Abstract
Human immunodeficiency virus (HIV) when integrated into a host chromosome exists in a transcriptionally inactive but replication-competent state. Such latent infection represents a major challenge to HIV eradication efforts because a permanent virus reservoir resided in the infected cell is able to spike the viral load on immune suppression or during interruption of highly active anti-retroviral therapy. Understanding the molecular mechanisms that control HIV proviral latency and its reactivation could provide new perspectives on host factors as therapeutic targets for abolishing cellular reservoirs of dormant HIV. Although the control of HIV latency is multifactorial, chromatin structure and the chromatin-associated transcriptional machinery are known to be important factors. For instance, transcription initiation of the HIV provirus involves a complex molecular interplay between chromatin-associated proteins and the virus-encoded trans-activator, Tat. The first part of this review discusses our current understanding of the elements involved in HIV transcriptional activation and viral mRNA elongation, mainly post-translational modifications of HIV Tat and its interactions with host chromatin-modifying enzymes and chromatin-remodeling complexes. The second part highlights new experimental therapeutic approaches aimed at administrating activators of HIV gene expression to reduce or eliminate the pool of latently HIV-infected cells.
Keywords: HIV, Tat, Transcription, Histone acetylation
1. Introduction
A major challenge that prevents elimination of human immunodeficiency virus (HIV) infection is the virus's ability to integrate into the human genome, thereby achieving a transcriptionally silent and permanent latent infection [1,2]. The course of HIV pathogenesis is marked by the creation of a transcriptionally inert proviral reservoir that is insensitive to anti-retroviral therapy and has capacity to trigger HIV load during interruption of anti-viral treatment [3]. Clearly, effective therapeutic targeting of HIV latency necessitates a thorough understanding of the basic mechanisms that govern long-term maintenance of latent HIV in resting memory CD4 T cells – the critical reservoir of transcriptionally silent provirus [4,5]. Further, the eruption of latent HIV provirus is attributed to progressively skewed and dysfunctional immune response, characteristics of the HIV-infected cells during early stages infection, and by the site of proviral integration within human genome [6]. The site of integration could be critical, as a provirus entrenched in heterochromatin is less likely to become activated as compared to a provirus in euchromatin [7,8]. Indeed, provirus existing within actively transcribing genes of euchromatin will support rapid HIV replication. Our knowledge of the molecular and cellular mechanisms that underlie the establishment of this remarkably stable proviral reservoir depends on appropriate in vitro model systems and in vivo animal models, which allows the dissection of the pathways that lead to transcriptional control of HIV replication. In particular, it is crucial to correlate the activation state of host cells with the transcriptional state of the provirus. Key elements to be examined are: the site of provirus integration, which is determined by the viral integrase, and the mechanisms that control gene expression at these chromatin loci, which are governed mainly by the activity of the HIV trans-activator protein, Tat [9].
An extensive network of molecular interactions between human chromatin-associated proteins and HIV proteins controls the transcriptional initiation of proviral genes from the single promoter of HIV, the long terminal repeat (LTR). During HIV activation, active recruitment of host chromatin-associated proteins, including chromatin-modifying enzymes and chromatin-remodeling complexes, which are also transcription cofactors, is initiated immediately upon integration of HIV into the human genome. The key molecule that effectively orchestrates coordination between the transcriptional machinery for viral replication is the HIV Tat protein. This critical role of Tat is highlighted in Tat-deprived HIV-infected cells, in which provirus can neither transcribe nor replicate in the nucleus [10]. Tat's action begins with its specific binding to the Tat-responsive element (TAR) motif on the LTR that serves as the epicenter for assembling the host's transcriptional machinery [11–13]. Simultaneously, Tat also recruits the CDK9/cyclin T1 complex to promote elongation of RNA polymerase II [14,15]. Although Tat itself is capable of exploiting the host's cellular proteome, extensive post-translational modification (PTM) of Tat adds further complexity to the molecular events involved in the activation of HIV gene transcription [16,17]. These diverse PTMs enhance Tat's ability to interact with many cellular factors in a regulatory role. Importantly, these PTM-based molecular interactions may serve as novel targets against which new therapeutic strategies to inhibit HIV activation can be devised. Furthermore, this approach may offer new insights as well as opportunities to overcome both the drug resistance that nullifies many current anti-HIV therapies including lack of a globally effective anti-HIV vaccine.
Regulation of gene transcription is governed, in part, by enzymes that catalyze acetylation and deacetylation of key lysine residues of histones and other cellular proteins [16,17]. Acetylation of lysines within nucleosomal histones is closely linked to the relaxation of chromatin structure that contributes to transcriptional activation [18–21]. Similarly, co-repressor complexes that inhibit transcription include proteins that possess deacetylase activity [22]. Many transcriptional co-activators such as human GCN5, CREB-binding protein (CBP), p300, p300/CBP-associated factor (PCAF) and steroid receptor co-activator-1 (SRC-1) possess intrinsic histone acetyl-transferase (HAT) activity that is critical for their function [23,24]. Lysine acetylation is also vital to the function of various non-histone proteins, including general and specific transcription factors, non-histone structural chromosomal proteins, and nuclear-import factors [25]. Site-specific lysine acetylation of transcription factors, such as tumor suppressor p53, regulates many diverse functions, including its DNA binding, protein–protein interactions, protein stability and nuclear localization [26,27]. Likewise, lysine acetylation of HIV Tat on multiple lysine residues helps Tat recruit cellular cofactors and enhance transcription from the LTR promoter. This notion is supported by studies that showed that histone deacetylase inhibitors, including trichostatin A (TSA), trapoxin, valproic acid and sodium butyrate, cause transcriptional activation of the HIV LTR [28]. Such acetylation-dependence of HIV transcriptional activation was reported in studies using different model systems, including ex vivo transiently or stably transfected HIV LTR promoter reporter constructs and latently HIV-1-infected cell lines, in vitro chromatin-reconstituted HIV templates, and in the context of de novo HIV infection [29,30]. The ability of the HIV promoter to respond specifically to TSA reflects a complex regulatory interplay between acetylation and transcriptional activation of the LTR. Taken together, these studies strongly suggest that the maintenance of HIV in human cells is dependent upon cellular factors that could serve as novel targets for new anti-HIV therapies.
2. Transcriptional activation of latent HIV by trans-activator Tat
HIV Tat has been shown to be the target of at least five HATs in HIV-infected cells, including p300/CBP, PCAF, GCN5, Tip60, and TAFII250 (Fig. 1A). CBP and p300 are close homologs and act as transcriptional co-activators; they function by interacting with promoter-binding transcription factors targeting the CREB site, as well as with HATs, including PCAF [11,12]. p300/CBP can acetylate preferentially lysines 14 and 18 of histone H3 as well as lysines 5 and 8 of histone H4. PCAF is closely related to GCN5 (about 70% sequence similarity) and tends to acetylate free or nucleosomal histones on lysine 14 of histone H3 or lysine 8 of histone H4 [11,12]. Relief of chromatin repression at the HIV LTR by Tat occurs prior to the onset of transcriptional initiation [31,32]. Molecular interactions of Tat with p300/CBP and PCAF can lead to acetylation of both Tat and nucleosomes on the LTR promoter, thus delivering a concerted and robust alteration of chromatin structure that results in activation of HIV gene expression [31]. It has been shown that the presence of Tat, p300/CBP and PCAF induce the acetylation of histones H3 and H4 [13,28,33,34]. Further assisting the activation of viral transcription is the interaction of Tat with TAFII250, which likely results in the interruption of transcription of other cellular genes [35].
Fig. 1.
The role of the HIV trans-activator Tat in latent HIV transcriptional activation. (A) Functional domains of HIV Tat, and an array of post-translational modifications that control its functions in HIV transcriptional activation. (B) Schematic diagram highlighting HIV Tat-mediated regulation of LTR activation and molecular interactions essential for latent HIV activation in host cells.
The interaction of Tat with Tip60 inhibits its HAT activity, resulting in a negative regulatory effect on Tip60-dependent genes, but preferentially facilitating the transcription of the HIV LTR [36]. The recruitment of p300/CBP and PCAF to the HIV LTR assists in chromatin remodeling through the formation of a permissive environment that allows the binding of basal transcription machinery such as TFIIB and TBP. In the absence of Tat, the LTR-bound nucleosomes are hypoacetylated (repressed); however, recruitment of Tat-associated HATs results in hyperacetylation (activated) of those nucleosomes [32]. Although several studies have identified and characterized PTMs of the Tat protein, the biochemical functions of only a few such modifications have been studied in detail. Moreover, an in-depth mechanistic understanding of both PTMs and the molecular basis of the Tat interactions that support HIV transcriptional activation remains to be developed.
3. Acetylation-mediated Interactions between HIV Tat and cellular proteins
Accumulating studies have revealed nearly 1500 molecular interactions between HIV and human proteins [37]. Viral proteins are known to compete with host proteins, thus disrupting the normal cellular protein–protein interaction network. Efforts to understand molecular interactions between HIV and cellular proteins have led to new insights into the regulation of cellular genes by HIV [38–40]. The functions for HIV Tat include binding to TAR, chromatin remodeling, P-TEFb complex-directed phosphorylation of RNA polymerase II, and transactivation of viral genes. Interestingly, Tat performs these functions in a sequentially triggered cascade that ultimately causes activation of HIV genes. Tat recruits the components of chromatin-remodeling complexes, particularly the histone modifying enzymes p300/CBP and PCAF, to initiate its own modification that subsequently results in alterations to the architecture of the LTR promoter to permit initiation of HIV replication (Fig. 1A).
Site-specific protein acetylation leads to recognition of acetylated-lysine (Kac) by bromodomains, a family of evolutionarily conserved protein modules present in many chromatin-associated proteins, including HATs [41–44]. We previously demonstrated that acetylation at lysine 50 on Tat (TatK50ac) enables recruitment of PCAF via its bromodomain [41]. Further, in the absence of Tat acetylation, PCAF binds to amino acid residues 20–40 within Tat [45]. Interestingly, acetylation of Tat at lysine 28 abrogates the Tat–PCAF interaction [45]. Acetylation at lysine 50 creates a new site for binding to PCAF and directs the formation of a ternary complex of Tat–PCAF–P-TEFb. Thus, differential lysine acetylation of Tat coordinates its interactions with co-activators, cyclin T1 and PCAF. Another study also suggests that acetylation of Tat by p300 leads to dissociation of cyclin T1 from TAR RNA and serves to transfer Tat to aid in the transcript-elongation function of RNA polymerase II [46]. In addition to the bromodomain of PCAF, it was speculated that the double bromodomain-containing BRD4 protein of the BET family also participates in transcript elongation from the LTR promoter. One study showed that BRD4, through its bromodomains, binds to nuclear factor kappa-B (NF-κB) through its acetylated-lysine 310 (NF-κBK310ac). Because NF-κB binds to its response elements in the LTR, it is also possible that the BRD4/NF-κBK310ac interaction could stimulate LTR activity [47].
4. Small-molecule inhibitors of HIV transcription
Several studies showed that small molecules can effectively modulate protein–protein interactions such as those between p53 and MDM2, Bcl2 and BAK-BH3, Myc and Max, and LFA and ICAM-1 [48]. At present, treatment of HIV-infected individuals is based on combinatorial therapy with inhibitors that target HIV reverse transcriptase (RT), protease, and/or gp41 [49]. Despite the notable success of this highly active anti-retroviral therapy (HAART) in reducing plasma viral loads to an undetectable level during HIV infection and slowing down clinical progression to acquired immunodeficiency syndrome (AIDS), HAART fails to completely eradicate the virus in HIV-infected individuals. This curative failure is mainly the result of a small pool of chronically HIV-infected resting CD4+ T cells containing integrated but transcriptionally dormant HIV provirus. Several targets in the HIV replicative cycle other than RT, protease and virus entry have been identified as possible intervention sites for anti-viral chemotherapy. Among these, HIV transcriptional regulation seems to be very attractive, as it would open the possibility to control HIV replication not only in acutely but also in chronically infected cells. In this way, inhibitors of HIV transcriptional regulation may have great potential in anti-HIV drug combination therapy because they could force the virus to slow its replication rate or even shut off virus replication altogether, thus affording life-long control of the HIV infection. Moreover, it may be also argued that the use of anti-retroviral drugs targeted at HIV transcriptional regulation would result in a lower incidence of drug resistance, because the regulation of HIV transcription requires the interplay between viral and cellular components.
HIV Tat also recruits the P-TEFb complex consisting of cyclin T1/CDK9 to the TAR RNA. Subsequently, CDK9 phosphorylates RNA polymerase II and Tat interacts with cyclin T1, a regulatory subunit of P-TEFb. Thus, the direct interaction between Tat and cyclin T1 is a possible target for anti-viral intervention. This interaction has been characterized functionally, but its molecular details remain elusive. Additionally, during activation of viral transcription, Tat interacts with the TAR RNA element. This Tat/TAR interaction is therefore also another potential target for anti-viral development. However, development of small molecules that disrupt a protein–RNA interaction appears to have distinct challenges [50–53]. A number of researchers have attempted various screening strategies to identify chemical molecules able to disrupt the Tat–TAR RNA interaction [54–56]. These screens, unfortunately, have not yet succeeded to identify lead compounds that could be developed into potential drugs.
HIV transcriptional initiation is a complex and multistage process that requires the concerted actions of viral and cellular proteins. The nucleosomal positioning at the viral transcription initiation site and its disruption upon transcriptional activation suggests that chromatin plays an essential role in the suppression of HIV expression during latency as well as during post-integration of the HIV genome into the host chromosome. Reactivation of the HIV provirus may be mediated by the displacement of histone deacetylases in response to the recruitment of HATs by NF-κB or the viral trans-activator Tat [57]. Additionally, productive transcription of the HIV provirus requires the recruitment of CDK9 to the viral promoter by Tat. Although these crucial functions of Tat are indispensable for HIV replication, the implications of Tat reach far beyond, both inside and outside the cell. Tat has the unusual property of being able to freely enter and be released from cells without losing its activity, enabling it to up-regulate numerous genes, including HIV gene expression. As such, targeting Tat provides interesting possibilities for therapeutic intervention in HIV infection.
An important and attractive therapeutic approach is based on interference of the interaction between Tat and the bulge of TAR RNA [58,59]. Numerous Tat–TAR inhibitors have been designed and are represented in several classes, including peptoid molecules, Tat peptide mimetics, quinolone derivatives, polyamide oligomers, arginine-aminoglycoside conjugates, intercalators, and a large group of small RNA-binding molecules [54,56,60]. Another approach to sequester Tat's function is based on targeting its crucial interactions with CDK9 by using CDK inhibitors such as flavopiridol and R-roscovitine [61,62]. Moreover, R-roscovitine is able to selectively induce apoptosis of HIV-infected cells without viral release, which opens important opportunities for this class of CDK inhibitors. Tat-mediated transcription could also be inhibited by curcumin and HR73, which interfere with acetylation and deacetylation of Tat, respectively [63]. These recent approaches to disrupt Tat functions emphasize the importance of PTMs in the regulation of Tat. Therefore, different anti-HIV strategies that have recently been developed based on directly or indirectly targeting Tat, provide promise for combination anti-viral strategies. Once HIV replication is shut down and plasma viral loads reach undetectable levels upon combination therapy, HIV transcription inhibitors may potentially be able to keep the virus in its dormant state and secure the latent HIV reservoir.
5. Interception of PCAF recruitment by the acetylated HIV Tat complex
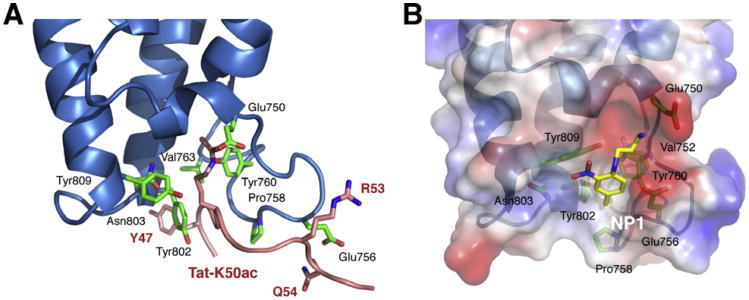
Our earlier work demonstrated that the TatK50ac moiety generated by the acetyl-transferase activity of p300/CBP serves as a binding site for recruiting the PCAF bromodomain (Fig. 2A) [41,64]. Structural analysis of this molecular interaction by using nuclear magnetic resonance (NMR) spectroscopy revealed that TatK50ac intercalates into the hydrophobic pocket of the PCAF bromodomain. The selectivity of the bromodomain for TatK50ac is established by PCAF interactions with the HIV Tat residues flanking lysine 50. Further, structural knowledge of the PCAF bromodomain/TatK50ac recognition has guided the development of promising small molecule compounds that block this specific interaction (Fig. 1B) [65]. The lead compounds have been shown to inhibit the activation of HIV LTR-driven luciferase reporter gene expression in cultured cells [49,65].
Fig. 2.
Structural basis of ligand recognition of the PCAF bromodomain. (A) Structure of the PCAF bromodomain in a complex with a HIV TatK50ac peptide showing the acetyl-lysine binding site for the peptide. (B) Surface representations highlighting a small-molecule inhibitor bound to the acetyl-lysine binding pocket in the PCAF bromodomain.
Novel targets for the management of HIV infection have become increasingly relevant in view of the extensive drug resistance and side effects of current treatments. Target structure-based drug design with the use of X-ray crystallography, NMR, and mass spectrometry techniques have led to the identification of a new class of drugs targeting different stages of the HIV life cycle. These agents include chemokine receptor antagonists and integrase inhibitors, which were recently approved for HIV treatment, and numerous other agents directed toward previously untested targets such as viral maturation, zinc finger protein, CDK, Tat–TAR interaction, as well as agents acting on the proviral DNA [66]. In addition, anti-CD4 monoclonal antibody, antisense oligonucleotides, and oxidizers of the HIV lipid envelope have also been reported [67,68]. Despite these new developments and the hope that they provide to patients and clinicians in the fight against HIV/AIDS, eradication of the disease still remains a challenge.
6. Future perspectives
The development of anti-HIV drugs began soon after the discovery of HIV 25 years ago. Since then, substantial progress has been made, but uncertainties persist about the best way to prevent and manage this challenging disease. Despite the availability of 23 approved anti-retroviral drugs [66], there is continued interest in developing new agents for the treatment of HIV/AIDS. This has prompted consideration of alternative mechanisms for exploitation. Protein–protein interactions play an important role in most biological processes, from intracellular communication to programmed cell death, and therefore represent a large and new class of therapeutic targets [69]. The wealth of information obtained from genomics and proteomics studies has revealed many key protein–protein interactions involved in the pathogenesis of various human diseases, including HIV/AIDS. Nevertheless, these interactions have been largely ignored in favor of conventional approaches to drug development that have focused on classical targets such as enzymes and receptors. Given the new advances in our understanding of the complexity of HIV biology and the importance of host-virus interactions at the chromatin level for latent HIV activation, it is expected that targeting protein–protein interactions will boost our chances to identify and validate new anti-viral targets to combat HIV/AIDS.
Acknowledgments
This work was supported by grants from the National Institutes of Health (M.-M.Z. and S.M.).
References
- 1.Trono D, et al. Science. 2010;329(5988):174–180. doi: 10.1126/science.1191047. [DOI] [PubMed] [Google Scholar]
- 2.Margolis DM. Curr HIV/AIDS Rep. 2010;7(1):37–43. doi: 10.1007/s11904-009-0033-9. [DOI] [PubMed] [Google Scholar]
- 3.Keedy KS, Margolis DM. Trends Pharmacol Sci. 2010;31(5):206–211. doi: 10.1016/j.tips.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho DD, et al. Nature. 1995;373(6510):123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 5.Wei X, et al. Nature. 1995;373(6510):117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 6.Marcello A. Retrovirology. 2006;3:7. doi: 10.1186/1742-4690-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han A, et al. J Virol. 2004;78(12):6122–6133. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siliciano JD, Siliciano RF, Antimicrob J. Chemother. 2004;54(1):6–9. doi: 10.1093/jac/dkh292. [DOI] [PubMed] [Google Scholar]
- 9.Schroder AR, et al. Cell. 2002;110(4):521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 10.Garber ME, Jones KA. Curr Opin Immunol. 1999;11(4):460–465. doi: 10.1016/S0952-7915(99)80077-6. [DOI] [PubMed] [Google Scholar]
- 11.Deng L, et al. Virology. 2000;277(2):278–295. doi: 10.1006/viro.2000.0593. [DOI] [PubMed] [Google Scholar]
- 12.Deng L, et al. Virology. 2001;289(2):312–326. doi: 10.1006/viro.2001.1129. [DOI] [PubMed] [Google Scholar]
- 13.Benkirane M, et al. J Biol Chem. 1998;273(38):24898–24905. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]
- 14.Garber ME, Wei P, Jones KA. Cold Spring Harb Symp Quant Biol. 1998;63:371–380. doi: 10.1101/sqb.1998.63.371. [DOI] [PubMed] [Google Scholar]
- 15.Wei P, et al. Cell. 1998;92(4):451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 16.Dormeyer W, et al. Anal Bioanal Chem. 2003;376(7):994–1005. doi: 10.1007/s00216-003-2058-z. [DOI] [PubMed] [Google Scholar]
- 17.Ott M, et al. Curr Biol. 1999;9(24):1489–1492. doi: 10.1016/s0960-9822(00)80120-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Tini M, Evans RM. Curr Opin Cell Biol. 2001;13(2):218–224. doi: 10.1016/s0955-0674(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 19.Hebbes TR, et al. Nucleic Acids Res. 1992;20(5):1017–1022. doi: 10.1093/nar/20.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenuwein T, Allis CD. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 21.Strahl BD, Allis CD. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 22.Fischle W, et al. Biochem Cell Biol. 2001;79(3):337–348. [PubMed] [Google Scholar]
- 23.Roth SY, Denu JM, Allis CD. Annu Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 24.Sterner DE, Berger SL. Microbiol Mol Biol Rev. 2000;64(2):435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glozak MA, et al. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Berger SL. Curr Opin Cell Biol. 1999;11(3):336–341. doi: 10.1016/S0955-0674(99)80046-5. [DOI] [PubMed] [Google Scholar]
- 27.Kouzarides T. EMBO J. 2000;19(6):1176–1179. doi: 10.1093/emboj/19.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiernan RE, et al. EMBO J. 1999;18(21):6106–6118. doi: 10.1093/emboj/18.21.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Lint C, et al. EMBO J. 1996;15(5):1112–1120. [PMC free article] [PubMed] [Google Scholar]
- 30.Van Lint C, Emiliani S, Verdin E. Gene Expr. 1996;5(4–5):245–253. [PMC free article] [PubMed] [Google Scholar]
- 31.Marcello A, et al. EMBO J. 2003;22(9):2156–2166. doi: 10.1093/emboj/cdg205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lusic M, et al. EMBO J. 2003;22(24):6550–6561. doi: 10.1093/emboj/cdg631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hottiger MO, Nabel GJ, Virol J. 1998;72(10):8252–8256. doi: 10.1128/jvi.72.10.8252-8256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marzio G, et al. Proc Natl Acad Sci USA. 2001;98(11):6342–6347. doi: 10.1073/pnas.111031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weissman JD, et al. Proc Natl Acad Sci USA. 1998;95(20):11601–11606. doi: 10.1073/pnas.95.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Creaven M, et al. Biochemistry. 1999;38(27):8826–8830. doi: 10.1021/bi9907274. [DOI] [PubMed] [Google Scholar]
- 37.Romani B, Engelbrecht S, Glashoff RH. J Gen Virol. 2009 doi: 10.1099/vir.0.016303-0. [DOI] [PubMed] [Google Scholar]
- 38.He N, et al. Mol Cell. 2010;38(3):428–438. doi: 10.1016/j.molcel.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobhian B, et al. Mol Cell. 2010;38(3):439–451. doi: 10.1016/j.molcel.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tahirov TH, et al. Nature. 2010;465(7299):747–751. doi: 10.1038/nature09131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mujtaba S, et al. Mol Cell. 2002;9(3):575–586. doi: 10.1016/s1097-2765(02)00483-5. [DOI] [PubMed] [Google Scholar]
- 42.Mujtaba S, et al. Mol Cell. 2004;13(2):251–263. doi: 10.1016/s1097-2765(03)00528-8. [DOI] [PubMed] [Google Scholar]
- 43.Mujtaba S, Zeng L, Zhou MM. Oncogene. 2007;26(37):5521–5527. doi: 10.1038/sj.onc.1210618. [DOI] [PubMed] [Google Scholar]
- 44.Zeng L, Zhou MM. FEBS Lett. 2002;513(1):124–128. doi: 10.1016/s0014-5793(01)03309-9. [DOI] [PubMed] [Google Scholar]
- 45.Bres V, et al. EMBO J. 2002;21(24):6811–6819. doi: 10.1093/emboj/cdf669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaehlcke K, et al. Mol Cell. 2003;1(1):167–176. doi: 10.1016/s1097-2765(03)00245-4. [DOI] [PubMed] [Google Scholar]
- 47.Yang Z, et al. Mol Cell. 2005;19(4):535–545. doi: 10.1016/j.molcel.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 48.Nishioka K, Reinberg D. Methods. 2003;31(1):49–58. doi: 10.1016/s1046-2023(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 49.Pan C, et al. Chem. 2007;50(10):2285–2288. doi: 10.1021/jm070014g. [DOI] [PubMed] [Google Scholar]
- 50.Hermann T. Angew Chem Int Ed Engl. 2000;39(11):1890–1904. doi: 10.1002/1521-3773(20000602)39:11<1890::aid-anie1890>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 51.Hermann T. Biochimie. 2002;84(9):869–875. doi: 10.1016/s0300-9084(02)01460-8. [DOI] [PubMed] [Google Scholar]
- 52.Hermann T. Biopolymers. 2003;70(1):4–18. doi: 10.1002/bip.10410. [DOI] [PubMed] [Google Scholar]
- 53.Hermann T, Westhof E. Comb Chem High Throughput Screen. 2000;3(3):219–234. doi: 10.2174/1386207003331652. [DOI] [PubMed] [Google Scholar]
- 54.Gelman MA, et al. Org Lett. 2003;5:3563–3565. doi: 10.1021/ol034977v. [DOI] [PubMed] [Google Scholar]
- 55.Hamma T, et al. Bioorg Med Chem Lett. 2003;13(11):1845–1848. doi: 10.1016/s0960-894x(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 56.Hwang S, et al. J Biol Chem. 2003;278(40):39092–39103. doi: 10.1074/jbc.M301749200. [DOI] [PubMed] [Google Scholar]
- 57.Stevens M, De Clercq E, Balzarini J. Med Res Rev. 2006;26(5):595–625. doi: 10.1002/med.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He M, et al. Bioorg Med Chem Lett. 2005;15(17):3978–3981. doi: 10.1016/j.bmcl.2005.01.068. [DOI] [PubMed] [Google Scholar]
- 59.Yang M. Curr Drug Targets Infect Disord. 2005;5(4):433–444. doi: 10.2174/156800505774912901. [DOI] [PubMed] [Google Scholar]
- 60.Marcheschi RJ, Mouzakis KD, Butcher SE. ACS Chem Biol. 2009;4(10):844–854. doi: 10.1021/cb900167m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumli S, et al. EMBO J. 2008;27(13):1907–1918. doi: 10.1038/emboj.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Canduri F, et al. Med Chem. 2008;4(3):210–218. doi: 10.2174/157340608784325205. [DOI] [PubMed] [Google Scholar]
- 63.Barthelemy S, et al. Res Virol. 1998;149(1):43–52. doi: 10.1016/s0923-2516(97)86899-9. [DOI] [PubMed] [Google Scholar]
- 64.Dhalluin C, et al. Nature. 1999;399(6735):491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 65.Zeng L, et al. J Am Chem Soc. 2005;127(8):2376–2377. doi: 10.1021/ja044885g. [DOI] [PubMed] [Google Scholar]
- 66.Flexner C. Nat Rev Drug Discov. 2007;6(12):959–966. doi: 10.1038/nrd2336. [DOI] [PubMed] [Google Scholar]
- 67.Jabado N, et al. Eur J Immunol. 1994;24(11):2646–2652. doi: 10.1002/eji.1830241112. [DOI] [PubMed] [Google Scholar]
- 68.Jabado N, et al. Eur J Immunol. 1997;27(2):397–404. doi: 10.1002/eji.1830270209. [DOI] [PubMed] [Google Scholar]
- 69.Ryan DP, Matthews JM. Curr Opin Struct Biol. 2005;15(4):441–446. doi: 10.1016/j.sbi.2005.06.001. [DOI] [PubMed] [Google Scholar]