Abstract
Background
Ventilator-associated pneumonia (VAP) is a leading cause of morbidity and mortality in patients hospitalized in intensive care units. Recent studies suggest that dental plaque biofilms serve as a reservoir for respiratory pathogens. The goal of this study was to determine the genetic relationship between strains of respiratory pathogens first isolated from the oral cavity and later isolated from bronchoalveolar lavage fluid from the same patient undergoing mechanical ventilation with suspected VAP.
Methods
Plaque and tracheal secretion samples were obtained on the day of hospital admission and every other day thereafter until discharge from the intensive care unit from 100 patients who underwent mechanical ventilation. Bronchoalveolar lavage was performed for 30 patients with suspected VAP. Pulse-field gel electrophoresis and multilocus sequence typing were used to determine the genetic relatedness of strains obtained from oral, tracheal, and bronchoalveolar lavage samples.
Results
Isolates of Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter species, and enteric species recovered from plaque from most patients were indistinguishable from isolates recovered from bronchoalveolar lavage fluid (i.e., had >95% similarity of pulse-field gel electrophoresis patterns). Nearly one-half of the Pseudomonas strains showed identical genetic profiles between patients, which suggested a common environmental source of infection.
Conclusions
Respiratory pathogens isolated from the lung are often genetically indistinguishable from strains of the same species isolated from the oral cavity in patients who receive mechanical ventilation who are admitted to the hospital from the community. Thus, dental plaque serves as an important reservoir for respiratory pathogens in patients who undergo mechanical ventilation.
Trial registration
ClinicalTrials.gov identifier: NCT00123123
Ventilator-associated pneumonia (VAP) is an important infection in patients hospitalized in intensive care units (ICUs) who receive mechanical ventilation [1]. VAP occurs in such patients >48 h after intubation [2]. Common VAP pathogens include Staphylococcus aureus and gram-negative bacteria, including Pseudomonas aeruginosa and Enterobacteriaceae [3]. Gram-negative bacteria account for 60% of all hospital-acquired pneumonia [4]. The mortality rate associated with VAP can be as high as 76% [5], especially among patients with life-threatening illness. The prevalence of VAP is 10%–65% among patients who receive mechanical ventilation, with mortality rates of 24%–76%, depending on the study method, the patient population, and the mode of treatment [1, 6–8]. VAP can prolong the duration of mechanical ventilation by 14–32 days and the length of stay in the ICU by 11.7 days, resulting in an increase in total hospital charges of >$150,000 per patient [9].
Dental plaque is a complex and dynamic biofilm that forms on supragingival and subgingival tooth surfaces, oral mucosal surfaces (especially the tongue), and dental restorations [10]. Recent molecular studies have suggested that >700 species of bacteria colonize dental plaque [11]. It is well known that a lack of oral hygiene allows uninterrupted microbial ecological succession and, therefore, increased microbial diversity within dental plaque [12, 13]. Plaque formation is also fostered by reduced salivary function, which is often a sequelae of xerostomic medications in critical care patients [14]. Such conditions may favor colonization by respiratory pathogens. Indeed, several studies have suggested that dental plaque may serve as a reservoir for respiratory pathogens in patients in the ICU, but not in community-dwelling subjects [15–19].
In the present study, a molecular epidemiologic analysis was used to determine the genetic relationship between respiratory pathogen strains isolated from the oral cavity of patients in the ICU who underwent mechanical ventilation and strains isolated from tracheal secretions and bronchoalveolar lavage (BAL) fluid obtained from the same patient.
PATIENTS, MATERIALS, AND METHODS
Study population
Subjects for this prospective study were recruited for a randomized clinical trial that tested the effect of chlorhexidine gluconate oral rinse on oral colonization by respiratory pathogens (ClinicalTrials.gov identifier: NCT0012 3123). The University at Buffalo Human Subjects Institutional Review Board approved the study protocol. Subjects were recruited from among patients admitted to the Trauma ICU (TICU) of Erie County Medical Center from 14 February 2005 through 15 May 2006. All participants or their surrogate provided informed consent. Patients with the following conditions were excluded from the study: (1) witnessed aspiration (inhaled food or other foreign objects); (2) postobstructive pneumonia; (3) short-term treatment for drug overdose; (4) treatment of community-acquired pneumonia; (5) diagnosed blood-clotting abnormality; (6) age <18 years; (7) pregnancy; and (8) admission from another ICU or readmission to the TICU.
Pneumonia was diagnosed using the clinical pulmonary infection score system, as follows: (1) body temperature scored as 0 (36.5°C–38.4°C), 1 (38.5°C–39°C), or 2 (<36.0°C or >39.0°C); (2) leukocytosis scored as 0 (4000–11,000 cells/mL), 1 (11,000–17,000 cells/mL), or 2 (>17,000 cells/mL); (3) new radiographic infiltrate scored as 0 (none), 1 (patchy), or 2 (localized); (4) secretions scored as 0 (none to minimal), 1 (moderate), or 2 (large amount); and (5) PaO2/FiO2 scored as 0 (≥330 mm Hg) or 2 (<330 mm Hg). If the clinical pulmonary infection score was ≥6, BAL fluid was collected as described below. Confirmation of pneumonia was defined as detection of ≥104 bacterial colony-forming units/mL of a potential respiratory pathogen in a BAL specimen. The Acute Physiology and Chronic Health Evaluation II score was performed as described elsewhere [20].
Oral examination
An oral examination was performed and dental plaque samples were collected on the day of admission to the TICU and every 48 h thereafter for up to 21 days or until the patient was discharged from the unit; the examination was performed with a standard dental mirror and portable dental light. The number of teeth were enumerated (table 1). The plaque index was performed for 6 teeth, as follows: upper right first molar, upper right central incisor, upper left first bicuspid, lower left first molar, lower left central incisor, and lower right first bicuspid. If an index tooth was missing or if obstructions precluded the examination of specific teeth, a tooth close to the missing or obstructed tooth was scored. Only a single score (0, no plaque; 1, plaque seen only on tip of an explorer passed over the tooth surface; 2, plaque obvious to the naked eye; 3, gross deposits of plaque) was assigned to indicate the surface with the most plaque. A mean score was calculated from the 6 teeth for each subject at each time point.
Table 1.
Demographic and clinical characteristics of 18 patients with ventilator-associated pneumonia.
| Patient no. | Sex | Age, years | Plaque score | No. of teeth | APACHE II | Target species isolated from SG, TS, BAL |
|---|---|---|---|---|---|---|
| 12 | M | 24 | 1 | 30 | 21 | Escherichia coli, Acinetobacter baumannii |
| 15 | M | 79 | ND | 0a | 18 | Staphylococcus aureus |
| 21 | M | 41 | ND | ND | 15 | Enterobacter aerogenes, Pseudomonas aeruginosa |
| 30 | F | 32 | ND | ND | 23 | Enterobacter cloacae |
| 41 | M | 44 | 2 | 10 | 5 | S. aureus, E. coli, Klebsiella pneumoniae |
| 48 | M | 65 | 1 | 17 | 24 | Klebsiella oxytoca, Serratia marcescens |
| 50 | M | 62 | 3 | 10 | 11 | P. aeruginosa |
| 59 | M | 26 | 1 | 16 | 17 | Methicillin-resistant S. aureus, P. aeruginosa |
| 61 | M | 28 | 2 | 32 | 2 | E. aerogenes |
| 67 | M | 42 | ND | ND | 5 | E. coli |
| 69 | F | 67 | ND | 0a | 19 | E. aerogenes |
| 74 | F | 45 | ND | 32 | 23 | S. aureus |
| 76 | M | 48 | ND | 28 | 22 | K. pneumoniae |
| 84 | F | 20 | ND | 32 | 20 | S. aureus |
| 86 | F | 76 | 1 | 6 | 13 | P. aeruginosa |
| 88 | M | 25 | 1 | 32 | 23 | P. aeruginosa |
| 89 | F | 53 | 1 | 32 | 21 | P. aeruginosa |
| 94 | M | 20 | 0 | 32 | 14 | S. aureus |
NOTE. APACHE, Acute Physiology and Chronic Health Evaluation; BAL, bronchoalveolar lavage fluid; ND, not determined; SG, supragingival plaque; TS, tracheal secretion.
Patient was edentulous.
Oral, tracheal, and lung microbial sampling
Samples were collected from supragingival dental plaque and tracheal secretions every 48 h. Supragingival dental plaque samples were collected with a sterile curette and were dispersed into 2 mL of sterile normal saline. When plaque could not be collected (e.g., from edentulous subjects or because of obstruction from tubes), the buccal mucosa was sampled by rubbing with a sterile swab. Tracheal secretion samples were obtained by instillation of sterile normal saline with use of a sterile, protected, distally blinded alveolar catheter placed into the endotracheal tube. BAL samples were obtained from patients who showed a clinical pulmonary infection score ≥6 and who displayed symptoms that were consistent with pneumonia by instillation of sterile normal saline with use of a plugged-telescoping catheter (CombiCath; Plastimed) via flexible bronchoscopy.
Samples were immediately transported on ice to the clinical microbiology laboratory, were diluted, were plated, and were incubated for 72 h at 37°C in 5% carbon dioxide. The growth of ≥1 species of a target pathogen (S. aureus, methicillin-resistant S. aureus, P. aeruginosa, Acinetobacter species, Enterobacter species, Escherichia coli, Klebsiella species, and Serratia marcescens [1]) was assessed on sheep blood agar (to isolate S. aureus) and MacConkey agar (for isolation of P. aeruginosa and gram-negative bacilli). Strains were identified using standard microbiological techniques.
PFGE
Bacterial genomic DNA was prepared from each strain [21, 22], and PFGE was performed as a modification of a method described elsewhere [23]. A single bacterial colony was streaked onto appropriate agar and cultured overnight at 37°C. Cells were transferred into 3 mL of PIV (1000 mmol/L sodium chloride, 10 mmol/L Tris, pH 8.0) and were adjusted to optical density at 600 nm of 1.0–1.2. Two hundred μL of cells was incubated at 37°C for 2 min and then was mixed with 200 μL of melted 1.6% (weight-to-volume) InCert agarose (FMC) in phosphate buffer IV (equilibrated to 60°C). S. aureus was suspended in 200 μL of melted 1% (weight-to-volume) Seakem Gold agarose (FMC) containing 2 μL of lysostaphin solution (Sigma) (1 mg/mL). The homogenized mixture was placed in wells of a disposable plug mold (100 μL in each well; Bio-Rad), placed at 10°C for 10 min to solidify, and was then placed in a screened cap tube (Bio-Rad) containing 5 mL of fresh erythrocyte lysis buffer (6 mmol/L Tris hydrochloride, pH 8.0, 1 mol/L sodium chloride, 100 mmol/L EDTA, pH 8.0, 0.5% Brij-58, 0.2% sodium deoxycholate, 0.5% sodium lauroylsar-cosine, ribonuclease A [3 μL; Sigma]) and Readi-Lyse lysozyme (2 μL; Epicentre). The buffer was removed and replaced with 2 mL of ESP solution (500 mmol/L EDTA, pH 9.0, 1% sodium lauroylsarcosine, 0.5 mg/mL proteinase K). The plugs were incubated with agitation at 55°C for 2 h, and the ESP solution was decanted. The ESP lysis step was omitted for S. aureus. The plugs were washed in TE buffer (10 mmol/L Tris hydrochloride, 10 mmol/L EDTA, pH 8.0) for 15 min at 50°C 3 times and were then stored at 4°C until used.
For in-plug restriction enzyme digestion, 1 plug was placed into a sterile microcentrifuge tube with 500 μL of restriction enzyme buffer (445 μL of sterile distilled water, 50 μL of the appropriate 10 × NEbuffer [New England BioLabs]), and 5 μL of bovine serum albumin (New England BioLabs) for 30 min at room temperature. After aspiration of restriction enzyme buffer, 196 μL of fresh restriction enzyme buffer was added to 4 μL of restriction enzyme at 37°C for 2–4 h, as recommended by the manufacturer (S. aureus, SmaI; P. aeruginosa, NheI or SpeI; Acinetobacter baumannii, ApaI; E. coli and other gram-negative bacilli, XbaI).
PFGE gels were run as described elsewhere [8, 24–27] with use of a contour-clamped homogenous electric field apparatus (CHEF DR-II; Bio-Rad,). Gels were stained with ethidium bromide and were photographed using AlphaEase FC and Alpha-Imager (Alpha Innotech). For all gels, λ DNA ladder (Bio-Rad) was used as molecular size markers.
PFGE gel images were analyzed with BioNumerics software (Applied Maths). Each gel was normalized to the appropriate global standard strain (S. aureus NCTC 8325, P. aeruginosa PAO1, or E. coli K12). S. aureus strains were also compared with the same internal standard provided by Dr. Fred McDougal (Centers for Disease Control and Prevention; Atlanta, GA) for reference strains in the Centers for Disease Control and Prevention national collection. Percentage similarities were calculated by Dice coefficients, and phylogenetic trees were constructed using the unpaired group mean arithmetic-averages clustering method (band tolerance, 1.25; optimization, 0.5%). A cutoff value of 90% similarity was applied to define the PFGE type and to type for supplementary multilocus sequencing. Interpretation of relatedness of the PFGE band patterns was conducted on the basis of the recent consensus criteria [28].
Multilocus sequence typing (MLST) was performed as described elsewhere [29]. Only closely related PFGE types (showing 2- or 3-band differences) or indistinguishable PFGE types (as positive controls) were chosen for MLST characterization. Internal fragments of 7 housekeeping genes (400–500 base pairs in length) were sequenced for each species, and each sequence (allele) was compared with the sequences of known alleles at the appropriate MLST Web site [30, 31]. The following alleles were studied: arcC, aroE, glpF, gmk, pta, tpi, and yqiL for S. aureus; acsA, aroE, guaA, mutL, nuoD, ppsA, and trpE for P. aeruginosa; and adk, fumC, gyrB, icd, mdh, purA, and recA for E. coli. MLST allele names and sequence types were obtained from the MLST Web site. Each different sequence was assigned as a distinct allele. The sequence type or allelic profile for each isolate was determined on the basis of the combination of 7 alleles.
The PCR protocol and primer sequence for the 7 MLST loci were obtained from the MLST Web site [30]. All MLST allele sequences used for subsequencing were wrapped by a minimum of 1 forward and 1 reverse overlapping, high-quality sequence read (minimum quality score of 35). Forward and reverse reads for the 7 MLST loci from each isolate were assembled from the read pairs with use of modified TIGR Assembler software (J. Craig Venter Institute). The assembled sequence was oriented in the 5′→3 direction with the flanking sequence removed. Sequence chromatograms for unique alleles were deposited in the MLST database [30].
Methicillin resistance (mecA) and Panton-Valentine leu-kocidin (PVL) typing
Multiplex PCR for mecA and PVL typing was performed as described by McClure et al. [32], with a modification in the primer Luk-PV-2 (5′-GCATCAASTGTAT-TGGATAGCAAAAGC-3′), which was substituted to account for single-nucleotide polymorphisms at the 8 nucleotides.
RESULTS
Clinical characteristics of patients with VAP
One hundred patients who underwent mechanical ventilation were enrolled in the study. Target respiratory pathogens were recovered from the oral cavities of 60 (60%) of 100 patients, and BAL specimens yielded respiratory pathogens from 30 (50%) of these 60 patients. Of these 30 patients, 18 (60%) were selected for further molecular analysis because they were colonized by ≥1 target pathogen at all 3 sample sites (i.e., mouth, trachea, and lung). The clinical characteristics of these patients and the corresponding isolated pathogens are summarized in table 1. Three patients (patients 12, 21, and 48) harbored 2 pathogen species at all 3 sites, and 1 patient (patient 59) harbored 3 species present at all 3 sites.
PFGE analysis of isolates
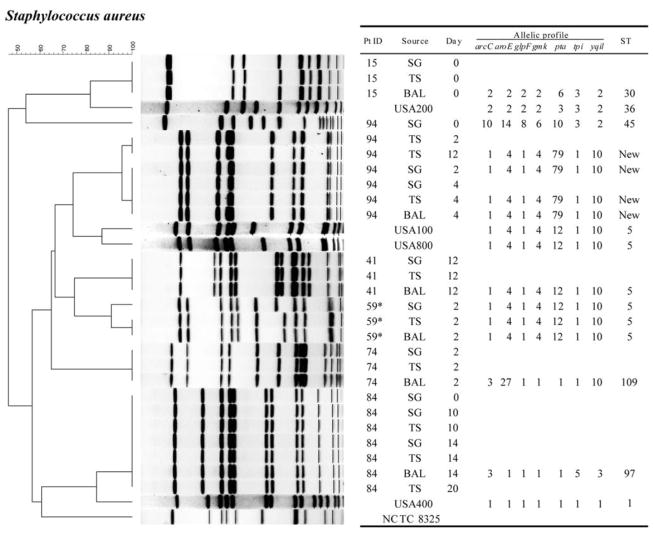
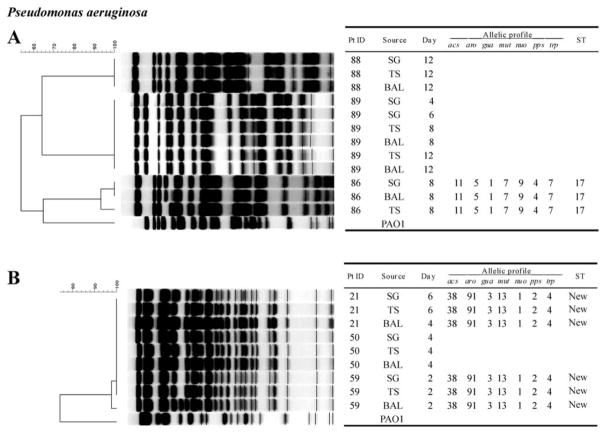
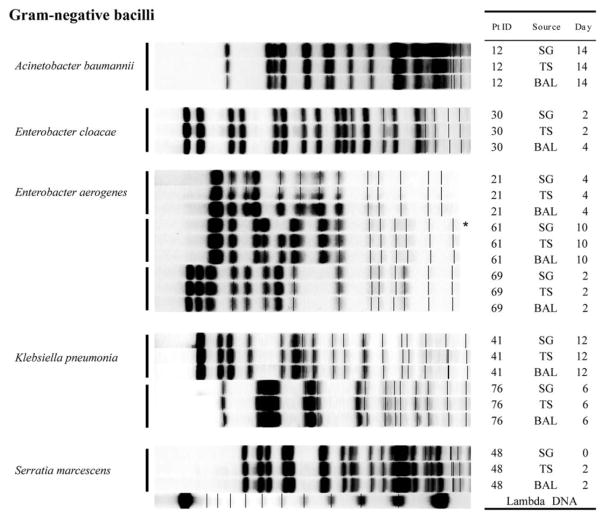
A total of 83 PFGE band patterns were obtained from 26 isolates of S. aureus (figure 1), 21 isolates of P. aeruginosa (figure 2), 12 isolates of E. coli (figure 3), and 24 isolates of other gram-negative bacilli (figure 4). With a single exception, comparison of strains of the same species obtained from different sites within a single patient showed no difference in PFGE pattern (i.e., ≥90% similarity index). The exception involved 1 patient (patient 94) who harbored 2 clones of S. aureus.
Figure 1.
PFGE patterns with dendrogram for Staphylococcus aureus isolates. A genetic similarity index scale is shown above the dendrogram. Patient identification (Pt ID), sample site (Source), number of days after admission to the intensive care unit that the strain was isolated (Day), and multilocus sequence type (ST) are provided. USA100, USA200, USA400, USA800, and NCTC 8325 strains from the Centers for Disease Control and Prevention were used as reference strains. *Methicillin-resistant Staphylococcus aureus. For genotyping conditions, see Patients, Materials, and Methods. BAL, bronchoalveolar lavage; SG, supragingival dental plaque; TS, tracheal secretion.
Figure 2.
PFGE patterns with dendrogram for Pseudomonas aeruginosa isolates. A genetic similarity index scale is shown above the dendrogram. Patient identification (Pt ID), sample site (Source), number of days after admission to the intensive care unit that the strain was isolated (Day), and multilocus sequence type (ST) are provided. P. aeruginosa PAO1 strain was used as the reference strain. To obtain optimal PFGE band patterns, different conditions of pulse time and running hours were used. For running conditions, see Patients, Materials, and Methods. BAL, bronchoalveolar lavage; SG, supragingival dental plaque; TS, tracheal secretion.
Figure 3.
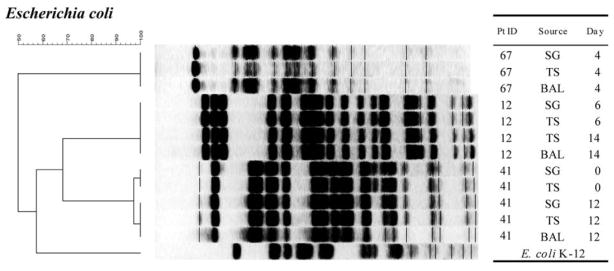
PFGE patterns with dendrogram for Escherichia coli isolates. A genetic similarity index scale is shown above the dendrogram. Patient identification (Pt ID), sample site (Source), and number of days after admission to the intensive care unit that the strain was isolated (Day) are provided. Escherichia coli K-12 strain was used as the reference strain. For running conditions, see Patients, Materials, and Methods. BAL, bronchoalveolar lavage; SG, supragingival dental plaque; TS, tracheal secretion.
Figure 4.
PFGE patterns with dendrogram for other potentially pathogenic gram-negative bacilli. Patient identification (Pt ID), sample site (Source), number of days after admission to the intensive care unit that the strain was isolated (Day) are provided. λ DNA was used as the reference for standardization. For running conditions, see Patients, Materials, and Methods. BAL, bronchoalveolar lavage; SG, supragingival dental plaque; TS, tracheal secretion.
The relatedness of strains isolated from the mouth at baseline to other strains isolated from the same patient’s mouth, trachea, and/or lung at some point after baseline was studied by PFGE analysis of isolates from each site collected over time. Two patients with S. aureus colonization (patients 84 and 94; figure 1), 1 patient with P. aeruginosa colonization (patient 89; figure 2), and 2 patients with E. coli colonization (patients 12 and 41; figure 3) were selected for PFGE analysis. The PFGE profiles from strains of the same species obtained from the same patient showed no differences in band patterns over time (≥90% similarity index). Also, 1 patient with S. aureus colonization (patient 84) maintained the same clone for up to 20 days (figure 1). Moreover, a strain of S. aureus that was isolated from the mouth at baseline appeared to later colonize the trachea and lung (where it was isolated on days 10, 14, and 20). Similar results were found for P. aeruginosa (figure 2) and E. coli (figure 3).
PFGE patterns were compared between strains that were isolated from different individuals. Overall, isolates from a given individual appeared to be clonally independent from those isolated from other patients. The exception was P. aeruginosa (figure 2); several P. aeruginosa strains isolated from different patients appeared to share identical PFGE profiles.
MLST was performed on selected isolates that were either indistinguishable by PFGE (as positive controls) or closely related (showing 2- or 3-band differences by PFGE). In addition, heterogeneous strains (i.e., those with >7-band differences) were also studied. A total of 26 MLST sequence types were obtained from 12 isolates of S. aureus (figure 1), 9 isolates of P. aeruginosa (figure 2), and 5 isolates of E. coli (data not shown). Overall, the same sequence types were found in isolates with indistinguishable PFGE types isolated from different sites and isolated at different times from the same patient (figures 1 and 2). Conversely, distinctly unrelated PFGE types always showed different sequence types. However, in a few cases, sequence types were found not to be as discriminatory as PFGE types. For example, the methicillin-resistant S. aureus reference strains [8] USA100 and USA800, demonstrated by PFGE to be in a different cluster from the isolates obtained from patients 41 and 59, were also shown by MLST to share the same allelic profiles (sequence type 5) as clones from patients 41 and 59.
mecA and PVL typing of S. aureus
Only strains from patient 59 were positive for mecA, which was consistent with the results of Kirby-Bauer antibiotic susceptibility testing (figure 1). However, no isolates were positive for PVL. This is not surprising, because reference methicillin-resistant S. aureus strains obtained from individuals with health care–associated infections (USA100, USA200, and USA800) were also negative for PVL [33]. Moreover, none of the clinical strains from TICU clustered with the PVL-positive USA300 isolates, which are known to be associated with community-associated disease.
DISCUSSION
In the present study, oral respiratory pathogen isolates were mostly genetically indistinguishable from tracheal and BAL isolates recovered from the same patient. These results concur with those of earlier studies that have found that the oral cavity may serve as a reservoir for respiratory pathogens in patients who receive mechanical ventilation [34–38]. These findings further support a previous report involving 49 elderly patients who were admitted to the hospital from a nursing home [39]; in that report, molecular genotyping found that 9 of 13 isolates recovered from BAL fluid were genetically matched with strains recovered from dental plaque from the same patient.
In addition, a more recent study examined the oropharynx as a reservoir for respiratory species with use of 16S ribosomal DNA analysis [40]. This culture-independent molecular approach identified not only pathogens known to be associated with VAP, such as Pseudomonas species and Enterobacteriaceae, but also various as-yet-uncultivated species in both the oral cavity and BAL specimens.
The pathogenesis of pneumonia involves aspiration of pathogens into the lower airway and the inability of the host defenses to clear them [41]. In VAP, an artificial surface, such as an endotracheal tube, bypasses anatomic barriers, such as the glottis and larynx, between the oropharynx and trachea and facilitates the transport of bacteria from the oral cavity. The results reported here support the mouth as a likely portal of entry for passage of bacteria into the lung [42].
In the present study, most patients were admitted to the hospital as a consequence of trauma (e.g., motor vehicle accident or closed head trauma). These subjects were likely to be healthy before entering the TICU, with normal oral flora and a sterile lower respiratory tract. Results of the present study convincingly demonstrate that pathogens either emerged from the resident oral flora or were acquired from the environment after admission to the hospital. The organisms then spread from the mouth into the trachea and subsequently into the lung to initiate infection. Other recent findings have also demonstrated strong correlations between oropharyngeal colonization and subsequent positive BAL samples [19].
In the present study, the same clone of P. aeruginosa was shared among 3 of 6 patients (figure 2B), whereas unique clones were obtained from the other 3 patients (figure 2A). This is consistent with recent studies that show environmental cross-acquisition to be responsible for at least 50% of P. aeruginosa infections in ICUs [43–45]. Thus, P. aeruginosa is often transmitted from patient to patient or from a common source in the ICU. All of the other target pathogens (S. aureus and other gram-negative bacteria) were clones that were unique to each patient. The heterogeneity of most of the strains recovered from patients in ICUs suggests an endogenous origin, which is consistent with the findings of earlier studies [46]. In addition, all of the S. aureus isolates that were recovered were PVL negative; this is consistent with the absence in the ICU of isotype USA300 and sequence type 8 strains, which are features commonly observed in community-associated methicillin-resistant S. aureus isolates.
In conclusion, our results show that, in patients who underwent mechanical ventilation who were admitted to hospital from the community, respiratory pathogens from the lung are often genetically indistinguishable from strains isolated from the oral cavity; this provides convincing evidence that dental plaque serves as an important reservoir for respiratory pathogens in these patients. Several randomized controlled trials have suggested that interventions to improve oral hygiene in patients who receive mechanical ventilation reduces the risk of VAP [47–49]. Future studies should assess optimal interventions to reduce respiratory pathogen colonization of the mouth and thereby reduce the risk of pneumonia for patients hospitalized in ICUs.
Acknowledgments
We thank Dr. Daniel Amsterdam of the Department of Laboratory Medicine, Erie County Medical Center Healthcare Network (Buffalo, NY), and his staff, for isolation and identification of target bacterial strains; Dr. Timothy F. Murphy of the Division of Infectious Diseases, Veterans Affairs Western New York Healthcare System (Buffalo, NY), and his staff (Nina Johnson and Aimee L. Brauer), for providing excellent technical support and discussion regarding PFGE; study nurses Angela Vacanti and Noreen Frawley, for enrolling patients and sample collection; and Ann Gill of the Center of Excellence in Bioinformatics and Life Sciences (Buffalo, NY), for discussion regarding MLST analysis.
Financial support. National Institute of Dental and Craniofacial Research (grant DE014685).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 2.Torpy JM, Lynm C, Glass RM. JAMA patient page: ventilator-associated pneumonia. JAMA. 2007;297:1616. doi: 10.1001/jama.297.14.1616. [DOI] [PubMed] [Google Scholar]
- 3.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Guidelines for prevention of nosocomial pneumonia. MMWR Morb Mortal Weekly Rep. 1997;46 (RR-1):1–79. [PubMed] [Google Scholar]
- 5.Salata RA, Lederman MM, Shlaes DM, et al. Diagnosis of nosocomial pneumonia in intubated, intensive care unit patients. Am Rev Respir Dis. 1987;135:426–32. doi: 10.1164/arrd.1987.135.2.426. [DOI] [PubMed] [Google Scholar]
- 6.Craven DE, Barber TW, Steger KA, Montecalvo MA. Nosocomial pneumonia in the 1990s: update of epidemiology and risk factors. Semin Respir Infect. 1990;5:157–72. [PubMed] [Google Scholar]
- 7.Vincent JL, Bihari DJ, Suter PM, et al. The prevalence of nosocomial infection in intensive care units in Europe: results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. JAMA. 1995;274:639–44. [PubMed] [Google Scholar]
- 8.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–20. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–21. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 10.ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94:1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 11.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–7. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 12.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–87. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 13.Marsh PD. Dental plaque as a biofilm and a microbial community: implications for health and disease. BMC Oral Health. 2006;6(Suppl 1):S14. doi: 10.1186/1472-6831-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Epstein JB, Sroussi H. Hyposalivation in elderly patients. J Can Dent Assoc. 2006;72:841–6. [PubMed] [Google Scholar]
- 15.Johanson WG, Pierce AK, Sanford JP. Changing pharyngeal bacterial flora of hospitalized patients: emergence of gram-negative bacilli. N Engl J Med. 1969;281:1137–40. doi: 10.1056/NEJM196911202812101. [DOI] [PubMed] [Google Scholar]
- 16.Valenti WM, Trudell RG, Bentley DW. Factors predisposing to oro-pharyngeal colonization with gram-negative bacilli in the aged. N Engl J Med. 1978;298:1108–11. doi: 10.1056/NEJM197805182982002. [DOI] [PubMed] [Google Scholar]
- 17.Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20:740–5. doi: 10.1097/00003246-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Fourrier F, Duvivier B, Boutigny H, Roussel-Delvallez M, Chopin C. Colonization of dental plaque: a source of nosocomial infections in intensive care unit patients. Crit Care Med. 1998;26:301–8. doi: 10.1097/00003246-199802000-00032. [DOI] [PubMed] [Google Scholar]
- 19.Berdal JE, Bjornholt J, Blomfeldt A, Smith-Erichsen N, Bukholm G. Patterns and dynamics of airway colonisation in mechanically-ventilated patients. Clin Microbiol Infect. 2007;13:476–80. doi: 10.1111/j.1469-0691.2006.01678.x. [DOI] [PubMed] [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 21.Goering RV. Pulsed-field gel electrophoresis. In: Persing DH, Tenover FC, Versalovic J, et al., editors. Molecular microbiology, diagnostic principles and practice. Washington, DC: ASM Press; 2004. pp. 185–96. [Google Scholar]
- 22.Centers for Disease Control and Prevention. One-day (24–28 h) standardized laboratory protocol for molecular subtyping of Escherichia coli O157:H7, non-typhoidal Salmonella serotypes, and Shigella sonnei by pulsed field gel electrophoresis. [Accessed January 2006];2004 Available at: http://www.cdc.gov/pulsenet/protocols.htm.
- 23.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–71. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 24.Murchan S, Kaufmann ME, Deplano A, et al. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J Clin Microbiol. 2003;41:1574–85. doi: 10.1128/JCM.41.4.1574-1585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romling U, Tummler B. Achieving 100% typeability of Pseudomonas aeruginosa by pulsed-field gel electrophoresis. J Clin Microbiol. 2000;38:464–5. doi: 10.1128/jcm.38.1.464-465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bohm H, Karch H. DNA fingerprinting of Escherichia coli O157:H7 strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1992;30:2169–72. doi: 10.1128/jcm.30.8.2169-2172.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gautom RK. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coli O157:H7 and other gram-negative organisms in 1 day. J Clin Microbiol. 1997;35:2977–80. doi: 10.1128/jcm.35.11.2977-2980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tenover FC, Arbeit RD, Goering RV, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–9. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–15. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. [Accessed February 2004];Multi locus sequence typing home page. Available at: http://www.mlst.net.
- 31.Pseuomonas aeruginosa MLST database. [Accessed February 2004]; Available at: http://pubmlst.org/paeruginosa/
- 32.McClure JA, Conly JM, Lau V, et al. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leu-kocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci. J Clin Microbiol. 2006;44:1141–4. doi: 10.1128/JCM.44.3.1141-1144.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tenover FC, McDougal LK, Goering RV, et al. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44:108–18. doi: 10.1128/JCM.44.1.108-118.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scannapieco FA, Mylotte JM. Relationships between periodontal disease and bacterial pneumonia. J Periodontol. 1996;67(Suppl 10):1114–22. doi: 10.1902/jop.1996.67.10s.1114. [DOI] [PubMed] [Google Scholar]
- 35.Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793–802. doi: 10.1902/jop.1999.70.7.793. [DOI] [PubMed] [Google Scholar]
- 36.Shay K, Scannapieco FA, Terpenning MS, Smith BJ, Taylor GW. Nosocomial pneumonia and oral health. Spec Care Dentist. 2005;25:179–87. doi: 10.1111/j.1754-4505.2005.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 37.Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006;77:1465–82. doi: 10.1902/jop.2006.060010. [DOI] [PubMed] [Google Scholar]
- 38.Raghavendran K, Mylotte JM, Scannapieco FA. Nursing home-associated pneumonia, hospital-acquired pneumonia and ventilator-associated pneumonia: the contribution of dental biofilms and periodontal inflammation. Periodontol 2000. 2007;44:164–77. doi: 10.1111/j.1600-0757.2006.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Solh AA, Pietrantoni C, Bhat A, et al. Colonization of dental plaques: a reservoir of respiratory pathogens for hospital-acquired pneumonia in institutionalized elders. Chest. 2004;126:1575–82. doi: 10.1378/chest.126.5.1575. [DOI] [PubMed] [Google Scholar]
- 40.Bahrani-Mougeot FK, Paster BJ, Coleman S, et al. Molecular analysis of oral and respiratory bacterial species associated with ventilator-associated pneumonia. J Clin Microbiol. 2007;45:1588–93. doi: 10.1128/JCM.01963-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verghese A, Berk SL. Bacterial pneumonia in the elderly. Medicine (Baltimore) 1983;62:271–85. doi: 10.1097/00005792-198309000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Leibovitz A, Dan M, Zinger J, Carmeli Y, Habot B, Segal R. Pseudomonas aeruginosa and the oropharyngeal ecosystem of tube-fed patients. Emerg Infect Dis. 2003;9:956–9. doi: 10.3201/eid0908.030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertrand X, Thouverez M, Talon D, et al. Endemicity, molecular diversity and colonisation routes of Pseudomonas aeruginosa in intensive care units. Intensive Care Med. 2001;27:1263–8. doi: 10.1007/s001340100979. [DOI] [PubMed] [Google Scholar]
- 44.Grundmann H, Barwolff S, Tami A, et al. How many infections are caused by patient-to-patient transmission in intensive care units? Crit Care Med. 2005;33:946–51. doi: 10.1097/01.ccm.0000163223.26234.56. [DOI] [PubMed] [Google Scholar]
- 45.Agodi A, Barchitta M, Cipresso R, Giaquinta L, Romeo MA, Denaro C. Pseudomonas aeruginosa carriage, colonization, and infection in ICU patients. Intensive Care Med. 2007;33:1155–61. doi: 10.1007/s00134-007-0671-6. [DOI] [PubMed] [Google Scholar]
- 46.Waters V, Larson E, Wu F, et al. Molecular epidemiology of gram-negative bacilli from infected neonates and health care workers’ hands in neonatal intensive care units. Clin Infect Dis. 2004;38:1682–7. doi: 10.1086/386331. [DOI] [PubMed] [Google Scholar]
- 47.DeRiso AJ, 2nd, Ladowski JS, Dillon TA, Justice JW, Peterson AC. Chlorhexidine gluconate 0. 12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556–61. doi: 10.1378/chest.109.6.1556. [DOI] [PubMed] [Google Scholar]
- 48.Fourrier F, Dubois D, Pronnier P, et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double-blind placebo-controlled multi-center study. Crit Care Med. 2005;33:1728–35. doi: 10.1097/01.ccm.0000171537.03493.b0. [DOI] [PubMed] [Google Scholar]
- 49.Koeman M, van der Ven AJ, Hak E, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006;173:1348–55. doi: 10.1164/rccm.200505-820OC. [DOI] [PubMed] [Google Scholar]