Abstract
The tailbud is a population of stem cells in the posterior embryonic tail. During zebrafish development, these stem cells give rise to the main structures of the embryo's posterior body, including the tail somites. Progenitor cells reside in the tailbud for variable amounts of time before they exit and begin to differentiate. There must be a careful balance between cells that leave the tailbud and cells that are held back in order to give rise to later somites. However, this meticulous process is not well understood. A gene that has shed some light on this area is the t-box transcription factor spadetail (spt). When spt is mutated, embryos develop an enlarged tailbud and are only able to form roughly half of their somites. This phenotype is due to the fact that some of the somitic precursors are not able to leave the tailbud or differentiate. Another factor involved in tail morphogenesis is the Bone Morphogenetic Protein (BMP) pathway. BMPs are important for many processes during early development, including cell migration. Chordino (chd) is a secreted protein that inhibits BMP signaling. BMPs are upregulated in chd mutants, however, these mutants are able to form organized somites. In embryos where chd and spt are mutated, somites are completely absent. These double mutants also develop a large tailbud due to the accumulation of progenitor cells that are never able to leave or differentiate. To study the dynamics of cells in the tailbud and their role in somite formation we have analyzed the genetic factors and pathway interactions involved, conducted transplant experiments to look at behavior of mutant cells in different genetic backgrounds, and used time lapse microscopy to characterize cell movements and behavior in wild type and mutant tailbuds. These data suggest that spt expression and BMP inhibition are both required for somitic precursors to exit the tailbud. They also elucidate that chd;spt tailbud mesodermal progenitor cells (MPC) behave autonomously and their dynamics within the tailbud are drastically different than WT MPCs.
Keywords: spadetail, BMP, tailbud, somitogenesis, mesoderm progenitors
Introduction
In zebrafish, mesodermal precursors are continuously generated both during and subsequent to gastrulation in order to form trunk and tail somites (Agathon et al., 2003; Kimmel et al., 1990; Szeto and Kimelman, 2006). A careful balance between proliferation, migration, and differentiation among these precursors must be struck to ensure that as some progenitors differentiate and contribute to somite formation, others are maintained in an undifferentiated state in order to contribute to somites formed later in development. The BMP signaling pathway has been shown to be essential for proper specification and patterning of mesodermal progenitors(Myers et al., 2002;Row and Kimelman, 2009; Szeto and Kimelman, 2004, 2006). During gastrulation, a gradient of BMP activity is established by the complex interplay between BMP ligands, expressed most highly on the ventral side of the embryo, and secreted BMP inhibitors such as Chordin and Noggin, which are expressed dorsally(Dal-Pra et al., 2006; Furthauer et al., 1999;Myers et al., 2002;von der Hardt et al., 2007). This BMP gradient not only patterns mesodermal cell fates along the dorsal/ventral (DV) axis, but also regulates morphogenetic movements during gastrulation, ensuring that, for example, lateral mesodermal precursors converge towards the dorsal midline where they can contribute to trunk somites, while the ventral-most progenitors are directed to the tailbud, where they will subsequently form tail somites(Ho and Kane, 1990; Kanki and Ho, 1997;Kimmel et al., 1990; Myers et al., 2002;von der Hardt et al., 2007)
Patterning and morphogenesis of trunk and tail mesoderm is also under the control of members of the t-box family of transcription factors: no tail(ntl), brachyury(bra), and spadetail(spt) (Griffin et al., 1998). ntl and bra function redundantly to maintain a population of mesodermal progenitors that contribute to the somites of the posterior trunk and tail(Martin and Kimelman, 2008). ntl and bra form a positive regulatory loop with two Wnt genes, wnt3a and wnt8a (Martin and Kimelman, 2008). In embryos lacking both ntl and bra or both wnt3a and wnt8a, only the anteriormost 8-10 somites are formed(Martin and Kimelman, 2008; Shimizu et al., 2005;Thorpe et al., 2005). It has been proposed that this phenotype reflects the failure to maintain a population of mesodermal progenitor cells. In the absence of ntl/bra or wnt3a/wnt8a, the initial population of progenitors is quickly exhausted, leading to a severely truncated embryo(Martin and Kimelman, 2008, 2009; Thorpe et al., 2005)
spt is required for the formation of trunk somites. spt mutant embryos have only a few scattered muscle cells in the trunk, but no somites(Kimmel et al., 1989). The tailbud of spt embryos is significantly enlarged (the “spade” phenotype) although tail somites are formed normally. Detailed cellular analysis has shown that in spt mutants, trunk somite precursors, rather than converging towards the dorsal midline, are instead carried by epiboly movements to the vegetal pole, where they contribute to the enlarged tailbud(Ho and Kane, 1990). Curiously, these misplaced trunk mesodermal progenitors are unable to exit the tailbud and contribute to tail somites, remaining trapped in the tailbud through the completion of tail development. This phenotype indicates a key difference between ‘endogenous’ tail mesodermal progenitors derived from the ventral margin, which exit the tailbud normally in spt mutants, and the misplaced progenitors derived from the lateral margin, which cannot(Ho and Kane, 1990). The nature of the difference between these two populations of mesodermal progenitors remains unclear, although one possibility is that exposure to different levels of BMP during pregastrula stages could play a role. BMP signaling occurring between 4-5 hours post fertilization (hpf) is thought to program a subset of mesodermal progenitors to move to the tailbud and begin forming somites only later, during tail development (Szeto and Kimelman, 2006). Normally, these cells derive only from the ventral margin, where BMP activity is highest(Ho and Kane, 1990;Kimmel et al., 1990;Myers et al., 2002; Pyati et al., 2005). It may be that cells derived from the lateral margin, where BMP signaling is lower, are in some way not competent to respond to later cues that govern exit from the tailbud.
Some insight into the mechanisms governing tailbud exit comes from genetic analysis of double mutants between spt and one eyed pinhead(oep), an essential cofactor in Nodal signaling, which has uncovered a role for spt and Nodal in this process(Griffin and Kimelman, 2002; Gritsman et al., 1999; Zhang et al., 1998). As in spt embryos, formation of tail somites in oep mutants occurs normally (Hammerschmitt et al., 1996a). However, in spt;oep double mutant embryos, a dramatic defect in posterior mesoderm development is observed. Not only are the scattered muscle cells observed in spt single mutants completely absent, but tail somitic muscle is also missing. These embryos fail to downregulate the expression of mesodermal progenitor marker genes such as ntl and wnt8a in the tailbud, leading to the suggestion that in spt;oep embryos, progenitor cells are ‘locked’ into an undifferentiated state, and, being unable to progress along a differentiation program, are unable to leave the tailbud(Kelly et al., 1995; Griffin et al., 1998; Griffin and Kimelman, 2002; Schulte-Merker et al., 1992, 1994). Intriguingly, it has been shown that induced overexpression of a constitutively active BMP receptor construct (caBMPR) early in tail development resulted in embryos with expanded ntl expression in the tailbud and a transient defect in tail extension (Row and Kimelman, 2009). This result suggests that BMP signaling, in addition to an early role setting aside a population of mesodermal progenitors for tail development, might act later, during tail development, in governing the exit of these cells from the tailbud. This effect may not be direct, as overexpression of caBMPR led to a downregulation of secreted Wnt inhibitors in the presomitic mesoderm just anterior to the tailbud(Row and Kimelman, 2009). There are multiple Wnts expressed in the tailbud, both those that signal through β-catenin, such as wnt3a and wnt8a, as well as noncanonical Wnts like pipetail(ppt)/wnt5, which has been shown to regulate cell movements during gastrulation(Clements et al., 2009; Kelly et al., 1995; Lekven et al., 2001; Liu et al., 1999; Rauch et al., 1997). caBMPR overexpression does lead to increased nuclear β-catenin, indicating that the observed decrease in the expression of secreted Wnt inhibitors does have an effect on Wnt responsiveness in the tailbud(Row and Kimelman, 2009).
However, much remains unclear concerning the role of BMP in regulating exit from the tailbud. Neither chordino nor ogon mutant embryos, which carry mutations in the secreted BMP inhibitors Chordin and Sizzled, respectively, show any defects in tail somite formation, although it is possible that BMP activity is not sufficiently elevated in these mutants to cause a tailbud exit phenotype (Hammerschmidt et al., 1996b, 1996c;Martyn and Schulte-Merker, 2003; Miller-Bertoglio et al., 1999; Schulte-Merker et al., 1997). Also, it is unknown how BMP signaling ties in with the previously described roles for spt and Nodal signaling in governing exit from the tailbud.
To address these open questions, we have undertaken an analysis of tail development in WT and spt mutant embryos in which BMP signaling has been altered. We have found that chd;spt double mutant embryos exhibit a dramatic defect in somitic mesoderm development highly reminiscent of spt;oep embryos, with a nearly complete absence of muscle cells in the trunk and a total block in tail development, leading to the formation of an enormous tailbud. We show that BMP is functioning during post-gastrulation stages in this process. We also use transplantation and imaging techniques to show that spt;caBMPR-expressing cells are autonomously unable to leave the tailbud. Lastly, we show chd;spt tailbud cells exhibit drastically different behavior and morphology than cells in wild type zebrafish tailbuds.
Material and Methods
Fish Lines and Maintenance
Zebrafish were raised using standard techniques. Wild type fish used were AB. We used sptb104 and a new allele of chd which arose via spontaneous mutation and was phenotypically indistinguishable from previously described b215 allele (Fisher and Halpern, 1999; Griffin et al., 1998). Transgenic flh:eGFP were a generous gift of Marnie Halpern (Gamse et al., 2003). Mutant alleles were maintained in heterozygous fish that were outcrossed to wild type lines. Single and double mutants were identified based on evident phenotypes.
Morpholino and RNA Injection
Morpholinos, RNA, and fluorescent dyes were injected at the one cell stage. Spt, chd, oep, p53, and standard control morpholinos (Lekven et al., 2001; Nasevicius and Ekker, 2000; Ramel et al., 2005) were designed and obtained from Gene Tools LLC, Philomath, OR USA. They were diluted in Danieau's buffer prior to injection. sptMO was injected at 3mg/mL and chdMO was injected at 2 mg/mL when injected singly. In combinations they were both injected at 3 mg/mL along with p53 MO 1mg/mL. oep MO was injected at 2 mg/mL, both individually and in combination with spt, though in the latter case, 1mg/mL of p53 was added. mRNA's were constructed using mMessage mMachine kit (Ambion). They were diluted in RNAse free water prior to injection. mGFP was injected at 50 ug/mL. caBMPR(Macias-Silva et al, 1998) was injected at 4 ug/mL. Dextran Rhodamine B and dextran Alexa Fluor 488 (Invitrogen) were diluted in 0.2M KCl. Dextran Rhodamine B was injected at 500ug/mL and dextran Alexa Fluor 488 was injected at 50 ug/mL.
In Situ Hybridization and Antibody Staining
Digoxigenin labled RNA probes were used for in situ hybridizations using standard methods (Oxtoby and Jowett, 1993). For ntn1b and tbx6 reactions tbx6 was labeled with fluoroscein and incubated with anti-fluoroscein antibody after ntn1b NBT/BCIP color reaction. Embryos were washed and then stained with fast red to detect tbx6-expressing cells(Hauptmann and Gerster, 1994). Probes used were myoD(Weinberg et al., 1996), papc(Yamamoto et al., 1998), ntn1b(Strahle et al., 1997), and tbx6(Hug et al., 1997). P-smad 1/5/8 antibody (Cell Signaling Technology) was used at 1:100 overnight incubation. Secondary antibody was Alexa Fluor 488 anti-rabbit (Invitrogen) at 1:500.
Dorsomorphin Treatment
Dechorinated embryos were treated at 12hpf with 63μM of dorsomorphin using 5 mg/ml stock solution in DMSO (AMPK inhibitor, Compound C : Calbiochem). Embryos were treated overnight at 28°C and fixed at 24hpf.
Transplantations
Cell transplants were performed prior to gastrulation (30-50% epiboly). Labeled cells were removed from donor embryos using a manual microinjector (Sutter Instruments Co.). Cells were then transplanted to the ventral lateral margin of transgenic flh:eGFP embryos at corresponding stages. Embryos were mounted in 30% methyl cellulose during transplantation and placed in Ringers solution with penicillin and streptomycin for recovery afterward. Transplants were screened post gastrulation to look for fluorescence in the tailbud. Only embryos with fluorescence in the tailbud were screened after somitogenesis.
Time Lapse Imaging and Cell Measurements and Tracking
Time lapse was performed using a laser scanning confocal microscope (Zeiss LSM 5 Pascal, KSU Microscope Facility). Embryos were mounted in low melt agarose and methyl cellulose. Scans were recorded every 90 seconds. Images were merged and compiled into videos using ImageJ. Cell movements were demonstrated using manual cell tracking in ImageJ. Cell length and width were measured using ImageJ draw and measure tools(Rasband, 1997-2009). Cells posterior of the notochord were used for measurement data. Length was measured perpendicular to the notochord and width was measured parallel to the notochord of embryos.
Results
Phenotype of chd;spt embryos
To approach the question of what allows cells to exit the tailbud and pattern somites we began by looking at spadetail(spt) mutants (Fig1b). An outstanding question regarding these mutants is why endogenous muscle precursor cells (those derived from the ventral margin) are able to leave the tailbud when tail somite development commences, but ectopic precursors (those from the lateral margin) remain trapped. Szeto and Kimelman suggested that exposure to high levels of BMP signaling during gastrulation directs muscle progenitors to adopt a tail somite identity (2006). One possible explanation for the spt phentotype, then, is that the laterally derived precursors are not exposed to sufficiently high levels of BMP during gastrulation to specify them as tail somite progenitors. These ectopic cells may then be unable to respond to cues within the tailbud that direct their exit during tail somitogenesis.
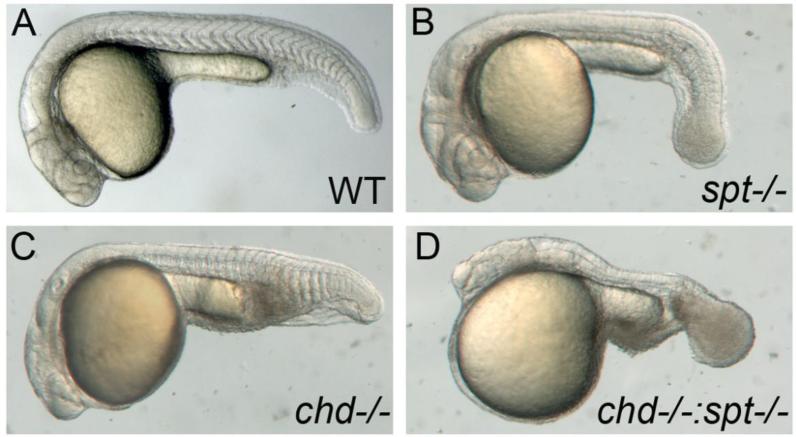
Figure 1. Spt and chd expression are required for tail somite formation.
Live embryos were photographed at 24hpf. All images are lateral views with left being anterior and posterior to the right. WT embryos have somites throughout the trunk and tail (A). spt mutants lack trunk somites and have an enlarged tailbud (B). chd embryos are ventralized and have reduced trunk somites(C). spt and chd were crossed to see if spt phenotype could be rescued. Instead chd;spt mutants exhibit an even larger tailbud and no visible somites (D).
If this were the case, we reasoned that by increasing the levels of BMP signaling during gastrulation, we might be able to reprogram the lateral muscle progenitors from a trunk somite fate to a tail somite fate, perhaps allowing them to exit the tailbud properly. To test this idea, we constructed a double mutant line between spt and chordino(chd). chd mutant embryos exhibit higher levels of BMP signaling during gastrulation, and have slightly smaller trunk somites and enlarged tail somites, though all cells are able to exit the tailbud normally (Schulte-Merker et al., 1997) (Fig1c).
If exposure to higher levels of BMP were able to direct misplaced spt muscle precursors to exit the tailbud, we would expect to see rescue of the enlarged ‘spade’ tail phenotype. In contrast, we observed a dramatic enhancement of the tailbud phenotype in chd;spt embryos (Fig1d). These embryos exhibited a significantly enlarged tailbud compared to spt single mutants (Fig1b), and a nearly complete failure to generate any tail somites. We confirmed the absence of somites by staining chd;sptdouble mutant embryos with myoD(Weinberg et al., 1996),(Fig2a-d). In most embryos (92%, n=65), we observed only a few scattered myoD-positive cells in the trunk and tail, and no organized somites at all. In rare cases (8%), chd;spt embryos made 2-3 small somites in the tail.
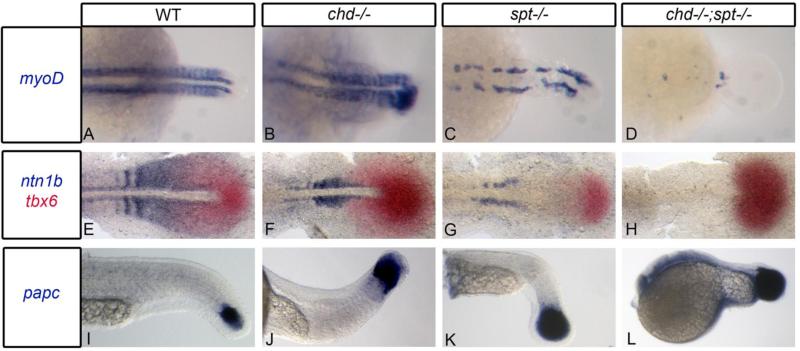
Figure 2. Chd;spt mutants produce mesodermal progenitor cells in the tailbud but are not able to form somites.
A-D, Dorsal view of myoD expression in 24hpf embryos. A-C, WT, chd, and spt embryos form organized somites even if it is only in the tail (spt). chd;spt mutants do not form organized somites. (60/65 have no organized somites. The remaining 5 only had 2-3 somites form.) (D). E-H, dorsal view of ntn1b expressing muscle cells and tbx6 expressing MPCs in 11hpf embryos. E-G, WT, chd, and spt embryos have MPCs in tailbud as well as differentiated muscle cells outside the tailbud. chd;spt embryos have an accumulation of MPCs in the tailbud and no differentiated muscle cells. I-L, lateral view of papc expression in 24hpf embryos. WT and mutant embryos have MPCs in the tailbud with spt and chd;spt embryos having a large accumulation of progenitor cells.
Production of MPCs in chd;spt embryos
One possible explanation for the lack of somites in chd;spt embryos could be that they are not producing somitic progenitor cells. We scored embryos for the presence of mesodermal progenitor cells (MPCs), as well as differentiating muscle cells outside the tailbud by in situ hybridization. We used ntn1b to label MPCs that had exited the tailbud and tbx6 as a marker for progenitor cells within the tailbud (Hug et al., 1997;Strahle et al., 1997). In wild type, chd, and spt embryos we observed MPCs in the tailbud as well as anterior to the tailbud (Fig2e-g). In contrast, in chd;spt mutants, we saw an accumulation of MPCs in the tailbud with a complete absence of muscle cells anterior to the tailbud (Fig2h). We also used papc to label progenitor cells in WT and mutant embryos at a later stage(Yamamoto et al., 1998). All backgrounds contained MPCs in the tailbud, with double mutants again having a large accumulation of progenitor cells in the tailbud (Fig2i-l). Therefore, chd;spt embryos are able to produce MPCs; these cells are simply not able to leave the tailbud and differentiate to form somites. This indicates that spt and chd redundantly promote exit of MPCs from the tailbud.
BMP activity levels in chd and oep mutant backgrounds
Previous studies have shown that a similar tailbud phenotype results when spt is knocked down in combination with one-eyed pinhead (oep). oep;spt mutants have an enlarged tailbud and lack somites (Griffin and Kimelman, 2002). Oep is a required co-receptor in the Nodal pathway and has been indicated to act as an upstream inhibitor of BMP (Kiecker et al, 2000). Interestingly, fgf8, a transcriptional target of Nodal signaling in late blastula stage embryos, has been shown to inhibit the transcription of BMP ligands(Furthauer et al., 1997, 2004). This raises the possibility that Nodal signaling might regulate tailbud exit via inhibition of BMP activity. We therefore tested whether oep and/or spt;oep embryos exhibited elevated levels of BMP signaling in the tailbud, using an anti-phospho Smad-1,5,8 (p-Smad) antibody.
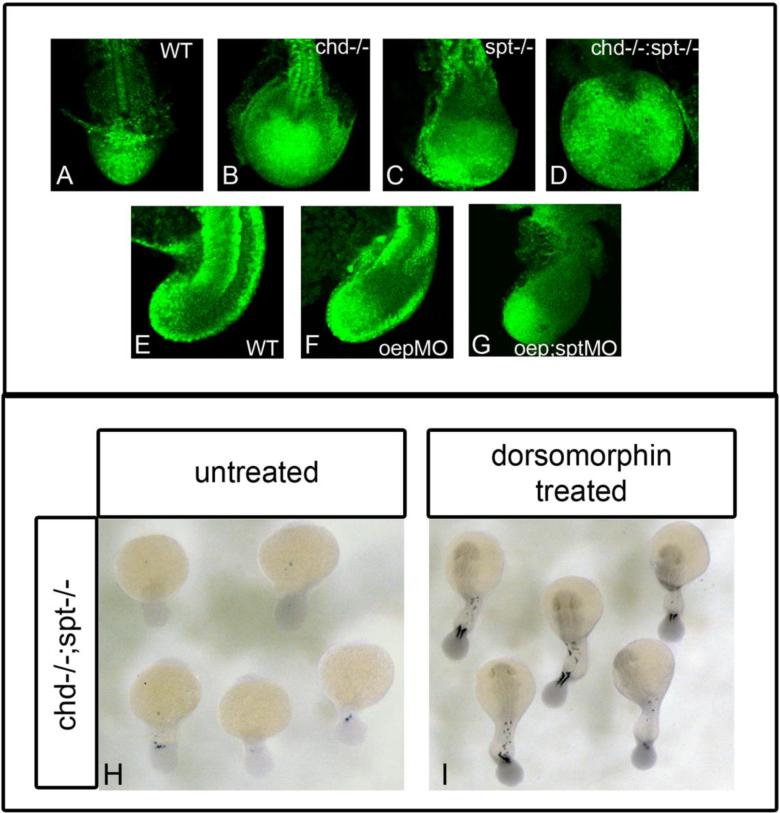
We looked at levels of active BMP in the tailbuds of WT, single, and double mutants. We observed that both chd mutant(Fig3b) and oepMO(Fig3f) embryos had higher levels of p-Smad staining in their tailbuds compared to WT (Fig3a,e). chd-/-;spt-/-(Fig3d) and oep;sptMO (Fig3g) embryos also had high expression in their tailbuds, although this expression seemed concentrated in patches. These data suggest that both oep and chd mutants have high levels of BMP activity in their tailbuds. However, this result also indicates that merely having high levels of BMP does not interfere with tailbud exit, as oep and chd single mutants have normal tails. Only when high levels of BMP activity are combined with the absence of spt function is tailbud exit impaired.
Figure 3. BMP inhibition is required for cells to exit the tailbud and form somites.
P-smad 1/5/8 antibody was used to detect BMP activity in the tailbud of embryos during somitiogenesis. A-D, dorsal view of P-smad 1/5/8 antibody fluorescence in 17hpf (16 som) embryos; E-G are lateral views of P-smad 1/5/8 staining in 17hpf embryos. WT embryos have normal levels of active BMP (A, E). Chd-/- (B) and oepMO (F) embryos have elevated levels of BMP, possibly due to the roles of chd and oep in BMP inhibition. chd-/-;spt-/- (D) and oepMO;sptMO (G) embryos also have higher levels of BMP in the tailbud, however, elevated levels are found in only some areas in the tailbud. Elevated Bmp activity and lack of spt leads to phenotypes where progenitor cells cannot exit the tailbud to form somites. H-I, dorsal view of myoD expression in 24hpf embryos:chd;spt embryos treated with dorsomorphin, a small molecule inhibitor of BMP, at 12hpf were partially rescued and able to produce tail somites. (23/25 had 5-9 somites) (I), whereas untreated chd;spt embryos do not produce somites (1/26 had 5-9 somites) (H).
Timing of BMP requirement in tailbud exit
Our phospho-Smad staining results suggested that elevated BMP levels could contribute to the tailbud phenotype in oep;spt embryos. If this were the case, we reasoned that we may be able to rescue the tailbud phenotype of oep;spt double mutants by inhibiting BMP by other means. To test this possibility, we used dorsomorphin, a small molecule inhibitor of the BMP pathway. Dorsomorphin selectively inhibits BMP type I receptors, blocking downstream phosphorylation of Smad proteins(Yu et al., 2008). Treating oep;spt embryos with dorsomorphin should rescue them to a spt phenotype if BMP inhibition is the only role oep is playing in tailbud exit. We also treated chd;spt embryos in parallel. We treated embryos at several stages with dorsomorphin, then assessed phenotypic rescue by staining embryos with myoD to score for the presence of somitic muscle. When treated at gastrulation and pre-somitogenesis stages no rescue was observed in chd;spt or oep;spt embryos (data not shown). However, when treated during early somitogenesis, at the 6 somite stage, partial rescue was observed in chd;spt embryos. Most embryos were able to form some tail somites (Fig3h-i). (23/25 chd;spt treated embryos had 5-9 somites whereas 1/26 untreated chd;spt had 5-9 somites). In contrast, we observed no significant rescue of the oepMO;sptMO double mutant by any regimen of dorsomorphin treatment (data not shown, oepMO;sptMO nontreated, n=215; oepMO;sptMO dorsomorphin treated n=349). These results suggest that inhibition of BMP by chd, in combination with spt activity are required for cells to exit the tailbud, and that the role of oep is independent of BMP signaling. These results also indicate that inhibition of BMP is required at the 6 somite stage (12hpf). Inhibition of BMP at this timepoint is required in order for MPCs to exit the tailbud and form tail somites.
BMP and spt regulation of tailbud exit is cell autonomous
Next we addressed the question of whether BMP regulates tailbud exit in a cell-autonomous or non-cell autonomous fashion. We performed transplantation studies to examine cell behavior in genetically mixed backgrounds. To generate spt donor cells or host embryos, we used sptMO, and to generate cells autonomously experiencing high levels of BMP, we injected embryos with caBMPR mRNA (Macias-Silva et al., 1998). chd mutant cells could not be used due to the fact that Chd is a secreted protein and transplanted chd-/-cells would be exposed to Chordin secreted from WT neighboring cells. We used doses of caBMPR mRNA that mimicked the chd phenotype when injected into embryos at the 1 cell stage (data not shown).
For these experiments, donor embryos were labeled with rhodamine-dextran and donor cells were transplanted at 30-50% epiboly into unlabeled host embryos. We used transgenic flh:eGFP host embryos so that the dorsally localized GFP expression could be used as a marker of the dorsal side of the embryo. This enabled us to target donor cells to the ventral margin even at pre-gastrula stages, allowing for efficient incorporation of donor cells into the tailbud. Transplants were screened post-gastrulation to verify that transplanted cells were localized exclusively in the tailbud. Only transplants with obvious fluorescence localized to the tailbud were used for screening after somitogenesis. Once somitogenesis was complete, embryos were examined with confocal microscopy to see if transplanted cells were able to contribute to somites or if they remained in the tailbud.
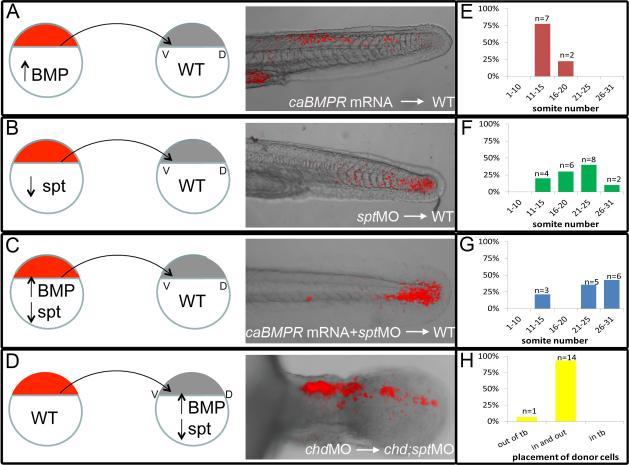
As expected, caBMPR donor cells were able to leave the tailbud in WT embryos and contribute to tail somites (n=9; Fig 4a). Donor cells were found in a range of somites, the most anterior somite containing donor cells was used to categorize recipient embryos. Somites were labeled 1-31 with 1 being the most anterior and 31 being the most posterior somite. Out of 9 recipient WT embryos, 7 had caBMPR donor cells in somites 11-15 and 2/9 had donor cells in somites 16-20 (Fig 4e). We also observed that transplanted sptMO cells were able to leave the tailbud of WT hosts and contribute to anterior and posterior tail somites (n=20; Fig4b, 10/20 had donor cells in somites 11-20 and 10/20 had donor cells in somites 21-31, Fig 4f). However, caBMPR mRNA + sptMO injected-cells were not able to efficiently leave the tailbud. Most cells remained in the tailbud at 48hpf (n=14; Fig4c). Occasionally a few fluorescent cells could be found in posterior tail somites and 3 of 14 embryos had a few cells in the anterior tail somites(Fig 4g). It is possible that these cells may not have been efficiently expressing caBMPR and/or had sufficient knockdown of spt, although we note that most chd;spt double mutant embryos have a few cells that are able to exit the tailbud.
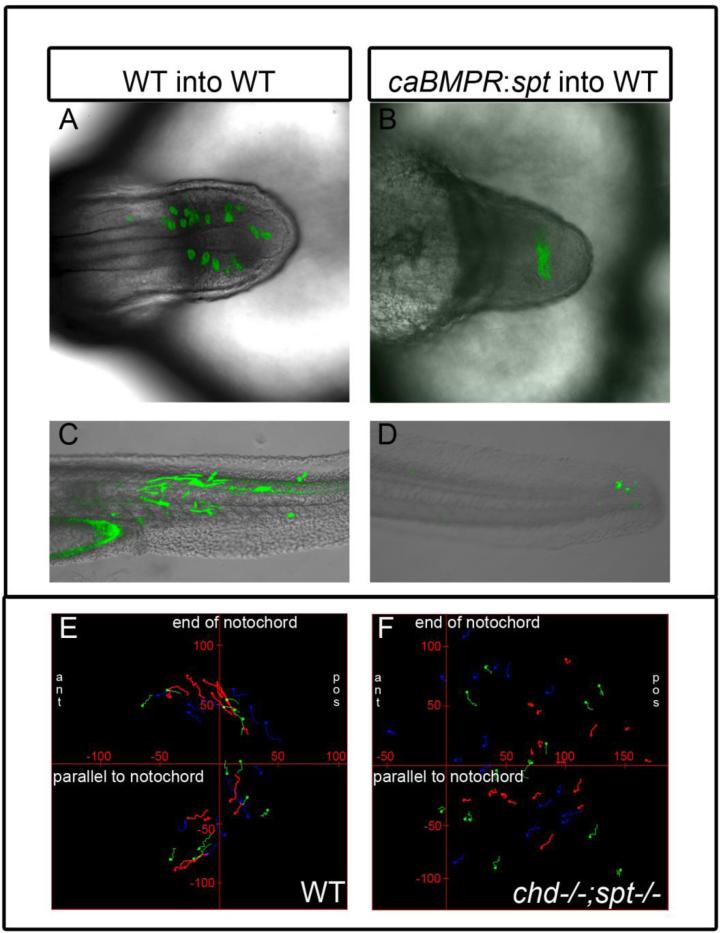
Figure 4. Ability to exit the tailbud is a cell autonomous fate decision.
Cell transplants were performed at 30-50% epiboly stages as diagramed above. Labeled donor cells were placed on the ventral lateral margin of unlabeled host embryos. A-D, Live embryos were photographed using confocal microscopy at 30-48hpf. E-H, Recipient embryos were scored based on the most anterior somite containing donor cells. Somite 1 being the most anterior and 31 being the most posterior. Cells injected with caBMPR mRNA 4ug/mL and placed in a WT host were able to exit the tailbud and differentiate in posterior and anterior tail somites, n=9 (A, E). Cells injected with sptMO 3ug/mL were also able to leave the tailbud in WT background and contribute to tail somites, n=20(B, F). Cells injected with caBMPRmRNA +sptMO were not able to efficiently leave the tailbud in WT backgrounds. A few donor cells could be occasionally found in posterior tail somites and 3 embryos had donor cells in anterior tail somites. However, the majority of transplanted cells were still in the tailbud at 48hpf, n=14 (C, G). When chdMO cells were placed in a chdMO;sptMO background they were able to exit the tailbud and migrate anteriorly, n=15 (D, H).
To test whether cells expressing spt(spt +/+) are able to leave the tailbud of chd;spt hosts, we used chdMO donor cells. In the presence of spadetail, chdMO should not affect the ability of cells to exit the tailbud. We used these cells to exclude the possibility that wild type donor cells might secrete enough Chd to create a localized region of relatively normal levels of BMP activity that might affect cell behavior. When chdMO cells were placed in a double mutant background, they were able to exit the tailbud (n=15; Fig 4d, h). Taken together, our transplant experiments suggest that the ability of a cell to exit the tailbud is an autonomous cell function.
In a separate set of experiments, we characterized behavior of transplanted cells in different backgrounds at time points during and post somitogenesis. For these experiments, donor embryos were labeled by injecting mGFP mRNA at the 1 cell stage, and WT lines were used for host embryos instead embryos from the flh:eGFP line. As a control, we examined the behavior of wild type cells transplanted in wild type hosts. Donor cells freely intermixed with host cells and left the tailbud at different times (Fig 5a). Embryos were screened at 18hpf and 48hpf to determine the placement of transplanted cells. Transplanted cells were differentiated as muscle cells and were found in a range of tail somites (n=11; Fig 5c). Double mutant cells (caBMPR mRNA + sptMO) in WT background were not able to efficiently exit the tailbud. Further, they did not intermix with wild type host cells, instead remaining together in a tight clump (Fig 5b). When scored at 48 hours, caBMPR mRNA + sptMO donor cells were seen in the very tip of the tail, lacking muscle morphology (14/18 embryos had transplanted cells exclusively in the tailbud at 48hpf (Fig 5d). As seen previously, chdMO cells in double mutant background (chdMO;sptMO) did mix with double mutant tailbud cells and some were able to exit the tailbud (n=2, FigS1a). Usually some cells would exit and some would remain behind. The cells that were able to leave the tailbud seemed to be due to tail extension even though extension in double mutants is severely reduced. As expected, double (chdMO;sptMO) into double mutant (chdMO;sptMO) transplants showed intermixing of donor cells and host cells, although the donor cells did not exit the tailbud (n=2, FigS1b). Taken together, our observations indicate that exit of MPCs from the tailbud is a cell autonomous process. Further, the clumping behavior of caBMPR mRNA + sptMO cells in a wild type background suggests that these cells may differ in their adhesive properties from the surrounding wild type cells.
Figure 5. Cell movements are perturbed in cells lacking spadetail and experiencing high BMP levels.
A-B, Top-down view of tailbud at 18hpf with transplanted WT(A) and caBMPR mRNA + sptMO(B) cells in WT background. C-D Lateral view of 48hpf embryos with transplanted WT (C, n=11) and caBMPR mRNA + sptMO (D, n=18) in a WT background. Transplanted WT cells are able to exit the tailbud and contribute to somites (A, C). caBMPR mRNA + sptMO cells remain in the tailbud forming a tight cluster and do not intermix with WT cells or form somites(B,D). E-F, Time lapse images were made of mGFP labeled embryos. Stills were taken every 90 seconds for 1-2 hours of development at 14 somite stage. Manual cell tracking was performed for 10-30 consecutive frames using ImageJ and placed on graphs with X axis parallel to the notochord and Y axis perpendicular to the end of the notochord, tick marks on axis are 50um. Tailbud cells in WT embryos (n=3) display movement away from the midline and anterior migration to form tail somites (E). chd:spt tailbud (n=3) cells do not display a uniform pattern of movement and their migration paths are much shorter (F).
Cell movement inchd;spt mutant tailbuds are perturbed
We next used time lapse imaging to look at detailed cell movements in the tailbuds of WT and mutant embryos. This allowed us to look at dynamics that take place as tail extension and somite formation occurs. Embryos were labeled with membrane targeted GFP by injecting mRNA at the one cell stage. Embryos with strong expression in the tailbud were mounted in low melt agarose and used for confocal microscopy time lapse. Agarose was cleared from around the tail to allow for proper extension. Tailbud cells posterior of the notochord were measured at early and late somitogenesis stages. Length was measured perpendicular to the notochord and width was measured parallel to the notochord (FigS2). WT movements occurred as previously described by Kanki and Ho (1997). As the tail extends, the notochord moves posteriorly and cells in the posterior tailbud move away from the midline and migrate anteriorly where they form somites. Cells are polarized perpendicular to the midline which corresponds with their movement away from the midline. WT tailbud cells have 1.7:1 length to width ratio just prior to tail somite formation (10 somites; cells=102, 3 individuals, Movie1) and a 2.3:1 ratio during later stages (20-22 somites; cells=55, 2 ind.). chd mutants display similar cell movements but are not as drastically polarized if at all. They have a 1.1:1 length to width ratio at 10 somite stage (cells=105, 2 ind, Movie2) and a 1.4:1 ratio at 22 somite stage (cells=60, 2 ind.). This may be attributed to the fact that chd tailbuds are larger and contain more cells that WT tailbuds until late stages of somitogenesis. spt mutant embryos exhibit similar subduction movements to WT during early tail somite formation; however at later stages (18-25 somites) cells were not as dynamic and didn't exhibit obvious movement away from the midline. Most cells in the tailbud at these stages are likely trunk precursors and will remain in the tailbud. spt mutant cells display a 1.2:1 length to width ratio at 10 somite stage (cells=95, 3 ind, Movie3) and a 1.3:1 ratio at 22 somite stage (cells=100, 3 ind). They are not as polarized or active as wild type cells and the few that are may be ones that will form tail somites. In chd;spt mutants the cell movements were dramatically different from WT. The notochord did not penetrate and extend into the tailbud. Instead, it moved the entire mass of cells posteriorly as it extended. Cells in the tailbud seem very cohesive and held together as a unit. They are still dynamic and intermixing but do not seem to leave the tailbud. These cells do not appear to be polarized. They exhibit a 1:1 length to width ratio prior to when tail somites would usually be starting to form (cells=95, 3 ind.Movie4) and 1.2:1 ratio when later stages of somitogenesis should be taking place (cells=100, 2 ind). Cell movements in WT and double mutants were illustrated by using manual cell tracking in ImageJ (Fig5e-f). These results indicate that cell movements in chd;spt mutant tailbuds are perturbed and abnormal. Cells are not polarized nor do they have a uniform migration pattern when spadetail is not expressed and BMP signaling is increased.
Discussion
Role of spadetail and BMP signaling in fating tail somites
A number of signaling pathways are involved in the specification of tail somites. The exact roles and complex interactions of these pathways and their components remain unclear. Our results show that spadetail function and appropriate levels of BMP signaling are required for cells to properly exit the tailbud and differentiate into tail somites.
In the absence of spt function, trunk MPCs migrate inappropriately into the tailbud, where they are never able to leave. Cells that would normally form tail somites are able to leave and do so while cells that would have formed trunk somites are stuck in the tailbud (Ho and Kane, 1990). We show through chd;spt mutants that high BMP does not rescue the spt phenotype and instead causes a more severe defect. Presomitic trunk cells that are stuck in the tailbud are not reprogrammed to a muscle fate. Instead, high BMP in the spadetail background leads to no formation of trunk or tail somites. However, high BMP alone does not lead to such a phenotype. Chd mutants form trunk and tail somites but are slightly ventralized which results in expanded posterior tissues including a few posterior somites (Schulte-Merker et al., 1997). Only in combination with spt does this phenotype occur.
Cells that accumulate in the tailbud of chd;spt mutants are multipotent progenitor cells based on in situ hybridizations we performed using progenitor markers such as ntn1b, papc and tbx6(Hug et al., 1997;Strahle et al., 1997; Yamamoto et al., 1998). High BMP may affect proper communication between cells, proper polarization, migration, or a number of molecular cues required for tailbud exit. Presomitic trunk precursors may behave differently in the tailbud due to a sensitive time window that is missed and needed in order to respond to molecular cues that lead to proper migration and differentiation. They may also have unique surface molecules which affect their ability to respond to ligands or molecules in the tailbud or may possess adhesive qualities which inhibit their mobility.
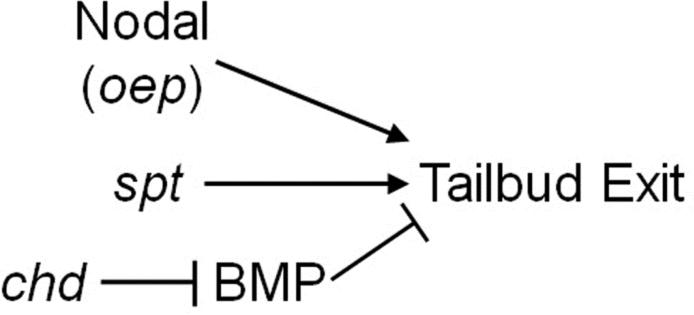
Inhibition of Nodal signaling combined with the spadetail mutation leads to a nearly identical phenotype to that observed in chd;spt mutants(Griffin and Kimelman 2002). Oep dependent Nodal signaling has been shown to inhibit the BMP pathway during gastrulation, and we indeed observe elevated levels of BMP signaling in the tailbuds of both oep and oep;spt mutants(Kiecker et al., 2000). However, our failure to rescue oep;spt mutants by inhibiting BMP signaling with dorsomorphin suggests that Nodal signaling does not regulate tailbud exit via regulation of BMP signaling. Our data are consistent with BMP and Nodal acting through independent mechanisms, though both in conjunction with spt function, to control exit of MPCs from the tailbud (Figure 6).
Figure 6. Tailbud exit requires expression of spadetail in combination with BMP inhibition or with Nodal signaling.
Spadetail, Nodal signaling and BMP inhibition are required for MPCs to properly exit the tailbud to form muscle cells. oep and chd mutant MPCs are able to exit the tailbud and form somites. spt mutants are only able to form tail somites while trunk somite precursors remain trapped in their tailbud. Nodal and Chordin each act in conjunction with Spadetail to control this process. Loss of oep and chd in spadetail mutants results in the inability to form somites. However, oep and chd seem to be using independent mechanisms, with Chordin's crucial role in this process being to inhibit BMP signaling.
Wnt signaling and tailbud exit
Wnt signaling has long been known to play a role in the differentiation and migration of progenitor cells. Canonical Wnt signaling has been found to play a role in determining cell fates within the tailbud (Martin and Kimelman, 2012). However, its exact role in tailbud exit is still unclear. Row and Kimelman used a heat shock inducible, constitutively active BMP receptor (caBMPR) to show that high levels of BMP signaling impede the exit of MPCs from the tailbud (2009). BMP is proposed to accomplish this, at least in part, by negatively regulating the expression of secreted Wnt inhibitors in the anterior tailbud. The activity of these inhibitors is thought to restrict Wnt activity to the posterior part of the tailbud. High levels of BMP result in the loss of expression of Wnt inhibitors, which is predicted to result in higher levels of both canonical and noncanonical Wnt signaling in the tailbud(Row and Kimelman, 2009). BMP regulates the activity of at least the canonical Wnt signaling pathway in the tailbud, although whether Wnt signaling regulates tailbud exit is still uncertain.
Rac and Rho have been shown to function downstream of non-canonical Wnt signaling (reviewed in Schlessinger et al., 2009). These RhoGTPases can be important for cell polarity, migration, and adhesion. Therefore, they may play a possible role in the ability of cells to exit the tailbud. Rho and Rho-associated kinase are involved in myosin phosphorylation in cell blebbing and migration (Amano et al., 1996; Blaser et al., 2006; Kimura et al., 1996). However, Rho dependent myosin phosphorylation needed for cell blebbing does not seem to be involved in the regulation of cell blebbing in spt-/- tailbuds (Row et al., 2011). Rac and Rho have also been shown to be required for establishment of cadherin dependent cell-cell adhesion and actin recruitment and remodeling (Braga et al., 1997). spt-/- tailbud cells have been shown to be more adhesive than wild type cells, however there is no obvious difference in cadherin levels between the two (Row et al., 2011). At this time, the specific role of Rac and Rho in mesodermal progenitor cells in the tailbud has yet to be characterized.
Cell behavior in the tailbud
Our transplant experiments show that the ability to exit the tailbud is a cell autonomous function. Specifically, transplanted wild type cells were able to exit the tailbud irrespective of the host background; they were even able to leave the tailbuds of chd;spt host embryos, despite the severe defects in extension of the embryo and nearly complete absence of any host cells exiting the tailbud.
In contrast, transplanted caBMPR mRNA+ sptMO cells failed to exit the tailbud when transplanted into wild type host embryos. Further analysis showed that wild type cells transplanted in wild type background did intermix with other cells in the tailbud and exited the tailbud normally. caBMPR mRNA+ sptMOcells did not mix with the wild type cells in the tailbud. Instead, they formed a cluster of cells that never left. This may be due to surface adhesion proteins that caused them to form a cohesive group or their hindered ability polarize, migrate or respond to cues needed to exit the tailbud.
Our time lapse analyses also lend to these possibilities, as they indicate abnormal cell movement within chd;spt mutant tailbuds. As described by Kanki and Ho, cells in the posterior tailbud converge away from the midline moving laterally and then anteriorly where they are incorporated into a specific somite (1997). Cells leave the tailbud in a timely manner as tail extension occurs and ones that remain behind actively divide in the tailbud to provide progenitor cells to form later somites. Wild type tailbud cells exhibited such movement as indicated by cell tracking. WT cells were polarized in the direction of these movements and very dynamic until incorporated into a somite. Cells in chd and spt tailbuds were not as dramatically polarized as wild type tailbud cells. This could be due to high BMP in chd tailbuds. It may also be due to the large size of chd tailbuds prior to tail somite formation. Differences in spt tailbuds could be a result of the mixture of presomitic trunk precursors that will remain in the tailbud and presomitic tail precursors that will exit the tailbud. chd;spt mutants were not polarized perhaps owing to high BMP, high number of cells in the tailbud, inability to move past a certain point, or inability to respond to molecular cues in order to exit. Chd;spt tailbud cells were slightly polarized at later time points likely because cells are overcrowded and cramped for room as the notochord extended posteriorly and pushed against the anterior border of the tailbud. These cells did not exhibit uniform migration patterns or organized movements when tracked. The exact cellular mechanisms responsible for the ability of cells to exit the tailbud are elusive at this point. Future studies may be able to determine what players are involved and how cell polarity, adhesion, and migration are regulated and employed in this process.
Conclusions
In summary, we have characterized a novel double mutant in which the t-box transcription factor, spadetail, and the BMP inhibitor, chordin, are non-functional. These double mutants have an enlarged tailbud due to an accumulation of mesodermal progenitor cells. These cells are not able to properly exit the tailbud and differentiate into somitic muscle cells. This phenotype is similar to oep;spt mutants. We were able to show that BMP inhibition is crucial at 12hpf for tailbud exit to occur properly. Chd;spt mutants phenotypes were partially rescued at this stage when treated with a small molecule inhibitor of BMP. Oep;spt mutants were not rescued suggesting an alternative mechanism for oep's role in this process. Transplantation studies indicate the ability of cells to exit the tailbud is an autonomous function. Time lapse data revealed wild type MPCs polarize away from the midline and move laterally and then anteriorly in order to exit the tailbud and form somites. Chd;spt MPCs did not exhibit organized migration patterns and scarcely polarized, if at all. They remain as a unified group of cells throughout tail extension yet they are still dynamic and intermixing within the tailbud. Studies with chd;spt mutants will aid in further understanding of tail formation and cues needed for tailbud exit and somitogenesis during embryonic development.
Supplementary Material
Highlights.
Chordin and Spadetail are required for somite formation
Spadetail and BMP inhibition are required for cells to exit tailbud
Exiting the tailbud is a cell autonomous function
Acknowledgements
We would like to thank Michael Veeman and Rollie Clem for helpful suggestions on the manuscript and submission. We also thank Marnie Halpern for the generously sharing the flh:eGFP zebrafish line. This work was supported by funding from the Terry C. Johnson Center for Basic Cancer Research and NIH Grant (P20 RR016475) from the INBRE program of the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agathon A, Thisse C, Thisse B. The Molecular Nature of the Zebrafish Tail Organizer. Nature. 2003;424:448–452. doi: 10.1038/nature01822. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and Activation of Myosin by Rho-Associated Kinase (Rho-Kinase). J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Blaser H, Reichman-Fried M, Castanon I, Dumstrei K, Marlow FL, Kawakami K, Solnica-Krezel L, Heisenberg CP, Raz E. Migration of Zebrafish Primordial Germ Cells: A Role for Myosin Contraction and Cytoplasmic Flow. Dev Cell. 2006;11:613–627. doi: 10.1016/j.devcel.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. The Small GTPases Rho and Rac are Required for the Establishment of Cadherin-Dependent Cell-Cell Contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements WK, Ong KG, Traver D. Zebrafish wnt3 is Expressed in Developing Neural Tissue. Dev Dyn. 2009;238:1788–1795. doi: 10.1002/dvdy.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal-Pra S, Furthauer M, Van-Celst J, Thisse B, Thisse C. Noggin1 and Follistatin-like2 Function Redundantly to Chordin to Antagonize BMP Activity. Dev Biol. 2006;298:514–526. doi: 10.1016/j.ydbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Fisher S, Halpern ME. Patterning the Zebrafish Axial Skeleton Requires Early Chordin Function. Nat Genet. 1999;23:442–446. doi: 10.1038/70557. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Van Celst J, Thisse C, Thisse B. Fgf Signalling Controls the Dorsoventral Patterning of the Zebrafish Embryo. Development. 2004;131:2853–2864. doi: 10.1242/dev.01156. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Thisse B, Thisse C. Three Different Noggin Genes Antagonize the Activity of Bone Morphogenetic Proteins in the Zebrafish Embryo. Dev Biol. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Thisse C, Thisse B. A Role for FGF-8 in the Dorsoventral Patterning of the Zebrafish Gastrula. Development. 1997;124:4253–4264. doi: 10.1242/dev.124.21.4253. [DOI] [PubMed] [Google Scholar]
- Gamse JT, Thisse C, Thisse B, Halpern ME. The Parapineal Mediates Left-Right Asymmetry in the Zebrafish Diencephalon. Development. 2003;130:1059–1068. doi: 10.1242/dev.00270. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kimelman D. One-Eyed Pinhead and Spadetail are Essential for Heart and Somite Formation. Nat Cell Biol. 2002;4:821–825. doi: 10.1038/ncb862. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Amacher SL, Kimmel CB, Kimelman D. Molecular Identification of Spadetail: Regulation of Zebrafish Trunk and Tail Mesoderm Formation by T-Box Genes. Development. 1998;125:3379–3388. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- Gritsman K, Zhang J, Cheng S, Heckscher E, Talbot WS, Schier AF. The EGF-CFC Protein One-Eyed Pinhead is Essential for Nodal Signaling. Cell. 1999;97:121–132. doi: 10.1016/s0092-8674(00)80720-5. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nusslein-Volhard C. Mutations Affecting Morphogenesis during Gastrulation and Tail Formation in the Zebrafish, Danio Rerio. Development. 1996a;123:143–151. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nusslein-Volhard C. Dino and Mercedes, Two Genes Regulating Dorsal Development in the Zebrafish Embryo. Development. 1996b;123:95–102. doi: 10.1242/dev.123.1.95. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija GN, McMahon AP. Genetic Analysis of Dorsoventral Pattern Formation in the Zebrafish: Requirement of a BMP-Like Ventralizing Activity and its Dorsal Repressor. Genes Dev. 1996c;10:2452–2461. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- Hauptmann G, Gerster T. Two-Color Whole-Mount in Situ Hybridization to Vertebrate and Drosophila Embryos. Trends Genet. 1994;10:266. doi: 10.1016/0168-9525(90)90008-t. [DOI] [PubMed] [Google Scholar]
- Ho RK, Kane DA. Cell-Autonomous Action of Zebrafish Spt-1 Mutation in Specific Mesodermal Precursors. Nature. 1990;348:728–730. doi: 10.1038/348728a0. [DOI] [PubMed] [Google Scholar]
- Hug B, Walter V, Grunwald DJ. Tbx6, a Brachyury-Related Gene Expressed by Ventral Mesendodermal Precursors in the Zebrafish Embryo. Dev Biol. 1997;183:61–73. doi: 10.1006/dbio.1996.8490. [DOI] [PubMed] [Google Scholar]
- Kanki JP, Ho RK. The Development of the Posterior Body in Zebrafish. Development. 1997;124:881–893. doi: 10.1242/dev.124.4.881. [DOI] [PubMed] [Google Scholar]
- Kelly GM, Greenstein P, Erezyilmaz DF, Moon RT. Zebrafish wnt8 and wnt8b Share a Common Activity but are Involved in Distinct Developmental Pathways. Development. 1995;121:1787–1799. doi: 10.1242/dev.121.6.1787. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Muller F, Wu W, Glinka A, Strahle U, Niehrs C. Phenotypic Effects in Xenopus and Zebrafish Suggest that One-Eyed Pinhead Functions as Antagonist of BMP Signalling. Mech Dev. 2000;94:37–46. doi: 10.1016/s0925-4773(00)00329-4. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Warga RM, Schilling TF. Origin and Organization of the Zebrafish Fate Map. Development. 1990;108:581–594. doi: 10.1242/dev.108.4.581. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Kane DA, Walker C, Warga RM, Rothman MB. A Mutation that Changes Cell Movement and Cell Fate in the Zebrafish Embryo. Nature. 1989;337:358–362. doi: 10.1038/337358a0. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of Myosin Phosphatase by Rho and Rho-Associated Kinase (Rho-Kinase). Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 Encodes Two wnt8 Proteins on a Bicistronic Transcript and is Required for Mesoderm and Neurectoderm Patterning. Dev Cell. 2001;1:103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in Vertebrate Axis Formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Macias-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific Activation of Smad1 Signaling Pathways by the BMP7 Type I Receptor, ALK2. J Biol Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Canonical Wnt Signaling Dynamically Controls Multiple Stem Cell Fate Decisions during Vertebrate Body Formation. Dev Cell. 2012;22:223–232. doi: 10.1016/j.devcel.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Wnt Signaling and the Evolution of Embryonic Posterior Development. Curr Biol. 2009;19:R215–9. doi: 10.1016/j.cub.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Regulation of Canonical Wnt Signaling by Brachyury is Essential for Posterior Mesoderm Formation. Dev Cell. 2008;15:121–133. doi: 10.1016/j.devcel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn U, Schulte-Merker S. The Ventralized Ogon Mutant Phenotype is Caused by a Mutation in the Zebrafish Homologue of Sizzled, a Secreted Frizzled-Related Protein. Dev Biol. 2003;260:58–67. doi: 10.1016/s0012-1606(03)00221-5. [DOI] [PubMed] [Google Scholar]
- Miller-Bertoglio V, Carmany-Rampey A, Furthauer M, Gonzalez EM, Thisse C, Thisse B, Halpern ME, Solnica-Krezel L. Maternal and Zygotic Activity of the Zebrafish Ogon Locus Antagonizes BMP Signaling. Dev Biol. 1999;214:72–86. doi: 10.1006/dbio.1999.9384. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Bmp Activity Gradient Regulates Convergent Extension during Zebrafish Gastrulation. Dev Biol. 2002;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective Targeted Gene ‘Knockdown’ in Zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Oxtoby E, Jowett T. Cloning of the Zebrafish Krox-20 Gene (Krx-20) and its Expression during Hindbrain Development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyati UJ, Webb AE, Kimelman D. Transgenic Zebrafish Reveal Stage-Specific Roles for Bmp Signaling in Ventral and Posterior Mesoderm Development. Development. 2005;132:2333–2343. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- Ramel MC, Buckles GR, Baker KD, Lekven AC. WNT8 and BMP2B Co-Regulate Non-Axial Mesoderm Patterning during Zebrafish Gastrulation. Dev Biol. 2005;287:237–248. doi: 10.1016/j.ydbio.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, MD, USA: 1997-2009. http://rsb.info.nih.gov/ij. [Google Scholar]
- Rauch GJ, Hammerschmidt M, Blader P, Schauerte HE, Strahle U, Ingham PW, McMahon AP, Haffter P. Wnt5 is Required for Tail Formation in the Zebrafish Embryo. Cold Spring Harb Symp Quant Biol. 1997;62:227–234. [PubMed] [Google Scholar]
- Row RH, Maitre JL, Martin BL, Stockinger P, Heisenberg CP, Kimelman D. Completion of the Epithelial to Mesenchymal Transition in Zebrafish Mesoderm Requires Spadetail. Dev Biol. 2011;354:102–110. doi: 10.1016/j.ydbio.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row RH, Kimelman D. Bmp Inhibition is Necessary for Post-Gastrulation Patterning and Morphogenesis of the Zebrafish Tailbud. Dev Biol. 2009;329:55–63. doi: 10.1016/j.ydbio.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K, Hall A, Tolwinski N. Wnt Signaling Pathways Meet Rho GTPases. Genes Dev. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The Zebrafish Organizer Requires Chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, van Eeden FJ, Halpern ME, Kimmel CB, Nusslein-Volhard C. No Tail (Ntl) is the Zebrafish Homologue of the Mouse T (Brachyury) Gene. Development. 1994;120:1009–1015. doi: 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Ho RK, Herrmann BG, Nusslein-Volhard C. The Protein Product of the Zebrafish Homologue of the Mouse T Gene is Expressed in Nuclei of the Germ Ring and the Notochord of the Early Embryo. Development. 1992;116:1021–1032. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Bae YK, Muraoka O, Hibi M. Interaction of Wnt and Caudal-Related Genes in Zebrafish Posterior Body Formation. Dev Biol. 2005;279:125–141. doi: 10.1016/j.ydbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Strahle U, Fischer N, Blader P. Expression and Regulation of a Netrin Homologue in the Zebrafish Embryo. Mech Dev. 1997;62:147–160. doi: 10.1016/s0925-4773(97)00657-6. [DOI] [PubMed] [Google Scholar]
- Szeto DP, Kimelman D. The Regulation of Mesodermal Progenitor Cell Commitment to Somitogenesis Subdivides the Zebrafish Body Musculature into Distinct Domains. Genes Dev. 2006;20:1923–1932. doi: 10.1101/gad.1435306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto DP, Kimelman D. Combinatorial Gene Regulation by Bmp and Wnt in Zebrafish Posterior Mesoderm Formation. Development. 2004;131:3751–3760. doi: 10.1242/dev.01236. [DOI] [PubMed] [Google Scholar]
- Thorpe CJ, Weidinger G, Moon RT. Wnt/beta-Catenin Regulation of the Sp1-Related Transcription Factor sp5l Promotes Tail Development in Zebrafish. Development. 2005;132:1763–1772. doi: 10.1242/dev.01733. [DOI] [PubMed] [Google Scholar]
- von der Hardt S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg CP, Hammerschmidt M. The Bmp Gradient of the Zebrafish Gastrula Guides Migrating Lateral Cells by Regulating Cell-Cell Adhesion. Curr Biol. 2007;17:475–487. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Weinberg ES, Allende ML, Kelly CS, Abdelhamid A, Murakami T, Andermann P, Doerre OG, Grunwald DJ, Riggleman B. Developmental Regulation of Zebrafish MyoD in Wild-Type, no Tail and Spadetail Embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Amacher SL, Kim SH, Geissert D, Kimmel CB, De Robertis EM. Zebrafish Paraxial Protocadherin is a Downstream Target of Spadetail Involved in Morphogenesis of Gastrula Mesoderm. Development. 1998;125:3389–3397. doi: 10.1242/dev.125.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin Inhibits BMP Signals Required for Embryogenesis and Iron Metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Talbot WS, Schier AF. Positional Cloning Identifies Zebrafish One-Eyed Pinhead as a Permissive EGF-Related Ligand Required during Gastrulation. Cell. 1998;92:241–251. doi: 10.1016/s0092-8674(00)80918-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.