Abstract
Background
Clinical immunology has traditionally relied on accurate phenotyping of the patient’s immune dysfunction for the identification of a candidate gene or genes for sequencing and molecular confirmation. Although this is also true for other branches of medicine, the marked variability in immune-related phenotypes and the highly complex network of molecules that confer normal host immunity are challenges that clinical immunologists often face in their quest to establish a specific genetic diagnosis.
Objective
We sought to identify the underlying genetic cause in a consanguineous family with chronic inflammatory bowel disease–like disorder and combined immunodeficiency.
Methods
We performed exome sequencing followed by autozygome filtration.
Results
A truncating mutation in LPS-responsive beige-like anchor (LRBA), which abolished protein expression, was identified as the most likely candidate variant in this family.
Conclusion
The combined exome sequencing and autozygosity mapping approach is a powerful tool in the study of atypical immune dysfunctions. We identify LRBA as a novel immunodeficiency candidate gene the precise role of which in the immune system requires future studies.
Keywords: LPS-responsive beige-like anchor (LRBA), chronic diarrhea, common variable immunodeficiency, autoimmunity
Immune-related disorders are important contributors to disease burden in human subjects not only in their rare Mendelian forms (eg, immunodeficiency disorders) but also in their frequently encountered “common” forms (eg, autoimmune disorders) through the complex interaction of genes in oligogenic or polygenic models with the environment.1 The relative ease with which Mendelian immunologic disorders can be studied has been an important factor in propelling gene discovery in this category of diseases, in which medically actionable information can be provided to patients and their families once disease-causing mutations are identified. The identification of the likely candidate gene is usually contingent on careful immunologic phenotyping, which involves clinically validated functional assays of the various components of the immune system.2 However, marked variability in the clinical phenotype of many of these disorders often complicates the clinician’s ability to order relevant immunologic assays that can facilitate the identification of the correct candidate gene.3 In addition, although an impressive number of Mendelian immune dysfunction genes has been identified in the past few years,4 many more are yet to be discovered. Thus, even when a specific immunologic defect is identified clinically, the underlying genetic heterogeneity will still pose a significant diagnostic challenge.
Until recently, linkage analysis and autozygosity mapping of families with immune disorders has been one of the most powerful tools in identifying novel disease genes that underlie known or atypical immune disorders, and much has been learned about factors that contribute to the normal development and maintenance of the immune system through these discoveries. The recent availability of massively parallel (next-generation) sequencing has revolutionized the gene discovery process in Mendelian disorders, including those that involve immune dysfunction.5 The ability to identify the underlying causative mutation even in simplex cases by using these sequencing techniques has obviated the historical requirement of large and/or multiple consanguineous pedigrees.
Common variable immunodeficiency (CVID) is a heterogeneous group of disorders with variable age of onset and is characterized by hypogammaglobulinemia and poor antibody responses. CVID affects approximately 1:25,000 white subjects. Patients with CVID have low serum IgG and IgA levels, and about half of the patients have reduced serum IgM levels.2,6 Autoimmunity affects up to 25% of these patients and might be the presenting feature in some. The most common autoimmune complications are hematologic, namely thrombocytopenia and hemolytic anemia. Up to half of the patients with CVID will have chronic diarrhea with malabsorption. This might be secondary to infection, granuloma formation, celiac disease, or intestinal inflammation with features of Crohn disease.7
In this study we describe an apparently novel immune deficiency and immune dysregulation phenotype in 5 affected members who belonged to 2 branches of a large consanguineous pedigree. The phenotype is characterized by an immunodeficiency with features of CVID, immune dysregulation, or both manifesting as inflammatory bowel disease (IBD) with chronic diarrhea, autoimmune cytopenia, and EBV-induced lymphoproliferative disease. Combined exome sequencing and autozygome filtration in this consanguineous family revealed a null mutation in LPS-responsive beige-like anchor (LRBA), which segregates with the disease. This report links LRBA to a human disease and suggests an important role of LRBA in maintaining normal B-cell, and possibly other immune cell, function.
METHODS
Human subjects
All patients were fully evaluated by a clinical immunologist who is certified by the American Board of Allergy and Immunology (A.A. or S.A.). Their immunologic assessment included immunoglobulin levels, lymphocyte subset enumeration, measurement of specific antibody titers to antigens, and T-cell proliferation determination by using a tritiated thymidine incorporation assay. Patients and their relatives were recruited by using written informed consent approved by the Internal Review Board of King Saud University and KFSHRC (RAC #2121053).
Autozygosity analysis
DNA samples from affected and unaffected members of the family were genotyped on an Axiom Chip platform per the manufacturer’s protocol (Affymetrix, Santa Clara, Calif). Runs of homozygosity of greater than 2 Mb that span 107 single nucleotide polymorphisms were used as surrogates of autozygosity (ie identical by descent) given the consanguineous nature of the family determined by using autoSNPa.8 The entire set of autozygosity blocks (autozygome) was determined for each affected member, and potential overlap between the autozygomes of affected members was pursued.
Exome sequencing
Exome capture was performed with the TruSeq Exome Enrichment kit (Illumina, San Diego, Calif), according to the manufacturer’s protocol. Samples were prepared as an Illumina sequencing library, and in the second step the sequencing libraries were enriched for the desired target by using the Illumina Exome Enrichment protocol. The captured libraries were sequenced with the Illumina HiSeq2000 Sequencer. The reads were mapped against UCSC hg19 (http://genome.ucsc.edu/) by using BWA (http://bio-bwa.sourceforge.net/). The single nucleotide polymorphisms and indels were detected by using SAMTOOLS (http://samtools.sourceforge.net/).
Immunophenotyping and proliferation response to mitogens
PBMCs were isolated on a Ficoll-Paque PLUS gradient (GE Healthcare, Fairfield, Conn). Lymphocytes were surface stained with anti-human CD3, CD4, CD8, CD19, CD16, and CD56 (BioLegend, San Diego, Calif) and analyzed with an LSRFortessa flow cytometer (BD Biosciences, San Jose, Calif) and FlowJo software (Tree Star, Ashland, Ore). PBMCs were placed in aliquots in 96-well tissue-culture plates at a density of 2 × 105 cells per well and stimulated with 1 μg/mL PHA (Sigma-Aldrich, St Louis, Mo), 1 μg/mL concanavalin A (ConA), or 10 ng/mL anti-CD3 mAb (OKT3; eBioscience, San Diego, Calif). Cultures were pulsed at 96 hours with tritiated thymidine (1 μCi per well), and tritiated thymidine uptake into DNA was determined 18 hours later.
PCR and RT-PCR
The coding sequence of LRBA exon 44 (NM_006726) was amplified by using specially designed primers on genomic DNA. In addition, cDNA primers were also used to check for the stability of the aberrant transcript. The source of RNAwas lymphoblasts extracted with the QIAamp RNA Mini Kit (Qiagen, Germantown, Md) and DNase treated with the RNase-Free DNase Set (Qiagen), according to the manufacturer’s recommendations, and used for cDNA synthesis with the iScript cDNA synthesis kit and Poly T oligonucleotide primers (Applied Biosystems, Carlsbad, Calif). Amplicons were sequenced on DNA Analyzer 3700xl (Applied Biosystems).
Immunoblot analysis
Cell lysates were prepared with RIPA buffer (Sigma) containing a protease inhibitor cocktail mix (Roche, Mannheim, Germany). Samples were normalized by measuring on a SmartSpec Plus spectrophotometer (Bio-Rad Laboratories, Hercules, Calif) using the Protein Assay Dye Reagent (Bio-Rad). Laemmli buffer (Bio-Rad Laboratories) with 5% β-mercaptoethanol was added to the samples. These were then boiled, electrophoresed on a 12% SDS-PAGE (National Diagnostics, Charlotte, NC), and transferred onto a polyvinylidene difluoride membrane (Hybond; GE Healthcare Biosciences, Pittsburgh, Pa). Membranes were blocked for 1 hour in PBS-Tween containing 5% nonfat milk at room temperature and incubated with a primary rabbit antibody raised against amino acid residues 907-1038 of human LRBA (ie, N-terminal to the truncation [no. ab121765; Abcam, Cambridge, Mass]), according to the manufacturer’s instructions, and then incubated with horseradish peroxidase–conjugated secondary antibodies (Pierce, Cheshire, United Kingdom). Immunosignals were detected by using SuperSignal West Pico Chemiluminescent (Pierce). β-Actin antibody was used as a loading control (Cell Signaling, Danvers, Mass).
RESULTS
Identification of a novel immunodeficiency phenotype
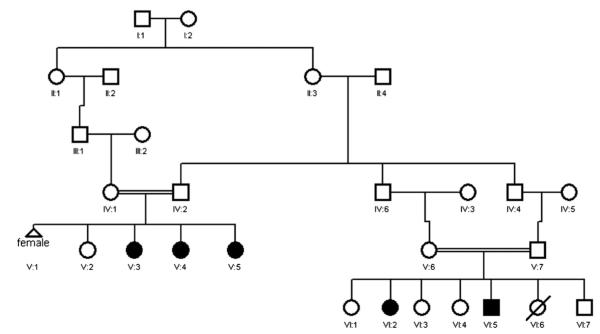
The family pedigree of the 5 affected patients is shown in Fig 1. The index patient (VI:5) is a 7-year-old male offspring of first-cousin parents and has 1 affected sister (VI:2). The 3 other patients (V:3, V:4, and V:5) are sisters, also offspring of second-cousin parents. The index patient’s mother is a paternal cousin of the 3 affected sisters. Thus a common set of grandparents is shared between the 2 nuclear families. Chronic diarrhea was the common clinical feature among all 5 affected family members. Below is a clinical summary of each of the 5 affected members.
FIG 1.
Pedigree of a multiplex consanguineous family with a novel phenotype of CVID, immune dysregulation, or both characterized by IBD. Solid symbols denote affected status.
Patient VI:5 is a 7-year-old boy with chronic diarrhea since infancy. He had onset of nonbloody diarrhea at 2 months of age that got worse after introducing solid foods. Duodenal biopsy performed at 3 years of age showed partial villous blunting with intraepithelial lymphocytic infiltration. Results of serologic tests for celiac disease were negative, and diarrhea persisted despite trial of a gluten-free diet. The interpretation of a repeat duodenal biopsy at 6 years of age was similar to the previous one, and a colonic biopsy specimen showed patchy thickening of the subepithelial collagenous plate, mucin depletion, and expansion of the lamina propria by lymphocytic infiltration, which is suggestive of collagenous colitis. His history was also remarkable for autoimmune pancytopenia at 3 years of age, which responded to intravenous immunoglobulin (IVIG) replacement therapy and a short course of prednisone. He also had 2 episodes of EBV-associated lymphoproliferative disease with intense infiltration with B cells evident on histopathologic analysis of lymph node biopsy specimens (see Fig E1 in this article’s Online Repository at www.jacionline.org) at ages 5 and 6 years. The EBV PCR titer was high on both occasions, a lymph node biopsy specimen showed marked follicular lymphoid hyperplasia with prominent germinal centers, and lymph nodes were mostly infiltrated by B cells, as shown by using CD20 immunohistochemical staining (see Fig E1). EBV-associated lymphoproliferative episodes were transient and improved spontaneously with no treatment. He had no history of recurrent infections. Immunologic investigations revealed normal serum IgG, IgA, IgM, and IgE levels and normal numbers of CD3+ T lymphocytes, CD4+ and CD8+ T-cell subsets, B cells, and natural killer (NK) cells. He had a normal T-cell proliferation to the mitogens PHA and ConA (Table I) and a normal increase in antibody titers after vaccination with tetanus toxoid, diphtheria, toxoid and Haemophilus influenzae capsular antigens (data not shown).
TABLE I.
Immunologic studies
| Patient VI:5 | Patient VI:2 | Patient V:3 | Patient V:4 | Patient V:5 | |
|---|---|---|---|---|---|
| Serum immunoglobulins | |||||
| IgG (660-1350 mg/dL) | 1,760 | 1,330 | 200 | 218 | 419 |
| IgM (56-350 mg/dL) | 52 | 159 | 187 | 68 | 93 |
| IgA (70-312 mg/dL) | 79 | 332 | <22 | <35 | <0.93 |
| IgE (1.5-114 IU/mL) | 99 | 12.5 | <20 | ND | 22 |
| Lymphocytes (cells/μL) | |||||
| CD3+ cells (700-2100) | 2,700 (73%) | 1,575 (79%) | 823 (72%) | 1,089 (77%) | 1,557 (79%) |
| CD4+ cells (300-1400) | 1,290 (35%) | 941 (10%) | 342 (30%) | 596 (42%) | 1,060 (54%) |
| CD8+ cells (200-900) | 1,590 (43%) | 700 (35%) | 617 (54%) | 540 (38%) | 0.628 (32%) |
| CD19+ cells (100-500) | 520 (14%) | 200 (10%) | 91 (8%) | 43 (3%) | 196 (10%) |
| CD16/CD56+ cells (90-600) | 370 (10%) | 120 (6%) | 194 (17%) | 142 (10%) | 216 (11%) |
| Proliferation (cpm)* | |||||
| PHA patient (control subject) | 245,369 (128,631) | 193,826 (128,631) | 5,461 (53,398) | 9,594 (53,398) | 2,547 (53,398) |
| ConA patient (control subject) | 139,516 (97,565) | 109,047 (97,565) | ND | ND | ND |
| Anti-CD3 patient (control subject) | ND | ND | 22,683 (41,866) | 4,928 (41,866) | 3,550 (41,866) |
Values in parentheses are values for healthy adult control subjects. Percentages indicate the percentage of the individual subsets in the lymphocyte gate.
ND, Not done.
Measurement of T-cell proliferation for patients VI.5 and VI.2 was done in a different laboratory than measurement of proliferation for patients V.3, V.4, and V.5. The results of a healthy control subject examined the same day are shown in parentheses.
Patient VI:2 is a 19-year-old woman with a history of nephrotic syndrome diagnosed at the age of 2 years and treated with steroids until she was 8 years old. Shortly after stopping steroids, she started to have mucous nonbloody stools, which persisted on a gluten-free diet. Duodenal biopsy performed at 16 years of age revealed partial villous atrophy and marked inflammation, and colonic biopsy performed at the same time showed mild chronic colitis with lymphocytic infiltration of the lamina propria (Fig 2, A and B). She was started on prednisone and azathioprine with poor compliance, and her diarrhea persisted. Her history was remarkable for megaloblastic anemia caused by vitamin B12 deficiency, which improved after starting monthly intramuscular vitamin B12 injections. She also had evidence of growth hormone deficiency and is currently receiving human growth hormone replacement. She had no history of recurrent infections. Clubbing was noted on her clinical examination. Immunologic investigation at 17 years of age revealed normal serum IgG, IgA, IgM, and IgE levels and normal numbers of CD3+ T lymphocytes, CD4+ and CD8+ T-cell subsets, B lymphocytes, and NK cells. She had a normal T-cell proliferation to the mitogens PHA and ConA (Table I) and a normal increase in antibody titers after vaccination with pneumococcal vaccine (data not shown).
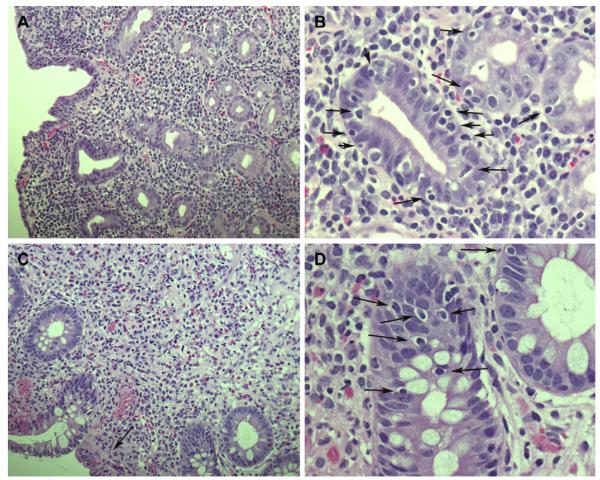
FIG 2.
Histology of patients’ intestinal biopsy specimens. A, Duodenal biopsy specimen from patient VI:2 showing marked villous atrophy and increased chronic inflammatory lymphoplasma cells in the lamina propria (hematoxylin and stain, original magnification ×20). B, High-power view of a duodenal biopsy specimen from patient VI:2 showing increased intraepithelial lymphocytes (arrows; hematoxylin and eosin stain, original magnification ×60). C, Colonic biopsy specimen from patient V:4 (transverse colon) showing a remarkable increase in mixed acute and chronic inflammatory cell numbers in the lamina propria and a decreased number of crypts and fibrosis, mainly subepithelial (arrow; hematoxylin and eosin stain, original magnification ×20). D, High-power view of a colonic biopsy specimen from patient V:4 (transverse colon) showing increased intraepithelial lymphocyte numbers (arrows; hematoxylin and eosin stain, original magnification ×60).
Patient V:3 is a 22-year-old woman with a history of recurrent otitis media and pneumonia since the age of 4 years. She eventually experienced bilateral bronchiectasis and finger clubbing. She also had nonmucous and nonbloody chronic diarrhea and poor appetite since the age of 18 years, which resulted in poor weight gain. A large-bowel biopsy specimen showed mucosal inflammation with lymphocytic infiltration but no granulomas or ulcerations. Her chronic diarrhea improved on oral prednisone (1 mg/kg/d). She also had a history of recurrent arthritis in the large joints, mainly the knees. Synovial fluid analysis was consistent with inflammation, with no evidence of infection. Her investigations revealed low serum IgG and IgA levels and decreased B-cell numbers but normal numbers of T cells, T-cell subsets, and NK cells (Table I). T-cell proliferation in response to PHA and anti-CD3 mAb was markedly reduced (Table I).
Patient V:4 is a 19-year-old woman who has had chronic nonbloody and nonmucous diarrhea since the age of 3 years, which did not improve on a gluten-free diet. Her large-bowel biopsy was similar to that of her older sister (Fig 2, C and D). Duodenal biopsy showed villous atrophy. She had autoimmune thrombocytopenia and autoimmune hemolytic anemia at the age of 13 years, both of which responded to treatment with steroids and rituximab. She has no history of recurrent infections. Her investigations at the age of 14 years, when she was referred to us, revealed low serum IgG and IgA levels and decreased B-cell numbers but normal numbers of T cells, T-cell subsets, and NK cells (Table I). T-cell proliferation to PHA and anti-CD3 mAb was markedly reduced (Table I).
Patient V:5 is a 15-year-old girl who has had chronic diarrhea since she was 2 years old, with no blood or mucus in stool. She has no history of recurrent infections or autoimmune hematologic manifestations. Her investigations revealed low serum IgG and IgA levels and normal numbers of T cells, T-cell subsets, B cells, and NK cells (Table I). T-cell proliferation in response to PHA and anti-CD3 mAb was markedly reduced (Table I).
All 3 sisters (patients V:3, V:4, and V:5) were started on IVIG replacement immediately after the diagnosis of hypogammaglobulinemia was made. Serum antibody titers were not available for them before institution of IVIG replacement therapy.
Identification of a null LRBA mutation in a family with a novel immunodeficiency/immune dysregulation syndrome
Because the affected members from the 2 branches of the family share a common set of grandparents and because the disease affects both sexes, we hypothesized that their phenotype is autosomal recessive and caused by inheriting 2 copies of an ancestral haplotype that harbors an autosomal recessive mutation (ie, autozygosity).9 Therefore we implemented autozygosity mapping as a tool to identify the entire set of autozygous blocks within the genome (autozygome) and searched for autozygosity blocks, which are exclusively shared by the affected members, because such blocks are highly likely to harbor the disease-causing mutation. Because patients VI:3 and VI:5 were recruited first, autozygome analysis was initially carried out on these 2 patients, and several autozygous intervals were found to be exclusively shared between them when compared with their unaffected siblings.
To identify the causative mutation within these blocks of autozygosity, exome sequencing was performed on patient VI:5, and the results were filtered as outlined in the scheme shown in Fig 3. We identified 1245 homozygous variants (of >80,000 total exomic variants) that reside within the shared autozygome. By only considering coding/splicing variants (synonymous changes were excluded), we were left with just 13 variants, of which only 1 was found to be novel (ie, absent in public dbSNP and our in-house collection of 183 Saudi exomes). Thus our strategy effectively narrowed the search to a single variant in exon 44 of LRBA. This 2-bp deletion, NM_006726:c.6657_6658del, predicts a frameshift and premature truncation of the protein p.(Glu2219Aspfs*3). Segregation of this variant with the disease was confirmed within this family by means of Sanger sequencing.
FIG 3.
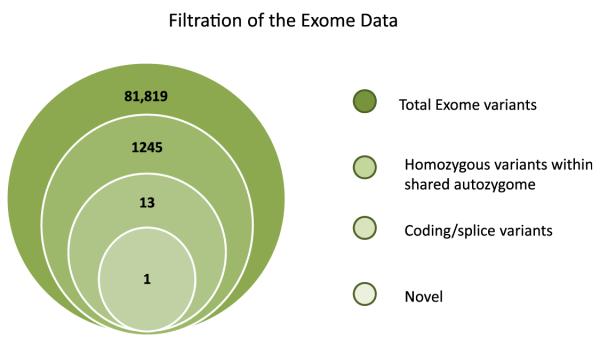
Filtration scheme of the exome variants identified in patient VI:5. Note the dramatic reduction in the number of candidate variants when filtered by the coordinates of the autozygosity intervals that are exclusively shared between the affected members. Only 1 of the 13 coding/splicing variants was novel (ie, not found in publically available single nucleotide polymorphism databases or our in-house 183 Saudi exomes).
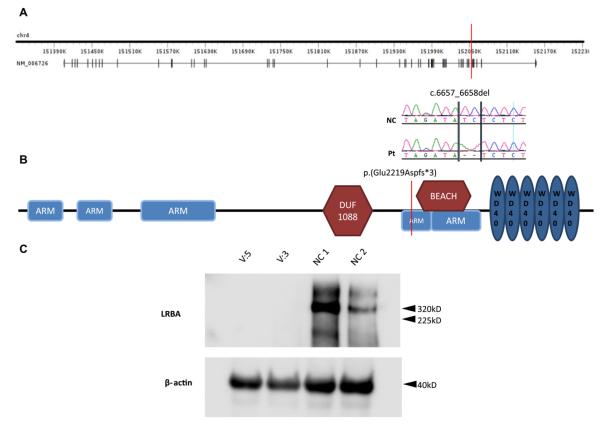
The structure of the LRBA gene and LRBA protein and the location of the mutation in the gene and its product are shown in Fig 4. The protein has 5 Armadillo (ARM) domains, 3 of which are located at the N terminus, a beige and Chediak-Higashi syndrome (BEACH) domain, and 5 WD40 repeat (WD40) domains located at the C-terminus. The BEACH and WD domains in LRBA are homologous to those in the protein encoded by the CHS1 gene, which is mutated in patients with Chediak-Higashi Syndrome (CHS). The truncating mutation in our patients removes the BEACH and WD domains, which are known to be functionally important in the CHS protein, which is also known as lysosomal trafficking regulator (LYST).10,11 To examine the effect of the mutation on the RNA and protein level, we performed RT-PCR on patients’ lymphoblasts and showed that there is no evidence of significant nonsense-mediated decay (see Fig E2 in this article’s Online Repository at www.jacionline.org). Western blot analysis revealed a 320-kDa band in lymphoblast lysates from 2 healthy donors, which corresponds in size to LRBA. In contrast, this band was absent from lymphoblast lysates of the patients. More importantly, patients’ lymphoblast lysates lacked a 247-kDa band that would correspond to the putative truncated 2220-amino-acid mutant protein, suggesting that the truncated mutant protein is rapidly degraded and unstable. Thus the mutation we describe appears to be nullimorphic in nature.
FIG 4.
A, Genomic structure of the LRBA gene. The location of the mutation is denoted by the red bar, and the genomic sequence around it in the patient (Pt) and control subject (NC) is shown under it. B, Schematic structure of the LRBA protein. Note that the truncating mutation removes the BEACH and WD40 domains at the C-terminus, which are functionally important in CHS/LYST. ARM, Armadillo; DUF, domain of unknown function. C, Western blot analysis reveals the absence of the hypothetical 247-kDa truncated LRBA in 2 patients’ cell lines compared with the 320-kDa normal LRBA band seen in both control cell lines (NC 1 and NC 2).
DISCUSSION
We report a novel immune deficiency phenotype, immune dysregulation phenotype, or both, which segregates with a truncating mutation in LRBA.
All 5 patients in the family studied had early onset of colitis and IBD-like presentation. Intestinal inflammation was documented by histology of colonic biopsy specimens in the 4 patients from whom a colonic biopsy specimen was obtained. Early-onset colitis is often a presentation of immune deficiency, immune dysregulation, or both. The dense lymphocytic infiltrate observed on histopathologic examination of the intestinal mucosa, as well as the dramatic response to steroids in the 3 compliant patients, lead us to speculate that autoimmunity might underlie the chronic intestinal inflammation in our patients. The fact that 2 of the patients had normal T-cell function and all patients lacked other manifestations of graft-versus-host disease makes it unlikely that the colitis was due to maternal cell engraftment.
Three of the patients (V:3, V:4, and V:5) were given a diagnosis of combined immunodeficiency (CID) based on low serum IgG and IgA levels and poor T-cell proliferation to mitogens. Only one of the 3 patients (V:3) had recurrent sinopulmonary infections, which were complicated by bronchiectasis. Another sister (V:4) had autoimmune hemolytic anemia and thrombocytopenia. The third sister (V:5) had neither recurrent infections nor cytopenia. The numbers of circulating T cells and T-cell subsets were normal in all 3 sisters. However, the CD4/CD8 ratio was reversed in one and low in the other. Two of the sisters had low B-cell numbers. All 3 sisters had a decreased T-cell response to PHA and anti-CD3. It has been shown that a significant number of patients with CVID have a low CD4+ cell count and a decreased CD4/CD8 ratio.12 Previous work has also shown that patients with CVID could exhibit suppressed T-cell proliferative responses to stimuli that include recall antigens, such as tetanus toxoid or purified protein derivative, superantigen, and IL-2.13,14 Agarwal et al15 investigated specifically patients with CVID with IBD and found that they have reduced proliferative responses to mitogens and reduced production of cytokines (IL-2 and IL-10) in response to stimulation with anti-CD3 and anti-CD28. The immunologic profile of the 3 sisters is consistent with these observations. However, because specific antibody titers were not available on them before IVIG replacement, the diagnosis of CID, rather than CVID, was made.
The 2 affected cousins of the 3 sisters, siblings VI.2 and VI.5, had normal serum IgG and IgA levels, normal antibody titers, normal T- and B-cell numbers, and normal proliferation response to PHA and ConA. However, early-onset colitis, recurrent EBV-induced lymphoproliferative disease, and autoimmune thrombocytopenia in patient VI.5 are strongly suggestive of immune dysregulation. Similarly, childhood-onset colitis and vitamin B12 deficiency, possibly caused by anti–parietal cell antibodies, are suggestive of immune dysregulation in patient VI.2. Given that these 2 siblings are 7 and 19 years of age and that they have the same mutation in the LRBA gene as their affected cousins, it is possible that they might develop with age a picture of CID or CVID and T-cell dysfunction. Alternatively, modifier genes might account for the development of CID in the 3 sisters who belong to the other branch of the family. It is not clear at present whether the nephrotic syndrome and growth hormone deficiency in patient VI.2 are part of the clinical spectrum of LRBA deficiency.
The variability of the clinical and immunologic phenotype in the 5 patients is likely to be secondary to genetic, epigenetic, and environmental factors, as is often the case in patients with primary immune deficiency diseases. Orange et al16 showed, using a genome-wide association approach in 363 patients with CVID, that disease heterogeneity could be related to several variations in the genetic background, including variations in the MHC region, a hyperpolymorphic region of DNA.
Thus far, mutations in 7 genes, inducible costimulator (ICOS),17 transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI),18,19 CD19,20 B-cell activating factor receptor (BAFFR),21 CD81,22 CD20,23 and CD21,24 have been found to cause CVID. However, more than 80% of patients with CVID have no identifiable mutation in these genes, a testimony to the marked genetic heterogeneity that characterizes this disorder. This study shows that LRBA can be mutated in a subset of patients with features of CVID, adding a potential novel genetic cause for CVID.
The product of the LRBA gene is a cytosolic protein expressed in many tissues. LRBA was shown to translocate on activation of B cells to the membrane of vesicles that include the trans-Golgi network, lysosomes, and endoplasmic reticulum, and to plasma membrane.25 LRBA was also shown to be involved in endocytosis of ligand-activated receptors rather than in vesicular exocytosis.25,26 Endocytosis is important in both lymphocyte receptor–mediated activation and inhibition.27 It is possible that impaired receptor internalization in LRBA-deficient lymphocytes might lead to a defect in B-cell activation and immunoglobulin synthesis and impaired T-cell activation. In addition, failure of signaling by inhibitory receptors that depend on receptor internalization might lead to failure of immune tolerance and autoimmunity. Interestingly, it was shown that defects in LRBA decrease the sensitivity of tumor cells to apoptosis.28 Thus it is tantalizing to speculate that autoimmunity observed in our patients might be caused by a defect in the elimination of autoreactive lymphocytes, resulting in inefficient peripheral, central, or both tolerance and autoimmunity.
LRBA is partly homologous to CHS/LYST, the protein defective in patients with CHS.25 The 2 proteins share the BEACH and WD40 domains located at their carboxy terminus. The truncating mutation we report in LRBA removes both of these domains.29 Patients with CHS present early in childhood with partial hair and skin albinism, neurologic dysfunction, bleeding diathesis, and recurrent infections and eventually have an accelerated phase of hemophagocytic lymphohistiocytosis. They also have giant granules in their peripheral blood leukocytes and bone marrow, indicating failure of lysosomal degranulation.30 Thus, the CHS phenotype is clearly different from that of our patients. Although LRBA appears to be involved in endocytosis, CHS/LYST plays an important role in vesicle trafficking and exocytosis. This difference might explain the difference in phenotypes between patients with CHS and our patients. Further work is needed to determine the precise role of LRBA in the immune system.
Our observations suggest that LRBA deficiency should be considered in patients with CID or CVID, as well as in children with early-onset colitis and autoimmunity. Long-term follow-up and additional reports of patients with LRBA mutations will delineate the full phenotypic spectrum of disease caused by LRBA deficiency.
Supplementary Material
FIG E1. A, Lymph node (left axilla) shows marked follicular lymphoid hyperplasia with prominent germinal centers (hematoxylin and eosin stain, original magnification ×20). B, The same lymph node. A CD20 immunohistochemistry stain specific for B cells (Novocastra, Newcastle Upon Tyne, United Kingdom) shows dense staining (CD20 marker, immunohistochemical stain, original magnification ×20).
FIG E2.. RT-PCR analysis shows no evidence of significant nonsense-mediated decay affecting the aberrant LRBA transcript in patients because it appears to have comparable abundance with the normal transcript seen in control subjects.
Clinical implications: LRBA deficiency should be considered in patients with CID and/or early-onset colitis and autoimmunity.
Acknowledgments
This work was supported in part by an intramural fund from KFSHRC (to F.S.A.), National Institutes of Health grants AI-076210 and AI094017 (to R.S.G.), the Perkin-Elmer Foundation (to R.S.G.), and the Dubai-Harvard Foundation for Medical Research (to R.S.G. and F.S.A.).
We thank the family for their enthusiastic participation. We also thank all health care providers who helped in the management of these patients. We are indebted to the Genotyping and Sequencing Core Facilities at KFSHRC for their technical help. We also thank Ms Mais Hashem and Ms Niema Ibrahim for their help as research coordinators and Ms Tarfa Alshiddi for her help in establishing lymphoblast cell lines.
Abbreviations used
- BEACH
Beige and Chediak-Higashi syndrome
- CHS
Chediak-Higashi syndrome
- CID
Combined immunodeficiency
- ConA
Concanavalin A
- CVID
Common variable immunodeficiency
- IBD
Inflammatory bowel disease
- IVIG
Intravenous immunoglobulin
- LRBA
LPS-responsive beige-like anchor
- LYST
Lysosomal trafficking regulator
- NK
Natural killer
Footnotes
Disclosure of potential conflict of interest: N. Adly has received grants from and is employed by the Research Center at King Faisal Specialist Hospital & Research Centre. R. S. Geha has received grants from the National Institutes of Health, is employed by Children’s Hospital Boston, has patents for Quickchange and Stratogene, and receives royalties for the book Pediatric Allergy Cases in Immunology. F. S. Alkuraya has received grants from the Dubai Harvard Foundation for Medical Research. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Fischer A. Human primary immunodeficiency diseases. Immunity. 2007;27:835–45. doi: 10.1016/j.immuni.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham-Rundles C. Human B cell defects in perspective. Immunol Res. doi: 10.1007/s12026-012-8318-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salzer U, Unger S, Warnatz K. Common variable immunodeficiency (CVID): exploring the multiple dimensions of a heterogeneous disease. Ann N Y Acad Sci. 2012;1250:41–9. doi: 10.1111/j.1749-6632.2011.06377.x. [DOI] [PubMed] [Google Scholar]
- 4.Al-Herz W, Casanova J-L, Chapel H, Conley ME, Cunningham-Rundles C, Etzioni A, et al. Primary immunodeficiency diseases: an update on the classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency. Front Primary Immunodef. 2011;2:1–26. doi: 10.3389/fimmu.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12:745–55. doi: 10.1038/nrg3031. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, Resnick ES, Cunningham-Rundles C. Perspectives on common variable immune deficiency. Ann N Y Acad Sci. 2011;1246:41–9. doi: 10.1111/j.1749-6632.2011.06338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park MA, Li JT, Hagan JB, Maddox DE, Abraham RS. Common variable immunodeficiency: a new look at an old disease. Lancet. 2008;372:489–502. doi: 10.1016/S0140-6736(08)61199-X. [DOI] [PubMed] [Google Scholar]
- 8.Carr IM, Flintoff KJ, Taylor GR, Markham AF, Bonthron DT. Interactive visual analysis of SNP data for rapid autozygosity mapping in consanguineous families. Hum Mutat. 2006;27:1041–6. doi: 10.1002/humu.20383. [DOI] [PubMed] [Google Scholar]
- 9.Alkuraya FS. Autozygome decoded. Genet Med. 2010;12:765–71. doi: 10.1097/GIM.0b013e3181fbfcc4. [DOI] [PubMed] [Google Scholar]
- 10.Ward DM, Shiflett SL, Kaplan J. Chediak-Higashi syndrome: a clinical and molecular view of a rare lysosomal storage disorder. Curr Mol Med. 2002;2:469–77. doi: 10.2174/1566524023362339. [DOI] [PubMed] [Google Scholar]
- 11.Zarzour W, Kleta R, Frangoul H, Suwannarat P, Jeong A, Kim SY, et al. Two novel CHS1 (LYST) mutations: clinical correlations in an infant with Chediak-Higashi syndrome. Mol Genet Metab. 2005;85:125–32. doi: 10.1016/j.ymgme.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Moratto D, Gulino AV, Fontana S, Mori L, Pirovano S, Soresina A, et al. Combined decrease of defined B and T cell subsets in a group of common variable immunodeficiency patients. Clin Immunol. 2006;121:203–14. doi: 10.1016/j.clim.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Fischer MB, Hauber I, Eggenbauer H, Thon V, Vogel E, Schaffer E, et al. A defect in the early phase of T-cell receptor-mediated T-cell activation in patients with common variable immunodeficiency. Blood. 1994;84:4234–41. [PubMed] [Google Scholar]
- 14.Stagg AJ, Funauchi M, Knight SC, Webster AD, Farrant J. Failure in antigen responses by T cells from patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 1994;96:48–53. doi: 10.1111/j.1365-2249.1994.tb06228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal S, Smereka P, Harpaz N, Cunningham-Rundles C, Mayer L. Characterization of immunologic defects in patients with common variable immunodeficiency (CVID) with intestinal disease. Inflamm Bowel Dis. 2011;17:251–9. doi: 10.1002/ibd.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orange JS, Glessner JT, Resnick E, Sullivan KE, Lucas M, Ferry B, et al. Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127:1360–7. e6. doi: 10.1016/j.jaci.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimbacher B, Hutloff A, Schlesier M, Glocker E, Warnatz K, Drager R, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4:261–8. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 18.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37:820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 19.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37:829–34. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 20.van Zelm MC, Reisli I, van der Burg M, Castano D, van Noesel CJ, van Tol MJ, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354:1901–12. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 21.Warnatz K, Salzer U, Rizzi M, Fischer B, Gutenberger S, Bohm J, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibodydeficiency syndrome in humans. Proc Natl Acad Sci U S A. 2009;106:13945–50. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Zelm MC, Smet J, Adams B, Mascart F, Schandene L, Janssen F, et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest. 2010;120:1265–74. doi: 10.1172/JCI39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuijpers TW, Bende RJ, Baars PA, Grummels A, Derks IA, Dolman KM, et al. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120:214–22. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiel J, Kimmig L, Salzer U, Grudzien M, Lebrecht D, Hagena T, et al. Genetic CD21 deficiency is associated with hypogammaglobulinemia. J Allergy Clin Immunol. 2012;129:801–10. e6. doi: 10.1016/j.jaci.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Wang JW, Howson J, Haller E, Kerr WG. Identification of a novel lipopolysaccharide-inducible gene with key features of both A kinase anchor proteins and chs1/beige proteins. J Immunol. 2001;166:4586–95. doi: 10.4049/jimmunol.166.7.4586. [DOI] [PubMed] [Google Scholar]
- 26.de Souza N, Vallier LG, Fares H, Greenwald I. SEL-2, the C. elegans neurobeachin/LRBA homolog, is a negative regulator of lin-12/Notch activity and affects endosomal traffic in polarized epithelial cells. Development. 2007;134:691–702. doi: 10.1242/dev.02767. [DOI] [PubMed] [Google Scholar]
- 27.Yuseff MI, Lankar D, Lennon-Dumenil AM. Dynamics of membrane trafficking downstream of B and T cell receptor engagement: impact on immune synapses. Traffic. 2009;10:629–36. doi: 10.1111/j.1600-0854.2009.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JW, Gamsby JJ, Highfill SL, Mora LB, Bloom GC, Yeatman TJ, et al. Deregulated expression of LRBA facilitates cancer cell growth. Oncogene. 2004;23:4089–97. doi: 10.1038/sj.onc.1207567. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan J, De Domenico I, Ward DM. Chediak-Higashi syndrome. Curr Opin Hematol. 2008;15:22–9. doi: 10.1097/MOH.0b013e3282f2bcce. [DOI] [PubMed] [Google Scholar]
- 30.Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu Rev Genomics Hum Genet. 2008;9:359–86. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIG E1. A, Lymph node (left axilla) shows marked follicular lymphoid hyperplasia with prominent germinal centers (hematoxylin and eosin stain, original magnification ×20). B, The same lymph node. A CD20 immunohistochemistry stain specific for B cells (Novocastra, Newcastle Upon Tyne, United Kingdom) shows dense staining (CD20 marker, immunohistochemical stain, original magnification ×20).
FIG E2.. RT-PCR analysis shows no evidence of significant nonsense-mediated decay affecting the aberrant LRBA transcript in patients because it appears to have comparable abundance with the normal transcript seen in control subjects.