Abstract
Infection with the gastric bacterial pathogen Helicobacter pylori is typically contracted in early childhood and often persists for decades. The immunomodulatory properties of H. pylori that allow it to colonize humans persistently are believed to also account for H. pylori’s protective effects against allergic and chronic inflammatory diseases. H. pylori infection efficiently reprograms dendritic cells (DCs) toward a tolerogenic phenotype and induces regulatory T cells (Tregs) with highly suppressive activity in models of allergen-induced asthma. We show here that two H. pylori virulence determinants, the γ-glutamyl transpeptidase GGT and the vacuolating cytotoxin VacA, contribute critically and nonredundantly to H. pylori’s tolerizing effects on murine DCs in vitro and in vivo. The tolerance-promoting effects of both factors are independent of their described suppressive activity on T cells. Isogenic H. pylori mutants lacking either GGT or VacA are incapable of preventing LPS-induced DC maturation and fail to drive DC tolerization as assessed by induction of Treg properties in cocultured naive T cells. The Δggt and ΔvacA mutants colonize mice at significantly reduced levels, induce stronger T-helper 1 (Th1) and T-helper 17 (Th17) responses, and/or trigger more severe gastric pathology. Both factors promote the efficient induction of Tregs in vivo, and VacA is required to prevent allergen-induced asthma. The defects of the Δggt mutant in vitro and in vivo are phenocopied by pharmacological inhibition of the transpeptidase activity of GGT in all readouts. In conclusion, our results reveal the molecular players and mechanistic basis for H. pylori-induced immunomodulation, promoting persistent infection and conferring protection against allergic asthma.
Keywords: bacterial virulence factors, hygiene hypothesis, persistent bacterial infection, human microbiota, persistence strategies
The bacterial pathogen Helicobacter pylori persistently colonizes the gastric mucosa of humans. It is typically acquired in early childhood (1) and, in the absence of antibiotic therapy, may persist for the entire lifespan of the host (2, 3). The extraordinary ability of H. pylori to resist a vigorous adaptive immune response driven in large part by T-helper 1 (Th1) and/or T-helper 17 (Th17)-polarized effector T cells (4, 5) has been attributed to its perfect adaptation to—and manipulation of—the human innate and adaptive immune systems (6). H. pylori has colonized its human host for at least 60,000 y (7) and during this long period of coevolution has evolved elaborate ways to systemically manipulate adaptive immune responses and to promote its persistence through the preferential induction of regulatory T-cell (Treg) over T-effector cell responses. Treg-predominant responses are characteristic of heavily colonized but asymptomatic carriers (4) and of children with particularly mild forms of Helicobacter-associated gastritis (8). Several recent functional studies using experimentally infected animals have implicated Tregs and dendritic cells (DCs) with “tolerogenic” activity in mediating the local and systemic immunomodulatory effects of H. pylori infection (9–12). The depletion of Tregs in a genetic model resulted in spontaneous clearance of the infection (9) and greatly improved the efficacy of an H. pylori vaccine (10). H. pylori-induced Tregs differentiate in the periphery as a result of their priming by tolerogenic DCs (13), which convert naive T cells into FoxP3+ Tregs through antigen presentation in the absence of costimulatory signals or cytokines (13, 14). We have shown recently that H. pylori exposure reprograms DCs toward a tolerance-promoting phenotype in vitro and in vivo; H. pylori-experienced DCs fail to induce T-cell effector functions, but rather acquire the ability to induce FoxP3 and CD25 expression in cocultured naive T cells (12). Consistent with a critical role for DCs in the development of H. pylori-specific immune tolerance, the systemic depletion of DCs breaks tolerance and facilitates clearance of the bacteria (12).
Here, we describe the role of two H. pylori virulence determinants, the vacuolating cytotoxin VacA and the γ-glutamyl transpeptidase GGT, in DC reprogramming and in the development of immune tolerance in vitro and in vivo. We show that both factors independently interfere with DC maturation and thereby contribute to DC tolerization. Specific deletion of the ggt and vacA genes or the pharmacological inhibition of GGT activity impair the ability of H. pylori to tolerize DCs in vitro and in vivo and to generate Tregs with suppressive activity. The isogenic Δggt and ΔvacA mutants fail to colonize mice persistently and have lost the ability to induce immune tolerance. In conclusion, we have identified here a unique immunomodulatory mechanism of H. pylori that involves specific targeting and tolerogenic reprogramming of DCs and possibly explains the extraordinary ability of these bacteria to persist in their mammalian host and, at the same time, to confer protection against allergen-specific T-cell responses and asthma.
Results
Tolerogenic Reprogramming of DCs by H. pylori Depends on the Virulence Determinants γ-Glutamyl Transpeptidase and Vacuolating Cytotoxin.
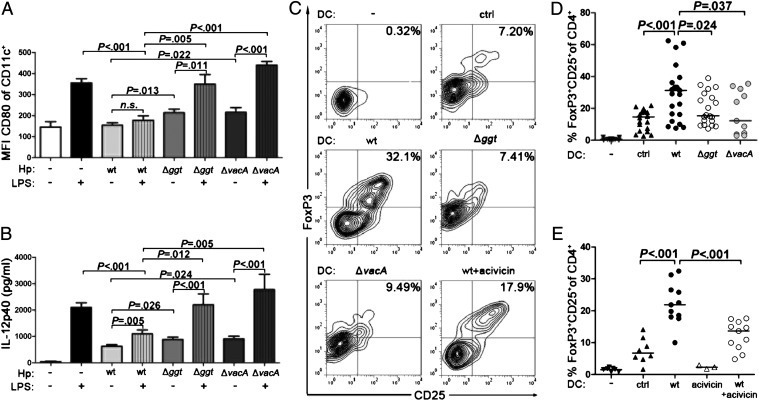
To assess the contribution of specific H. pylori virulence determinants to DC tolerization, we compared various isogenic mutants to the respective parental wild-type strains with respect to their ability to (i) induce DC maturation and to (ii) actively suppress LPS-induced DC maturation. Mutants lacking a functional type IV secretion system, or one of several adhesins, did not differ from the corresponding wild-type strains in these respects (shown representatively for a ΔcagPAI mutant and a ΔbabA mutant in Fig. S1A). In contrast, isogenic mutants lacking either of two secreted virulence factors, GGT (Fig. S1B) or VacA, induced DC maturation more efficiently than wild-type bacteria and showed a reduced ability to inhibit LPS-induced DC maturation as assessed by CD80 expression and IL-12 secretion (Fig. 1 A and B), both of which are well-accepted indicators of DC maturation (13). Similar results were obtained in three different strain backgrounds (Fig. 1 A and B and Fig. S1 C and D) and confirm a previous report linking VacA to impaired DC maturation (15). The combined deletion of ggt and vacA did not produce additive or synergistic effects (Fig. S1C).
Fig. 1.
VacA and the enzymatic activity of GGT are required for DC tolerization. Bone marrow DCs were infected with H. pylori strains PMSS1, PMSS1Δggt, or PMSS1ΔvacA at a multiplicity of infection (MOI) of 50 and/or treated with 0.5 μg/mL E. coli LPS for 16 h before the analysis of CD11c and CD80 expression (A) and of IL-12p40 secretion by ELISA (B). Data are pooled from four independent experiments and represented as means ± SEM. MFI, mean fluorescence intensity. (C–E) Bone marrow DCs were infected as described in A and B; acivicin was added to the infections at 5 μg/mL where indicated. After 16 h, bacteria were killed with antibiotics. DCs were cocultured with CD4+CD25− T cells for 3 d in the presence of rTGF-β, rIL-2, and anti-CD3ε mAb before the flow cytometric analysis of CD4, CD25, and FoxP3 expression. Representative plots of the CD4+ gate are shown in C. Pooled results from four (PMSS1ΔvacA) to seven (PMSS1Δggt) independent experiments are shown in D and from three independent wild-type infections performed with or without acivicin in E; in D and E, each symbol represents one coculture and horizontal lines indicate the medians. Uninfected DCs (ctrl), acivicin-treated DCs (acivicin), and T cells cultured in the absence of DCs (−) served as controls.
To obtain definitive proof that the semimature status of H. pylori-experienced DCs is required for their tolerance-promoting activity, we measured FoxP3 and CD25 expression in cocultured naive T cells. Bone marrow-derived DCs that had been exposed to H. pylori efficiently induced T-cellular FoxP3/CD25 expression, which was abrogated by the forced (partial) maturation of H. pylori-infected DCs by simultaneous treatment with increasing doses of Escherichia coli LPS (Fig. S2 A and B). To assess the effects of LPS treatment in vivo, C57BL/6 mice were infected with H. pylori during the neonatal period, that is, at a time when H. pylori exposure is known to induce immune tolerance (9), and then subjected to twice-weekly sublethal i.p. doses of E. coli LPS. The LPS treatment induced DC maturation in the gut-draining mesenteric lymph nodes, the sites of H. pylori-specific T-cell priming (Fig. S2C). LPS-treated mice controlled the infection more effectively as determined by colony counting (Fig. S2D) and exhibited higher gastric mucosal leukocyte and CD4+ T-cell infiltration than untreated infected controls (Fig. S2 E and F); a trend toward more Th1 and Th17 infiltration and higher inflammation scores was seen as well (Fig. S2 G–I). In summary, the results suggest that the forced maturation of DCs with sublethal LPS breaks H. pylori-specific, neonatally acquired immune tolerance.
To examine the functional contribution of VacA and GGT to H. pylori-induced DC tolerogenicity in vitro, bone marrow-derived DCs were infected overnight (o/n) with either Δggt or ΔvacA mutant bacteria or the corresponding parental wild-type strain and then cocultured with naive T cells. DCs that had been exposed to the mutant bacteria were much less capable of inducing T-cellular CD25 and FoxP3 expression than wild-type–infected DCs; this was true in all three strain backgrounds analyzed (Fig. 1 C and D and Fig. S3 A and B). Again, a double mutant lacking both GGT and VacA did not exhibit a stronger phenotype than the single mutants (Fig. S3A). The differential ability of wild-type and mutant bacteria to tolerize DCs could not be linked to differential effects on DC viability (Fig. S3C). To clarify whether the catalytic activity of the enzyme is required for GGT’s tolerogenic effects, we infected DCs with wild-type H. pylori in the presence or absence of acivicin, a small-molecule inhibitor that blocks H. pylori GGT and also GGTs from other species. The tolerogenic activity of H. pylori-infected DCs was strongly reduced by exposure to acivicin (Fig. 1 C and E), an effect that could not be attributed to adverse effects of the inhibitor on H. pylori growth. Interestingly, treatment with recombinant GGT was sufficient to tolerize otherwise naive DCs (Fig. S3D). The combined results obtained with the isogenic mutants, the GGT inhibitor, and recombinant GGT thus suggest that VacA and GGT both contribute to DC tolerogenicity, the latter through its enzymatic transpeptidase activity.
Efficient Gastric Colonization by H. pylori and Tolerogenic Reprogramming of DCs in Vivo Requires VacA and GGT.
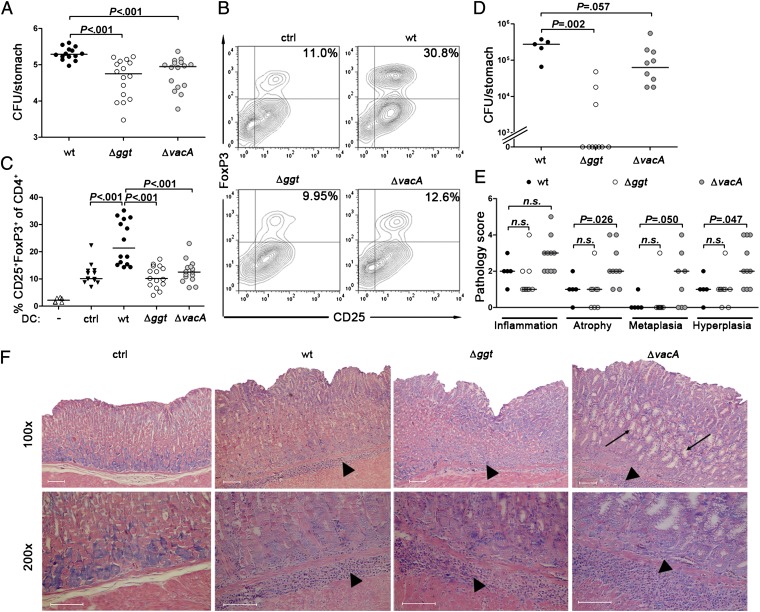
Both VacA and GGT have previously been shown to contribute to the ability of H. pylori to colonize the gastric mucosa of experimentally infected mice (16, 17). We have used the isogenic mutants generated for this study in the PMSS1 background to revisit the earlier findings and to examine a possible role of VacA and GGT in colonization and in tolerogenic DC reprogramming in vivo. Consistent with the earlier reports, both the Δggt and the ΔvacA mutant exhibited significant defects in colonizing mice relative to the parental PMSS1 strain, with average colonization levels reduced by approximately one order of magnitude compared with those of the wild-type bacteria at 1 mo postinfection (p.i.) (Fig. 2A). The mutants’ lower colonization levels were accompanied by lower Treg counts and somewhat stronger Th1 and Th17 responses in the mesenteric lymph nodes (MLNs) of the mutant-infected relative to wild-type–infected animals (Fig. S4 A–E), suggesting that both mutants elicited more pronounced pathogen-specific T-effector cell responses. To assess whether the three strains differentially affected DC tolerogenicity, DCs were immunomagnetically isolated from single-cell MLN preparations of individual infected mice and subjected to the same coculture protocol with naive T cells as outlined earlier. Whereas DCs from wild-type–infected mice efficiently induced T-cellular FoxP3/CD25 expression, this was not observed with DCs from uninfected controls, or with DCs from mice infected with the Δggt and the ΔvacA mutants (Fig. 2 B and C). Similar results were obtained with a second, independently generated ΔvacA mutant in the PMSS1 background, both in terms of its colonization defect and its inability to tolerize DCs in vivo (Fig. S4 F and G). To confirm the phenotypes of both mutants in longer-term infections, and to assess possible differential effects on gastric histopathology, mice from the same infected cohorts were analyzed 2 mo p.i. At this later time point, 7 of the 10 Δggt-infected mice had cleared the infection, and the ΔvacA-infected animals still exhibited somewhat lower colonization levels than the wild-type–infected controls (Fig. 2D). Interestingly, whereas the wild-type– and Δggt-infected animals exhibited mild to moderate inflammation and beginning preneoplastic changes at most, the ΔvacA-infected animals were characterized by strongly aggravated preneoplastic pathology (i.e., gastric atrophy, hyperplasia, and metaplasia; Fig. 2 E and F). Both VacA and GGT thus contribute critically to DC tolerization in vitro and in vivo, and this likely explains the stronger H. pylori-specific Th1/Th17 responses, the more efficient clearance of the mutant bacteria, and the more pronounced gastric pathology observed with the ΔvacA mutant. To assess which strain—and which strain’s effects on DCs—would dominate in coinfections, we infected mice with either ΔvacA and wild-type or ΔvacA and Δggt bacteria. Interestingly, the “ΔvacA phenotype” clearly dominated over the “wild-type phenotype,” with wild-type colonization levels in coinfections reduced significantly over wild-type colonization levels in single infections (Fig. S4F). The effect of ΔvacA bacteria also clearly dominated over the tolerizing effects of wild-type bacteria on DCs, both in vivo (as assessed with isolated MLN DCs) and in vitro (as assessed with infected bone marrow DCs) (Fig. S4 G and H). Neither of the two gene deletion mutants (ΔvacA and Δggt) was able to rescue the other mutant’s phenotype in vitro or in vivo, confirming that both factors act on nonredundant pathways to induce DC tolerogenicity (Fig. S4 G and H).
Fig. 2.
VacA and GGT are required for gastric colonization and DC tolerization in vivo. (A–F) C57BL/6 mice were infected at 6 wk of age with H. pylori PMSS1, PMSS1Δggt, or PMSS1ΔvacA for 1 (A–C) or 2 mo (D–F). Colony forming units (CFU) per stomach are shown in A and D. CD11c+ MLN DCs were immunomagnetically isolated from all mice killed at 1 mo p.i., cocultured with T cells, and subjected to flow cytometric analysis of CD4, CD25, and FoxP3 expression. CD25+FoxP3+ cells in the CD4+ gate are shown in B for representative donors and in C for all mice. Data in A–C are pooled from three independent experiments. DCs from uninfected mice (ctrl) and T cells cultured in the absence of DCs (−) served as controls. (E) Pathology scores assigned for inflammation, atrophy, intestinal metaplasia, and epithelial hyperplasia. (F) Representative micrographs of H&E-stained sections at 100 and 200× magnification. Arrowheads point to areas with inflammation; arrows indicate intestinal metaplastic glands. Uninfected controls are shown for comparison. (Scale bars, 100μm.) In A and C–E, each symbol represents one mouse; note that the mice in D–F are from the same infected cohort as a subset of mice shown in A–C.
DC-Mediated, Neonatally Acquired Immune Tolerance to H. pylori Infection Requires VacA and the Enzymatic Activity of GGT.
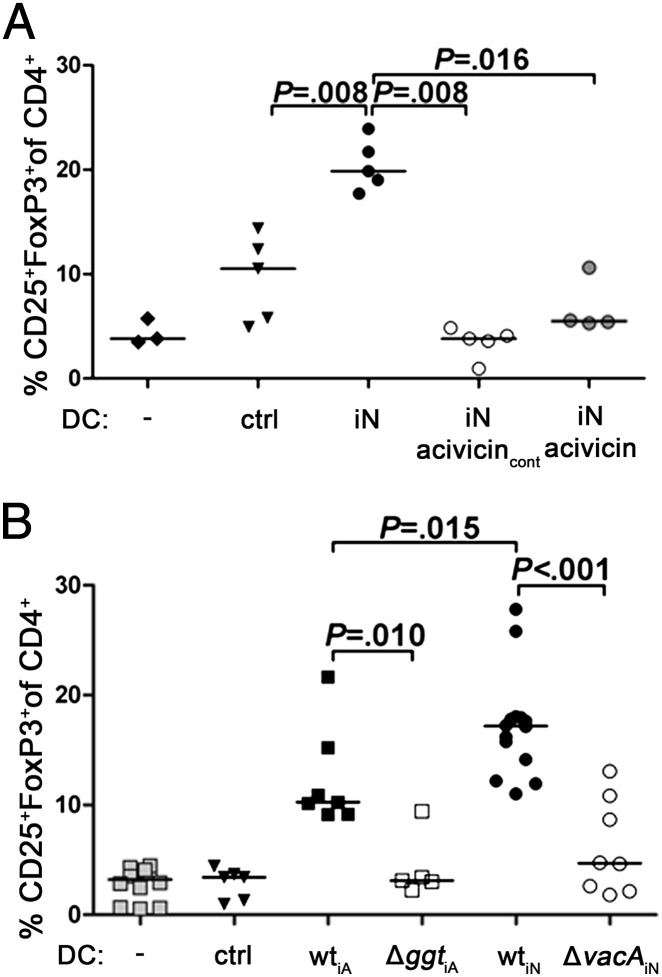
The outcome of the H. pylori–host interaction in experimentally infected mice is determined in large part by the age at the time of infection. Mice that are experimentally infected as neonates develop only mild gastritis and are completely protected from atrophy and preneoplasia (9). We have previously attributed this differential disease susceptibility to the development of Treg-mediated, peripheral immune tolerance in neonatally infected mice (9). To assess the contribution of GGT and VacA to neonatally acquired immune tolerance, we infected newborn mice with either wild-type PMSS1, PMSS1Δggt, or PMSS1ΔvacA and assessed their colonization levels at various time points p.i. Interestingly, PMSS1Δggt completely failed to colonize neonatal mice: no colonies were retrieved at 1 mo p.i. (Fig. S5A). Mice infected with H. pylori ΔvacA were colonized, but at lower levels than their wild-type–infected counterparts at 1, 2, and 4 mo p.i. (Fig. S5A); again, MLN DCs from ΔvacA-infected donors were significantly less tolerogenic than DCs from wild-type–infected donors (Fig. S5B). To assess whether the enzymatic activity of GGT is required for the development and maintenance of neonatally acquired tolerance to the infection, we infected mice at 1 wk of age and either subjected them to regular i.p. doses of the GGT inhibitor acivicin throughout the 6-wk infection or to acivicin treatment during the last 2 wk only. Acivicin treatment led to a significant reduction in colonization in both treatment arms (Fig. S5C) and abrogated DC tolerogenicity irrespective of the duration of the treatment (Fig. 3A). In conclusion, GGT, via its enzymatic activity, contributes critically to neonatal colonization by targeting DCs and promoting immune tolerance to H. pylori infection.
Fig. 3.
VacA and the enzymatic activity of GGT contribute to neonatally acquired immune tolerance independently of T cells. (A) C57BL/6 mice were infected with H. pylori PMSS1 as neonates (iN) for 6 wk. One group received acivicin continuously every other day starting from the day of infection (acivicincont). Another group received acivicin only during the last 2 wk of infection. CD11c+ MLN DCs were cocultured with CD4+CD25− T cells, and cocultures were stained for CD4, CD25, and FoxP3. CD25 and FoxP3 staining of the CD4+ gate is shown for all donors. (B) TCR-β−/− mice were infected with H. pylori PMSS1, PMSS1Δggt, or PMSS1ΔvacA at 7 d (iN) or 6 wk of age (iA, infected as adults) for 1 mo. CD25+FoxP3+ cells in the CD4+ gate are shown for DC/T-cell cocultures of all donors. Data in B are pooled from two studies. In A and B, DCs from uninfected mice (ctrl) and T cells cultured in the absence of DCs (−) served as controls.
Both VacA and GGT have been implicated before in immunomodulation by H. pylori. The immunomodulatory activity of both factors has been attributed to suppressive effects on human T cells (18–20); murine T cells, in contrast, are known to be resistant to VacA (21). To examine whether the tolerogenic effects of H. pylori on DCs in vivo are dependent on T cells, we infected mice lacking α/β T cells due to a targeted deletion of the T-cell receptor (TCR) β-chain with wild-type and mutant H. pylori PMSS1 as neonates and/or as adults. As noted before (9), the colonization efficiency of wild-type bacteria does not differ between adult and neonatally infected TCR-β−/− mice (Fig. S5D). H. pylori ΔvacA also colonizes neonatally infected mice at high levels; in contrast, the Δggt mutant failed to colonize any of the neonatally infected mice analyzed, despite normal colonization of adult-infected animals (Fig. S5D). DCs from TCR-β−/− mice infected with wild-type bacteria were substantially more tolerogenic than DCs from uninfected TCR-β−/− donors; this was particularly true for DCs from neonatally infected TCR-β−/− donors (Fig. 3B). Interestingly, DCs from ΔvacA-infected or Δggt-infected TCR-β−/− donors were incapable of inducing FoxP3 and CD25 expression in T cells (Fig. 3B), despite normal colonization levels. Similar results were obtained with DCs from infected OT-II mice that transgenically express a TCR specific for ovalbumin and therefore lack a normal diverse T-cell repertoire (Fig. S6). The combined results suggest that VacA and GGT are critically involved in DC tolerization independent of their effects on T cells.
H. pylori-Mediated Protection Against Airway Inflammation and Asthma Depends on VacA.
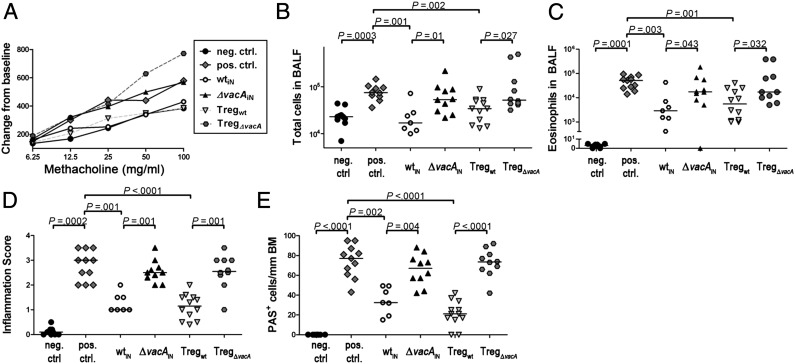
A beneficial consequence of neonatal infection with H. pylori is the protection against allergen-specific T-cell responses and asthma (22). To assess the contribution of VacA to the H. pylori-mediated protection against airway hyper-responsiveness and lung inflammation in a mouse model of allergen-induced asthma, newborn mice were infected with either wild-type or ΔvacA H. pylori and then sensitized and subsequently challenged with the allergen ovalbumin (Δggt H. pylori could not be examined in this model because of their inability to colonize neonatal mice; Fig. S5A). Uninfected control mice subjected to the allergen sensitization/challenge protocol were characterized by airway hyper-responsiveness to methacholine and bronchoalveolar immune cell infiltration, especially of eosinophils (Fig. 4 A–C and Fig. S7 A–D), as well as histologically evident lung inflammation and goblet cell metaplasia (Fig. 4 D and E and Fig. S7E). Neonatal infection with wild-type H. pylori, but not the ΔvacA mutant, efficiently prevented all symptoms of allergen-induced asthma (Fig. 4 A–E and Fig. S7). Similarly, the adoptive transfer of MLN-derived CD4+CD25+ Tregs (12, 22) isolated from wild-type– but not ΔvacA-infected donors to naive recipients before allergen challenge conferred protection against allergic airway disease (Fig. 4 A–E and Fig. S7). We conclude from the combined results that neonatal infection with wild-type, but not VacA-deficient, bacteria yields highly suppressive Tregs, which on the one hand suppress clearance of the bacteria and promote persistent infection and on the other hand efficiently suppress allergen-induced asthma.
Fig. 4.
The H. pylori-mediated protection against asthma and generation of Tregs with protective activity depends on VacA. C57BL/6 mice were sensitized and challenged with ovalbumin to induce airway inflammation. Two groups of mice were neonatally infected with either wild-type PMSS1 (wtiN) or PMSS1ΔvacA (ΔvacAiN) before allergen sensitization. Two additional groups received CD4+CD25+ MLN T cells from neonatally wild-type– or PMSS1ΔvacA-infected donors (Tregwt or TregΔvacA) just before challenge. Negative controls were challenged without prior sensitization. (A) Airway hyper-responsiveness as assessed with increasing doses of methacholine. (B and C) Total cells and eosinophils in 1 mL of bronchoalveolar lavage fluid (BALF). (D) Tissue inflammation as assessed on H&E-stained tissue sections. (E) Goblet cell metaplasia as assessed on periodic acid Schiff (PAS)-stained sections. Data in A–E are pooled from three independent studies.
Discussion
Several recent studies have independently documented the distinct ability of H. pylori to reprogram DCs toward a tolerogenic phenotype in vitro and in vivo, ensuring persistence of the bacteria and cross-protecting against chronic inflammatory and autoimmune diseases (11, 12, 23, 24). H. pylori-experienced DCs appear to preferentially prime Treg over Th1 or Th17 responses and fail to produce proinflammatory cytokines (11, 12, 24). Here, we extend these findings and implicate two H. pylori virulence determinants, VacA and GGT, in DC tolerization. Both factors were previously known to share several important properties: They both facilitate murine colonization (16, 17), inhibit human T-cell activation (18–20, 25), and induce epithelial cell apoptosis (26, 27). The evidence now provided here documents a unique role for VacA and GGT in DC tolerization and links the tolerizing effects of both factors on DCs to persistence: (i) The H. pylori-induced inhibition of DC maturation depends on the (nonredundant) activity of both factors; (ii) the induction of Treg properties in naive T cells by H. pylori-experienced DCs likewise depends on both factors, and strains lacking either VacA or GGT due to targeted gene deletion (iii) fail to colonize mice at wild-type levels, and, in the case of the ΔvacA mutant (iv) induce somewhat stronger Th1 and Th17 responses and gastric pathology and fail to protect against allergen-induced asthma. The defects of the Δggt mutant in vitro and in vivo are phenocopied by pharmacological inhibition of the transpeptidase activity of GGT in all readouts, suggesting that the enzymatic activity of GGT is required for its immunomodulatory effects. Interestingly, the tolerance-promoting effects of VacA- and GGT-proficient H. pylori on DCs were as pronounced in mouse strains with defective T-cell compartments as in wild-type mice, ruling out a critical contribution of α/β T cells to DC tolerization.
We found the phenotype of the Δggt mutant to differ depending on the age of the mice at the time of infection. Whereas adult-infected animals support initial colonization but have largely cleared the Δggt mutant by 2 mos p.i., no mutant bacteria could ever be retrieved from neonatally infected mice, independent of the T-cell proficiency of the host. This observation argues that GGT has two mechanistically distinct roles in colonization and in persistence, enabling initial colonization of the specific environment of the juvenile stomach and acting as an immunomodulator of the adult immune system. Both effects of GGT depend on its enzymatic activity. Exactly how GGT and VacA prevent DC maturation and promote DC tolerization remains to be clarified in detail. It is likely that both factors act on entirely different pathways. VacA has been described to prevent phagosome maturation in macrophages (28) and to prevent autophagy in epithelial cells (29), both of which could also be true in DCs and might explain their failure to respond properly to ΔvacA H. pylori. Interestingly, the tolerizing activity of VacA does not seem to be linked to the vacuolating cytotoxicity attributed to some of its variants. Strains expressing the toxic VacA variant s1/m1 (P12, G27) were not more, or less, tolerogenic in vitro than strain PMSS1 expressing nontoxic s2/m2 VacA; rather, our data suggest that VacA’s essential function in establishing immune tolerance and persistence is distinct from its vacuolating activity but may account for the conservation of a vacA gene in all analyzed H. pylori isolates. Because PMSS1 harbors the nontoxic s2/m2 variant of VacA, our studies do not allow us to draw conclusions with respect to the role of cytotoxic VacA in gastric pathology. Despite a clear association of toxigenic forms of VacA with an increased risk of peptic ulceration and gastric cancer in humans (30), other experimental studies in rodents have not detected a role for VacA in gastritis (17) or gastric dysplasia and cancer (31, 32).
Despite its extraordinary host adaptation, H. pylori is lost from Western populations at an astonishing rate: In the United States, for example, H. pylori prevalence has declined from >50% in birth cohorts born at the beginning of the 20th century to ∼10% in cohorts born at its end (33). A series of epidemiological studies has documented an inverse epidemiological association between H. pylori infection and asthma and other allergic and chronic inflammatory disease manifestations, especially in children and young adults (34–36). Using an experimental model of allergic airway disease induced by ovalbumin-specific sensitization and challenge, we found that H. pylori infection protects mice against the clinical and histopathological symptoms of asthma (22). The same H. pylori factors facilitating persistent infection also appear to be required for immune tolerance and protection against asthma. Indeed, it is conceivable that the immunomodulatory properties of H. pylori and of its persistence determinants can be harnessed to prevent or treat allergies and possibly other chronic inflammatory diseases that are of increasing public health importance in societies from which H. pylori is disappearing.
Materials and Methods
H. pylori Strains and Culture Conditions.
The following previously published or newly generated strains of H. pylori were used: PMSS1 (9), PMSS1ΔvacA (generated by consecutive natural transformation of G27ΔvacA gDNA into H. pylori strain SS1, and of SS1ΔvacA gDNA into H. pylori strain PMSS1), PMSS1Δggt (generated by natural transformation of G27Δggt gDNA) (20), G27 (37), G27ΔvacA (deficient for the entire vacuolating cytotoxin gene; generously provided by H. Kusters, Department of Microbiology, University of Utrecht Medical Center, Utrecht, The Netherlands), P12, P12Δggt, P12ΔvacA, and P12Δggt/ΔvacA (obtained by replacing a P12ΔvacA mutant’s entire ggt gene by a kanamycin resistance cassette and subsequent selection on chloramphenicol and kanamycin plates). All P12 strains are described in ref. 38. H. pylori was grown on agar and in liquid culture as described (5).
Animal Experimentation, Assessment of H. pylori Colonization, and Gastric Histopathology.
C57BL/6 wild-type, BL/6.TCR-β−/−, and OT II TCR-transgenic mice were purchased from Charles River Laboratories. All mice were bred at a University of Zurich specific pathogen-free facility. Mixed-sex groups were infected at either 7 d or 6 wk of age with one orogastric dose of ∼2 × 107 cfu of H. pylori PMSS1 (9). E. coli LPS (Serotype 0111:B4; Sigma-Aldrich) was administered i.p. every other day at 1 μg/g body weight. Acivicin (Santa Cruz Biotechnology) was administered i.p. every other day at 2 mg/kg body weight. Stomachs were retrieved and dissected longitudinally into equally sized pieces. For the quantitative assessment of H. pylori colonization, one stomach section was homogenized in Brucella broth and serial dilutions were plated for colony counting as described (9). For the quantitative assessment of gastric histopathology, H&E-stained, paraffin-embedded stomach sections were scored on a scale of 0–6 for the parameters chronic inflammation, atrophy, epithelial hyperplasia, and metaplasia, as first proposed by Dixon et al. (39) and modified as described in detail previously (5). All gastric histopathology images were taken at 100 or 200× final magnification on a Leica Leitz DMRB microscope equipped with a DFC 420C camera. Protocols used for the preparation of murine gastric single cell suspensions and their flow cytometric analysis and for allergen-induced asthma and adoptive T-cell transfers are provided in SI Materials and Methods. All animal experimentation described here was reviewed and approved by the Zurich Cantonal veterinary office (63/2008 and 170/2009 to A.M.).
Preparation of Murine Bone Marrow DCs and MLN DCs and DC/T-Cell Cocultures.
For generation of bone marrow DCs, bone marrow isolated from the hind legs of donor mice was seeded at 50,000 cells per well in 96-well plates in RPMI/10% (vol/vol) FCS and 4 ng/mL GM-CSF and cultured for 5 d. E. coli LPS (Serotype 0111:B4; Sigma-Aldrich) was added at 0.5 μg/mL final concentration, unless stated otherwise, to induce bone marrow DC maturation. For the isolation of MLN DCs, MLNs of individual mice were digested in 1 mg/mL collagenase (Sigma-Aldrich) for 30 min at 37 °C with shaking before filtering through a cell strainer (40 μm; BD Biosciences) and immunomagnetic isolation using mouse-specific CD11c microbeads (Miltenyi Biotec). Bone marrow DC cultures were infected overnight with wild-type H. pylori or the respective ΔvacA and Δggt isogenic mutants at an MOI of 50; bacteria were killed with 200 U of penicillin and 0.2 mg of streptomycin per mL for 6 h before the addition of T cells. CD4+CD25− T cells were prepared from single-cell suspensions of naive C57BL/6 spleens by immunomagnetic sorting (R&D Systems). DCs were cocultured with CD4+CD25− T cells at a ratio of 1:2 (0.5 × 105 DC to1 × 105 T cells) in RPMI containing 10% FCS, 10 ng/mL rTGF-β (PeproTech), 10 ng/mL rIL-2 (R&D Systems), and 1 μg/mL anti-CD3ε (BD Bioscience). After 72 h of coculture, the cells were stained first for CD4 (CD4-FITC; Biolegend) and CD25 (CD25-PB; BD Bioscience) and then, after fixation and permeabilization, for FoxP3 (FoxP3-APC; eBioscience). The percentage of CD4+FoxP3+CD25+ T cells was assessed by FACS. IL12p40 production by DCs was assessed by ELISA (BD Bioscience). Statistical analyses are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Karen Otteman and Katrin Koch for help with constructing the PMSS1ΔvacA mutant strain and Esther Kohler for expert technical assistance. This work was funded by the Swiss National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1211248110/-/DCSupplemental.
References
- 1.Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: Independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol. 2009;104(1):182–189. doi: 10.1038/ajg.2008.61. [DOI] [PubMed] [Google Scholar]
- 2.Morelli G, et al. Microevolution of Helicobacter pylori during prolonged infection of single hosts and within families. PLoS Genet. 2010;6(7):e1001036. doi: 10.1371/journal.pgen.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suerbaum S, Josenhans C. Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol. 2007;5(6):441–452. doi: 10.1038/nrmicro1658. [DOI] [PubMed] [Google Scholar]
- 4.Robinson K, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut. 2008;57(10):1375–1385. doi: 10.1136/gut.2007.137539. [DOI] [PubMed] [Google Scholar]
- 5.Sayi A, et al. The CD4+ T cell-mediated IFN-gamma response to Helicobacter infection is essential for clearance and determines gastric cancer risk. J Immunol. 2009;182(11):7085–7101. doi: 10.4049/jimmunol.0803293. [DOI] [PubMed] [Google Scholar]
- 6.Müller A, Oertli M, Arnold IC. H. pylori exploits and manipulates innate and adaptive immune cell signaling pathways to establish persistent infection. Cell Commun Signal. 2011;9(1):25. doi: 10.1186/1478-811X-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linz B, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445(7130):915–918. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PR, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology. 2008;134(2):491–499. doi: 10.1053/j.gastro.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Arnold IC, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011;140(1):199–209. doi: 10.1053/j.gastro.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hitzler I, Oertli M, Becher B, Agger EM, Müller A. Dendritic cells prevent rather than promote immunity conferred by a helicobacter vaccine using a mycobacterial adjuvant. Gastroenterology. 2011;141(1):186–196, 196.e1. doi: 10.1053/j.gastro.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Kao JY, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology. 2010;138(3):1046–1054. doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oertli M, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest. 2012;122(3):1082–1096. doi: 10.1172/JCI61029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6(12):1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 15.Kim JM, et al. Stimulation of dendritic cells with Helicobacter pylori vacuolating cytotoxin negatively regulates their maturation via the restoration of E2F1. Clin Exp Immunol. 2011;166(1):34–45. doi: 10.1111/j.1365-2249.2011.04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chevalier C, Thiberge JM, Ferrero RL, Labigne A. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol Microbiol. 1999;31(5):1359–1372. doi: 10.1046/j.1365-2958.1999.01271.x. [DOI] [PubMed] [Google Scholar]
- 17.Salama NR, Otto G, Tompkins L, Falkow S. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect Immun. 2001;69(2):730–736. doi: 10.1128/IAI.69.2.730-736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boncristiano M, et al. The Helicobacter pylori vacuolating toxin inhibits T cell activation by two independent mechanisms. J Exp Med. 2003;198(12):1887–1897. doi: 10.1084/jem.20030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebert B, Fischer W, Weiss E, Hoffmann R, Haas R. Helicobacter pylori vacuolating cytotoxin inhibits T lymphocyte activation. Science. 2003;301(5636):1099–1102. doi: 10.1126/science.1086871. [DOI] [PubMed] [Google Scholar]
- 20.Schmees C, et al. Inhibition of T-cell proliferation by Helicobacter pylori gamma-glutamyl transpeptidase. Gastroenterology. 2007;132(5):1820–1833. doi: 10.1053/j.gastro.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Sewald X, et al. Integrin subunit CD18 Is the T-lymphocyte receptor for the Helicobacter pylori vacuolating cytotoxin. Cell Host Microbe. 2008;3(1):20–29. doi: 10.1016/j.chom.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Arnold IC, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121(8):3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luther J, et al. Helicobacter pylori DNA decreases pro-inflammatory cytokine production by dendritic cells and attenuates dextran sodium sulphate-induced colitis. Gut. 2011;60(11):1479–1486. doi: 10.1136/gut.2010.220087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YH, Gorvel JP, Chu YT, Wu JJ, Lei HY. Helicobacter pylori impairs murine dendritic cell responses to infection. PLoS ONE. 2010;5(5):e10844. doi: 10.1371/journal.pone.0010844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundrud MS, Torres VJ, Unutmaz D, Cover TL. Inhibition of primary human T cell proliferation by Helicobacter pylori vacuolating toxin (VacA) is independent of VacA effects on IL-2 secretion. Proc Natl Acad Sci USA. 2004;101(20):7727–7732. doi: 10.1073/pnas.0401528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KM, et al. Gamma-glutamyltranspeptidase of Helicobacter pylori induces mitochondria-mediated apoptosis in AGS cells. Biochem Biophys Res Commun. 2007;355(2):562–567. doi: 10.1016/j.bbrc.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 27.Boquet P, Ricci V. Intoxication strategy of Helicobacter pylori VacA toxin. Trends Microbiol. 2012;20(4):165–174. doi: 10.1016/j.tim.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Zheng PY, Jones NL. Helicobacter pylori strains expressing the vacuolating cytotoxin interrupt phagosome maturation in macrophages by recruiting and retaining TACO (coronin 1) protein. Cell Microbiol. 2003;5(1):25–40. doi: 10.1046/j.1462-5822.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 29.Raju D, et al. Vacuolating cytotoxin and variants in Atg16L1 that disrupt autophagy promote Helicobacter pylori infection in humans. Gastroenterology. 2012;142(5):1160–1171. doi: 10.1053/j.gastro.2012.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palframan SL, Kwok T, Gabriel K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front Cell Infect Microbiol. 2012;2:92. doi: 10.3389/fcimb.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franco AT, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68(2):379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogura K, et al. Virulence factors of Helicobacter pylori responsible for gastric diseases in Mongolian gerbil. J Exp Med. 2000;192(11):1601–1610. doi: 10.1084/jem.192.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7(12):887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amberbir A, et al. 2011. Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy 41(10):1422–1430.
- 35.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198(4):553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: A meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2010;16(6):1077–1084. doi: 10.1002/ibd.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Censini S, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93(25):14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fassi Fehri L, et al. Helicobacter pylori induces miR-155 in T cells in a cAMP-Foxp3-dependent manner. PLoS ONE. 2010;5(3):e9500. doi: 10.1371/journal.pone.0009500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.