Neuropathy affects up to 50% of people with diabetes and is a major risk factor for foot ulceration and amputation (1). The etiology is multifactorial, and currently there is no satisfactory treatment except maintenance of good glycemic control (2), thereby highlighting the importance of identifying novel therapeutic targets.
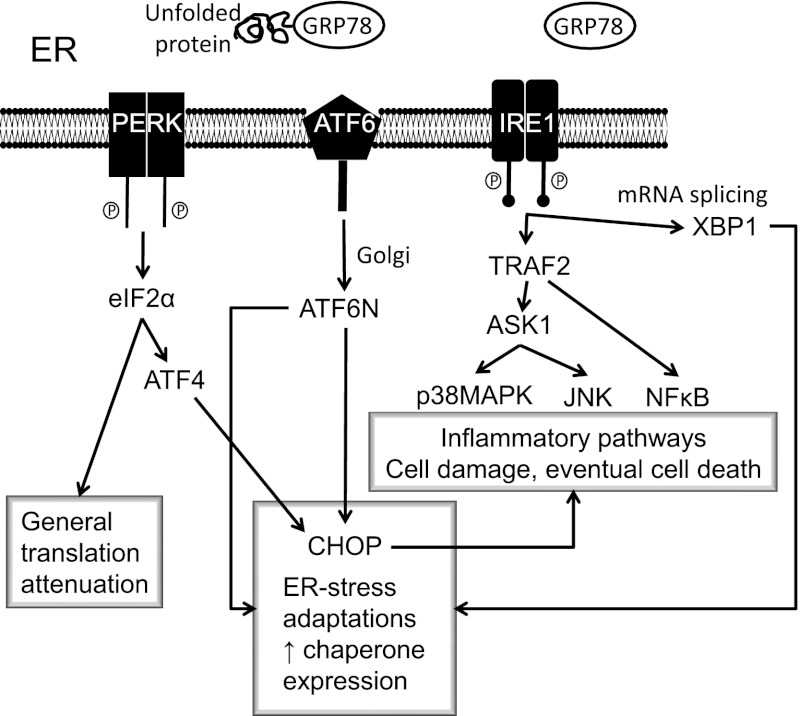
The endoplasmic reticulum (ER) forms a membranous network throughout the cytosol and is contiguous with the outer nuclear membrane. The ER has three major functions: 1) synthesis, folding, and maturation of proteins; 2) Ca2+ storage; and 3) lipid biosynthesis. The ER provides a Ca2+-rich oxidizing environment for the formation of disulphide bonds and accurate protein folding. This process is controlled by Ca2+-dependent molecular chaperones and protein-folding enzymes such as glucose-regulated protein 78/immunoglobulin binding protein (GRP78/BiP), glucose-regulated protein 94, and protein disulfide isomerase. Folding to produce the native protein conformation is a complex, energy-requiring process. This process may be disrupted in pathological conditions, resulting in a buildup of misfolded and potentially toxic proteins that give rise to ER stress. Homeostatic responses to ER stress comprise the unfolded protein response (UPR). The UPR consists of signaling systems (Fig. 1) that are based on three transmembrane stress sensors. 1) The first is PKR-like eukaryotic initiation factor 2A kinase (PERK). Normally, PERK is bound to the chaperone GRP78/BiP, but as unfolded proteins accumulate GRP78/BiP dissociates, thereby activating PERK, which then phosphorylates eukaryotic initiation factor 2α, ultimately leading to general inhibition of protein translation and synthesis. 2) GRP78/BiP also dissociates from inositol-requiring enzyme 1, which mediates mRNA splicing reaction for the transcription factor X box-binding protein 1. In turn, this participates in the increased production of ER chaperones and CAAT/enhancer-binding protein homologous protein (CHOP), which contributes to ER-dependent degradative processes, including cell death if ER stress is severe and prolonged. 3) Activating transcription factor 6 translocates to the Golgi apparatus, producing another transcription factor, activating transcription factor 6N, which stimulates chaperone and protein folding enzyme expression. In addition, the UPR activates proinflammatory pathways based on nuclear factor-κB (NF-κB), p38 mitogen-activated protein kinase (p38MAPK), and c-Jun N-terminal kinase (JNK) signaling. ER stress can profoundly affect cellular signaling and viability and plays a key role in neurodegenerative diseases, ischemia-reperfusion injury, cardiovascular diseases, cancer, and diabetes/obesity (3–5). Within the context of diabetes complications, ER stress has been linked to nephropathy (6), retinopathy (7), endothelial dysfunction (8), and cognitive decline (9).
FIG. 1.
ER stress and the unfolded protein response. When ER function is stressed, for example by impaired Ca2+ homeostasis or accumulation of poorly folded proteins, this causes the UPR. The initial response attempts to restore normal function by halting protein synthesis and increasing chaperone production. ER stress also causes activation of proinflammatory pathways, and if prolonged this can lead to cell damage and apoptotic cell death. ASK1, apoptosis signal-regulating kinase 1; ATF4, activating transcription factor 4; ATF6, activating transcription factor 6; CHOP, CAAT/enhancer-binding protein homologous protein; eIF2α, eukaryotic translation initiation factor; GRP78, glucose-regulated protein 78; IRE1, inositol-requiring enzyme 1; JNK, c-Jun N-terminal kinase; NF-κB, nuclear factor-κB; PERK, PKR-like eukaryotic initiation factor 2A kinase; TRAF2, tumor necrosis factor receptor–associated factor family protein 2; XBP1, X box-binding protein 1.
In this issue of Diabetes, Lupachyk et al. (10) examined the role of ER stress in the development of peripheral neuropathy in streptozotocin-induced diabetic rodents. Two experimental models were used. The first used rats with 12 weeks’ diabetes duration. In these experiments, groups of diabetic and nondiabetic rats were treated with one of two structurally dissimilar chemical chaperones (trimethylamine oxide [TMAO] or 4-phenylbutyric acid [PBA]) to counteract ER stress by promoting normal protein folding (11). Markers of the UPR were examined in sciatic nerve by Western blot. Results showed that diabetes caused upregulation of phospho-PERK, phospho-eukaryotic initiation factor 2α, inositol-requiring enzyme 1, CHOP, GRP78/BiP, and ER oxidase 1α. These results were largely suppressed by TMAO, and no effects were observed in nondiabetic rats. There were similar findings for spinal cord and for treatment with PBA. These data establish the presence of ER stress in neural tissue of diabetic rats. Cellular localization by immunohistochemistry would provide further important information: the data demonstrated that chaperone treatment is an effective strategy and is in line with the literature in other disease models (12) including diabetic nephropathy (13). Importantly, there was no obvious action of the drugs on glycemic control.
Blocking ER stress with chaperones partially protected against the development of decrements in sciatic motor nerve conduction velocity and completely prevented digital nerve sensory nerve conduction velocity deficits in diabetic rats. Behavioral measures of reflex foot withdrawal to potentially noxious stimuli showed reduced thermal and mechanical responses (hypoalgesia), and enhanced sensitivity to touch (tactile allodynia) with diabetes. These abnormal responses were approximately halved by both treatments. Additional dose-response information would be required to assess whether maximal treatment benefits were being achieved in these experiments. In addition, although TMAO prevented intraepidemal nerve fiber loss in diabetic rats, the behavioral results suggest that although distal dying-back of nerves was attenuated, the fibers might not have maintained completely normal function.
The second set of experiments compared neuropathic effects in CHOP−/− mice with those in their wild-type counterparts. Twelve weeks of diabetes doubled CHOP levels in sciatic nerve of wild-type mice, whereas CHOP was undetectable in the transgenic mice. There was a marked attenuation of motor and sensory nerve conduction velocity deficits, thermal hypoalgesia, and intraepidermal nerve fiber loss in the diabetic CHOP−/− mice. Intriguingly, diabetes-induced mechanical hypoalgesia and tactile allodynia remained at similar levels in both CHOP−/− and wild-type mice. This differs from the positive effects of chaperone treatment in rats on these measures, plausibly because different aspects of ER stress and the UPR were targeted.
Interestingly, nerve tissue markers of oxidative/nitrosative stress were elevated by diabetes in both rats and mice and were also markedly attenuated by TMAO treatment or CHOP knockout. This is consistent with findings for diabetic nephropathy (13), in which PBA treatment reduced markers of oxidative stress in kidney, serum, and urine. Upregulation of folding capacity as part of the UPR would increase oxidative stress; hydrogen peroxide is a by-product of S-S bond formation during protein folding (14). As oxidative/nitrosative stress can trigger ER stress, there is an element of positive feedback that could produce a self-reinforcing disease cycle.
Lupachyk et al. (10) have established ER stress as a contributor to diabetic neuropathy and highlight that various elements of the UPR are potentially novel therapeutic targets. Further research is needed to clarify their relative clinical and scientific importance. Moreover, the relevance of the “alarm response” of the UPR, with its accompanying activation of proinflammatory pathways based on NF-κB, p38MAPK, and JNK, and the role of proapoptotic and necrotic cell changes also need to be assessed. NF-κB cascade and p38MAPK inhibition correct measures of diabetic neuropathy in rats (15,16). Given the role of ER stress in cardiovascular disease (5,8) and the contribution of reduced nerve blood flow and endoneurial hypoxia to the pathogenesis of diabetic neuropathy (2,17), further investigation is required to assess whether UPR modulation improves nerve perfusion. Finally, experiments were preventive, and there was incomplete protection of nerve function; it would be of practical interest to ascertain whether ER stress inhibition could reverse established neuropathic changes.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
The author thanks Prof. Mary A. Cotter, University of Aberdeen, for thoughtful comments on the manuscript.
Footnotes
See accompanying original article, p. 944.
REFERENCES
- 1.Boulton AJM. Management of diabetic peripheral neuropathy. Clin Diabetes 2005;23:9–15 [Google Scholar]
- 2.Cameron NE, Eaton SEM, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 2001;44:1973–1988 [DOI] [PubMed] [Google Scholar]
- 3.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev 2006;86:1133–1149 [DOI] [PubMed] [Google Scholar]
- 4.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010;140:900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res 2010;107:1071–1082 [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Zhang R, Torreggiani M, et al. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol 2010;176:2163–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, Zhang SX. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Müller cell-derived inflammatory cytokine production in diabetes. Diabetes 2012;61:492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basha B, Samuel SM, Triggle CR, et al. Endothelial dysfunction in diabetes mellitus: possible involvement of endoplasmic reticulum stress? Exp Diab Res 2012; 2012:481840 [DOI] [PMC free article] [PubMed]
- 9.Sims-Robinson C, Zhao S, Hur J, Feldman EL. Central nervous system endoplasmic reticulum stress in a murine model of type 2 diabetes. Diabetologia 2012;55:2276–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lupachyk S, Watcho P, Stavniichuk R, Shevalye H, Obrosova IG. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes 2013;62:944–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006;313:1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraskiewicz H, FitzGerald U. InterfERing with endoplasmic reticulum stress. Trends Pharmacol Sci 2012;33:53–63 [DOI] [PubMed] [Google Scholar]
- 13.Luo ZF, Feng B, Mu J, et al. Effects of 4-phenylbutyric acid on the process and development of diabetic nephropathy induced in rats by streptozotocin: regulation of endoplasmic reticulum stress-oxidative activation. Toxicol Appl Pharmacol 2010;246:49–57 [DOI] [PubMed] [Google Scholar]
- 14.Higa A, Chevet E. Redox signaling loops in the unfolded protein response. Cell Signal 2012;24:1548–1555 [DOI] [PubMed] [Google Scholar]
- 15.Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets 2008;9:60–67 [DOI] [PubMed] [Google Scholar]
- 16.Price SA, Agthong S, Middlemas AB, Tomlinson DR. Mitogen-activated protein kinase p38 mediates reduced nerve conduction velocity in experimental diabetic neuropathy: interactions with aldose reductase. Diabetes 2004;53:1851–1856 [DOI] [PubMed] [Google Scholar]
- 17.Tuck RR, Schmelzer JD, Low PA. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain 1984;107:935–950 [DOI] [PubMed] [Google Scholar]