Abstract
The aim of this study was to evaluate the pretreatment hepatoprotective effect of the extract of marine-derived fungus Trichurus spiralis Hasselbr (TS) isolated from Hippospongia communis sponge on hepatotoxicity. Twenty-eight male Sprague-Dawley rats were divided into four groups (n = 7). Group I served as −ve control, group II served as the induced group receiving subcutaneously for seven days 0.25 mg heavy metal mixtures, group III received (i.p.) TS extract of dose 40 mg for seven days, and group IV served as the protected group pretreated with TS extract for seven days as a protection dose, and then treated with the heavy metal-mixture. The main pathological changes within the liver after heavy-metal mixtures administrations marked hepatic damage evidenced by foci of lobular necrosis with neutrophilic infiltration, adjacent to dysplastic hepatocytes. ALT and AST measurements show a significant increase in group II by 46.20% and 45.12%, respectively. Total protein, elevated by about 38.9% in induction group compared to the −ve control group, in contrast to albumin, decreased as a consequence of metal administration with significant elevation on bilirubin level. The results prove that TS extract possesses a hepatoprotective property due to its proven antioxidant and free-radical scavenging properties.
1. Introduction
1.1. Heavy Metals as Major Toxicological Problems
A number of trace metals are used by living organisms to stabilize protein structures, facilitate electron transfer reactions, and catalyze enzymatic reactions [1]. For example, copper (Cu), zinc (Zn), and iron (Fe) are essential as constituents of the catalytic sites of several enzymes [2]. However, other metals, such as lead (Pb), mercury (Hg), and cadmium (Cd) may displace or substitute for essential trace metals and interfere with proper functioning of enzymes and associated cofactors. Metals are usually present at low (or very low) concentrations in the oceans [1]. In coastal waters, metals can occur at much higher concentrations, probably due to inputs from river systems [3]. Close to urban centers, metal pollution has been associated with sewage outlets [4, 5].
Metal-induced toxicity is very well reported in the literature [6, 7]. One of the major mechanisms behind heavy metal toxicity has been attributed to oxidative stress. A growing amount of data provide evidence that metals are capable of interacting with nuclear proteins and DNA, causing oxidative deterioration of biological macromolecules [6, 7]. One of the best evidence supporting this hypothesis is provided by the wide spectrum of nucleobase products, typical for the oxygen attack on DNA in cultured cells and animals [7, 8].
Cadmium (Cd) is listed by the US Environmental Protection Agency as one of 126 priority pollutants. The most dangerous characteristic of cadmium is that it accumulates throughout a lifetime. Cadmium accumulates mostly in the liver and kidney, and has a long biological half-life time of 17 to 30 years in humans [9, 10].
Lead is known to induce a broad range of physiological, biochemical, and behavioral dysfunctions in laboratory for animals and humans [11, 12], including central and peripheral nervous systems [13], hemopoietic system [14], cardiovascular system [15], kidneys [16], liver [17], male and female reproductive systems [18, 19].
Mercury is a transition metal. It promotes the formation of reactive oxygen species (ROS) such as hydrogen peroxides. These ROS enhance the peroxides and reactive hydroxyl radicals [20, 21]. Mercuric chloride is an inorganic compound that is used in agriculture as a fungicide in medicine as topical antiseptic and disinfectant and in chemistry as an intermediate in the production of other mercury compounds [22]. Poisoning from environmental sources usually arises from contaminated drinking water as well as plant and animal sourced food products. The metal has been reported to be highly prone to bioaccumulation, leading to biomagnification along the food chain [23]. The absorption, distribution, metabolism, excretion, and toxic dynamics of mercury have been reported to depend on the form and oxidation states [23]. The forms of mercury which are important from a toxicological point of view are elemental (vapor), inorganic salts, and organic salts of mercury. Ingestion of inorganic mercury salts such as mercuric chloride had been reported to cause mainly severe gastrointestinal irritation and renal failure [24]. The toxic effects of an organic and elemental mercury have also been widely reported [25]. Several epidemiologic studies had been conducted on the exposure of humans to mercury through fish and marine mammals' consumption in different geographical areas [26].
Cobalt and nickel are essential trace metals in the human diet. Also, they are major components of the alloys employed in the plate and screw used for connecting bones in orthopedic surgery and in the manufacture of artificial organs [27].
Cobalt is also used as coloring agents for pottery, ceramics, and glass. However, excessive amounts of these transitional metal ions are toxic. For example, cobalt and nickel salts have been reported to induce convulsions [28]; and to cause DNA strand breaks [29]; and to be organ toxic [30]. Cobalt salts are thought to promote the oxidation of reduced glutathione [31] to produce the reduction on a number of hepatic hem proteins such as cytochrome P450, and to interfere with heme metabolism by accelerating its breakdown and inhibiting its synthesis [32]. In addition, numerous authors have studied the impact of nickel on health. Nickel can cause dermatitis to certain persons [33]. Particles of nickel may cause some morphological transformations in numerous cellular systems and chromosomal aberrations [34]. Cobalt was also found obviously harmful on the prenatal development of mice, rats, and rabbits [35].
Nickel breaks down the immunity by affecting the T-cell system and suppresses the activity of natural killer cells in rats and mice [36, 37]. Nickel has been shown to interact with a number of trace elements that include iron, zinc, copper, manganese, sodium, and potassium [38, 39]. Nickel mobilizes and promotes the excretion of copper, zinc, and manganese from organs and promotes storage of chromium in organs [40].
The salts of nickel as particles of nickel can be allergens and carcinogens in man while forming the oxygenated radicals [41]. This cytotoxicity was investigated in numerous microorganisms [42]. Nickel was also found to be responsible for many sexual disorders [43].
1.2. Marine Natural Product as Potent Detoxifier Agent
Over the last forty years, sponge (phylum Porifera) has been identified as an excellent source of unique marine natural products, having a high incidence of biologically active compounds than any single marine phylum [44]. The exploration of microorganisms living inside invertebrates is one of the most exciting strategies to solve the pressing supply issue inherent to marine drug discovery. Marine microorganisms, including fungi, have shown to be the potential source of pharmacologically active metabolites, because of their capability to adapt and survive in the marine environment, and to produce unique secondary metabolites [45].
Fungi are known to tolerate and detoxify metals by several mechanisms, including valence transformation, extra- and intracellular precipitation, and active uptake [46, 47]. The high surface to the volume ratio of microorganisms and their ability to detoxify metals are among the reasons that they are considered as a potential alternative to synthetic resins for remediation of dilute solutions of metals and solid wastes [48, 49].
Metal resistance is defined as the ability of an organism to survive metal toxicity by means of a mechanism produced in direct response to metal species concerned. Biological mechanisms implicated in fungal survival include extracellular precipitation, complexion and crystallization, transformation of metals, biosorption to cell wall and pigments, decreased transport or impermeability, efflux, intracellular compartmentation, and sequestration [47, 50–54].
2. Materials and Methods
2.1. Materials
2.1.1. Sampling
Samples of honeycomb sponge (Hippospongia communis) were collected from the Egyptian western region of the Mediterranean Sea (from Sidi-Krir to El-Salloum) by dragging ships.
2.1.2. Isolation of Sponge-Derived Fungi
To get rid of nonspecific fungal propagules from seawater column on sponge and jellyfish surfaces, animal tissues were rinsed three times with sterile seawater. The surface of the sample was disinfected with 70% ethanol for 2 minutes. The inner tissue was taken out with a scalpel and forceps and then cut into small cubes approx. 0.5 cm3. A total of 15–20 cubes of each sample were placed on isolation media.
All isolation and culture maintaining media for marine taxa were prepared by sea water (SW) and isolation media basically were supplemented with Rose bengal (1/15,000) and chloramphenicol (50 ppm) for suppression of bacterial growth. Five media were adopted for isolation after Atlas (2004) they were: Sea Water Rose bengal Chloramphenicol Agar (SWRCA), Sea Water Czapeks Yeast Extract Agar (SWCYA), Sea Water Oatmeal agar (SWOA), Sea Water Agar (SWA), and Sea Water Potato Dextrose Agar (SWPDA).
For maintaining cultures and for proper identification, pure cultures of isolated fungi were grown on standard media such as Vegetable Agar (V8), Oatmeal Agar (OA), Malt Extract Agar (MEA), Potato Dextrose Agar (PDA), and Potato Carrot Agar (PCA).
2.1.3. Identification of Isolates
Taxonomic identification using morphology characteristics of fungal isolates down to the species level on standard media was mainly based on the following identification keys: Raper and Thom [55], Pitt [56] for Penicillium (on Czapek Yeast Extract Agar (CYA) and Malt-Extract Agar (MEA)); Raper and Fennell [57] for Aspergillus (on Czapek Agar (CZ)); Ellis [58, 59] for dematiaceous hyphomycetes (Potato Carrot Agar (PCA)); Booth [60] for Fusarium (Potato Dextrose Agar (PDA)); von Arx [61], Domsch et al. [62], Watanabe [63] for miscellaneous fungi (on MEA, PDA, CYA); von Arx et al. [64] and Cannon [65] for Chaetomium (Oat Meal Agar + Lupin Stem (OA + LUP)). The systematic arrangement follows the latest system of classification appearing in the 10th edition of Ainsworth and Bisby's Dictionary of the fungi [66] and Species Fungorum website (http://www.speciesfungorum.org/Names/Names.asp).
2.2. Preparation of Marine Fungus Extract
Regarding fungal diversity of Hippospongia communis a total number of 18 taxa were encountered which only one ascosporic species were recorded. Trichurus spiralis Hasselbr. has been selected as a promising taxon for in vitro and in vivo biochemical assays. Preparative-scale production (0.5 L) was carried out in 1 L Erlenmeyer flasks contained potato dextrose extract (Difco) for 2 weeks at 28°C in a shaking incubator at 102 rpm. Pellets were homogenized and centrifuged by using cooling centrifuge at 8000 rpm for 2 min at 4°C. Resultant mixtures were extracted with ethyl acetate (1 × 50 mL), the organic fractions were combined, and the solvent removed at reduced pressure and 35°C. Residues were redissolved in DMSO for further bioassay. The steps of isolation and extraction of fungi and secondary metabolites are shown in Figure 1.
Figure 1.
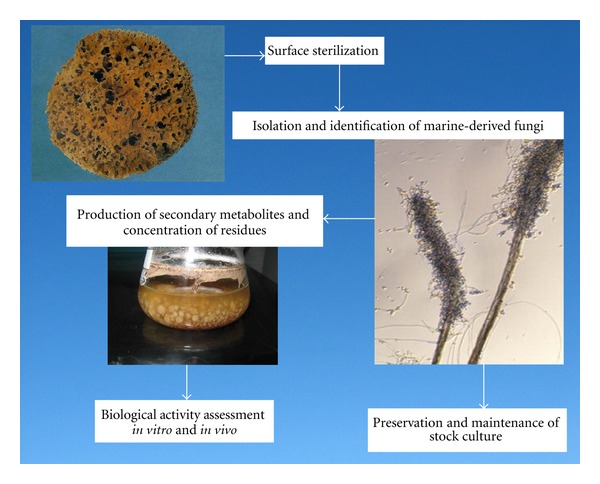
Schematic overview on important steps involved in the isolation of fungi from marine sponge and in preparation of their secondary metabolites.
2.3. Animals and Treatment
A total number of 28 adults white male, Sprague Dawley rats, weighing from 100 to 120 g, were obtained from the animal house of the Faculty of Veterinary Medicine, Assiut University. They were kept in plastic cages, each cage containing five animals. They were maintained under standard laboratory conditions of temperature (about 33 ± 3°C), humidity (20 ± 2%), and duration of light (7:00 a.m. to 7:00 p.m.)/dark (7:00 p.m. to 7:00 a.m.) cycles and were fed on standard rodent chow, with water provided ad libitum. After 1 week of acclimatization to the laboratory environment, the rats were divided into the following groups:
-
Group I: received saline solution subcutaneously for one week and served as the negative (–ve) control group.
-
Group II: received subcutaneously for one week; 0.25 mg /100 gm body weight/day of the heavy metal mixture (Ni, Cd, Co and Hg chloride and Pb acetate) and served as an induced toxicity group.
-
Group III: received intraperitoneal (i.p); 40 mg/100 g/body weight/day, of Trichurus spiralis extract (the most effective fungal extract) dose for one week and served as a positive (+ve) control group.
-
Group IV: received i.p dose of Trichurus spiralis extract as in group III (40 mg/100 g/body weight/day), for one week as a protection dose before administration of heavy metal mixture (Ni, Cd, Co and Hg chloride and Pb acetate) dose as mentioned in group III. This group served as a protective group.
2.4. Biochemical Profiling for Fungus Extract
2.4.1. Elemental Analysis of Trichurus spiralis Extract
The fungal extract subjected to elemental analysis instruments to determine hydrogen, carbon, nitrogen, and sulfur ratio (Elemental analysis CHNS elementary, Vario EL III, Germany).
2.4.2. Determination of Total Phenolic Content in (T.S) Fungus Extract
Total phenolic compounds in the fungal extract were determined by the method of [67].
2.4.3. Determination of Total Flavonoid Content in (T.S) Fungus Extract
Total flavonoid content was determined by a colorimetric method of [68].
2.4.4. Diphenyle-α-Picrylhydrazyl (DPPH) Radical Scavenging Effect of (T.S) Fungus Extract
DPPH radical scavenging assay of the total extract was performed by using the previously established modified methodology of [69, 70]:
| (1) |
2.4.5. Determination of Thiobarbituric Acid Reactive Substance Method Using TBARS Assay for (T.S) Fungus Extract
The method used was adapted from [71] and modified by K.M. Fisch:
| (2) |
2.5. Biochemical Assay in Serum
The appropriate kits (Bio diagnostic kits) were used for the determination of serum total protein according to [72], aminotransferase activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) according to [73]. Determination of albumin level was determined according to the method of [74]. Determination of total bilirubin was determined by the method described by [75].
2.6. Histopathology
The fixed liver tissues in formalin were dehydrated in ascending grades of alcohol, then cleaned by immersing the tissues in xylene for 1 h (three times), followed by impregnation in melted paraffin, in wax, then in oven at 60°C for 1 h. The specimens were embedded in paraffin and were left to solidify at RT. Using a rotatory microtone, sections of 5 μm thick were cut and mounted on clean glass slides. Sections were stained with hematoxylin and eosin (H&E) and examined for any histopathological changes [76].
2.7. Statistical Analysis
The data were given as individual values and as means (X) ± standard deviation (SD) for 7 animals in each group. Comparisons between the means of various treatment groups were analyzed using least significant difference (LSD) test. Differences were considered significant at P < 0.05. All statistical analyses were performed using the statistical software SPSS, version 11.5.
3. Result
The biochemical profile for Trichurus spiralis fungus extract show the higher ratio of sulfur content in the elemental analysis as shown in Figure 2, where the flavnoids content in fungal extract is higher than phenolic as shown in Figure 3. The antioxidant capacity using DPPH assay and inhibition of lipid peroxidation using TABRS in vitro show higher antioxidant capacity by 85% and 78.80%, respectively, as shown in Figure 4.
Figure 2.
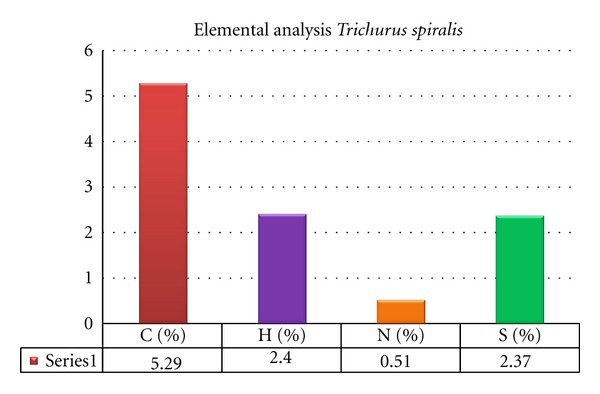
The elemental analysis of Trichurus spiralis extract as percentages.
Figure 3.
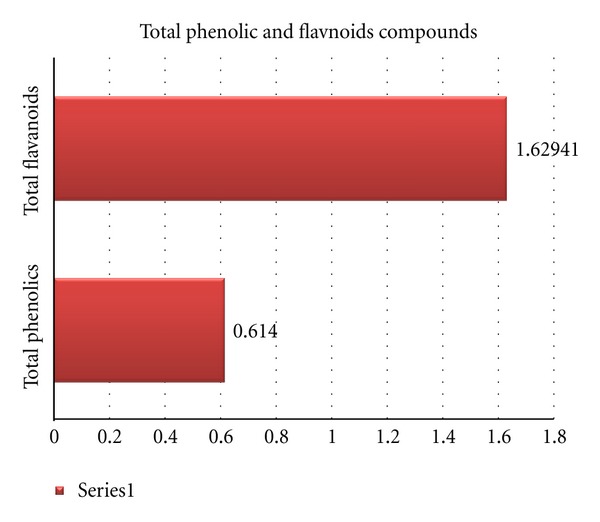
Total phenolic and flavnoids content of Trichurus spiralis extract.
Figure 4.
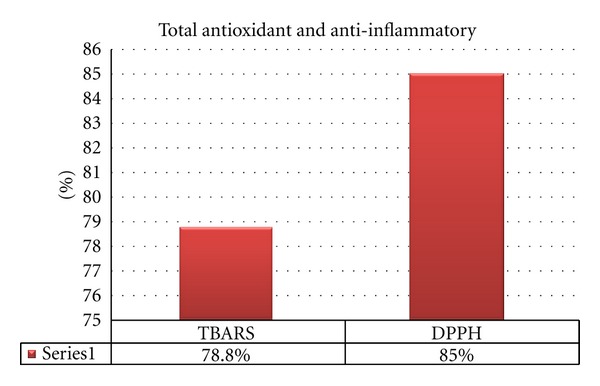
Total antioxidant capacity of Trichurus spiralis extract (6 mg/mL). (The percent of Trichurus spiralis inhibition to word oxidative stress and lipid peroxidation in vitro).
3.1. Mortality Rate
The courses of mortality rate for each group are shown in Figure 5. In the group II (induction group), four of 12 animals died by day 6: two of them died within the first 48 h, and the two others died by 72 and 144 h following induction of heavy metal mixtures. In the group IV (protection group), two rats died in first 72 h (after marine fungal extract and heavy metal administration), where the third died by 120 h. although, there were no differences at the other two groups, group I (−ve control) and group III (+ve control).
Figure 5.
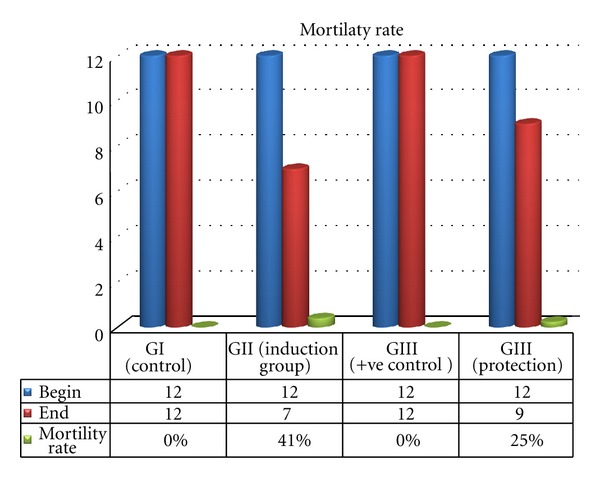
Mortality rat for each group during the study course.
3.1.1. Histological Findings
The results of rats liver histopathological studies are shown in Figures 6, 7(a)–7(h), 8 and 9 for the induced toxicity group (Group II) compared to other groups (I, III, and IV). The figures showed that group I (−ve control), and group III showed normal liver with no remarkable pathological changes in (Figures 6 GI and 8 GIII). The rat's liver of individual induced toxicity group, group II (treated with the heavy metal mixture, 0.25 mg/100 gm b.wt/7 day), showed different proportional to histopathological changes appeared as an extensive loss of hepatic architecture and large amount of damage as shown in Figures 7(a)–7(h) and summarized in Table 1.
Figure 6.
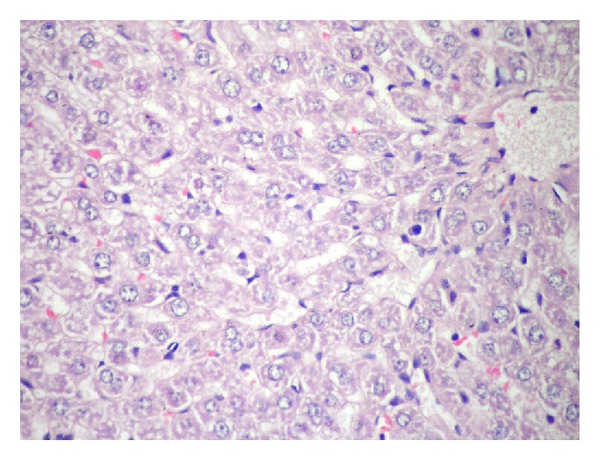
The normal liver showing hepatic architecture formed of cords of hepatocytes separated by hepatic sinusoids (H&E 400x).
Figure 7.
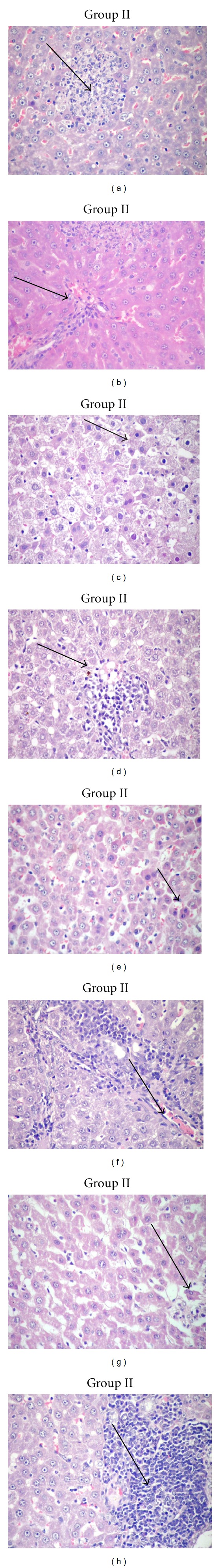
((a) and (b)) Light microscopic observations on the histological liver structures of two individual rats from induced toxicity group (group II (a) and (b)). ((c) and (d)) Light microscopic observations on the histological liver structures of two individual rats from induced toxicity group (group II (c) and (d)). ((e) and (f)) Light microscopic observations on the histological liver structures of two individual rats from induced toxicity group (group II (e) and (f)). ((g) and (h)) Light microscopic observations on the histological liver structures of two individual rats from induced toxicity group (group II (g) and (h)).
Figure 8.
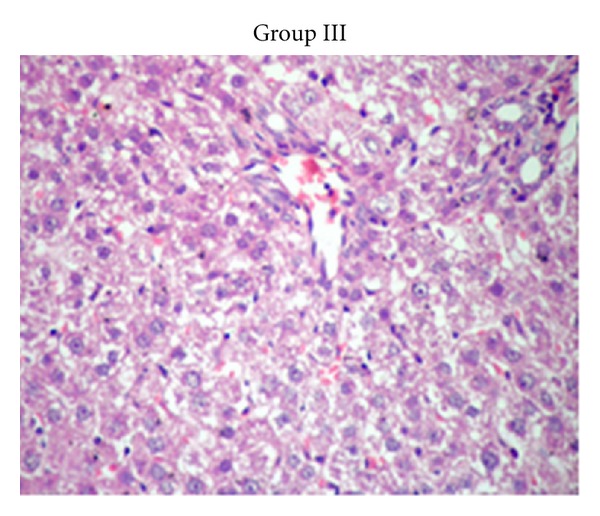
The liver showing normal hepatic architecture (H&E 400x).
Figure 9.
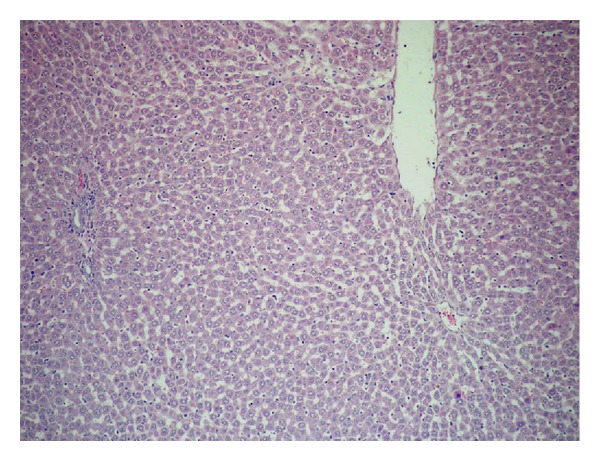
The liver showing preserved hepatic architecture with, mild portal inflammatory infiltrate and frequent apoptotic figures, group IV. (H&E 200x).
Table 1.
Livers of rats in the −ve control group showed normal histopathological appearance, where livers of rats treated with heavy metal mixtures that showed many histopathological changes are listed in the following table.
| Figure | Histopathological change |
|---|---|
| (a) | Marked hepatic damage evidenced by foci of lobular necrosis with neutrophilic infiltration, adjacent to dysplastic hepatocytes, congested sinusoids, and frequent apoptotic nuclei |
| (b) | Marked large cell dysplasia of hepatocytes with focal necrosis and mild portal inflammatory infiltrate |
| (c) | Liver showing hydro pic changes in hepatocytes, and moderate portal lymphoplasmacytic infiltrate |
| (d) | Liver showing marked parenchymal hydro pic changes with apoptosis and adjacent regenerative hepatocytes with binucleated cells |
| (e) | Liver showing degenerative changes with frequent apoptotic cells at the same time evidence of beginning regeneration is seen with the appearance of binucleated cells |
| (f) | Section in the liver showing moderate portal lymphoplasmacytic infiltration with mild interface hepatitis. (H&E 400x) |
| (g) | Section in the liver showing intense heavy portal lymphoplasmacytic inflammatory infiltrate and dyspalstic changes of hepatocytes. (H&E 400x) |
| (h) | Liver showing coagulative necrosis and karyopickosis of the hepatocytes together with evidence of regeneration, multinucleated |
Some animals with the group IV, protected group (treated with fungal extract prior to their treatment by the heavy metal mixture), showed normal hepatic architecture, while others showed preserved hepatic architecture with mild portal inflammatory infiltrate and frequent apoptotic (Figure 9 G IV).
The Effect of Trichurus Spiralis Extract on the Activities of Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) in the Serum of Rats, Treated with Heavy Metal Mixture (Induced Toxicity)
Levels of both ALT and AST in the group IV showed nonsignificantly increase and /or decrease when compared to their corresponding values either of group, I or group III (positive control group), as shown in Table 2 and Figures 10 and 11, respectively.
Table 2.
Effect of Trichurus Spiralis extract on the activities of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
| Group | ALT (U/mL) Mean ± SD |
P 1 | AST (U/mL) Mean ± SD |
P 1 |
|
| ||||
| I (−ve control group) | 80.91 ± 8.35 | 85.32 ± 8.16 | ||
| II (induced group) | 119.28 ± 26.58 | <0.001* | 117.79 ± 26.56 | 0.003* |
| III (+ve control group) | 75.18 ± 8.04 | 0.471 | 78.05 ± 13.54 | 0.470 |
| IV (protected group) | 65.45 ± 3.85 | 0.060 | 63.37 ± 20.54 | 0.037* |
Number of rats for each group = 7, P 1: P value of LSD test between −ve control group and other groups.
Figure 10.
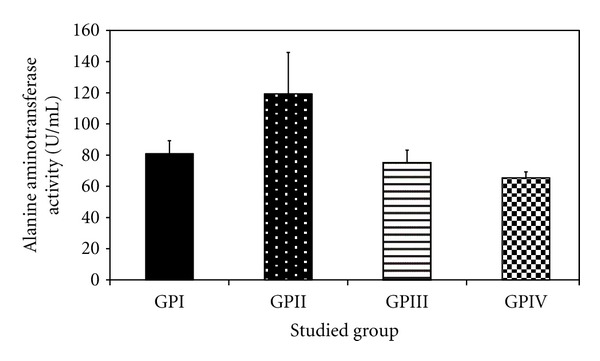
Effect of Trichurus spiralis extract on the activity of alanine aminotransferase (ALT, mean ± SD) in rat sera of induced toxicity group comparing to other groups.
Figure 11.
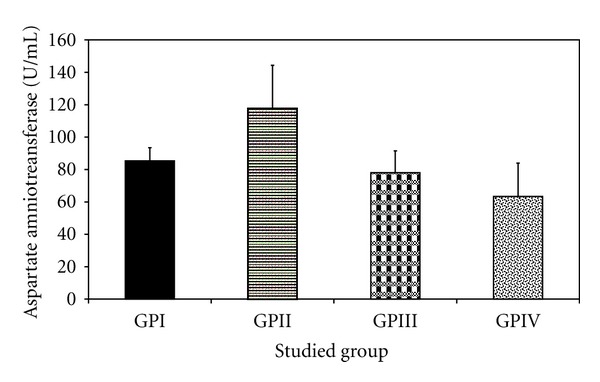
Effect of Trichurus spiralis extract on the activity of aspartate aminotransferase (AST, mean ± SD) in rat sera of induced toxicity group comparing to other groups.
In rats treated with the heavy metal mixture in group II, the activity of serum ALT (119.28 ± 26.58 U/L, P < 0.001) and AST (117.79 ± 26.58 U/L, P = 0.003) were significantly increased than that of −ve control group (group I) rats (85.32 ± 8.16), respectively. In contrast, the fungus extract pretreated group (group IV) at 40 mg/100 g b.wt/day for 7 days had a significantly lower ALT (65.45 ± 3.85) and AST (63.37 ± 20.54), when compared to group II, at P < 0.003.
The Effect of Trichurus Spiralis Extract on the Levels of Total Protein, Albumin and Bilirubin
The levels of total protein and total bilirubin are found to be significantly increased in the heavy metal mixture treated group (group II) comparing to their corresponding values of –ve control group (group I) by about 38.9% and 20 times, respectively. The albumin value in group II showed a nonsignificant decrease by 14.16% compared to group I (−ve control group).
Administration of fungus extract prior to heavy metal mixture injection (group IV) showed a significant decrease in total bilirubin, compared to group II by 87.3% (0.12 ± 0.05 versus 0.95 ± 0.5 g/dL, P < 0.001), while the level of total protein shows a non-significant decrease compared to −ve control group. Also it showed a nonsignificant increase in albumin level between group IV and group I.
The levels of total protein, total bilirubin and albumin in group I, III, and IV showed a non-significant increase and/or decrease, when compared to each other, at P < 0.05, as shown in Table 3 and Figures 12, 13, and 14, respectively.
Table 3.
Effect of Trichurus spiralis extract on the serum levels of total protein, albumin, and total bilirubin of induced toxicity groups of rats.
| Mean ± SD Groups |
I (−ve control group) |
II (Induced group) |
III (+ve control group) |
IV (Protected group) |
|---|---|---|---|---|
| Total protein (g/dL) | 4.60 ± 1.67 | 6.39 ± 1.06 | 5.65 ± 1.08 | 5.53 ± 0.56 |
| P 1 | 0.008* | 0.101 | 0.146 | |
| Albumin (g/dL) | 3.46 ± 0.30 | 2.97 ± 0.40 | 5.92 ± 1.87 | 3.89 ± 0.50 |
| P 1 | 0.369 | <0.001* | 0.436 | |
| Total biluribin (g/dL) | 0.04 ± 0.04 | 0.95 ± 0.50 | 0.04 ± 0.02 | 0.12 ± 0.05 |
| P 1 | <0.001* | 0.992 | 0.571 |
Number of rats for each group = 7, P 1: P value of LSD test between control group and other groups.
Figure 12.
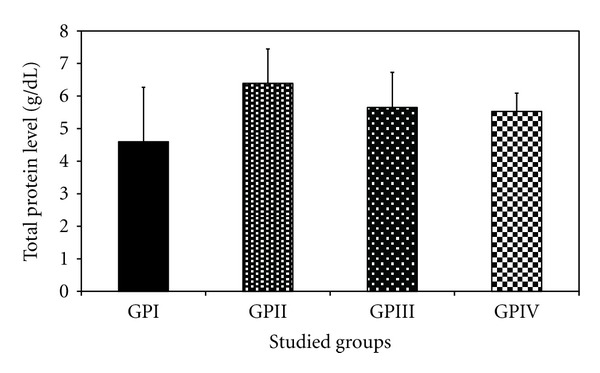
Effect of Trichurus spiralis extract on the level of total protein (mean ± SD) in rat sera of induced toxicity group compared to other groups.
Figure 13.
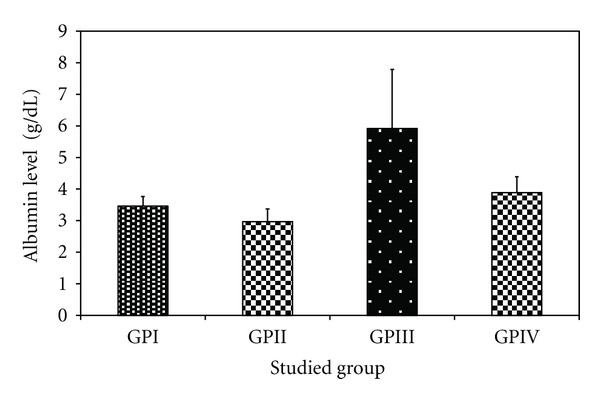
Effect of Trichurus spiralis extract on the level of albumin (mean ± SD) in rat sera of induced toxicity group compared to other groups.
Figure 14.
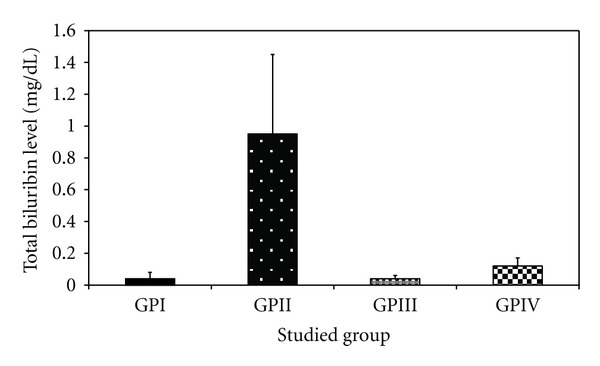
Effect of Trichurus spiralis extract on the level of total bilirubin (mean ± SD) in rat sera of induced toxicity group compared to other groups.
4. Discussion
Liver damage mainly occurs due to excessive alcohol consumption, viral infections; and as a consequence of drug adverse effects. Nowadays, liver diseases constitute a major medical problem of worldwide proportions [77, 78].
There are approximately 35 heavy metals in our environment. Heavy metals become toxic when they are not metabolized, which allows them to accumulate in several organs leading to tissue damage due to their toxicity [79, 80]. According to ASTDR (2005–2007), the most known pollutants in our environment are Cd, Co, Hg, Ni, and Pb. On the other hand, liver tissues are the factory of biological metabolism in mammals and act as the master player in the detoxification process. Liver tissues are also a victim for heavy metal toxicity. So, our in vivo study are designed to investigate the hepatoprotective effect of trichuris extract isolated from the marine sponge, against the heavy-metal mixture of Cd, Co, Hg, Ni chloride, and Pb acetate.
Hepatic system is the major organ system involved in metabolism, detoxification, and excretion of various endogenous and exogenously administered/ingested substances, like xenobiotics, pollutants, and so forth [81]. The physiological activity in the liver results is due to to the generation of highly reactive free radicals; which covalently bonds with membrane lipids, causing lipid preoxidation. Lipid preoxidation alters the membrane permeability and causes tissue damage [81]. Since the liver is involved in various biochemical reactions; it is prone to be attacked by the free radicals and cell necrosis resulted. However, inbuilt antioxidant systems like superoxide dismutase (SOD), reduced glutathione (GSH), and so forth protect the tissue from free radical attack [12]. Excessive release of ROS powers over this system resulted in organ damage. Strengthening of inbuilt protective mechanisms or exogenous administration of antioxidants may be useful in the protection of the organs from ROS damage. In spite of phenomenal growth of the allopathic system of medicine, synthetic antioxidant/organs protectants are not available. Hence, researchers worldwide are engaged in searching for organ protective, such as hepatoprotectants drugs from natural origin [82–84].
Natural products are of considerable importance for the discovery of new therapeutic agents [85]. Apart from plants, bacteria and fungi are the most important producers of such compounds [86]. For a long time neglected as a group of producers of natural products, marine microorganisms have more recently been isolated from a variety of marine habitats such as sea water, sediments, algae, and different animals, to discover new natural products [87, 88]. In particular, sponges which are filter feeders and accumulate high numbers of microorganisms have attracted attention [89, 90]. Consistently, fungi isolated from sponges account for the highest number (28%) of novel compounds reported from marine isolates of fungi [45]. Marine isolates of fungi evidently are a rich source of chemically diverse natural products, which have not been consequently exploited so far [91].
Fungi, like all living organisms, have evolved a set of mechanisms that control and respond to the uptake and accumulation of heavy metals. Possible interactions between toxic metals and fungi include: (a) production and secretion of organic acids, polysaccharides, melanin, or proteins and subsequent binding/complexion and/or precipitation of metal ions [47, 92–94], (b) metal binding to cell walls [95], (c) transport of metal cations [95–97], (d) chemical transformation of metals [47], (e) organelle compartmentation [95–97], and (f) synthesis of thiol-containing compounds, such as the non-proteinacious glutathione, phytochelatins, and the metallothioneins proteins of families 8–13 (fungi I–VI MTs), which can sequester metal ions [47, 98–101]. Among microorganisms, fungi biomass is known to possess excellent metal-binding properties which offer the advantages of having the high percentage of cell wall material [102].
Metal-induced toxicity is very well reported in literatures [6, 12]. These metals generate reactive species, which in turn may cause neurotoxicity, hepatotoxicity and nephrotoxicity in humans and animals [8, 103]. The aminotransferase are intracellular enzymes, which are active in the operating the reversible exchange of amino acids between alpha-amino and alph-keto acids. As all the naturally occurring amino acids can undergo aminotransferase reaction, this class of intracellular enzyme (aminotransferases) represent an important link between protein and carbohydrate's metabolism. It is now well authorized that the liver has an important function through the regulation of trace element metabolism [104, 105]. Further trace elements serve as cofactors for many enzymes in numerous metabolic pathways, therefore, changes in the distribution of these essential and toxicological consequences with regard to the metabolism of other metals [105, 106]. Those metals which are essential for maintenance of the structural and functional integrality of the living organisms are found in all living systems and are conserved within strict concentration limits in the systems [107].
However, imbalance in the supply of any of these essential elements in the body can have both nutritional and toxicological consequences with regard to the metabolism of other metals. They can further be responsible for the development of clinical signs of trace elements deficiencies or can modify the susceptibility to metal toxicity [108]. It will insinuate that metals which have similar chemical, and physical properties, would often interact biologically and antagonize or embellish each other's function [109]. That is also confirmed in our study on the activity of aminotransferases, total protein, albumin, and bilirubin, which is confirmed by histopathology of liver as shown in Tables 2 and 3 and Figures 10, 11, 12, 13, and 14.
ALT and AST in this study show a significant increase as the consequence of heavy-metal administration in group II (induction group) by 46.20% and 45.12%, respectively. This result in agreement with previous studied, which carried on exposure to mercury chloride [110–114], and Ni [115]. Furthermore, this agrees with several investigators which reported that Cd and Pd administration increase aminotransferase, especially ALT, as a result of the necrotic lesion in the liver [114, 116, 117]. As seen in Figure 7(b), on the other hand, lead overloads stimulated oxidative damage in the liver tissue by causing oxidation of lipid. These enzymes cause liver injury [118]; this was confirmed in this study by the histopatholgical result of liver tissue induced by the heavy metal mixture alone (group II) as seen in Figures 7(a)–7(h).
Total protein, is elevated by about 38.9% in blood serum induction group compared to control group (−ve group). This observation may be as a result of the injury inflicted on the liver; thereby making the proteins synthesized in the liver and spill out into the blood [116]. Also, this result is compatible with [119, 120]. Possible explanation for protein elevation is due to toxic insult of mercury that leads to induce a number of stress proteins [119, 120]. These large groups of proteins include heat shock proteins (HSPs) and glucose regulated proteins (GRPs). As reported in [120], an enhanced de novo synthesis of several stress proteins when chick embryos, were exposed to mercury.
In contrast, albumin and protein are predominately produced within the liver, decreased as a consequence of metal elevation. This suggests that the heavy metals, like cadmium and lead, occurs when present in toxic concentrations in the system, impair the protein synthesis in liver [116].
Bilirubin is also regarded as a member of an antioxidant family even if it is known to have toxic effects at high concentration [121, 122]. Bilirubin has been regarded for many years as cytotoxic, mainly because of its associations with neonate jaundice at high concentrations [123]. It is only since the early 1990s that a physiological role for bilirubin as potent antioxidant has emerged. Reference [124] noted that bilirubin possesses strong antioxidant potential against peroxyl radicals. However, high level bilirubin may exacerbate oxidative stress [122]. Reference [125] showed that the increase of bilirubin formation due to activation of HO-2 (constitutive isoform of HO) protects against hydrogen-peroxide-induced neurotoxicity. It has been also demonstrated that intracellular bilirubin concentrations can be locally and temporarily increased by induction of HO-1 (inducible isoform of HO) or rapid activation of HO-2, so as to resist short- and long-lasting oxidative stress [123].
It has been proposed that the specific induction of HO-1 by various forms of oxidative stress, for example, different heavy metals, CCL4, and aminoacetophenone, was part of the defensive mechanism mounted by cells against stress injury, to decrease the levels of potential pro-oxidants and to increase the concentrations of active bile pigments that can act as antioxidants [126, 127]. HO-1 upregulation is followed by an increased bilirubin production, altogether determining the adaptive response of cells to oxidative stress [126].
Some natural antioxidant products have been shown to protect cells from oxidative injury [128], the high antioxidant capacity of fungal extract as shown in Figure 4, due to its high total phenolic and flavonoid content shown in Figure 3, which is confirmed in vitro. From the in vitro results, the Trichurus extract has high flavonoid content, where flavonoids are best known for their antioxidant properties and may act in vitro as reducing agents, hydrogen donors, free radical quenchers, and metal ion chelators [129].
It has been demonstrated previously that fungi, as well as algae are potentially biosorbent, for heavy metals [130–132]. This fact has also been confirmed in the present study.
According to [133], this general chelating ability of phenolic compounds is probably related to the high nucleophilic character of the aromatic rings, rather than to be specific chelating groups within the molecule. This agrees with our results, as the extract shows high total polyphenol content (phenolic and flavonoid content) as shown in Figure 3. There is another mechanism underlying their antioxidant ability. Metal ions decompose lipid hydroperoxide (LOOH) by the hemolytic cleavage at the O–O bond and give lipid alkoxyl radicals, which initiate free radical chain oxidation. Phenolic antioxidants inhibit lipid peroxidation by trapping the lipid alkoxyl radical; and that was confirmed by our results, as the fungal extract showed a high antioxidant activity and a high inhibition ratio to lipid peroxidation in vitro, by a percentage of 70.80% and 85%, respectively, as shown in Figure 4. This activity depends on the structure of the molecules, the number, and position within the hydroxyl group in the molecules [134]. Many flavonoids have also been found to possess hepatoprotective activity [135].
Reference [136] show that phenolic (especially flavonoids) is able to alter peroxidation kinetics, by modifying the lipid packing order. They stabilize membranes by decreasing the membrane fluidity (in a concentration-dependent manner) and hinder the diffusion of free radicals and restrict per oxidative reaction [136, 137]. According to [138], in addition to the known protein-binding capacity of flavanols and procyanidins, they can interact with membrane phospholipids through hydrogen bonding, to the polar head groups of phospholipids. As a consequence, these compounds can be accumulated at the membranes' surface, both outside and inside the cells.
For this activity, polyphenols possess an ideal structural chemistry and have been shown to be more effective in vitro than vitamins E and C on the molar basis [139]. Many beneficial pharmacological properties have been attributed to flavonoids, including antioxidant, anti-inflammatory, anticarcinogenic, chemo preventive, and cytochrome-P450-inhibitory activities [140, 141].
In addition to high total phenolic and flavonoid content for fungal extract, the fungal extract shows high sulfur content, where sulfur is an essential component in normal physiological function and is incorporated into amino acids, proteins, enzymes, and micronutrients [142]. Humans satisfy their nutritional needs of sulfur by consuming plants and animals [143]. The high content of sulfur due to marine chemodiversty is also heightened due to the composition of sea water, which has itself a concentration of halides in sea water of 1900 mg/L Cl−, 65 mg/L Br−, 5 × 10−4, and I/IO3 −, which are reflected by the number of compounds incorporating these elements and sulfated compounds that can account for by the relatively high concentration of sulfur, 2700 mg/L seawater. That is confirmed in our study as shown in Figure 2 [144].
Marine natural product in general, and especially marine fungi, can be good hepatoprotective candidates in using the heavy metal as a toxicology model. AS in the bioassay-directed searching for the hepato-protective agents from natural sources, employing the closely relevant model system to human liver toxicosis, could be an effective way to identify therapeutically applicable agents [145].
5. Conclusion
In conclusion, there is a beneficial influence of the investigated fungus extract against heavy-metal mixtures-intoxicated rats. We could confirm that this extract possesses hepatoprotective property due to its proven antioxidant and free radical scavenging properties, in addition to its high sulfur content. However, other possible mechanisms such as inhibition of antioxidant enzymes, induction of oxidative stress, and the influence on different signal pathways in liver cells should not be neglected. Further investigations of these matters are warranted, particularly that of fungus extract, as well as elucidation of compounds that are responsible for such activities and their effect on liver antioxidant capacity, which should be carried out.
Acknowledgments
All the authors thanks go to Dr. Amani Kasem, Department of Pathology, Medical Research Institute of Alexandria, for giving them such an honorable opportunity to explain the histopathological result. They also owe thanks to Gihan S. Soliman for critical reading and grammatical review of the paper.
References
- 1.Ash C, Stone R. A question of dose. Science. 2003;300(5621, article 925) [Google Scholar]
- 2.Allan R. Mining and metals in the environment. Journal of Geochemical Exploration. 1997;58(2-3):95–100. [Google Scholar]
- 3.Morillo J, Usero J, Gracia I. Heavy metal distribution in marine sediments from the Southwest coast of Spain. Chemosphere. 2004;55(3):431–442. doi: 10.1016/j.chemosphere.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 4.Chen TB, Zheng YM, Lei M, et al. Assessment of heavy metal pollution in surface soils ofurban parks in Beijing, China. Chemosphere. 2005;60(4):542–551. doi: 10.1016/j.chemosphere.2004.12.072. [DOI] [PubMed] [Google Scholar]
- 5.Wannaz ED, Carreras HA, Pérez CA, Pignata ML. Assessment of heavy metal accumulation in two species of Tillandsia in relation to atmospheric emission sources in Argentina. Science of the Total Environment. 2006;361(1–3):267–278. doi: 10.1016/j.scitotenv.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Leonard SS, Harris GK, Shi X. Metal-induced oxidative stress and signal transduction. Free Radical Biology and Medicine. 2004;37(12):1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Flora SJS, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian Journal of Medical Research. 2008;128(4):501–523. [PubMed] [Google Scholar]
- 8.Chen F, Ding M, Castranova V, Shi XL. Carcinogenic metals and NF-κB activation. Molecular and Cellular Biochemistry. 2001;222(1-2):159–171. [PubMed] [Google Scholar]
- 9.Shimada H, Yasutake A, Hirashima T, et al. Strain difference of cadmium accumulation by liver slices of inbred Wistar-Imamichi and Fischer 344 rats. Toxicology In Vitro. 2008;22(2):338–343. doi: 10.1016/j.tiv.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Draz E, El- Kelany R, El- Nimr T, Badawy A, Zakaria S. role of selenium and vitamin E in occupational exposure to heavy metals (mercury, lead, and cadmium ) impact of working in lamp factory. Mansoura Journal of Forensic Medicine and Clinical Toxicology. 2009;17(2) [Google Scholar]
- 11.Ruff HA, Markowitz ME, Bijur PE, Rosen JF. Relationships among blood lead levels, iron deficiency, and cognitive development in two-year-old children. Environmental Health Perspectives. 1996;104(2):180–185. doi: 10.1289/ehp.96104180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flora SJS, Flora G, Saxena G. Environmental occurrence, health effects and management of lead poisoning. In: Cascas SB, Sordo J, editors. Lead Chemistry, Analytical Aspects, Environmental Impacts and Health Effects. Amsterdam, The Netherlands: Elsevier; 2006. pp. 158–228. [Google Scholar]
- 13.Dressier J, Kim KA, Chakraborti T, Goldstein G. Molecular mechanisms of lead neurotoxicity. Neurochemical Research. 1999;24(4):595–600. doi: 10.1023/a:1022596115897. [DOI] [PubMed] [Google Scholar]
- 14.Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations < 10 μg/dL in US children and adolescents. Public Health Reports. 2000;115(6):521–529. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalil-Manesh F, Gonick HC, Weiler EWJ, Prins B, Weber MA, Purdy RE. Lead-induced hypertension: possible role of endothelial factors. American Journal of Hypertension. 1993;6(9):723–729. doi: 10.1093/ajh/6.9.723. [DOI] [PubMed] [Google Scholar]
- 16.Damek-Poprawa M, Sawicka-Kapusta K. Histopathological changes in the liver, kidneys, and testes of bank voles environmentally exposed to heavy metal emissions from the steelworks and zinc smelter in Poland. Environmental Research. 2004;96(1):72–78. doi: 10.1016/j.envres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Sharma RP, Street JC. Public health aspects of toxic heavy metals in animal feeds. Journal of the American Veterinary Medical Association. 1980;177(2):149–153. [PubMed] [Google Scholar]
- 18.Lancranjan I, Popescu HI, GAvănescu O, Klepsch I, Serbănescu M. Reproductive ability of workmen occupationally exposed to lead. Archives of Environmental Health. 1975;30(8):396–401. doi: 10.1080/00039896.1975.10666733. [DOI] [PubMed] [Google Scholar]
- 19.Ronis MJJ, Badger TM, Shema SJ, et al. Endocrine mechanisms underlying the growth effects of developmental lead exposure in the rat. Journal of Toxicology and Environmental Health A. 1998;54(2):101–120. doi: 10.1080/009841098158944. [DOI] [PubMed] [Google Scholar]
- 20.Miller DM, Lund BO, Woods JS. Reactivity of Hg(II) with superoxide: evidence for the catalytic dismutation of superoxide by Hg(II) Journal of Biochemical Toxicology. 1991;6(4):293–298. doi: 10.1002/jbt.2570060409. [DOI] [PubMed] [Google Scholar]
- 21.Hussain S, Rodgers DA, Duhart HM, Ali SF. Mercuric chloride-induced reactive oxygen species and its effect on antioxidant enzymes in different regions of rat brain. Journal of Environmental Science and Health B. 1997;32(3):395–409. doi: 10.1080/03601239709373094. [DOI] [PubMed] [Google Scholar]
- 22.NTP. Technical Reports Series. 408. National Toxicology Programme; 2003. Toxicology and carcinogenesis studies of mercuric chloride in F Rats and B6C3F mice (gavage studies) [PubMed] [Google Scholar]
- 23.Nwakocaha AC, Ejebe DE, Nwangwa EK, Ekene N, Akonoghrere R, Ukwu J. The effects of bitter Kola supplemented diet on hepatotoxicity of mercury in Wistar rats. Journal of Applied Sciences and Environmental Management. 2010;14(1):89–95. [Google Scholar]
- 24.Klaasen CD. Godman's and Gilman's the Pharmacological Basis of Therapeutics. 9th edition. New York, NY, USA: McGraw Hill; 1999. Heavy metals and heavy metal antagonists; pp. 1164–1169. [Google Scholar]
- 25.Wikipedia. Mercury poisoning. Wikipedia the Free Encyclopedia, May 2009, http://en.wikipedia.org/wiki/Mercury_poisoning.
- 26.Bakir F, Damluji SF, Amin Zaki I, et al. Methylmercury poisoning in Iraq: an interuniversity report. Science. 1973;181(4096):230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 27.Kocijan A, Milošev I, Pihlar B. Cobalt-based alloys for orthopaedic applications studied by electrochemical and XPS analysis. Journal of Materials Science: Materials in Medicine. 2004;15(6):643–650. doi: 10.1023/b:jmsm.0000030204.08616.3d. [DOI] [PubMed] [Google Scholar]
- 28.Papp A, Fehér O, Erdélyi L. The ionic mechanism of the pentylenetetrazol convulsions. Acta Biologica Hungarica. 1987;38(3-4):349–361. [PubMed] [Google Scholar]
- 29.Christie NT, Tummolo DM. The effect of Ni(II) on DNA replication. Biological Trace Element Research. 1989;21:3–12. doi: 10.1007/BF02917231. [DOI] [PubMed] [Google Scholar]
- 30.Xie J, Funakoshi T, Shimada H, Kojima S. Effects of chelating agents on testicular toxicity in mice caused by acute exposure to nickel. Toxicology. 1995;103(3):147–155. doi: 10.1016/0300-483x(95)03134-2. [DOI] [PubMed] [Google Scholar]
- 31.Iscan M, Coban T, Eke BC. Differential combined effect of cadmium and nickel on hepatic and renal glutathione S-transferases of the guinea pig. Environmental Health Perspectives. 1994;102(9):69–72. doi: 10.1289/ehp.94102s969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura M, Yasukochi Y, Minakami S. Effect of cobalt on heme biosynthesis in rat liver and spleen. Journal of Biochemistry. 1975;78(2):373–380. doi: 10.1093/oxfordjournals.jbchem.a130917. [DOI] [PubMed] [Google Scholar]
- 33.Cogoluenhes VC, Chambrier C, Michallet M, et al. Energy expenditure during allogeneic and autologous bone marrow transplantation. Clinical Nutrition. 1998;17(6):253–257. doi: 10.1016/s0261-5614(98)80316-6. [DOI] [PubMed] [Google Scholar]
- 34.Coen N, Mothersill C, Kadhim M, Wright EG. Heavy metals of relevance to human health induced genomic instability. Journal of Pathology. 2001;195(3):293–299. doi: 10.1002/path.950. [DOI] [PubMed] [Google Scholar]
- 35.Szakmary E, Ungvary G, Hudak A, et al. Effects of cobalt sulfate on prenatal development of mice, rats, and rabbits, and on early postnatal development of rats. Journal of Toxicology and Environmental Health A. 2001;62(5):367–386. doi: 10.1080/152873901300018110. [DOI] [PubMed] [Google Scholar]
- 36.Goutet M, Ban M, Binet S. Effects of nickel sulfate on pulmonary natural immunity in Wistar rats. Toxicology. 2000;145(1):15–26. doi: 10.1016/s0300-483x(99)00216-4. [DOI] [PubMed] [Google Scholar]
- 37.Condevaux F, Guichard J, Forichon A, Aujoulat M, Descotes J. Compared effects of morphine and nickel chloride on NK cell activity in vitro in rats and monkeys. Journal of Applied Toxicology. 2001;21(5):431–434. doi: 10.1002/jat.776. [DOI] [PubMed] [Google Scholar]
- 38.Alcón MP, Arola L, Mas A. Response to acute nickel toxicity in rats as a function of sex. Biology of Metals. 1991;4(3):136–140. doi: 10.1007/BF01141303. [DOI] [PubMed] [Google Scholar]
- 39.Rai LC, Raizada M, Mallick N, Husaini Y, Singh AK, Dubey SK. Effect of four heavy metals on the biology of Nostoc muscorum. Biology of Metals. 1990;2(4):229–234. doi: 10.1007/BF01141365. [DOI] [PubMed] [Google Scholar]
- 40.Henry AS, Marian M, Alexis PN. Life term effects of nickel in rats: survival, tumors, and Interactions with trace elementsand tissue levels. Journal of Nutrition. 1974;104:239–243. doi: 10.1093/jn/104.2.239. [DOI] [PubMed] [Google Scholar]
- 41.Lansdown ABG. Physiological and toxicological changes in the skin resulting from the action and interaction of metal ions. Critical Reviews in Toxicology. 1995;25(5):397–462. doi: 10.3109/10408449509049339. [DOI] [PubMed] [Google Scholar]
- 42.Wu LF, Navarro C, de Pina K, Quenard M, Mandrand MA. Antagonistic effect of nickel on the fermentative growth of Escherichia coli K-12 and comparison of nickel and cobalt toxicity on the aerobic and anaerobic growth. Environmental Health Perspectives. 1994;102(3):297–300. doi: 10.1289/ehp.94102s3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakroun H, Hfaidh N, Makni-Ayadi F, Guermazi F, Kammoun A, Elfeki A. Nickel and fertility in the rat. Sexologies. 2002;12:1–4. [Google Scholar]
- 44.Harper MK, Bugni TS, Copp BR, et al. Introduction to the chemical ecology of marine natural products. In: McCIintock JB, Baker BJ, editors. Marine Chemical Ecology. Boca Raton, Fla, USA: CRC Press, LLC; 2001. pp. 3–69. [Google Scholar]
- 45.Bugni TS, Ireland CM. Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Natural Product Reports. 2004;21(1):143–163. doi: 10.1039/b301926h. [DOI] [PubMed] [Google Scholar]
- 46.Ashida J. Adaptation of fungi to metal toxicants. Annual Review of Phytopathology. 1965;3:153–174. [Google Scholar]
- 47.Gadd GM. Interactions of fungi with toxic metals. New Phytologist. 1993;124(1):25–60. [Google Scholar]
- 48.Kapoor A, Viraraghavan T, Cullimore DR. Removal of heavy metals using the fungus Aspergillus niger . Bioresource Technology. 1999;70(1):95–104. [Google Scholar]
- 49.Magyarosy A, Laidlaw R, Kilaas R, Echer C, Clark D, Keasling J. Nickel accumulation and nickel oxalate precipitation by Aspergillus niger . Applied Microbiology and Biotechnology. 2002;59(2-3):382–388. doi: 10.1007/s00253-002-1020-x. [DOI] [PubMed] [Google Scholar]
- 50.Ross IS. Some effects of heavy metals on fungal cells. Transactions of the British Mycological Society. 1975;64:175–193. [Google Scholar]
- 51.Gadd GM. Heavy metal accumulation by bacteria and other microorganisms. Experientia. 1990;46(8):834–840. [Google Scholar]
- 52.Gadd GM. Fungi and yeast metal accumulation. In: Ehrlich HL, Brierley CL, editors. Microbiol Mineral Recovery. New York, NY, USA: McGraw-Hill; 1990. pp. 249–276. [Google Scholar]
- 53.Gadd GM. Molecular biology and biotechnology of microbial interaction with organic and inorganic heavy metal compounds. In: Herbert RA, Sharp RT, editors. Molecular Biology and Biotechnology of Extreamophiles. Glasgow, UK: Blackie and Sons; 1992. pp. 225–257. [Google Scholar]
- 54.Mehra RK, Winge DR. Metal ion resistance in fungi: molecular mechanisms and their related expression. Journal of Cellular Biochemistry. 1991;45(1):30–40. doi: 10.1002/jcb.240450109. [DOI] [PubMed] [Google Scholar]
- 55.Raper KB, Thom CA. A Manual of the Penicillia. Baltimore, Md, USA: Williams & Wilkins; 1949. [Google Scholar]
- 56.Pitt JI. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces. London, UK: Academic Press; 1980. [Google Scholar]
- 57.Raper KB, Fennell DI. The Genus Aspergillus. Baltimore, Md, USA: Williams & Wilkins; 1965. [Google Scholar]
- 58.Ellis MB. Dematiaceous Hyphomycetes. Kew, Australia: Commonwealth Mycological Institute; 1971. [Google Scholar]
- 59.Ellis MB. More Dematiaceoua Hyphomycetes. Kew, Australia: Commonwealth Mycological Institute; 1976. [Google Scholar]
- 60.Booth C. The Genus Fusarium. Kew, Australia: Commonwealth Mycological Institute; 1971. [Google Scholar]
- 61.von Arx JA. The Genera of Fungi Sporulating in Pure Culture. 3rd edition. Vaduz, Liechtenstein: J. Cramer; 1981. [Google Scholar]
- 62.Domsch KH, Gams W, Anderson TH. Compendium of Soil Fungi. Eching, Germany: IHW; 2007. [Google Scholar]
- 63.Watanabe T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species. 2nd edition. Boca Raton, Fla, USA: CRC Press; 2002. [Google Scholar]
- 64.von Arx JA, Guarro J, Figueras MJ. The ascomycete genus Chaetomium. Beihefte zur Nova Hedwigia. 1986;84:1–162. [Google Scholar]
- 65.Cannon PF. A revision of Achaetomium, Achaetomiella and Subramaniula, and some similar species of Chaetomium . Transactions of British Mycological Society. 1986;87(1):45–76. [Google Scholar]
- 66.Kirk P, Cannon PF, Minter DW, Stalpers JA. AinSworth & Bisby’s Dictionary of the Fungi. 10th edition. Wallingford, UK: CAB International; 2008. [Google Scholar]
- 67.Taga MS, Miller EE, Pratt DE. Chia seeds as a source of natural lipid antioxidants. Journal of the American Oil Chemists’ Society. 1984;61(5):928–931. [Google Scholar]
- 68.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry. 1999;64(4):555–559. [Google Scholar]
- 69.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. [Google Scholar]
- 70.Amarowicz R, Naczk M, Shahidi F. Antioxidant activity of various fractions of non-tannin phenolics of Canula hulls. Journal of Agricultural and Food Chemistry. 2000;48(7):2755–2759. doi: 10.1021/jf9911601. [DOI] [PubMed] [Google Scholar]
- 71.Wallin B, Rosengren B, Shertzer HG, Camejo G. Lipoprotein oxidation and measurement of thiobarbituric acid reacting substances formation in a single microtiter plate: Its use for evaluation of antioxidants. Analytical Biochemistry. 1993;208(1):10–15. doi: 10.1006/abio.1993.1002. [DOI] [PubMed] [Google Scholar]
- 72.Lowry OH, Rosehmugh NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 73.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 74.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clinica Chimica Acta. 1971;31(1):87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- 75.Walters MI, Gerarde HW. An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchemical Journal. 1970;15(2):231–243. [Google Scholar]
- 76.Griffith J, Farris E. The osseous system. In: Griffith J, Farris E, editors. The Rat in Laboratory Investigation. Philadelphia, Pa, USA: J.B. Lippincottco; 1942. pp. 418–419. [Google Scholar]
- 77.Negi AS, Kumar JK, Luqman S, Shanker K, Gupta MM, Khanuja SP. Recent advances in plant hepatoprotectives: a chemical and biological profile of some important leads. Medicinal Research Reviews. 2008;28(5):746–772. doi: 10.1002/med.20115. [DOI] [PubMed] [Google Scholar]
- 78.Kukić-Marković J, Dobrić S, aćević J, Topić A, Marin P, Petrović S. Hepatoprotective activity of Stachys extracts against CCl4-induced hepatotoxicity in rats. Planta Medica. 2009;75, article PJ37 [Google Scholar]
- 79.Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure—a review of the literature and a risk estimate. Scandinavian Journal of Work, Environment and Health. 1998;24:1–51. [PubMed] [Google Scholar]
- 80.Trinchella F, Riggio M, Filosa S, Volpe MG, Parisi E, Scudiero R. Cadmium distribution and metallothionein expression in lizard tissues following acute and chronic cadmium intoxication. Comparative Biochemistry and Physiology C. 2006;144(3):272–278. doi: 10.1016/j.cbpc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe M, Henmi K, Ogawa K, Suzuki T. Cadmium-dependent generation of reactive oxygen species and mitochondrial DNA breaks in photosynthetic and non-photosynthetic strains of Euglena gracilis . Comparative Biochemistry and Physiology C. 2003;134(2):227–234. doi: 10.1016/s1532-0456(02)00253-3. [DOI] [PubMed] [Google Scholar]
- 82.Kumar G, Banu GS, Murugesan AG. Effect of Helicteres isora bark extracts on heat antioxidant status and lipid peroxidation in streptozotocin diabetic rats. Journal of Applied Biomedicine. 2008;6(2):89–95. [Google Scholar]
- 83.Kumar P, Rao D, Lakshmayya G, Ramachandra S. Antioxidant and hepatoprotective activity of tubers of Momordica tuberosa Cogn. against CCl4 induced liver injury in rats. Indian Journal of Experimental Biology. 2008;46(7):510–513. [PubMed] [Google Scholar]
- 84.Periasamy M, Pavankumar, Venkata Gangadhar V, Jeeva T, Anandhan R, Sengottuvelu S. Hepatoprotective and antioxidant activity of “Euphorbia Ligularia” against carbon tetrachloride induced hepatotoxicity in Wistar rats. International Journal of Research in Pharmaceutical and Biomedical Sciences. 2012;3(1) [Google Scholar]
- 85.Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. Journal of Natural Products. 2007;70(3):461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 86.Harvey AL. Natural products in drug discovery. Drug Discovery Today. 2008;13:894–901. doi: 10.1016/j.drudis.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 87.Blunt JW, Copp BR, Munro MHG, Northcote PT, Prinsep MR. Marine natural products. Natural Product Reports. 2006;26(2):170–244. doi: 10.1039/b805113p. [DOI] [PubMed] [Google Scholar]
- 88.König GM, Kehraus S, Seibert SF, Abdel-Lateff A, Müller D. Natural products from marine organisms and their associated microbes. ChemBioChem. 2006;7(2):229–238. doi: 10.1002/cbic.200500087. [DOI] [PubMed] [Google Scholar]
- 89.Imhoff JF, Stöhr R. Sponge-associated bacteria: general overview and special aspects of bacteria associated with Halichondria panicea . Progress in Molecular and Subcellular Biology. 2003;37:35–57. doi: 10.1007/978-3-642-55519-0_2. [DOI] [PubMed] [Google Scholar]
- 90.Gao Z, Li B, Zheng C, Wang G. Molecular detection of fungal communities in the hawaiian marine sponges Suberites zeteki and Mycale armata. Applied and Environmental Microbiology. 2008;74(19):6091–6101. doi: 10.1128/AEM.01315-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiese J, Ohlendorf B, Blümel M, Schmaljohann R, Imhoff JF. Phylogenetic identification of fungi isolated from the Marine Sponge Tethya aurantium and identification of their secondary metabolites. Marine Drugs. 2011;9(4):561–585. doi: 10.3390/md9040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sayer JA, Gadd GM. Binding of cobalt and zinc by organic acids and culture filtrates of Aspergillus niger grown in the absence or presence of insoluble cobalt or zinc phosphate. Mycological Research. 2001;105(10):1261–1267. [Google Scholar]
- 93.Martino E, Franco B, Piccoli G, Stocchi V, Perotto S. Influence of zinc ions on protein secretion in a heavy metal tolerantstrain of the ericoid mycorrhizal fungus Oidiodendron maius . Molecular and Cellular Biochemistry. 2002;231(1-2):179–185. doi: 10.1023/a:1014485420186. [DOI] [PubMed] [Google Scholar]
- 94.Baldrian P. Interactions of heavy metals with white-rot fungi. Enzyme and Microbial Technology. 2003;32(1):78–91. [Google Scholar]
- 95.Blaudez D, Jacob C, Turnau K, et al. Differential responses of ectomycorrhizal fungi to heavy metals in vitro. Mycological Research. 2000;104(11):1366–1371. [Google Scholar]
- 96.Li ZS, Lu YP, Zhen RG, Szczypka M, Thiele DJ, Rea PA. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(1):42–47. doi: 10.1073/pnas.94.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clemens S, Bloss T, Vess C, Neumann D, Nies DH, Zur Nieden U. A transporter in the endoplasmic reticulum of Schizosaccharomyces pombe cells mediates zinc storage and differentially affects transition metal tolerance. Journal of Biological Chemistry. 2002;277(20):18215–18221. doi: 10.1074/jbc.M201031200. [DOI] [PubMed] [Google Scholar]
- 98.Cervantes C, Gutierrez-Corona F. Copper resistance mechanisms in bacteria and fungi. FEMS Microbiology Reviews. 1994;14(2):121–137. doi: 10.1111/j.1574-6976.1994.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 99.Binz PA, Kägi JHR. Metallothionein: molecular evolution and classification. In: Klaassen C, editor. Metallothionein IV. Basel, Switzerland: Birkhäuser; 1999. pp. 7–13. [Google Scholar]
- 100.Miersch J, Tschimedbalshir M, Bärlocher F, et al. Heavy metals and thiol compounds in Mucor racemosus and Articulospora tetracladia . Mycological Research. 2001;105(7):883–889. [Google Scholar]
- 101.Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annual Review of Plant Biology. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 102.Das N, Vimala R, Karthika P. Biosorption of heavy metals—an overview. Indian Journal of Biotechnology. 2008;7(2):159–169. [Google Scholar]
- 103.Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biology and Medicine. 1995;18(2):321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 104.Failla ML, Kiser RA. Hepatic and renal metabolism of copper and zinc in the diabetic rat. The American Journal of Physiology. 1983;244(2):E115–E121. doi: 10.1152/ajpendo.1983.244.2.E115. [DOI] [PubMed] [Google Scholar]
- 105.Dhawan D, Goel A. Further evidence for zinc as a hepatoprotective agent in rat liver toxicity. Experimental and Molecular Pathology. 1995;63(2):110–117. doi: 10.1006/exmp.1995.1035. [DOI] [PubMed] [Google Scholar]
- 106.Dhawan DK, Goel A. Protective role of zinc on rat liver function in long-term toxicity induced by carbon tetrachloride. Journal of Trace Elements in Experimental Medicine. 1994;7(1):1–9. [Google Scholar]
- 107.McCall JT, Goldstein NP, Smith LH. Implications of tract metals in human diseases. Federation Proceedings. 1971;30(3):1011–1015. [PubMed] [Google Scholar]
- 108.Hill CH, Matrone G. Chemical parameters in the study of in vivo and in vitro interactions of transition elements. Federation Proceedings. 1970;29(4):1474–1481. [PubMed] [Google Scholar]
- 109.Singh B, Dhawan D, Nehru B, et al. Impact of lead pollution on the status of other trace metals in blood and alterations in hepatic functions. Biological Trace Element Research. 1994;40(1):21–29. doi: 10.1007/BF02916817. [DOI] [PubMed] [Google Scholar]
- 110.Kumar Sharma M, Kumar M, Kumar A. Ocimum sanctum aqueous leaf extract provides protection against mercury induced toxicity in Swiss albino mice. Indian Journal of Experimental Biology. 2002;40(9):1079–1082. [PubMed] [Google Scholar]
- 111.Reus IS, Bando I, Andrés D, Cascales M. Relationship between expression of HSP70 and metallothionein and oxidative stress during mercury chloride induced acute liver injury in rats. Journal of Biochemical and Molecular Toxicology. 2003;17(3):161–168. doi: 10.1002/jbt.10074. [DOI] [PubMed] [Google Scholar]
- 112.Sobutskii MP, Kovanko EG, Liutinskii SI, Ivanov SD. Effect of age and gender on genotoxic and biochemical indexes in animal blood after low doses of radiation-mercury exposures. Advances in Gerontology. 2007;20(2):91–96. [PubMed] [Google Scholar]
- 113.Jadhav SH, Sarkar SN, Patil RD, Tripathi HC. Effects of subchronic exposure via drinking water to a mixture of eight water-contaminating metals: a biochemical and histopathological study in male rats. Archives of Environmental Contamination and Toxicology. 2007;53(4):667–677. doi: 10.1007/s00244-007-0031-0. [DOI] [PubMed] [Google Scholar]
- 114.Wadaan MAM. Effects of mercury exposure on blood chemistry and liver histopathology of male rats. Journal of Pharmacology and Toxicology. 2009;4(3):126–131. [Google Scholar]
- 115.Pari L, Prasath A. Efficacy of caffeic acid in preventing nickel induced oxidative damage in liver of rats. Chemico-Biological Interactions. 2008;173(2):77–83. doi: 10.1016/j.cbi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 116.Dabak D, Gazuwa SY, Uborn GA. Hepatoprotective: potential of calcium and magnesium against cadmium and lead induce hepatotoxicty in Wistar rats. Asian Journal of Biotechnology. 2009;1(1):12–19. [Google Scholar]
- 117.Chaurasia SS, Kar A. Protective effects of vitamin E against lead-induced deterioration of membrane associated type-I iodothyronine 5′-monodeiodinase (5′D-I) activity in male mice. Toxicology. 1997;124(3):203–209. doi: 10.1016/s0300-483x(97)00155-8. [DOI] [PubMed] [Google Scholar]
- 118.Taki Y, Shimahara Y, Isselhard W. Derangement of hepatic energy metabolism in lead-sensitized endotoxicosis. European Surgical Research. 1985;17(3):140–149. doi: 10.1159/000128459. [DOI] [PubMed] [Google Scholar]
- 119.Goering PL, Fisher BR, Noren BT, Papaconstantinou A, Rojko JL, Marler RJ. Mercury induces regional and cell-specific stress protein expression in rat kidney. Toxicological Sciences. 2000;53(2):447–457. doi: 10.1093/toxsci/53.2.447. [DOI] [PubMed] [Google Scholar]
- 120.Papaconstantinou AD, Brown KM, Noren BT, McAlister T, Fisher BR, Goering PL. Mercury, cadmium, and arsenite enhance heat shock protein synthesis in chick embryos prior to embryotoxicity. Birth Defects Research B. 2003;68(6):456–464. doi: 10.1002/bdrb.10044. [DOI] [PubMed] [Google Scholar]
- 121.Hagymàsi K, Kocsis I, Lengyel G, Sipos P, Fehér J, Blàzovics A. Further evidence of altered redox status of hyperbilirubinemic patients: role of bilirubin in Gilbert syndrome. Acta Biologica Szegediensis. 2003;47(1–4):131–134. [Google Scholar]
- 122.Berrahal AA, Nehdi A, Hajjaji N, Gharbi N, El-Fazâa S. Antioxidant enzymes activities and bilirubin level in adult rat treated with lead. Comptes Rendus—Biologies. 2007;330(8):581–588. doi: 10.1016/j.crvi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 123.Tomaro ML, Batlle AMDC. Bilirubin: its role in cytoprotection against oxidative stress. International Journal of Biochemistry and Cell Biology. 2002;34(3):216–220. doi: 10.1016/s1357-2725(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 124.Stocker R, McDonagh AF, Glazer AN, Ames BN. Antioxidant activities of bile pigments: biliverdin and bilirubin. Methods in Enzymology. 1990;186:301–309. doi: 10.1016/0076-6879(90)86123-d. [DOI] [PubMed] [Google Scholar]
- 125.Doré S, Takahashi M, Ferris CD, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(5):2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clark JE, Foresti R, Green CJ, Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochemical Journal. 2000;348(3):615–619. [PMC free article] [PubMed] [Google Scholar]
- 127.Ossola JO, Kristoff G, Tomaro ML. Heme oxygenase induction by menadione bisulfite adduct-generated oxidative stress in rat liver. Comparative Biochemistry and Physiology C. 2000;127(1):91–99. doi: 10.1016/s0742-8413(00)00133-x. [DOI] [PubMed] [Google Scholar]
- 128.Tarasub N, Tarasub C, Devakul W, Ayutthaya N, Suramana T. Effects of quercetin on acute toxicity of rat spleen and chromosome aberrations in bone marrow induced by nickel chloride. 2000, http://www.med.tu.ac.th/medJournal/TUmed74/TuM7_4page321.pdf.
- 129.Shahidi F, Wanasundara PK. Phenolic antioxidants. Critical Reviews in Food Science and Nutrition. 1992;32(1):67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 130.Volesky B, May-Phillips HA. Biosorption of heavy metals by Saccharomyces cerevisiae . Applied Microbiology and Biotechnology. 1995;42(5):797–806. doi: 10.1007/BF00171964. [DOI] [PubMed] [Google Scholar]
- 131.Veglio’ F, Beolchini F. Removal of metals by biosorption: a review. Hydrometallurgy. 1997;44(3):301–316. [Google Scholar]
- 132.Volesky B. Biosorption process simulation tools. Hydrometallurgy. 2003;71(1-2):179–190. [Google Scholar]
- 133.Moran JF, Klucas RV, Grayer RJ, Abian J, Becana M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radical Biology and Medicine. 1997;22(5):861–870. doi: 10.1016/s0891-5849(96)00426-1. [DOI] [PubMed] [Google Scholar]
- 134.Milić BL, Djilas SM, Čanadanović-Brunet JM. Antioxidative activity of phenolic compounds on the metal-ion breakdown of lipid peroxidation system. Food Chemistry. 1998;61(4):443–447. [Google Scholar]
- 135.Narayana KR, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian Journal of Pharmacology. 2001;33(1):2–16. [Google Scholar]
- 136.Arora A, Byrem TM, Nair MG, Strasburg GM. Modulation of liposomal membrane fluidity by flavonoids and isoflavonoids. Archives of Biochemistry and Biophysics. 2000;373(1):102–109. doi: 10.1006/abbi.1999.1525. [DOI] [PubMed] [Google Scholar]
- 137.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Verstraeten SV, Keen CL, Schmitz HH, Fraga CG, Oteiza PI. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radical Biology and Medicine. 2003;34(1):84–92. doi: 10.1016/s0891-5849(02)01185-1. [DOI] [PubMed] [Google Scholar]
- 139.Rice-Evans C, Miller N. Measurement of the antioxidant status of dietary constituents, low density lipoproteins and plasma. Prostaglandins Leukotrienes and Essential Fatty Acids. 1997;57(4-5):499–505. doi: 10.1016/s0952-3278(97)90435-x. [DOI] [PubMed] [Google Scholar]
- 140.Kühnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Review of Nutrition and Dietetics. 1976;24:117–191. [PubMed] [Google Scholar]
- 141.Nikolic D, van Breemen R. New metabolic pathways for flavanones catalyzed by rat liver microsomes. The American Society for Pharmacology and Experimental Therapeutics. 2004;32(4):387–397. doi: 10.1124/dmd.32.4.387. [DOI] [PubMed] [Google Scholar]
- 142.Atmaca G. Antioxidant effects of sulfur-containing amino acids. Yonsei Medical Journal. 2004;45(5):776–788. doi: 10.3349/ymj.2004.45.5.776. [DOI] [PubMed] [Google Scholar]
- 143.Battin EE, Brumaghim JL. Antioxidant activity of sulfur and selenium: a review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochemistry and Biophysics. 2009;55(1):1–23. doi: 10.1007/s12013-009-9054-7. [DOI] [PubMed] [Google Scholar]
- 144.Fenical W. Natural products chemistry in the marine environment. Science. 1982;215(4535):923–928. doi: 10.1126/science.215.4535.923. [DOI] [PubMed] [Google Scholar]
- 145.Oh H, Kim DH, Cho JH, Kim YC. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. Journal of Ethnopharmacology. 2004;95(2-3):421–424. doi: 10.1016/j.jep.2004.08.015. [DOI] [PubMed] [Google Scholar]


