Abstract
External guide sequences (EGSs) are RNA molecules that can bind to a target mRNA and direct ribonuclease P (RNase P), a tRNA processing enzyme, for specific cleavage of the target mRNA. Using an in vitro selection procedure, we have previously generated EGS variants that efficiently direct human RNase P to cleave a target mRNA in vitro. In this study, we constructed EGSs from a variant to target the overlapping region of the mRNAs coding for human cytomegalovirus (HCMV) capsid scaffolding protein (CSP) and assemblin, which are essential for viral capsid formation. The EGS variant was about 40-fold more active in directing human RNase P to cleave the mRNA in vitro than the EGS derived from a natural tRNA. Moreover, a reduction of about 98% and 75% in CSP/assemblin gene expression and a reduction of 7000- and 250-fold in viral growth were observed in HCMV-infected cells that expressed the variant and the tRNA-derived EGS, respectively. Our study shows that the EGS variant is more effective in blocking HCMV gene expression and growth than the tRNA-derived EGS. Moreover, these results demonstrate the utility of highly active EGS RNA variants in gene targeting applications including anti-HCMV therapy.
Keywords: RNase P, external guide sequence, gene therapy, cytomegalovirus, gene targeting
Introduction
Human cytomegalovirus (HCMV) is a human herpesvirus and causes serious clinical manifestations in newborns and immunocompromised populations such as AIDS patients.1 For example, this virus is the leading cause of congenital infections associated with mental retardation in newborns.2 Drug toxicity and the emergence of drug-resistant virus strains associated with current antiviral drug therapy3-6 have posed a need for developing new drugs and strategies for treatment and prevention of infections by HCMV as well as other herpesviruses. HCMV capsid scaffolding protein (CSP) completely overlaps with and is within the 3′ coding sequence of another viral capsid protein, assemblin.7 Both CSP and assemblin are believed to be essential for HCMV capsid formation and viral replication.8,9 Furthermore, homologous proteins have been found in other herpesviruses and are conserved among all herpesviruses.1 Thus, CSP and assemblin may serve as targets for novel drug development to combat HCMV infection.
Nucleic acid-based gene interference strategies, such as antisense oligonucleotides, ribozymes or DNAzymes, and RNA interference (RNAi), represent powerful research tools and promising therapeutic agents for human diseases.10,11 Each of these approaches has its own advantages and limitations in terms of targeting efficacy, sequence specificity, toxicity, and delivery efficiency in vivo. Ribonuclease P (RNase P) has been proposed as a novel RNA-based gene interference strategy for knocking down gene expression.12,13 By catalyzing a hydrolysis reaction to remove the leader sequence of a precursor tRNA, RNase P is responsible for the maturation of 5′ termini of all tRNAs, which account for about 2% of total cellular RNA.12,14,15 Human RNase P has at least ten polypeptides and a RNA subunit (H1 RNA).16 One of the unique features of RNase P is its ability to recognize the structures, rather than the sequences of the substrates, which allows the enzyme to hydrolyze different natural substrates in vivo or in vitro. Accordingly, any complex of two RNA molecules that resembles a tRNA molecule can be recognized and cleaved by RNase P (Fig. 1A and B).17,18 One of the RNA molecules is called the external guide sequence (EGS). In principle, an mRNA sequence can be targeted for RNase P cleavage by using EGSs to hybridize with the target RNA and direct RNase P to the site of cleavage. Subsequent studies have shown that expression of EGSs can modulate gene expression in bacteria and in mammalian cells.18-23 We have previously shown that EGS RNAs derived from a natural tRNA efficiently directed human RNase P to cleave the mRNA sequence encoding the thymidine kinase (TK) of herpes simplex virus 1 (HSV-1) in vitro.24,25 A reduction of 75–80% in the TK mRNA and protein expression was observed in HSV-1 infected cells expressing the EGS RNAs.
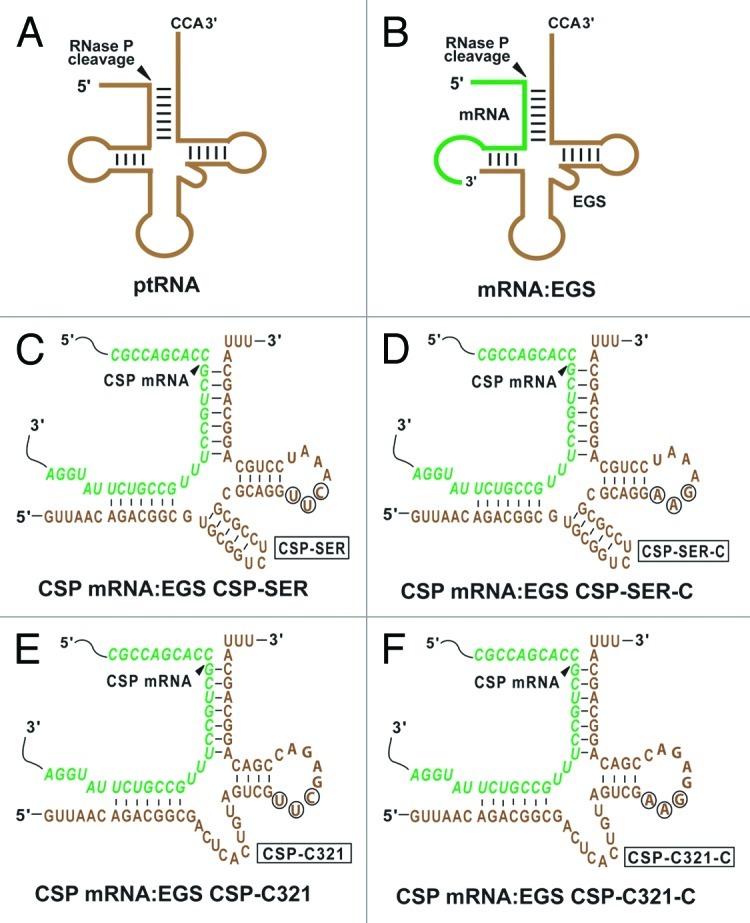
Figure 1. Substrates for RNase P. (A) A natural substrate (ptRNA). (B) A hybridized complex of an EGS and a target RNA (e.g., mRNA) that resembles the structure of a tRNA. (C, D, E, and F) Complexes between CSP mRNA sequence and EGS CSP-SER, CSP-SER-C, CSP-C321, and CSP-C321-C, respectively. The sequences of CSP-SER and CSP-SER-C that were equivalent to the T-stem and loop, and variable region of a tRNA molecule were derived from tRNAser, while those of CSP-C321 and CSP-C321-C were from EGS variant C321. The site of cleavage by RNase P is marked with an arrowhead. The EGS sequence was shown in brown color. Only the exact sequence of the CSP mRNA around the targeting site was shown (in green).
Targeted cleavage of mRNA by human RNase P provides a unique approach to inactivate any RNA of known sequence expressed in vivo. The in vitro efficiency of EGS-induced RNase P cleavage as well as its efficacy in vivo needs to be improved in order to develop EGSs for practical use in vivo. Using an in vitro selection procedure, we have recently isolated novel EGS variants that direct RNase P to cleave TK mRNA in vitro more efficiently than those derived from a natural tRNA sequence.25 Little is currently known about how these EGS RNA variants increase their activities in directing RNase P to cleave a target mRNA. Equally unclear is whether these engineered EGS RNAs are more effective in blocking HCMV gene expression and replication in cultured cells than the EGS derived from a natural tRNA. In this study, one of these EGS variants was used to target the overlapping region (CSP mRNA) of the mRNAs encoding HCMV capsid scaffolding protein (CSP) and assemblin, which are essential for viral capsid formation and replication.1 We investigated the activity of the EGS in inducing RNase P to cleave the target mRNA and its efficacy in inhibiting HCMV gene expression and growth in cultured cells. Our results provide direct evidence to suggest that engineered EGS RNAs are more effective in blocking HCMV CSP/assemblin expression and growth than the EGS derived from the natural tRNA sequence, leading to an inhibition of up to 98% in HCMV CSP expression and a reduction of up to 7,000 fold in HCMV growth in cultured cells. These results demonstrate the potential of generating highly active EGS variants and using them as a research tool and as a therapeutic agent for gene-targeting applications.
Results
RNase P-mediated cleavage of the HCMV CSP mRNA sequence induced by EGSs in vitro
It is important to select the target regions of the CSP mRNA that are accessible to EGS binding as most intracellular RNAs are usually associated with proteins and are present in folded conformations. We have employed an in vivo mapping assay with dimethyl sulfate (DMS)26,27 to examine the accessibility of the CSP mRNA. In this assay, human U373MG cells were infected with HCMV and then incubated with culture media that contained DMS. DMS entered the cells and modified the nucleotides of the mRNA regions that were accessible. Total mRNAs were isolated and those regions of the CSP mRNA that were modified by DMS were mapped by primer extension assays in the presence of reverse transcriptase. A position, 218 nucleotides downstream from the translation initiation codon, was chosen as the cleavage site for EGSs. This site appeared to be one of the regions most accessible to DMS modification (data not shown) and is presumably accessible to EGS binding.
In our previous studies, an in vitro selection procedure was used to isolate EGS RNA variants that are more efficient in directing human RNase P for cleavage of the TK mRNA sequence than the EGS derived from a natural tRNA sequence.25 However, little is known about whether these variants are also highly active when they are used to target another mRNA. To address these issues, we chose variant C321 for the study because the EGS RNAs derived from this variant are among the most active EGSs in inducing RNase P to cleave the TK as well as the CSP mRNA sequences in vitro (Table 1). By covalently linking the EGS domain of C321 to the targeting sequences that are complementary to the CSP mRNA, we constructed EGS CSP-C321, which resembles a part of a tRNA structure and contains a T-loop, a T-stem, and a variable region but not the anticodon region that is dispensable for EGS activity (Fig. 1E).
Table 1. Kinetic parameters [Vmax(apparent), Km(apparent), and Vmax(apparent)/Km(apparent)] in the RNase P-mediated cleavage reactions of ptRNAser or csp38 in the presence of different EGSs.
| Substrate | Km (µM) |
Vmax (apparent) (pmol·min−1) |
Vmax(apparent)/Km(apparent) (pmol·µM−1·min−1) |
Kd (µM) |
|---|---|---|---|---|
| ptRNASer |
0.018 ± 0.005 |
0.050 ± 0.015 |
2.8 ± 0.5 |
|
| CSP mRNA(csp38) |
|
|
|
|
| +CSP-SER |
0.50 ± 0.08 |
0.025 ± 0.010 |
0.050 ± 0.010 |
1.5 ± 0.4 |
| +CSP-SER-C |
ND |
ND |
< 0.001 |
1.5 ± 0.4 |
| |
|
|
|
|
| +CSP-C321 |
0.37 ± 0.08 |
0.82 ± 0.30 |
2.2 ± 0.4 |
0.030 ± 0.005 |
| +CSP-C321-C | ND | ND | < 0.001 | 0.029 ± 0.005 |
The values shown are the average derived from triplicate experiments.
Another EGS, CSP-SER, which was derived from the natural tRNAser sequence, was also constructed in a similar way and included in the study (Fig. 1C). Two additional EGSs, CSP-C321-C and CSP-SER-C, were also constructed and derived from CSP-C321 and CSP-SER, respectively, and contained point mutations (5′-UUC-3′ - > AAG) at the three highly conserved positions in the T-loop of these EGSs (Fig. 1D and F). These nucleotides, found in most of the known natural tRNA sequences,28 are believed to be important for the interactions between the tRNA domains and human RNase P.12,15 EGSs carrying these mutations have been shown to preclude RNase P recognition and exhibited little activity in directing RNase P-mediated cleavage.19,25,29
EGS RNAs were synthesized in vitro from the DNA sequence templates by T7 RNA polymerase and subsequently incubated with human RNase P and substrate csp38, which contains the target CSP mRNA sequence. CSP-SER and CSP-C321 efficiently induced human RNase P-mediated cleavage of the CSP mRNA sequence in vitro as apparent cleavage of the substrate was observed in the presence of these EGSs (Fig. 2, lanes 2 and 3). In contrast, no cleavage of csp38 by RNase P was observed in the absence of these EGSs (Fig. 2, lane 1). To further study the activity of the EGS in vitro, we performed detailed kinetic analyses in the presence of different EGSs and determined the values of Km(apparent) and Vmax(apparent) as well as the overall cleavage efficiency [Vmax(apparent)/Km(apparent)] for the cleavage reactions. CSP-C321 was highly efficient in directing human RNase P to cleave csp38 and was at least 40–fold more active than CSP-SER (Table 1). An increase in the cleavage rate of RNase P may be due to additional tertiary interactions that may potentially stabilize the mRNA-EGS complex. If this is the case, it is expected that the binding affinity of the EGS variant (i.e., CSP-C321) to the CSP mRNA sequence may be better than that of the EGS (i.e., CSP-SER) derived from the natural tRNA sequence. We determined the binding affinities of EGS CSP-C321 and CSP-SER to substrate csp38 by measuring the dissociation constant (Kd) with gel-shift assays for the separation of substrate-EGS complexes in polyacrylamide gels under non-denaturing conditions. Our results showed that CSP-C321 exhibited about 50 times higher binding affinity to csp38 than CSP-SER (Table 1). Because both CSP-C321 and CSP-SER have the same antisense sequences to csp38 (Fig. 1C and 1E), these results suggest that the increased binding affinity and the stability of the substrate-EGS complex in the presence CSP-C321 is probably due to the additional tertiary interactions introduced by this EGS.
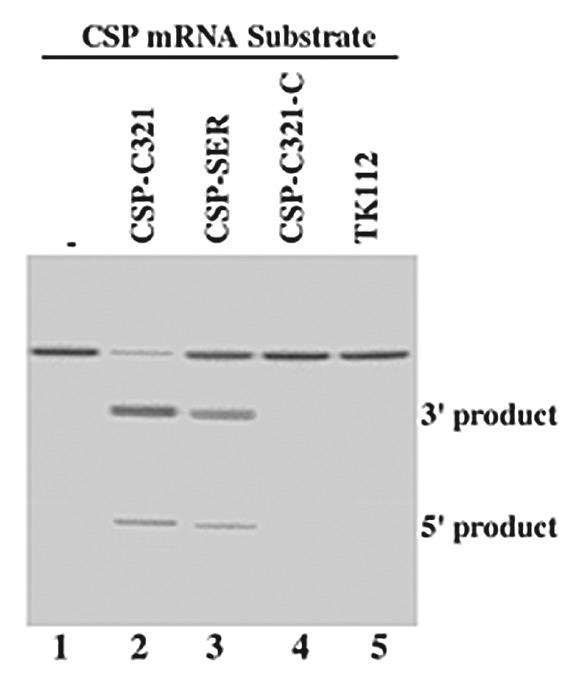
Figure 2. Cleavage of CSP mRNA sequence (substrate csp38) by human RNase P in the presence of different EGSs. No EGS was added to the reaction mixture in lane 1. EGS CSP-SER (10 nM) (lane 3), CSP-C321 (2 nM) (lane 2), CSP-C321-C (10 nM) (lane 4), and TK112 (10 nM) (lane 5) were incubated with [32P]-labeled CSP RNA substrate (20 nM) and human RNase P (2 units) at 37°C in a volume of 10 μl for 45 min in buffer A (50 mM Tris, pH 7.4, 100 mM NH4Cl, and 10 mM MgCl2). Experimental details can be found in Materials and Methods.
In the presence of control EGSs CSP-SER-C and CSP-C321-C, cleavage of csp38 by human RNase P was barely detected (Fig. 2, lanes 4, data not shown) and was at least 1x103-fold slower than the cleavage in the presence of CSP-C321 (Table 1). CSP-C321-C and CSP-SER-C contained the same antisense sequence to the CSP mRNA sequence as CSP-C321 and CSP-SER (Fig. 1D and F), and exhibited similar binding affinities to csp38 as CSP-C321 and CSP-SER, respectively, when assayed in vitro (Table 1). Therefore, CSP-C321-C and CSP-SER-C can be used as controls for the antisense effect of these EGSs.
Expression of the engineered EGSs in human cells
The DNA sequences coding for CSP-SER, CSP-SER-C, CSP-C321, and CSP-C321-C were cloned into retroviral vector LXSN and placed under the control of the small nuclear U6 RNA promoter, which has previously been shown to express EGS RNAs efficiently.18,24,30 This promoter is transcribed by RNA polymerase III, and its transcripts are highly expressed and primarily localized in the nucleus.18,24,31 To construct cell lines that express EGS RNAs, we transfected amphotropic packaging cells (PA317) with LXSN-EGS DNAs to produce retroviral vectors that contained the genes for EGS RNAs. Human U373MG cells were then infected with these vectors, and cells expressing the EGSs were cloned.
We also constructed an additional cell line, which expressed EGS TK112 that targeted the mRNA for thymidine kinase (TK) of herpes simplex virus (HSV-1).24 No RNase P-mediated cleavage of substrate csp38 in the presence of TK112 was observed in vitro (Fig. 2, lane 5). We used this EGS to determine whether EGS RNA with an incorrect guide sequence could target CSP mRNA in tissue culture. Our MTT assays revealed that the constructed lines and a control line in which cells were transfected with LXSN vector DNA alone were indistinguishable in terms of their growth and viability for up to three months (data not shown), suggesting that the expression of the EGSs did not appear to exhibit significant cytotoxicity.
The level of EGS RNA in the constructed cell lines was determined by Northern analysis, using that of H1 RNA, the RNA subunit of human RNase P, which is exclusively localized in the nuclei,16 as the internal control (Fig. 3). The EGS RNAs were readily expressed in the nuclei as they were detected in the nuclear RNA fractions (Fig. 3, data not shown). Only the cell lines that expressed similar levels of these EGS RNAs were used for further studies in tissue culture.
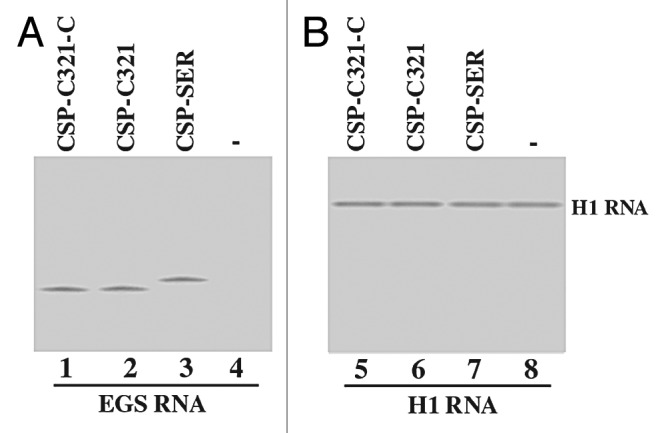
Figure 3. EGS RNA expression in cultured cells. Northern analyses were performed using nuclear RNA fractions isolated from parental U373MG cells (-, lanes 4 and 8) and a cloned cell line that expressed CSP-SER (lanes 3 and 7), CSP-C321 (lanes 2 and 6), and CSP-C321-C (lanes 1 and 5). Equal amounts of each RNA sample (30 μg) were separated on 2% agarose gels that contained formaldehyde, transferred to nitrocellulose membranes, and hybridized to a [32P]-radiolabeled probe that contained the DNA sequence coding for EGS CSP-SER/CSP-C321 (lanes 1–4) or H1 RNA (lanes 5–8), the RNA subunit of human RNase P and a nuclear RNA.12 The hybridized products corresponding to the full-length retroviral transcripts (~6kb), transcribed from the LTR promoter, are at the top of the gel and are not shown.
The activity of EGS for inhibiting viral CSP and assemblin expression
To study the EGS activities in cultured cells, cells were infected with HCMV at a multiplicity of infection (MOI) of 0.05–1. Total RNAs were isolated from the infected cells at 8–72 h postinfection, and Northern analyses were used to determine the expression levels of CSP and assemblin mRNAs. The level of the 5 kb long viral immediate-early transcript (5 kb RNA), expression of which is not regulated by CSP or assemblin under the assay conditions,1 was used as an internal control for the quantitation of expression of CSP and assemblin mRNAs (Fig. 4A and B). In addition, the expression level of human H1 RNA was also used as a control and was similar in each lane (Fig. 4C). These results suggested that an equal amount of cellular RNAs was present in each lane of the gel. We observed a reduction of about 98% and 75% (average of three experiments) in the expression level of CSP/assemblin mRNAs in cells that expressed CSP-C321 and CSP-SER, respectively (Table 2). In contrast, a reduction of less than 10% in the expression level of these two mRNAs was observed in cells that expressed CSP-SER-C, CSP-C321-C, or TK112. These results suggest that the significant reduction of the target mRNA expression in cells that expressed CSP-SER and CSP-C321 was due to the RNase P-mediated cleavage directed by the EGS. The low level of inhibition found in cells that expressed CSP-SER-C and CSP-C321-C was probably due to an antisense effect because these two control EGSs exhibited similar binding affinity to the target sequence as CSP-SER and CSP-C321 but did not direct RNase P for targeted cleavage.
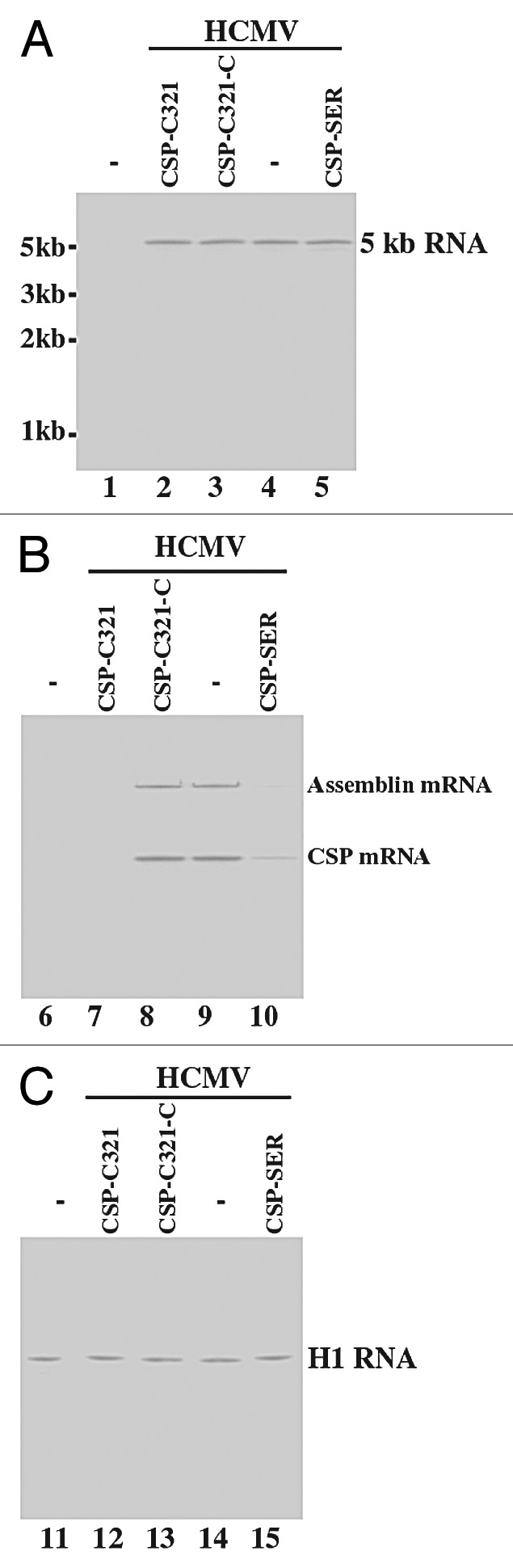
Figure 4. Expression of HCMV mRNAs as determined by Northern analysis. Cells (1x106) were either mock-infected (lanes 1, 6, and 11) or infected with HCMV (MOI = 1) (lanes 2–5, 7–10, and 12–15) and were harvested at 36 h postinfection. Northern analyses were performed using RNA isolated from parental U373MG cells (-, lanes 1, 4, 6, 9, 11, and 14) and cell lines that expressed CSP-SER (lanes 5, 10, and 15), CSP-C321-C (lanes 3, 8, and 13), and CSP-C321 (lanes 2, 7, and 12). Equal amounts of each RNA sample (30 μg) were separated on agarose gels that contained formaldehyde, transferred to a nitrocellulose membrane, and hybridized to a [32P]-radiolabeled probe that contained the DNA sequence of the HCMV 5 kb transcript (lanes 1–5), CSP mRNA (lanes 6–10), and human H1 RNA (lanes 11–15).
Table 2. Levels of inhibition of viral gene expression in the cells expressing CSP-SER, CSP-SER-C, CSP-C321, CSP-C321-C, and TK112, as compared with that in the parental U373MG cells that did not express an EGS (U373MG).
| Viral gene class |
EGS RNA |
||||||
|---|---|---|---|---|---|---|---|
| U373-MG | TK112 | CSP-SER-C | CSP-C321-C | CSP-SER | CSP-C321 | ||
| IE2 mRNA |
α |
0% |
1% |
1% |
1% |
2% |
1% |
| US2 mRNA |
β |
0% |
0% |
0% |
0% |
1% |
1% |
| CSP mRNA |
γ |
0% |
0% |
8% |
7% |
75 ± 8% |
98 ± 7% |
| Assemblin mRNA |
γ |
0% |
0% |
7% |
8% |
75 ± 8% |
98 ± 8% |
| IE1 protein |
α |
0% |
0% |
0% |
1% |
0% |
0% |
| UL44 protein |
β,γ |
0% |
0% |
1% |
0% |
0% |
0% |
| CSP protein |
γ |
0% |
1% |
8% |
7% |
75 ± 9% |
98 ± 8% |
| Assemblin protein |
γ |
0% |
0% |
7% |
8% |
75 ± 7% |
98 ± 9% |
| UL83 protein | γ | 0% | 0% | 0% | 0% | 0% | 1% |
The values shown are the means derived from triplicate experiments and values for the standard deviation that were less than 5% are not shown.
To examine the protein levels of CSP and assemblin in EGS-expressing cells, proteins were isolated from cells at 24–72 h postinfection, separated in SDS-polyacrylamide gels, and transferred to membranes, and reacted with antibodies against CSP and assemblin (Fig. 5B, data not shown). The double bands in Figure 5B represent the full length and processed CSP protein.7 The membranes were also stained with a monoclonal antibody against human actin (anti-Actin) (Fig. 5A). The expression of actin served as an internal control for the quantitation of CSP and assemblin protein expression. A reduction of about 98% and 75% in the level of CSP/assemblin proteins was observed in cells that expressed EGS CSP-C321 and CSP-SER, respectively, while a reduction of less than 10% was detected in cells that expressed control EGSs (Table 2). The low level of reduction in the expression level of CSP and assemblin proteins observed in cells that expressed CSP-SER-C or CSP-C321-C was presumably due to the antisense effect of the EGS. We note that the levels of reduction in the amount of the CSP/PR mRNAs correlated well with those in the amount of the CSP/PR proteins.
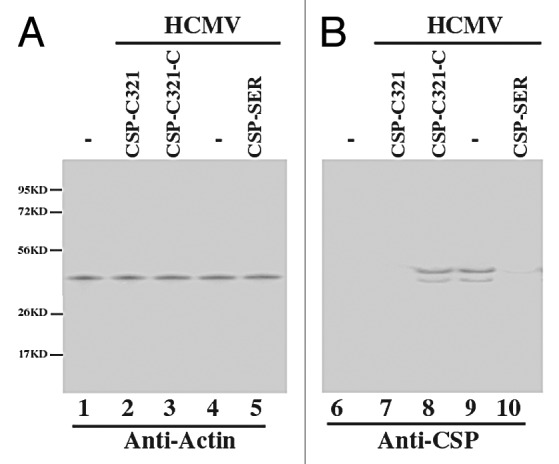
Figure 5. Expression of human actin and HCMV proteins as assayed by western blot analysis. Protein samples were isolated from the parental U373MG cells or EGS-expressing cells that were either mock-infected (lanes 1 and 6) or infected with HCMV (MOI = 0.5–1) (lanes 2–5 and 6–10) for 48 h, separated in either SDS-polyacrylamide gels, transferred to membranes, and reacted with antibodies against human actin (A) and HCMV CSP (B). The double bands in (B) represent the full length and processed CSP protein.7
The activity of EGS for inhibiting HCMV growth by targeting CSP mRNA
To study the activity of EGS for inhibiting HCMV replication, cells were infected by HCMV at an MOI of 1–5. Virus stocks were prepared from the infected cultures (cells and culture medium together) at 1 d intervals through 7 d postinfection and the number of plaque forming units (PFU) was determined by measuring the viral titer on human foreskin fibroblasts. After 5 d postinfection, a reduction of at least 7,000 and 250 fold in viral yield was observed in cells that expressed EGS CSP-C321 and CSP-SER, respectively (Fig. 6). No significant reduction was found in cells that expressed the control EGSs CSP-SER-C, CSP-C321-C, or TK112 (Fig. 6, data not shown). These results suggest that the engineered EGS that was generated by the in vitro selection procedure is about 30 times more effective in inhibiting HCMV growth than the EGS derived from a natural tRNA sequence.
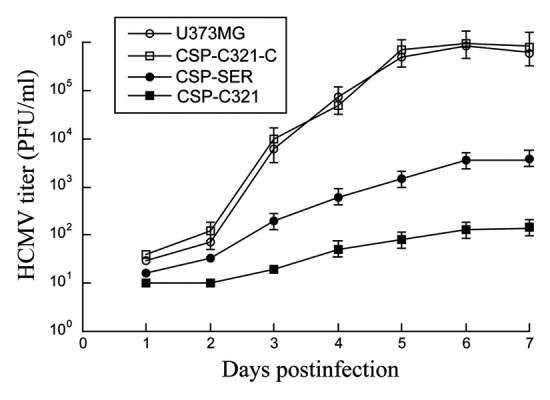
Figure 6. Growth of HCMV in U373MG cells and the EGS-expressing cell lines. Cells (5x105) were infected with HCMV at a MOI of 2. Virus stocks were prepared from the infected cells at 1 d intervals through 7 d postinfection. The PFU count was determined by measurement of the viral titer on human foreskin fibroblasts. The standard deviation is indicated by the error bars. These values are derived from the means from triplicate experiments.
Specific inhibition of HCMV capsid formation by EGS RNA-directed targeting of CSP mRNA
Inhibition of HCMV CSP expression is expected to lead to a reduction of HCMV lytic replication as the CSP is essential for viral capsid assembly.1 Meanwhile, it is possible that the observed reduction of viral replication in the EGS-expressing cells is not necessarily due to specific EGS RNA-directed RNase P cleavage of CSP and assemblin mRNAs but is due to other effects of the EGS on viral lytic replication that are unrelated to the consequence of the EGS-directed cleavage or the inhibition of viral CSP or assemblin expression, such as blocking the expression of viral immediate-early genes. To exclude these possibilities and further study the specificity of the EGS-directed cleavage, we performed two sets of experiments to investigate the step of the viral lytic cycle that is blocked in the cells that expressed CSP-SER and CSP-C321.
In the first set of experiments, we examined the expression of other viral genes in the cells. Inhibition of CSP and assemblin expression is not expected to affect the expression of other viral genes, including immediate-early (α), early (β), and late (γ) genes, which are not regulated by the CSP or the assemblin.1 We performed Northern analyses to determine the expression levels of the IE2 (an α transcript) and US2 mRNA (a β transcript). Moreover, we performed Western analyses to determine the expression level of viral protein UL44, a viral late (βγ) protein and UL83, a viral late (γ) protein. We observed no significant difference in the expression level of these genes in cells that expressed different EGS RNAs (Table 2). Thus, the expression of CSP-SER and CSP-C321 appeared to specifically inhibit the expression of CSP and assemblin, and did not affect overall viral gene expression.
In the second set of experiments, we investigated whether viral genomic replication as well as capsid maturation is affected in the cells that expressed CSP-SER and CSP-C321 RNAs. Total DNA was isolated from HCMV-infected cell lysates. The level of intracellular viral DNA was determined by PCR detection of HCMV IE1 sequence, using the level of β-actin DNA as the internal control. The amount of the intracellular viral DNA detected by the PCR assay represents the replication level of the viral genome because HCMV only replicates in an episomal form and does not integrate its DNA into the host genome.1 To examine viral capsid formation, we assayed the level of encapsidated viral DNA in order to determine the level of mature capsid formed in the infected cells. We isolated DNA samples from HCMV-infected cell lysates that were treated with DNase I. The encapsidated viral DNAs are expected to be resistant to DNase I digestion while those that are not packaged in the capsid are expected to be susceptible to degradation. We determined the level of intracellular “encapsidated” viral DNA by PCR detection of HCMV IE1 sequence from the DNase I-treated samples.
When assaying the DNA samples from cell lysates that were not treated with DNase I, no significant difference in the level of total intracellular (both encapsidated and uncapsidated) viral DNA was found in the EGS-expressing cells (Fig. 7, lanes 1–4). These results suggested that a reduction of CSP and assemblin expression by the EGS does not affect the step of viral genome replication. When the DNase I-treated samples were assayed, however, the level of “encapsidated” DNA was significantly reduced in the cells that expressed CSP-SER and even lower in cells that expressed CSP-C321, compared with those in cells that did not express any EGS RNAs or expressed control EGS CSP-SER, CSP-C321, or TK112 (Fig. 7, lanes 5 and 6). These observations suggest that EGS-mediated inhibition of CSP and assemblin expression does not affect the replication of viral DNA but blocks the event(s) during capsid formation. Moreover, the engineered EGS (i.e., CSP-C321) was more effective in blocking HCMV capsid formation than the EGS (i.e., CSP-SER) derived from a natural tRNA.
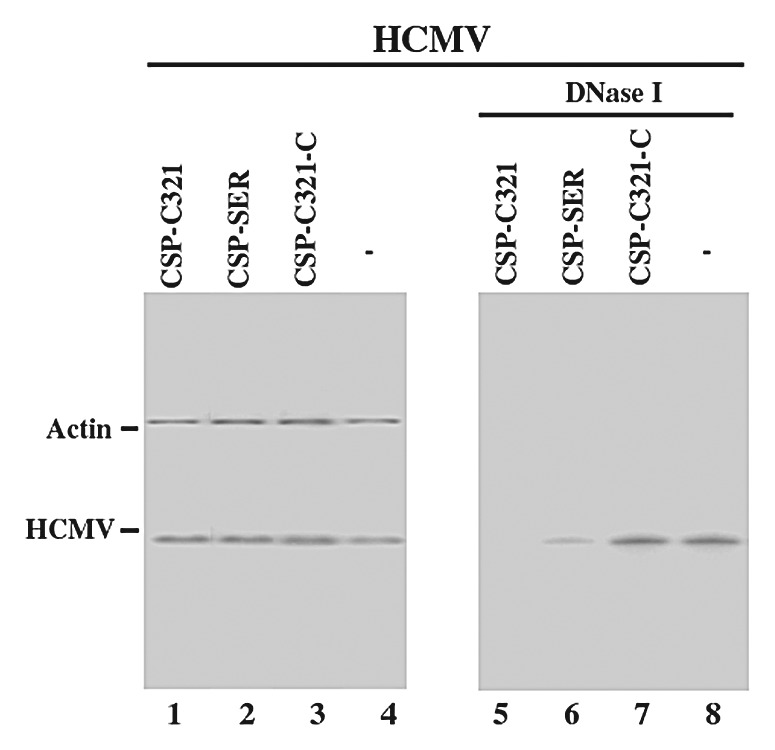
Figure 7. Level of encapsidated (B) and total intracellular (A) viral DNA as determined by semi-quantative PCR. Total DNA (lanes 1–4) or DNase I-treated DNA samples (lanes 5–8) were isolated from cells that either did not express an EGS (-, lanes 4 and 8) or express EGS CSP-SER (lanes 2 and 6), CSP-C321 (lanes 1 and 5), or CSP-C321-C (lanes 3 and 7). Cells were infected with HCMV at MOI of 2. We determined the levels of viral IE1 sequence by PCR using human actin DNA sequence as the internal controls. The radiolabeled PCR products were separated in 4% nondenaturing polyacrylamide gels. The amplification by PCR was within the linear range.
Discussion
EGS-based technology represents an attractive and promising approach for gene inactivation. This technology utilizes endogenous RNase P to generate highly efficient and specific cleavage of the target RNA.12,13 Furthermore, RNase P-mediated cleavage directed by EGSs is highly specific and does not generate “irrelevant cleavage” that is usually associated with RNase H-mediated cleavage induced by conventional antisense oligonucleotides.20 Further studies to improve the efficacy of the EGSs in inhibiting gene expression, including the construction of highly active EGS variants, will facilitate the development of the EGS technology for practical gene-targeting applications.
Little is currently known about the rate-limiting step of EGS-targeting reaction in cultured cells. Equally unclear is whether the efficacy of the EGSs can be improved, and if so, how it can be improved. In the present study, we constructed EGS RNAs that targeted an accessible region of the CSP mRNA. Moreover, we used the small nuclear U6 RNA promoter expression cassette to express the EGSs in the nuclear compartment. This design would increase the probability for the EGS RNAs to bind to their target mRNA sequence and co-localize with human RNase P, which is exclusively localized in the nuclei.12 Under the described settings, we postulated that the efficacy of EGS technology in cultured cells is dictated by the catalytic efficiency (Vmax/Km) of RNase P-mediated cleavage directed by the EGS. If this is the case, increasing the activity of EGS in directing RNase P cleavage may lead to more effective inhibition of the target mRNA expression in cultured cells.
Our results showed that an engineered EGS variant generated by the in vitro selection procedure (i.e., CSP-C321) is about 40 times more active [Vmax(apparent)/Km(apparent)] in directing RNase P to cleave CSP mRNA sequence in vitro than the EGS (i.e., CSP-SER) derived from the natural tRNAser sequence. Moreover, we showed that CSP-C321 inhibited CSP/assemblin expression in cultured cells by about 98% and was more effective than CSP-SER, which reduced CSP/assemblin expression by about 75%. A reduction of about 7,000-fold in viral growth was observed in the CSP-C321-expressing cells while a reduction of about 250-fold was observed in cells that expressed CSP-SER. In contrast, a reduction of less than 10% in the CSP/assemblin expression level and viral growth was observed in cells that expressed CSP-C321-C, CSP-SER-C, or TK112. In the experiments with the control EGSs, CSP-C321-C and CSP-SER-C exhibited similar binding affinity to csp38 as CSP-C321 and CSP-SER, respectively, but were inactive in directing RNase P-mediated cleavage due to the presence of the mutations at the T-loop that precluded RNase P recognition (Figs. One and 2, Table 1). These results suggest that the observed inhibition of HCMV gene expression and growth with CSP-C321 and CSP-SER is primarily attributed to the specific targeted RNase P-mediated cleavage induced by these two EGSs as opposed to the antisense effect or other nonspecific effects of the EGSs. Furthermore, our study indicates that the EGS (i.e., CSP-C321) that is more active [Vmax(apparent)/Km(apparent)] in inducing RNase P to cleave CSP mRNA sequence in vitro is also more effective in inhibiting HCMV gene expression and growth in cultured cells. These results support our hypothesis that increasing the activity of EGS in directing RNase P cleavage may lead to improved efficacy in inhibiting gene expression in cultured cells.
In vitro selection has been widely used to generate highly active ribozymes and functional RNA molecules that have increased activity.32-34 Furthermore, this procedure has been used to generate EGS molecules that direct human RNase P to cleave the mRNA encoding chloramphenicol acetyltransferase (CAT).29 Using an EGS RNA variant selected from a pool of EGS molecules containing randomized sequences, we, in this study, showed that an EGS selected in vitro with increased targeting activity also exhibited improved efficacy in inhibiting HCMV CSP/assemblin expression and viral growth in cultured cells. Thus, our study provides a direction for the engineering and generation of highly active EGS molecules by carrying out selection procedures and manipulation of the EGS domain to interact with the mRNA substrates. Our results suggest that the enhanced stability of the mRNA-EGS complexes may possibly contribute to the increased targeting activity of CSP-C321. CSP-C321 bound to substrate csp38 at least 50 fold better than CSP-SER (Table 1). Previous studies on tRNA molecules indicated that tertiary interactions between variable region and D-loop are important for maintaining the tRNA conformation and RNase P cleavage.12,14,15 Given the fact that, in the csp38-CSP-C321 complex, the 3′ region of csp38 can be considered equivalent to the D-loop in a tRNA (Fig. 1E), it is conceivable that the additional interactions between csp38 and CSP-C321 stabilize the mRNA-EGS complex and result in an enhanced binding affinity and increased targeting activity of the EGS. Further characterization of the cleavage reactions of this as well as other EGS variants should provide insights into the mechanism of how an EGS RNA efficiently directs RNase P to cleave an mRNA substrate.
In our current study, cell lines constitutively expressing EGS RNAs were first constructed and then infected with HCMV. To determine if EGS RNAs can be used for treating pre-existing HCMV infection, cells can be infected prior to the expression or delivery of EGS. It may not be possible to investigate the activity of EGS in treating pre-existing HCMV infection using the EGS-expressing clonal cell lines generated in our study. However, we believe that the constructed EGS RNAs should be effective in blocking HCMV replication in cells that are infected with HCMV prior to EGS expression since the EGSs would efficiently induce RNase P-mediated cleavage of the CSP mRNA and significantly inhibit the expression of CSP in these cells.
HCMV is a member of the human herpesvirus family, which includes seven other viruses such as HSV and Epstein-Barr virus.1 Each of these viruses can engage in lytic replication as well as establish latent infections. HCMV CSP and assemblin are essential for viral replication and their homologous proteins have been found in other herpesviruses and are conserved among all herpesviruses,1 suggesting that these proteins may serve as the ideal targets for drug development for the treatment and prevention of infections by HCMV as well as other herpesviruses. Our study here indicates that EGS RNA-mediated inhibition of the expression of HCMV CSP and assemblin leads to a significant (~7,000-fold) reduction of viral growth, and is consistent with the notion that blocking the expression of these two proteins should yield effective antiviral therapy. To further evaluate their anti-HCMV activity, we can deliver and express the EGSs in the monocyte/macrophage-lineage cells where HCMV is believed to establish latent infection.1 In these experiments, we will determine whether the EGSs can abolish viral gene expression in these cells and prevent HCMV reactivation from latent infection into lytic replication. These studies will further facilitate the development of the EGS-based technology for general gene-targeting applications.
Materials and Methods
Viruses, cells and antibodies
Human primary foreskin fibroblasts (HFFs), astrocytoma U373MG cells, and PA317 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum. The propagation of HCMV (AD169) in HFFs and U373MG cells was performed as described previously.8,35 The monoclonal antibodies c1202, c1203, and c1205, which react with HCMV proteins UL44, IE1/IE2, and UL83, were purchased from Goodwin Institute for Cancer Research. The monoclonal antibody against human actin was purchased from Sigma Inc. The anti-rabbit polyclonal antibodies against HCMV capsid scaffolding protein (CSP) and assemblin were kindly provided by Annette Meyer of Pfizer Inc. and John Wu of Promab, Inc.
Mapping of the accessible regions of viral mRNA in HCMV infected cells
U373MG cells were infected with HCMV at a multiplicity of infection (MOI) of 5 for 8–24 h prior to the treatment with dimethyl sulfate (DMS). Cells were washed with fresh DMEM media once, and then incubated with 5 mL of fresh media that contain 1% of DMS for 5–10 min.26,27 After the incubation, the DMS media were immediately aspirated. The cells were washed 3 times with phosphate-buffered saline (PBS) that contained 1 mM β –mercaptoethanol, and then lysed by adding cell lysis buffer (150 mM NaCl, 10 mM Tris–HCl pH 7.4, 1.5 mM MgCl2, 0.2% NP40).26,27 The lysates were transferred to an Eppendorf tube and immediately spun in a microcentrifuge for 10 sec at 4°C. The supernatant containing the cellular lysate was transferred to another tube. Total RNAs were isolated by phenol-chloroform extraction of the supernatant of the cellular lysate 3 times, followed by ethanol precipitation. To carry out primer extension assays to identify the DMS modification sites, oligonucleotide primers of 20 nt complementary to several regions of the targeted RNA were synthesized chemically and 5′-labeled by T4 polynucleotide kinase in the presence of γ-[32P]-ATP. Total cellular RNA (10 μg) was mixed with 50,000 cpm of the primer in a volume of 8 μL, heated to 90°C for 2 min, and cooled down to allow them to be annealed to each other, and then added with 4 μL 5 × RT buffer, 2 μL 10 mM dATP, 2 μL 10 mM dTTP, 2 μL 10 mM dGTP, 2 μL 10 mM dCTP, 0.5 μL RNasin, and 1 μL AMV reverse transcriptase. The primer extension reactions were preceded for 2 h at 42°C. The reaction products were extracted with phenol chloroform, then precipitated by cold ethanol, and finally separated in 8% denaturing gels. Analyses of the gels using a STORM840 Phosphorimager revealed the sites that blocked the primer extension reaction by reverse transcription, which represented the potential locations modified by DMS.
Construction of plasmids, EGS RNAs and RNA substrate for studies in vitro
The DNA sequence that encodes substrate csp38 was constructed by PCR using pGEM3zf(+) as a template and oligonucleotides AF25 (5′-GGAATTCTAATACGACTCACTATAG-3′) and sCSP1 (5′-CGGGATCCATAAGACGGCAAAGGCAGCGGTGCTGGCGCCTATAGTGAGTCGTATTA-3′) as 5′ and 3′ primers, respectively. The DNA sequences coding for the EGSs were synthesized by the polymerase chain reaction (PCR), using construct pTK112 DNA24 or pC321 as the templates, and were cloned under the control of the T7 RNA polymerase promoter. All oligonucleotides used as PCR primers were synthesized in a DNA synthesizer. To construct pCSP-SER, the 5′ and 3′ primer were oligoCSP-SER-5 (5′- GGAATTCTAATACGACTCACTATAGGTTAACAGACGGCGTGCGGTCTCC-3′) and oligoCSP-SER-3 (5′-AAGCTTTAAATGCTGCCTGCAGGATTTGAACCTGCGCGCG-3′), respectively. To construct pCSP-SER-C, the 5′ and 3′ primer were oligoCSP-SER-5 and the oligoCSP-SER-C-3 (5′- AAGCTTTAAATGCTGCCTGCAGGATTTCTTCCTGCGCGCG -3′), respectively. To construct pCSP-C321, the 5′ and 3′ primer were oligoCSP-C321–5 (5′-GGAATTCTAATACGACTCACTATAGGTTAACAGACGGCGACTCACTGT-3′) and oligoCSP-C321–3 (5′-AAGCTTTAAATGCTGCCTGTCGGTCTCGAACGACTACAGTGAGTC-3′), respectively. To construct pCSP-C321-C, the 5′ and 3′ primer were oligoCSP-C321–5 and the oligoCSP-C321-C-3 (5′-AAGCTTTAAATGCTGCCTGTCGGTCTCCTTCGACTACAGTGAGTC-3′), respectively.
RNase P assay and in vitro studies
Human RNase P was prepared from HeLa cellular extracts as described previously.19,24 The EGSs and [32P]-labeled cap38 were incubated with human RNase P at 37°C in buffer A (50 mM Tris, pH 7.4, 100 mM NH4Cl, and 10 mM MgCl2). Cleavage products were separated in denaturing gels and analyzed with a STORM840 phosphorimager. Assays to determine kinetic parameters were performed under multiple turnover conditions, as described previously.25,29 Aliquots were withdrawn from reaction mixtures at regular intervals and analyzed in polyacrylamide-urea gels, and the values of Km(apparent) and Vmax(apparent) were obtained from Lineweaver-Burk double-reciprocal plots.25,29,36
Assaying for the binding between EGSs and CSP mRNA sequence in vitro was performed as described previously.19,24,37 In brief, using a gel shift approach, various concentrations of EGSs were preincubated in buffer B (50 mM Tris, pH 7.5, 100 mM NH4Cl, 10 mM MgCl2, 3% glycerol, 0.1% xylene cyanol, 0.1% bromophenol blue) for 10 min before mixing with an equal volume of different concentrations of substrate RNA preheated under identical conditions. The samples were incubated for 10–120 min to allow binding, then loaded on a 5% polyacrylamide gel, and run at 10 Watts.37 The values of the equilibrium dissociation constant (Kd) were then extrapolated from a graph plotting percent of product bound vs. EGS concentration.25 The values were the average of three experiments.
Constructing EGS-expressing cells
Cell lines expressing different EGSs were constructed following the procedures described previously.30,35 In brief, amphotropic PA317 cells were transfected with retroviral vector DNAs (LXSN-CSP-SER, LXSN-CSP-SER-C, LXSN-CSP-C321, LXSN-CSP-C321-C, and LXSN-TK112). Forty-eight hours post transfection, culture supernatants that contained retroviral vectors were collected and used to infect U373MG cells. At 48–72 h postinfection, cells were incubated in culture medium that contained 600 μg/ml neomycin. Cells were subsequently selected in the presence of neomycin for two weeks and neomycin-resistant cells were cloned.
The cytotoxicity of the EGS expression was assessed by an MTT assay (Sigma). Parental U373MG cells and cells expressing different EGS RNAs were grown in 96-well plates. At different periods of time, 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma) (5 mg/mL in PBS) was added to each well, and cell viability was determined following the manufacture’s recommendation. The absorbance was measured at 570 nm on a microplate reader. All experiments were performed in four wells and repeated three times. Furthermore, the morphology of the cells at different periods of time was examined using a Nikon TE300 microscope.
To assay the expression of the EGSs, we isolated both nuclear and cytoplasmic RNA fractions from EGS-expressing cells as described previously.24,35 In these experiments, cells were lysed in buffer A (10 mM HEPES [pH 7.4], 10 mM KCl, 1 mM dithiothreitol, 0.6% NP-40) at 4°C for 10 min. After centrifugation at 1,000 x g for 5 min, the supernatant was collected as the cytoplasmic fraction and the nuclear fraction was prepared by resuspending the remaining pellet in buffer B (20 mM HEPES [pH 7.4], 150 mM NaCl, 1 mM dithiothreitol). The purity of nuclear and cytoplasmic fractions was assessed by immunoblotting for the presence of cytoplasmic protein actin and the nuclear protein histone H1. The RNA samples were separated in a 2.5% agarose gel that contained formaldehyde, transferred to a nitrocellulose membrane, hybridized with the [32P]-radiolabeled DNA probes that contained the DNA sequences coding for CSP-C321 and CSP-SER, and finally analyzed with a STORM840 phosphorimager. The radiolabeled DNA probes used to detect EGS RNAs were synthesized from constructs pCSP-C321 and pCSP-SER, by using a random primed labeling kit (Roche Applied Science).
Assaying the level of viral gene expression and growth
To determine the level of the inhibition of viral growth, 5x105 cells were either mock-infected or infected with HCMV at an MOI of 1–5. The cells and medium were harvested at 1, 2, 3, 4, 5, 6, and 7 d postinfection and viral stocks were prepared by adding 10% skim milk followed by sonication. The titers of the viral stocks were determined by performing plaque assays on human foreskin fibroblasts.8,35 The values obtained were the average from triplicate experiments.
Northern and Western analyses were performed to study the level of viral mRNA and proteins. T-25 flasks of cells (approximately 106 cells) were either mock-infected or infected with HCMV with the multiplicity of infection (MOI) as specified in the Result section. The infected cells were incubated for 8–72 h and viral mRNAs or proteins were isolated as described previously.19,24 To measure the levels of viral immediate-early (IE) transcripts, some of the cells were also treated with 100 μg/ml cycloheximide prior to and during infection. The RNA fractions were separated in 1% agarose gels that contained formaldehyde, transferred to a membrane, hybridized with the [32P]-radiolabeled DNA probes that contained the HCMV or human H1 DNA sequences, and analyzed with a STORM840 Phosphorimager. The DNA probes used to detect human H1 RNA, HCMV immediate-early 5kb RNA transcript, IE2 mRNA, US2 mRNA, and CSP and assemblin mRNA were synthesized from plasmids pH1 RNA, pCig27, pIE2, pCig38, and pCSP, respectively.
In Western analysis experiments, the polypeptides from cell lysates were separated on either SDS/7.5% polyacrylamide gels or SDS/9% polyacrylamide gels cross-linked with N,N”methylenebisacylamide, and then transferred electrically to nitrocellulose membranes. We stained the membranes using the antibodies against HCMV proteins and human actin in the presence of a chemiluminescent substrate (GE Healthcare), and analyzed the stained membranes with a STORM840 phosphorimager. Quantitation was performed in the linear range of RNA and protein detection.
Assaying the level of viral genome replication
5x105 cells grown on six-well plates were mock-infected or infected with HCMV. After a 1.5 h incubation at 37°C, the inoculum was removed and the cells were further incubated and harvested at 48–96 h postinfection. Total and encapsidated (DNase I-treated) DNAs were isolated as described38 and used as the PCR DNA templates. Viral DNA was detected by PCR amplification of the viral immediate-early IE1 sequence, using human β-actin sequence as the internal control. The 5′ and 3′ primers used to amplify the HCMV IE1 sequence were CMV3 (5′-CCAAGCGGCCTCTGATAACCAAGCC-3′) and CMV4 (5′-CAGCACCATCCTCCTCTTCCTCTGG-3′), respectively while those to amplify the actin sequence were Actin5 (5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′) and Actin3 (5′-CTAGAAGCATTGCGGTGGCAGATGGAGGG-3′), respectively.39 The PCR reaction consisted of 20 cycles with denaturation at 94°C for 1 min, followed by primer annealing at 47°C for 1 min and extension at 72°C for 1 min. The last cycle was again an extension at 72°C for 10 min. PCR cycles and other conditions were optimized to assure that the amplification was within the linear range.
The PCR reactions were performed in the presence of α-[32P]-dCTP. The radiolabeled DNA samples were separated on polyacrylamide gels and then scanned with a STORM840 phosphorimager. We also generated a standard (dilution) curve by amplifying different dilutions of the template DNA. The plot of counts for both HCMV and β-actin vs dilutions of DNA did not reach a plateau for the saturation curve (data not shown) under the conditions described above, indicating that quantitation of viral DNA could be accomplished. Moreover, the ratio of viral DNA to β-actin remained constant with respect to each DNA dilution in the standard curve, suggesting that the assay is adequately accurate and reproducible. The PCR results were derived from three independent experiments.
Acknowledgments
We are grateful to Yong Bai, Paul Rider, Ed Yang, and Vincent Sheu for technical assistance and invaluable suggestions. The authors are indebted to Annette Meyer (Pfizer, Inc., Ann Arbor, MI) and John Wu (Promab, Inc., Richmond, CA) for anti-HCMV antibodies. X. J. is a recipient of a China Graduate Student Scholarship from the Chinese Ministry of Education. Y.B. was partially supported by a predoctoral Block grant from University of California at Berkeley. This research has been supported by grants from NIH (RO1-AI041927, RO1-AI050468, RO1-DE014145, and RO1-DE014842).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/21724
References
- 1.Mocarski ES, Shenk T, Pass RF. Cytomegalovirus. In: Knipe DM, Howley PM, Griffin DE, Martin MA, Lamb RA, Roizman B, et al., eds. Fields Virology. Philadelphia, Pa.: Lippincott-William & Wilkins, 2007:2701-72. [Google Scholar]
- 2.Britt WJ. Congenital cytomegalovirus infection. In: Hitchcock H, MacKay T, Wasserheit JN, eds. Sexually transmitted diseases and adverse outcomes of pregnancy. Washington D.C.: ASM Press, 1999:269-81. [Google Scholar]
- 3.Baldanti F, Simoncini L, Talarico CL, Sarasini A, Biron KK, Gerna G. Emergence of a ganciclovir-resistant human cytomegalovirus strain with a new UL97 mutation in an AIDS patient. AIDS. 1998;12:816–8. [PubMed] [Google Scholar]
- 4.Baldanti F, Underwood MR, Stanat SC, Biron KK, Chou S, Sarasini A, et al. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J Virol. 1996;70:1390–5. doi: 10.1128/jvi.70.3.1390-1395.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baldanti F, Underwood MR, Talarico CL, Simoncini L, Sarasini A, Biron KK, et al. The Cys607-->Tyr change in the UL97 phosphotransferase confers ganciclovir resistance to two human cytomegalovirus strains recovered from two immunocompromised patients. Antimicrob Agents Chemother. 1998;42:444–6. doi: 10.1128/aac.42.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drew WL. Cytomegalovirus infection in patients with AIDS. Clin Infect Dis. 1992;14:608–15. doi: 10.1093/clinids/14.2.608-a. [DOI] [PubMed] [Google Scholar]
- 7.Welch AR, Woods AS, McNally LM, Cotter RJ, Gibson W. A herpesvirus maturational proteinase, assemblin: identification of its gene, putative active site domain, and cleavage site. Proc Natl Acad Sci U S A. 1991;88:10792–6. doi: 10.1073/pnas.88.23.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, et al. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci U S A. 2003;100:14223–8. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu D, Silva MC, Shenk T. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc Natl Acad Sci U S A. 2003;100:12396–401. doi: 10.1073/pnas.1635160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scherer LJ, Rossi JJ. Approaches for the sequence-specific knockdown of mRNA. Nat Biotechnol. 2003;21:1457–65. doi: 10.1038/nbt915. [DOI] [PubMed] [Google Scholar]
- 11.Santoro SW, Joyce GF. A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci U S A. 1997;94:4262–6. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopalan V, Altman S. RNase P:structure and catalysis. In: Gesteland R, Cech T, Atkins J, eds. The RNA World. Cold Spring Harbor: Cold Spring Harbor Laboratory Press, 2006:Chapter 6.1(online only at http://rna.cshl.edu).
- 13.Kim K, Liu F. Inhibition of gene expression in human cells using RNase P-derived ribozymes and external guide sequences. Biochim Biophys Acta. 2007;1769:603–12. doi: 10.1016/j.bbaexp.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans D, Marquez SM, Pace NR. RNase P: interface of the RNA and protein worlds. Trends Biochem Sci. 2006;31:333–41. doi: 10.1016/j.tibs.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Marvin MC, Engelke DR. Broadening the mission of an RNA enzyme. J Cell Biochem. 2009;108:1244–51. doi: 10.1002/jcb.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gopalan V, Vioque A, Altman S. RNase P: variations and uses. J Biol Chem. 2002;277:6759–62. doi: 10.1074/jbc.R100067200. [DOI] [PubMed] [Google Scholar]
- 17.Forster AC, Altman S. External guide sequences for an RNA enzyme. Science. 1990;249:783–6. doi: 10.1126/science.1697102. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Y, Hwang ES, Altman S. Targeted cleavage of mRNA by human RNase P. Proc Natl Acad Sci U S A. 1992;89:8006–10. doi: 10.1073/pnas.89.17.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu J, Trang P, Kim K, Zhou T, Deng H, Liu F. Effective inhibition of Rta expression and lytic replication of Kaposi’s sarcoma-associated herpesvirus by human RNase P. Proc Natl Acad Sci U S A. 2004;101:9073–8. doi: 10.1073/pnas.0403164101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma M, Benimetskaya L, Lebedeva I, Dignam J, Takle G, Stein CA. Intracellular mRNA cleavage induced through activation of RNase P by nuclease-resistant external guide sequences. Nat Biotechnol. 2000;18:58–61. doi: 10.1038/81113. [DOI] [PubMed] [Google Scholar]
- 21.Guerrier-Takada C, Li Y, Altman S. Artificial regulation of gene expression in Escherichia coli by RNase P. Proc Natl Acad Sci U S A. 1995;92:11115–9. doi: 10.1073/pnas.92.24.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hnatyszyn H, Spruill G, Young A, Seivright R, Kraus G. Long-term RNase P-mediated inhibition of HIV-1 replication and pathogenesis. Gene Ther. 2001;8:1863–71. doi: 10.1038/sj.gt.3301606. [DOI] [PubMed] [Google Scholar]
- 23.Plehn-Dujowich D, Altman S. Effective inhibition of influenza virus production in cultured cells by external guide sequences and ribonuclease P. Proc Natl Acad Sci U S A. 1998;95:7327–32. doi: 10.1073/pnas.95.13.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawa D, Wang J, Yuan Y, Liu F. Inhibition of viral gene expression by human ribonuclease P. RNA. 1998;4:1397–406. doi: 10.1017/S1355838298980918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou T, Kim J, Kilani AF, Kim K, Dunn W, Jo S, et al. In vitro selection of external guide sequences for directing RNase P-mediated inhibition of viral gene expression. J Biol Chem. 2002;277:30112–20. doi: 10.1074/jbc.M200183200. [DOI] [PubMed] [Google Scholar]
- 26.Liu F, Altman S. Inhibition of viral gene expression by the catalytic RNA subunit of RNase P from Escherichia coli. Genes Dev. 1995;9:471–80. doi: 10.1101/gad.9.4.471. [DOI] [PubMed] [Google Scholar]
- 27.Zaug AJ, Cech TR. Analysis of the structure of Tetrahymena nuclear RNAs in vivo: telomerase RNA, the self-splicing rRNA intron, and U2 snRNA. RNA. 1995;1:363–74. [PMC free article] [PubMed] [Google Scholar]
- 28.Jühling F, Mörl M, Hartmann RK, Sprinzl M, Stadler PF, Pütz J. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37(Database issue):D159–62. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan Y, Altman S. Selection of guide sequences that direct efficient cleavage of mRNA by human ribonuclease P. Science. 1994;263:1269–73. doi: 10.1126/science.8122108. [DOI] [PubMed] [Google Scholar]
- 30.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- 31.Bertrand E, Castanotto D, Zhou C, Carbonnelle C, Lee NS, Good P, et al. The expression cassette determines the functional activity of ribozymes in mammalian cells by controlling their intracellular localization. RNA. 1997;3:75–88. [PMC free article] [PubMed] [Google Scholar]
- 32.Gold L, Polisky B, Uhlenbeck O, Yarus M. Diversity of oligonucleotide functions. Annu Rev Biochem. 1995;64:763–97. doi: 10.1146/annurev.bi.64.070195.003555. [DOI] [PubMed] [Google Scholar]
- 33.Szostak JW. In vitro genetics. Trends Biochem Sci. 1992;17:89–93. doi: 10.1016/0968-0004(92)90242-2. [DOI] [PubMed] [Google Scholar]
- 34.Joyce GF. Directed molecular evolution. Sci Am. 1992;267:90–7. doi: 10.1038/scientificamerican1292-90. [DOI] [PubMed] [Google Scholar]
- 35.Trang P, Lee M, Nepomuceno E, Kim J, Zhu H, Liu F. Effective inhibition of human cytomegalovirus gene expression and replication by a ribozyme derived from the catalytic RNA subunit of RNase P from Escherichia coli. Proc Natl Acad Sci U S A. 2000;97:5812–7. doi: 10.1073/pnas.100101797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu F, Altman S. Differential evolution of substrates for an RNA enzyme in the presence and absence of its protein cofactor. Cell. 1994;77:1093–100. doi: 10.1016/0092-8674(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 37.Pyle AM, McSwiggen JA, Cech TR. Direct measurement of oligonucleotide substrate binding to wild-type and mutant ribozymes from Tetrahymena. Proc Natl Acad Sci U S A. 1990;87:8187–91. doi: 10.1073/pnas.87.21.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matusick-Kumar L, Hurlburt W, Weinheimer SP, Newcomb WW, Brown JC, Gao M. Phenotype of the herpes simplex virus type 1 protease substrate ICP35 mutant virus. J Virol. 1994;68:5384–94. doi: 10.1128/jvi.68.9.5384-5394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daftarian PM, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-alpha. J Immunol. 1996;157:12–20. [PubMed] [Google Scholar]


