SUMMARY
Sirtuins extend lifespan across species, although the role in nematodes and fruitflies is controversial. Whether sirtuins can reverse aging-associated degeneration is unknown. Tissue-specific stem cells persist throughout the entire lifespan to repair and maintain tissues, but their self-renewal and differentiation potential become dysregulated with aging. We show that SIRT3, a mammalian sirtuin that regulates the global acetylation landscape of mitochondrial proteins and reduces oxidative stress, is highly enriched in hematopoietic stem cells (HSCs) where it regulates a stress response. SIRT3 is dispensable for HSC maintenance and tissue homeostasis at a young age under homeostatic conditions, but is essential under stress or at an old age. Importantly, SIRT3 is suppressed with aging, and SIRT3 upregulation in aged HSCs improves their regenerative capacity. Our study illuminates the plasticity of mitochondrial homeostasis controlling stem cell and tissue maintenance during the aging process, and shows that aging-associated degeneration can be reversed by a sirtuin.
Keywords: SIR2, sirtuin, SIRT3, mitochondria, aging, rejuvenation, hematopoietic stem cell, oxidative stress, ROS
INTRODUCTION
Aging is a multifaceted degenerative process. Remarkably, lifespan can be extended by single gene mutations (Kenyon, 2010). A key regulator of organismal longevity is SIR2 (silencing information regulator 2). An extra copy of SIR2 extends lifespan in yeast, worms, and flies (Guarente, 2007). However, its role in worms and flies has recently become controversial (Banerjee et al., 2012; Burnett et al., 2011). In mammals, there are seven SIR2 homologs (sirtuins), SIRT1-7, localized in various cellular compartments (Finkel et al., 2009). Recently, mice overexpressing SIRT6 have been shown to have increased lifespan (Kanfi et al., 2012), providing additional evidence that the role of SIR2 in lifespan extension is conserved throughout evolution. However, it is unclear whether sirtuins can reverse, as opposed to simply slow, aging-associated degeneration.
A hallmark of aging is compromised tissue maintenance (Rando, 2006). Tissue-specific stem cells self-renew and persist throughout an organism’s lifespan to repair and maintain tissues. The self-renewal potential and differentiation capacity of stem cells become dysregulated with age (Rossi et al., 2008; Sahin and Depinho, 2010). Stem cell aging is thought to be due to cumulative cellular and genomic damages, resulting in permanent cell cycle arrest, apoptosis, or senescence (Janzen et al., 2006; Rossi et al., 2008; Sahin and Depinho, 2010). A major source of cellular damage is reactive oxygen species (ROS), a natural byproduct of cellular respiration (Balaban et al., 2005). ROS levels in stem cells increase dramatically with age (Ito et al., 2006). Deficient intracellular management of ROS results in increased stem cell cycling and apoptosis, as well as compromised self-renewal and differentiation, resembling essential aspects of aged stem cells (Ito et al., 2004; Ito et al., 2006; Miyamoto et al., 2007; Paik et al., 2009; Renault et al., 2009; Tothova et al., 2007). Despite compelling evidence supporting the essential role of ROS in regulating stem cell aging, outstanding questions still remain unanswered. How do ROS levels increase with age in stem cells? Is stem cell aging a chronic result of cumulative oxidative damage or an acute effect of increased ROS levels? Is ROS-induced physiological stem cell aging and tissue degeneration reversible?
Approximately 90% of cellular ROS are produced in the mitochondria (Balaban et al., 2005). ROS levels are thought to increase with age due to the accumulation of damaged mitochondria in a self-perpetuating cycle. ROS-induced impairment of mitochondria results in increased ROS production, which in turn leads to further mitochondrial damage. However, nutrient intake and numerous genetic mutations alter the rate of aging with concomitant alteration of mitochondrial metabolism and ROS accumulation, suggesting that mitochondrial homeostasis is amenable to regulation during the aging process (Balaban et al., 2005).
Metabolic pathways are coordinated through reversible acetylation of metabolic enzymes in response to nutrient availability (Shin et al., 2011). The sirtuin family has emerged as key regulators of the nutrient-sensitive metabolic regulatory circuit. Prominently, SIRT3 regulates the global acetylation landscape of mitochondrial proteins, and SIRT3-initiated metabolic adaptations enhance mitochondrial management of ROS. SIRT3 increases the activity of antioxidants, such as superoxide dismutase 2 (SOD2) and reduced glutathione, and promotes ROS scavenging (Qiu et al., 2010; Someya et al., 2010; Tao et al., 2010). Additionally, SIRT3 initiates metabolic reprogramming toward more efficient electron transport and fuel usage away from carbohydrate catabolism, which are thought to result in reduced ROS production (Ahn et al., 2008; Hirschey et al., 2010). Thus, SIRT3 provides a unique tool to understand mitochondrial metabolism and management of ROS.
In this study, we use hematopoietic stem cells (HSCs) to identify SIRT3 as an essential regulator of physiological stem cell aging. SIRT3 is downregulated with age, contributing to increased ROS levels in aged HSCs. Evidence is provided to show that increased ROS levels in aged HSCs have acute effects on HSC function and that ROS-mediated physiological HSC aging is reversible. These data shed new light on the plasticity of mitochondrial homeostasis in stem cell maintenance and tissue homeostasis during the aging process and have profound implications for understanding aging and rejuvenation.
RESULTS
SIRT3 deficiency has no effect on the HSC pool at a young age
We compared the expression of SIRT3 in various immunophenotypically-defined subpopulations of mouse bone marrow (BM) cells (HSC: Lin−c-Kit+Sca1+CD150+CD48−, multipotent progenitors (MPP): Lin−c-Kit+Sca1+CD150−CD48−, hematopoietic stem/progenitor cells (HSPC or LKS): Lin−c-Kit+Sca1+, myeloid progenitors (MP): Lin−c-Kit+Sca1−, common lymphoid progenitors (CLP): Lin−IL7Rα+c-kitmed/Sca1med, differentiated blood cells: Lin+) (Kiel et al., 2005). Strikingly, SIRT3 mRNA levels were about 3000-fold higher in HSCs and MPPs than in differentiated blood cells (Figure 1A–C). In contrast to the high expression levels of SIRT3, the expression of the other mitochondrial sirtuins, SIRT4 and SIRT5, was too low to be detected in HSCs (data not shown). Thus, SIRT3 is highly enriched in HSPCs and its expression decreases dramatically in differentiated hematopoietic cells.
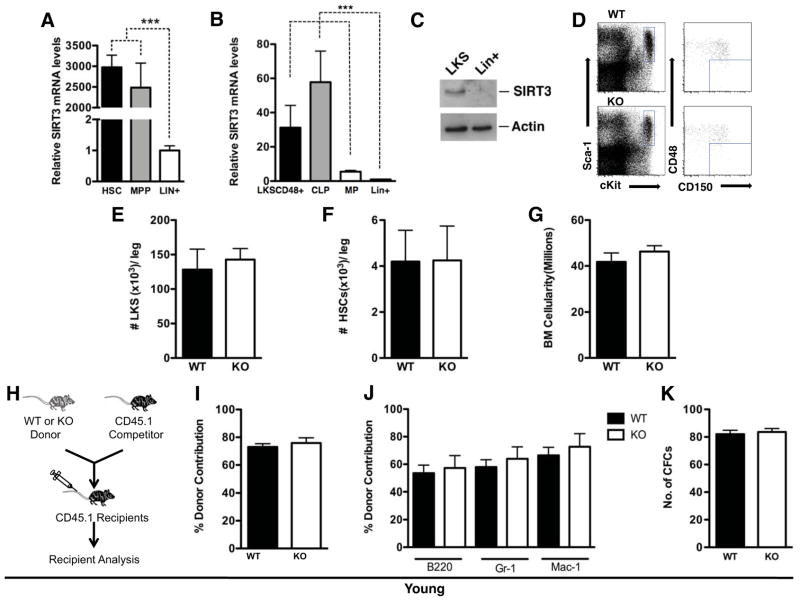
Figure 1. SIRT3 is highly enriched in HSCs and SIRT3 deficiency does not affect the HSC pool at a young age.
A, B, C. BM subpopulations were isolated based on cell surface markers. SIRT3 expression levels were quantified by real time PCR (A, B) or western blot (C). n=5. D, E, F. The frequency of HSPCs and HSCs in the BMs of young mice was determined via flow cytometry (n=3). Flow cytometry plots are gated on Lin− BM cells. Data presented are the numbers of specified cell populations per leg. G. The numbers of total BM cellularity per leg of young WT and SIRT3 KO mice (n=3). H, I, J. BM transplantation. Schematic representation of competitive transplantation assays using BM cells from young WT and SIRT3 KO mice as donors (H). Data shown are the percentage of total donor-derived cells (I) and donor-derived individual lineages (J) in the peripheral blood of the recipients. Donors: n=3. Recipients: n=15. K. The number of colonies formed in a colony-forming assay using BM cells of young WT and SIRT3 KO mice (n=6). Error bars represent standard errors. ***: p<0.001.
To assess the functional role of SIRT3 in HSCs, we compared the quantity and quality of HSCs in wild type (WT) and SIRT3 knockout (KO) mice. SIRT3 KO mice fed ad libitum do not have overt phenotypes at a young age (Lombard et al., 2007). In young animals (3-month old), no difference in the number of immunophenotypically-defined enriched HSPCs or highly enriched HSCs was observed between WT and SIRT3 KO mice (Figure 1D, E, F). BM cellularity was also comparable (Figure 1G). To assess whether SIRT3 affects HSC regeneration capacity in vivo, we performed a competitive transplantation assay. Donor BM cells were transplanted with an equal number of CD45.1+ competitor BM cells to reconstitute the hematopoietic compartment of lethally irradiated recipient mice (Figure 1H). BM cells from young WT and SIRT3 KO mice were equally adept at hematopoietic reconstitution (Figure 1I). When differentiated, HSCs give rise to all blood cell types including myeloid and lymphoid lineages. To determine whether SIRT3 regulates lineage differentiation, we assayed donor-derived mature hematopoietic subpopulations in the transplanted recipients. No significant difference was observed in the percentages of B cells (B220+), granulocytes (Gr1+), and macrophages (Mac-1+) in the blood (Figure 1J). Additionally, we performed in vitro colony-forming assays in which isolated BM mononuclear cells (MNCs) were cultured in methylcellulose medium supplemented with growth factors. The numbers of colonies derived from young WT and SIRT3 KO BM cells were comparable (Figure 1K). Thus, SIRT3 is not required to maintain the HSC pool size and regenerative capacity at a young age.
SIRT3 deficiency results in reduced HSC pool at an old age
Given that SIRT3 functions to trigger mitochondrial reprogramming toward reduced oxidative stress (Qiu et al., 2010; Someya et al., 2010; Tao et al., 2010), we investigated whether SIRT3 regulates HSCs under conditions of elevated oxidative stress, such as aging (Ito et al., 2006). The size of both HSPC and HSC compartments were 50% smaller in aged (18–24-month old) SIRT3 KO mice compared to their WT littermates (Figure 2A, B, C). BM cellularity of aged SIRT3 KO mice was 15% lower (Figure 2D). The reconstitution ability of donor cells from aged SIRT3 KO mice decreased 30% in comparison to age-matched WT controls, with B cells, T cells, granulocytes, and macrophages all significantly reduced (Figure 2E, F). The reduced reconstitution capacity of aged SIRT3-deficient cells is not due to compromised homing (Figure S1A, B). It is worth noting that cell surface markers can only enrich HSCs, and HSCs are ultimately defined by function. Thus, reduced HSC pool size defined by cell surface markers and reduced reconstitution capacity cannot be compared against each other quantitatively. The reduced reconstitution capacity of aged SIRT3-deficient BM may result from both reduced HSC pool size and reduced function per HSC. In a colony-forming assay, aged SIRT3 KO BM cells gave rise to 20% fewer colonies than WT controls (Figure 2G). Thus, SIRT3 is required to maintain HSC pool size and regenerative capacity at an old age.
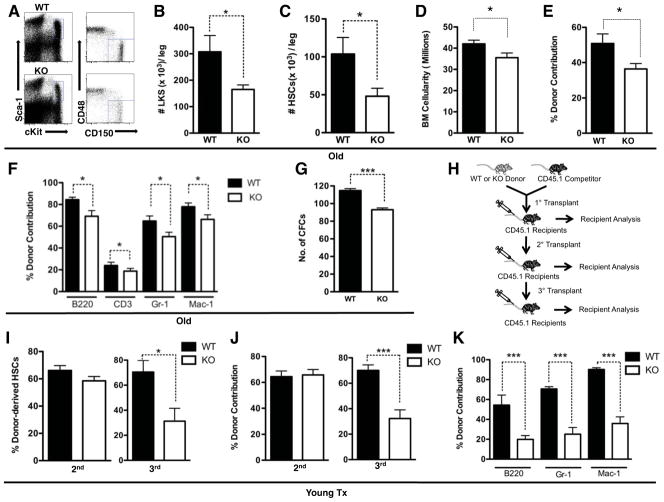
Figure 2. SIRT3 regulates HSC self-renewal at an old age or under transplantation stress.
A, B, C. The frequency of HSPCs and HSCs in the BM of aged mice determined via flow cytometry (n=4). Data presented are the numbers of specified cell populations per leg. D. The numbers of total BM cellularity per leg of aged WT and SIRT3 KO mice (n=4). E, F. Competitive transplantation using aged mice as donors. The percentage of total donor-derived cells (E) and donor-derived individual lineages (F) in the peripheral blood of the recipients are shown. Donors: n=3. Recipients: n=15. G. The number of colonies formed in a colony-forming assay using BM cells of aged WT and SIRT3 KO mice (n=6). H–K. Schematic representation of competitive serial transplantation assays. BM cells from the competitive transplant recipients were used as donors for the next round of transplantation (H). Data shown are the percentage of donor-derived HSCs (LKSCD150+) in the BM (I), total donor-derived cells (J) and donor-derived individual lineages (K) in the peripheral blood using BM cells from young WT and SIRT3 KO mice as donors. Donors: n=3. Recipients: n=15. Error bars represent standard errors. *: p<0.05. ***:p<0.001. See also Figure S1.
SIRT3 deficiency causes compromised HSC self-renewal upon serial transplantation stress
A hallmark of stem cells is their ability to self-renew, allowing them to maintain and repair tissues throughout life. HSCs are able to reconstitute lethally irradiated hosts in secondary and tertiary transplants. ROS levels in HSCs increase modestly after the primary and the secondary transplants but increase dramatically after the tertiary transplant (Ito et al., 2006). We investigated whether SIRT3 is required to sustain HSC function upon serial transplantation (Figure 2H). No difference was observed in HSC self-renewal and hematopoietic reconstitution derived from BM cells of young WT or SIRT3 KO mice in the secondary transplant recipients (Figure 2I, J). However, in the tertiary transplant, SIRT3 KO BM cells resulted in a 50% reduction in HSC self-renewal and reconstitution (Figure 2I, J, K). Together with the results from aged mice, these data suggest that SIRT3 is required to preserve HSCs under oxidative stress conditions, such as aging and serial transplantation.
SIRT3 regulates HSCs autonomously
We next investigated whether the HSC defects observed in the SIRT3 KO mouse model are due to HSC-autonomous effects of SIRT3 or to a non-autonomous role of SIRT3, e.g. the role of SIRT3 in regulating the HSC microenvironment or the niche. The transplantation studies comparing WT and SIRT3 KO BM-derived donors suggest that SIRT3 acts cell-autonomously to maintain HSC self-renewal (Figure 2H–K). Additionally, when aged WT donors were transplanted into lethally irradiated WT or SIRT3 KO mice, comparable HSC self-renewal, reconstitution, and differentiation were observed (Figure S1C–F). Thus, SIRT3 is not required in the niche to support HSC function. Furthermore, SIRT3 overexpression increased the colony-forming activity of aged SIRT3 KO cells by 25% (Figure S1G). These data suggest that the functional defects of HSCs derived from SIRT3 KO mice can be rescued by SIRT3, providing additional support that SIRT3 regulates HSCs autonomously.
SIRT3 reduces oxidative stress in HSCs
We next assessed whether SIRT3 regulates HSPC function by reducing oxidative stress. While HSPCs from young WT and SIRT3 KO mice had comparable ROS levels (Figure S2A), increased ROS levels were detected in HSPCs of aged SIRT3 KO mice compared to WT controls under homeostatic conditions (Figure S2B) and under transplant stress (Figure 3A). Thus, SIRT3 reduces oxidative stress in HSPCs under stress.
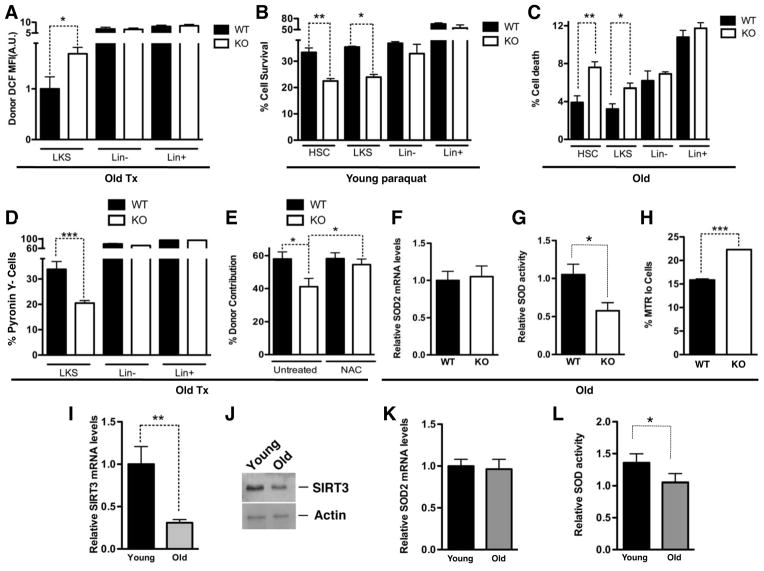
Figure 3. SIRT3 regulates mitochondrial metabolic homeostasis in HSCs and SIRT3 reduces with age.
A. Intracellular ROS levels were determined by H2DCFDA staining in various subpopulations of BMs of old WT and SIRT3 KO mice in a transplant setting. MFI: mean fluorescence intensity. n=4. B. BM cells isolated from WT and SIRT3 KO mice were treated with paraquat and cell survival in various cell populations were scored by flow cytometry (HSC: LKSCD150+). n=3. C. Dead cells were quantified in various subpopulations of BM cells of old WT and SIRT3 KO mice by propidium iodide staining (HSC: LKSCD150+). n=4. D. Cycling of BM cells derived from old mice was assessed in transplant recipients using PY staining. n=4. E. Competitive transplantation assays using BM cells from old WT or SIRT3 KO mice as donors. Recipient mice were either untreated or supplemented with NAC throughout the entire experiment. Data shown are the percentage of donor-derived cells in the peripheral blood. Donors: n=3. Recipients: n=15. F, G. SOD2 mRNA levels (F) and the enzymatic activity (G) in HSPCs of old WT and SIRT3 KO mice were determined. n=4. H. Dysfunctional non-respiring mitochondria in HSCs of old WT and SIRT3 KO mice were determined by MitoTracker Green (MTG) and MitoTracker Red (MTR) staining. I, J. HSPCs were isolated from the BM of young or old mice. SIRT3 expression levels were quantified by real time PCR (I) and western blotting (J) (n=3). K, L. SOD2 mRNA levels (K) and the enzymatic activity (L) in the HSPCs of young and old mice were determined. n=4. Error bars represent standard errors. *: p<0.05. **: p<0.005. ***: p<0.001. See also Figure S2.
We next investigated whether SIRT3 promotes oxidative stress resistance in HSCs. BM cells from WT and SIRT3 KO mice were cultured with or without paraquat, a superoxide-generating compound. The cell survival rates for the SIRT3 KO HSC and LKS populations were 37% lower than WT controls (Figure 3B), suggesting that SIRT3 promotes HSC survival in response to oxidative stress. Next, we determined whether the defects in SIRT3-deficient HSCs are due to an increase in cell death. No significant difference was detected between various BM cell populations of young WT and SIRT3 KO mice (Figure S2C). However, in aged SIRT3 KO mice, the percentage of dead cells in the HSC and HSPC populations doubled relative to the WT controls, but no difference in the Lin- and Lin+ fractions was observed (Figure 3C, S2D), consistent with the SIRT3 expression pattern in these populations (Figure 1A).
HSCs are normally maintained in a quiescent state, which protects HSCs from losing their self-renewal capacity (Rossi et al., 2008). Oxidative stress drives HSCs out of quiescence (Ito et al., 2006; Miyamoto et al., 2007; Tothova et al., 2007). We evaluated cell cycling by staining with Ki67, a cell proliferation marker, and with Pyronin Y (PY), a chemical that stains double-stranded RNA. PY negative populations quantitatively represent quiescent cells (Miyamoto et al., 2007). No difference in cell cycling was noted in HSCs of WT and SIRT3 KO mice (Figure S2E–H). However, differences in cycling were observed under stress. There was a 40% reduction in PY negative HSPCs derived from SIRT3 KO BM compared to WT controls in a transplant setting (Figure 3D, S2I). Thus, SIRT3 deficiency results in increased cycling and reduced survival, which may account for the compromised HSC self-renewal.
To determine whether increased oxidative stress is the underlying cause of compromised function in HSCs lacking SIRT3, we examined whether antioxidant treatment could restore the repopulating ability of aged SIRT3 KO cells. We performed a competitive transplant with BM cells from aged WT and SIRT3 KO mice, and the transplant recipients were supplemented daily with the antioxidant N-acetyl-L-cysteine (NAC), which has been shown to effectively reduce ROS levels in HSCs (Miyamoto et al., 2007). NAC treatment rescued reconstitution defects of aged SIRT3 KO HSCs (Figure 3E), demonstrating that oxidative stress indeed compromises HSC function in the absence of SIRT3.
SIRT3 regulates mitochondrial metabolism in HSCs
We next determined how SIRT3 regulates mitochondrial metabolism in HSPCs. SOD2, a key mitochondrial antioxidant, is a substrate of SIRT3 (Qiu et al., 2010; Tao et al., 2010). We tested whether SIRT3 reduces oxidative stress in HSCs by activating SOD2. SIRT3 enhances the enzymatic activity of SOD2 via a posttranscriptional mechanism (Qiu et al., 2010; Tao et al., 2010). Consistently, SOD2 mRNA levels were comparable in WT and SIRT3 KO HSPCs (Figure 3F). However, the enzymatic activity was 50% lower in SIRT3 KO HSPCs compared to WT controls (Figure 3G). To determine the effects of oxidative stress on mitochondrial function, we used two mitochondria-specific labels to distinguish respiring (MitoTracker Red) versus total (MitoTracker Green) mitochondria. Dysfunctional non-respiring mitochondria (low MitoTracker Red relative to MitoTracker Green) increase with age ((Balaban et al., 2005) and Figure S2J). There was a 40% increase in dysfunctional non-respiring mitochondria in aged SIRT3 KO HSCs compared to the age-matched WT control (Figure 3H). Thus, SIRT3 regulates mitochondrial metabolic homeostasis and integrity in HSPCs.
SIRT3 expression reduces with age in HSCs
Next, we examined how SIRT3 is regulated with age. SIRT3 mRNA levels were 70% lower in HSPCs of old mice compared to those in young mice (Figure 3I, J). To determine whether SIRT3 activity is also decreased with age, we compared expression and the enzymatic activity of SOD2 in HSPCs of young and old mice. SOD2 mRNA levels were unchanged with age (Figure 3K). However, the enzymatic activity was reduced by 30% in aged HSPCs compared to young HSPCs (Figure 3L). Together, these data indicate that SIRT3 expression and activity decrease with age and that suppression of SIRT3-mediated mitochondrial homeostasis contributes to increased ROS in aged HSCs.
SIRT3 upregulation rescues functional defects of aged HSCs
We next determined whether SIRT3 upregulation is sufficient to rescue the functional defects of aged HSCs and reverse aging-associated degeneration. Consistent with the role of SIRT3 in reducing oxidative stress, SIRT3 overexpression reduced the ROS levels in aged HSCs (Figure 4A, S3A, B). While the expression of p19 and BAX increased in HSPCs of aged SIRT3 KO mice compared to WT controls, SIRT3 overexpression in aged HSPCs suppressed their expression (Figure 4B). Two assays confirmed functional rescue of aged HSCs by SIRT3 upregulation. In a colony-formation assay, SIRT3 overexpression increased the colony-forming activity of aged HSCs by 40% (Figure 4C). In a competitive transplantation assay, SIRT3 overexpression resulted in a 5-fold increase in functional reconstitution, with B cells, granulocytes, and macrophages all increased (Figure 4D, E, F). Interestingly, SIRT3 overexpression did not reduce cellular ROS levels and improve the functional capacity of young HSCs (Figure S3C, D), consistent with the observation that SIRT3 is required to maintain HSC self-renewal in aged but not young mice (Figure 1, 2). Together, these data indicate that forced SIRT3 expression can reduce oxidative stress and rejuvenate aged HSCs.
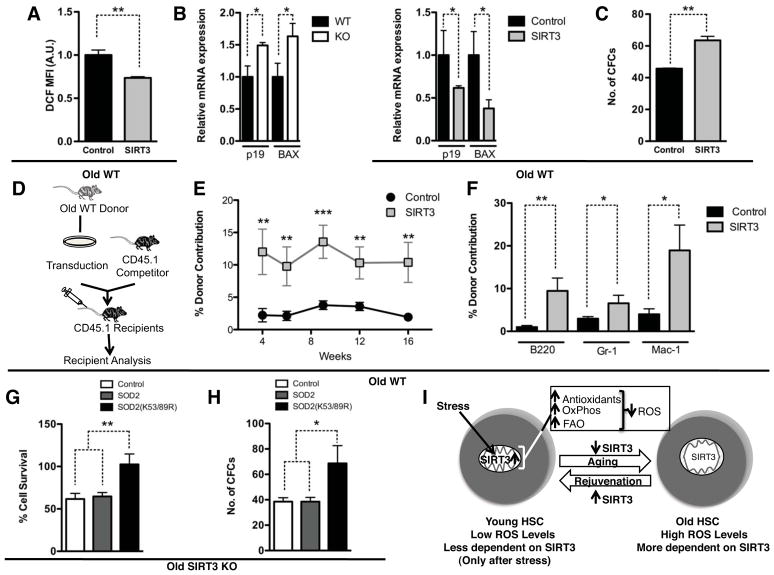
Figure 4. SIRT3 overexpression rescues functional defects of aged HSCs.
A–F. SIRT3 was overexpressed in Lin− cells isolated from old mice via lentiviral transduction. The cellular ROS levels in the HSC population (LKSCD150+) were determined by H2DCFDA staining (n=3) (A). MFI: mean fluorescence intensity. The expression of p19 and BAX was compared in LKS cells of aged WT and SIRT3 KO mice, and aged LKS cells transduced with control or SIRT3 lentivirus by RT-PCR (B). Colony-forming activity was determined in a colony-forming assay (n=6) (C). Schematic representation of a competitive transplantation assay to compare in vivo reconstitution activity of HSCs transduced with control lentivirus or SIRT3 lentivirus (D). Data shown are the percentage of total donor-derived cells (E) and donor-derived individual lineages (F) in the peripheral blood at 4, 6,10, 12, 16 weeks posttransplant. Donor: n=3. Recipients: n=15. G, H. Lin− cells isolated from old SIRT3 KO mice were infected with a control virus, WT SOD2 virus, or SOD2 K53/89R virus. Cells were treated with paraquat and HSC survival was scored by flow cytometry (G). n=3. Colony-forming activity was determined in a colony-forming assay (H). n=6. I. A proposed model on stem cell aging and rejuvenation regulated by SIRT3-mediated mitochondrial homeostasis. Error bars represent standard errors. *: p<0.05. **: p<0.005. ***: p<0.001. See also Figure S3.
SIRT3 deacetylates critical lysine residues on SOD2 and improves the antioxidative activity of SOD2 (Qiu et al., 2010; Tao et al., 2010). We next examined whether constitutively deacetylated SOD2 can improve the functional capacity of aged HSCs without SIRT3. WT or mutant SOD2 with lysines 53 and 89 mutated to arginines (K53/89R) to mimic the constitutively deacetylated form were ectopically expressed via lentiviral infection in Lin− cells isolated from aged SIRT3 KO mice. Infected cells were treated with paraquat to assay oxidative stress resistance. Compared to control virus, SOD2 K53/89R improved the survival of HSCs by 67% while WT SOD2 had no effect (Figure 4G). Furthermore, in a colony-formation assay, SOD2 K53/89R increased colony forming activity by 75% while WT SOD2 infected cells showed comparable activity as cells infected with control virus (Figure 4H). These data suggest that constitutively deacetylated SOD2 bypasses SIRT3 to improve the function of aged HSCs and provide additional support that reducing oxidative stress improves the functional capacity of aged HSCs.
Discussion
The study presented here provides important insights into mitochondrial metabolism in stem cell maintenance, and illuminates the previously underappreciated plasticity of mitochondrial homeostasis in stem cell maintenance and tissue homeostasis during the aging process. Using oxidative stress as a readout for various mitochondrial processes regulated by SIRT3, we show that SIRT3-mediated mitochondrial homeostasis is essential for HSC maintenance under stress (Figure 1, 2), and that this regulatory program is downregulated with age (Figure 3I–L). Together, these data suggest that suppression of SIRT3-mediated mitochondrial homeostasis contributes to increased oxidative stress in aged HSCs. This regulatory process complements the view that passive accumulation of damaged mitochondria with age results in increased ROS and underlies the plasticity of mitochondrial homeostasis in stem cell maintenance and tissue homeostasis (Figure 4I).
The more surprising finding of our study is that upregulation of SIRT3 rescues functional defects of aged HSCs (Figure 4), providing direct evidence that physiological stem cell aging can be an acute casualty of high levels of oxidative stress and that oxidative stress-induced physiological stem cell aging and tissue degeneration are reversible. Although we do not rule out the possibility that chronic oxidative damage to cellular components contributes to the functional decline of aged stem cells, our data suggest that ROS-initiated signaling events are the likely regulators of physiological stem cell aging, providing the basis for reducing oxidative stress to rejuvenate aged stem cells and improve tissue regeneration.
It is intriguing that HSC defects are only apparent in aged but not young SIRT3 KO mice (Figure 1, 2). This is consistent with our observation that SIRT3 preserves HSC function by reducing oxidative stress (Figure 3A–E). Young mice have low levels of cellular ROS, which can be managed by antioxidants in the absence of SIRT3 (Figure S2A). With advancing age or stress, such as serial transplants, the levels of ROS increase (Ito et al., 2006). High levels of cellular ROS require a more robust antioxidative defense system. SIRT3 mediates metabolic reprogramming to reduce ROS production and enhances the antioxidant system to counteract oxidative stress (Figure 3A, B). These data suggest that SIRT3-mediated mitochondrial homeostasis is particularly important for stem cell maintenance under stress conditions. With advancing age, oxidative stress increases in HSCs, and aged HSCs rely more on the SIRT3-mediated mitochondrial stress response. However, as SIRT3 is downregulated in aged HSCs, this stress response becomes less effective, further contributing to increased oxidative stress.
In summary, we have shown that SIRT3 regulates stress-responsive mitochondrial homeostasis, and more importantly, SIRT3 upregulation rejuvenates aged HSCs. We speculate that SIRT3 may regulate stem cells in other tissues. Given that adult stem cells are thought to be central to tissue maintenance and organismal survival, SIRT3 may promote organismal longevity by maintaining the integrity of tissue-specific stem cells. Future studies will determine the effect of SIRT3 on lifespan. While evidence is emerging to indicate that mammalian sirtuins slow aging (Kanfi et al., 2012), our study demonstrates that a sirtuin can also reverse aging-associated degeneration. The understanding of the plasticity of mitochondrial homeostasis in stem cell maintenance and tissue homeostasis should provide new insights into mammalian aging and rejuvenation, and the development of novel approaches for regenerative medicine.
EXPERIMENTAL PROCEDURES
Mice
SIRT3−/− mice have been described (Lombard et al., 2007). All mice were housed on a 12:12-hr light:dark cycle at 25°C. All animal procedures were in accordance with the animal care committee at the University of California, Berkeley.
Flow Cytometry and Cell Sorting
BM cells were obtained by crushing the long bones with sterile PBS without calcium and magnesium supplemented with 2% FBS. Lineage staining contained a cocktail of biotinylated anti-mouse antibodies to Mac-1α (CD11b), Gr-1 (Ly-6G/C), Ter119 (Ly-76), CD3, CD4, CD8a (Ly-2), and B220 (CD45R) (BD Biosciences). For detection or sorting, we used streptavidin conjugated to PerCP, c-Kit-APC, CD48-FITC, CD150-PE-Cy7, and Sca-1-Pacific blue (Biolegend). For congenic strain discrimination, anti-CD45.1-PE or PerCP and anti-CD45.2 FITC or PE-Cy7 antibodies (BD Biosciences) were used. For cell cycle analysis, Pyronin Y staining was performed as described (Miyamoto et al., 2007) and Ki-67 staining was performed according to the manufacturer’s recommendation (BD Biosciences). For cell death, we used propidium iodide staining (Biolegend) with the same antibodies as described above, except Streptavidin-APC-Cy7, c-Kit-FITC, and CD150-APC (Biolegend). ROS levels were detected using H2DCFDA (Invitrogen) at 10 μM for 30 minutes at 37° in the dark (Miyamoto et al., 2007). MitroTracker Red and MitoTracker Green (Invitrogen) were used according to manufacturer’s instructions. All data were collected on an LSRII (Beckon, Dickinson) and data analysis performed with FlowJo (Treestar). For cell sorting, lineage depletion was performed according to the manufacture’s instructions (Miltenyi Biotech). Cells were sorted using a Cytopeia INFLUX Sorter.
Transplantation Assays
5×105 BM cells from WT or SIRT3 KO CD45.2 littermates were mixed with 5×105 CD45.1 B6.SJL (Jackson Laboratory) competitor cells and injected into lethally irradiated (950 Gy) B6.SJL recipient mice. For the niche experiment, 1×106 BM cells from WT CD45.1 B6.SJL were injected into lethally irradiated WT or SIRT3 KO CD45.2 recipients. Multilineage reconstitution was assayed by flow cytometry analysis of peripheral blood 12 to 16 weeks post transplant. For the serial transplantation assay, cells were pooled from all the recipients derived from the same donor. 1×106 whole BM cells were transplanted into each secondary recipient (Miyamoto et al., 2007). Homing efficiency was analyzed as described (Christopherson et al., 2004).
Cell culture
2×104 BM cells were used for colony formation assay in methylcellulose-based medium (Stem Cell Technologies). For paraquat treatment, 1×106 BM cells were cultured in StemSpan SFEM (Stem Cell Technologies) supplemented with 20 ng/mL thrombopoietin, 100 ng/mL SCF, and 100 ng/mL FLT3 ligand (Invitrogen), and 1% Penicillin Streptomycin (Invitrogen), with 200 uM paraquat for 2 days. BM cells were stained with cell surface markers for various cell populations and counted via flow cytometry.
Gene expression
mRNA was extracted from sorted BM subpopulations using Trizol (Invitrogen). Reverse transcription was performed using qScript™ cDNA SuperMix (Quanta Biosciences). Gene expression was determined by real time PCR using Eva qPCR SuperMix kit (BioChain Institute) on an ABI StepOnePlus. GAPDH or b-actin was used as an internal control. Primer sequences are in table S1.
SOD Activity
SOD activity was measured as described (Qiu et al., 2010).
Lentiviral Transduction
Stem and progenitor cells from an aged mouse were enriched using a lineage depletion kit (Miltenyi Biotec). Lineage depleted BM cells were pre-stimulated for 24 hours in StemSpan SFEM (Stem Cell Technologies) supplemented with 20 ng/mL thrombopoietin, 100 ng/mL SCF, and 100 ng/mL FLT3 ligand (Invitrogen), and 1% Penicillin Streptomycin (Invitrogen). SIRT3, SOD2, SOD2 K53/89R were cloned into pFUGW lentiviral construct. Lentivirus was produced as described (Qiu et al., 2010), concentrated by centrifugation, and resuspended with supplemented StemSpan SFEM media. The lentiviral media was added to the enriched stem and progenitor cells, spinoculated for 90 minutes at 270 × g in the presence of 8μg/ml polybrene. This process was repeated 24 hours later with a fresh batch of lentiviral media. After an additional 24 hours, 5× 104 transduced cells were mixed with 5× 105 competitor cells (CD45.1) for competitive transplantation. 750 transduced cells were used for colony formation assay. 2.5×105 transduced cells were used for paraquat treatment.
Statistical analysis
Student’s t-test was used for statistical analyses and null hypotheses were rejected at 0.05. The error bars represent standard errors.
Supplementary Material
HIGHLIGHTS.
SIRT3 is highly enriched in HSCs and is suppressed in differentiated cells
SIRT3 regulates HSC self-renewal under stress or at an old age
SIRT3 regulates mitochondrial metabolic homeostasis and reduces ROS in HSCs
SIRT3 is suppressed with age and its upregulation rejuvenates aged HSCs
Acknowledgments
We thank F. Alt and H. Cheng for reagents. This work is supported by Searle Scholars Program (D.C.), the Hellman Family Faculty Funds (D.C.), the Ellison Medical Foundation (D.C.), the NIH (D.C. R01AG040990), UCOP TRDRP (D.C.), American Heart Association (D.C.), the Siebel Stem Cell Institute (D.C., X.Q., M.M.), the CIRM (X.Q.), and the NSF (J.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Banerjee KK, Ayyub C, Ali SZ, Mandot V, Prasad NG, Kolthur-Seetharam U. dSir2 in the Adult Fat Body, but Not in Muscles, Regulates Life Span in a Diet-Dependent Manner. Cell Rep. 2012 doi: 10.1016/j.celrep.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, Orfila AM, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012 doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Paik JH, Ding Z, Narurkar R, Ramkissoon S, Muller F, Kamoun WS, Chae SS, Zheng H, Ying H, Mahoney J, Hiller D, Jiang S, Protopopov A, Wong WH, Chin L, Ligon KL, DePinho RA. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie Restriction Reduces Oxidative Stress by SIRT3-Mediated SOD2 Activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- Renault VM, Rafalski VA, Morgan AA, Salih DA, Brett JO, Webb AE, Villeda SA, Thekkat PU, Guillerey C, Denko NC, Palmer TD, Butte AJ, Brunet A. FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell. 2009;5:527–539. doi: 10.1016/j.stem.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Zhang D, Chen D. Reversible Acetylation of Metabolic Enzymes Celebration: SIRT2 and p300 Join the Party. Mol Cell. 2011;43:3–5. doi: 10.1016/j.molcel.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.