Abstract
Glutathione is a tripeptide involved in various aspects of plant metabolism. This study investigated the effects of the reduced form of glutathione (GSH) applied to specific organs (source leaves, sink leaves, and roots) on cadmium (Cd) distribution and behaviour in the roots of oilseed rape plants (Brassica napus) cultured hydroponically. The translocation ratio of Cd from roots to shoots was significantly lower in plants that had root treatment of GSH than in control plants. GSH applied to roots reduced the Cd concentration in the symplast sap of root cells and inhibited root-to-shoot Cd translocation via xylem vessels significantly. GSH applied to roots also activated Cd efflux from root cells to the hydroponic solution. Inhibition of root-to-shoot translocation of Cd was visualized, and the activation of Cd efflux from root cells was also shown by using a positron-emitting tracer imaging system (PETIS). This study investigated a similar inhibitory effect on root-to-shoot translocation of Cd by the oxidized form of glutathione, GSSG. Inhibition of Cd accumulation by GSH was abolished by a low-temperature treatment. Root cells of plants exposed to GSH in the root zone had less Cd available for xylem loading by actively excluding Cd from the roots. Consequently, root-to-shoot translocation of Cd was suppressed and Cd accumulation in the shoot decreased.
Key words: Cadmium (Cd), glutathione, oilseed rape (Brassica napus), oxidized form of glutathione (GSSG), positron-emitting tracer imaging system (PETIS), reduced form of glutathione, transport, xylem.
Introduction
Cd is a toxic heavy metal that is released into the environment through human agricultural, industrial, and urban activities, resulting in Cd pollution of significant areas of farmland worldwide (McLaughlin et al., 1999). Cd causes serious human health problems when it enters the food chain (Obata and Umebayashi, 1997). To reduce Cd accumulation in crop plants, it is necessary to elucidate the mechanisms of Cd uptake and distribution. These mechanisms are not fully understood to date.
So far, various approaches have been followed to reduce Cd accumulation in crops. One of these attempts focuses on the removal of Cd from soils using plants, a technique called phytoremediation (Salt et al., 1998). However, it often takes many years to restore Cd-polluted soils by phytoremediation. It is necessary to establish novel, faster technologies to meet the demands of the market.
Previously, Nakamura et al. (2005) found that glutathione (GSH) concentrations in the phloem sap of oilseed rape plants increase under Cd treatment. GSH has a variety of physiological functions in removal of reactive oxygen species (Noctor and Foyer, 1998), heavy metal detoxification (Mendoza-Cózatl et al., 2005), and sugar metabolism (Ito et al., 2003). Additionally, GSH is a precursor of phytochelatins (PCs) (Rauser, 1995), which play important roles in vacuolar heavy metal sequestration (Salt and Rauser, 1995) and Cd transport from roots to shoots (Gong et al., 2003). These findings suggest that GSH might have a significant impact on the behaviour of Cd in plants. The Cd-dependent increase in GSH in the sieve tubes could be due to an increased requirement for GSH in sink tissues of Cd-treated plants. From this aspect, this study considered that GSH applied to specific parts of a plant might have significant effects on the behaviour of Cd throughout whole plants.
This study investigated the effects of applying GSH to roots, sink leaves, and source leaves of oilseed rape plants on Cd accumulation and Cd behaviour. Additionally, a positron-emitting tracer imaging system (PETIS) was employed to observe Cd movement in whole plants. PETIS enables the visualization of the transport and accumulation of positron-emitters in the plant non invasively (Fujimaki, 2007). The present study is built on this study group’s previous success in visualizing Cd movement in rice plants by monitoring 107Cd as a positron emitter (Fujimaki et al., 2010). To demonstrate that glutathione is functioning via some physiological processes, but not by simple chemical binding between Cd and GSH, two experiments were performed. First, the effects of GSSG applied to roots on Cd accumulation in plants were investigated. Glutathione is generally known to have two different chemical forms: a reduced form (GSH) and an oxidized form (GSSG). GSSG lacks some ability to bind with Cd due to its molecular structure. Second, the effects of low-temperature treatment were examined. At low temperatures, only physiological processes should be suppressed in the roots.
Materials and methods
Plant materials
Seeds of oilseed rape (Brassica napus L. cv. Nourin No. 16, Kaneko Seed, Gumma, Japan) were germinated in Petri dishes and then grown on floating nets in 1.5 l plastic containers with nutrient solution (modified Hoagland solution, described in Nakamura et al., 2008). After 1 week, seedling density was reduced to four seedlings per container. The containers were kept in a growth chamber at 24/18 °C 16/8 light/dark cycle (about 180 µmol m–2 s–1). Nutrient solutions were aerated continuously and renewed once a week. Four-week-old plants were treated with Cd and glutathione for 2 days (short-term) and 2 weeks (long-term). For the collection of xylem and phloem sap, plants were grown following the method of Nakamura et al. (2005).
Cd and glutathione treatment
Plants were exposed to 0 or 10 µM Cd (CdCl2) for 2 days (short-term treatment) or 2 weeks (long-term treatment). GSH was applied to roots, source leaves, and sink leaves, while the plants were exposed to Cd. In the root treatment, 1mM GSH and GSSG (final concentration) was added to the nutrient solution. In the leaf treatment, 20mM GSH in a foliar application solution (10mM MES-NaOH, pH 6.1, 0.01%, w/v, Triton X-100) was applied with a brush. The total amount of GSH applied to sink and source leaves was 2.5 µmol and 5.0 µmol, respectively, per application. GSH was applied to three sink or source leaves per plant, twice daily for 5 days before harvest.
Measurement of heavy metal (Cd, Fe, Mn, and Zn) accumulation in plants
After harvest, seedlings were divided into shoots and roots. The roots were washed in 0.1M HNO3 and deionized water, and were blotted with paper towels. Shoots and roots were dried at 105 °C for 24h, weighed, and then digested in 6ml of a 5:1 (v/v) mixture of HNO3 and H2O2 in a microwave oven (ETHOS1600, Milestone, Italy). Heavy metal concentrations in the digested solution were measured by an inductively coupled plasma atomic emission spectrometer (ICP-AES, IRIS Duo, Nippon Jarrell-Ash, Japan), and the heavy metal content of shoots and roots were calculated. The Cd translocation ratio indicates the ratio of Cd translocated to shoots compared to the Cd absorbed by plants.
Collection of symplast sap, xylem sap, and phloem sap from oilseed rape plants and measurement of heavy metal concentrations in these saps
After Cd and GSH treatment, root cell sap (symplast sap) was collected by centrifugation, following the method of Yu et al. (1999). Heavy metal concentrations (Cd, Fe, Mn, and Zn) in these cell saps were measured by an atomic absorption photometer (AA-6100, Shimadzu, Japan). After Cd and GSH treatment, xylem sap and phloem sap were collected following the method of Nakamura et al. (phloem sap 2005; xylem sap 2008). Heavy metal concentrations in xylem sap and phloem sap were also measured in the manner described above.
Effects of GSH on Cd efflux from root cells
Three-week-old oilseed rape plants were transferred to 1.5 l plastic containers (four plants per container) with a nutrient solution plus 10 µM CdCl2. After 24h Cd treatment, plant roots were rinsed briefly in deionized water, followed by a desorption of apoplastic Cd in a desorbing solution containing 5mM MES-KOH (pH 6.0), 1mM K2HPO4, and 0.5mM Ca(NO3)2 for 10min. Then each plant was transferred to a light-shielding bottle with 0.6ml nutrient solution for 24h. After 24h treatment, each nutrient solution was collected and root freshweights (FW) were measured. Cd concentration in solution was determined by an atomic absorption photometer (AA280Z, Varian, USA) and Cd efflux from roots was calculated.
HPLC analysis of GSH and phytochelatins (PC2, PC3, and PC4) in the root tissues
Two-week-old oilseed rape plants were treated with 10 µM CdCl2, 1mM GSH and 1mM GSSG. After 2 days, plant roots were rinsed briefly in deionized water and a desorbing solution, as described above, for 10min. Then root FWs were measured. About 0.3g each sample was ground in liquid nitrogen using a mortar and pestle. GSH and PCs were extracted using 1.5ml extract solution containing 1.5mM diethylenetriamine pentaacetic acid and 1.5mM Na2S2O3 in 5.0% (w/v) 5-sulphosalicylic acid. The supernatant was collected after centrifugation at 15,000 g for 10min at 4 °C. GSH and PC concentration in the supernatants were measured immediately using HPLC system (Class 10 vp system, Shimadzu, Kyoto, Japan). GSH and PCs were separated on a C18 column (LiChrospher 100 RP-18 e, 250×4mm, particle size 5 µm, Merck Millipore, Darmstadt, Germany) using the method of de Knecht et al. (1994) with some modifications. The eluent was derivatized with 75 µM 5,5’-dithiobis(2-nitrobenzoic acid) in 50mM potassium phosphate buffer (pH 7.6), which was added at a flow rate of 0.75ml min–1. Derivatized materials were detected with an ultraviolet detector (wavelength 412nm). Identification of GSH and PCs was achieved by comparison of retention times with authentic standards. GSH was purchased from Wako Pure Chemical Industries (Osaka, Japan). Phytochelatin standards (PC2, PC3, and PC4) were purchased from Bonac Corporation (Fukuoka, Japan).
Non-invasive imaging of Cd by PETIS
107Cd, a positron emitter with a half-life of 6.5h, was produced as previously described (Ishioka et al., 2006; Fujimaki et al. 2010). Purified 107Cd was dissolved in an appropriate volume of 0.5mM CaCl2 with or without 1mM glutathione (GSH or GSSG). In these PETIS experiments, 2-week-old seedlings, grown hydroponically in a growth chamber under controlled growth conditions, were used. Test plants were set in an acrylic root box which was devised for PETIS experiments (Ishikawa et al., 2011). PETIS experiments were started by adding a total of 10 µM Cd including 107Cd to 0.5mM CaCl2 solution. Images of the 107Cd distribution were obtained every 4 minutes for 36h.
Low-temperature treatment experiment
Four-week-old seedlings, cultivated hydroponically, were used in these experiments. Seedlings were pretreated in 0.5mM CaC12 for 24h before Cd treatment. The seedlings were treated with 10 µM Cd in 0.5mM CaCl2 or 10 µM Cd + 1mM GSH in 0.5mM CaCl2 for 2h at 2 or 24 °C. After harvest, the Cd content in shoots and roots was measured following the method described above.
Statistical analysis
Analytical data were analysed by Student’s t-test at a significance level of 0.05.
Results
Effects of GSH applied to different organs on Cd accumulation
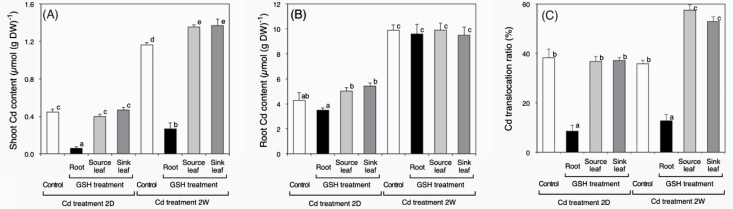
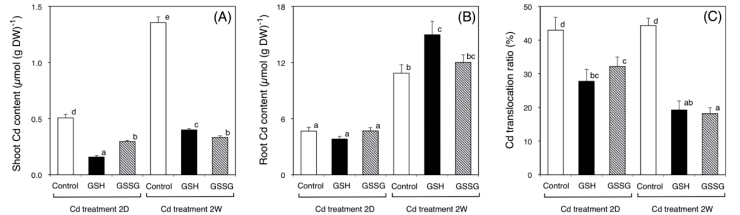
To examine the effects of GSH applied to different organs on Cd accumulation, Cd content of oilseed rape plants were measured after 2 days (short-term treatment) and 2 weeks (long-term treatment) of Cd exposure. At the time of harvest, plants that had undergone the short-term treatment showed no obvious symptoms or changes in dryweight (DW) (Supplementary Fig. S1, available at JXB online). However, plants treated with cadmium but without GSH for 2 weeks developed chlorosis and had a significant decrease in shoot dryweight (Supplementary Fig. S1). In the short-term treatment, the Cd contents in shoots were about 0.4 µmol (g DW)–1 both in control plants and plants treated with GSH onto their leaves (Fig. 1A). In contrast, Cd content was roughly 4-fold lower in plants exposed to GSH in the root zone (Fig. 1A). In the long-term treatment, the Cd content in the shoots of control plants and plants that had received GSH on their leaves was about 1.2 and about 1.4 µmol (g DW)–1, respectively (Fig. 1A). The shoot Cd content of plants exposed to GSH in the root zone was also roughly 4-fold lower (Fig. 1A). However, there was no significant difference in the Cd content of the roots in short-term and long-term treatments (Fig. 1B). Cd translocation ratios were calculated from these experimental results. The Cd translocation ratio was about 10% when GSH was applied to plant roots (Fig. 1C). This ratio was significantly lower than that of control plants and plants to which GSH was applied to their leaves (Fig. 1C). In the long-term treatment, these ratios from plants in which GSH was applied to leaves were about 1.5-fold higher (Fig. 1C). In these experiments, the Fe, Mn, and Zn contents of plants harvested after treatments were also measured (Supplementary Fig. S2). Compared with non-treated plants, the Fe, Mn, and Zn contents in shoots and roots of plants that had received GSH in the root zone were unaffected by the short-term Cd treatment. Compared with non-treated plants, Fe and Zn contents in the shoots of plants that were treated with GSH increased significantly under long-term Cd treatment. Compared with non-treated plants, the Fe, Mn, and Zn contents in roots of plants exposed to GSH also increased significantly under the long-term treatment.
Fig. 1.
(A, B) Cd contents in the shoots (A) and roots (B) of oilseed rape plants harvested after the indicated treatments. (C) Cd translocation ratio of oilseed rape plants, calculated from the Cd content in shoots and roots and their dryweights. Data are means ± SE (n > 8). Means labelled with different letters are significantly different according to Student’s t-test (P < 0.05).
Effects of GSH in the root zone on Cd behaviour
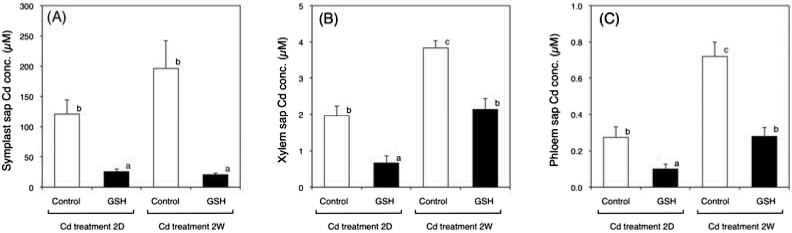
To understand more in detail the effects of GSH in the root zone on Cd behaviour throughout the entire plant, the Cd distribution in the root was investigated. In short-term treatment experiments, Cd concentration in the symplast sap collected from control plants was about 120 µM, whereas that from GSH-treated plants was reduced to about 30 µM (Fig. 2A). A similar trend was observed in the long-term treatment (Fig. 2A). In contrast, Fe, Mn, and Zn concentrations in the symplast sap did not differ significantly by these treatments (Supplementary Fig. S3). To examine the long-distance transport of Cd throughout the plant, xylem and phloem saps were collected from the Cd-treated plants as well as the Cd plus GSH-treated plants. The Cd concentration in the xylem sap from control plants was about 2.0 µM; this concentration was reduced to approximately one-half in GSH-treated plants (Fig. 2B). The concentrations of Fe, Mn, and Zn in the xylem sap were not affected by the GSH treatment (Supplementary Fig. S3). Similarly, Cd concentration in the phloem sap decreased significantly in GSH-treated plants (Fig. 2C), whereas the levels of Fe, Mn, and Zn mostly remained constant; only Fe decreased somewhat after short-term Cd treatment (Supplementary Fig. S3).
Fig. 2.
Cd concentration in the symplast sap (A), xylem sap (B), and phloem sap (C), collected from oilseed rape plants after treatments as indicated. Data are means ± SE (n > 4). Means labelled with different letters are significantly different according to Student’s t-test (P < 0.05).
HPLC analysis of GSH and PCs (PC2, PC3, and PC4) in root tissues
GSH and PCs have effects on the radial transport of Cd in root tissues of plants (Sanità di Toppi and Gabbrielli, 1999). Therefore, the GSH and PC contents in root tissues were determined to investigate the effects of glutathione treatment. GSH and PC levels in root tissues are shown in Table 1. The GSH content was about 0.16 µmol (g FW)–1 in control plants. The GSH content in root tissues increased slightly in response to Cd and glutathione treatment. Biosynthesis of PCs was induced by Cd treatment. PC2, PC3, and PC4 contents in root tissues were about 0.18, 0.27, and 0.18 µmol (g FW)–1, respectively. However, glutathione treatment did not increase the PC content in root tissues; the PC content was slightly lower, but not significantly, when GSH and GSSG were applied to the roots.
Table 1.
Contents of GSH and phytochelatins (PC2, PC3, and PC4) in roots of oil seed rape plants exposed to Cd, GSH, and GSSG.Two-week-old seedlings were transferred to each treatment solution for 2 days. The GSH and PC contents of roots were calculated from results of HPLC analysis and their root freshweights as µmol (g FW)–1. Data are means ± SE (n = 4). –, under detection limit.
| Treatment | GSH | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| Control | 0.16±0.04 | – | – | – |
| 10 µM Cd | 0.18±0.02 | 0.18±0.02 | 0.27±0.03 | 0.18±0.03 |
| 10 µM Cd + 1mM GSH | 0.20±0.02 | 0.13±0.01 | 0.19±0.02 | 0.11±0.02 |
| 10 µM Cd + 1mM GSSG | 0.23±0.01 | 0.13±0.01 | 0.19±0.01 | 0.10±0.01 |
Effect of GSH on Cd efflux from root cells
The effects of GSH in the root zone on Cd efflux from root cells were investigated (Fig. 3). Cd efflux from roots of control plants was about 120 nmol (g FW)–1 (Fig. 3). Meanwhile, that from GSH-treated plants was about 150 nmol (g FW)–1 (Fig. 3). Cd efflux from roots was activated significantly by GSH in the root zone.
Fig. 3.
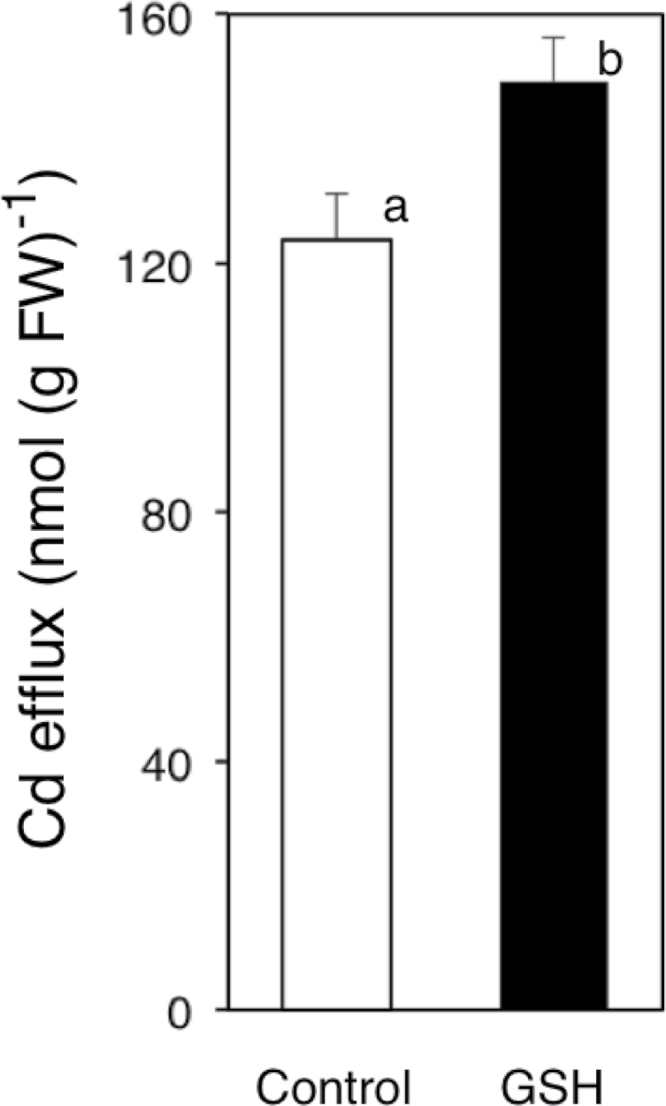
Efflux of Cd from root cells of oilseed rape plants. Cd amount released from root to nutrient solution with or without GSH for 24h was monitored. Experimental results are indicated as the amount of Cd in the nutrient solution normalized by root freshweight. Data are means ± SE (n = 10). Means labelled with different letters are significantly different according to Student’s t-test (P < 0.05).
Analysis of 107Cd absorption, transport, and accumulation by PETIS
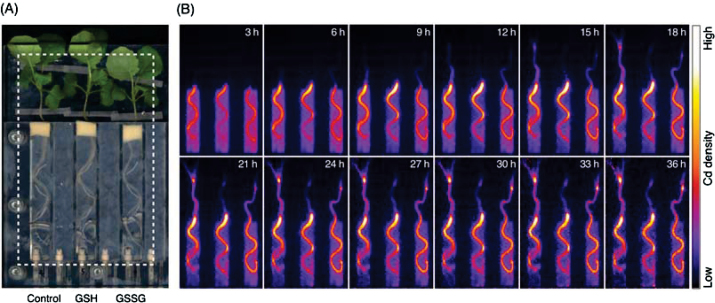
Cd movement in plants was analysed by PETIS. Fig. 4A shows a typical field of view; a representative time series of PETIS images is presented in Fig. 4B. It should be noted that all the 107Cd values and intensities in the graphs and images from PETIS experiments in this paper have already been decay-corrected. That is, the figures directly indicate dynamics of the non-radioactive Cd. 107Cd signals appeared in the shoot base region at 6h after the addition of Cd to the root medium and accumulated gradually in control plants and GSSG-treated plants (Fig. 4B; plants on the left and right in each image). In contrast, no 107Cd signal appeared at 6h in the shoots of plants additionally exposed to GSH (Fig. 4B; plants on the centre in each image). In GSH-treated plants, 107Cd appeared with a delay compared to control plants and plants exposed to GSSG, and the signal remained weaker throughout the period of observation (Fig. 4B). In GSSG-treated plants, 107Cd signal also remained weaker than that of control plants and stronger than that of GSH-treated plants (Fig. 4B). In shoots of control plants and GSSG-treated plants, strong 107Cd signals appeared at the node where petioles occurred. Animation movies of these images are also available in Supplementary Fig. S4.
Fig. 4.
PETIS imaging of transport and accumulation of Cd in oilseed rape plants. (A) Field of view (rectangle) of a representative PETIS experiment. (B) Time series of PETIS images showing the 107Cd signal (0–36h) after decay correction. Each image shown is a composite of 45 original images collected every 4min. All plants are exposed to 107Cd in the root medium; plants on the centre and right were exposed additionally to GSH and GSSG, respectively.
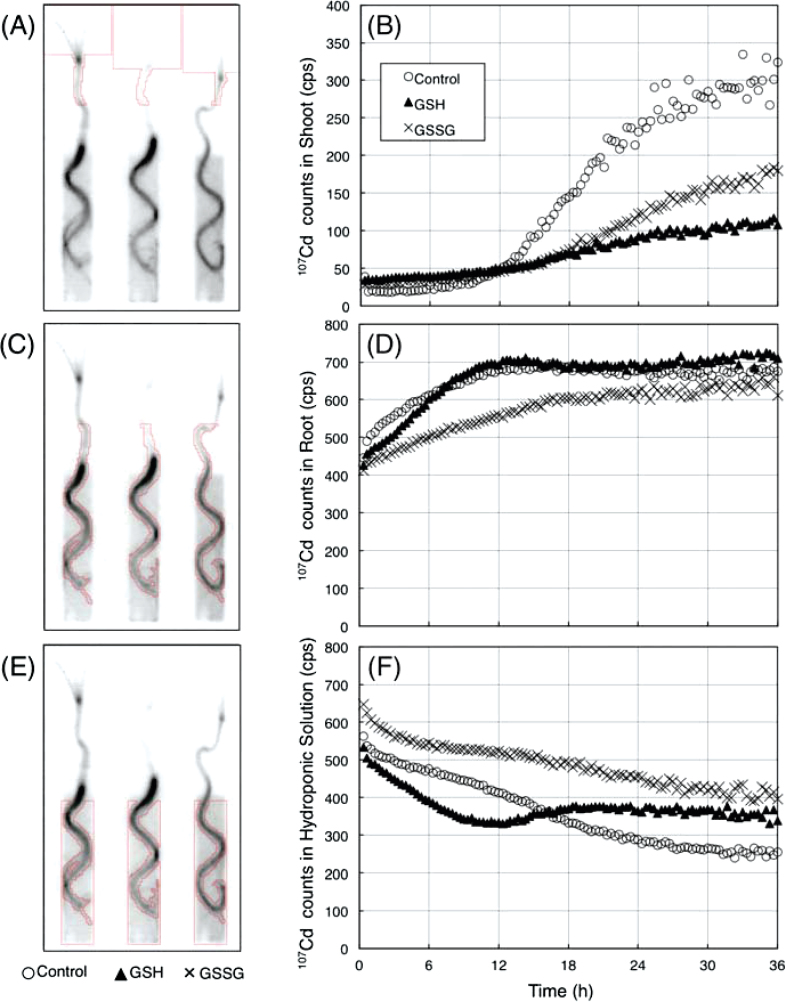
Fig. 5A C, and E show the regions of interest (ROI) to be analysed. Time courses of 107Cd signals were established in the shoot (ROI-1), root (ROI-2) and solution (ROI-3) (Fig. 5B, D, F). In ROI-1 (plant shoots), 107Cd signals in each plant increased gradually until about 10h after 107Cd feeding (Fig. 5B). After that, 107Cd signals in the shoots of control plants increased more sharply than those in glutathione-treated plants (Fig. 5B). In ROI-2 (plant roots), 107Cd accumulated following its addition to the root medium, but less rapidly with GSSG treatment than the control (Fig. 5D). There was no difference in the pattern of 107Cd accumulation in control plant roots and GSH-treated plant roots (Fig. 5C). In these roots, 107Cd signal increased continuously about 12h after Cd feeding. Afterwards, 107Cd signal reached equilibrium (Fig. 5D). In ROI-3 (0.5mM CaCl2 solution without or with GSSG), there was a significant difference in the pattern of 107Cd decrease in the solution. In control plants and GSSG-treated plants, 107Cd signal in the solution decreased continuously (Fig. 5F). However, 107Cd signal in the solution with 1mM GSH shifted to increase at 12–18h and reached equilibrium after the initial decrease (Fig. 5F).
Fig. 5.
Time-course analyses of Cd dynamics in shoot, root, and solution: regions of interest (ROIs) for time-course analyses. (A, C, E) Red squares in each panel indicate ROI-1 (A, shoot), ROI-2 (C, root), and ROI-3 (E, solution). (B, D, F) Time courses of 107Cd signal in ROI-1 (B), ROI-2 (D), and ROI-3 (F). Each graph indicates the intensity of 107Cd signal after decay correction.
Effect of GSSG applied to roots on Cd accumulation
To examine the effects of GSSG applied to roots on Cd accumulation, the Cd contents were also measured in shoots and roots after 2 days (short-term treatment) and 2 weeks (long-term treatment) of Cd exposure. There were no differences in the dryweights of shoots and roots between GSH-treated plants and GSSG-treated plants (data not shown). In the short-term treatment, the Cd content in the shoots of GSH-treated plants was about 0.16 µmol (g DW)–1 (Fig. 6A). The shoot Cd content of plants exposed to GSSG in the root zone was roughly 2-fold higher (Fig. 6A). In the long-term treatment, the Cd content in the shoots of GSH- and GSSG-treated plants was about 0.40 and 0.33 µmol (g DW)–1, respectively (Fig. 6A). Compared to control plants, the Cd contents in shoots decreased significantly after glutathione treatments (Fig. 6A). There was no significant difference in the root Cd content in GSH-treated plants and GSSG-treated plants in short-term and long-term treatments (Fig. 6B). In the short-term treatment, Cd translocation ratios from GSSG-treated plants were a little higher, but not different significantly (Fig. 6C); however, in the long-term treatment, these ratios were nearly the same (Fig. 6C). No inhibitory effects of GSSG on the Fe, Mn, and Zn contents in shoots and roots were found in these experiments (data not shown).
Fig. 6.
Cd contents in the shoots (A), roots (B), and Cd translocation ratio (C) of oilseed rape plants harvested after GSH or GSSG treatments as indicated. Data are means ± SE (n > 7). Means labelled with different letters are significantly different according to Student’s t-test (P < 0.05).
Effects of GSH in the root zone on the Cd distribution at different temperatures
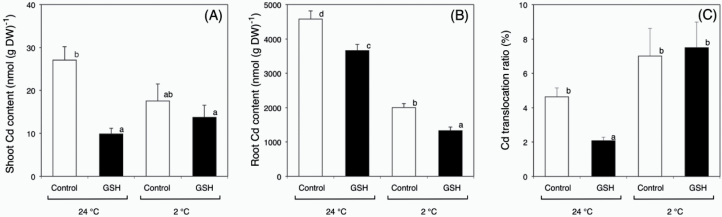
To investigate the effects of chemical interaction between Cd and GSH on Cd accumulation in plants in more detail, plants were treated with Cd and GSH at a low temperature. The shoot Cd content of control plants and GSH-treated plants at 24 °C was about 27 and 10 nmol (g DW)–1, respectively (Fig. 7A). There was no significant difference in the Cd content in shoots of control plants and GSH-treated plants at 2 °C (Fig. 7B). The root Cd content of control plants was significantly higher than that of GSH-treated plants at 2 and 24 °C (Fig. 7B). At 24 °C, Cd translocation ratios from the control plants were roughly 2-fold higher (Fig. 7C); however, at 2 °C, there was no difference in these ratios (Fig. 7C). These results indicated that the inhibitory effects of Cd accumulation in the shoots were abolished by low-temperature treatments.
Fig. 7.
Cd contents in the shoots (A), roots (B), and Cd translocation ratio (C) of oilseed rape plants harvested after different temperature (2 and 24 °C) treatments as indicated. Data are means ± SE (n > 8). Means labelled with different letters are significantly different according to Student’s t-test (P < 0.05).
Discussion
GSH applied to roots inhibits Cd translocation and accumulation
GSH applied to roots inhibited Cd translocation from roots to shoots and Cd accumulation in the shoot (Fig. 1A). On the other hand, GSH applied to leaves did not reduce Cd accumulation in shoots (Fig. 1A). On the contrary, Cd accumulation in the shoot in the long-term Cd treatment was slightly promoted by GSH application to leaves (Fig. 1A). Unlike the experimental results of Cd accumulation in shoots, GSH had no effect on Cd accumulation in roots, regardless of the type of application (Fig. 1B). GSH applied to roots had a significant effect on Cd translocation (Fig. 1C). From these experimental results it is concluded that GSH present in the root zone affects Cd translocation from roots to shoots.
Effects of GSH on Cd behaviour in plants
The Cd concentration in the symplast sap decreased significantly when GSH was present (Fig. 2A). In these experiments, the Cd concentration in the xylem sap was also reduced significantly by GSH treatment (Fig. 2B), suggesting that the amount of Cd loaded into the xylem decreased, thereby reducing Cd accumulation in the shoots of GSH-treated plants. Cytosolic Cd concentrations in roots have a large effect on xylem loading of Cd (Uraguchi et al., 2009; Ishikawa et al., 2011; Ueno et al., 2011). GSH applied to roots might activate some processes in which the intracellular Cd concentration decreases in roots. The Cd concentration in the phloem sap showed a similar trend as that in the xylem sap (Fig. 2C). Thus, it is unlikely that the reduced Cd levels in the shoot observed in this study are due to Cd export from shoots via the phloem.
How is GSH functioning to reduce the amount of Cd in the cytosol of root cells? Currently, two possibilities are seen. (1) Activation of Cd extrusion from root cells. ABC transporters can act as Cd extrusion pumps (Kim et al., 2007). On the basis of in vitro experiments, thiol compounds have been proposed to activate Cd efflux systems in cucumber root cells (Migocka et al., 2011), and it is conceivable that GSH might activate Cd extrusion in roots of intact oilseed rape plants. (2) GSH could promote the sequestration of Cd in the vacuoles of root cells. Cd is detoxified by accumulation and storage in vacuoles (Mendoza-Cózatl et al., 2011). PC, which is synthesized from GSH, is involved in the detoxification process. GSH also may be directly involved in the sequestration of Cd (Li et al., 1997). To determine which of the two possible mechanisms are operative in living plants, this study tried to evaluate Cd efflux from and PC content in root cells.
Effect of GSH on Cd efflux from root cells
Cd efflux from root cells was significantly activated by GSH applied to roots (Fig. 3). In the brassica family, cation efflux transporters have been reported (Xu et al., 2009; Lang et al., 2011). Further studies are needed to identify and characterize Cd efflux transporters that are activated by GSH in the roots of oilseed rape plants.
GSH and PCs in root tissues
To investigate the effects of GSH and PCs on Cd radial transport, the GSH and PC contents in root tissues were measured. The experimental results indicated that the GSH content of root tissues increased slightly by glutathione treatment (Table 1). Synthesis of PCs was induced by Cd treatment. In contrast to the result with the GSH treatment, the PC content of root tissues decreased slightly by glutathione treatment (Table 1). That is, glutathione, applied to roots, does not seem to have any effect on increasing the PC content of root tissues. PCs are related to Cd translocation from roots to shoots (Gong et al., 2003). Therefore, a decrease in the PC levels in root tissues might trigger the inhibition of Cd translocation. A PC transporter, localized in the tonoplast membrane, was recently identified (Mendoza-Cózatl et al., 2010). There remains a possibility that sequestration of PCs into vacuoles is activated by GSH, exceeding the biosynthesis of PCs. It will be necessary to investigate the distribution of Cd, GSH, and PCs in root cells in detail in order to elucidate the molecular mechanisms for the inhibition of Cd translocation from roots to shoots.
Dynamic analysis of Cd translocation to shoots in intact plants
The PETIS method enabled visualization of Cd transport and accumulation in whole plants. The inhibitory effect of GSH treatment on Cd translocation from roots to shoots was clearly observed and provided hints at the effects of GSH on these processes (Fig. 4B). The 107Cd signal accumulated in the nodes of oilseed rape plants (Fig. 4B). 107Cd also accumulated significantly in the shoot bases of rice plants where nodes are assembled (Fujimaki et al., 2010). Therefore, it seems that the nodes, from which vascular tissues branch, play an important role in Cd distribution throughout the entire plant. During the initial 10 hours after 107Cd feeding, there were no significant differences in the pattern of decrease of 107Cd signals in the culture solution of control plants and GSH-treated plants; however, 107Cd signals in the culture solution stopped decreasing and shifted to increasing only in the GSH-treated plants after that (Fig. 5F). Afterwards, the 107Cd signal in this solution reached an equilibrium (Fig. 5F). These experimental results supported the notion that Cd extrusion from root cells was activated by GSH. In GSH-treated plants, a response time of about 10 hours was required to change the pattern of 107Cd accumulation (Fig. 5B, D, F). This long response time supported the hypothesis that some physiological responses to GSH must occur in the roots or shoots of oilseed rape plants. These responses might have a special effect on Cd radial transport in roots of oilseed rape plants.
Effects of chemical binding between Cd and GSH on Cd behaviour in roots
GSH is known to bind Cd directly and form bis(glutathionato)cadmium (Cd-GS2) (Nocito et al., 2007). This Cd-GS2 complex strongly reduces Cd bioavailability. To verify that the reduced Cd accumulation in shoots was caused mainly by physiological functions of GSH and not by chemical binding between GSH and Cd, two experiments were performed. The first was an experiment using GSSG, which is formed by oxidizing GSH. Under these experimental conditions (oxidative conditions), GSSG in hydroponic solution is present without binding Cd. GSSG lacks the ability to bind Cd because GSSG lacks free thiol groups. However, GSSG applied to roots also had an inhibitory effect on Cd accumulation in the shoots (Fig. 6A). Additionally, the effects of GSSG increased as the treatment periods became longer (Fig. 6A). These results demonstrated that glutathione applied to roots triggered some physiological process in plant roots. The results of short-term treatment experiments indicated that there were some differences in the effects of GSH and GSSG on Cd behaviour (Fig. 6A). These differences were also confirmed by PETIS experiments. The 107Cd accumulation pattern in roots differed depending on the presence of GSH or GSSG (Figs. 4 and 5). In future research, it will be necessary to elucidate how GSH and GSSG are metabolized and function in root cells. The second experiment was a low-temperature treatment experiment. In this experiment, a significant difference in the Cd translocation ratio observed at 24 °C disappeared at 2 °C (Fig. 7C). At 2 °C, only physiological processes in plant roots should be suppressed. These experimental results also indicated that GSH triggers some physiological processes. These experimental results demonstrated that inhibition of Cd translocation from roots to shoots is highly dependent on the physiological processes triggered by GSH rather than chemical binding between Cd and GSH.
Effects of GSH on other heavy metal behaviour in plants
ZIP family proteins, which are involved in Cd absorption by roots, are also related to the membrane transport of Fe, Mn, and Zn (Guerinot, 2000). Therefore, the present study examined the effects of GSH on the transport and accumulation of these heavy metals. Intriguingly, the presence of GSH in the root medium had no restrictive influence on the behaviour of other heavy metals in the plant (Supplementary Fig. S2). Fe, Mn, and Zn concentrations in the symplast sap or xylem sap did not change with GSH treatment (Supplementary Fig. S3). These experimental results demonstrated that GSH, applied to roots, selectively inhibited Cd translocation from roots to shoots.
The work reported here demonstrated that GSH, applied to roots, activated Cd efflux from root cells to the outer culture and decreased the level of Cd in the cytosol of root cells, as well as Cd loading into the xylem. Consequently, the Cd content in the shoots selectively decreased about 4-fold lower than that of control plants. The future elucidation of the molecular mechanisms underlying Cd efflux from root cells will establish new agronomic practices without gene manipulation to realize farm products with low Cd contents.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Dryweights of shoots and roots of oilseed rape plants harvested after various treatments.
Supplementary Fig. S2. Fe, Mn, and Zn contents in shoots and roots from oilseed rape plants harvested after various treatments.
Supplementary Fig. S3. Fe, Mn, and Zn concentrations in the symplast sap, xylem sap, and phloem sap collected from oilseed rape plants after various treatments.
Supplementary Fig. S4. Animation movie of transport and accumulation of Cd in oilseed rape plants.
Acknowledgements
We gratefully thank Dr. Ken’ichi Ogawa (Research Institute for Biological Sciences Okayama) for providing us with GSSG and Mr. H. Suto (Tokyo Nuclear Services Co., Ltd) for his technical assistance in producing 107Cd by irradiation. This study was supported in part by the Grant-in-Aid for Scientific Research (no. 19380185, 23380194 to S.N. and no. 17380194, 23380155 to S.F.).
References
- de Knecht JA, van Dillen M, Koevoets PLM, Schat H, Verkleij JAC, Ernst WHO. 1994. Phytochelatins in cadmium-sensitive and cadmium-tolerant Silene vulgaris chain length distribution and sulfide incorporation. Plant Physiology 104, 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimaki S. 2007. The positron emitting tracer imaging system (PETIS), a most-advanced imaging tool for plant physiology. ITE Letters on Batteries, New Technologies and Medicine 8, 404–413 [Google Scholar]
- Fujimaki S, Suzui N, Ishioka NS, Kawachi N, Ito S, Chino M, Nakamura S. 2010. Tracing cadmium from culture to spikelet: non-invasive imaging and quantitative characterization of absorption, transport and accumulation of cadmium in an intact rice plant. Plant Physiology 152, 1796–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J-M, Lee DA, Schroeder JI. 2003. Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis. Proceedings of the National Academy of Sciences, USA 100, 10118–10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML. 2000. The ZIP family of metal transporters. Biochimica et Biophysica Acta 1465, 190–198 [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Suzui N, Ito-Tanabata S, Ishii S, Igura M, Abe T, Kuramata M, Kawachi N, Fujimaki S. 2011. Real-time imaging and analysis of differences in cadmium dynamics in rice cultivars (Oryza sativa) using positron-emitting107Cd tracer. BMC Plant Biology 11, 172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishioka NS, Fujimaki S, Suzui N, Watanabe S, Matsuhashi S. 2006. Production of positron-emitting cadmium tracer for plant study. JAEA Review 2005-001, 277–279 [Google Scholar]
- Ito H, Iwabuchi M, Ogawa K. 2003. The sugar-metabolic enzymes aldolase and triose-phosphate isomerase are targets of glutathionylation in Arabidopsis thaliana: detection using biotinylated glutathione. Plant and Cell Physiology 44, 655–660 [DOI] [PubMed] [Google Scholar]
- Kim D-Y, Bovet L, Maeshima M, Martinoia E, Lee Y. 2007. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. The Plant Journal 50, 207–218 [DOI] [PubMed] [Google Scholar]
- Lang M, Hao M, Fan Q, Wang W, Mo S, Zhao W, Zhou J. 2011. Functional characterization of BjCET3 and BjCET4, two new cation-efflux transporters from Brassica juncea L. Journal of Experimental Botany 62, 4467–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z-S, Lu Y-P, Zhen R-G, Szczypka M, Thiele DÄ, Rea PÄ. 1997. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proceedings of the National Academy of Sciences, USA 94, 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin MJ, Parker DR, Clarke JM. 1999. Metals and micronutrients – food safety issues. Field Crops Research 60, 143–163 [Google Scholar]
- Mendoza-Cózatl D, Loza-Tavera H, Hernández-Navarro A, Moreno-Sánchez R. 2005. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiology Reviews 29, 653–671 [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Jobe TO, Hauser F, Schroeder JI. 2011. Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Current Opinion in Plant Biology 14, 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Zhai Z, Jobe TO, et al. 2010. Tonoplast-localized Abc2 transporter mediates phytochelatin accumulation in vacuoles and confers cadmium tolerance. Journal of Biological Chemistry 285, 40416–40426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migocka M, Papierniak A, Kosatka E, Kłobus G. 2011. Comparative study of the active cadmium efflux systems operating at the plasma membrane and tonoplast of cucumber root cells. Journal of Experimental Botany 62, 4903–4916 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nakamura S, Akiyama C, Sasaki T, Hattori H, Chino M. 2008. Effect of cadmium on the chemical composition of xylem exudate from oilseed rape plants (Brassica napus L.). Soil Science and Plant Nutrition 54, 118–127 [Google Scholar]
- Nakamura S, Maruyama K, Watanabe A, Hattori H, Chino M. 2005. Response of glutathione in the sieve tube of Brassica napus L. to cadmium treatment. In: Saito K, De Kok LJ, Stulen I, Hawkesford MJ, Schnug E, eds, Sulfur transport and assimilation in plants in the post genomic era. Leiden, NL: Backhuys Publishers, pp 229–232 [Google Scholar]
- Nocito FF, Lancilli C, Giacomini B, Sacchi GA. 2007. Sulfur metabolism and cadmium stress in higher plants. Plant Stress 1, 142–156 [Google Scholar]
- Noctor G, Foyer CH. 1998. ASCORBATE AND GLUTATHIONE: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49, 249–279 [DOI] [PubMed] [Google Scholar]
- Obata H, Umebayashi M. 1997. Effects of cadmium on mineral nutrient concentrations in plants differing in tolerance for cadmium. Journal of Plant Nutrition 20, 97–105 [Google Scholar]
- Rauser WE. 1995. Phytochelatins and related peptides (structure, biosynthesis, and function). Plant Physiology 109, 1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Rauser WE. 1995. MgATP-dependent transport of phytochelatins across the tonoplast of oat roots. Plant Physiology 107, 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Smith RD, Raskin I. 1998. PHYTOREMEDIATION. Annual Review of Plant Physiology and Plant Molecular Biology 49, 643–668 [DOI] [PubMed] [Google Scholar]
- Sanità di Toppi L, Gabbrielli R. 1999. Response to cadmium in higher plants. Environmental and Experimental Botany 41, 105–130 [Google Scholar]
- Ueno D, Koyama E, Yamaji N, Ma JF. 2011. Physiological, genetic, and molecular characterization of a high-Cd-accumulating rice cultivar, Jarjan. Journal of Experimental Botany 62, 2265–2272 [DOI] [PubMed] [Google Scholar]
- Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S. 2009. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. Journal of Experimental Botany 60, 2677–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chai T, Zhang Y, Lang M, Han L. 2009. The cation-efflux transporter BjCET2 mediates zinc and cadmium accumulation in Brassica juncea L. leaves. Plant Cell Reports 28, 1235–1242 [DOI] [PubMed] [Google Scholar]
- Yu Q, Tang C, Chen Z, Kuo J. 1999. Extraction of apoplastic sap from plant roots by centrifugation. New Phytologist 143, 299–304 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.