Abstract
Human trials of formaldehyde-inactivated respiratory syncytial virus (FI-RSV) vaccine in 1966–1967 caused disastrous worsening of disease and death in infants during subsequent natural respiratory syncytial virus (RSV) infection. The reasons behind vaccine-induced augmentation are only partially understood, and fear of augmentation continues to hold back vaccine development. We now show that mice vaccinated with FI-RSV show enhanced local recruitment of conventional CD4+ T cells accompanied by a profound loss of regulatory T cells (Tregs) in the airways. This loss of Tregs was so complete that additional depletion of Tregs (in transgenic depletion of regulatory T-cell mice) produced no additional disease enhancement. Transfer of conventional CD4+ T cells from FI-RSV–vaccinated mice into naive RSV-infected recipients also caused a reduction in airway Treg responses; boosting Tregs with IL-2 immune complexes failed to restore normal levels of Tregs or to ameliorate disease. However, delivery of chemokine ligands (CCL) 17/22 via the airway selectively recruited airway Tregs and attenuated vaccine-augmented disease, reducing weight loss and inhibiting local recruitment of pathogenic CD4+ T cells. These findings reveal an unexpected mechanism of vaccine-induced disease augmentation and indicate that selective chemoattraction of Tregs into diseased sites may offer a novel approach to the modulation of tissue-specific inflammation.
Keywords: chemokines, lung infection, bronchiolitis
Respiratory syncytial virus (RSV) causes common colds in adults but is the major cause of infantile bronchiolitis (1) and is characterized by an intense local inflammatory response to infection. RSV is estimated to cause 34 million cases of lung infection annually, 3.4 million hospitalizations, and the deaths of up to 199,000 children younger than 5 y of age worldwide (2). Between 25% and 40% of previously healthy RSV-infected infants develop signs of lower respiratory tract infection that may develop into viral bronchitis, bronchiolitis, or pneumonia (3). Those recovering from severe disease are at high risk of recurrent wheeze in later childhood, and RSV is increasingly recognized as an important cause of winter deaths in the elderly. Despite this enormous disease burden, there is still no vaccine for human use.
Trials of formaldehyde-inactivated RSV (FI-RSV) vaccine in 1966–1967 caused disastrous worsening of disease and deaths in infants during subsequent natural RSV infection (3). The mechanisms that cause disease augmentation are incompletely understood, but include aberrant Th2-mediated disease triggered by generation of carbonyl groups (4), defective Toll-like receptor signaling (5), and induction of poorly neutralizing antibody (6). Although it is clear that CD4 T cells play a major pathogenic role, little is known how these responses are regulated.
CD4+CD25hiforkhead box p3 (Foxp3)+ regulatory T cells (Treg) play a crucial role in controlling immune responses. Human genetic Treg defects causes multiorgan inflammatory disease (7, 8), and depletion of Treg in mice can lead to an analogous inflammatory syndrome mainly affecting the gut and skin (9, 10). Tregs are widely distributed in lymphoid and nonlymphoid sites and express a variety of chemoattractant receptors and adhesion molecules that determine their migration in and out of tissue compartments (11). Among the various homing and migration receptors, chemokine receptors (CCR) 4/8 expression on human Tregs seems particularly important (12), suggesting that the ligands CCL1, CCL17, and CCL22 may guide the migration of Tregs to specific areas and thus suppress local inflammation (13–15). Most remarkably, mice lacking CCR4 expression on Tregs develop lymphocytic infiltration and severe inflammatory disease of the skin and lungs (16), and CCL22-directed Treg recruitment has been used to prevent murine autoimmune diabetes (17).
We now show that mice vaccinated with FI-RSV exhibit an almost complete absence of Tregs in the airways during RSV infection. Moreover, selective recruitment of Tregs by chemokine administration of CCL17/22 reestablished a local airway Treg population, therefore attenuating the pathogenic effects of vaccine-induced CD4 T cells. The use of appropriate chemokines to draw regulatory cells into inflamed tissues offers a possible approach to the treatment of inflammatory disorders.
Results
FI-RSV Vaccination Attenuates Airway Treg Responses to RSV Infection.
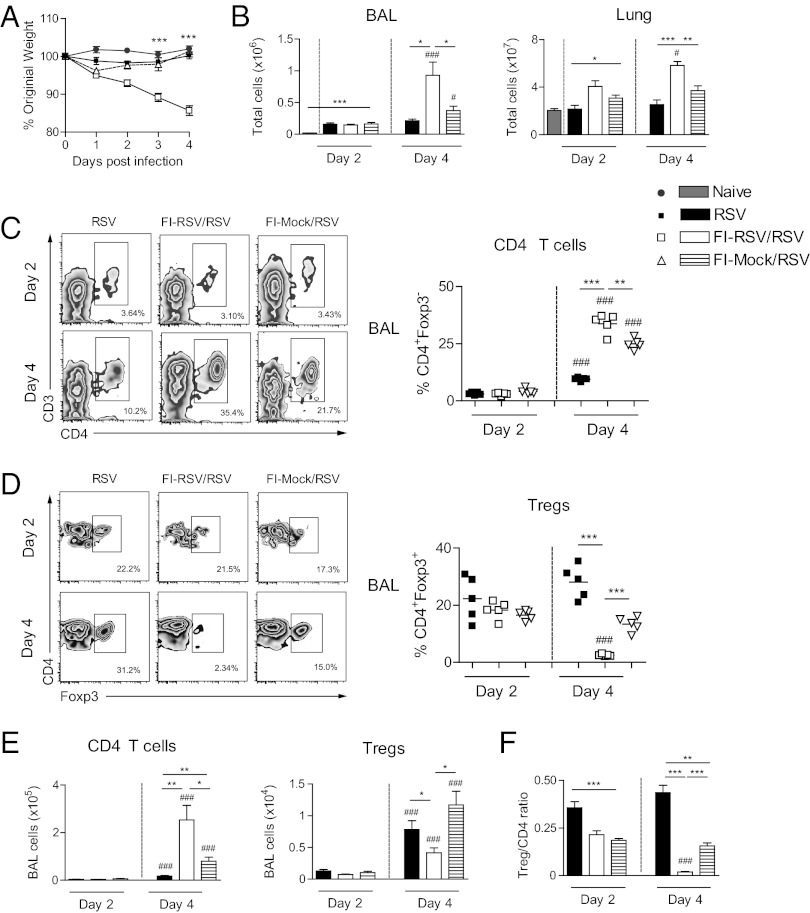
Mice vaccinated with FI-RSV showed augmented disease and weight loss after RSV infection (Fig. 1A). This was accompanied by elevated total cell numbers on day 4 after RSV infection in bronchoalveolar lavage (BAL) and lung (Fig. 1B). Flow cytometric analysis of the BAL cells showed a significant increase of CD4 T cells (CD3+CD4+Foxp3−) (Fig. 1 C and E) but a remarkable loss (both in frequency and numbers) of Tregs (Fig. 1 D and E) in FI-RSV–vaccinated mice compared with unvaccinated control mice infected with RSV. The loss of Tregs in FI-RSV–vaccinated mice was evident as early as day 2, but was virtually complete by day 4 (Fig. 1 D and E). RSV-infected mice vaccinated with formalin-treated, mock-infected Hep-2 cell material (FI-Mock) showed a significant but less pronounced increase of CD4 T cells and a slight drop in Treg frequencies on day 4 postinfection compared with unvaccinated controls (Fig. 1 C–E); such effects have previously been noted and ascribed to anti-cell responses because both the virus and the vaccine stocks were grown on Hep-2 cells (18). However, the ratio of Tregs to CD4 T cells was most markedly reduced in the airways of FI-RSV–vaccinated mice (Fig. 1F). Uninfected mice showed no cell efflux into the airways, regardless of vaccination with FI-RSV or FI-Mock. The effects were most obvious in the BAL cells, but similar changes in Treg proportions and numbers were detected in the lung cells (Fig. S1 A and B). However, there was no significant loss of Tregs in local draining lymph nodes (Fig. S1C), indicating that the effect is tissue-specific and confined to the site of infection.
Fig. 1.
FI-RSV vaccination attenuates BAL Treg responses during RSV infection. Naive, FI-RSV–, or FI-Mock–vaccinated mice were infected intranasally with RSV (day 0). (A) Illness was monitored daily by weight for 4 d after RSV infection. On day 4, BAL fluid was obtained and cells extracted. (B) Total numbers of cells in the BAL and lung were enumerated on days 2 and 4. (C) Frequencies of Foxp3– gated CD3+CD4+ T cells (CD4 T cells) and (D) CD3+CD4+ gated Foxp3+ T cells (Tregs) were quantified using flow cytometry on days 2 and 4 after RSV infection. (E) Total cell numbers of CD4 T cells and Tregs on days 2 and 4 after RSV infection. (F) Ratio of total number of Tregs to total number of CD4 T cells in the BAL on days 2 and 4 after RSV infection. Graphs show data from one of two independent experiments with five mice per group. Results are presented as means ± SEM. The significance of results between the groups was analyzed by two-tailed, unpaired Student t test. *P < 0.05, **P < 0.01, ***P < 0.001 was used to compare different groups on one day point, and #P < 0.05, ##P < 0.01, ###P < 0.001 for comparing one group at different day points.
Because disease enhancement was associated with reduced numbers of Treg cells, we attempted to reduce disease severity by administration of immune complexes of IL-2/anti–IL-2 (IL-2 Cx) before RSV infection. This procedure has been shown to selectively activate and expand Tregs without significantly affecting conventional effector CD4 T cells (19, 20), causing active suppression of immune activation and disease in vitro and in vivo and reducing lung inflammation in RSV disease (20). These IL-2 immune complexes increased the proportion of Tregs in the airways at day 2 of FI-RSV–vaccinated, RSV-infected mice (e.g., Fig. 1D and Fig. S1 D and E), but the benefits were only transient and Tregs showed a marked decline by day 4 (Fig. S1 D and E). Despite this treatment, the proportion of disease-associated conventional CD4 T cells increased during this period, just as it did in mice without IL-2 Cx injections (e.g., Fig. S1E and Fig. 1C).
Although it was previously demonstrated that Tregs can be starved of IL-2 produced by effector T cells during acute infection (21), our results suggest that IL-2 withdrawal is not the cause of the observed “crash” in Tregs.
Selective Treg Depletion Does Not Lead to Additional Enhancement of Disease.
Bacterial artificial chromosome-transgenic depletion of regulatory T-cell (DEREG) mice express diphtheria toxin (DT) receptor and EGFP under the control of the foxp3 gene locus, allowing selective depletion of Foxp3+ Treg cells by DT injection (22). Nondepleted FI-RSV–vaccinated DEREG mice respond to RSV infection similarly to WT mice. We have shown that two consecutive injections of DT into DEREG mice causes virtually complete Treg depletion, resulting in considerable disease enhancement after RSV infection (20). However, depletion of Tregs from FI-RSV–vaccinated, RSV-infected DEREG mice did not produce any additional enhancement of disease (Fig. S2 A–C) and failed to increase the numbers or CD4 T cells in the lung or BAL on day 2 or 4 after RSV infection (Fig. S2 B and C). Thus, the functional deficit of Tregs caused by FI-RSV vaccination is effectively complete because depletion of Tregs had no significant additional effect.
Vaccine-Primed CD4 T Cells Attenuate Airway Treg Responses.
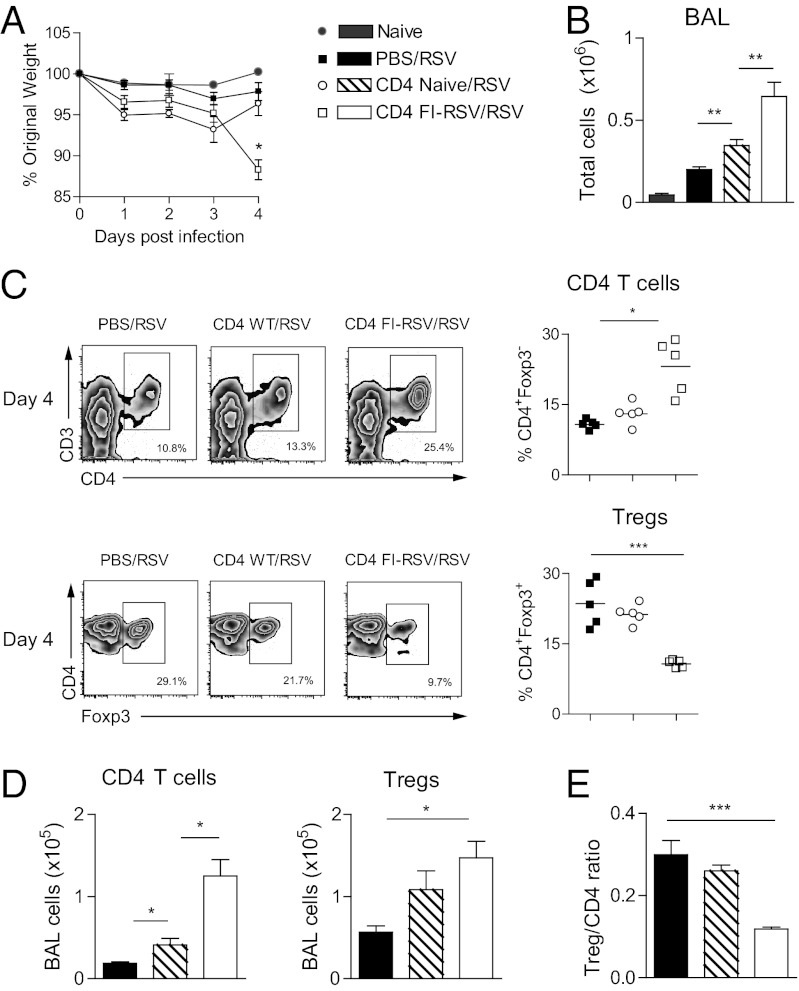
To determine the role of CD4 T cells in causing the loss of airway Tregs after RSV infection of primed mice, naive mice were injected intravenously with purified CD4 T cells from spleen and mesenteric lymph nodes derived from either FI-RSV–vaccinated or naive mice. Mice that received CD4 T cells from FI-RSV–vaccinated mice displayed enhanced weight loss and increased cellular influx into the airways on day 4 after RSV infection (Fig. 2 A and B). Flow cytometric analysis of the BAL showed a significant increase of CD4 T cells but a remarkable loss of Treg frequencies on day 4 postinfection in RSV-infected mice that received CD4 T cells from FI-RSV–vaccinated mice compared with controls (Fig. 2 C and D). This also led to a significant decrease of the Treg/CD4 T-cell ratio (Fig. 2E). Adoptive transfer of naive CD4 T cells also led to increased cellular influx into the airways on day 4 postinfection (Fig. 2 B and D), probably because of large numbers of partially activated CD4 T cells reaching the lungs after i.v. injection, but these effects were significantly less marked than those of CD4 cells from FI-RSV–vaccinated mice. Therefore, primed and activated CD4 T cells (especially those induced by FI-RSV vaccination) are responsible for the attenuation of airway Treg responses after RSV challenge.
Fig. 2.
Transferred CD4 T cells attenuate airway Treg responses. CD4 T cells from naive or FI-RSV–vaccinated mice were transferred i.v. into naive recipients on day −3, with RSV infection on day 0 and BAL harvest on day 4. (A) Illness was monitored daily by weight, displayed as percentage of original weight. (B) Total numbers of BAL cells. (C) Frequencies of Foxp3– gated CD3+CD4+ T cells (CD4 T cells) and Tregs quantified using flow cytometer. (D) Total calculated numbers of CD4 T cells and Tregs. (E) Ratio of Tregs to CD4 T cells. One representative study of two independent experiments with five mice per group is shown, presented as means ± SEM. The significance of results between the groups was analyzed by two-tailed, unpaired Student t test. *P < 0.05, **P < 0.01, ***P < 0.001.
CCR4 Expression by Tregs and Chemokine Levels in the Airways.
To further examine the causes of Treg deficiency in the airways of mice with augmented disease, we studied chemokine receptor expression and chemokine production by the infected airways. CCR4 plays a pivotal role in Treg migration in both man and mouse and expression of CCR4 on Tregs is required for appropriate migration to the skin or respiratory tract (15, 16, 23). Mice lacking CCR4 on Tregs develop lymphocytic infiltration and inflammation of the lung and skin with local influx of CD4 T cells and granulocytes (16). This pattern of pathology is reminiscent of the inflammation seen in the lungs of FI-RSV–vaccinated children or mice infected with RSV (24).
We therefore analyzed the expression of CCR4 on airway Tregs. After RSV infection, airway Tregs of all experimental groups showed greater expression of CCR4 than conventional CD4 T cells, either by flow cytometry (Fig. S3 A and B) or by quantitative PCR analysis of gene expression on sorted cells from BAL (Fig. S3C) or lung (Fig. S3D). Therefore, the observed defect in Treg recruitment cannot be explained by reduced chemokine receptor expression on Tregs.
Because CCR4 is present and expressed on Tregs regardless of priming, we investigated the possibility that a lack of the specific chemokines binding to CCR4 (i.e., CCL17/22) might explain the reduced Treg recruitment in vaccinated RSV-infected mice. We attempted to measure levels of CCL17 and CCL22 in the airway fluid in the different groups (Fig. S3E). In most of the groups, CCL17 was under the detection limit. However, airway fluid from IL-2 Cx–injected mice contained higher levels of CCL17 and CCL22 on day 2 postinfection. Levels of both these chemokines decreased by day 4 postinfection at the time of declining Treg numbers, compatible with the possibility that the increased Treg frequencies in the airways of IL-2 Cx–treated mice on day 2 postinfection were due to CCL17/22-mediated chemoattraction of Treg trafficking into the inflamed airways (16). There was no significant difference in levels of CCL22 between the other groups (Fig. S3E). These findings suggest that CCL17/22 may play a role in attracting Tregs to the airway and modulating disease, but that our methods of detecting these chemokines may not be sufficiently sensitive to fully elucidate their role.
Administration of CCL17/22 via the Airways Reestablishes Treg Population.
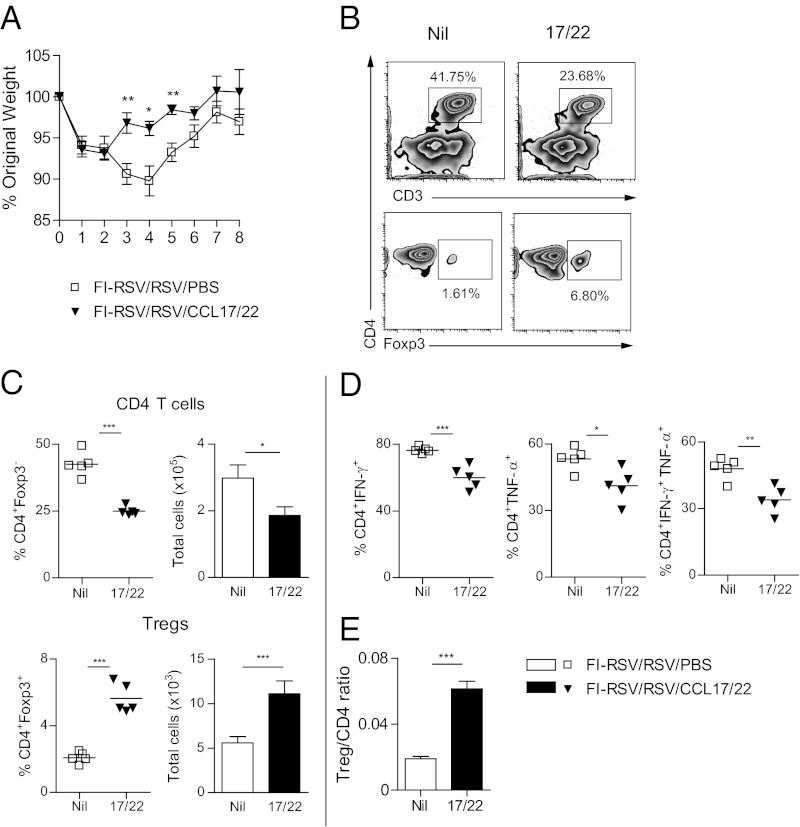
Because Tregs displayed CCR4 and we were unable to show whether there was a defect in chemokine production, we next tested the effect of delivering CCL17/22 via the airway. Because topical vaginal application of CXCL9/10 recruits herpes virus–specific tissue-resident primed memory T cells to the genital mucosa (25), we reasoned that delivering CCL17/22 via the airway might recruit Tregs and thus reduce disease severity. We gave a single intranasal (i.n.) dose of CCL17/22 on day 2 after RSV challenge and found that this greatly reduced weight loss up to day 8 postinfection (Fig. 3A), while increasing Treg recruitment into the BAL (Fig. 3 B and C). This was accompanied by a marked reduction in conventional CD4 T cells compared with PBS control mice (Fig. 3 B and C). Administration of either CCL17 or CCL22 alone was much less effective (Fig. S4A), suggesting that these two chemokines act synergistically. CCL17/22 administration also reduced the proportion of CD4 T cells that produced IFN-γ and/or TNF-α (Fig. 3D and Fig. S4B) and significantly increased the ratio of Tregs to CD4 T cells (Fig. 3E), but did not affect total cell numbers in the lung or BAL or the recruitment of granulocytes to the airway (Fig. S4C). We could not detect IL-17– or IL-4–producing CD4 T cells in the lung or airways at any time by flow cytometry, suggesting that Tregs predominantly modulate Th1, rather than the Th2 or Th17 CD4 T-cell subsets in this situation. Although the Treg recruitment to the airways in FI-RSV–vaccinated mice was inhibited for at least 8 d postinfection, the increase of Tregs in the airways after chemokine treatment was sustained at least until day 8 postinfection (Fig. S5 A–C). Notably, the weight loss recovery already started by day 5 after RSV infection as soon as the number of FI-RSV–specific CD4 T cells declined. There was no significant difference between levels or total numbers of CD4 T cells between CCL17/22-treated and CCL17/22-untreated mice on day 8 postinfection (Fig. S5 B and C). However, CCL17/22 administration did not affect mice undergoing primary RSV infection, in which case the balance of regulatory and disease-causing cells is already under appropriate control.
Fig. 3.
Effects of chemoattraction of Tregs by CCL17/22 administration via the airway. BALB/c mice were vaccinated with FI-RSV and infected 3 wk later with RSV i.n. (day 0). Mice were given a mixture of 0.5 µg CCL17 and 0.5 µg CCL22, or PBS i.n. on day 2, and BAL cells were harvested from 5 mice per group on day 4 and weight monitored to day 8 in the remainder. (A) Daily weight after RSV infection as percentage of original weight. (B) Frequencies of Tregs and Foxp3− gated CD4 T cells by flow cytometry in mice with (17/22) or without (“nil”) intranasal CCL17/22. (C) Frequencies (Left) and total numbers (Right) of Tregs and CD4 T cells. (D) Frequencies of CD4+IFN-γ+ T cells, CD4+TNF-α+ T cells, and CD4+TNF-α+IFN-γ+ T cells in the BAL on day 4 postvaccination and RSV infection. (E) Ratio of total number of Tregs to total number of CD4 T cells in the BAL on day 4 after RSV infection. One representative study of two independent experiments with five mice per group is shown. Results are presented as means ± SEM. The significance of results between the groups was analyzed by two-tailed, unpaired Student t test. *P < 0.05, **P < 0.01, ***P < 0.001.
These results show clearly that selective chemoattraction of Tregs to the airway by administration of CCL17/22 reduces the recruitment and activity of conventional CD4 T cells and reverses the enhanced lung inflammation resulting from vaccination with FI-RSV.
We next examined whether neutralization of CCL17/22 by i.p. injection of anti-chemokine antibodies on day 1 after RSV infection (with or without chemokine instillation on day 2 postinfection) was able to prevent Treg recruitment into the airways and the consequent effects (Fig. S6). Anti-CCL17/22 (but not goat IgG isotype control) prevented the effects of CCL17/22 treatment, reducing Treg and increasing CD4 T-cell frequencies on day 4 postinfection. Importantly, anti-CCL17/22 treatment further decreased Treg frequencies in the airways in vaccinated mice compared with vaccinated control mice, emphasizing the important role of CCL17/22 in Treg recruitment even under these conditions.
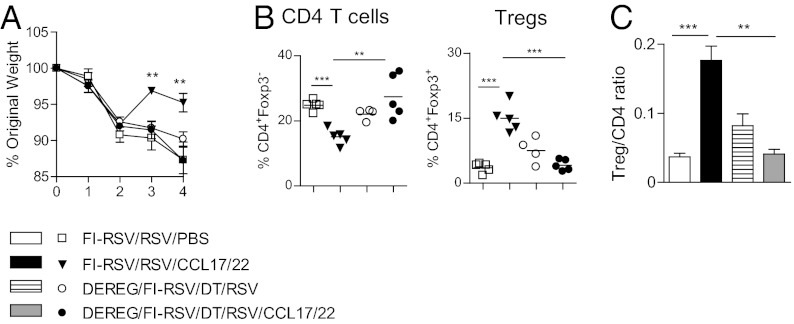
To demonstrate that additional recruited Tregs in the airways of FI-RSV vaccinated mice treated with CCL17/22 are directly responsible for the decrease in CD4 T cells after RSV infection, FI-RSV vaccinated DEREG mice were depleted of Tregs by injections of DT, infected with RSV, and treated with CCL17/22. Treg depleted, FI-RSV vaccinated DEREG mice and nondepleted vaccinated mice, CCL17/22 treated or untreated, served as controls. We found that only nondepleted mice given CCL17/22 showed significantly reduced weight loss after RSV infection accompanied by increased Treg recruitment and by a marked reduction in CD4 T cells and Treg/CD4 T-cell ratio in the BAL (Fig. 4 A–C). Therefore, Treg recruitment is necessary for the beneficial effects of administration of CCL17/22 via the airway.
Fig. 4.
Recruited Tregs are necessary to control CD4 T cells response in the airways. BALB/c WT or DEREG mice or were vaccinated with FI-RSV and infected 3 wk later with RSV i.n. (day 0). When indicated, mice were treated i.n. with a mixture of 0.5 µg of both CCL17 and 22, or PBS on day 2 after RSV infection or/ and depleted of Tregs by diphtheria toxin injections on days −2, −1, and 2. All mice were killed on day 4 and BAL cells obtained. (A) Daily weights as percentage of original weight. (B) Frequencies of CD4 T cells and Tregs by flow cytometry. (C) Ratio of numbers of Tregs to CD4 T cells. One representative study of two independent experiments with five mice per group is shown. Results are presented as means ± SEM. The significance of results between the groups was analyzed by two-tailed, unpaired Student t test. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Studying vaccine-augmented disease, our results show that RSV-infected mice suffering the effects of exuberant RSV-specific CD4 T-cell memory responses show a marked reduction in airway Treg cells, with a virtually complete loss of immune regulatory function. The “crash” in Tregs can be partially reproduced by adoptive transfer of activated CD4 T cells. Most remarkably, selective recruitment of disease-attenuating Tregs by the administration of CCL17/22 into the airways rapidly restored local immunoregulation and caused a sustained reduction in disease severity, even if the chemokines were delivered after virus-induced inflammation was at its peak. Together, these results show that Tregs play a key role in determining the balance between the beneficial and disease-enhancing components of cellular immunity.
Consideration of the site at which different components of the immune response act is essential in understanding this complex but informative disease model. It is known that generalized depletion of Tregs (either with anti-CD25 antibody or in genetically modified mice) enhances RSV-induced disease and increases T-cell activation at multiple sites (20, 26–28). We now show that an almost complete local depletion of Tregs occurs in the airways of vaccine-sensitized RSV-infected mice, and that this crash in local Tregs allows the effect of disease-causing T cells to become dominant. Whereas Treg depletion during primary RSV infection causes disease enhancement, depletion of Tregs in FI-RSV–vaccinated DEREG mice caused no further disease enhancement during RSV infection. Thus, the functional deficit of Tregs in the airways was virtually complete under these conditions and additional depletion of Tregs had no significant additional effect. The disappearance of Tregs, which was most evident in the airways, was seen also in the lung but not in the spleen or draining nodes. The loss of Tregs in FI-RSV–vaccinated mice was evident as early as day 2, but was virtually complete by day 4 and continued to at least day 8 after RSV infection. Thus, attenuation of Treg responses is focused at the site of enhanced disease.
To explain our observations, we suggest that primed conventional CD4 T cells cause a loss of chemoattraction of Tregs (29), probably by an action on the cells that make CCL17 [e.g., airway epithelial cells and dendritic cells (DCs)] and CCL22 (DCs and macrophages; illustrated diagrammatically in Fig. S7). This explanation is supported by our finding that passive transfer of CD4 T cells from vaccinated mice led to reduced Treg recruitment and is compatible with the known effects of CD4 T cells on innate immune responses (30). Although T-cell subsets that do not express Foxp3 can have suppressive functions (31) and might play an important role in controlling lung inflammation, our studies indicate that variations in FoxP3+ cells rather than additional regulatory subsets sufficiently explain the observed changes in disease in FI-RSV–vaccinated mice. However, clearly it is important to investigate the role of additional suppressive mechanism in future studies.
Recent studies suggested an important role for CCR4 in human and mouse Treg migration (15, 16, 23). Notably, Sather et al. showed that CCR4 expression on Tregs is required for appropriate migration to the skin or respiratory tract, and that mice lacking CCR4 on Tregs develop lymphocytic infiltration and inflammation of the lung and skin with local influx of CD4 T cells and granulocytes (16). This chronic and progressive disease bears close similarity to the acute inflammatory disease that we observe in FI-RSV–vaccinated mice after infection with RSV (24). However, we found that FI-RSV–vaccinated mice had similar levels of CCR4 expression on lung and airway Tregs to those seen in mice undergoing primary infection with RSV, in which no crash in Tregs occurs. Thus, we do not believe that down-regulation of CCR4 on Tregs is responsible for their failure to accumulate in enhanced disease.
Because we could not detect CCL17 in the BAL or lung homogenates and found only low levels of CCL22 in the lungs, we were unable to show directly whether a lack chemoattraction was responsible for the crash or Tregs. However, soluble mediators can be difficult to detect, especially if they are rapidly taken up, destroyed, or bound to cells. It has recently been found that adenovirus recombinant expression of CCL22 in pancreatic islets recruits Tregs and causes long-term protection from autoimmune diabetes in NOD mice (17) and that local administration of chemokines can “pull” activated virus–specific cells into the vaginal mucosa (25). To test a similar approach we administered the chemokines CCL17/ 22 via the airway in the hope of attracting Tregs into the site of infection and attenuating disease. A single dose of this chemokine mixture was indeed effective, even if administered on day 2 after RSV challenge (a time at which enhanced disease is already well established in FI-RSV–vaccinated mice), showing that administration of additional CCL17/22 via the airway can reestablish a local Treg population and attenuate vaccine-enhanced disease.
The implication of these findings is that selective recruitment of Tregs by appropriate chemokine administration might rapidly reduce the severity of inflammation and disease, even when inflammation is well established, offering a therapeutic avenue in the treatment of tissue-specific inflammation. Although the lung is unique in allowing chemokine delivery via the airway, it is possible that disease at other sites (e.g., the skin, joints, vagina) might also respond favorably to the local administration of chemokines that selectively recruit regulatory T cells to the site of inflammation.
Materials and Methods
Ethics Statement.
All mouse experiments were ethically approved by the Imperial College Central Biological Services (CBS) ethics committee and performed in accordance with approved UK Home Office guidelines (Project License No. PPL 70/6785).
Mice, Virus Stocks and Infection, and FI-RSV Vaccination.
Plaque-purified human RSV (type A2 strain, ATCC) was grown in HEp-2 cells. FI-RSV was prepared as described previously (4). In brief, RSV was grown in HEp-2 cells, flasks were frozen and thawed, and cells harvested and pooled. The cell suspension was sonicated for 3 min on ice and spun at 1,000 g for 10 min at 4 °C. A 40% (vol/vol) formalin solution was added to the supernatant to give a final concentration of 1:4,000 (2.5 μL of formalin per each 4 mL of virus stock) and incubated for 72 h at 37 °C, 5% CO2. After, the supernatant was centrifuged at 50,000 g for 1 h at 4 °C and the pellet diluted (1:25 of the starting volume) in serum-free medium. Aluminum hydroxide (12 μL per 1 mL of supernatant) was added and the suspension shaken for 30 min at room temperature before centrifugation at 1,000 g for 30 min. The final pellet was resuspended 1:4 in PBS (i.e., 1:100 of the starting volume) and stored frozen at −80 °C.
Age- and sex-matched 6- to 10-week-old BALB/c mice (Harlan) or DEREG mice (22) on BALB/c background were lightly anesthetized and infected i.n. with 106 focus-forming units RSV in 100 µL. For FI-RSV vaccination, BALB/c mice were injected intramuscularly (i.m.) with 50 μL FI-RSV (3 mg/mL protein). Three weeks later, mice were infected with RSV as described above.
IL-2 Cx Injections.
IL-2 Cx were obtained as described (19) by mixing 1 μg rmIL-2 (Peprotech) and 5 μg anti-IL-2 (Clone JES6-1A12; eBioscience) and incubating at 37 °C for 30 min. Age- and sex-matched BALB/c mice received daily i.p. injections of IL-2 Cx or PBS for 3 consecutive days (−3, −2, and −1) before RSV infection (20).
DT Injections.
DEREG mice (22) were injected with 0.75 µg DT (Merck) in PBS i.p. on days −2 and −1 and days 2 and 5 after RSV infection to induce and maintain Foxp3+ T-cell depletion as previously described (20).
Chemokine and Antibody Administration.
Chemokine administration was performed by i.n. instillation of 500 ng CCL17 and 22 (R&D Systems) in 100 µL PBS under light anesthesia, ensuring deep lung inhalation on day 2 postinfection. For neutralization of CCL17 and 22, mice were injected with one dose i.p. of 20 µg anti-CCL17 and anti-CCL22 or IgG isotype control (goat anti-mouse antibodies, R&D Systems) in 200 µL PBS on day 1 after RSV infection.
Adoptive Cell Transfer.
BALB/c mice were injected i.m. with 50 μL FI-RSV. Three weeks later, isolation of CD4 T cells from spleen and mesenteric lymph nodes was performed using a negative CD4 T-cell isolation kit II (Miltenyi) and the Auto MACS pro (Miltenyi). Purity was confirmed by flow cytometry and was ≥90%. Purified CD4 T cells (27 × 106/mouse) were transferred i.v. into naive recipients. These mice were infected with RSV i.n. 3 d later.
Real-Time PCR.
Lung and BAL CD4 T cells (CD4+GFP−) and Tregs (CD4+GFP+) from FI-RSV–vaccinated and RSV-infected DEREG mice were sorted on a FACS Aria II (BD). Total RNA was isolated from purified T cells using the Qiagen RNeasy Micro Kit with on-column DNase digestion using the RNase-Free DNase set (according to the manufacturer’s protocol). cDNA was generated using the SuperScript III FirstStrand Synthesis SuperMix for RT-PCR and oligodT primers (Invitrogen), according to the manufacturer’s protocol. cDNA was used as a template for quantitative real-time PCR using TaqMan Gene Expression Assay (Applied Biosystems) for mouse CCR4. PCR and analysis was performed using a 7500 Fast Realtime PCR System (Applied Biosystems). Gene expression was calculated relative to GAPDH by the formulary 1/2ΔΔCT.
Cell Collection and Preparation.
BAL was carried out using 1 mL PBS containing 12 mM lidocaine flushing the lungs three times. To obtain a single cell suspension, lymph nodes were mashed through a cell strainer and lungs were processed with the gentleMax dissociator (Miltenyi Biotech) according to the manufacturer’s protocol using Collagenase D (50 μg/mL, Sigma). Total cell counts were determined by flow cytometry using Count Bright counting beads (Invitrogen) and dead cells were excluded by staining for 7-amino-actinomycin D (7-AAD, Sigma). For determination of cellular composition in the BAL, cells were transferred onto a microscope slides (Cytospin, Thermo Scientific) and stained with H&E (Reagena, Gamidor).
Flow Cytometry.
For flow analysis, the LIVE/DEAD Fixable Red Dead cell stain kit (Invitrogen) was used to exclude dead cells. Cells were incubated with FcγIII/II receptor antibody (BD Biosciences) diluted in PBS containing 1% BSA and 5 mM EDTA and were subsequently labeled with the following antibodies (from BD Biosciences unless otherwise stated): PE-Cy7 or V500 conjugated anti-CD3 (145-2C11), Pacific Blue conjugated anti-CD4 (RM4-5), and PE conjugated anti-CCR4 (2G12; Biolegend). For intracellular staining for Foxp3, the Foxp3 staining kit (eBioscience) using Allophycocyanin (APC) or fluorescein isothiocyanate (FITC) conjugated anti-Foxp3 (FJK-16s; eBioscience) was used following manufacturer’s recommendations. To detect intracellular IFN-γ production, cells were stimulated with 100 ng/mL Phorbol 12-myristate 13-acetate (PMA) and 1 μg/mL ionomycin in complete RPMI. After 1 h incubation, monensin (Golgi Stop, BD) was added. After two additional hours of incubation, cell surface staining was followed by intracellular staining with Percp Cy 5.5 anti–IFN-γ (XMG1), PE anti–TNF-α (MP6-XT22), APC anti–IL-4 (11B1), or Alexa 700 anti–IL-17 (TC11-18H1) using the Foxp3 staining kit (eBioscience). Cells were acquired on a LSR II (BD) with data analyzed using Flow Jo software. Cells were gated for live cells, singlets, and lymphocytes before analysis of indicated markers.
Chemokine Detection.
CCL17 and CCL22 levels in the BAL were measured by ELISA following manufacturer’s recommendations (R&D). The concentration of cytokines in each sample was determined according to the standard curve. The detection limit for CCL17 was 31.2 pg/mL and for CCL22 7.8 pg/mL.
Statistical Analysis.
Results are presented as means ± SEM. The significance of results between the groups was analyzed by two-tailed, unpaired Student t test (*P < 0.05, **P < 0.01, ***P < 0.001) (Prism software; Graph-Pad Software Inc.). P values of <0.05 were considered significant.*P < 0.05, **P < 0.01, ***P < 0.001 was used to compare different groups on one time point and #P < 0.05, ##P < 0.01, ###P < 0.001 was used for comparing one experimental group at different time points.
Supplementary Material
Acknowledgments
We thank T. Sparwasser for providing DEREG mice and K. Webster and J. Sprent for providing protocols and advice on the preparation and administration of the IL-2 complexes. We also thank S. Makris for help with experiments. This work was supported by the Centre of Respiratory Infections, the Medical Research Council (MRC) and Asthma UK Centre in Allergic Mechanisms of Asthma, Wellcome Trust Programme 087805/Z/08/Z (P.J.O.), and MRC career development award, Grant G0800311 (to C.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217580110/-/DCSupplemental.
References
- 1.Hall CB, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth RL, Openshaw PJ. Bronchiolitis. Lancet. 2006;368(9532):312–322. doi: 10.1016/S0140-6736(06)69077-6. [DOI] [PubMed] [Google Scholar]
- 4.Moghaddam A, et al. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12(8):905–907. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- 5.Delgado MF, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15(1):34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy BR, et al. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24(2):197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 8.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Kim J, et al. Cutting edge: Depletion of Foxp3+ cells leads to induction of autoimmunity by specific ablation of regulatory T cells in genetically targeted mice. J Immunol. 2009;183(12):7631–7634. doi: 10.4049/jimmunol.0804308. [DOI] [PubMed] [Google Scholar]
- 10.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 11.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iellem A, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194(6):847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas J, et al. Specific recruitment of regulatory T cells into the CSF in lymphomatous and carcinomatous meningitis. Blood. 2008;111(2):761–766. doi: 10.1182/blood-2007-08-104877. [DOI] [PubMed] [Google Scholar]
- 14.Soler D, et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol. 2006;177(10):6940–6951. doi: 10.4049/jimmunol.177.10.6940. [DOI] [PubMed] [Google Scholar]
- 15.Hirahara K, et al. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177(7):4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 16.Sather BD, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204(6):1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montane J, et al. Prevention of murine autoimmune diabetes by CCL22-mediated Treg recruitment to the pancreatic islets. J Clin Invest. 2011;121(8):3024–3028. doi: 10.1172/JCI43048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boelen A, et al. Both immunisation with a formalin-inactivated respiratory syncytial virus (RSV) vaccine and a mock antigen vaccine induce severe lung pathology and a Th2 cytokine profile in RSV-challenged mice. Vaccine. 2000;19(7-8):982–991. doi: 10.1016/s0264-410x(00)00213-9. [DOI] [PubMed] [Google Scholar]
- 19.Webster KE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: Induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206(4):751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loebbermann J, et al. Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol. 2012;5(2):161–172. doi: 10.1038/mi.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31(5):772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahl K, et al. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204(1):57–63. doi: 10.1084/jem.20061852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan Q, et al. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med. 2007;204(6):1327–1334. doi: 10.1084/jem.20062076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Openshaw PJ, Tregoning JS. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev. 2005;18(3):541–555. doi: 10.1128/CMR.18.3.541-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491(7424):463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruckwardt TJ, Bonaparte KL, Nason MC, Graham BS. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J Virol. 2009;83(7):3019–3028. doi: 10.1128/JVI.00036-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulton RB, Meyerholz DK, Varga SM. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J Immunol. 2010;185(4):2382–2392. doi: 10.4049/jimmunol.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DC, et al. CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J Virol. 2010;84(17):8790–8798. doi: 10.1128/JVI.00796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pease JE. Targeting chemokine receptors in allergic disease. Biochem J. 2011;434(1):11–24. doi: 10.1042/BJ20101132. [DOI] [PubMed] [Google Scholar]
- 30.Strutt TM, McKinstry KK, Swain SL. Control of innate immunity by memory CD4 T cells. Adv Exp Med Biol. 2011;780:57–68. doi: 10.1007/978-1-4419-5632-3_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loebbermann J, et al. IL-10 regulates viral lung immunopathology during acute respiratory syncytial virus infection in mice. PLoS ONE. 2012;7(2):e32371. doi: 10.1371/journal.pone.0032371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.