not be calculated and may represent a potential hazard to aquatic organisms. Compounds marked with (*) indicate inconsistent detection.
Results from the PBT Profiler were also significant when estimating the persistence of these compounds in the environment. It was noted that all compounds had half-life values of ≥30 days. Upon correlating these findings with their respective log KOW values, it was found that the tendency to accumulate in solids was in part due to the high hydrophobicity of some compounds, whereas forces other than hydrophobic interactions are presumed to govern partitioning of compounds such as cocaine and oxycodone. In mesocosm studies containing soil-biosolids mixtures, Walters et al. (2010) experimentally showed that the half-lives of compounds applied to soils in the form of biosolids were much greater than half-lives predicted by fate models and laboratory studies using addition of neat chemicals to soils. By leveraging the archived soil/biosolids samples from the aforementioned mesocosm study, we were able to compute half-lives for four compounds whose loss over time followed first-order kinetics. These analytes were amitriptyline, paroxetine, propranolol and sertraline (Fig. 3).
4.4. Risk assessments and data gaps
Of all PPCPs detected in this study, three groups of compounds that have been noted for their pharmacodynamic effects on humans were found to occur uniformly in the high ppb range either as parent compounds or as metabolites. These were antidepressants (n = 5), β-blockers (n = 2) and narcotic analgesics (n = 3). Owing to an absence of terrestrial field studies, experimental and modeled values had to be relied upon as best estimates for potential hazards caused by the presence of PPCPs in the environment. Studies have demonstrated the ability of lipophilic compounds to undergo bioaccumulation and biomagnification within terrestrial food chains (Higgins et al., 2007; Kinney et al., 2008). Given the magnitude of annual loading reported in this study and the lack of bio-accumulation studies for these newly detected PPCPs, further work in this area appears to be warranted.
Of particular significance were the calculated half-lives for the compounds amitriptyline, paroxetine, propranolol and sertraline (Table 2). While values were absent for paroxetine and sertraline, the experimental t1/2 value was noted to exceed the predicted t1/2 by at least a factor of 4 for amitriptyline and by a factor of 29 for propranolol. Correlating the experimental t1/2 value with the individual annual loading rate proved crucial in gauging the magnitude of potential exposure to these pharmaceuticals.
Apart from this, sequestered compounds tend to occur as mixtures and not discreetly in biosolids and soils. Thus, greater emphasis should be placed on the effects of pharmaceutical mixtures on soil-dwelling organisms. Although effort has gone into studying the effects of antibiotics on soil microorganisms and the development of resistant species, the environmental pressures exerted by these compounds especially in solid matrices have not been thoroughly investigated. This lack of information prevents the completion of a comprehensive risk assessment for all compounds.
5. Conclusions
Twenty-six compounds were newly detected in archived biosolids samples, and are predicted to enter terrestrial environments in the U.S. through biosolids application at a combined rate of 5–15 tons yr−1. The majority of these compounds have not been extensively investigated with regards to occurrence and effects in the environment and exposure pathways to humans. This study further demonstrated that consistent results can be obtained when analyzing archived biosolids from national sampling campaigns by using a mega composite approach. This implies that long-term storage of samples in the freezer at temperatures of −20 °C or less does not significantly impact the analytes examined here, and that the mixing of composite samples from thawed slurries, although being challenging, can be performed such that consistent results are obtained. This finding is noteworthy as this mega composite sampling approach could help to dramatically reduce the cost of environmental monitoring on the regional and national scale. While it has been established that mean concentrations of several PPCPs in U.S biosolids have remained fairly constant over the years, the detection of a set of new compounds in biosolids warrants analysis of more recent biosolids samples in order to establish a trend, with regards to both their occurrence and concentrations detected.
Supplementary Material
Acknowledgments
This project was supported in part by Award Numbers R01ES015445 and 1R01ES020889 from the National Institute of Environmental Health Sciences (NIEHS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health (NIH).
Abbreviations
- KOW
n-Octanol/Water Partitioning Coefficient
- MDL
Method Detection Limit
- NEBRA
North East Biosolids and Residuals Association
- PPCPs
Pharmaceuticals and Personal Care Products
- RPD
Relative Percent Difference
- ppm
Parts per Million
- ppb
Parts per Billion
- ppt
Parts per Trillion
Appendix A. Supplementary data
Supplementary data related to this article can be found online at http://dx.doi.org/10.1016/j.watres.2012.06.017.
References
- Barnes KK, Christenson SC, Kolpin DW, Focazio MJ, Furlong ET, Zaugg SD, Meyer MT, Barber LB. Pharmaceuticals and other organic wastewater contaminants within a leachate plume downgradient of a municipal landfill. Ground Water Monitoring and Remediation. 2004;24 (2):119–126. [Google Scholar]
- Barron L. Occurrence and Fate of Pharmaceuticals and Personal Care Products within Sewage Sludge and Sludge-enriched Soils: Summary of Findings. Dublin City University, Norwegian Institute for Water Research (NIVA), and Masaryk University, Czech Republic; Dublin, Ireland: 2009. p. 3. available: http://www.epa.ie/downloads/pubs/research/waste/STRIVE_34_PPCPs_Summary_Findings.pdf. [Google Scholar]
- Bartelt-Hunt SL, Snow DD, Damon T, Shockley J, Hoagland K. The occurrence of illicit and therapeutic pharmaceuticals in wastewater effluent and surface waters in Nebraska. Environmental Pollution. 2009;157 (3):786–791. doi: 10.1016/j.envpol.2008.11.025. [DOI] [PubMed] [Google Scholar]
- Batt AL, Kostich MS, Lazorchak JM. Analysis of ecologically relevant pharmaceuticals in wastewater and surface water using selective solid-phase extraction and UPLC-MS/MS. Analytical Chemistry. 2008;80 (13):5021–5030. doi: 10.1021/ac800066n. [DOI] [PubMed] [Google Scholar]
- Bendz D, Paxeus NA, Ginn TR, Loge FJ. Occurrence and fate of pharmaceutically active compounds in the environment, a case study: Hoje River in Sweden. Journal of Hazardous Materials. 2005;122 (3):195–204. doi: 10.1016/j.jhazmat.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Bound JP, Voulvoulis N. Household disposal of pharmaceuticals as a pathway for aquatic contamination in the United Kingdom. Environmental Health Perspectives. 2005;113 (12):1705–1711. doi: 10.1289/ehp.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglioni S, Baganti R, Fanelli R, Pomati F, Calamari D, Zuccato E. Removal of pharmaceuticals in sewage treatment plants in Italy. Environmental Science & Technology. 2006;40 (1):357–363. doi: 10.1021/es050991m. [DOI] [PubMed] [Google Scholar]
- Chiaia AC, Banta-Green C, Field J. Eliminating solid phase extraction with large-volume injection LC/MS/MS: analysis of illicit and legal drugs and human urine indicators in U.S. wastewaters. Environmental Science & Technology. 2008;42 (23):8841–8848. doi: 10.1021/es802309v. [DOI] [PubMed] [Google Scholar]
- Chu S, Metcalfe CD. Analysis of paroxetine, fluoxetine, and norfluoxetine in fish tissues using pressurized liquid extraction, mixed mode solid phase extraction cleanup and liquid chromatography – tandem mass spectrometry. Journal of Chromatography A. 2007;1163 (1–2):112–118. doi: 10.1016/j.chroma.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Daughton CG. Illicit drugs: contaminants in the environment and utility in forensic epidemiology. Reviews of Environmental Contamination and Toxicology. 2011;210:59–110. doi: 10.1007/978-1-4419-7615-4_3. [DOI] [PubMed] [Google Scholar]
- De Lannoy GJM, Verhoest NEC, Houser PR, Gish TJ, Van Meirvenne M. Spatial and temporal characteristics of soil moisture in an intensively monitored agricultural field (OPE3) Journal of Hydrology. 2006;331 (3–4):719–730. [Google Scholar]
- Eggen T, Moeder M, Arukwe A. Municipal landfill leachates: a significant source for new and emerging pollutants. Science of the Total Environment. 2010;408 (21):5147–5157. doi: 10.1016/j.scitotenv.2010.07.049. [DOI] [PubMed] [Google Scholar]
- Gobel A, Thomsen A, Mcardell CS, Joss A, Giger W. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environmental Science & Technology. 2005;39 (11):3981–3989. doi: 10.1021/es048550a. [DOI] [PubMed] [Google Scholar]
- Gonzáles-Mariño I, Quintana JB, Rodriguez I, Cela R. Determination of drugs of abuse in water by solid-phase extraction, derivatisation and gas chromatography–ion trap-tandem mass spectrometry. Journal of Chromatography A. 2010;1217 (11):1748–1760. doi: 10.1016/j.chroma.2010.01.046. [DOI] [PubMed] [Google Scholar]
- Gottschall N, Topp E, Metcalfe C, Edwards M, Payne M, Kleywegt S, Russell P, Lapen DR. Pharmaceutical and personal care products in groundwater, subsurface drainage, soil, and wheat grain, following a high single application of municipal biosolids to a field. Chemosphere. 2012 Jan 31; doi: 10.1016/j.chemosphere.2011.12.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Halling-Sorensen B, Nielsen SN, Lanzky PF, Ingerslev F, Lutzhoft HCH, Jorgensen SE. Occurrence, fate and effects of pharmaceutical substances in the environment – a review. Chemosphere. 1998;36 (2):357–394. doi: 10.1016/s0045-6535(97)00354-8. [DOI] [PubMed] [Google Scholar]
- Heidler J, Halden RU. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere. 2007;66 (2):362–369. doi: 10.1016/j.chemosphere.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Higgins CP, McLeod PB, MacManus-Spencer LA, Luthy RG. Bioaccumulation of perfluorochemicals in sediments by the aquatic oligochaete Lumbriculus variegatus. Environmental Science & Technology. 2007;41 (13):4600–4606. doi: 10.1021/es062792o. [DOI] [PubMed] [Google Scholar]
- Howard PH, Muir DCG. Identifying new persistent and bioaccumulative organics among chemicals in commerce. Environmental Science & Technology. 2010;44 (7):2277–2285. doi: 10.1021/es903383a. [DOI] [PubMed] [Google Scholar]
- Huerta-Fontela M, Galceran MT, Ventura F. Ultraperformance liquid chromatography–tandem mass spectrometry analysis of stimulatory drugs of abuse in wastewater and surface waters. Analytical Chemistry. 2007;79 (10):3821–3829. doi: 10.1021/ac062370x. [DOI] [PubMed] [Google Scholar]
- Hummel D, Löffler D, Fink D, Ternes TA. Simultaneous determination of psychoactive drugs and their metabolites in aqueous matrices by liquid chromatography mass spectrometry. Environmental Science & Technology. 2006;40 (23):7321–7328. doi: 10.1021/es061740w. [DOI] [PubMed] [Google Scholar]
- IMS Health. 2009 http://www.rxlist.com/script/main/hp.asp.
- Jelic A, Gros M, Ginebreda A, Cespedes-Sánchez R, Ventura F, Petrovic M, Barcelo D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Research. 2011;45 (3):1165–1176. doi: 10.1016/j.watres.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Jones-Lepp TL, Stevens R. Pharmaceuticals and personal care products in biosolids/sewage sludge: the interface between analytical chemistry and regulation. Analytical and Bioanalytical Chemistry. 2007;387 (4):1173–1183. doi: 10.1007/s00216-006-0942-z. [DOI] [PubMed] [Google Scholar]
- Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Research. 2008;42 (13):3498–3518. doi: 10.1016/j.watres.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Kinney CA, Furlong ET, Zaugg SD, Burkhardt MR, Werner SL, Cahill JD, Jorgensen GR. Survey of organic wastewater contaminants in biosolids destined for land application. Environmental Science & Technology. 2006;40 (23):7207–7215. doi: 10.1021/es0603406. [DOI] [PubMed] [Google Scholar]
- Kinney CA, Furlong ET, Kolpin DW, Burkhardt MR, Zaugg SD, Werner SL, Bossio JP, Benotti MJ. Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environmental Science & Technology. 2008;42 (6):1863–1870. doi: 10.1021/es702304c. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams 1999–2000: a national reconnaissance. Environmental Science & Technology. 2002;36 (6):1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Langdon KA, Warne MStJ, Kookana RS. Aquatic hazard assessment for pharmaceuticals, personal care products, and endocrine-disrupting compounds from biosolids-amended land. Integrated Environmental Assessment and Management. 2010;6 (4):663–676. doi: 10.1002/ieam.74. [DOI] [PubMed] [Google Scholar]
- Lapen DR, Topp E, Metcalfe CD, Lic H, Edwards M, Gottschall N, Bolton P, Curnoed W, Payne M, Beck A. Pharmaceutical and personal care products in tile drainage following land application of municipal biosolids. Science of the Total Environment. 2008;399 (1–3):52–59. doi: 10.1016/j.scitotenv.2008.02.025. [DOI] [PubMed] [Google Scholar]
- McClellan K, Halden RU. Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA national sewage sludge survey. Water Research. 2010;44 (2):658–668. doi: 10.1016/j.watres.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao XS, Metcalfe CD. Determination of pharmaceuticals in aqueous samples using positive- and negative-voltage switching microbore liquid chromatography/electrospray ionization tandem mass spectrometry. Journal of Mass Spectrometry. 2003;38 (1):27–34. doi: 10.1002/jms.394. [DOI] [PubMed] [Google Scholar]
- Nagarnaik P, Batt A, Boulanger B. Concentrations and mass loadings of cardiovascular pharmaceuticals in healthcare facility wastewaters. Journal of Environmental Monitoring. 2010;12 (11):2112–2119. doi: 10.1039/c0em00216j. [DOI] [PubMed] [Google Scholar]
- North East Biosolids and Residuals Association. A National Biosolids Regulation, Quality, End Use & Disposal Survey 2007 [Google Scholar]
- Postigo C, López de Alda MJ, Barceló D. Drugs of abuse and their metabolites in the Ebro River basin: occurrence in sewage and surface water, sewage treatment plants removal efficiency, and collective drug usage estimation. Environment International. 2010;36 (1):75–84. doi: 10.1016/j.envint.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Phillips PJ, Smith SG, Kolpin DW, Zaugg SD, Buxton HT, Furlong ET, Esposito K, Stinson B. Pharmaceutical formulation facilities as sources of opioids and other pharmaceuticals to wastewater-treatment-plant effluents. Environmental Science & Technology. 2010;44 (13):4910–4916. doi: 10.1021/es100356f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjenović J, Petrovic M, Barcelo D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Research. 2009;43 (3):831–841. doi: 10.1016/j.watres.2008.11.043. [DOI] [PubMed] [Google Scholar]
- Ramirez AJ, Brain RA, Usenko S, Mottaleb MA, O’Donnell JG, Stahl LL, Wathen JB, Snyder BD, Pitt JL, Perez-Hurtado P, Dobbins LL, Brooks BW, Chambliss CK. Occurrence of pharmaceuticals and personal care products in fish: results of a national pilot study in the United States. Environmental Toxicology and Chemistry. 2009;28 (12):2587–2597. doi: 10.1897/08-561.1. [DOI] [PubMed] [Google Scholar]
- Richards SM, Cole SE. A toxicity and hazard assessment of fourteen pharmaceuticals to Xenopus laevis larvae. Ecotoxicology. 2006;15 (8):647–656. doi: 10.1007/s10646-006-0102-4. [DOI] [PubMed] [Google Scholar]
- Scheurer M, Ramil M, Metcalfe CD, Groh S, Ternes TA. The challenge of analyzing beta-blocker drugs in sludge and wastewater. Analytical and Bioanalytical Chemistry. 2010;396 (2):845–856. doi: 10.1007/s00216-009-3225-7. [DOI] [PubMed] [Google Scholar]
- Schultz MM, Higgins CP, Huset CA, Luthy RG, Barofsky DF, Field JA. Fluorochemical mass flows in a municipal wastewater treatment facility. Environmental Science & Technology. 2006;40 (23):7350–7357. doi: 10.1021/es061025m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz MM, Furlong ET. Trace analysis of antidepressant pharmaceuticals and their select degradates in environmental aquatic matrices by LC/ESI/MS/MS. Analytical Chemistry. 2008;80 (5):1756–1762. doi: 10.1021/ac702154e. [DOI] [PubMed] [Google Scholar]
- Ternes TA. Occurrence of drugs in German sewage treatment plants and rivers. Water Research. 1998;32 (11):3245–3260. [Google Scholar]
- Ternes TA, Bonerz M, Herrmann N, Teiser B, Andersen HR. Irrigation of treated wastewater in Braunschweig, Germany: an option to remove pharmaceuticals and musk fragrances. Chemosphere. 2007;66 (5):894–904. doi: 10.1016/j.chemosphere.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Topp E, Monteiro SC, Beck A, Coelho BB, Boxall ABA, Duenk PW, Kleywegt S, Lapen DR, Payne M, Sabourin L, Li HX, Metcalfe CD. Runoff of pharmaceuticals and personal care products following application of biosolids to an agricultural field. Science of the Total Environment. 2008;396 (1):52–59. doi: 10.1016/j.scitotenv.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Trenholm RA, Vanderford BJ, Holady JC, Rexing DJ, Snyder SA. Broad range analysis of endocrine disruptors and pharmaceuticals using gas chromatography and liquid chromatography tandem mass spectrometry. Chemosphere. 2006;65 (11):1990–1998. doi: 10.1016/j.chemosphere.2006.07.004. [DOI] [PubMed] [Google Scholar]
- USEPA. Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS. Washington, D.C: 2007. EPA-821-R-08–002. [Google Scholar]
- USEPA. Sewage Sludge Use and Disposal Rule (40 CFR Part 503) – Fact Sheet. U.S. Environmental Protection Agency; Washington, DC: 1992. EPA-882-F-92-002. Office of Water, Fact Sheet WH-556. [Google Scholar]
- Walters E, McClellan K, Halden RU. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Research. 2010;44 (20):6011–6020. doi: 10.1016/j.watres.2010.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RT, Cook JC. Exposure to pharmaceuticals present in the environment. Drug Information Journal. 2007;41 (2):133–141. [Google Scholar]
- Wu CX, Witter JD, Spongberg AL, Czakowski KP. Occurrence of selected pharmaceuticals in an agricultural landscape, western Lake Erie basin. Water Research. 2009;43 (14):3407–3416. doi: 10.1016/j.watres.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Wu CX, Spongberg AL, Witter JD, Fang M, Ames A, Czajkowlski KP. Detection of pharmaceuticals and personal care products in agricultural soils receiving biosolids application. Clean-Soil Air Water. 2010;8 (3):230–237. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.