Abstract
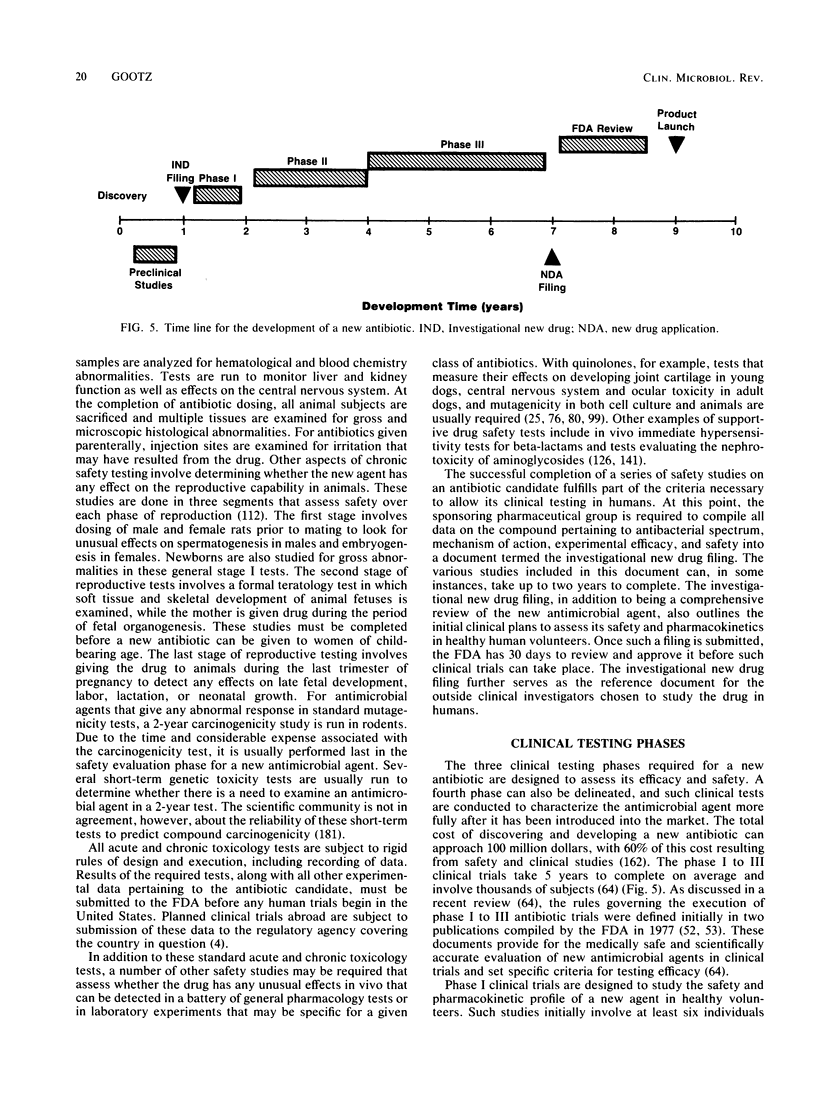
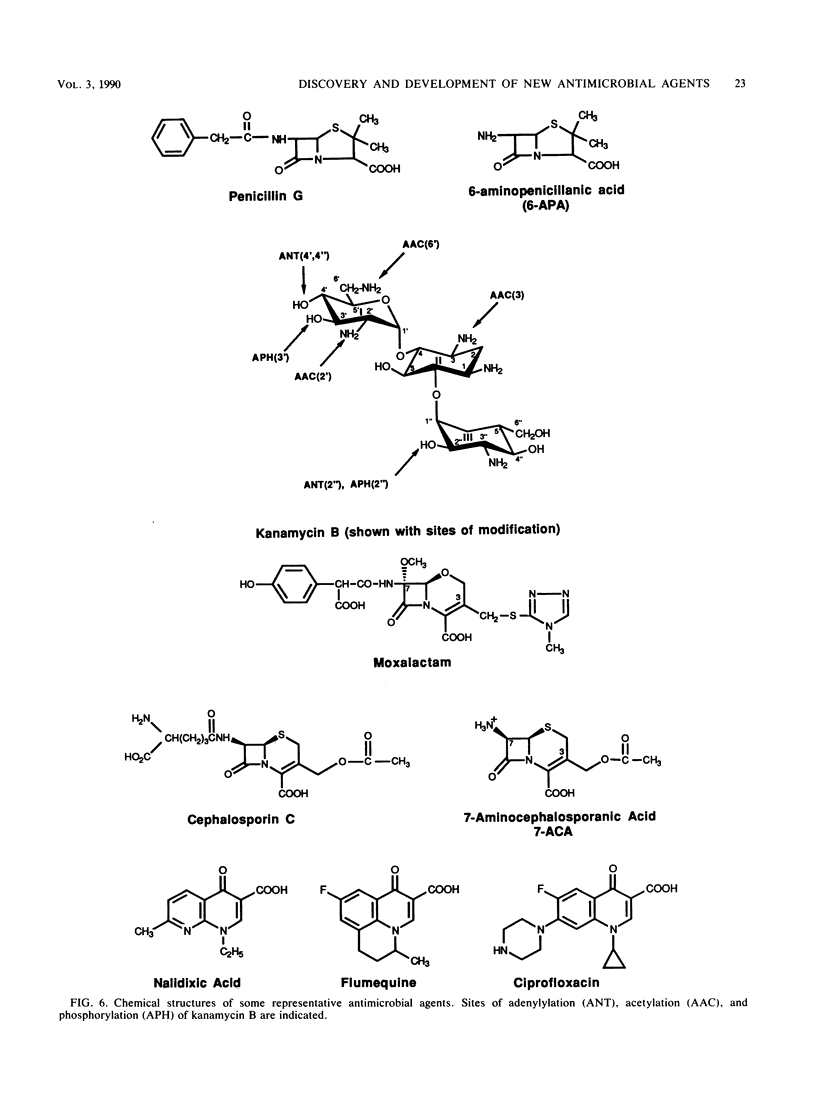
The unprecedented growth in the number of new antibiotics over the past two decades has been the result of extensive research efforts that have exploited the growing body of knowledge describing the interactions of antibiotics with their targets in bacterial cells. Information gained from one class of antimicrobial agents has often been used to advance the development of other classes. In the case of beta-lactams, information on structure-activity relationships gleaned from penicillins and cephalosporins was rapidly applied to the cephamycins, monobactams, penems, and carbapenems in order to discover broad-spectrum agents with markedly improved potency. These efforts have led to the introduction of many new antibiotics that demonstrate outstanding clinical efficacy and improved pharmacokinetics in humans. The current review discusses those factors that have influenced the rapid proliferation of new antimicrobial agents, including the discovery of new lead structures from natural products and the impact of bacterial resistance development in the clinical setting. The development process for a new antibiotic is discussed in detail, from the stage of early safety testing in animals through phase I, II, and III clinical trials.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM E. P., NEWTON G. G. The structure of cephalesporin C. Biochem J. 1961 May;79:377–393. doi: 10.1042/bj0790377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E. P. A glimpse of the early history of the cephalosporins. Rev Infect Dis. 1979 Jan-Feb;1(1):99–105. doi: 10.1093/clinids/1.1.99. [DOI] [PubMed] [Google Scholar]
- Acar J. F., Buu-Hoi A. Y. Resistance patterns of important gram-positive pathogens. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):41–47. doi: 10.1093/jac/21.suppl_c.41. [DOI] [PubMed] [Google Scholar]
- Barza M. Imipenem: first of a new class of beta-lactam antibiotics. Ann Intern Med. 1985 Oct;103(4):552–560. doi: 10.7326/0003-4819-103-4-552. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Lindsay P., Yih J., Hirano L., Lee D., Blomquist I. K. Efficacy of ciprofloxacin in experimental aortic valve endocarditis caused by a multiply beta-lactam-resistant variant of Pseudomonas aeruginosa stably derepressed for beta-lactamase production. Antimicrob Agents Chemother. 1986 Oct;30(4):528–531. doi: 10.1128/aac.30.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. M., Plowman D. Mechanisms of ampicillin resistance in Haemophilus influenzae from respiratory tract. Lancet. 1980 Feb 9;1(8163):279–280. doi: 10.1016/s0140-6736(80)90778-3. [DOI] [PubMed] [Google Scholar]
- Benbrook D. M., Miller R. V. Effects of norfloxacin on DNA metabolism in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986 Jan;29(1):1–6. doi: 10.1128/aac.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Structure-activity relationships among the aminoglycoside antibiotics: role of hydroxyl and amino groups. Antimicrob Agents Chemother. 1973 Oct;4(4):402–409. doi: 10.1128/aac.4.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. A., Zonszein J., Villamena P., Mittman N. Infectious diseases of the thyroid gland. Rev Infect Dis. 1983 Jan-Feb;5(1):108–122. doi: 10.1093/clinids/5.1.108. [DOI] [PubMed] [Google Scholar]
- Birnbaum J., Stapley E. O., Miller A. K., Wallick H., Hendlin D., Woodruff H. B. Cefoxitin, a semi-synthetic cephamycin: a microbiological overview. J Antimicrob Chemother. 1978 Jul;4(B):15–32. doi: 10.1093/jac/4.suppl_b.15. [DOI] [PubMed] [Google Scholar]
- Blaser J., Stone B. B., Groner M. C., Zinner S. H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987 Jul;31(7):1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock B. V., Edelstein P. H., Meyer R. D. Prospective comparative study of efficacy and toxicity of netilmicin and amikacin. Antimicrob Agents Chemother. 1980 Feb;17(2):217–225. doi: 10.1128/aac.17.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box S. J., Brown A. G., Gilpin M. L., Gwynn M. N., Spear S. R. MM 42842, a new member of the monobactam family produced by Pseudomonas cocovenenans. II. Production, isolation and properties of MM 42842. J Antibiot (Tokyo) 1988 Jan;41(1):7–12. doi: 10.7164/antibiotics.41.7. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H. Interspecies scaling, allometry, physiological time, and the ground plan of pharmacokinetics. J Pharmacokinet Biopharm. 1982 Apr;10(2):201–227. doi: 10.1007/BF01062336. [DOI] [PubMed] [Google Scholar]
- Brabin B. J. Epidemiology of infection in pregnancy. Rev Infect Dis. 1985 Sep-Oct;7(5):579–603. doi: 10.1093/clinids/7.5.579. [DOI] [PubMed] [Google Scholar]
- Brion N., Barge J., Godefroy I., Dromer F., Dubois C., Contrepois A., Carbon C. Gentamicin, netilmicin, dibekacin, and amikacin nephrotoxicity and its relationship to tubular reabsorption in rabbits. Antimicrob Agents Chemother. 1984 Feb;25(2):168–172. doi: 10.1128/aac.25.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Butterworth D., Cole M., Hanscomb G., Hood J. D., Reading C., Rolinson G. N. Naturally-occurring beta-lactamase inhibitors with antibacterial activity. J Antibiot (Tokyo) 1976 Jun;29(6):668–669. doi: 10.7164/antibiotics.29.668. [DOI] [PubMed] [Google Scholar]
- Bush K., Freudenberger J. S., Sykes R. B. Interaction of azthreonam and related monobactams with beta-lactamases from gram-negative bacteria. Antimicrob Agents Chemother. 1982 Sep;22(3):414–420. doi: 10.1128/aac.22.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cama L. D., Leanza W. J., Beattie T. R., Christensen B. G. Substituted penicillin and cephalosporin derivatives. I. Stereospecific introduction of the C-6(7) methoxy group. J Am Chem Soc. 1972 Feb 23;94(4):1408–1410. doi: 10.1021/ja00759a089. [DOI] [PubMed] [Google Scholar]
- Cartwright S. J., Waley S. G. beta-Lactamase inhibitors. Med Res Rev. 1983 Oct-Dec;3(4):341–382. doi: 10.1002/med.2610030402. [DOI] [PubMed] [Google Scholar]
- Chambers H. F. Methicillin-resistant staphylococci. Clin Microbiol Rev. 1988 Apr;1(2):173–186. doi: 10.1128/cmr.1.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M., Radwanski E., Loebenberg D., Lin C. C., Oden E., Symchowicz S., Gural R. P., Miller G. H. Interspecies pharmacokinetic scaling of Sch 34343. J Antimicrob Chemother. 1985 Jun;15 (Suppl 100):227–233. doi: 10.1093/jac/15.suppl_c.227. [DOI] [PubMed] [Google Scholar]
- Counts G. W. Cefoxitin: its role in treatment and prophylaxis of obstetric and gynecologic infections. Rev Infect Dis. 1988 Jan-Feb;10(1):76–91. doi: 10.1093/clinids/10.1.76. [DOI] [PubMed] [Google Scholar]
- Courtright J. B., Turowski D. A., Sonstein S. A. Alteration of bacterial DNA structure, gene expression, and plasmid encoded antibiotic resistance following exposure to enoxacin. J Antimicrob Chemother. 1988 Feb;21 (Suppl B):1–18. doi: 10.1093/jac/21.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., East S. J., Cornford R. J., Walker L. A. Properties of spontaneous Enterobacter cloacae mutants with temperature-conditional derepression of type I beta-lactamase synthesis. J Antimicrob Chemother. 1987 Apr;19(4):417–427. doi: 10.1093/jac/19.4.417. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Ross G. W., Boulton M. G. Effect of 7-alpha methoxy substitution of cephalosporins upon their affinity for the penicillin-binding proteins of E. coli K12. Comparison with antibacterial activity and inhibition of membrane bound model transpeptidase activity. J Antimicrob Chemother. 1979 Jul;5(4):391–398. doi: 10.1093/jac/5.4.391. [DOI] [PubMed] [Google Scholar]
- Daschner F. D., Just H. M., Jansen W., Lorber R. Netilmicin versus tobramycin in multi-centre studies. J Antimicrob Chemother. 1984 Jan;13 (Suppl A):37–45. doi: 10.1093/jac/13.suppl_a.37. [DOI] [PubMed] [Google Scholar]
- Daum S. J., Lemke J. R. Mutational biosynthesis of new antibiotics. Annu Rev Microbiol. 1979;33:241–265. doi: 10.1146/annurev.mi.33.100179.001325. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Davis B. D. Mechanism of bactericidal action of aminoglycosides. Microbiol Rev. 1987 Sep;51(3):341–350. doi: 10.1128/mr.51.3.341-350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedrick R. L. Animal scale-up. J Pharmacokinet Biopharm. 1973 Oct;1(5):435–461. doi: 10.1007/BF01059667. [DOI] [PubMed] [Google Scholar]
- Dekker A. W., Rozenberg-Arska M., Verhoef J. Infection prophylaxis in acute leukemia: a comparison of ciprofloxacin with trimethoprim-sulfamethoxazole and colistin. Ann Intern Med. 1987 Jan;106(1):7–11. doi: 10.7326/0003-4819-106-1-7. [DOI] [PubMed] [Google Scholar]
- Duthu G. S. Interspecies correlation of the pharmacokinetics of erythromycin, oleandomycin, and tylosin. J Pharm Sci. 1985 Sep;74(9):943–946. doi: 10.1002/jps.2600740907. [DOI] [PubMed] [Google Scholar]
- Eisenach K. D., Reber R. M., Eitzman D. V., Baer H. Nosocomial infections due to kanamycin-resistant, (R)-factor carrying enteric organisms in an intensive care nursery. Pediatrics. 1972 Sep;50(3):395–402. [PubMed] [Google Scholar]
- Eliopoulos G. M., Moellering R. C., Jr Azlocillin, mezlocillin, and piperacillin: new broad-spectrum penicillins. Ann Intern Med. 1982 Nov;97(5):755–760. doi: 10.7326/0003-4819-97-5-755. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Saunders J. R., Richmond M. H., Falkow S. Relationships among some R plasmids found in Haemophilus influenzae. J Bacteriol. 1977 Jul;131(1):356–362. doi: 10.1128/jb.131.1.356-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L. J., Goldenberg R. I., Poretz D. M. Combined ceftriaxone and surgical therapy for osteomyelitis in hospital and outpatient settings. Am J Surg. 1984 Oct 19;148(4A):1–4. [PubMed] [Google Scholar]
- FINLAND M., FRANK P. F., WILCOX C. In vitro susceptibility of pathogenic staphylococci to seven antibiotics. Am J Clin Pathol. 1950 Apr;20(4):325–334. doi: 10.1093/ajcp/20.4.325. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Hanson C. W., Stamm J. M., Vojtko C., Shipkowitz N. L., St Martin E. The frequency of in-vitro resistance development to fluoroquinolones and the use of a murine pyelonephritis model to demonstrate selection of resistance in vivo. J Antimicrob Chemother. 1987 Apr;19(4):449–465. doi: 10.1093/jac/19.4.449. [DOI] [PubMed] [Google Scholar]
- Finland M. Emergence of antibiotic resistance in hospitals, 1935-1975. Rev Infect Dis. 1979 Jan-Feb;1(1):4–22. doi: 10.1093/clinids/1.1.4. [DOI] [PubMed] [Google Scholar]
- Franco J. A., Eitzman D. V., Baer H. Antibiotic usage and microbial resistance in an intensive care nursery. Am J Dis Child. 1973 Sep;126(3):318–321. doi: 10.1001/archpedi.1973.02110190280006. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Joris B., Varetto L., Crine M. Structure-activity relationships in the beta-lactam family: an impossible dream. Biochem Pharmacol. 1988 Jan 1;37(1):125–132. doi: 10.1016/0006-2952(88)90764-2. [DOI] [PubMed] [Google Scholar]
- Gardner I. D. The effect of aging on susceptibility to infection. Rev Infect Dis. 1980 Sep-Oct;2(5):801–810. doi: 10.1093/clinids/2.5.801. [DOI] [PubMed] [Google Scholar]
- Gargallo D., Moros M., Coll R., Esteve M., Parés J., Xicota M. A., Guinea J. Activity of E-3846, a new fluoroquinolone, in vitro and in experimental cystitis and pyelonephritis in rats. Antimicrob Agents Chemother. 1988 May;32(5):636–641. doi: 10.1128/aac.32.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Cimarusti C. M., Sykes R. B. Binding of monobactams to penicillin-binding proteins of Escherichia coli and Staphylococcus aureus: relation to antibacterial activity. Antimicrob Agents Chemother. 1983 Jan;23(1):98–104. doi: 10.1128/aac.23.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Sykes R. B. Mode of action of azthreonam. Antimicrob Agents Chemother. 1982 Jun;21(6):950–956. doi: 10.1128/aac.21.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. N. Guidelines for evaluating new antimicrobial agents. J Infect Dis. 1987 Dec;156(6):934–941. doi: 10.1093/infdis/156.6.934. [DOI] [PubMed] [Google Scholar]
- Girard A. E., Girard D., English A. R., Gootz T. D., Cimochowski C. R., Faiella J. A., Haskell S. L., Retsema J. A. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987 Dec;31(12):1948–1954. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladue R. P., Bright G. M., Isaacson R. E., Newborg M. F. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989 Mar;33(3):277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Jackson D. B., Sherris J. C. Development of resistance to cephalosporins in clinical strains of Citrobacter spp. Antimicrob Agents Chemother. 1984 May;25(5):591–595. doi: 10.1128/aac.25.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C. Characterization of beta-lactamase induction in Enterobacter cloacae. Antimicrob Agents Chemother. 1983 Jan;23(1):91–97. doi: 10.1128/aac.23.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Goering R. V. Resistance to cefamandole: derepression of beta-lactamases by cefoxitin and mutation in Enterobacter cloacae. J Infect Dis. 1982 Jul;146(1):34–42. doi: 10.1093/infdis/146.1.34. [DOI] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Sanders W. E., Jr In vitro activity of furazlocillin (Bay k 4999) compared with those of mezlocillin, piperacillin, and standard beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Jun;15(6):783–791. doi: 10.1128/aac.15.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granich G. G., Krogstad D. J. Ion pair high-performance liquid chromatographic assay for ceftriaxone. Antimicrob Agents Chemother. 1987 Mar;31(3):385–388. doi: 10.1128/aac.31.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gural R., Lin C., Chung M., Oden E., Digiore C. Oral absorption and tolerance in man, of a new penem antibiotic, Sch 29428. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):239–243. doi: 10.1093/jac/9.suppl_c.239. [DOI] [PubMed] [Google Scholar]
- Haley R. W., Hightower A. W., Khabbaz R. F., Thornsberry C., Martone W. J., Allen J. R., Hughes J. M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982 Sep;97(3):297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Malpartida F., Kieser H. M., Ikeda H., Duncan J., Fujii I., Rudd B. A., Floss H. G., Omura S. Production of 'hybrid' antibiotics by genetic engineering. Nature. 1985 Apr 18;314(6012):642–644. doi: 10.1038/314642a0. [DOI] [PubMed] [Google Scholar]
- Jacobs J. Y., Livermore D. M., Davy K. W. Pseudomonas aeruginosa beta-lactamase as a defence against azlocillin, mezlocillin and piperacillin. J Antimicrob Chemother. 1984 Sep;14(3):221–229. doi: 10.1093/jac/14.3.221. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Medeiros A. A., O'Brien T. F., Pinto M. E., Jiang H. Broad-spectrum, transmissible beta-lactamases. N Engl J Med. 1988 Sep 15;319(11):723–724. doi: 10.1056/NEJM198809153191114. [DOI] [PubMed] [Google Scholar]
- Kahan F. M., Kropp H., Sundelof J. G., Birnbaum J. Thienamycin: development of imipenen-cilastatin. J Antimicrob Chemother. 1983 Dec;12 (Suppl 500):1–35. doi: 10.1093/jac/12.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- Kahan J. S., Kahan F. M., Goegelman R., Currie S. A., Jackson M., Stapley E. O., Miller T. W., Miller A. K., Hendlin D., Mochales S. Thienamycin, a new beta-lactam antibiotic. I. Discovery, taxonomy, isolation and physical properties. J Antibiot (Tokyo) 1979 Jan;32(1):1–12. doi: 10.7164/antibiotics.32.1. [DOI] [PubMed] [Google Scholar]
- Kirby B. D., Busch D. F., Citron D. M., Finegold S. M. Cefoxitin for treatment of infections due to anaerobic bacteria. Rev Infect Dis. 1979 Jan-Feb;1(1):113–117. doi: 10.1093/clinids/1.1.113. [DOI] [PubMed] [Google Scholar]
- Kirby W. M. EXTRACTION OF A HIGHLY POTENT PENICILLIN INACTIVATOR FROM PENICILLIN RESISTANT STAPHYLOCOCCI. Science. 1944 Jun 2;99(2579):452–453. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- Kobayashi G. S., Travis S. J., Medoff G. Comparison of fluconazole and amphotericin B in treating histoplasmosis in immunosuppressed mice. Antimicrob Agents Chemother. 1987 Dec;31(12):2005–2006. doi: 10.1128/aac.31.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEPPER M. H., DOWLING H. F., JACKSON G. G., HIRSCH M. M. Epidemiology of penicillin- and aureomycin-resistant staphylococci in a hospital population. AMA Arch Intern Med. 1953 Jul;92(1):40–50. doi: 10.1001/archinte.1953.00240190052003. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lindquist S., Normark S. Induction of chromosomal beta-lactamase expression in enterobacteria. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):43–50. doi: 10.1093/jac/18.supplement_c.43. [DOI] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCHESNEY E. W., FROELICH E. J., LESHER G. Y., CRAIN A. V., ROSI D. ABSORPTION, EXCRETION, AND METABOLISM OF A NEW ANTIBACTERIAL AGENT, NALIDIXIC ACID. Toxicol Appl Pharmacol. 1964 May;6:292–309. doi: 10.1016/0041-008x(64)90070-5. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Hopwood D. A. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. 1984 May 31-Jun 6Nature. 309(5967):462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- Mandell L. A. Effects of antimicrobial and antineoplastic drugs on the phagocytic and microbicidal function of the polymorphonuclear leukocyte. Rev Infect Dis. 1982 May-Jun;4(3):683–697. doi: 10.1093/clinids/4.3.683. [DOI] [PubMed] [Google Scholar]
- Maura A., Pino A. Evaluation of the DNA-damaging and mutagenic activity of oxolinic and pipemidic acids by the granuloma pouch assay. Mutagenesis. 1988 Sep;3(5):397–401. doi: 10.1093/mutage/3.5.397. [DOI] [PubMed] [Google Scholar]
- McAlpine J. B., Tuan J. S., Brown D. P., Grebner K. D., Whittern D. N., Buko A., Katz L. New antibiotics from genetically engineered actinomycetes. I. 2-Norerythromycins, isolation and structural determinations. J Antibiot (Tokyo) 1987 Aug;40(8):1115–1122. doi: 10.7164/antibiotics.40.1115. [DOI] [PubMed] [Google Scholar]
- Meulemans A., Vicart P., Mohler J., Vulpillat M., Pocidalo J. J. Continuous sampling for determination of pharmacokinetics in rat cerebrospinal fluid. Antimicrob Agents Chemother. 1986 Dec;30(6):888–891. doi: 10.1128/aac.30.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdalof B. H. Methods for obtaining drug time course data from individual small laboratory animals: serial microblood sampling and assay. Drug Metab Rev. 1976;5(2):295–310. doi: 10.3109/03602537609029981. [DOI] [PubMed] [Google Scholar]
- Mizen L., Woodnutt G., Kernutt I., Catherall E. J. Simulation of human serum pharmacokinetics of ticarcillin-clavulanic acid and ceftazidime in rabbits, and efficacy against experimental Klebsiella pneumoniae meningitis. Antimicrob Agents Chemother. 1989 May;33(5):693–699. doi: 10.1128/aac.33.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody J. A., Peterson L. R., Gerding D. N. In vitro activities of ureidopenicillins alone and in combination with amikacin and three cephalosporin antibiotics. Antimicrob Agents Chemother. 1984 Aug;26(2):256–259. doi: 10.1128/aac.26.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody M. M., de Jongh C. A., Schimpff S. C., Tillman G. L. Long-term amikacin use. Effects on aminoglycoside susceptibility patterns of gram-negative bacilli. JAMA. 1982 Sep 10;248(10):1199–1202. doi: 10.1001/jama.248.10.1199. [DOI] [PubMed] [Google Scholar]
- Mordenti J. Forecasting cephalosporin and monobactam antibiotic half-lives in humans from data collected in laboratory animals. Antimicrob Agents Chemother. 1985 Jun;27(6):887–891. doi: 10.1128/aac.27.6.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordenti J. Pharmacokinetic scale-up: accurate prediction of human pharmacokinetic profiles from animal data. J Pharm Sci. 1985 Oct;74(10):1097–1099. doi: 10.1002/jps.2600741017. [DOI] [PubMed] [Google Scholar]
- Nagarajan R., Boeck L. D., Gorman M., Hamill R. L., Higgens C. E., Hoehn M. M., Stark W. M., Whitney J. G. Beta-lactam antibiotics from Streptomyces. J Am Chem Soc. 1971 May 5;93(9):2308–2310. doi: 10.1021/ja00738a035. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Cefoxitin: an overview of clinical studies in the United States. Rev Infect Dis. 1979 Jan-Feb;1(1):233–239. doi: 10.1093/clinids/1.1.233. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Labthavikul P. The in-vitro activity of a novel penem FCE 22101 compared to other beta-lactam antibiotics. J Antimicrob Chemother. 1985 Sep;16(3):305–313. doi: 10.1093/jac/16.3.305. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Comparative studies of cefoxitin and cephalothin: an overview. Rev Infect Dis. 1979 Jan-Feb;1(1):144–151. doi: 10.1093/clinids/1.1.144. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The emergence of bacterial resistance and its influence on empiric therapy. Rev Infect Dis. 1983 Mar-Apr;5 (Suppl 1):S9–20. doi: 10.1093/clinids/5.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Jonsson M. Comparative in vitro antibacterial activity of Sch 34343, a novel penem antibiotic. Antimicrob Agents Chemother. 1985 Jan;27(1):128–131. doi: 10.1128/aac.27.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S., Kitao C., Sadakane N. The microbial transformation of tylosin by the spiramycin-producing strain, Streptomyces ambofaciens KA-1028. J Antibiot (Tokyo) 1980 Aug;33(8):911–912. doi: 10.7164/antibiotics.33.911. [DOI] [PubMed] [Google Scholar]
- Phillips I., Culebras E., Moreno F., Baquero F. Induction of the SOS response by new 4-quinolones. J Antimicrob Chemother. 1987 Nov;20(5):631–638. doi: 10.1093/jac/20.5.631. [DOI] [PubMed] [Google Scholar]
- Price K. E. Aminoglycoside research 1975-1985: prospects for development of improved agents. Antimicrob Agents Chemother. 1986 Apr;29(4):543–548. doi: 10.1128/aac.29.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rake J. B., Gerber R., Mehta R. J., Newman D. J., Oh Y. K., Phelen C., Shearer M. C., Sitrin R. D., Nisbet L. J. Glycopeptide antibiotics: a mechanism-based screen employing a bacterial cell wall receptor mimetic. J Antibiot (Tokyo) 1986 Jan;39(1):58–67. doi: 10.7164/antibiotics.39.58. [DOI] [PubMed] [Google Scholar]
- Reeves D. S., Bywater M. J., Holt H. A. Comparative in-vitro activity of Sch 29482, a new penem antibiotic. J Antimicrob Chemother. 1982 Feb;9 (Suppl 100):31–40. doi: 10.1093/jac/9.suppl_c.31. [DOI] [PubMed] [Google Scholar]
- Reyes M. P., Lerner A. M. Current problems in the treatment of infective endocarditis due to Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):314–321. doi: 10.1093/clinids/5.2.314. [DOI] [PubMed] [Google Scholar]
- Robertson M. H. Tetracycline-resistant beta-haemolytic streptococci in South-west Essex: decline and fall. Br Med J. 1973 Oct 13;4(5884):84–85. doi: 10.1136/bmj.4.5884.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolinson G. N. 6-APA and the development of the beta-lactam antibiotics. J Antimicrob Chemother. 1979 Jan;5(1):7–14. doi: 10.1093/jac/5.1.7. [DOI] [PubMed] [Google Scholar]
- Rosin E., Ebert S., Uphoff T. S., Evans M. H., Schultz-Darken N. J. Penetration of antibiotics into the surgical wound in a canine model. Antimicrob Agents Chemother. 1989 May;33(5):700–704. doi: 10.1128/aac.33.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman R. L., Peterson P. K. Immunodeficiency of the elderly. Rev Infect Dis. 1987 Nov-Dec;9(6):1127–1139. doi: 10.1093/clinids/9.6.1127. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance during therapy with the newer beta-lactam antibiotics: role of inducible beta-lactamases and implications for the future. Rev Infect Dis. 1983 Jul-Aug;5(4):639–648. doi: 10.1093/clinids/5.4.639. [DOI] [PubMed] [Google Scholar]
- Sanders C. V., Greenberg R. N., Marier R. L. Cefamandole and cefoxitin. Ann Intern Med. 1985 Jul;103(1):70–78. doi: 10.7326/0003-4819-103-1-70. [DOI] [PubMed] [Google Scholar]
- Sanders W. E., Jr, Sanders C. C. Sisomicin: a review of eight years' experience. Rev Infect Dis. 1980 Mar-Apr;2(2):182–195. doi: 10.1093/clinids/2.2.182. [DOI] [PubMed] [Google Scholar]
- Savani D. V., Perfect J. R., Cobo L. M., Durack D. T. Penetration of new azole compounds into the eye and efficacy in experimental Candida endophthalmitis. Antimicrob Agents Chemother. 1987 Jan;31(1):6–10. doi: 10.1128/aac.31.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y., Hanano M., Sugiyama Y., Iga T. Prediction of the disposition of beta-lactam antibiotics in humans from pharmacokinetic parameters in animals. J Pharmacokinet Biopharm. 1984 Jun;12(3):241–261. doi: 10.1007/BF01061720. [DOI] [PubMed] [Google Scholar]
- Schindler P. W., König W., Chatterjee S., Ganguli B. N. Improved screening for beta-lactam antibiotics. A sensitive, high-throughput assay using DD-carboxypeptidase and a novel chromophore-labeled substrate. J Antibiot (Tokyo) 1986 Jan;39(1):53–57. doi: 10.7164/antibiotics.39.53. [DOI] [PubMed] [Google Scholar]
- Schwartz R., Rodriguez W., Khan W., Ross S. The increasing incidence of Ampicillin-resistant Haemophilus influenzae. A cause of otitis media. JAMA. 1978 Jan 23;239(4):320–323. [PubMed] [Google Scholar]
- Simon H. J., Yin E. J. Microbioassay of antimicrobial agents. Appl Microbiol. 1970 Apr;19(4):573–579. doi: 10.1128/am.19.4.573-579.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. R., Ambinder R., Lipsky J. J., Petty B. G., Israel E., Levitt R., Mellits E. D., Rocco L., Longstreth J., Lietman P. S. Cefotaxime compared with nafcillin plus tobramycin for serious bacterial infections. A randomized, double-blind trial. Ann Intern Med. 1984 Oct;101(4):469–477. doi: 10.7326/0003-4819-101-4-469. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V., Zimmermann W. Binding of thienamycin and clavulanic acid to the penicillin-binding proteins of Escherichia coli K-12. Antimicrob Agents Chemother. 1977 Sep;12(3):406–409. doi: 10.1128/aac.12.3.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley E. O., Birnbaum J., Miller A. K., Wallick H., Hendlin D., Woodruff H. B. Cefoxitin and cephamycins: microbiological studies. Rev Infect Dis. 1979 Jan-Feb;1(1):73–89. doi: 10.1093/clinids/1.1.73. [DOI] [PubMed] [Google Scholar]
- Stapley E. O., Jackson M., Hernandez S., Zimmerman S. B., Currie S. A., Mochales S., Mata J. M., Woodruff H. B., Hendlin D. Cephamycins, a new family of beta-lactam antibiotics. I. Production by actinomycetes, including Streptomyces lactamdurans sp. n. Antimicrob Agents Chemother. 1972 Sep;2(3):122–131. doi: 10.1128/aac.2.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Swabb E. A. Modern beta-lactam antibiotics. Pharmacol Ther. 1985;29(3):321–352. doi: 10.1016/0163-7258(85)90007-5. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Cimarusti C. M., Bonner D. P., Bush K., Floyd D. M., Georgopapadakou N. H., Koster W. M., Liu W. C., Parker W. L., Principe P. A. Monocyclic beta-lactam antibiotics produced by bacteria. Nature. 1981 Jun 11;291(5815):489–491. doi: 10.1038/291489a0. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Wells J. S. Screening for beta-lactam antibiotics in nature. J Antibiot (Tokyo) 1985 Jan;38(1):119–121. doi: 10.7164/antibiotics.38.119. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., Baker C. N., Kirven L. A., Swenson J. M. Susceptibility of ampicillin-resistant Haemophilus influenzae to seven penicillins. Antimicrob Agents Chemother. 1976 Jan;9(1):70–73. doi: 10.1128/aac.9.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C. The development of antimicrobial resistance in staphylococci. J Antimicrob Chemother. 1988 Apr;21 (Suppl 100):9–17. doi: 10.1093/jac/21.suppl_c.9. [DOI] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuye A. In vitro activity and beta-lactamase stability of N-formimidoyl thienamycin compared to that of second and third generation cephalosporins. Chemotherapy. 1982;28(4):267–275. doi: 10.1159/000238089. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN M. J., LUEDEMANN G. M., ODEN E. M., WAGMAN G. H., ROSSELET J. P., MARQUEZ J. A., CONIGLIO C. T., CHARNEY W., HERZOG H. L., BLACK J. GENTAMICIN, A NEW ANTIBIOTIC COMPLEX FROM MICROMONOSPORA. J Med Chem. 1963 Jul;6:463–464. doi: 10.1021/jm00340a034. [DOI] [PubMed] [Google Scholar]
- Wells J. S., Hunter J. C., Astle G. L., Sherwood J. C., Ricca C. M., Trejo W. H., Bonner D. P., Sykes R. B. Distribution of beta-lactam and beta-lactone producing bacteria in nature. J Antibiot (Tokyo) 1982 Jul;35(7):814–821. doi: 10.7164/antibiotics.35.814. [DOI] [PubMed] [Google Scholar]
- Wick W. E., Preston D. A., White W. A., Gordee R. S. Compound 64716, a new synthetic antibacterial agent. Antimicrob Agents Chemother. 1973 Oct;4(4):415–420. doi: 10.1128/aac.4.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson A. E. The sensitivity of gonococci to penicillin. J Antimicrob Chemother. 1977 May;3(3):197–198. doi: 10.1093/jac/3.3.197. [DOI] [PubMed] [Google Scholar]
- Wise R. Norfloxacin--a review of pharmacology and tissue penetration. J Antimicrob Chemother. 1984 May;13 (Suppl B):59–64. doi: 10.1093/jac/13.suppl_b.59. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Norfloxacin: a new targeted fluoroquinolone antimicrobial agent. Ann Intern Med. 1988 Feb;108(2):238–251. doi: 10.7326/0003-4819-108-2-238. [DOI] [PubMed] [Google Scholar]
- Woodruff H. B., Hernandez S., Stapley E. O. Evolution of an antibiotic screening programme. A tribute to Justo Martinez Mata. Hindustan Antibiot Bull. 1979 Feb;21(3):71–84. [PubMed] [Google Scholar]
- Yao R. C., Mahoney D. F. Enzyme-linked immunosorbent assay for the detection of fermentation metabolites: aminoglycoside antibiotics. J Antibiot (Tokyo) 1984 Nov;37(11):1462–1468. doi: 10.7164/antibiotics.37.1462. [DOI] [PubMed] [Google Scholar]
- Young S. S. Do short-term tests predict rodent carcinogenicity? Science. 1988 Sep 2;241(4870):1232–1233. doi: 10.1126/science.3413488. [DOI] [PubMed] [Google Scholar]
- Zak O., Lang M., Cozens R., Konopka E. A., Mett H., Schneider P., Tosch W., Scartazzini R. Penems: in vitro and in vivo experiments. J Clin Pharmacol. 1988 Feb;28(2):128–135. doi: 10.1002/j.1552-4604.1988.tb05736.x. [DOI] [PubMed] [Google Scholar]


