Abstract
Background
Although in vitro studies have identified numerous possible targets, the molecules that mediate the in vivo effects of volatile anesthetics remain largely unknown. The mammalian ryanodine receptor (Ryr) is a known halothane target, and we hypothesized that it has a central role in anesthesia.
Methods
Gene function of the Drosophila Ryr (dRyr) was manipulated in the whole body or in specific tissues using a collection of mutants and transgenes, and responses to halothane were measured with a reactive climbing assay. Cellular responses to halothane were studied using Ca2+ imaging and patch clamp electrophysiology.
Results
Halothane potency strongly correlates with dRyr gene copy number, and missense mutations in regions known to be functionally important in mammalian Ryrs gene cause dominant hypersensitivity. Tissue-specific manipulation of dRyr shows that expression in neurons and glia, but not muscle, mediates halothane sensitivity. In cultured cells, halothane-induced Ca2+ efflux is strictly dRyr-dependent, suggesting a close interaction between halothane and dRyr. Ca2+ imaging and electrophysiology of Drosophila central neurons reveal halothane-induced Ca2+ flux that is altered in dRyr mutants and correlates with strong hyperpolarization.
Conclusions
In Drosophila, neurally-expressed dRyr mediates a substantial proportion of halothane's anesthetic effects in vivo, is potently activated by halothane in vitro, and activates an inhibitory conductance. Our results provide support for Ryr as an important mediator of immobilization by volatile anesthetics.
Introduction
Volatile general anesthetics are simple organic compounds that rapidly and reversibly suppress responsiveness to external stimuli in all animals, while at the same time leaving basal functions intact. These properties have rendered anesthetics indispensable in the clinic, and made them attractive tools for studying the control of arousal. One obstacle to progress is that, despite considerable effort over many years, the molecular pathways through which volatile anesthetics act remain largely unknown. In vitro studies have identified many proteins whose activity is affected by clinical concentrations of volatile general anesthetics1, but validating these candidates in vivo has proven difficult.
Studies in genetic model organisms such as worms, flies and mice have been instrumental in identifying novel targets of volatile anesthetics, and have provided in vivo validation of candidates identified in vitro2-6. These mutants illustrate several features of the molecular pathways mediating anesthesia. First, voltage-insensitive (“leak”) ion channels, which act to hyperpolarize neurons, have repeatedly appeared in genetic screens for targets of volatiles2,3,5,7 suggesting that they act, at least in part, through changes in voltage and resistance across the plasma membrane. Second, the response to each anesthetic compound is affected differently by a given mutant, suggesting the presence of agent-specific pathways2,8. Finally, mutations in these genes reduce, but do not eliminate sensitivity to volatile anesthetics, indicating that additional anesthetic targets remain unidentified.
The ryanodine receptor (Ryr), a large-conductance channel that mediates release of Ca2+ from internal stores, is known to be activated by volatile anesthetics, especially halothane9,10. However, the study of the interaction between halothane and Ryr has been largely limited to muscle, where halothane potently induces malignant hyperthermia, a pathology defined by inappropriate activation of Ryr in susceptible humans and swine11. Despite the clear connection between Ryr and halothane, the role of neurally-expressed Ryr in the immobilizing effects of halothane is unexplored.
In this study we use Drosophila genetics to test the hypothesis that Ryr mediates halothane anesthesia. We find that dRyr is a major determinant of halothane's anesthetic effects in flies and that these effects are mediated by expression of dRyr in the nervous system, but not muscle.
Materials and Methods
Fly Stocks and Genetic Analysis
All flies were reared on cornmeal-molasses medium and were maintained at 25°C in a 12 h light–dark cycle. The following fly strains were used in this study: dRyrk04913, Appl-GAL4, nrv2-GAL4, cn1 l(2)44Fp1 bw1 sp1/SM6a, cn1 l(2)44Fl1 bw1 sp1/SM6a, l(2)44Fa3/CyO, l(2)44Ff1/CyO, l(2)44Fg1/CyO, l(2)44Fh1/CyO, l(2)44Fj1/CyO (Drosophila Stock Center, Bloomington, IN); dRyrGS21220 (Drosophila Genetic Resource Center, Kyoto, Japan); UAS-dRyrRNAi (10844R-3, National Institute of Genetics, Japan), UAS-GCaMP3 was a generous gift from Howard Hughes Medical Institute, Janelia Farm, Ashburn VA; elav-GAL4 was a generous gift from Ravi Allada, Ph.D, Professor, University of Chicago, IL; repo-GAL4 is from Chi-Hon Lee, Ph.D., Senior Investigator, National Institute of Child Health and Human Development, Bethesda, MD; MHC-GAL4 was from Benjamin White, Ph.D., Senior Investigator, National Institute of Mental Health, Bethesda, MD; ShakB-GAL4 and Canton-S were from the lab stock collection. dRyrk04913, dRyrGS21220, dRyrΔ25, elav-GAL4, nrv2-GAL4, and UAS-dRyrRNAi were made congenic with Canton-S by outcrossing for seven generations. As described previously12, dRyrk04913 was homozygous lethal. However, the lethality was not rescued by the genomic duplication dRyr24D03, indicating that it resulted from a closely linked mutation outside the dRyr locus.
Behavioral assays of anesthesia are subject to a number of confounding factors, including genetic background and subtle environmental variations. To avoid such confounding factors, all comparisons were made within experiments run in parallel over the same time, and only between groups matched for genetic background.
The deletion mutant dRyrΔ25 was generated by excising the region between transposons P{XP}d03686 and P{XP}d03830 (Exelixis Stock Collection, Boston, MA), using flippase-mediated recombination at flippase recombination target sites present in these elements13. The deletion was verified using PCR amplification and DNA sequencing.
The genomic duplication dRyr24D03 was generated by integrating the P[acman] clone CH321-24D03 (Bellen laboratory, Baylor College of Medicine), into the VK33 docking site on the third chromosome via ΦC31-mediated site-specific integration14. UAS-dRyr-V5 was generated by cloning a 15.6kb PacI-PsiI fragment from DRyR-15B (kindly provided by Daniel Cordova, M.Sc., Senior Biochemist, Dupont Crop Protection, Newark, DE) into the pUAST vector, and injecting the construct into Canton-S flies that carried the w1118 mutation. All injections were performed by Rainbow Transgenic Flies, Inc. (Camarillo, CA).
Genomic Sequencing to Identify dRyr Mutation Sites
The genomic DNA libraries from both control and mutant strains used in this study were constructed and sequenced in accordance with the manufacturer's protocols (Illumina, San Diego, CA). Briefly, one microgram of genomic DNA was sheared by sonication, end-repaired, A-tailed, adapter-ligated, size-fractionated by gel electrophoresis, and PCR-amplified. Paired-end 101-cycle sequencing on a HiSeq 2000 instrument (Illumina, San Diego, CA) yielded an average of 20 million reads (range 13-30 million) equivalent to ~29-fold coverage (range, 19-43-fold). Reads were aligned to the D. melanogaster reference genome (dm3, Berkeley Drosophila Genome Project Release 5) with BFAST15. SAMTools16 were used to call sequence variants, and non-synonymous mutations were identified using ANNOVAR17.
To generate a list of unique candidate mutations, we filtered sequence variants in the mutant strains against those present in the control strain. This process revealed that only dRyr contained sequence variants in all of the dRyr alleles carrying the EMS mutations. Pairwise alignments were carried out with mammalian Ryr isoforms using BLASTP.
Tissue Homogenates and Western Blots
After flies were tested in the reactive climbing assay, 200 fly heads or 50-100 whole flies from each genotype were used to prepare homogenates. The samples were homogenized in 100-200 μl of homogenization buffer (0.25 M Sucrose, 10 mM Tris, 1 mM EDTA) and protease inhibitors (Roche, Indianapolis, IN). The homogenates were centrifuged for 10 min at 1000 × g at 4°C. The supernatant was centrifuged again for 40 min at 48,000 × g at 4°C to extract membrane protein. The pellet was resuspended (50 mM Tris (pH 7.4), 150 mM NaCl, 1mM EDTA and protease inhibitors), and 20 to 30 μg of protein was processed, electrophoresed, and transferred according to supplier's instructions (Invitrogen, Carlsbad, CA). Blots were probed with anti-dRyr (rabbit polyclonal, a generous gift from Daniel Cordova, M.Sc. (Senior Biochemist, Dupont Crop Protection, Newark, DE) at a 1:1000 dilution, and anti-Na, K-ATPase (mouse mAbα5, Developmental Studies Hybridoma Bank, Iowa City, IA) at 1.3 ng/mL. Secondary antibodies were peroxidase-linked and used according to the supplier's instructions. Blots were developed with the Enhanced Chemiluminescence detection system (GE HealthCare, Piscataway, NJ). For quantification, four independent homogenates were prepared for each genotype, and two aliquots of each were analyzed, yielding eight blots for each genotype. Intensities of dRyr bands were measured and normalized to those of Na, K-ATPase using ImageJ.
Reactive Climbing Assay
The reactive climbing assay, also known as the distribution test, was performed as described previously18. Male flies aged 3-7 days were collected and sorted under carbon dioxide anesthesia, and allowed to recover for one day. Without further anesthesia, flies were loaded into testing vials (10 per vial), consisting of 50 ml tubes perforated to allow gas exchange. Testing vials were equilibrated in a chamber with a constant flow of a fixed concentration of volatile anesthetics - halothane (Sigma, St. Louis, MO), sevoflurane and enflurane (RxElite, Boise, ID), and isoflurane (Baxter Healthcare Corp, Deerfield, IL) - for 35-45 min. Following equilibration, the flies were tapped down quickly to the bottom of the vial several times at 30 second intervals. After the last round of tapping, the flies were allowed to climb for one minute, after which the proportion remaining at the conical bottom of the testing vial (fraction down) was recorded. Flies were tested with three or four more concentrations of anesthetic, progressively increased by 0.05% with each successive test, and equilibrated for 35-45 minutes before each test. Naïve flies of the same genotype were then tested at the four or five higher concentrations in order to produce the full concentration-response curve of 8-10 concentrations, covering the full range from 0% down to 100% down. The process was repeated twice with naïve flies. In total, this yielded a sample size of 9 for each combination of drug and genotype.
Countercurrent Locomotion Assay
The countercurrent assay, was performed as described previously19. Typically, a group of 30 male flies of each genotype, aged 3-7 days, were loaded into the start tube, tapped to the bottom, and given 10 seconds to climb up into a transfer tube at top. The flies in each transfer tube were then shifted into the next base tube by banging the device. This is repeated 4 more times at 10 second intervals, and at the end, the flies in each base tube were counted. A transfer probability, Pt, is calculated by the following formula:
The experiment was performed three to five times for each genotype.
Sf9 Cell Culture and Flow Cytometry
Sf9 cells stably-transfected with dRyr20 were a generous gift from Daniel Cordova, M. Sc. (Senior Biochemist, Dupont Crop Protection, Newark, DE). Wild-type Sf9 cells were purchased from Invitrogen (Carlsbad, CA). Cultures were maintained at 28°C in SF900-II serum-free medium (Invitrogen), without antibiotics, and subcultured at confluence by sloughing, about twice weekly.
To prepare Sf9 cells for Ca2+ imaging, they were suspended, centrifuged, washed in Ca2+ imaging saline (in mM: 130 NaCl, 5.4 KCl, 1.2 MgCl2, 1 CaCl2, 4.2 NaHCO3, 7.3 NaH2PO4, 10 Glucose, 63 Sucrose20), then centrifuged and resuspended in 5 ml Ca2+ imaging saline containing 1 μM of the acetoxymethyl (AM) ester of a Fluo Calcium indicator (Fluo-5N-AM in most experiments), plus Pluronic F-127 (Invitrogen). Cells were incubated in Fluo-AM for 30-45 min, and then washed in Ca2+ imaging saline for a minimum of 30 minutes before cytometry. Aliquots containing ~0.5 × 106 cells were placed in styrene tubes, treated with anesthetics and/or thapsigargin (Tocris Bioscience, Minneapolis, MN), and fluorescence of the Fluo indicator was measured on a BD FACScan (Becton-Dickinson, Franklin Lakes, NJ). Debris and dead cells were gated out by size and fluorescence due to propidium iodide uptake, respectively. Intracellular Ca2+ concentration was calibrated at the end of the experiment by sequential addition of ionomycin (Tocris) and EGTA, calculated as described by Tsien et al21, using Kd = 2.3 μM for Fluo-5F and 90 μM for Fluo-5N. Offline analysis of cytometry data was carried out using Flow-Jo 7.6.1 (Tree Star Inc., Ashland OR). Anesthetic concentrations in solution were converted to partial atmospheric pressures using Ostwald water/gas partition coefficients.
Ca2+ Imaging in Larval Central Nervous System
Changes in internal Ca2+ were visualized in the cell body of motoneuron RP2 (MNISN1s) using the genetically encoded Ca2+ indicator GCaMP322 driven by ShakB-GAL4. The central neruvous system was dissected from the larva, brain lobes removed, and mounted dorsal side down on a polyornithine-coated 25 mm coverslip, which formed the bottom of the recording chamber (Warner Instruments, Hamden CT), mounted in a custom-fabricated plate that fit into the stage of a Nikon C-1 inverted confocal microscope (Nikon Instruments, Tokyo, Japan) . This setup allowed for superfusion from above, while the preparation was imaged using a 60X oil immersion objective from below. The preparation was scanned at 1 Hz, with the laser at minimum power and the pinhole opened to 100 μm.
Recording solution (in mM: 70 NaCl, 5 KCl, 0.3 CaCl2, 4 MgCl2, 10 NaHCO3,5 trehalose, 115 sucrose, 5 HEPES, pH 7.223) was perfused at 2 ml/min using a peristaltic pump, with solutions selected via remote-controlled solenoid valve. Anesthetics were vortexed into solution for 1-2 min and perfused from sealed reservoirs. Concentrations of anesthetic in contact with cells were determined via headspace analysis of 50 μl samples of solutions collected from the chamber, using an Agilent 6850 gas chromatograph24,25. Ca2+ imaging data were analyzed using Nikon EZ-C1 software. Because of their superficial positions, RP2s in multiple segments could be visualized at once, allowing analysis of 4-8 cells per preparation. The entire cell body of each cell in the focal plane of a given preparation was defined as a region of interest, and the average pixel intensity vs. time was determined. Because the background signal in regions outside of RP2 somata was essentially nil, background subtraction was unnecessary. ΔF/F was calculated by dividing the intensity at a given time by the average of samples 1-10, subtracting 1 to set the initial value to zero, and multiplying by 100 to generate a percentage change.
Electrophysiology
Whole cell recordings were performed largely as described previously24. The external solution was identical (in mM: 118 NaCl, 2 NaOH, 2 KCl, 4 MgCl2, 0.5 CaCl2, 60 sucrose, 5 trehalose, 5 HEPES, pH 7.1, osmolality 300-305 mosmoles/kg). The internal solution differed by the addition of Mg-ATP and GTP-Tris (152 K-gluconate, 2 NaCl, 0.1 CaCl2, 10 HEPES, 1 EGTA, 4 Mg-ATP, 0.6 GTP-Tris, 8.4 KOH, pH 7.2, 290-295 mosmoles/kg). A larval central nervous system, expressing UAS-mcd8-GFP in RP2 under the control of ShakB-GAL4 was mounted on a polyornithine-coated chip of coverslip. The region of the sheath around RP2 somata in segment A5 was softened with collagenase type XIV (Sigma) and removed manually with a suction pipette. Gigaseals were formed under the guidance of Nomarski optics, and membrane voltage was recorded using an AxoPatch 200B in current-clamp mode. Recording parameters and solutions were controlled, and data were recorded using pClamp 8. Drugs were dissolved as described above under “Ca2+ Imaging in Larval Central Nervous System” and perfused at 2 ml/min.
Statistical Analysis
Reactive climbing data were analyzed largely as described previously18. In brief, the data were entered into SPSS13 (SPSS Inc, Chicago, IL) and fitted with the concentration-response relationship: fraction down = Cn/(Cn+EC50n), using logit analysis 26,27. Variable C is the anesthetic concentration, n reflects the steepness of the curve and is shared between genotypes for each experiment, and EC50 is the anesthetic concentration at the midpoint of the curve. Each vial of flies was used for 4-5 anesthetic concentrations, so that the full range of eight concentrations required a pair of vials. EC50 was determined for each pair of vials, for which the percent shift in EC50 was derived using the following formula: .
Flow cytometry and GCaMP3 Fluorescence data were analyzed statistically in Sigmaplot 11.0. For fitting of concentration-response curves for Fluo-5 fluorescence, data were normalized by defining percentage of untreated cells with elevated [Ca2+]i as 0, those treated with saturating concentrations of anesthetic as 1, and the resulting relationships were fitted with a 3-parameter Hill equation. Concentration-response curves for GCaMP3 fluorescence were plotted and analyzed as for Fluo-5, but using ΔF/F values. A few preparations that responded with exaggerated Ca2+ flux to halothane concentrations above 2.0 mM were excluded from the concentration-response analysis by Dixon's test for outliers.
All data fit the assumptions for parametric statistics. Differences between two groups were assessed using Student's t-test. Comparisons involving single factor were performed using one-way ANOVA. The analysis of multiple genotypes and anesthetics described in “dRyr Mutations Have Weaker Effects on Other Volatile Anesthetics” was performed with two-way ANOVA. The Bonferroni-Dunn test was used for post-hoc analysis. Because of the conservative nature of the Bonferroni correction, P values were equal to 1 in a small number of cases.
Results
Ryanodine Receptor Insertion Mutants Are Resistant to Halothane
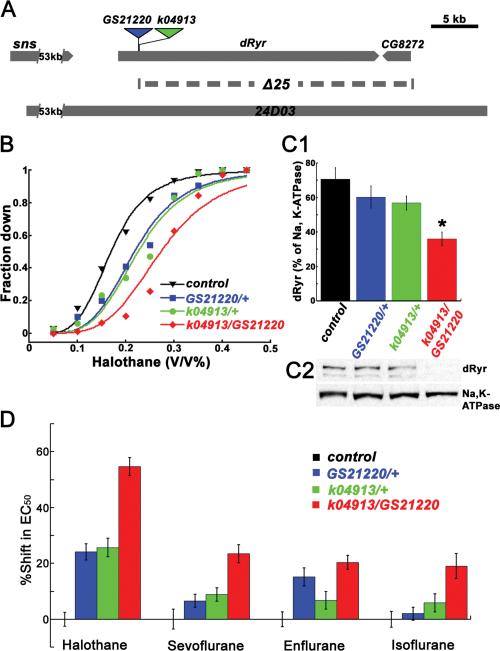
The Drosophila genome contains a single Ryr gene at cytological position 44F28,12. In our intial tests of the role of dRyr in the response to the volatile anesthetic halothane, we used dRyrk04913, a hypomorphic mutant allele resulting from the insertion of a transposon 399 bases upstream of the start codon of dRyr 12, and dRyrGS21220, 9 bases upstream of dRyrk04913 (Figure 1A). dRyrGS21220 is homozygous viable, whereas, as described previously12, dRyrk04913 is lethal, presumably due to a tightly linked mutation outside the dRyr locus (see Methods for details). Given the close proximity of the transposons in the two alleles, we expected them to produce similar phenotypes.
Figure 1.
dRyr mutants display preferential resistance to halothane. (A) Genetic map of dRyr region. The insertion sites of dRyrGS21220 (blue triangle) and dRyrk04913 (green triangle) are diagrammed above the genomic structure. The dashed line below the dRyr gene indicates the genomic segment deleted in dRyrΔ25. The genomic extent of CH321-24D03, which was used to generate the duplication strain dRyr24D03, is represented by the grey bar at the bottom. (B) dRyr mutants show resistance to the volatile anesthetic halothane in the reactive climbing assay. Canton-S flies (control, black triangles) respond to halothane in a concentration-dependent fashion (solid line is a fit of the data in the logit model). The concentration-response curves of dRyrGS21220/+ (blue squares) and dRyrk04913/+ (green circles) shift to the right relative to the control, indicating increased resistance. dRyrk04913/dRyrGS21220 (red diamonds) shows an even stronger halothane resistance. (C) dRyr protein expression is decreased in dRyr mutants. (C1) Quantification of dRyr, normalized to Na,K-ATPase. Values are mean ± SEM, with statistical significance indicated by an asterisk (*; P = 0.0005). (n = 8). (C2) A representative western blot. (D) dRyr mutants affect halothane anesthesia in preference to the effects of other anesthetics. Data are plotted as the shift in EC50, the concentration at which half of the flies are down, compared to the wildtype. dRyr mutants, dRyrGS21220/+ (blue); dRyrk04913/+ (green); and dRyrk04913/dRyrGS21220 (red) have a strong effect on halothane sensitivity, a weak effect on the sensitivity to sevoflurane, enflurane, and isoflurane. Detailed comparisons are described in Results. Values are mean ± SEM.
We examined the response of the mutants to halothane using a reactive climbing assay that evaluates the flies’ righting/climbing reflex following mechanical agitation in the presence of anesthetic18. Under these conditions, wild-type Canton-S males exhibited a halothane-dependent decrease in locomotor activity, resulting in an increase in the proportion failing to climb (“Fraction Down;” Figure 1B, black curve), with flies lying immobile at the bottom of the vial at the highest anesthetic concentrations. For heterozygous dRyrGS21220/+ and dRyrk04913/+, and the transheterozygote dRyrk04913/dRyrGS21220, the concentration-response curves shifted to the right, indicating resistance to halothane relative to the wildtype. The effective concentrations (vol/vol) at which 50% of flies were down (EC50s) were 0.22%, 0.22%, and 0.27% for dRyrGS21220/+, dRyrk04913/+, and dRyrk04913/dRyrGS21220, respectively, all of which were significantly higher than that of the wild-type control (0.17% vol/vol; Figure 1B; all P < 0.0001, two-way ANOVA and Bonferroni-Dunn post-hoc tests). In separate experiments, the EC50 for dRyrGS21220 homozygotes (0.27%) was also significantly higher than matched controls (0.20%;P < 0.0001, Student's t-test).
Reduction in halothane sensitivity was paralleled by reduction in dRyr protein levels (Figure 1C). There was a significant effect of genotype on protein levels (P = 0.0009, one-way ANOVA), and the allelic combination that reduced halothane sensitivity the most, dRyrk04913/dRyrGS21220, reduced dRyr protein levels significantly. dRyr protein levels in the heterozygotes were intermediate between the wildtype and dRyrk04913/dRyrGS21220, but Bonferroni-Dunn post-hoc tests failed to demonstrate significant differences from control. We conclude that the insertion alleles reduce dRyr expression, but that anesthetic sensitivity discriminates between alleles more effectively than do immunoblots.
dRyr Mutations Have Weaker Effects on Other Volatile Anesthetics
Previous work identified target genes for anesthetic action that have a distinct preference for halothane over other anesthetic agents, implying the presence of agent-specific pathways2,8. To determine the level of anesthetic specificity of dRyr, we assayed the responses of dRyr mutants to sevoflurane, enflurane, and isoflurane, and compared them to the response to halothane using a two-way ANOVA (Generalized Linear Model; Figure 1D). There was a significant effect of anesthetic agent on the magnitude of shifts in EC50, with halothane having the strongest effect (P < 0.0001, two-way ANOVA). In Bonferroni-Dunn post-hoc tests, dRyrk04913/ dRyrGS21220 mutants were resistant to sevoflurane, enflurane, and isoflurane compared to wild-type controls. dRyrk04913/+ produced small shifts in the EC50s for sevoflurane, enflurane, and isoflurane that were not significantly different from wild-type controls, and were significantly smaller than the effect of this allele on halothane. dRyrGS21220/+ had mixed effects. It showed significant resistance to enflurane, which was not significantly different from that for halothane, but did not significantly affect the response to sevoflurane or isoflurane. dRyr mutants were therefore more resistant to the anesthetic effects of halothane than to those of other volatile agents, implicating dRyr as a component of the pathway(s) that regulates halothane sensitivity.
Halothane Sensitivity Correlates with dRyr Copy Number
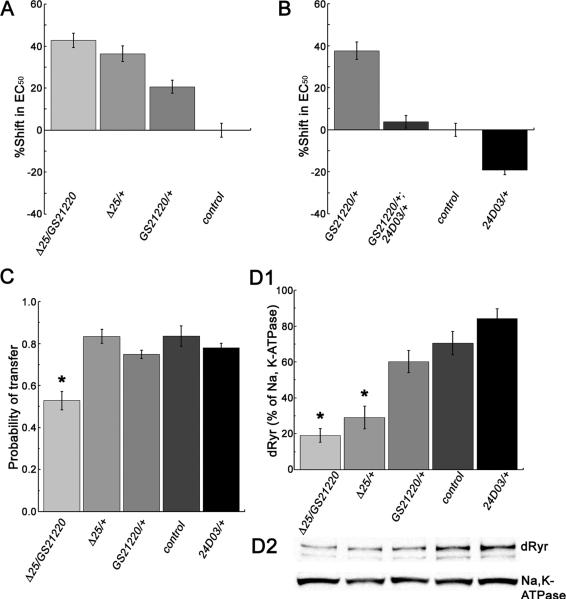
To explore the relationship between dRyr gene copy number, dRyr protein levels, and halothane sensitivity more systematically, we generated flies with the dRyr gene deleted or duplicated. The deletion dRyrΔ25 was created by excising the region between two transposable elements using flippase-flippase recombination target mediated recombination (Figure 1A). The resulting lethal allele removes all but the first exon of dRyr and all of CG8272, a gene of unknown function. This allele produces significant resistance to halothane, both in a heterozygous state and in combination with the hypomorphic allele RyrGS21220 (Figure 2A; P < 0.0001 and P < 0.0001, one-way ANOVA and Bonferroni-Dunn post-hoc tests). In dRyrΔ25/+ animals, which possess exactly one copy of dRyr, halothane EC50 is 36% higher than wild-type controls. Trans-heterozygous dRyrΔ25/ dRyrGS21220 mutants shift halothane EC50 by 43% (Figure 2A), which is significantly higher than the wildtype but not different from dRyrΔ25/+. As might be expected for a hypomorphic mutation, dRyrGS21220/+ had intermediate effects, significantly higher than the wildtype and lower than dRyrΔ25/+ or dRyrΔ25/ dRyrGS21220.
Figure 2.
Sensitivity to halothane follows dRyr copy number. (A) Reduced dRyr copy number is associated with resistance to halothane. Heterozygous dRyr deletion mutant dRyrΔ25/+ (medium grey), carrying one copy of dRyr, is resistant to halothane compared to the control, Canton-S, which carries two copies. dRyrΔ25/dRyrGS21220 transheterozygotes (light grey) are resistant compared to the wildtype and dRyrGS21220/+ (dark gray). (B) The dRyr duplication strain dRyr24D03/+ (black), carrying three copies of dRyr, is more sensitive to halothane than its control (VK33 without insertion, carrying two copies of dRyr). Introducing dRyr24D03 into insertion mutant dRyrGS21220 (dRyrGS21220/+;dRyr24D03/+, dark grey) rescues the resistant phenotype of dRyrGS21220/+ (gray) to normal levels. Values are mean ± SEM (C) In the absence of anesthetic dRyrΔ25/+ (grey), dRyrGS21220/+ (dark grey), and dRyr24D03(black) display normal locomotor activity, while RyrΔ25/dRyrGS21220 (light grey) is significantly less active than control flies. Values are mean ± SEM (n = 3-5), and asterisk denotes statistical significance (P = 0.0006, one-way ANOVA and Bonferroni-Dunn post-hoc tests). (D) dRyr copy number affects dRyr protein expression. (D1) Quantification of dRyr protein levels. Asterisks denote statistically significant difference from the wildtype (P < 0.0001 and P < 0.0001, one-way ANOVA and Bonferroni-Dunn post-hoc tests). Values are mean ± s.e.m. (n = 8) (D2) A representative western blot, showing trend of increasing dRyr expression with the number of dRyr copies.
To increase dRyr gene copy number, we inserted an additional genomic copy of dRyr into chromosome 3 to generate the dRyr duplication strain, dRyr24D03 (Figure 1A). dRyr24D03 rescued the halothane resistant phenotype of dRyrGS21220/+ (Figure 2B), with the shift in EC50 of dRyrGS21220/+; dRyr24D03/+ significantly reduced from dRyrGS21220/+ (P < 0.0001, one-way ANOVA and Bonferroni-Dunn post-hoc tests), and not different from the wildtype. Combining dRyr24D03 into a wild-type background, resulting in three copies of dRyr, caused significant hypersensitivity to halothane (i.e. a shift in EC50 of -19%, Figure 2B). Additional copies of dRyr24D03 produced lethality, suggesting that no more than three copies of the gene can be tolerated.
Importantly, the mutants’ altered response to halothane did not result from non-specific hyperactivity or arousal, in that all performed normally or worse than wild-type animals when tested for locomotion and responsiveness to mechanical stimulation in the absence of anesthetic (Figure 2C).
As was the case for the dRyr insertion mutants (Figure 1C), there was a significant effect of dRyr genotype on dRyr protein levels (Figure 2D; P < 0.0001, one-way ANOVA). In Bonferroni-Dunn post-hoc tests, dRyr protein was strongly and significantly reduced in dRyrΔ25/+ and dRyrΔ25/ dRyrGS21220. The increase in dRyr protein in dRyr24D03/+ did not reach significance.
Taken together, our results demonstrate that halothane sensitivity follows dRyr gene copy number, and protein levels. We conclude that dRyr is a limiting factor for halothane-induced anesthesia and a likely target of halothane.
dRyr Point Mutations Change Halothane Sensitivity
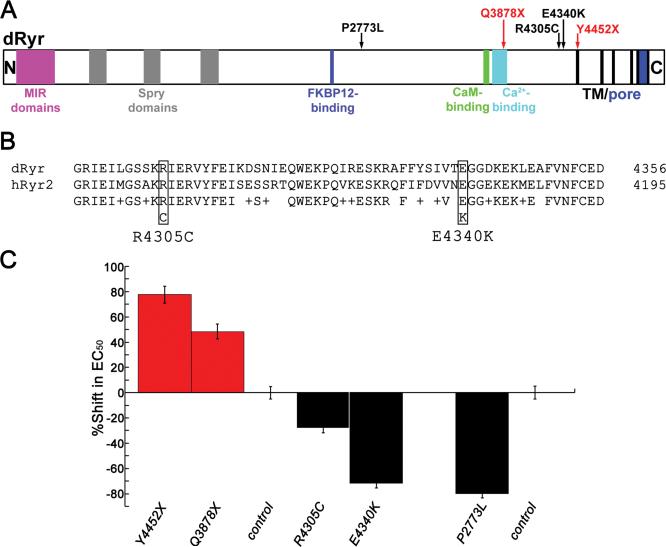
Although copy number variation in humans is well-documented, most disease-associated mutations in human Ryr (hRyr) genes are amino acid substitutions that lead to dominant alleles11,29. dRyr is 45-47% identitical in primary sequence to the three mammalian Ryrs and contains conserved protein domains important to Ryr function (Figure 3A), indicating that Ryr activity and regulation may be conserved across species.
Figure 3.
Point mutations in dRyr change halothane sensitivity of Drosophila. (A) Schematic illustration shows predicted motifs in the primary sequence of Drosophila Ryr, including MIR domains, SPRY domains, FKBP12- binding domain, Ca2+-binding domain, calmodulin-binding domain, six trans-membrane segments (TM), and the Ca2+ pore. Sites of amino acid alterations (arrows) in the five dRyr alleles described in the main text. Red arrows indicate nonsense mutations and black arrows indicate missense mutations. (B) Alignment of dRyr (top line; accession NP_476994.1) and the human cardiac Ryr, hRyr2 (second line; accession NP_001026.2), in the region spanning the missense alleles dRyrR4305C and dRyrE4340K. Sites of amino acid substitutions are indicated by boxes. In the third line, identities between the sequences are indicated by the amino acid symbol, conservative substitutions by plus signs, and non-conservative substitutions by blank spaces. (C) Point mutations in dRyr alter halothane sensitivity. Nonsense mutations dRyrY4452X/+ and dRyrQ3878X/+ (red) are resistant to halothane, whereas missense mutations dRyrR4305C/+, dRyrE4340K/+, and dRyrP2773L/+ (black) display hypersensitivity to halothane. All mutant values are significantly different from their matched wild-type controls (P = 0.0043 for dRyrR4305C/+ and P < 0.0001 for all others, one-way ANOVA and Bonferroni-Dunn post-hoc tests). Values are mean ± SEM.
Previous work had identified a collection of lethal EMS mutants in region 44D-45F 30,31. Using NextGen sequencing, we determined that five of these mutants (l(2)44Fa3, l(2)44Fg1, l(2)44Fh1, l(2)44Fj1, and l(2)44Fp1) altered the sequence of dRyr (Table 1; Figure 3A, 3B). Two of these dRyr alleles contained nonsense mutations at positions corresponding to amino acid 3878 (Q3878X) in l(2)44Fa3, and at 4452 (Y4452X) in l(2)44Fj1. In both alleles, mutations introduced a stop codon before the transmembrane region containing the ion channel, producing truncated and non-functional dRyrs. As expected for loss of function alleles, heterozygotes were resistant to halothane (dRyrY4452X/+, 78% shift, dRyrQ3878X/+, 48% shift compared to genetically matched control strain l(2)44Ff1; Figure 3C; P < 0.0001 and P < 0.0001, one-way ANOVA and Bonferroni-Dunn post-hoc tests) and both failed to complement the lethal phenotype of dRyrΔ25. The relative magnitude of halothane resistance in the point mutants appeared to be larger than that of the deletion allele dRyrΔ25 (Figure 2A), but differences in genetic backgrounds of the animals in the two experiments make comparisons problematic.
Table 1.
Point Mutations Identified in dRyra
| Mutantb | Mutation in dRyr mRNAc | Amino acid changed | Halothane phenotype |
|---|---|---|---|
| l(2)44Fa3 | C11786T | Q3878X | Resistance |
| l(2)44Fj1 | T13510A | Y4452X | Resistance |
| l(2)44Fg1 | C13067T | R4305C | Hypersensitivity |
| l(2)44Fh1 | G13172A | E4340K | Hypersensitivity |
| l(2)44Fp1 | C8472T | P2773L | Hypersensitivity |
dRyr, Drosophila ryanodine receptor
l(2)44F, lethal mutation on chromosome 2, cytological position 44F
dRyr transcript variant D (NM_057646.2)
dRyr isoform D (NP_476994.1)
The three other alleles were missense mutations, leading to substitutions of highly conserved amino acids: R4305C in l(2)44Fg1, E4340K in l(2)44Fh1, and P2773L in l(2)44Fp1 (Table 1, Figure 3A, 3B). All of the missense mutations were lethal in combination with dRyrΔ25, two of the mutations are located in a region associated with dominant channelopathies in hRyr2 (see “Ryr and Neuropathology” in Discussion for details), and all exhibited dominant hypersensitivity to halothane (Figure 3C). Halothane sensitivity was enhanced in dRyrR4305C/+ (-28% shift in EC50 P = 0.0043, one-way ANOVA and Bonferroni-Dunn post-hoc tests), and strongly increased in dRyrE4340K/+ and dRyrP2773L/+ (-72% and -80%, respectively; P < 0.0001, one-way ANOVA and Bonferroni-Dunn post-hoc tests; Figure 3C). Thus, the missense mutations in dRyr produced dominant effects on halothane anesthesia in Drosophila, resembling the gain of function phenotype caused by an extra copy of dRyr (Fig 2B).
dRyr Function in the Nervous System is Required for Normal Halothane Sensitivity
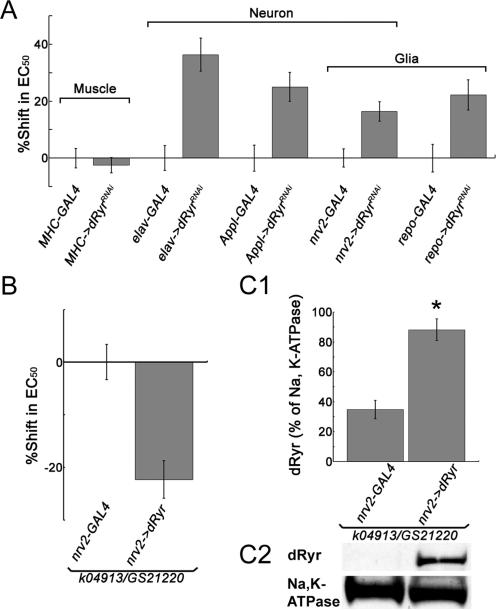
In the human disease malignant hyperthermia, mutations in hRyr1 are associated with a potentially fatal condition in which skeletal muscle Ryrs activate in response to volatile anesthetics, particularly halothane. Because the reactive climbing assay is based on locomotion, and could therefore be influenced by dRyr action in muscle, we determined the site of action of dRyr by driving the expression of double stranded RNA (UAS-dRyrRNAi) using tissue-specific GAL4 drivers. Importantly, driving UAS-dRyrRNAi with the muscle-specific driver MHC-GAL4, failed to alter halothane sensitivity compared to controls carrying MHC-GAL4 alone (Figure 4A; P = 1, two-way ANOVA and Bonferroni-Dunn post-hoc tests). By contrast, flies expressing UAS-dRyrRNAi under the control of the pan-neuronal drivers elav-GAL4 and Appl-GAL4 were significantly resistant to halothane (36% and 25% shift in EC50, respectively; Figure 4A; P < 0.0001 and P = 0.0094), suggesting dRyr function is required in neurons for normal halothane sensitivity. Interestingly, driving UAS-dRyrRNAi expression in glia with repo-GAL4 also produced significant resistance to halothane (22% shift in EC50; P = 0.0373), indicating that dRyr is required in glia as well as neurons for halothane anesthesia. When driven by nrv2-GAL4, which expresses broadly but not ubiquitously in neurons and glia, UAS-dRyrRNAi produced a small (16 %), but not statistically significant shift in EC50 (Figure 4A; P = 0.0862).
Figure 4.
dRyr expressed in the nervous system is required for normal halothane sensitivity. (A) dRyr activity in the nervous system, but not in muscle, is required for normal halothane sensitivity in Drosophila. Flies expressing RNAi against dRyr (UAS-dRyrRNAi) under the control of the muscle-specific driver MHC-GAL4 (MHC->dRyrRNAi) are not different from controls (MHC-GAL4 alone) in the reactive climbing assay for halothane (P = 1, two-way ANOVA and Bonferroni-Dunn post-hoc tests). In contrast, elav->dRyrRNAi and Appl->dRyrRNAi flies, in which dRyr expression in neurons is specifically inhibited, display strong resistance to halothane compared to controls (elav-GAL4 and Appl-GAL4, respectively). Expressing UAS-dRyrRNAi using the glial-specific driver repo-GAL4 (repo->dRyrRNAi) significantly increases resistance to halothane (P = 0.0373 two-way ANOVA and Bonferroni-Dunn post-hoc tests). Broad expression in neurons and glia, driven by nrv2-GAL4 (nrv2->dRyrRNAi), does not cause significant resistance (P = 0.0862. two-way ANOVA and Bonferroni-Dunn post-hoc tests). Values are mean ± SEM. (B) dRyr expressed in the nervous system is sufficient for halothane sensitivity. Restoring dRyr expression in the nervous system using nrv2-GAL4 to drive UAS-dRyr rescues the resistant phenotype in mutant dRyrk04913/dRyrGS21220. (C) Restoring dRyr expression in the nervous system in rescue flies (dRyrk04913/dRyrGS21220; nrv2->dRyr) is confirmed by western blot of membrane extracts from fly heads. (C1) The bar graph shows that dRyr levels increase significantly (Asterisk) over the negative control (P < 0.0001, Student's t-test; n = 8). Values are mean ± s.e.m. (C2) Representative western blot. As shown in the bar graph, dRyr protein is present in the mutants, but not visible in this exposure.
To further establish the site of action of dRyr, we sought to restore halothane sensitivity in dRyr mutants by expressing a UAS-dRyr transgene in a selected cell types. Although expression of the rescue construct using the exclusively muscle-, neuron-, and glial-specific drivers proved lethal, expression in neurons and glia using nrv2-GAL4, restored sensitivity to halothane in transheterozygous dRyrk04913/dRyrGS21220 mutants (-22% shift in EC50; Figure 4B; P < 0.0001, Student's t-test). This manipulation also restored dRyr protein expression in the brains of rescued flies, as shown by western blots of head extracts (Figure 4C; P < 0.0001, Student's t-test).
In summary, tissue-specific knockdown and rescue experiments support the conclusion that dRyr functions in the nervous system and not muscle to control anesthetic responsiveness of Drosophila. In addition, the lethality resulting from UAS-dRyr overexpression in most tissues, combined with the observation that homozygous dRyr24D03 and dRyrΔ25 are lethal, indicates that temporal or spatial variation in dRyr expression levels are poorly tolerated, presumably because of its critical role in cellular calcium homeostasis.
Halothane Induces Ca2+ Release in dRyr-transfected Sf9 Cells
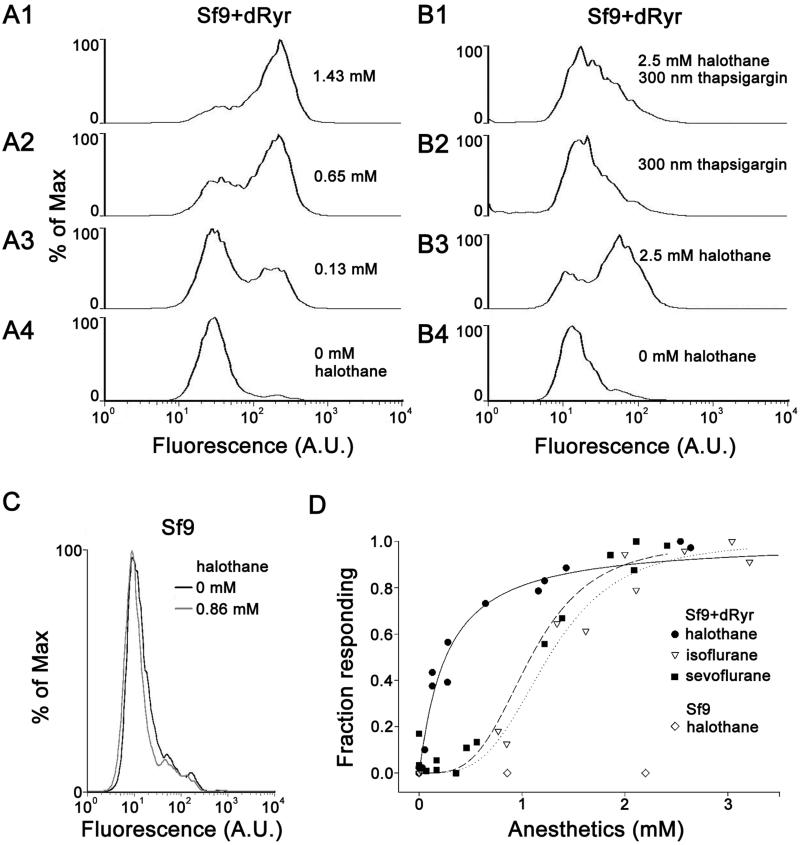
The results described in the previous section demonstrate that dRyr in the nervous system is required for full susceptibility to halothane, but the ability of halothane to activate the Drosophila Ryr has not been tested. We therefore assayed Ca2+ flux in Sf9 cells stably transfected with dRyr (Sf9+dRyr) 20, using the Ca2+-sensitive dye Fluo-5 and flow cytometry (Figure 5). Without halothane, the intensity of Fluo-5 fluorescence in Sf9+dRyr cells (Figure 5A4) corresponded to [Ca2+]i between 50-100 nM. Treatment with halothane concentrations greater than 1 mM shifted fluorescence to a much higher level in almost all transfected cells (Figure 5A1), with calibrated [Ca2+]i exceeding 100 μM. Pretreatment of transfected cells with 300 nM thapsigargin to deplete intracellular calcium stores blocked the halothane-induced [Ca2+]i increase (Figure 5B), confirming that the response to halothane depends on internal Ca2+ stores. Halothane had no effect on untransfected Sf9 cells (Figure 5C) even at high concentrations (Figure 5D, open diamonds), indicating that the response to halothane required dRyr.
Figure 5.
Halothane induces Ca2+ release from internal stores in Sf9 cells stably transfected with dRyr (Sf9+dRyr). Flow cytometry was used to measure [Ca2+]i in Sf9 cells loaded with Fluo5-AM. (A) Representative histograms from flow cytometry analysis of Sf9+dRyr cells treated with halothane. Plots show the number of cells at each level of fluorescence (arbitrary units, A.U.), normalized to the modal value (% of Max). (A1) Treatment with 1.43 mM halothane results in a high proportion of cells with high fluorescence intensity. (A2, A3) Intermedate concentrations of halothane shift the proportions of cells with high vs. low fluorescence. (A4) In the absence of halothane, Sf9+dRyr cells show low levels of fluorescence. Each histogram represents 10,000 cells. (B) Depletion of intracellular Ca2+ with thapsigargin blocks the halothane-induced [Ca2+]i increase in Sf9+dRyr cells. (B1) Pre-treatment with thapsigargin, which blocks the sarco/endoplasmic reticulum Ca2+ ATPase and results in store depletion, blocks the effect of halothane, compared to cells treated with thapsigargin alone (B2). (B3) Treatment of cells with a high concentration of halothane (2.5 mM), the majority show elevated fluorescence compared to untreated cells (B4). Note that a subpopulation of cells in this experiment did not respond to halothane. (C) Untransfected Sf9 cells do not respond to halothane. Fluorescence does not increase in Sf9 cells treated with 0.86 mM halothane (grey line), compared to untreated controls (black line). (D) Concentration-response curves for anesthetic-induced Ca2+ flux. Each data point represents the proportion of cells with elevated [Ca2+]i in a sample of 10,000 cells. The proportion of Sf9+dRyr cells with elevated [Ca2+]i increases as a function of anesthetic concentration, with halothane (filled circles) being many-fold more potent than sevoflurane (filled squares) or isoflurane (open triangles) in stimulating Ca2+ release via dRyr. Untransfected Sf9 cells do not respond to halothane (open diamonds), even at high concentrations (>2mM).
Curiously, individual Sf9+dRyr cells responded to halothane in an all-or-none manner, exhibiting either basal or maximal levels of [Ca2+]i at any given halothane concentration (Figure 5A2, 5A3). Concentration-response curves were thus analyzed as the proportion of cells producing elevated [Ca2+]i in response to drug application. Analyzed in this way, the EC50 for caffeine (1.42 ± 0.96 mM), for which the response is completely dependent on dRyr expression, was similar to the previously published value20. For anesthetics, concentration-response relationships showed strong selectivity for halothane (Figure 5D), with EC50 values of 0.26, 1.1 and 1.3 mM for halothane, sevoflurane, and isoflurane, respectively. Converted to partial pressures, these values correspond to 0.48%, 1.9%, and 2.6% atm, indicating that halothane is many-fold more potent than either sevoflurane or isoflurane. We propose that molecular interactions between halothane and the dRyr protein explain the selective sensitivity of dRyr mutants to halothane anesthesia in Drosophila (Figure 1D).
Halothane Increases [Ca2+]i and Hyperpolarizes Motoneuron RP2
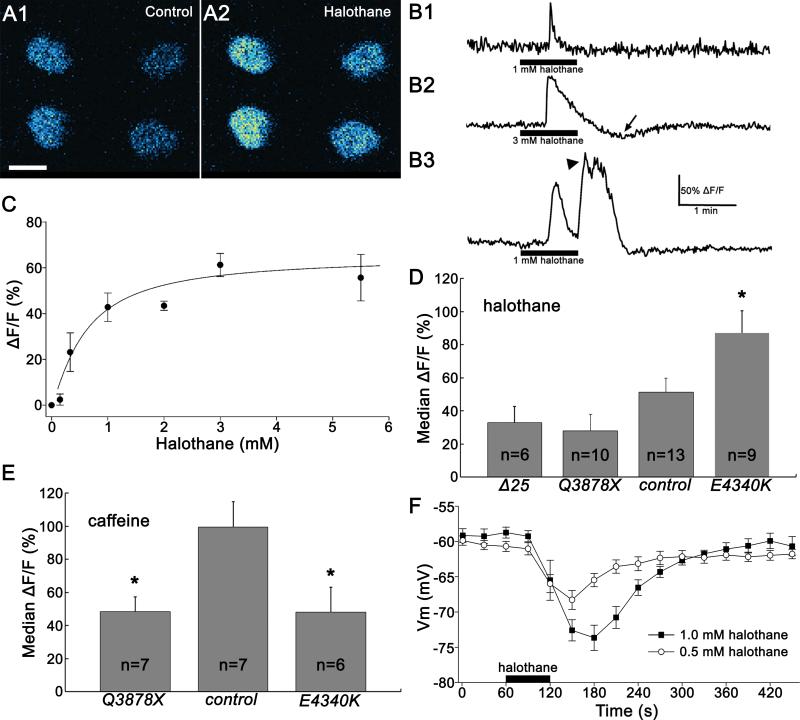
To examine the effects of halothane on Ca2+ flux in central neurons of Drosophila, we drove the expression of the genetically encoded Ca2+ indicator, GCaMP322, in larval motoneuron RP2 using ShakB-GAL4 . RP2 responded to halothane with robust increases in GCaMP3 fluorescence, clearly visible in unprocessed images (Figure 6A).
Figure 6.
Halothane increases [Ca2+]i and hyperpolarizes motoneuron RP2. (A) Halothane treatment increases [Ca2+]i in larval motoneuron RP2. Changes in [Ca2+]i in RP2 cell bodies are visualized using the genetically encoded Ca2+ indicator UAS-GCaMP3 driven by ShakB-GAL4. As shown in this false color image of four cells in two abdominal segments, [Ca2+]i increases with the addition of halothane (2.5 mM). Scale bar: 10 μm. (B) Representative traces of calcium transients observed in the somata of RP2 neurons with halothane treatment. The entire soma was defined as the region of interest, and fluorescence intensity was converted into a % change (ΔF/F) as described in Experimental Procedures. (B1) All cells produced an immediate response, with a sharp rise, that occurred within a short period of halothane entering the chamber. [Ca2+]i began to decay before halothane was removed. (B2) At higher concentrations, a pronounced undershoot, in which [Ca2+]i dropped below baseline, followed halothane removal (arrow). (B3) A minority of cells produced a delayed response at the time of halothane removal, consisting of a plateau with superimposed spiky transients (arrowhead). (C) The concentration-response relationship for the peak amplitude of the halothane-induced calcium transient response in RP2 neurons. RP2 responded to halothane in a concentration-dependent manner, with EC50 = 0.61 mM. Values are mean ± SEM (n = 3-8 preparations per data point). (D) dRyr mutations affect RP2's response to halothane. RP2 neurons in larvae heterozygous for dRyrE4340K, which causes hypersensitivity to halothane in adult flies, respond more strongly to 0.5 mM halothane than controls. In contrast, halothane appears to induce smaller responses in RP2 neurons heterozygous for the deletion mutant dRyrΔ25and the nonsense mutation dRyrQ3878X. Error bars represent s.e.m., and asterisk denotes statistical significance by one-way ANOVA and Bonferroni-Dunn post-hoc tests (P = 0.0484). (E) dRyr mutations reduce sensitivity to caffeine. RP2 motoneurons from larvae heterozygous for dRyrQ3878X and dRyrE4340K respond more weakly to 5 mM caffeine than controls (P = 0.0375 and P = 0.0453, one-way ANOVA and Bonferroni-Dunn post-hoc tests). (F) Halothane hyperpolarizes wild-type RP2 neurons. In whole-cell current clamp recordings, halothane application produces a strong hyperpolarization that is proportional to halothane concentration. Recovery is delayed at 1 mM compared to 0.5 mM halothane. Membrane potentials are recorded using whole-cell current clamp recording. Values are mean ± SEM (n = 5 - 7).
All cells produced an immediate response to halothane application that peaked during the short period of halothane entering the chamber (Figure 6B). The amplitude of this immediate response showed a robust concentration-response relationship (Figure 6C), with an EC50 for halothane of 0.61 mM. A pronounced undershoot, during which [Ca2+]i fell below resting levels, was routinely observed, particularly in response to high concentrations of halothane (Figure 6B2). A minority of cells produced an additional delayed response, consisting of a plateau overlain with spiky transients, at or near the time of halothane removal (Figure 6B3).
The response of RP2 to halothane was altered by mutations in dRyr (Figure 6D). In preparations heterozygous for the deletion mutant, dRyrΔ25, and the presumed truncation, dRyrQ3878X, the GCaMP3 responses to 0.5 mM halothane were reduced by >30% and >50%, respectively. Although consistent with a reduced response, these differences did not reach statistical significance (P = 1 and P = 0.6008, one-way ANOVA and Bonferroni-Dunn post-hoc tests). However, RP2 motoneurons heterozygous for point mutant dRyrE4340K, which causes dominant hypersensitivity in the reactive climbing assay (Fig 3C), responded to halothane with significantly larger Ca2+ flux (Figure 6D; P = 0.0484), indicating this allele enhances the response of dRyr to halothane. The hypersensitivity of dRyrE4340K for both reactive climbing and Ca2+ release suggest that it is a gain-of-function allele for halothane action.
The response of RP2 motoneurons to caffeine provided additional insight into the nature of the mutations. Wild-type RP2 neurons’ responses to caffeine were similar to that of other insect neurons20, with an EC50 of 5.4 ± 2.4 mM. When challenged with 5 mM caffeine, the change in fluorescence in dRyrQ3878X/+ was significantly lower than in the wildtype (Figure 6E; P = 0.0375, one-way ANOVA and Bonferroni-Dunn post-hoc tests). Surprisingly, the caffeine response of neurons heterozygous for dRyrE4340K was also smaller than normal (Figure 6E; P = 0.0453), to a similar extent as dRyrQ3878X. Therefore, the E to K substitution enhances the response to halothane, yet reduces the response to caffeine, indicating that the mutation selectively alters the response of dRyr depending on the nature of the signal. This conditional loss-of-function phenotype may also explain the lethality of dRyrE4340K/dRyrΔ25, in that the substitution may disable dRyr for a vital function.
In whole-cell, current clamp recordings of RP2, halothane evoked a strong, concentration-dependent hyperpolarization (Figure 6F). Importantly, there was no evidence of depolarization or spiking that would indicate Ca2+ flux through channels in the plasma membrane. Instead, the membrane hyperpolarized 10-15 mV, depending on the halothane concentration. Onset and recovery both required about 2 minutes, although recovery was delayed at the higher concentration. Therefore the Ca2+ flux demonstrated by imaging with GCaMP3 is paralleled by a robust hyperpolarization of the motoneuron, suggesting the activation of an inhibitory conductance.
Discussion
The molecular and cellular mechanisms of volatile anesthetic action have been the subject of intensive study for decades. This interest derives not only from the great medical importance of anesthesia, but also from the conviction that understanding the molecular and neural pathways that mediate anesthetic effects will provide insight into the nature of arousal and consciousness. Additional interest has been generated recently by the finding that general anesthesia contributes to various neuropathologies. Despite these convergent interests, the search for biologically relevant protein targets of volatile anesthetics has produced few confirmed candidates. The present study demonstrates that in Drosophila the ryanodine receptor (dRyr) is likely to represent such an anesthetic target. We show that neurally-expressed dRyr mediates the behavioral response to the volatile anesthetic halothane, with whole animal sensitivity to halothane anesthesia strongly dependent on gene dosage of dRyr. Point mutations in the dRyr gene that generate truncated, and therefore non-functional, proteins cause resistance to halothane, whereas missense mutations that alter highly conserved amino acids make flies more sensitive to the anesthetic. Our demonstration that the dRyr imparts halothane sensitivity to Sf9 cells, and that neurons in central nervous systems isolated from halothane-sensitive dRyr mutants exhibit elevated Ca2+ influx specifically in response to halothane, further support the conclusion that dRyr is a bona fide target of the anesthetic.
dRyr in the Nervous System Mediates Haltothane Sensitivity
The data presented here show that the potency of halothane is proportional to dRyr function. Reduction of function, as described for heterozygous dRyrK04913, dRyrGS21220, dRyrΔ25 or their heteroallelic combinations, causes resistance to halothane. Moreover, point mutants that are predicted to produce truncated, and therefore non-functional dRyr channels, also produce dominant resistance. Conversely, an additional genomic copy of dRyr confers hypersensitivity to halothane.
Importantly, tissue-specific knockdown and rescue experiments demonstrated that dRyr function in neurons and glia, but not muscle, is necessary for normal susceptibility to halothane anesthesia and that dRyr expression in these cells is sufficient to impart anesthetic sensitivity. The Ryr-dependent anesthetic phenotypes are therefore clearly distinct from those found in malignant hyperthermia, a condition associated with mutations that cause halothane hypersensitivity of the skeletal muscle isoform, Ryr1, in humans and swine. This conclusion is underscored by the finding that RNAi-mediated knockdown of dRyr expression in muscle fails to alter the halothane sensitivity of reactive climbing.
The observation that both the Drosophila Ryr and the mammalian muscle isoform of this receptor, Ryr1, mediate halothane-sensitive physiological processes suggests that halothane sensitivity is a general property of Ryrs. Indeed, in cardiac myocytes and neurons, which express predominantly Ryr2 and Ryr3, respectively, halothane induces Ryr-dependent Ca2+ flux from intracellular stores32-34. This is consistent with our observations that dRyr mediates halothane-dependent Ca2+ flux in Drosophila motoneurons, and supports a proposal that neural Ryrs mediate the immobilizing effects of anesthetics.
Mechanisms of Ryr Activation
While regulation of Ryr function is complex, two principle mechanisms have been defined for its activation: Ca2+-mediated activation and interaction with accessory proteins35. If halothane activates Ryr by elevating intracellular Ca2+ levels, it must do so either by promoting Ca2+ entry from the extracellular space or by causing release from intracellular stores. Flow cytometry results reported here show that elevation of internal Ca2+ is not observed in Sf9 cells, even at high levels of halothane, unless they have been transfected with dRyr. Moreover, it has been shown previously that halothane can activate isolated Ryr1 channels in membrane preparations36. This argues against activation of dRyr by Ca2+-induced Ca2+ release.
While it remains possible that halothane activates Ryr indirectly by interacting with one of its many accessory proteins, this explanation would require that Sf9 cells, which do not normally express detectable Ryr20, produce the critical, halothane-dependent accessory subunit(s) when transfected with dRyr. We instead favor the hypothesis that halothane interacts directly with the dRyr protein. The absence of the full complement of regulatory subunits in Sf9+dRyr cells may in fact explain the curious bimodal pattern of the response of Sf9+dRyr cells to halothane. If Sf9 cells do not express a protein such as sorcin, which has been postulated to help terminate Ca2+-induced Ca2+ release35, activation of dRyr by halothane could initiate a positive feedback loop of Ca2+ release, in which a small increase of cytoplasmic Ca2+ would initiate a flood of Ca2+ in the cytoplasm.
In contrast to the all-or-none response of Sf9+dRyr cells, RP2 motoneurons responded to halothane with transient, concentration-dependent Ca2+ flux. The transient nature of the signal suggests that either dRyrs inactivate in the continued presence of halothane, or that Ca2+ is rapidly removed from the cytoplasm. The transient reduction of GCaMP3 fluorescence below baseline levels upon removal of halothane is consistent with robust activation of mechanisms for extrusion and sequestration of intracellular Ca2+.
Downstream Effectors
Ca2+ released by halothane-dependent activation of dRyr must ultimately change neuronal excitability in order to contribute to the anesthetic state. Observations in mammals and worms show that neuronal hyperpolarization is a common feature of halothane anesthesia4,5, consistent with our electrophysiological recordings of RP2 motoneurons. The ultimate effector of Ryr activation is thus likely to be an inhibitory conductance, possibly carried by a K+ channel. Ca2+-activated K+ channels are obvious candidates, but it is interesting to speculate that the rapid and robust removal of excess Ca2+ from the cytoplasm may provide a novel route to K+ channel activation. Ca2+ extrusion by the plasma membrane Ca2+ ATPase, which exchanges internal Ca2+ for external H+, can result in cytoplasmic acidification37, which could in turn activate K2P channels, such as TREK-1, which are sensitive to low internal pH38. This mechanism could also provide an additional pathway for the actions of carbon dioxide, which is membrane permeant, causes acidification through the action of carbonic anhydrase, and acts as an anesthetic39. The merit of these and competing hypotheses remain to be tested, but the work described here shows that the genetic and physiological tools readily available in Drosophila should be useful in doing so.
Ryr and Neuropathology
The intimate relationship between dRyr activity and halothane anesthesia has possible implications regarding the association between volatile anesthetics and cytotoxicity, neurodegeneration, and cognitive deficits, pathologies often associated with dysregulation of intracellular Ca2+ levels40. It is also interesting in this regard that two of the three point mutations in dRyr that we found to enhance sensitivity to halothane are in a region associated with human cellular pathology.
dRyrE4340K is adjacent to a mutation in hRyr2 (i.e. N4178S) that is associated with multiple cases of catecholaminergic polymorphic ventricular tachycardia, a condition characterized by exercise-induced cardiac arrhythmia29. Perhaps most interestingly, dRyrR4305C is a substitution identical to that causing sudden cardiac death (hRyr2 R4144C)41. All three missense mutations increase the potency of halothane in our reactive climbing assay, and one enhances the halothane-evoked release of Ca2+ in RP2 motoneurons, suggesting shared mechanisms of Ryr-dependent Ca2+ dysregulation among different isoforms and across phyla. Of particular interest is the observation that the amino acid substitution in dRyrE4340K enhances sensitivity to halothane but blocks activation by caffeine, indicating that the mutant protein is not simply hyperactive. Similar mutations in neurally-expressed Ryr in humans may predispose individuals to the cytotoxic effects of anesthetics. In support of this hypothesis, halothane produces altered electroencephalographic activity during episodes of malignant hyperthermia in susceptible swine42.
The emerging picture based on investigation of the mechanisms of anesthesia in genetic model organisms is that, despite the identical endpoint of immobility, each volatile anesthetic is likely to act through its own collection of molecular targets. The collection of targets mediating halothane anesthesia now includes Ryr. At present it is unclear how completely the mechanisms uncovered in invertebrate models, such as flies and worms, will translate to mammalian anesthesia. In mammals, immobilization by volatile anesthetics is likely to be mediated by spinal circuitry4. The insect ventral nerve cord and motoneurons such as RP2 are functional analogs of the mammalian spinal cord and spinal motoneurons, respectively. However, the degree of homology between the segmental neuromeres of insects and mammals is not fully understood, and therefore the level of conservation of molecular targets of anesthetics remains to be established.
Remaining open questions regard the identities of the cells required to mediate immobility and the precise cellular sites of action. Our knockdown and rescue experiments manipulated dRyr expression in large numbers of neurons and glia, and it will be necessary to drive dRyr expression in subsets of these cells to determine whether anesthesia depends on the activity of a few cells or a large population. Furthermore, although halothane elicits Ca2+ flux in neuronal cell bodies that correlates with hyperpolarization, we have established neither the causal link between Ca2+ flux and membrane potential, nor the most important subcellular location (axon, dendrite, synapse, e.g.) of dRyr function. These questions provide fertile territory for subsequent investigations.
Summary.
The potency of halothane anesthesia paralleled gene dosage of the ryanodine receptor Ca2+ release channel in Drosophila mutants. Halothane-evoked Ca2+ flux in central neurons was correlated with hyperpolarization.
MS #201203110 – Final Boxed Summary Statement.
What we already know about this topic
Ryanodine receptors are involved in the pathogenesis of malignant hyperthermia, but their role in neurological effects of anesthetics is less clear
Model organisms provide a powerful genetics based approach to study anesthetic targets
What this article tells us that is new
Halothane immobilization of Drosophila flies was bidirectionally affected by mutations in the ryanodine receptor gene
Immobilization required neurally expressed ryanodine receptors and halothane increased intracellular calcium, a mechanism that might also be relevant to anesthetic actions in vertebrates
Acknowledgments
We thank David Ide, B.A. (Research Assistant, Research Services Branch, National Institute of Mental Health, National Institutes of Health, Bethesda, MD) and the Research Services Branch for construction of equipment, Daniel Cordova, M.Sc. (Senior Biochemist, Dupont Crop Protection, Newark, DE) for the anti-dRyr antibody, Sf9+dRyr cells and Ryr-V5 plasmid; Ted Usdin, Ph.D., M.D. (Senior Investigator, National Institute of Mental Health, National Institutes of Health, Bethesda, MD) for the use of culture facilities, Robert L. Scott, M.Sc. (Research Assistant, National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland) for technical assistance and comments on the manuscript, Songling Huang, Ph.D., (Senior Data Analyst, TurningPoint Global Solutions, Rockville, MD) and Gang Chen, Ph.D. (Mathematical Statistician, National Institute of Mental Health, National Institutes of Health, Bethesda, MD) for statistical consultation; Qun Gu, A.A. (Research Assistant, National Institute of Mental Health, National Institutes of Health, Bethesda, MD) for plasmid construction, Benjamin White, Ph.D. (Senior Investigator, National Institute of Mental Health, National Institutes of Health, Bethesda, MD) for use of equipment and help in assembling the manuscript, and Philip Morgan, M.D. (Professor, Seattle Children's Hospital, Seattle, WA) and Joseph Campbell, Ph.D. (Program Officer, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD) for helpful comments.
Source of Financial Support
Supported by the National Institute of Mental Health, Division of Intramural Research, National Institutes of Health, Department of Health and Human Services, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nature Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 2.Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, Lang-Lazdunski L, Widmann C, Zanzouri M, Romey G, Lazdunski M. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–95. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, Snutch TP, Morgan PG, Nash HA. A putative cation channel and its novel regulator: Cross-species conservation of effects on general anesthesia. Curr Biol. 2007;17:624–9. doi: 10.1016/j.cub.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Lazarenko RM, Willcox SC, Shu S, Berg AP, Jevtovic-Todorovic V, Talley EM, Chen X, Bayliss DA. Motoneuronal TASK channels contribute to immobilizing effects of inhalational general anesthetics. J Neurosci. 2010;30:7691–704. doi: 10.1523/JNEUROSCI.1655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singaram VK, Somerlot BH, Falk SA, Falk MJ, Sedensky MM, Morgan PG. Optical reversal of halothane-induced immobility in C. elegans. Curr Biol. 2011;21:2070–6. doi: 10.1016/j.cub.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metz LB, Dasgupta N, Liu C, Hunt SJ, Crowder CM. An evolutionarily conserved presynaptic protein is required for isoflurane sensitivity in Caenorhabditis elegans. Anesthesiology. 2007;107:971–82. doi: 10.1097/01.anes.0000291451.49034.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying SW, Werner DF, Homanics GE, Harrison NL, Goldstein PA. Isoflurane modulates excitability in the mouse thalamus via GABA-dependent and GABA-independent mechanisms. Neuropharmacology. 2009;56:438–47. doi: 10.1016/j.neuropharm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell JL, Gu Q, Guo D, Nash HA. Genetic effects in Drosophila on the potency of diverse general anesthetics: A distinctive pattern of altered sensitivity. J Neurogenet. 2009;23:412–21. doi: 10.3109/01677060903177800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connelly TJ, Coronado R. Activation of the Ca2+ release channel of cardiac sarcoplasmic reticulum by volatile anesthetics. Anesthesiology. 1994;81:459–69. doi: 10.1097/00000542-199408000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Akata T, Nakashima M, Izumi K. Comparison of volatile anesthetic actions on intracellular calcium stores of vascular smooth muscle: Investigation in isolated systemic resistance arteries. Anesthesiology. 2001;94:840–50. doi: 10.1097/00000542-200105000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Treves S, Anderson AA, Ducreux S, Divet A, Bleunven C, Grasso C, Paesante S, Zorzato F. Ryanodine receptor 1 mutations, dysregulation of calcium homeostasis and neuromuscular disorders. Neuromuscular Disord. 2005;15:577–87. doi: 10.1016/j.nmd.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan KMC, Scott K, Zuker CS, Rubin GM. The ryanodine receptor is essential for larval development in Drosophila melanogaster. Proc Natl Acad Sci. 2000;97:5942–5947. doi: 10.1073/pnas.110145997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, Huppert K, Tan LR, Winter CG, Bogart KP, Deal JE, Deal-Herr ME, Grant D, Marcinko M, Miyazaki WY, Robertson S, Shaw KJ, Tabios M, Vysotskaia V, Zhao L, Andrade RS, Edgar KA, Howie E, Killpack K, Milash B, Norton A, Thao D, Whittaker K, Winner MA, Friedman L, Margolis J, Singer MA, Kopczynski C, Curtis D, Kaufman TC, Plowman GD, Duyk G, Francis-Lang HL. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nature Genet. 2004;36:288–92. doi: 10.1038/ng1312. [DOI] [PubMed] [Google Scholar]
- 14.Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, Spokony R, Wan KH, Koriabine M, de Jong PJ, White KP, Bellen HJ, Hoskins RA. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nature Methods. 2009;6:431–4. doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homer N, Merriman B, Nelson SF. BFAST: an alignment tool for large scale genome resequencing. PLOS One. 2009;4:e7767. doi: 10.1371/journal.pone.0007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alone DP, Rodriguez JC, Noland CL, Nash HA. Impact of gene copy number variation on anesthesia in Drosophila melanogaster. Anesthesiology. 2009;111:15–24. doi: 10.1097/ALN.0b013e3181a3276c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc Natl Acad Sci. 1967;58:1112–9. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordova D, Benner EA, Sacher MD, Rauh JJ, Sopa JS, Lahm GP, Selby TP, Stevenson TM, Flexner L, Gutteridge S, Rhoades DF, Wu L, Smith RM, Tao Y. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pest Biochem Physiol. 2006;84:196–214. [Google Scholar]
- 21.Tsien RY, Pozzan T, Rink TJ. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982;295:68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- 22.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods. 2009;6:875–81. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y, Ueda A, Wu C-F. A modified minimal hemolymph-like solution, HL3.1, for physiological recordings at the neuromuscular junctions of normal and mutant Drosophila larvae. J Neurogenet. 2004;18:377–402. doi: 10.1080/01677060490894522. [DOI] [PubMed] [Google Scholar]
- 24.Sandstrom DJ. Isoflurane reduces excitability of Drosophila larval motoneurons by activating a hyperpolarizing leak conductance. Anesthesiology. 2008;108:434–46. doi: 10.1097/ALN.0b013e318164cfda. [DOI] [PubMed] [Google Scholar]
- 25.Sandstrom DJ. Isoflurane depresses glutamate release by reducing neuronal excitability at the Drosophila neuromuscular junction. J Physiol. 2004;558:489–502. doi: 10.1113/jphysiol.2004.065748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waud DR. On biological assays involving quantal responses. J Pharmacol Exp Ther. 1972;183:577–607. [PubMed] [Google Scholar]
- 27.Motulsky H, Christopoulos A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting. Oxford University Press; New York: 2004. [Google Scholar]
- 28.Hasan G, Rosbash M. Drosophila homologs of two mammalian intracellular Ca2+-release channels: identification and expression patterns of the inositol 1,4,5-triphosphate and the ryanodine receptor genes. Development. 1992;116:967–75. doi: 10.1242/dev.116.4.967. [DOI] [PubMed] [Google Scholar]
- 29.Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, Hofman N, Bikker H, van Tintelen JP, Mannens MM, Wilde AA, Ackerman MJ. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol. 2009;54:2065–74. doi: 10.1016/j.jacc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dockendorff TC, Robertson SE, Faulkner DL, Jongens TA. Genetic characterization of the 44D-45B region of the Drosophila melanogaster genome based on an F2 lethal screen. Mol Gen Genet. 2000;263:137–43. doi: 10.1007/s004380050040. [DOI] [PubMed] [Google Scholar]
- 31.Mohr SE, Boswell RE. Genetic analysis of Drosophila melanogaster polytene chromosome region 44D-45F: Loci required for viability and fertility. Genetics. 2002;160:1503–10. doi: 10.1093/genetics/160.4.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Z, Harrison SM, Steele DS. ATP-dependent effects of halothane on SR Ca2+ regulation in permeabilized atrial myocytes. Cardiovasc. Res. 2005;65:167–76. doi: 10.1016/j.cardiores.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Mody I, Tanelian DL, MacIver MB. Halothane enhances tonic neuronal inhibition by elevating intracellular calcium. Brain Res. 1991;538:319–23. doi: 10.1016/0006-8993(91)90447-4. [DOI] [PubMed] [Google Scholar]
- 34.Gomez RS, Guatimosim C, Barbosa J, Jr., Massensini AR, Gomez MV, Prado MA. Halothane-induced intracellular calcium release in cholinergic cells. Brain Res. 2001;921:106–14. doi: 10.1016/s0006-8993(01)03098-0. [DOI] [PubMed] [Google Scholar]
- 35.Kushnir A, Betzenhauser MJ, Marks AR. Ryanodine receptor studies using genetically engineered mice. FEBS lett. 2010;584:1956–65. doi: 10.1016/j.febslet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz-Sylvester PL, Porta M, Copello JA. Halothane modulation of skeletal muscle ryanodine receptors: Dependence on Ca2+, Mg2+, and ATP. Am J Physiol. 2008;294:C1103–12. doi: 10.1152/ajpcell.90642.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapp S, Luckermann M, Kaila K, Ballanyi K. Acidosis of hippocampal neurones mediated by a plasmalemmal Ca2+/H+ pump. NeuroReport. 1996;7:2000–4. doi: 10.1097/00001756-199608120-00029. [DOI] [PubMed] [Google Scholar]
- 38.Enyedi P, Czirják G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 39.Brosnan RJ, Eger EI, 2nd, Laster MJ, Sonner JM. Anesthetic properties of carbon dioxide in the rat. Anesth Analg. 2007;105:103–6. doi: 10.1213/01.ane.0000265556.69089.78. [DOI] [PubMed] [Google Scholar]
- 40.Wei H. The role of calcium dysregulation in anesthetic-mediated neurotoxicity. Anesth Analg. 2011;113:972–4. doi: 10.1213/ANE.0b013e3182323261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berge KE, Haugaa KH, Fruh A, Anfinsen OG, Gjesdal K, Siem G, Oyen N, Greve G, Carlsson A, Rognum TO, Hallerud M, Kongsgard E, Amlie JP, Leren TP. Molecular genetic analysis of long QT syndrome in Norway indicating a high prevalence of heterozygous mutation carriers. Scand J Clin Lab Inv. 2008;68:362–8. doi: 10.1080/00365510701765643. [DOI] [PubMed] [Google Scholar]
- 42.Kochs E, Hoffman WE, Roewer N, Schulte am Esch J. Alterations in brain electrical activity may indicate the onset of malignant hyperthermia in swine. Anesthesiology. 1990;73:1236–42. doi: 10.1097/00000542-199012000-00023. [DOI] [PubMed] [Google Scholar]