Abstract
Monoclonal antibody (MAb) technology is well recognized as a significant development for producing specific serologic reagents to a wide variety of antigens in unlimited amounts. These reagents have provided the means for developing a number of highly specific and reproducible immunological assays for rapid and accurate diagnosis of an extensive list of diseases, including infectious diseases. The impact that MAbs have had in characterizing infectious disease pathogens, as well as their current and future applications for use in clinical microbiology laboratories, is reviewed. In addition, the advantages (and disadvantages) of the use of MAbs in a number of immunoassays, such as particle agglutination, radioimmunoassays, enzyme-linked immunosorbent assays, immunofluorescent-antibody assays, and immunohistology, are explored, including the use of these reagents in novel test system assays. Also, nucleic acid probe technology is compared with the use of MAbs from the perspective of their respective applications in the diagnosis of infectious disease agents. There is no question that hybridoma technology has the potential to alter significantly the methods currently used in most clinical microbiology laboratories.
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Adler-Storthz K., Kendall C., Kennedy R. C., Henkel R. D., Dreesman G. R. Biotin-avidin-amplified enzyme immunoassay for detection of herpes simplex virus antigen in clinical specimens. J Clin Microbiol. 1983 Dec;18(6):1329–1334. doi: 10.1128/jcm.18.6.1329-1334.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Suprun-Brown L., Kasmala L. Monoclonal antibody to a major surface glycoprotein immunogen differentiates isolates and subpopulations of Trichomonas vaginalis. Infect Immun. 1986 Apr;52(1):70–75. doi: 10.1128/iai.52.1.70-75.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambinder R. F., Charache P., Staal S., Wright P., Forman M., Hayward S. D., Hayward G. S. The vector homology problem in diagnostic nucleic acid hybridization of clinical specimens. J Clin Microbiol. 1986 Jul;24(1):16–20. doi: 10.1128/jcm.24.1.16-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Mann R. E., Gonzales C. Reactivity of monoclonal antibodies to Rickettsia rickettsii with spotted fever and typhus group rickettsiae. J Clin Microbiol. 1987 Jan;25(1):167–171. doi: 10.1128/jcm.25.1.167-171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. J., Godfrey E., McIntosh K., Hierholzer J. C. Comparison of a monoclonal antibody with a polyclonal serum in an enzyme-linked immunosorbent assay for detecting adenovirus. J Clin Microbiol. 1983 Sep;18(3):463–468. doi: 10.1128/jcm.18.3.463-468.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astaldi G. C., Wright E. P., Willems C., Zeijlemaker W. P., Janssen M. C. Increase of hybridoma formation by human lymphocytes after stimulation in vitro; effect of antigen, endothelial cells, and PWM. J Immunol. 1982 Jun;128(6):2539–2542. [PubMed] [Google Scholar]
- Barnes R. C., Suchland R. J., Wang S. P., Kuo C. C., Stamm W. E. Detection of multiple serovars of Chlamydia trachomatis in genital infections. J Infect Dis. 1985 Nov;152(5):985–989. doi: 10.1093/infdis/152.5.985. [DOI] [PubMed] [Google Scholar]
- Barnes R. C., Wang S. P., Kuo C. C., Stamm W. E. Rapid immunotyping of Chlamydia trachomatis with monoclonal antibodies in a solid-phase enzyme immunoassay. J Clin Microbiol. 1985 Oct;22(4):609–613. doi: 10.1128/jcm.22.4.609-613.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell D. M., Walsh E. E., Hruska J. F., Schnabel K. C., Hall C. B. Rapid detection of respiratory syncytial virus with a monoclonal antibody. J Clin Microbiol. 1983 Jun;17(6):1099–1101. doi: 10.1128/jcm.17.6.1099-1101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell T. A., Kuo C. C., Stamm W. E., Tam M. R., Stephens R. S., Holmes K. K., Grayston J. T. Direct fluorescent monoclonal antibody stain for rapid detection of infant Chlamydia trachomatis infections. Pediatrics. 1984 Aug;74(2):224–228. [PubMed] [Google Scholar]
- Bellamy K., Rousseau S. A., Gardner P. S. The development of an M antibody capture ELISA for rubella IgM. J Virol Methods. 1986 Nov;14(3-4):243–251. doi: 10.1016/0166-0934(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Belmaaza A., Hamel J., Mousseau S., Montplaisir S., Brodeur B. R. Rapid diagnosis of severe Haemophilus influenzae serotype b infections by monoclonal antibody enzyme immunoassay for outer membrane proteins. J Clin Microbiol. 1986 Sep;24(3):440–445. doi: 10.1128/jcm.24.3.440-445.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger M., Hammer M., Hoyer B., Gerin J. L. An assay for the detection of the DNA genome of hepatitis B virus in serum. J Med Virol. 1982;9(1):57–68. doi: 10.1002/jmv.1890090109. [DOI] [PubMed] [Google Scholar]
- Bibb W. F., Arnow P. M., Thacker L., McKinney R. M. Detection of soluble Legionella pneumophila antigens in serum and urine specimens by enzyme-linked immunosorbent assay with monoclonal and polyclonal antibodies. J Clin Microbiol. 1984 Sep;20(3):478–482. doi: 10.1128/jcm.20.3.478-482.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell D. E., Bartlett A., Voller A. Enzyme immunoassays for viral diseases. J Infect Dis. 1977 Oct;136 (Suppl):S274–S278. doi: 10.1093/infdis/136.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- Brams P., Pettijohn D. E., Brown M., Olsson L. In vitro B-lymphocyte antigen priming against both non-immunogenic and immunogenic molecules requiring low amounts of antigen and applicable in hybridoma technology. J Immunol Methods. 1987 Apr 2;98(1):11–22. doi: 10.1016/0022-1759(87)90430-3. [DOI] [PubMed] [Google Scholar]
- Brandsma J., Miller G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein-Barr viral DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6851–6855. doi: 10.1073/pnas.77.11.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson Y. J. The use of acyclovir in children. Pediatr Infect Dis. 1984 Jul-Aug;3(4):345–348. doi: 10.1097/00006454-198407000-00017. [DOI] [PubMed] [Google Scholar]
- Bullock S. L., Walls K. W. Evaluation of some of the parameters of the enzyme-linked immunospecific assay. J Infect Dis. 1977 Oct;136 (Suppl):S279–S285. doi: 10.1093/infdis/136.supplement_2.s279. [DOI] [PubMed] [Google Scholar]
- Caul E. O., Paul I. D. Monoclonal antibody based ELISA for detecting Chlamydia trachomatis. Lancet. 1985 Feb 2;1(8423):279–279. doi: 10.1016/s0140-6736(85)91056-6. [DOI] [PubMed] [Google Scholar]
- Chantler S., Evans C. J. Selection and performance of monoclonal and polyclonal antibodies in an IgM antibody capture enzyme immunoassay for rubella. J Immunol Methods. 1986 Feb 27;87(1):109–117. doi: 10.1016/0022-1759(86)90350-9. [DOI] [PubMed] [Google Scholar]
- Chou S. W., Roark L., Merigan T. C. DNA of 21 clinical cytomegalovirus strains detected by hybridization to cloned DNA fragments of laboratory strain AD-169. J Med Virol. 1984;14(3):263–268. doi: 10.1002/jmv.1890140310. [DOI] [PubMed] [Google Scholar]
- Clayton A. L., Beckford U., Roberts C., Sutherland S., Druce A., Best J., Chantler S. Factors influencing the sensitivity of herpes simplex virus detection in clinical specimens in a simultaneous enzyme-linked immunosorbent assay using monoclonal antibodies. J Med Virol. 1985 Nov;17(3):275–282. doi: 10.1002/jmv.1890170309. [DOI] [PubMed] [Google Scholar]
- Clayton A. L., Roberts C., Godley M., Best J. M., Chantler S. M. Herpes simplex virus detection by ELISA: effect of enzyme amplification, nature of lesion sampled and specimen treatment. J Med Virol. 1986 Sep;20(1):89–97. doi: 10.1002/jmv.1890200111. [DOI] [PubMed] [Google Scholar]
- Coulepis A. G., Veale M. F., MacGregor A., Kornitschuk M., Gust I. D. Detection of hepatitis A virus and antibody by solid-phase radioimmunoassay and enzyme-linked immunosorbent assay with monoclonal antibodies. J Clin Microbiol. 1985 Jul;22(1):119–124. doi: 10.1128/jcm.22.1.119-124.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue-Rolfe A., Kelley M. A., Bennish M., Keusch G. T. Enzyme-linked immunosorbent assay for shigella toxin. J Clin Microbiol. 1986 Jul;24(1):65–68. doi: 10.1128/jcm.24.1.65-68.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druyts-Voets E. Comparison of cytopathic effect and an enzyme-linked immunosorbent assay using monoclonal antibodies for typing herpes simplex virus isolates. Eur J Clin Microbiol. 1986 Aug;5(4):473–475. doi: 10.1007/BF02075715. [DOI] [PubMed] [Google Scholar]
- Echeverria P., Seriwatana J., Taylor D. N., Tirapat C., Chaicumpa W., Rowe B. Identification by DNA hybridization of enterotoxigenic Escherichia coli in a longitudinal study of villages in Thailand. J Infect Dis. 1985 Jan;151(1):124–130. doi: 10.1093/infdis/151.1.124. [DOI] [PubMed] [Google Scholar]
- Edberg S. C. Principles of nucleic acid hybridization and comparison with monoclonal antibody technology for the diagnosis of infectious diseases. Yale J Biol Med. 1985 Sep-Oct;58(5):425–442. [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H., Beer K. B., Sturge J. C., Watson A. J., Goldstein L. C. Clinical utility of a monoclonal direct fluorescent reagent specific for Legionella pneumophila: comparative study with other reagents. J Clin Microbiol. 1985 Sep;22(3):419–421. doi: 10.1128/jcm.22.3.419-421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein P. H. Evaluation of the Gen-Probe DNA probe for the detection of legionellae in culture. J Clin Microbiol. 1986 Mar;23(3):481–484. doi: 10.1128/jcm.23.3.481-484.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleberg N. C., Eisenstein B. I. The impact of new cloning techniques on the diagnosis and treatment of infectious diseases. N Engl J Med. 1984 Oct 4;311(14):892–901. doi: 10.1056/NEJM198410043111406. [DOI] [PubMed] [Google Scholar]
- Falkenberg F. W., Pierard D., Mai U., Kantwerk G. Polyclonal and monoclonal antibodies as reagents in biochemical and in clinical-chemical analysis. J Clin Chem Clin Biochem. 1984 Dec;22(12):867–882. [PubMed] [Google Scholar]
- Feldmeier H., Nogueira-Queiroz J. A., Peixoto-Queiroz M. A., Doehring E., Dessaint J. P., de Alencar J. E., Dafalla A. A., Capron A. Detection and quantification of circulating antigen in schistosomiasis by monoclonal antibody. II. The quantification of circulating antigens in human schistosomiasis mansoni and haematobium: relationship to intensity of infection and disease status. Clin Exp Immunol. 1986 Aug;65(2):232–243. [PMC free article] [PubMed] [Google Scholar]
- Ferns R. B., Tedder R. S. Detection of both hepatitis B e antigen and antibody in a single assay using monoclonal reagents. J Virol Methods. 1985 Jul;11(3):231–239. doi: 10.1016/0166-0934(85)90112-0. [DOI] [PubMed] [Google Scholar]
- Ferraro M. J., Kallas W. M., Welch K. P., Lau A. Y. Comparison of a new, rapid enzyme immunoassay with a latex agglutination test for qualitative detection of rubella antibodies. J Clin Microbiol. 1987 Sep;25(9):1722–1724. doi: 10.1128/jcm.25.9.1722-1724.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers R. S., Eckner K., Gabis D. A., Robison B. J., Mattingly J. A., Silliker J. H. Enzyme immunoassay for detection of Salmonella in foods: collaborative study. J Assoc Off Anal Chem. 1986 Sep-Oct;69(5):786–798. [PubMed] [Google Scholar]
- Fox P. C., Berenstein E. H., Siraganian R. P. Enhancing the frequency of antigen-specific hybridomas. Eur J Immunol. 1981 May;11(5):431–434. doi: 10.1002/eji.1830110516. [DOI] [PubMed] [Google Scholar]
- Fung J. C., Shanley J., Tilton R. C. Comparison of the detection of herpes simplex virus in direct clinical specimens with herpes simplex virus-specific DNA probes and monoclonal antibodies. J Clin Microbiol. 1985 Nov;22(5):748–753. doi: 10.1128/jcm.22.5.748-753.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G., Lundin D. J., Gladd M. F. Detection and speciation of common cell culture mycoplasmas by an enzyme-linked immunosorbent assay with biotin-avidin amplification and microporous membrane solid phase. In Vitro Cell Dev Biol. 1986 Aug;22(8):491–498. doi: 10.1007/BF02623451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilljam G., Sundqvist V. A., Linde A., Pihlstedt P., Eklund A. E., Wahren B. Sensitive analytic ELISAs for subclass herpes virus IgG. J Virol Methods. 1985 Mar;10(3):203–214. doi: 10.1016/0166-0934(85)90061-8. [DOI] [PubMed] [Google Scholar]
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984 Jun;19(6):917–919. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Antibody production by hybridomas. J Immunol Methods. 1980;39(4):285–308. doi: 10.1016/0022-1759(80)90230-6. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., Corey L., McDougall J. K., Tolentino E., Nowinski R. C. Monoclonal antibodies to herpes simplex viruses: use in antigenic typing and rapid diagnosis. J Infect Dis. 1983 May;147(5):829–837. doi: 10.1093/infdis/147.5.829. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., McDougall J., Hackman R., Meyers J. D., Thomas E. D., Nowinski R. C. Monoclonal antibodies to cytomegalovirus: rapid identification of clinical isolates and preliminary use in diagnosis of cytomegalovirus pneumonia. Infect Immun. 1982 Oct;38(1):273–281. doi: 10.1128/iai.38.1.273-281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes S. A., Nascimento J. P., Siqueira M. M., Krawczuk M. M., Pereira H. G., Russell W. C. In situ hybridization with biotinylated DNA probes: a rapid diagnostic test for adenovirus upper respiratory infections. J Virol Methods. 1985 Oct;12(1-2):105–110. doi: 10.1016/0166-0934(85)90012-6. [DOI] [PubMed] [Google Scholar]
- Graves D. C., McNabb S. J., Ivey M. H., Worley M. A. Development and characterization of monoclonal antibodies to Pneumocystis carinii. Infect Immun. 1986 Jan;51(1):125–133. doi: 10.1128/iai.51.1.125-133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Gustafsson B. Monoclonal antibody-based enzyme-linked immunosorbent assays for identification and serotyping of Vibrio cholerae O1. J Clin Microbiol. 1984 Dec;20(6):1180–1185. doi: 10.1128/jcm.20.6.1180-1185.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon M. W., Phillips D. J., Reimer C. B., Kendal A. P. Isotype-specific enzyme immunoassay for influenza antibody with monoclonal antibodies to human immunoglobulins. J Clin Microbiol. 1986 Dec;24(6):913–916. doi: 10.1128/jcm.24.6.913-916.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey C., Longbottom J. L. Development of a sandwich ELISA to detect IgG and IgG sub-class antibodies specific for a major antigen (Ag 7) of Aspergillus fumigatus. Clin Allergy. 1986 Jul;16(4):323–330. doi: 10.1111/j.1365-2222.1986.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Herrmann J. E., Armstrong A. S., Edberg S. C. Rapid methods for the immunodiagnosis of infectious diseases: recent developments. Yale J Biol Med. 1985 Sep-Oct;58(5):421–424. [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Blacklow N. R., Perron D. M., Cukor G., Krause P. J., Hyams J. S., Barrett H. J., Ogra P. L. Enzyme immunoassay with monoclonal antibodies for the detection of rotavirus in stool specimens. J Infect Dis. 1985 Oct;152(4):830–832. doi: 10.1093/infdis/152.4.830. [DOI] [PubMed] [Google Scholar]
- Higgins J. R., Pedersen N. C., Carlson J. R. Detection and differentiation by sandwich enzyme-linked immunosorbent assay of human T-cell lymphotropic virus type III/lymphadenopathy-associated virus- and acquired immunodeficiency syndrome-associated retroviruslike clinical isolates. J Clin Microbiol. 1986 Sep;24(3):424–430. doi: 10.1128/jcm.24.3.424-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfield P. E., Dougan G. DNA probes for microbial diagnosis. Med Lab Sci. 1985 Oct;42(4):352–360. [PubMed] [Google Scholar]
- Hill H. R., Matsen J. M. Enzyme-linked immunosorbent assay and radioimmunoassay in the serologic diagnosis of infectious diseases. J Infect Dis. 1983 Feb;147(2):258–263. doi: 10.1093/infdis/147.2.258. [DOI] [PubMed] [Google Scholar]
- Hoffmann B. E., Jungkind D. L., Haller G. J., Sharrar R., Baker R. A., Weisberg M. Evaluation of two rapid methods for the detection of herpes simplex virus antigen in patient specimens. Ann Clin Lab Sci. 1985 Sep-Oct;15(5):418–427. [PubMed] [Google Scholar]
- Holmberg H., Holme T., Krook A., Olsson T., Sjöberg L., Sjögren A. M. Detection of C polysaccharide in Streptococcus pneumoniae in the sputa of pneumonia patients by an enzyme-linked immunosorbent assay. J Clin Microbiol. 1985 Jul;22(1):111–115. doi: 10.1128/jcm.22.1.111-115.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsleth A., Bech-Thomsen N., Friis B. Detection by ELISA of IgG-subclass-specific antibodies in primary respiratory syncytial (RS) virus infections. J Med Virol. 1985 Aug;16(4):321–328. doi: 10.1002/jmv.1890160404. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Larsen S. H., Ståhlberg T., Terho P. Analysis and detection of chlamydial DNA. J Gen Microbiol. 1984 Dec;130(12):3159–3164. doi: 10.1099/00221287-130-12-3159. [DOI] [PubMed] [Google Scholar]
- Jones M. F., Smith T. F., Houglum A. J., Herrmann J. E. Detection of Chlamydia trachomatis in genital specimens by the Chlamydiazyme test. J Clin Microbiol. 1984 Sep;20(3):465–467. doi: 10.1128/jcm.20.3.465-467.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kennett R. H., Denis K. A., Tung A. S., Klinman N. R. Hybrid plasmacytoma production: fusions with adult spleen cells, monoclonal spleen fragments, neonatal spleen cells and human spleen cells. Curr Top Microbiol Immunol. 1978;81:77–91. doi: 10.1007/978-3-642-67448-8_13. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Wyatt R. G., Fernie B. F., Brandt C. D., Arrobio J. O., Jeffries B. C., Parrott R. H. Respiratory syncytial virus detection by immunofluorescence in nasal secretions with monoclonal antibodies against selected surface and internal proteins. J Clin Microbiol. 1983 Dec;18(6):1399–1404. doi: 10.1128/jcm.18.6.1399-1404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipstein F. A., Engert R. F., Houghten R. A., Rowe B. Enzyme-linked immunosorbent assay for Escherichia coli heat-stable enterotoxin. J Clin Microbiol. 1984 Jun;19(6):798–803. doi: 10.1128/jcm.19.6.798-803.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisley C. V., Bednarz-Prashad A. J., Pickering L. K. Detection of rotavirus in stool specimens with monoclonal and polyclonal antibody-based assay systems. J Clin Microbiol. 1986 May;23(5):897–900. doi: 10.1128/jcm.23.5.897-900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. A., Gill V., Swan J. C., Ognibene F., Shelhamer J., Parrillo J. E., Masur H. Prospective evaluation of a monoclonal antibody in diagnosis of Pneumocystis carinii pneumonia. Lancet. 1986 Jul 5;2(8497):1–3. doi: 10.1016/s0140-6736(86)92555-9. [DOI] [PubMed] [Google Scholar]
- Kramer S. M., Cremer N. E. Detection of IgG antibodies to measles virus using monoclonal antibodies for antigen-capture in enzyme immunoassay. J Virol Methods. 1984 May;8(3):255–263. doi: 10.1016/0166-0934(84)90020-x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Lambert J. P., Marbehant P., Marissens D., Zissis G. Monoclonal antibodies directed against different antigenic determinants of rotavirus. J Virol. 1984 Jul;51(1):47–51. doi: 10.1128/jvi.51.1.47-51.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry M. L., Zibello T. A., Hsiung G. D. Comparison of in situ hybridization and immunologic staining with cytopathology for detection and identification of herpes simplex virus infection in cultured cells. J Clin Microbiol. 1986 Dec;24(6):968–971. doi: 10.1128/jcm.24.6.968-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary J. J., Brigati D. J., Ward D. C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4045–4049. doi: 10.1073/pnas.80.13.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
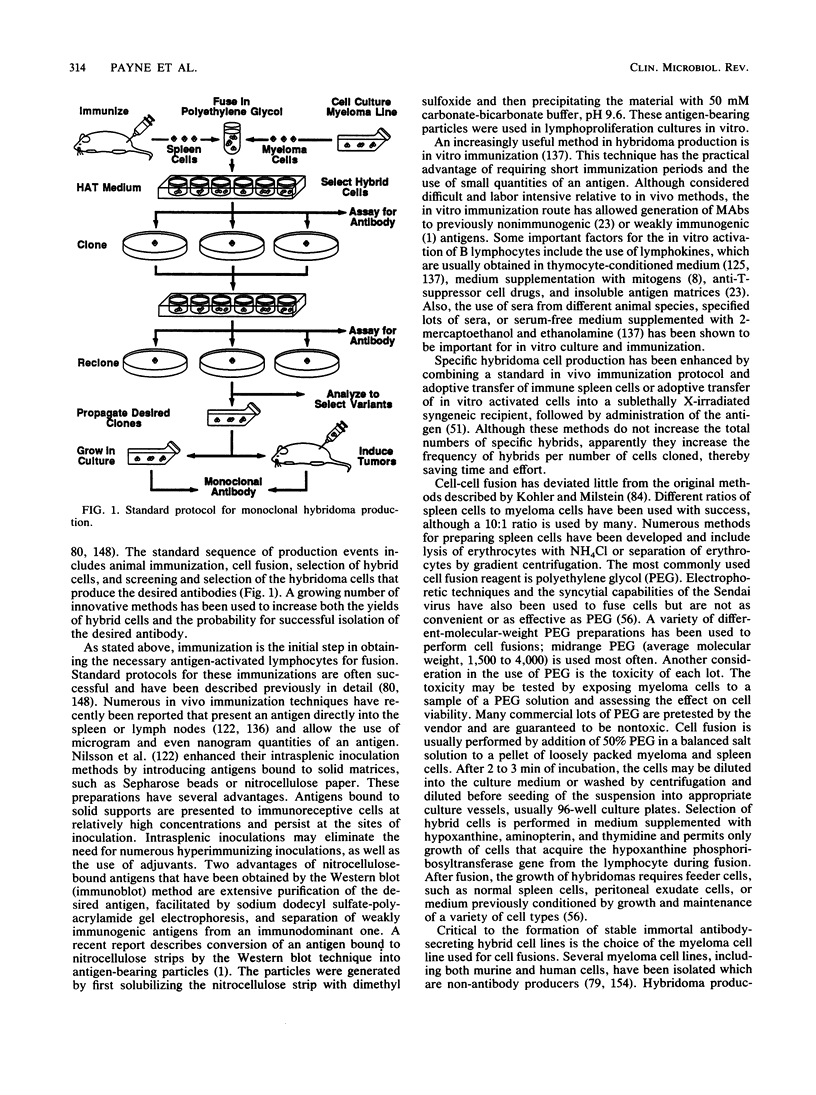
- Lee C. H., Bolinger C. D., Bartlett M. S., Kohler R. B., Wilde C. E., 3rd, Smith J. W. Production of monoclonal antibody against Pneumocystis carinii by using a hybrid of rat spleen and mouse myeloma cells. J Clin Microbiol. 1986 Mar;23(3):505–508. doi: 10.1128/jcm.23.3.505-508.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemesre J. L., Afchain D., Orozco O., Loyens M., Breniere F. S., Desjeux P., Carlier Y., Martin U., Nogueira-Queiroz J. A., Le Ray D. Specific and sensitive immunological diagnosis of Chagas' disease by competitive antibody enzyme immunoassay using a Trypanosoma cruzi-specific monoclonal antibody. Am J Trop Med Hyg. 1986 Jan;35(1):86–93. doi: 10.4269/ajtmh.1986.35.86. [DOI] [PubMed] [Google Scholar]
- Lim F., Sun A. M. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980 Nov 21;210(4472):908–910. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- Liu Y. S., Green A. A monoclonal-antibody enzyme immunoassay for detection of hepatitis B surface antigen with use of a biotin-avidin system. Clin Chem. 1985 Feb;31(2):202–205. [PubMed] [Google Scholar]
- Lukehart S. A., Tam M. R., Hom J., Baker-Zander S. A., Holmes K. K., Nowinski R. C. Characterization of monoclonal antibodies to Treponema pallidum. J Immunol. 1985 Jan;134(1):585–592. [PubMed] [Google Scholar]
- Lurain N. S., Thompson K. D., Farrand S. K. Rapid detection of cytomegalovirus in clinical specimens by using biotinylated DNA probes and analysis of cross-reactivity with herpes simplex virus. J Clin Microbiol. 1986 Nov;24(5):724–730. doi: 10.1128/jcm.24.5.724-730.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Wilkins T. D. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985 Jan;21(1):12–14. doi: 10.1128/jcm.21.1.12-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox C. W., Wilson R. A. High technology diagnostics: detection of enterotoxigenic Escherichia coli, using DNA probes. J Am Vet Med Assoc. 1986 Jan 1;188(1):57–59. [PubMed] [Google Scholar]
- Manil L., Motté P., Pernas P., Troalen F., Bohuon C., Bellet D. Evaluation of protocols for purification of mouse monoclonal antibodies. Yield and purity in two-dimensional gel electrophoresis. J Immunol Methods. 1986 Jun 10;90(1):25–37. doi: 10.1016/0022-1759(86)90379-0. [DOI] [PubMed] [Google Scholar]
- Manis R. D., Jr, Harris B., Geiseler P. J. Evaluation of Gonozyme, an enzyme immunoassay for the rapid diagnosis of gonorrhea. J Clin Microbiol. 1984 Oct;20(4):742–746. doi: 10.1128/jcm.20.4.742-746.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse M. J., Meulien P., Le Guern A., Kourilsky P. Monoclonal antibody detection of 2-acetyl-aminofluorene-modified DNA probes for the specific detection of nucleic acids in hybridization procedures. Ann Inst Pasteur Immunol. 1985 Nov-Dec;136D(3):231–243. doi: 10.1016/s0769-2625(85)80109-4. [DOI] [PubMed] [Google Scholar]
- Mayer K. H., Hanson E. Recurrent salmonella infection with a single strain in the acquired immunodeficiency syndrome. Confirmation by plasmid fingerprinting. Diagn Microbiol Infect Dis. 1986 Jan;4(1):71–76. doi: 10.1016/0732-8893(86)90059-3. [DOI] [PubMed] [Google Scholar]
- McKeating J. A., Stagno S., Stirk P. R., Griffiths P. D. Detection of cytomegalovirus in urine samples by enzyme-linked immunosorbent assay. J Med Virol. 1985 Aug;16(4):367–373. doi: 10.1002/jmv.1890160410. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Merten O. W., Reiter S., Scheirer W., Katinger H. Purification of HBsAg produced by the human hepatoma cell line PLC/PRE/5 by affinity chromatography using monoclonal antibodies and application for ELISA diagnostic. Dev Biol Stand. 1983;55:121–127. [PubMed] [Google Scholar]
- Milstein C., Galfre G., Secher D. S., Springer T. Monoclonal antibodies and cell surface antigens. Cell Biol Int Rep. 1979 Jan;3(1):1–16. doi: 10.1016/0309-1651(79)90063-8. [DOI] [PubMed] [Google Scholar]
- Morrow D. L., Kline J. B., Douglas S. D., Polin R. A. Rapid detection of group B streptococcal antigen by monoclonal antibody sandwich enzyme assay. J Clin Microbiol. 1984 Apr;19(4):457–459. doi: 10.1128/jcm.19.4.457-459.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Huq I., Alim A. R., So M., Samadpour-Motalebi M., Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980 Dec;142(6):892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- Murakami K., Yoshida T. Monoclonal antibodies against species-specific cephalosporinase of Pseudomonas aeruginosa. Eur J Biochem. 1985 Feb 1;146(3):693–697. doi: 10.1111/j.1432-1033.1985.tb08706.x. [DOI] [PubMed] [Google Scholar]
- Nahm M. H., Murray P. R., Clevinger B. L., Davie J. M. Improved diagnostic accuracy using monoclonal antibody group A streptococcal carbohydrate. J Clin Microbiol. 1980 Oct;12(4):506–508. doi: 10.1128/jcm.12.4.506-508.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasello M. A., Callihan D. R., Menegus M. A., Steigbigel R. T. A solid-phase enzyme immunoassay (Gonozyme) test for direct detection of Neisseria gonorrhoeae antigen in urogenital specimens from patients at a sexually transmitted disease clinic. Sex Transm Dis. 1985 Oct-Dec;12(4):198–202. doi: 10.1097/00007435-198510000-00006. [DOI] [PubMed] [Google Scholar]
- Nerurkar L. S., Miller N. R., Namba M., Monzon M., Brashears G., Scherba G., Sever J. L. Typing of herpes simplex virus by capture biotin-streptavidin enzyme-linked immunosorbent assay and comparison with restriction endonuclease analysis and immunofluorescence method using monoclonal antibodies. J Clin Microbiol. 1987 Jan;25(1):128–132. doi: 10.1128/jcm.25.1.128-132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell D. G. Monoclonal antibodies directed against the flagella of Campylobacter jejuni: cross-reacting and serotypic specificity and potential use in diagnosis. J Hyg (Lond) 1986 Jun;96(3):377–384. doi: 10.1017/s0022172400066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilheden E., Jeansson S., Vahlne A. Typing of herpes simplex virus by an enzyme-linked immunosorbent assay with monoclonal antibodies. J Clin Microbiol. 1983 Apr;17(4):677–680. doi: 10.1128/jcm.17.4.677-680.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson B. O., Svalander P. C., Larsson A. Immunization of mice and rabbits by intrasplenic deposition of nanogram quantities of protein attached to Sepharose beads or nitrocellulose paper strips. J Immunol Methods. 1987 May 4;99(1):67–75. doi: 10.1016/0022-1759(87)90033-0. [DOI] [PubMed] [Google Scholar]
- O'Connor C. G., Ashman L. K. Application of the nitrocellulose transfer technique and alkaline phosphatase conjugated anti-immunoglobulin for determination of the specificity of monoclonal antibodies to protein mixtures. J Immunol Methods. 1982 Oct 29;54(2):267–271. doi: 10.1016/0022-1759(82)90068-0. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Sanson A. J., Daniel T. M. Characterization of Mycobacterium tuberculosis antigen 5 epitopes by using a panel of 19 monoclonal antibodies. J Clin Microbiol. 1987 Mar;25(3):471–475. doi: 10.1128/jcm.25.3.471-475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossendorp F. A., De Boer M., Al B. J., Hilgers J., Bruning P. F., Tager J. M. Production of murine monoclonal antibodies against human thyroglobulin using an in vitro immunization procedure in serum-free medium. J Immunol Methods. 1986 Jul 24;91(2):257–264. doi: 10.1016/0022-1759(86)90487-4. [DOI] [PubMed] [Google Scholar]
- Patel K., Chittom A. L., Toshniwal R., Kocka F. E. Rapid commercial test for direct detection of group A streptococci in throat swabs. Eur J Clin Microbiol. 1987 Apr;6(2):193–194. doi: 10.1007/BF02018210. [DOI] [PubMed] [Google Scholar]
- Peiper S. C., Stass S. A. Markers of cellular differentiation in acute lymphoblastic leukemia. Arch Pathol Lab Med. 1982 Jan;106(1):3–8. [PubMed] [Google Scholar]
- Perine P. L., Totten P. A., Holmes K. K., Sng E. H., Ratnam A. V., Widy-Wersky R., Nsanze H., Habte-Gabr E., Westbrook W. G. Evaluation of a DNA-hybridization method for detection of African and Asian strains of Neisseria gonorrhoeae in men with urethritis. J Infect Dis. 1985 Jul;152(1):59–63. doi: 10.1093/infdis/152.1.59. [DOI] [PubMed] [Google Scholar]
- Peterson E., Schmidt O. W., Goldstein L. C., Nowinski R. C., Corey L. Typing of clinical herpes simplex virus isolates with mouse monoclonal antibodies to herpes simplex virus types 1 and 2: comparison with type-specific rabbit antisera and restriction endonuclease analysis of viral DNA. J Clin Microbiol. 1983 Jan;17(1):92–96. doi: 10.1128/jcm.17.1.92-96.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitjean F., Guillet J. G., Vray B., Hoebeke J., Tram C., Strosberg A. D. Standardization for diagnostic purposes of a monoclonal antibody against Legionella pneumophila. Dev Biol Stand. 1984;57:99–105. [PubMed] [Google Scholar]
- Philip A., Garton G. C. Comparative evaluation of five commercial systems for the rapid identification of pathogenic Neisseria species. J Clin Microbiol. 1985 Jul;22(1):101–104. doi: 10.1128/jcm.22.1.101-104.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polin R. A., Kennett R. Use of monoclonal antibodies in an enzyme-linked inhibition assay for rapid detection of streptococcal antigen. J Pediatr. 1980 Oct;97(4):540–544. doi: 10.1016/s0022-3476(80)80005-9. [DOI] [PubMed] [Google Scholar]
- Polin R. A. Monoclonal antibodies against microorganisms. Eur J Clin Microbiol. 1984 Oct;3(5):387–398. doi: 10.1007/BF02017358. [DOI] [PubMed] [Google Scholar]
- Pugh S. F., Slack R. C., Caul E. O., Paul I. D., Appleton P. N., Gatley S. Enzyme amplified immunoassay: a novel technique applied to direct detection of Chlamydia trachomatis in clinical specimens. J Clin Pathol. 1985 Oct;38(10):1139–1141. doi: 10.1136/jcp.38.10.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond Y., Suh M. Lymph node primary immunization of mice for the production of polyclonal and monoclonal antibodies. J Immunol Methods. 1986 Oct 23;93(1):103–106. doi: 10.1016/0022-1759(86)90439-4. [DOI] [PubMed] [Google Scholar]
- Reading C. L. Theory and methods for immunization in culture and monoclonal antibody production. J Immunol Methods. 1982 Sep 30;53(3):261–291. doi: 10.1016/0022-1759(82)90175-2. [DOI] [PubMed] [Google Scholar]
- Reiss E., de Repentigny L., Kuykendall R. J., Carter A. W., Galindo R., Auger P., Bragg S. L., Kaufman L. Monoclonal antibodies against Candida tropicalis mannan: antigen detection by enzyme immunoassay and immunofluorescence. J Clin Microbiol. 1986 Nov;24(5):796–802. doi: 10.1128/jcm.24.5.796-802.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rench M. A., Metzger T. G., Baker C. J. Detection of group B streptococcal antigen in body fluids by a latex-coupled monoclonal antibody assay. J Clin Microbiol. 1984 Nov;20(5):852–854. doi: 10.1128/jcm.20.5.852-854.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M. D., Warnock D. W. Enzyme-linked immunosorbent assay and its application to the serological diagnosis of fungal infection. Sabouraudia. 1983 Mar;21(1):1–14. doi: 10.1080/00362178385380031. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Cleveland P. H., Oxman M. N. A rapid enzyme immunofiltration technique using monoclonal antibodies to serotype herpes simplex virus. J Med Virol. 1982;9(4):299–305. doi: 10.1002/jmv.1890090408. [DOI] [PubMed] [Google Scholar]
- Richman D. D., Cleveland P. H., Redfield D. C., Oxman M. N., Wahl G. M. Rapid viral diagnosis. J Infect Dis. 1984 Mar;149(3):298–310. doi: 10.1093/infdis/149.3.298. [DOI] [PubMed] [Google Scholar]
- Ristaino P. A., Levine M. M., Young C. R. Improved GM1-enzyme-linked immunosorbent assay for detection of Escherichia coli heat-labile enterotoxin. J Clin Microbiol. 1983 Oct;18(4):808–815. doi: 10.1128/jcm.18.4.808-815.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin F. A., Kopecko D. J., Noon K. F., Baron L. S. Development of a DNA probe to detect Salmonella typhi. J Clin Microbiol. 1985 Oct;22(4):600–605. doi: 10.1128/jcm.22.4.600-605.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijs G., Kraai E. J., van Voorst Vader P. C., Schirm J., Schröder F. P. Rapid detection with monoclonal antibodies of Chlamydia trachomatis in urethral smears and urine sediments. Lancet. 1984 Apr 28;1(8383):960–961. doi: 10.1016/s0140-6736(84)92413-9. [DOI] [PubMed] [Google Scholar]
- Schachter J. Immunodiagnosis of sexually transmitted disease. Yale J Biol Med. 1985 Sep-Oct;58(5):443–452. [PMC free article] [PubMed] [Google Scholar]
- Schachter J., McCormack W. M., Smith R. F., Parks R. M., Bailey R., Ohlin A. C. Enzyme immunoassay for diagnosis of gonorrhea. J Clin Microbiol. 1984 Jan;19(1):57–59. doi: 10.1128/jcm.19.1.57-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff M. D., Roberts S., Thammana P. Monoclonal antibodies. J Infect Dis. 1981 Mar;143(3):346–351. doi: 10.1093/infdis/143.3.346. [DOI] [PubMed] [Google Scholar]
- Schuster V., Matz B., Wiegand H., Polack A., Corsten B., Neumann-Haefelin D. Nucleic acid hybridization for detection of herpes viruses in clinical specimens. J Med Virol. 1986 Jul;19(3):277–286. doi: 10.1002/jmv.1890190310. [DOI] [PubMed] [Google Scholar]
- Schuster V., Matz B., Wiegand H., Traub B., Kampa D., Neumann-Haefelin D. Detection of human cytomegalovirus in urine by DNA-DNA and RNA-DNA hybridization. J Infect Dis. 1986 Aug;154(2):309–314. doi: 10.1093/infdis/154.2.309. [DOI] [PubMed] [Google Scholar]
- Schwabe L. D., Small M. T., Randall E. L. Comparison of TestPack Strep A test kit with culture technique for detection of group A streptococci. J Clin Microbiol. 1987 Feb;25(2):309–311. doi: 10.1128/jcm.25.2.309-311.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shone C., Appleton N., Wilton-Smith P., Hambleton P., Modi N., Gatley S., Melling J. In vitro assays for botulinum toxin and antitoxins. Dev Biol Stand. 1986;64:141–145. [PubMed] [Google Scholar]
- Shone C., Wilton-Smith P., Appleton N., Hambleton P., Modi N., Gatley S., Melling J. Monoclonal antibody-based immunoassay for type A Clostridium botulinum toxin is comparable to the mouse bioassay. Appl Environ Microbiol. 1985 Jul;50(1):63–67. doi: 10.1128/aem.50.1.63-67.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Shuster E. A., Beneke J. S., Tegtmeier G. E., Pearson G. R., Gleaves C. A., Wold A. D., Smith T. F. Monoclonal antibody for rapid laboratory detection of cytomegalovirus infections: characterization and diagnostic application. Mayo Clin Proc. 1985 Sep;60(9):577–585. doi: 10.1016/s0025-6196(12)60979-3. [DOI] [PubMed] [Google Scholar]
- Singh-Naz N., Naz R. K. Development and application of monoclonal antibodies for specific detection of human enteric adenoviruses. J Clin Microbiol. 1986 May;23(5):840–842. doi: 10.1128/jcm.23.5.840-842.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S. A., Rua J. A., Spector D. H., McMillan R. Detection of human cytomegalovirus in clinical specimens by DNA-DNA hybridization. J Infect Dis. 1984 Jul;150(1):121–126. doi: 10.1093/infdis/150.1.121. [DOI] [PubMed] [Google Scholar]
- Sugasawara R. J., Prato C. M., Sippel J. E. Enzyme-linked immunosorbent assay with a monoclonal antibody for detecting group A meningococcal antigens in cerebrospinal fluid. J Clin Microbiol. 1984 Feb;19(2):230–234. doi: 10.1128/jcm.19.2.230-234.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Wikström M., Lindblad M., Holmgren J. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Oct;24(4):585–590. doi: 10.1128/jcm.24.4.585-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart R. T., Samloff I. M. Stable antibody-producing murine hybridomas. Science. 1983 Mar 11;219(4589):1228–1230. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- Tam M. R., Stamm W. E., Handsfield H. H., Stephens R., Kuo C. C., Holmes K. K., Ditzenberger K., Krieger M., Nowinski R. C. Culture-independent diagnosis of Chlamydia trachomatis using monoclonal antibodies. N Engl J Med. 1984 May 3;310(18):1146–1150. doi: 10.1056/NEJM198405033101803. [DOI] [PubMed] [Google Scholar]
- Thomas J. G., Dul M. J., Badger S., Grecco A., Immerman R. S., Jackson N., Jacob C. V. Multicenter clinical trial and laboratory utilization of an enzymatic detection method for gonococcal antigens. Am J Clin Pathol. 1986 Jul;86(1):71–78. doi: 10.1093/ajcp/86.1.71. [DOI] [PubMed] [Google Scholar]
- Thompson M. R., Brandwein H., LaBine-Racke M., Giannella R. A. Simple and reliable enzyme-linked immunosorbent assay with monoclonal antibodies for detection of Escherichia coli heat-stable enterotoxins. J Clin Microbiol. 1984 Jul;20(1):59–64. doi: 10.1128/jcm.20.1.59-64.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M. R., Jordan R. L., Luttrell M. A., Brandwein H., Kaper J. B., Levine M. M., Giannella R. A. Blinded, two-laboratory comparative analysis of Escherichia coli heat-stable enterotoxin production by using monoclonal antibody enzyme-linked immunosorbent assay, radioimmunoassay, suckling mouse assay, and gene probes. J Clin Microbiol. 1986 Nov;24(5):753–758. doi: 10.1128/jcm.24.5.753-758.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. E., Razdan M., Kuntsmann G., Aschenbach J. M., Evenson M. L., Bergdoll M. S. Detection of staphylococcal enterotoxins by enzyme-linked immunosorbent assays and radioimmunoassays: comparison of monoclonal and polyclonal antibody systems. Appl Environ Microbiol. 1986 May;51(5):885–890. doi: 10.1128/aem.51.5.885-890.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Holmes K. K., Handsfield H. H., Knapp J. S., Perine P. L., Falkow S. DNA hybridization technique for the detection of Neisseria gonorrhoeae in men with urethritis. J Infect Dis. 1983 Sep;148(3):462–471. doi: 10.1093/infdis/148.3.462. [DOI] [PubMed] [Google Scholar]
- Ungar B. L., Yolken R. H., Quinn T. C. Use of a monoclonal antibody in an enzyme immunoassay for the detection of Entamoeba histolytica in fecal specimens. Am J Trop Med Hyg. 1985 May;34(3):465–472. doi: 10.4269/ajtmh.1985.34.465. [DOI] [PubMed] [Google Scholar]
- Unger E. R., Budgeon L. R., Myerson D., Brigati D. J. Viral diagnosis by in situ hybridization. Description of a rapid simplified colorimetric method. Am J Surg Pathol. 1986 Jan;10(1):1–8. doi: 10.1097/00000478-198601000-00001. [DOI] [PubMed] [Google Scholar]
- Usuda S., Tsuda F., Gotanda T., Tachibana K., Nomura M., Okamoto H., Imai M., Nakamura T., Miyakawa Y., Mayumi M. A solid-phase enzyme immunoassay for the common and subtypic determinants of hepatitis B surface antigen with monoclonal antibodies. J Immunol Methods. 1986 Mar 13;87(2):203–210. doi: 10.1016/0022-1759(86)90532-6. [DOI] [PubMed] [Google Scholar]
- Uyeda C. T., Welborn P., Ellison-Birang N., Shunk K., Tsaouse B. Rapid diagnosis of chlamydial infections with the MicroTrak direct test. J Clin Microbiol. 1984 Nov;20(5):948–950. doi: 10.1128/jcm.20.5.948-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen M., Palva A., Laaksonen M., Halonen P., Söderlund H., Ranki M. Novel test for rapid viral diagnosis: detection of adenovirus in nasopharyngeal mucus aspirates by means of nucleic-acid sandwich hybridisation. Lancet. 1983 Feb 19;1(8321):381–383. doi: 10.1016/s0140-6736(83)91500-3. [DOI] [PubMed] [Google Scholar]
- Viscidi R. P., Connelly C. J., Yolken R. H. Novel chemical method for the preparation of nucleic acids for nonisotopic hybridization. J Clin Microbiol. 1986 Feb;23(2):311–317. doi: 10.1128/jcm.23.2.311-317.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi A., Lakeman A. D., Pereira L., Stagno S. Monoclonal antibodies for rapid diagnosis and typing of genital herpes infections during pregnancy. Am J Obstet Gynecol. 1983 Aug 1;146(7):813–815. doi: 10.1016/0002-9378(83)91083-9. [DOI] [PubMed] [Google Scholar]
- Volpi A., Whitley R. J., Ceballos R., Stagno S., Pereira L. Rapid diagnosis of pneumonia due to cytomegalovirus with specific monoclonal antibodies. J Infect Dis. 1983 Jun;147(6):1119–1120. doi: 10.1093/infdis/147.6.1119. [DOI] [PubMed] [Google Scholar]
- Wagner D., Ikenberg H., Boehm N., Gissmann L. Identification of human papillomavirus in cervical swabs by deoxyribonucleic acid in situ hybridization. Obstet Gynecol. 1984 Dec;64(6):767–772. [PubMed] [Google Scholar]
- Wells D. E., Reeves M. W., McKinney R. M., Graves L. M., Olsvik O., Bergan T., Feeley J. C. Production and characterization of monoclonal antibodies to toxic shock syndrome toxin 1 and use of a monoclonal antibody in a rapid, one-step enzyme-linked immunosorbent assay for detection of picogram quantities of toxic shock syndrome toxin 1. J Clin Microbiol. 1987 Mar;25(3):516–521. doi: 10.1128/jcm.25.3.516-521.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz R. A., Burkot T. R., Andre R. G., Rosenberg R., Collins W. E., Roberts D. R. Identification of Plasmodium vivax sporozoites in mosquitoes using an enzyme-linked immunosorbent assay. Am J Trop Med Hyg. 1985 Nov;34(6):1048–1054. doi: 10.4269/ajtmh.1985.34.1048. [DOI] [PubMed] [Google Scholar]
- Wolters G., Nelissen P., Kuijpers L. Improved ELISA for the detection of HBsAg. J Virol Methods. 1985 Apr;10(4):299–305. doi: 10.1016/0166-0934(85)90046-1. [DOI] [PubMed] [Google Scholar]
- Wood D. D., Staruch M. J. Control of the mitogenicity of muramyl dipeptide. Int J Immunopharmacol. 1981;3(1):31–44. doi: 10.1016/0192-0561(81)90043-6. [DOI] [PubMed] [Google Scholar]
- Wu B. The production of monoclonal antibodies against respiratory syncytial virus and its clinical applications. Clin Lab Med. 1985 Sep;5(3):589–613. [PubMed] [Google Scholar]
- Yolken R. H. Enzyme immunoassays for the detection of infectious antigens in body fluids: current limitations and future prospects. Rev Infect Dis. 1982 Jan-Feb;4(1):35–68. doi: 10.1093/clinids/4.1.35. [DOI] [PubMed] [Google Scholar]


