Abstract
Background
Smaller hippocampal volumes in major depressive disorder (MDD) have been linked with earlier onset, previous recurrences and treatment refractoriness. The aim of our study was to investigate metabolite abnormalities in the hippocampus associated with past depressive illness burden.
Methods
Glutamate/glutamine (Glx), N-acetylaspartate (NAA) and choline (Cho), potential markers of glial/neuronal integrity and membrane turnover, respectively, were measured in adults with depression and healthy controls using a 3 T magnetic resonance spectroscopy scanner. Voxels were placed in the head of the right and left hippocampus. We controlled for systematic differences resulting from volume-of-interest (VOI) tissue composition and total hippocampal volume.
Results
Our final sample comprised a total of 16 healthy controls and 52 adult patients with depression in different stages of the illness (20 treatment-resistant/chronic, 18 remitted-recurrent and 14 first-episode), comparable for age and sex distribution. Patients with treatment-resistant/chronic and remitted-recurrent depression had significantly lower levels of Glx and NAA than controls, especially in the right hippocampal region (p ≤ 0.025). Diminished levels of Glx were correlated with longer illness duration (left VOI r = −0.34, p = 0.01). By contrast, Cho levels were significantly higher in patients with treatment-resistant/chronic depression than those with first-episode depression or controls in the right and left hippocampus (up to 19% higher; all p ≤ 0.025) and were consistently related to longer illness duration (right VOI r = 0.30, p = 0.028; left VOI r = 0.38, p = 0.004) and more previous episodes (right VOI r = 0.46, p = 0.001; left VOI r = 0.44, p = 0.001).
Limitations
The cross-sectional design and the inclusion of treated patients are the main limitations of the study.
Conclusion
Our results support that metabolite alterations within the hippocampus are more pronounced in patients with a clinical evolution characterized by recurrences and/or chronicity and add further evidence to the potential deleterious effects of stress and depression on this region.
Introduction
The hippocampus is considered a crucial brain region within the limbic system, playing a determinant role in emotion regulation and in major depressive disorder (MDD).1 It is also a highly stress-sensitive structure, since a dysregulation of glucocorticoid secretion in stress-induced situations — and the accompanying increased activity of excitatory amino acid neuro-transmitters — could result in either potentially reversible remodelling or irreversible cell damage in the hippocampus of patients with MDD.2 There is considerable evidence suggesting that the hippocampus could be seriously affected in patients with depression. Two meta-analyses3,4 concluded that the hippocampus was bilaterally smaller in people with MDD than matched controls. Numerous studies have linked such volume reductions to greater severity of depression, younger age at onset of illness, greater number of previous episodes or nonresponsiveness to treatment.5
In contrast, little is known about the neuropathological hippocampal changes that underly the past burden of illness in patients with MDD. Few postmortem works have proven cellular loss in the hippocampus of depressed patients,6 but reports are consistent about more subtle abnormalities in neurons and glia, such as those showing reductions in neuronal soma size and in neuropil (i.e., the dense tangle of axon terminals, dendrites and glial cell processes).7 These deficiencies in the cellular integrity and potential cellular loss in the hippocampus should be indirectly detectable by proton magnetic resonance spectroscopy (MRS), as it is a noninvasive neuroimaging technique that allows in vivo quantification of diverse metabolites in localized brain regions. Glutamate/glutamine (Glx or Glu/Gln, respectively), choline-containing compounds (Cho) or N-acetylaspartate (NAA) are some of the metabolites measurable by MRS that are most commonly implicated in MDD.8 Glutamate-related metabolites mainly represent the intracellular pool contained in pyramidal glutamatergic neurons and glia, particularly in astrocyctes. Therefore, decreases on that signal may account for defects in these cellular lines.9 Resonance of Cho can be conceived as an indirect measure of membrane turnover.10 Primarily present in neurons, NAA is suggested to be a marker of neuronal viability and functionality.11
In last decade, a large and compelling body of literature on biochemical changes in MDD has appeared, but only a few works have focused on the hippocampus, sometimes leading to inconsistent conclusions. Two studies reported diminished levels of Glx within the head of the left hippocampus in severely ill patients with treatment-resistant depression12 and untreated patients with first-episode depression,13 although a subsequent study by Milne and colleagues14 was unable to confirm such a finding. With regard to Cho concentrations, patients with treatment-resistant depression15 or with previous recurrences14 have been shown to have a greater spectroscopic signal in the hippocampus, although other authors have reported low or normal Cho levels in patients with a current depressive episode, with subsequent increases after successful response to electroconvulsive therapy16 or pharmacological treatment.13 Previous studies of hippocampal levels of NAA have failed to observe deficiencies in depressed patients8 despite alterations in neuroplasticity and/or neurogenesis having been repeatedly suggested to underlie MDD.3,17 Nevertheless, some works have revealed increases in NAA levels associated with treatment response.12,13
Some of the inconsistencies among the studies can be attributed to methodological issues8 (e.g., the lack of consideration for structural differences on the region studied14) whereas others can be related to clinical differences of the samples included. In fact, the influence of relevant illness course variables (e.g., duration of illness, number of previous episodes, age at onset of illness) on metabolite changes has not been well established until now. Our group recently reported abnormalities in Glu, NAA and Cho that were consistently related to the course of illness within the ventromedial prefrontal cortex (vmPFC) of patients with MDD, supporting the idea that greater past illness burden (measured by longer illness duration, earlier onset of illness, recurrences and treatment resistance) entails more defects in cellular neurochemistry.18
The present study investigated whether alterations of Glx, NAA and Cho levels in the left and right hippocampus differed between patients with MDD in distinct stages of illness and healthy controls, as previously observed in the vmPFC. We hypothesized that a substantial past illness burden would imply more metabolite abnormalities in the medial temporal region, independent of mood state.
Methods
Participants
A group of right-handed adult patients with MDD (DSM-IV-TR criteria) underwent a specifically designed magnetic resonance protocol. We recruited them from the outpatient service of the Department of Psychiatry, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain, as part of a bigger project whose main purpose was to establish in vivo neuroimaging markers of clinical illness burden. Most of the patients included in the present study were different from those included in previous work by our group18 (only 11 patients were used in both studies), as the quality of spectra varied considerably from vmPFC to medial temporal volume-of-interest (VOI) locations. Patients were split into 3 different groups. One comprised depressed participants with greater past illness burden (≥ 3 previous episodes of MDD) who were euthymic (score < 8 on the Hamilton Rating Scale for Depression [HAM-D]19) in at least the 6 months preceding the study (remitted-recurrent group). The second group comprised patients who had a chronic depressive disorder and whose last episode had a duration of more than 2 years, with no response to several antidepressants (treatment-resistant/chronic group). The third group comprised patients with a newly diagnosed first episode of depression who were currently depressed and being treated at the time of scanning. The first-episode group was representative of depressed patients with low illness burden (i.e., no previous episodes, shorter illness duration and later age at onset of illness), and participants were of similar age to those in the rest of the groups. Likewise, we recruited right-handed healthy controls with comparable age and sex distribution to that in the patient groups from among restaurant staff, technical support workers and graduate students at Hospital de la Santa Creu i Sant Pau. Controls received monetary compensation for their participation. Exclusion criteria for controls were lifetime psychiatric diagnoses, first-degree relatives with psychiatric diagnoses and clinically important physical or neurologic illness. We conducted semistructured interviews for all participants to collect demographic and clinical information, including comorbid Axis I conditions according to DSM-IV-TR criteria. Experienced clinical staff assessed current depressive symptoms using the HAM-D. Participants with a history of head injury, neurologic illness, alcohol or substance abuse were excluded from the study. The study was approved by the Research Ethics Board of Hospital de la Santa Creu i Sant Pau and was carried out in accordance with the Declaration of Helsinki. All participants provided informed consent after a full explanation of the study protocol.
MRS scanning procedure
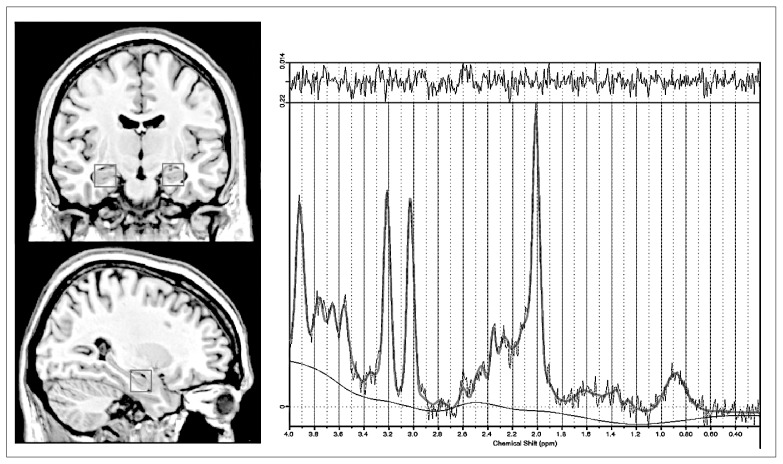
We obtained MRS images using a 3 T Philips Achieva scanner (software version 2.1.3.2) and a SENSE 8-channel head coil with a dedicated acquisition protocol. This included a 3-dimensional magnetization-prepared rapid-acquisition gradient echo (3D-MPRAGE) whole-brain sequence (turbo field echo, repetition time [TR] 6.7 ms, echo time [TE] 3.1 ms, voxel size 1 × 1 × 1.2), on which 1H-MRS (single-voxel spectroscopy with point resolved excitation spin-echo sequence; TR 2000 ms, TE 38 ms, numbers of signals averaging 128, VOI 2 × 2 × 2 cm, AutoWS-Prescan) images were obtained from the left and right medial temporal regions (Fig. 1, left side). First, borders of hippocampal voxels were aligned to the head of the hippocampus bilaterally as the anterior edge on the sagittal view. Then, the medial edge was set to be lateral to the medial border of the right and left hippocampus using the coronal series. Therefore, VOIs included the head of the hippocampus and surrounding white and grey matter, as well as cerebrospinal fluid (CSF).
Fig. 1.
Magnetic resonance images showing (left) the placement of the volumes of interest in a coronal and sagittal section of the medial temporal region and (right) a representative 1HMRS postprocessed with LCModel: PRESS acquisition at 3 T, with a repetition time of 2000 ms and echo time of 38 ms.
The 1H-MRS raw data were exported and then postprocessed using LCModel.20 This is an external reference method that provides concentrations (in millimoles) of the single metabolite peaks. For the purposes of the present study, we included the following metabolites: Glx (Glu/Gln peak, mainly dominated by Glu12), NAA (N-acetylaspartate + N-acetylaspartate-glutamate) and Cho (glycerophosphocholine and phosphocholine compounds). Apart from standard manufacturer’s quality assurance, weekly acquisitions of a home-built NAA phantom were also performed. All spectra were fit with LCModel (percent standard deviation [SD%] based on the Cramér–Rao lower bound), evaluated by an experienced observer (B.G.A.), and only good quality spectra (SD < 20% for quantifications of the main metabolites) were accepted (see Fig. 1, right side, for a representative 1H-MRS). We used absolute metabolite levels instead of ratios with creatine to avoid a bias through systematic drifts in the magnitude of the creatine resonance, as suggested by previous studies.18,21–24
VOI tissue segmentation and total hippocampal volume
To control for the effect of potential structural differences in the region studied, we assessed tissue composition within the VOIs and total hippocampal volume as follows. The amount of each tissue — grey matter, white matter and CSF — was quantified for each VOI, so the scans were first separated in the 3 different tissues using SPM8 software (Wellcome Trust Centre for Neuroimaging, www.fil.ion.ucl.ac.uk/spm) running under MATLAB 7.8.0 (MathWorks). We then manually reoriented the scans according to Montreal Neurological Institute space, aligning the anterior and posterior commissure in the same axis. When the images were segmented we used the “native space” option, which produces a tissue class image (c*) in the same anatomic space of the original nonsegmented image. After that, the VOI of 2 cm3 was resituated in the original images using ITK-SNAP software version 2.0.0.25 Finally, the mask of each participant was multiplied by his or her own image for the 3 required tissues using MATLAB, and then we quantified the volume of the resulting multiplied images. Following this procedure, we obtained the amount of grey matter, white matter and CSF of the left and right VOI. Measurement of hippocampal volumes was conducted automatically for all the participants using FreeSurfer image analysis software (http://surfer.nmr.mgh.harvard.edu/). Further details of the procedure used can be found elsewhere.26
Statistical analysis
We performed statistical analyses using SPSS software version 18. We compared the demographic and clinical characteristics of the groups using 1-way analysis of variance or non-parametric tests, as appropriate. Magnetic resonance spectrocopy measures were analyzed using multivariate analysis of covariance (MANCOVA; 1 per side), with group as the between-subjects factor, metabolite values as the dependent variables and total hippocampal volumes and VOI tissue differences as covariates. We considered these results to be significant at p < 0.05. Subsequently, metabolites exhibiting significant differences in the MANCOVA were included in bivariate correlation analyses to explore the influence of clinical variables. Given the exploratory purposes of the correlation analyses and the risk of type I errors associated with multiple comparisons, all patients with MDD were examined together, and we considered results to be significant at p < 0.01.
Results
We recruited 60 adult patients with MDD (20 in the remitted-recurrent group, 20 in the treatment-resistant/chronic group and 20 in the first-episode group) and 20 controls for participation in this study. After visual assessment of the spectra, 87.5% of the participants recruited had results that were adequate for analysis. The average SD% of included spectra was of 12% for Glx, 9% for NAA and 4.2% for Cho. An exploratory analysis showed that the assumption of homoscedasticity among study groups was satisfied for all metabolites (p > 0.05). On the basis of this assessment, our final sample included 52 patients with MDD (14 in the first-episode group, 18 in the remitted-recurrent group and 20 in the treatment-resistant/chronic group) and 16 healthy controls.
Demographic and clinical data are summarized in Table 1. After selection of good spectra, the 4 groups did not significantly differ in age or in sex. Antidepressant treatment was not distributed similarly across the patient groups. Treatment-resistant/chronic patients received more second-line antidepressants, antipsychotics, mood stabilizers and combined regimens than the other patients.
Table 1.
Participant demographic and clinical characteristics
| Group; mean (SD)* | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Control, n = 16 | First-episode depression, n = 14 | Remitted-recurrent depression, n = 18 | Treatment-resistant/chronic depression, n = 20 |
| Sex, % female | 61.1 | 62.5 | 88.9 | 80 |
| Age, yr | 43.4 (9.1) | 42.5 (8.7) | 47.3 (9) | 49.6 (7.6) |
| Years of education | 14.2 (3.1) | 13.8 (3.3) | 13.4 (3.1) | 13.2 (3.5) |
| Age at onset, yr†‡ | NA | 41.8 (8.7) | 26.3 (9.4) | 27.9 (8.5) |
| Duration of illness, mo†‡ | NA | 6.8 (4.3) | 245.8 (125.9) | 280.3 (142.4) |
| No. of previous episodes†‡ | NA | 1.0 (0) | 5.1 (4.7) | 6.0 (6.2) |
| HAM-D at scanning†§¶** | 2.8 (1.4) | 12.6 (7.4) | 2.6 (1.5) | 20.9 (4.6) |
| Treatment, % | ||||
| Treatment | ||||
| Antidepressant | ||||
| SSRI or SSNRI† | NA | 100 | 72.2 | 85.0 |
| TCA or MAOI‡ | NA | 6.3 | 16.7 | 40.0 |
| Othersঠ| NA | 6.3 | 22.2 | 55.0 |
| Combinationঠ| NA | 12.5 | 27.8 | 80.0 |
| No antidepressant | NA | 0 | 11.1 | 3.7 |
| Stabilizer‡ | NA | 0 | 16.7 | 30.0 |
| Antipsychoticঠ| NA | 6.3 | 11.1 | 40.0 |
| Benzodiazepine | NA | 50.0 | 33.0 | 50.0 |
Antipsychotic = mainly atypical antipsychotics associated with antidepressants; Combination = designs concomitant use of antidepressants with different mechanisms of action (e.g., SSRI with reboxetine); HAM-D = Hamilton Depression Rating Scale;19 MAOI = monoamine oxidase inhibitors; NA = not applicable; Others = noradrenaline reuptake inhibitors, noradrenaline and dopamine reuptake inhibitors, tetracyclic antidepressants, mirtazapine, metilfenidate or trazodone; SD = standard deviation; SSNRI = selective serotonin and noradrenaline reuptake inhibitors; SSRI = selective serotonin reuptake inhibitors; Stabilizer = includes anticonvulsants and mostly lithium; TCA = tricyclic antidepressant.
Unless otherwise indicated.
Significant differences between first-episode and remitted-recurrent depression.
Significant differences between first-episode and treatment-resistant/chronic depression.
Significant differences between first-episode depression and control.
Significant differences between treatment-resistant/chronic and remitted-recurrent depression.
Significant differences between treatment-resistant/chronic depression and control.
Table 2 displays absolute hippocampal volumes across the participant groups. There were significant group effects for the left (F = 4.5, p = 0.006) and the right hippocampus (F = 6.1, p = 0.001), with smaller volumes in the remitted-recurrent group (right p = 0.039) and the treatment-resistant/chronic group (right p < 0.001; left p = 0.004) than in the first-episode group. Table 2 also includes the percentage of white matter, grey matter and CSF segmentations of the selected VOIs. Both white and grey matter segments differed among the 4 groups (right VOI: F = 4.55, p = 0.006 and F = 3.13, p = 0.032, respectively; left VOI: F = 5.45, p = 0.002 and F = 4.96, p = 0.004, respectively). This difference indicates that patients with remitted-recurrent depression had a significantly higher proportion of grey matter and significantly lower proportion of white matter than healthy controls and patients with treatment-resistant/chronic depression. We found no differences in CSF among the 4 groups. Therefore, total hippocampal volumes and segmentation proportions of grey and white matter were included as covariates in the subsequent multivariate analyses.
Table 2.
Bilateral hippocampal volumes and proportions of grey matter, white matter and cerebrospinal fluid of left and right medial temporal voxels of interest
| Measure | Control, n = 16 | First-episode depression, n = 14 | Remitted-recurrent depression, n = 18 | Treatment-resistant/chronic depression, n = 20 |
|---|---|---|---|---|
| Hippocampal volume, mean (SD) mm3 | ||||
| Left | 4055.1 (419.7) | 4280.3 (482.4) | 3924.2 (494.5) | 3767.3 (460.5)* |
| Right | 4157.9 (323.1) | 4458.7 (427.8) | 4045.9 (568.4)† | 3858.3 (355.5)* |
| Tissue segmentation, % | ||||
| Left VOI | ||||
| Grey matter | 70.2 | 73.2 | 74.8ठ| 70.4 |
| White matter | 24.8 | 22.4 | 19.8ठ| 25.1 |
| Cerebrospinal fluid | 5.1 | 4.8 | 5.4 | 4.5 |
| Right VOI | ||||
| Grey matter | 69.3 | 70.2 | 73.4 | 70.2 |
| White matter | 25.9 | 24.6 | 21.2ठ| 25.0 |
| Cerebrospinal fluid | 4.9 | 5.2 | 5.4 | 4.8 |
SD = standard deviation; VOI = volumes of interest.
Significant differences between first-episode and treatment-resistant/chronic depression (left p = 0.004; right p < 0.001).
Significant differences between first-episode and remitted-recurrent depression (p = 0.039).
Significant differences between remitted-recurrent depression and controls (left VOI: grey matter p = 0.018, white matter p = 0.014; right VOI: white matter p = 0.009).
Significant differences between remitted-recurrent and treatment-resistant/chronic depression (left VOI: grey matter p = 0.011, white matter p = 0.003; right VOI: white matter p = 0.033).
Metabolite differences among groups
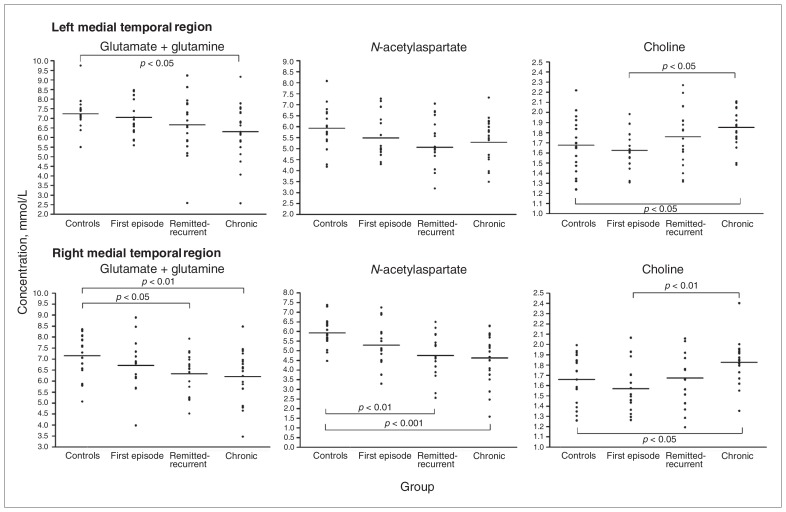
Metabolite concentrations in the medial temporal region of patients and controls are displayed in Figure 2. The 1-way MANCOVA for right VOI showed an effect of group (F = 3.9, p < 0.001), but no effect for either white matter (F = 0.7, p = 0.58), grey matter (F = 1.3, p = 0.28) or hippocampal volume (F = 1.2, p = 0.31). Between-subjects effects appeared for Glx (F = 2.7, p = 0.05), NAA (F = 6.6, p = 0.001) and Cho (F = 6.8, p < 0.001). Bonferroni post hoc analyses showed significantly lower values of Glx and NAA in patients with remitted-recurrent and treatment-resistant/chronic depression than in controls (p ≤ 0.025). Concentrations of Cho showed the opposite pattern (p < 0.001), where the highest values were observed in treatment-resistant/chronic depression and the lowest in first-episode depression and healthy controls (all p ≤ 0.034).
Fig. 2.
Absolute metabolite concentrations (glutamate/glutamine [Glx], N-acetylaspartate [NAA] and choline [Cho]) in left and right medial temporal regions of healthy controls and patients with first-episode, remitted-recurrent or treatment-resistant/chronic depression. Group means are displayed as horizontal lines. The p values are derived from Bonferroni post hoc tests. (Top) The Glx levels in patients with treatment-resistant/chronic depression were 14% lower than those in controls (p = 0.024, Cohen d = 0.81). The Cho concentrations in patients with treatment-resistant/chronic depression were 19% higher than those in patients with a first episode (p = 0.006, Cohen d = 1.89) and 12% higher than those in controls (p = 0.025, Cohen d = 0.80). Differences of NAA did not reach statistical significance. (Bottom) The Glx levels in patients with treatment-resistant/chronic depression were 15% lower than those in controls (p = 0.008, Cohen d = 1.04), and their NAA levels were 21% lower than those in controls (p < 0.001, Cohen d = 1.11). The Glx levels in patients with remitted-recurrent depression were 14% lower than those in controls (p = 0.025, Cohen d = 1.05), and their NAA levels were 17% lower than those in controls (p = 0.002, Cohen d = 1.04). The Cho concentrations in patients with treatment-resistant/chronic depression were 13% higher than those in patients with a first episode (p = 0.002, Cohen d = 0.9) and 6% higher than those in controls (p = 0.034, Cohen d = 0.4).
The 1-way MANCOVA for the left VOI also indicated a group effect (F = 2.6, p = 0.007), regardless of tissue composition (F = 1.2, p = 0.33 for white matter and F = 1.7, p = 0.17 for grey matter) or hippocampal volume (F = 1.1, p = 0.36). In this case, between-subject effects were significant for Glx (F = 2.7, p = 0.049) and Cho (F = 4.9, p = 0.004) and for NAA at a trend level (F = 2.5, p = 0.07). Cho levels were significantly higher in patients with treatment-resistant/chronic depression than in those with first-episode depression or in controls (p = 0.006 and p = 0.025, respectively), and Glx concentrations were significantly lower in patients with treatment-resistant/chronic depression than in controls (p = 0.024). Other post hoc analyses did not reach significance.
Influence of clinical variables
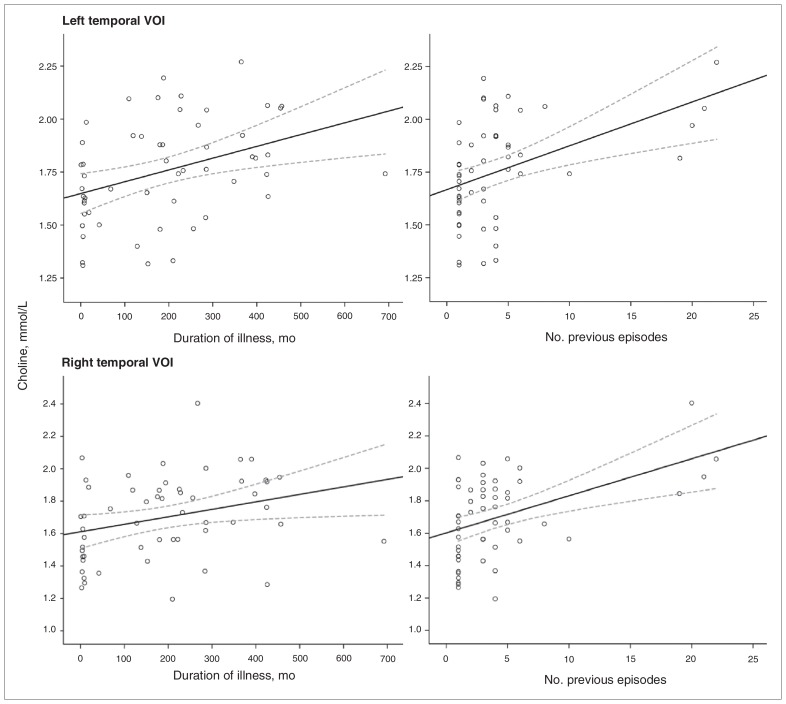
As shown in Figure 3, higher Cho concentrations were related to longer illness duration (right VOI r = 0.30, p = 0.028; left VOI r = 0.38, p = 0.004) and to more previous episodes (right VOI r = 0.46, p = 0.001; left VOI r = 0.44, p = 0.001). On the other hand, diminished levels of Glx were correlated with longer illness duration (left VOI r = −0.34, p = 0.008). To ensure that outliers were not responsible for the whole effect, we performed nonparametric Spearman rho correlations with the same variables, and the results were confirmed for Cho but not for Glx (data not shown). By contrast, concentrations of NAA did not correlate with any of the clinical variables (p > 0.10), nor was there a significant association between metabolite levels and HAM-D scores in our sample (p > 0.20).
Fig. 3.
Scatter plots showing correlation of choline levels within the left and right medial temporal regions of depressed patients (n = 52) with duration of illness and number of previous episodes. Bold and dotted lines represent the interpolation lines and their 95% confidence intervals, respectively. VOI = volumes of interest.
Discussion
Our data indicated that patients with MDD whose course of illness was characterized by multiple relapses or a chronic evolution presented lower levels of Glx and NAA than healthy controls, especially in the right medial temporal region. When patients with greater illness burden were compared with patients with a more benign course of MDD (i.e., first episode, later onset and shorter duration), differences emerged in both hemispheres for Cho concentrations, whereas patients with treatment-resistant/chronic illness displayed the highest levels. Changes of Glx and Cho peaks correlated in the opposite direction with a longer duration of illness, and higher Cho concentrations were also consistently associated with more previous depressive episodes.
Selected VOIs were situated in a medial temporal region that mainly included the head of the hippocampus. Converging evidence from clinical and preclinical studies underlines the central role of this highly stress-sensitive brain structure on MDD. Volumetric hippocampal reductions have been replicated in depressed patients, and they are preferentially observed in patients with an earlier onset of illness, recurrent evolution or treatment refractoriness,5 as confirmed by our present results.
On the other hand, scant literature on hippocampus neuropathological analyses of patients with MDD reveals increases in the mean densities of neurons and glia contrasting with reductions in neuronal soma size27 and diminished levels of neurotrophic and astrocyte viability markers, which have been putatively related to dysfunctional adult hippocampal neurogenesis.7 An earlier study observed neuronal loss in CA1 and CA4 regions, but the phenomenon, although convincing, was only moderate.6 However, reductions in neuropil seem to be prominent27 and were the most probable explanation for the cumulative hippocampal shrinkage.28 Whatever the deficiencies in the cellular integrity and neuropil, these would be likely to underlie the abnormalities in spectroscopic resonances reported herein. It has to be stressed that metabolic alterations found in our patients were evident even when we controlled for differences in total hippocampal volumes and VOI tissue composition.
Milne and colleagues14 did not find defects in Glx concentration in a sample of patients with recurrent MDD compared with healthy controls. In contrast, Michael and colleagues12 reported lower Glx levels in the hippocampus/amygdala of severely ill patients with treatment-resistant depression compared with healthy controls; the levels recovered after successful electroconvulsive therapy. Diminished levels of Glx were also described in a sample mainly composed of untreated patients with a first depressive episode.13 It has been assumed that Glx signal reflects the status of glia and pyramidal glutamatergic neurons.9 In the present study, the pattern of reduced Glx concentrations seen in the patients with treatment-resistant/chronic and remitted-recurrent depression was not apparent in patients presenting for a first episode, suggesting that long-lasting depressive history may have an important influence on the glial integrity and glutamatergic metabolism of the hippocampus. The presence of diminished Glx levels in patients with remitted-recurrent illness in our sample extends the previously reported findings, emphasizing the possibility that these abnormalities can remain within the hippocampus after resolution of a depressive episode.
A quite similar pattern of lower concentrations in chronic and recurrent forms of MDD was observed for NAA in our sample. N-acetylaspartate has been described as a sensitive marker of neuronal functionality/viability,10 and defects on that signal could reflect damage or loss of neurons, reductions of interneuronal neuropil, neuronal or axonal metabolic dysfunction or some combination of these processes.29 Reductions of NAA have been observed in patients with bipolar30 and pediatric depression,31 but there is no consistent agreement for altered NAA levels in the hippocampus in previous MRS studies of adult unipolar depression.8 The lack of control for VOI tissue composition in some of these studies might represent a serious limitation, which we attempted to correct herein. At least 2 independent groups reported increases of NAA concentrations after successful response to either electroconvulsive or pharmacological therapy.12,13 These findings suggest that antidepressant treatments could elicit neurotrophic effects, with a positive role in restoring neuronal integrity being therefore relevant for clinical recovery. Strikingly, 2 preclinical reports revealed decreased NAA concentrations associated with reductions of hippocampal volume and neurogenesis in chronically stressed animals, changes that were prevented with antidepressant treatment.32,33 A recent postmortem study in which quantification of metabolites was performed directly on brain tissue reported NAA decrements in different subcortical structures, including the hippocampus and amygdala, in patients with long-term, severe MDD.29 Although in the present study NAA did not correlate with any of the explored clinical variables, reduction of its levels in the right hippocampus was conspicuous, reaching up to 17% and 21% among patients with remitted-recurrent and treatment-resistant/chronic depression, respectively. These findings support the notion that hippocampal neuronal damage could be present in depression when the burden of illness is prominent, even in asymptomatic states.
The increased Cho signal seen bilaterally in patients with treatment-resistant/chronic depression compared with healthy controls and patients with first-episode depression represents one of the most intriguing findings. The Cho resonance mainly reflects changes in the concentrations of phosphocholine and glycerophosphocholine, a precursor and a degradation product, respectively, of membrane phospholipids. For this reason, it is considered a potential biomarker for the status of membrane metabolism.11 Our results are in agreement with those of previous studies that found increased Cho/creatine ratios in a sample of patients with treatment-resistant MDD15 and higher absolute Cho concentrations in patients with extensive past illness (but not in those with a first episode) than age-matched healthy controls.14 We also describe a robust association between higher Cho levels and longer duration of illness and more recurrences. This association provides preliminary evidence that increases in Cho concentrations might reveal an augmented membrane turnover related to past illness burden.
In contrast with the treatment-resistant/chronic group, our sample of patients with a first depressive episode displayed the lowest concentrations of Cho. Block and colleagues13 also reported marginally diminished baseline Cho peaks in untreated patients with a first episode of depression. Interestingly, they reported that low baseline Cho levels and a subsequent increase after 8 weeks of treatment were associated with good response. An earlier study16 had already described lower Cho concentrations that increased after a successful course of electroconvulsive therapy in patients with current, severe depression. Therefore, low Cho levels represent a marker of good response to treatment, but increments of such levels seem to be necessary for recovery from depression. However, in light of our results, excessive levels of Cho would mark resistance to treatment. From a neurobiological perspective, increases of Cho would reflect changes in membrane turnover, precipitated by either antidepressant treatment or endogenous mechanisms, as expression of the brain’s efforts to restore abnormal neural functioning. These changes, however, might end up being insufficient and even pathologically maintained in chronic stages of depression. In fact, it would be possible that increases in Cho peak occur at the expense of membrane precursors (as phosphocholine) during initial stages of treatment and at the expense of degradation products (as glycerophosphocholine) in patients with chronic depression that is unresponsive to treatment, although current MRS techniques do not allow this distinction to be made.
Abnormalities of Glx, NAA and Cho signal described herein within the hippocampus are in accordance with those reported in the vmPFC in a previous study of our group composed of patients in different stages of MDD.18 Alterations in vmPFC cellular neurochemistry were also consistently related to illness-course variables. The well-established structural and functional connection between the hippocampus, parahippocampal gyrus and prefrontal areas — all strongly linked with the physiopathology of mood disorders1 — may explain the similarity of metabolite alterations observed in our 2 studies. In fact, these areas, particularly the hippocampus, are known to play an important role in the response to stress, being extremely sensitive to its potential neurotoxic effects under chronic stress conditions. Glial and neuronal pathology and remodelling processes — as reflected by reductions of Glx and NAA and by increases in Cho, respectively — in both the vmPFC and medial temporal region of patients with MDD would then be considered a phenomenon associated with the burden of the disease.
Although other studies have reflected metabolite changes associated with treatment response or symptom severity, we did not obtain similar results. Our sample included a variety of patients, some of them entirely comparable in terms of age at onset of illness and illness duration but extremely different in terms of symptomatic state (treatment-resistant/chronic v. remitted-recurrent), who finally displayed notable similarities on metabolite abnormalities. This is a preliminary cross-sectional study designed to explore the role of past illness burden on brain metabolites, but we do not rule out the possibility of an association between metabolite resonances and symptoms from alternative approaches (e.g., analysis of specific groups of patients, longitudinal designs).
Limitations
There are some methodological limitations of this study that need to be mentioned. First, the hippocampus is a complex anatomic region that often entails spectral quality–related problems. However, MRS data were acquired using a 3 T magnetic resonance scanner, which provides a higher signal-to-noise ratio and better separation among metabolite peaks than commonly used 1.5 or 2 T scanners. Second, the present study was spatially limited to the anterior region of the hippocampus, and it remains to be clarified whether our results could be extrapolated to the whole structure. Nonetheless, we report data for both hemispheres in contrast to many previous works that have centred exclusively on the left temporal region.12–15 Third, a systematic bias in voxel placement/selection could have given rise to different tissue composition of the VOIs among the groups and accounted for differences in the measured spectra. To overcome this potential limitation, we controlled for VOI tissue segmentation in the main analyses. Fourth, the sample comprised a fairly large number of individuals, including healthy controls and a representative, well-characterized group of outpatients in different stages of illness, but the cross-sectional nature of the study should lead us to be cautious in interpreting the findings. Longitudinal studies are warranted to provide definitive evidence of the role of illness burden on brain metabolites in patients with MDD. Finally, the lack of a drug washout period represents an additional limitation. Given that the hypothesis to be tested required the inclusion of severely ill outpatients, there were inherent ethical challenges (e.g., patients’ conditions could worsen without medication). Moreover a washout period that was not long enough might have yielded metabolic changes related more to psychotropic withdrawal than with the illness, per se. To address these challenges, we decided to maintain medication in all patients and recruited an age- and sex-matched group of patients treated for a first episode as well as a non-treated healthy control group. The presence of treatment did not entail substantial metabolic differences between these latter groups. But, as might have been expected, patients with treatment-resistant/chronic illness more often received combined treatments (more than 1 antidepressant and/or atypical antipsychotics). For their part, patients with remitted-recurrent or first-episode depression received an almost equivalent antidepressant regimen, although the former group had a longer history of treatment. Nevertheless, the direction of the metabolic changes observed in our sample fit in with previous studies based on medication-free patients.12,13 Altogether, these comments suggest that the abnormalities described in the present study cannot be merely attributable to the treatment itself.
Conclusion
Our data support the notion that metabolite alterations within the hippocampus are more pronounced in patients with MDD whose clinical evolution is characterized by recurrences and/or chronicity. This adds further evidence to the link between hippocampal formation and the potential neurotoxic effects of stress and depression, suggesting that abnormalities in Glx, NAA and Cho spectra are closely related to the past burden of illness. Defects of glial/neuronal integrity and membrane turnover could underlie the metabolic changes reported herein, although further research is needed to establish the specific cellular processes involved in MDD over the course of the illness.
Acknowledgments
This study is funded by 2 grants of the Fondo de Investigación Sanitaria (FIS: PI10/00372 awarded to the Centro de Investigación Biomédica en Red de Salud Mental CIBERSAM, where M.J. Portella was the principal investigator; FIS: 07/00770 awarded to the Institut d’Investigació Biomédica Sant Pau, IIB Sant Paul, where B. Gómez-Ansón was the principal investigator) from the Instituto de Salud Carlos III, by the Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM) and by the Centro de Investigación Biomédica para Enfermedades Neurodegenerativas (CIBERNED). J. de Diego-Adeliño is funded by the Instituto de Salud Carlos III through a “Rio Hortega” research fellowship. M.J. Portella is funded by the Ministerio de Ciencia e Innovación of the Spanish Government and by the Instituto de Salud Carlos III through a “Miguel Servet” research contract (CP10-00393), cofinanced by the European Regional Development Fund (ERDF; 2007–2013). We thank the staff of the Department of Psychiatry and the Department of Neuroradiology of Hospital de la Santa Creu i Sant Pau who generously provided their time and experience. Finally, we also thank the patients who participated in the study for their cooperation.
Footnotes
Competing interests: As above for V. Pérez declares having received educational honoraria from Sanofi-Aventis, Lundbeck, Pfizer, Astra-Zeneca and Eli Lilly, and research funding from Boehringer-Ingelheim for this work. E. Alvarez has received consulting and educational honoraria from several pharmaceutical companies including Eli Lilly, Sanofi-Aventis, Lundbeck and Pfizer, and he has participated as main local investigator in clinical trials from Eli Lilly, Bristol-Myers and Sanofi-Aventis and also as national coordinator of clinical trials from Servier and Lundbeck. None declared for J. de Diego-Adeliño, M.J. Portella, B. Gómez-Ansón, O. López-Moruelo, M. Serra-Blasco, Y. Vives, D. Puigdemont and R. Pérez-Egea.
Contributors: J. de Diego-Adeliño, M.J. Portella, B. Gómez-Ansón and V. Pérez designed the study and acquired and analyzed the data. O. López-Moruelo, M. Serra-Blasco, Y. Vives, D. Puigdemont, R. Pérez-Egea and E. Alvarez also acquired the data. J. de Diego-Adeliño and M.J. Portella wrote the article, which all authors reviewed and approved for publication.
References
- 1.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–26. [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell S, Marriott M, Nahmias C, et al. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 4.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 5.McKinnon MC, Yucel K, Nazarov A, et al. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- 6.Lucassen PJ, Müller MB, Holsboer F, et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol. 2001;158:453–68. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43:947–61. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–94. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulanger Y, Labelle M, Khiat A. Role of phospholipase A(2) on the variations of the choline signal intensity observed by 1H magnetic resonance spectroscopy in brain diseases. Brain Res Brain Res Rev. 2000;33:380–9. doi: 10.1016/s0165-0173(00)00037-0. [DOI] [PubMed] [Google Scholar]
- 11.Moffett JR, Ross B, Arun P, et al. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michael N, Erfurth A, Ohrmann P, et al. Neurotrophic effects of electroconvulsive therapy: a proton magnetic resonance study of the left amygdalar region in patients with treatment-resistant depression. Neuropsychopharmacology. 2003;28:720–5. doi: 10.1038/sj.npp.1300085. [DOI] [PubMed] [Google Scholar]
- 13.Block W, Träber F, von Widdern O, et al. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12:415–22. doi: 10.1017/S1461145708009516. [DOI] [PubMed] [Google Scholar]
- 14.Milne A, MacQueena GM, Yucela K, et al. Hippocampal metabolic abnormalities at first onset and with recurrent episodes of a major depressive disorder: a proton magnetic resonance spectroscopy study. Neuroimage. 2009;47:36–41. doi: 10.1016/j.neuroimage.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Mervaala E, Föhr J, Könönen M, et al. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med. 2000;30:117–25. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- 16.Ende G, Braus DF, Walter S, et al. The hippocampus in patients treated with electroconvulsive therapy: a proton magnetic resonance spectroscopic imaging study. Arch Gen Psychiatry. 2000;57:937–43. doi: 10.1001/archpsyc.57.10.937. [DOI] [PubMed] [Google Scholar]
- 17.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 18.Portella MJ, de Diego-Adeliño J, Gómez-Ansón B, et al. Ventromedial prefrontal spectroscopic abnormalities over the course of depression: a comparison among first episode, remitted recurrent and chronic patients. J Psychiatr Res. 2011;45:427–34. doi: 10.1016/j.jpsychires.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–4. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- 21.Li BS, Wang H, Gonen O. Metabolite ratios to assumed stable creatine level may confound the quantification of proton brain MR spectroscopy. Magn Reson Imaging. 2003;21:923–8. doi: 10.1016/s0730-725x(03)00181-4. [DOI] [PubMed] [Google Scholar]
- 22.Gruber S, Frey R, Mlynárik V, et al. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H MRS at 3 Tesla. Invest Radiol. 2003;38:403–8. doi: 10.1097/01.rli.0000073446.43445.20. [DOI] [PubMed] [Google Scholar]
- 23.Frye MA, Watzl J, Banakar S, et al. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology. 2007;32:2490–9. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- 24.Brennan BP, Hudson JI, Jensen JE, et al. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology. 2010;35:834–46. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Resmini E, Santos A, Gómez-Anson B, et al. Verbal and visual memory performance and hippocampal volumes, measured by 3-Tesla magnetic resonance imaging, in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97:663–71. doi: 10.1210/jc.2011-2231. [DOI] [PubMed] [Google Scholar]
- 27.Stockmeier CA, Mahajan GJ, Konick LC, et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–50. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–60. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds LM, Reynolds GP. Differential regional N-acetylaspartate deficits in postmortem brain in schizophrenia, bipolar disorder and major depressive disorder. J Psychiatr Res. 2011;45:54–9. doi: 10.1016/j.jpsychires.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Yildiz-Yesiloglu A, Ankerst DP. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:969–95. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 31.MacMaster FP, Moore GJ, Russell A, et al. Medial temporal N-acetyl-aspartate in pediatric major depression. Psychiatry Res. 2008;164:86–9. doi: 10.1016/j.pscychresns.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Czéh B, Michaelis T, Watanabe T, et al. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Hart MG, Czéh B, de Biurrun G, et al. Substance P receptor antagonist and clomipramine prevent stress-induced alterations in cerebral metabolites, cytogenesis in the dentate gyrus and hippocampal volume. Mol Psychiatry. 2002;7:933–41. doi: 10.1038/sj.mp.4001130. [DOI] [PubMed] [Google Scholar]