Abstract
The mammalian response to stress involves the release of soluble products from the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis. Cells of the immune system respond to many of the hormones, neurotransmitters, and neuropeptides through specific receptors. The function of the immune system is critical in the mammalian response to infectious disease. A growing body of evidence identifies stress as a cofactor in infectious disease susceptibility and outcomes. It has been suggested that effects of stress on the immune system may mediate the relationship between stress and infectious disease. This article reviews recent psychoneuroimmunology literature exploring the effects of stress on the pathogenesis of, and immune response to, infectious disease in mammals.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrass C. K., O'Connor S. W., Scarpace P. J., Abrass I. B. Characterization of the beta-adrenergic receptor of the rat peritoneal macrophage. J Immunol. 1985 Aug;135(2):1338–1341. [PubMed] [Google Scholar]
- An S. H., DePauli F. J., Wright P., Ingram D. G. Characteristics of inapparent Aleutian disease virus infection in mink. Res Vet Sci. 1978 Mar;24(2):200–204. [PubMed] [Google Scholar]
- Ausiello C. M., Roda L. G. Leu-enkephalin binding to cultured human T lymphocytes. Cell Biol Int Rep. 1984 May;8(5):353–362. doi: 10.1016/0309-1651(84)90137-1. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Reisine T. D. Stress hormones: their interaction and regulation. Science. 1984 May 4;224(4648):452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Beard C. W., Mitchell B. W. Influence of environmental temperatures on the serologic responses of broiler chickens to inactivated and viable Newcastle disease vaccines. Avian Dis. 1987 Apr-Jun;31(2):321–326. [PubMed] [Google Scholar]
- Ben-Nathan D., Feuerstein G. The influence of cold or isolation stress on resistance of mice to West Nile virus encephalitis. Experientia. 1990 Mar 15;46(3):285–290. doi: 10.1007/BF01951768. [DOI] [PubMed] [Google Scholar]
- Ben-Nathan D., Lustig S., Danenberg H. D. Stress-induced neuroinvasiveness of a neurovirulent noninvasive Sindbis virus in cold or isolation subjected mice. Life Sci. 1991;48(15):1493–1500. doi: 10.1016/0024-3205(91)90187-g. [DOI] [PubMed] [Google Scholar]
- Ben-Nathan D., Lustig S., Feuerstein G. The influence of cold or isolation stress on neuroinvasiveness and virulence of an attenuated variant of West Nile virus. Arch Virol. 1989;109(1-2):1–10. doi: 10.1007/BF01310513. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Blackwell G. J., Flower R. J. Inhibition of phospholipase. Br Med Bull. 1983 Jul;39(3):260–264. doi: 10.1093/oxfordjournals.bmb.a071830. [DOI] [PubMed] [Google Scholar]
- Bonneau R. H., Sheridan J. F., Feng N. G., Glaser R. Stress-induced effects on cell-mediated innate and adaptive memory components of the murine immune response to herpes simplex virus infection. Brain Behav Immun. 1991 Sep;5(3):274–295. doi: 10.1016/0889-1591(91)90023-4. [DOI] [PubMed] [Google Scholar]
- Bonneau R. H., Sheridan J. F., Feng N. G., Glaser R. Stress-induced suppression of herpes simplex virus (HSV)-specific cytotoxic T lymphocyte and natural killer cell activity and enhancement of acute pathogenesis following local HSV infection. Brain Behav Immun. 1991 Jun;5(2):170–192. doi: 10.1016/0889-1591(91)90015-3. [DOI] [PubMed] [Google Scholar]
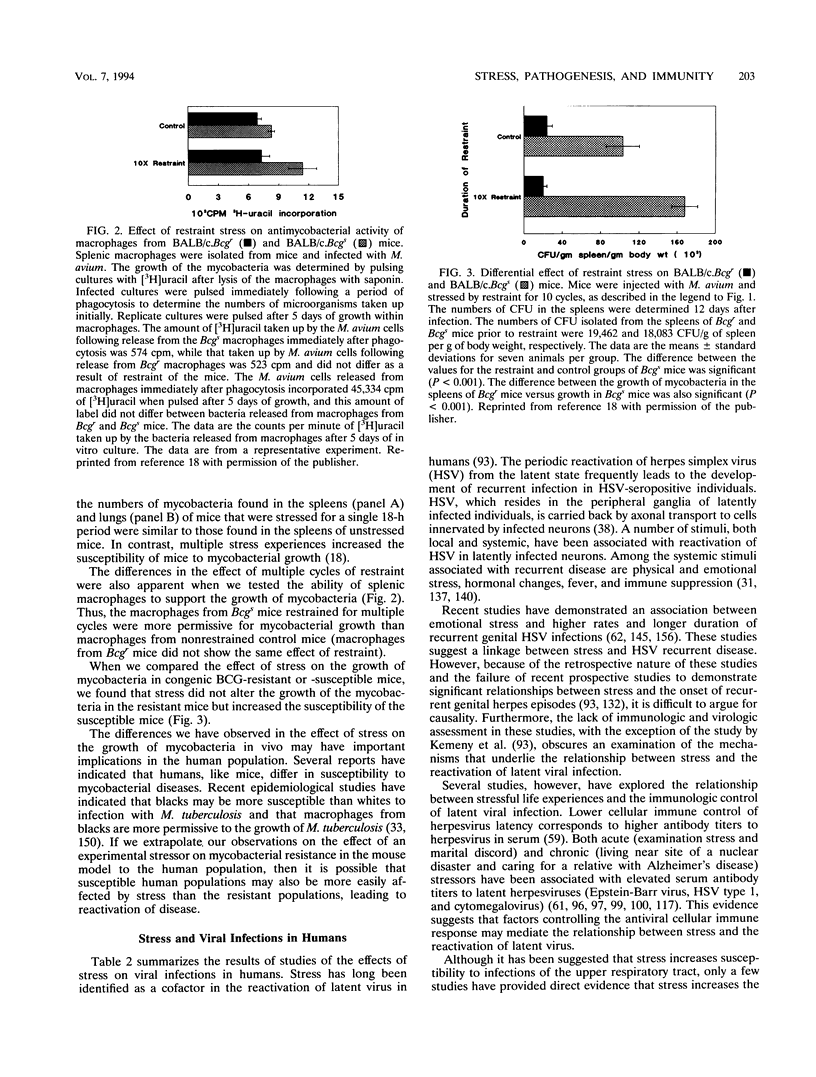
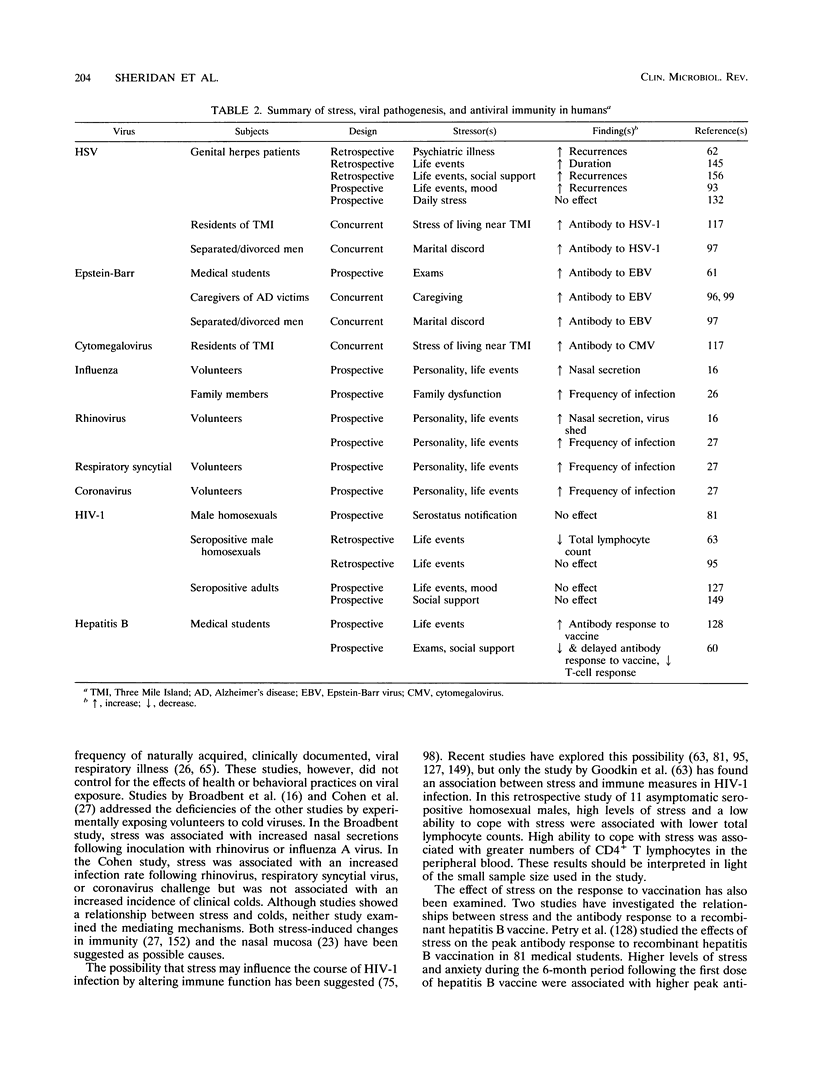
- Bonneau R. H., Sheridan J. F., Feng N., Glaser R. Stress-induced modulation of the primary cellular immune response to herpes simplex virus infection is mediated by both adrenal-dependent and independent mechanisms. J Neuroimmunol. 1993 Feb;42(2):167–176. doi: 10.1016/0165-5728(93)90007-l. [DOI] [PubMed] [Google Scholar]
- Bost K. L., Smith E. M., Wear L. B., Blalock J. E. Presence of ACTH and its receptor on a B lymphocytic cell line: a possible autocrine function for a neuroendocrine hormone. J Biol Regul Homeost Agents. 1987 Jan-Mar;1(1):23–27. [PubMed] [Google Scholar]
- Broadbent D. E., Broadbent M. H., Phillpotts R. J., Wallace J. Some further studies on the prediction of experimental colds in volunteers by psychological factors. J Psychosom Res. 1984;28(6):511–523. doi: 10.1016/0022-3999(84)90085-0. [DOI] [PubMed] [Google Scholar]
- Brooks W. H., Cross R. J., Roszman T. L., Markesbery W. R. Neuroimmunomodulation: neural anatomical basis for impairment and facilitation. Ann Neurol. 1982 Jul;12(1):56–61. doi: 10.1002/ana.410120111. [DOI] [PubMed] [Google Scholar]
- Brown D. H., Sheridan J., Pearl D., Zwilling B. S. Regulation of mycobacterial growth by the hypothalamus-pituitary-adrenal axis: differential responses of Mycobacterium bovis BCG-resistant and -susceptible mice. Infect Immun. 1993 Nov;61(11):4793–4800. doi: 10.1128/iai.61.11.4793-4800.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. L., Van Epps D. E. Opioid peptides modulate production of interferon gamma by human mononuclear cells. Cell Immunol. 1986 Nov;103(1):19–26. doi: 10.1016/0008-8749(86)90064-x. [DOI] [PubMed] [Google Scholar]
- COKER C. M. Cellular factors in acquired immunity to Trichinella spiralis, as indicated by cortisone treatment of mice. J Infect Dis. 1956 Mar-Apr;98(2):187–197. doi: 10.1093/infdis/98.2.187. [DOI] [PubMed] [Google Scholar]
- COKER C. M. Some effects of cortisone in mice with acquired immunity to Trichinella spiralis. J Infect Dis. 1956 Jan-Feb;98(1):39–44. doi: 10.1093/infdis/98.1.39. [DOI] [PubMed] [Google Scholar]
- Carr D. J., DeCosta B. R., Jacobson A. E., Rice K. C., Blalock J. E. Corticotropin-releasing hormone augments natural killer cell activity through a naloxone-sensitive pathway. J Neuroimmunol. 1990 Jun;28(1):53–61. doi: 10.1016/0165-5728(90)90040-t. [DOI] [PubMed] [Google Scholar]
- Carr D. J., Klimpel G. R. Enhancement of the generation of cytotoxic T cells by endogenous opiates. J Neuroimmunol. 1986 Jul;12(1):75–87. doi: 10.1016/0165-5728(86)90099-8. [DOI] [PubMed] [Google Scholar]
- Chester A. C. Psychological stress and the common cold. N Engl J Med. 1992 Feb 27;326(9):645–646. [PubMed] [Google Scholar]
- Chetverikova L. K., Frolov B. A., Kramskaya T. A., Polyak RYa Experimental influenza infection: influence of stress. Acta Virol. 1987 Sep;31(5):424–433. [PubMed] [Google Scholar]
- Chung H. T., Samlowski W. E., Daynes R. A. Modification of the murine immune system by glucocorticosteroids: alterations of the tissue localization properties of circulating lymphocytes. Cell Immunol. 1986 Sep;101(2):571–585. doi: 10.1016/0008-8749(86)90167-x. [DOI] [PubMed] [Google Scholar]
- Clover R. D., Abell T., Becker L. A., Crawford S., Ramsey C. N., Jr Family functioning and stress as predictors of influenza B infection. J Fam Pract. 1989 May;28(5):535–539. [PubMed] [Google Scholar]
- Cohen S., Tyrrell D. A., Smith A. P. Psychological stress and susceptibility to the common cold. N Engl J Med. 1991 Aug 29;325(9):606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin Microbiol Rev. 1989 Oct;2(4):360–377. doi: 10.1128/cmr.2.4.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey L., Spear P. G. Infections with herpes simplex viruses (2). N Engl J Med. 1986 Mar 20;314(12):749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Munck A., Smith K. A. Glucocorticoids and lymphocytes. I. Increased glucocorticoid receptor levels in antigen-stimulated lymphocytes. J Immunol. 1980 May;124(5):2430–2435. [PubMed] [Google Scholar]
- Crowle A. J., Elkins N. Relative permissiveness of macrophages from black and white people for virulent tubercle bacilli. Infect Immun. 1990 Mar;58(3):632–638. doi: 10.1128/iai.58.3.632-638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROZ B., LEBLOND C. P. Migration of proteins along the axons of the sciatic nerve. Science. 1962 Sep 28;137(3535):1047–1048. doi: 10.1126/science.137.3535.1047. [DOI] [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Skamene E. Pleiotropic effects of the Bcg gene. I. Antigen presentation in genetically susceptible and resistant congenic mouse strains. J Immunol. 1988 Apr 1;140(7):2395–2400. [PubMed] [Google Scholar]
- Denis M., Forget A., Pelletier M., Skamene E. Pleiotropic effects of the Bcg gene: III. Respiratory burst in Bcg-congenic macrophages. Clin Exp Immunol. 1988 Sep;73(3):370–375. [PMC free article] [PubMed] [Google Scholar]
- Dobbs C. M., Vasquez M., Glaser R., Sheridan J. F. Mechanisms of stress-induced modulation of viral pathogenesis and immunity. J Neuroimmunol. 1993 Nov-Dec;48(2):151–160. doi: 10.1016/0165-5728(93)90187-4. [DOI] [PubMed] [Google Scholar]
- Edwards E. A., Dean L. M. Effects of crowding of mice on humoral antibody formation and protection to lethal antigenic challenge. Psychosom Med. 1977 Jan-Feb;39(1):19–24. doi: 10.1097/00006842-197701000-00003. [DOI] [PubMed] [Google Scholar]
- Eguchi K., Kawakami A., Nakashima M., Ida H., Sakito S., Matsuoka N., Terada K., Sakai M., Kawabe Y., Fukuda T. Interferon-alpha and dexamethasone inhibit adhesion of T cells to endothelial cells and synovial cells. Clin Exp Immunol. 1992 Jun;88(3):448–454. doi: 10.1111/j.1365-2249.1992.tb06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Faith R. E., Liang H. J., Murgo A. J., Plotnikoff N. P. Neuroimmunomodulation with enkephalins: enhancement of human natural killer (NK) cell activity in vitro. Clin Immunol Immunopathol. 1984 Jun;31(3):412–418. doi: 10.1016/0090-1229(84)90093-x. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. Mechanisms of corticosteroid action on lymphocyte subpopulations. I. Redistribution of circulating T and b lymphocytes to the bone marrow. Immunology. 1975 Apr;28(4):669–680. [PMC free article] [PubMed] [Google Scholar]
- Felten S. Y., Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18(1):37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- Feng N., Pagniano R., Tovar C. A., Bonneau R. H., Glaser R., Sheridan J. F. The effect of restraint stress on the kinetics, magnitude, and isotype of the humoral immune response to influenza virus infection. Brain Behav Immun. 1991 Dec;5(4):370–382. doi: 10.1016/0889-1591(91)90032-6. [DOI] [PubMed] [Google Scholar]
- Filion L. G., Willson P. J., Bielefeldt-Ohmann H., Babiuk L. A., Thomson R. G. The possible role of stress in the induction of pneumonic pasteurellosis. Can J Comp Med. 1984 Jul;48(3):268–274. [PMC free article] [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidman S. B., Ader R., Grota L. J. Protective effect of noxious stimulation in mice infected with rodent malaria. Psychosom Med. 1973 Nov-Dec;35(6):535–537. doi: 10.1097/00006842-197311000-00008. [DOI] [PubMed] [Google Scholar]
- Friedman S. B., Glasgow L. A., Ader R. Differential susceptibility to a viral agent in mice housed alone or in groups. Psychosom Med. 1970 May-Jun;32(3):285–299. doi: 10.1097/00006842-197005000-00007. [DOI] [PubMed] [Google Scholar]
- Frohman E. M., Vayuvegula B., Gupta S., van den Noort S. Norepinephrine inhibits gamma-interferon-induced major histocompatibility class II (Ia) antigen expression on cultured astrocytes via beta-2-adrenergic signal transduction mechanisms. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1292–1296. doi: 10.1073/pnas.85.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs B. A., Albright J. W., Albright J. F. Beta-adrenergic receptors on murine lymphocytes: density varies with cell maturity and lymphocyte subtype and is decreased after antigen administration. Cell Immunol. 1988 Jul;114(2):231–245. doi: 10.1016/0008-8749(88)90318-8. [DOI] [PubMed] [Google Scholar]
- Galant S. P., Duriseti L., Underwood S., Insel P. A. Decreased beta-adrenergic receptors on polymorphonuclear leukocytes after adrenergic therapy. N Engl J Med. 1978 Oct 26;299(17):933–936. doi: 10.1056/NEJM197810262991707. [DOI] [PubMed] [Google Scholar]
- Gatmaitan B. G., Chason J. L., Lerner A. M. Augmentation of the virulence of murine coxsackie-virus B-3 myocardiopathy by exercise. J Exp Med. 1970 Jun 1;131(6):1121–1136. doi: 10.1084/jem.131.6.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S. C., Schwartz J. M., Milner R. J., Bloom F. E., Feldman J. D. beta-Endorphin enhances lymphocyte proliferative responses. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4226–4230. doi: 10.1073/pnas.79.13.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore W., Weiner L. P. Beta-endorphin enhances interleukin-2 (IL-2) production in murine lymphocytes. J Neuroimmunol. 1988 May;18(2):125–138. doi: 10.1016/0165-5728(88)90061-6. [DOI] [PubMed] [Google Scholar]
- Gilmore W., Weiner L. P. The opioid specificity of beta-endorphin enhancement of murine lymphocyte proliferation. Immunopharmacology. 1989 Jan-Feb;17(1):19–30. doi: 10.1016/0162-3109(89)90004-0. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J. K., Bonneau R. H., Malarkey W., Kennedy S., Hughes J. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom Med. 1992 Jan-Feb;54(1):22–29. doi: 10.1097/00006842-199201000-00005. [DOI] [PubMed] [Google Scholar]
- Glaser R., Kiecolt-Glaser J. K., Speicher C. E., Holliday J. E. Stress, loneliness, and changes in herpesvirus latency. J Behav Med. 1985 Sep;8(3):249–260. doi: 10.1007/BF00870312. [DOI] [PubMed] [Google Scholar]
- Goldmeier D., Johnson A. Does psychiatric illness affect the recurrence rate of genital herpes? Br J Vener Dis. 1982 Feb;58(1):40–43. doi: 10.1136/sti.58.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin K., Fuchs I., Feaster D., Leeka J., Rishel D. D. Life stressors and coping style are associated with immune measures in HIV-1 infection--a preliminary report. Int J Psychiatry Med. 1992;22(2):155–172. doi: 10.2190/BD46-F4JD-K8TW-RUFH. [DOI] [PubMed] [Google Scholar]
- Grabstein K., Dower S., Gillis S., Urdal D., Larsen A. Expression of interleukin 2, interferon-gamma, and the IL 2 receptor by human peripheral blood lymphocytes. J Immunol. 1986 Jun 15;136(12):4503–4508. [PubMed] [Google Scholar]
- Graham N. M., Douglas R. M., Ryan P. Stress and acute respiratory infection. Am J Epidemiol. 1986 Sep;124(3):389–401. doi: 10.1093/oxfordjournals.aje.a114409. [DOI] [PubMed] [Google Scholar]
- Green G. M., Kass E. H. The influence of bacterial species on pulmonary resistance to infection in mice subjected to hypoxia, cold stress, and ethanolic intoxication. Br J Exp Pathol. 1965 Jun;46(3):360–366. [PMC free article] [PubMed] [Google Scholar]
- Gross W. B., Domermuth C. H. Factors influencing the severity of Escherichia coli and avian adenovirus group II infections in chickens. Avian Dis. 1988 Oct-Dec;32(4):793–797. [PubMed] [Google Scholar]
- Gross W. B. Effect of a range of social stress severity on Escherichia coli challenge infection. Am J Vet Res. 1984 Oct;45(10):2074–2076. [PubMed] [Google Scholar]
- Gross W. B. Effect of adrenal blocking chemicals on viral and respiratory infections of chickens. Can J Vet Res. 1989 Jan;53(1):48–51. [PMC free article] [PubMed] [Google Scholar]
- Gross W. B. Effect of environmental stress on the responses of ascorbic-acid-treated chickens to Escherichia coli challenge infection. Avian Dis. 1988 Jul-Sep;32(3):432–436. [PubMed] [Google Scholar]
- Gross W. B., Falkinham J. D., 3rd, Payeur J. B. Effect of environmental-genetic interactions on Mycobacterium avium challenge infection. Avian Dis. 1989 Jul-Sep;33(3):411–415. [PubMed] [Google Scholar]
- Hall N. R., McClure J. E., Hu S. K., Tare N. S., Seals C. M., Goldstein A. L. Effects of 6-hydroxydopamine upon primary and secondary thymus dependent immune responses. Immunopharmacology. 1982 Oct;5(1):39–48. doi: 10.1016/0162-3109(82)90035-2. [DOI] [PubMed] [Google Scholar]
- Hamilton D. R. Immunosuppressive effects of predator induced stress in mice with acquired immunity to Hymenolepis nana. J Psychosom Res. 1974 Jun;18(3):143–153. doi: 10.1016/0022-3999(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Hassan N. F., Douglas S. D. Stress-related neuroimmunomodulation of monocyte-macrophage functions in HIV-1 infection. Clin Immunol Immunopathol. 1990 Feb;54(2):220–227. doi: 10.1016/0090-1229(90)90083-3. [DOI] [PubMed] [Google Scholar]
- Hermann G., Tovar C. A., Beck F. M., Allen C., Sheridan J. F. Restraint stress differentially affects the pathogenesis of an experimental influenza viral infection in three inbred strains of mice. J Neuroimmunol. 1993 Aug;47(1):83–94. doi: 10.1016/0165-5728(93)90287-9. [DOI] [PubMed] [Google Scholar]
- Holt P. S. Effect of induced molting on the susceptibility of White Leghorn hens to a Salmonella enteritidis infection. Avian Dis. 1993 Apr-Jun;37(2):412–417. [PubMed] [Google Scholar]
- Holt P. S., Porter R. E., Jr Microbiological and histopathological effects of an induced-molt fasting procedure on a Salmonella enteritidis infection in chickens. Avian Dis. 1992 Jul-Sep;36(3):610–618. [PubMed] [Google Scholar]
- Ilbäck N. G., Fohlman J., Friman G. Exercise in coxsackie B3 myocarditis: effects on heart lymphocyte subpopulations and the inflammatory reaction. Am Heart J. 1989 Jun;117(6):1298–1302. doi: 10.1016/0002-8703(89)90409-2. [DOI] [PubMed] [Google Scholar]
- Ilbäck N. G., Friman G., Beisel W. R., Johnson A. J., Berendt R. F. Modifying effects of exercise on clinical course and biochemical response of the myocardium in influenza and tularemia in mice. Infect Immun. 1984 Aug;45(2):498–504. doi: 10.1128/iai.45.2.498-504.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironson G., LaPerriere A., Antoni M., O'Hearn P., Schneiderman N., Klimas N., Fletcher M. A. Changes in immune and psychological measures as a function of anticipation and reaction to news of HIV-1 antibody status. Psychosom Med. 1990 May-Jun;52(3):247–270. doi: 10.1097/00006842-199005000-00001. [DOI] [PubMed] [Google Scholar]
- Irwin M. R., Hauger R. L. Adaptation to chronic stress. Temporal pattern of immune and neuroendocrine correlates. Neuropsychopharmacology. 1988 Sep;1(3):239–242. doi: 10.1016/0893-133x(88)90023-1. [DOI] [PubMed] [Google Scholar]
- Irwin M. R., Vale W., Britton K. T. Central corticotropin-releasing factor suppresses natural killer cytotoxicity. Brain Behav Immun. 1987 Mar;1(1):81–87. doi: 10.1016/0889-1591(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Irwin M., Hauger R. L., Brown M., Britton K. T. CRF activates autonomic nervous system and reduces natural killer cytotoxicity. Am J Physiol. 1988 Nov;255(5 Pt 2):R744–R747. doi: 10.1152/ajpregu.1988.255.5.R744. [DOI] [PubMed] [Google Scholar]
- JACKSON G. J. The effect of cortisone on Plasmodium berghei infections in the white rat. J Infect Dis. 1955 Sep-Oct;97(2):152–159. doi: 10.1093/infdis/97.2.152. [DOI] [PubMed] [Google Scholar]
- JOHNSSON T., LAVENDER J. F., HULTIN E., RASMUSSEN A. F., Jr THE INFLUENCE OF AVOIDANCE-LEARNING STRESS ON RESISTANCE TO COXSACKIE B VIRUS IN MICE. J Immunol. 1963 Nov;91:569–575. [PubMed] [Google Scholar]
- Johnson E. W., Blalock J. E., Smith E. M. ACTH receptor-mediated induction of leukocyte cyclic AMP. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1205–1211. doi: 10.1016/s0006-291x(88)81002-7. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Smith E. M., Torres B. A., Blalock J. E. Regulation of the in vitro antibody response by neuroendocrine hormones. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4171–4174. doi: 10.1073/pnas.79.13.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Torres B. A., Smith E. M., Dion L. D., Blalock J. E. Regulation of lymphokine (gamma-interferon) production by corticotropin. J Immunol. 1984 Jan;132(1):246–250. [PubMed] [Google Scholar]
- Johnson S. C., Zwilling B. S. Continuous expression of I-A antigen by peritoneal macrophages from mice resistant to Mycobacterium bovis (strain BCG). J Leukoc Biol. 1985 Nov;38(5):635–647. doi: 10.1002/jlb.38.5.635. [DOI] [PubMed] [Google Scholar]
- Kemeny M. E., Cohen F., Zegans L. S., Conant M. A. Psychological and immunological predictors of genital herpes recurrence. Psychosom Med. 1989 Mar-Apr;51(2):195–208. doi: 10.1097/00006842-198903000-00008. [DOI] [PubMed] [Google Scholar]
- Kern J. A., Lamb R. J., Reed J. C., Daniele R. P., Nowell P. C. Dexamethasone inhibition of interleukin 1 beta production by human monocytes. Posttranscriptional mechanisms. J Clin Invest. 1988 Jan;81(1):237–244. doi: 10.1172/JCI113301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. C., Foster C., Joseph J., Ostrow D., Wortman C., Phair J., Chmiel J. Stressful life events and symptom onset in HIV infection. Am J Psychiatry. 1991 Jun;148(6):733–738. doi: 10.1176/ajp.148.6.733. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Dura J. R., Speicher C. E., Trask O. J., Glaser R. Spousal caregivers of dementia victims: longitudinal changes in immunity and health. Psychosom Med. 1991 Jul-Aug;53(4):345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Fisher L. D., Ogrocki P., Stout J. C., Speicher C. E., Glaser R. Marital quality, marital disruption, and immune function. Psychosom Med. 1987 Jan-Feb;49(1):13–34. doi: 10.1097/00006842-198701000-00002. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Glaser R. Psychological influences on immunity. Implications for AIDS. Am Psychol. 1988 Nov;43(11):892–898. doi: 10.1037//0003-066x.43.11.892. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Glaser R., Shuttleworth E. C., Dyer C. S., Ogrocki P., Speicher C. E. Chronic stress and immunity in family caregivers of Alzheimer's disease victims. Psychosom Med. 1987 Sep-Oct;49(5):523–535. doi: 10.1097/00006842-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K., Kennedy S., Malkoff S., Fisher L., Speicher C. E., Glaser R. Marital discord and immunity in males. Psychosom Med. 1988 May-Jun;50(3):213–229. doi: 10.1097/00006842-198805000-00001. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Dunegan M. A. Modulation of macrophage-mediated tumoricidal activity by neuropeptides and neurohormones. J Immunol. 1985 Jul;135(1):350–354. [PubMed] [Google Scholar]
- Krieger D. T. Brain peptides: what, where, and why? Science. 1983 Dec 2;222(4627):975–985. doi: 10.1126/science.6139875. [DOI] [PubMed] [Google Scholar]
- Kusnecov A. V., Grota L. J., Schmidt S. G., Bonneau R. H., Sheridan J. F., Glaser R., Moynihan J. A. Decreased herpes simplex viral immunity and enhanced pathogenesis following stressor administration in mice. J Neuroimmunol. 1992 May;38(1-2):129–137. doi: 10.1016/0165-5728(92)90097-5. [DOI] [PubMed] [Google Scholar]
- Landmann R. M., Bürgisser E., Wesp M., Bühler F. R. Beta-adrenergic receptors are different in subpopulations of human circulating lymphocytes. J Recept Res. 1984;4(1-6):37–50. doi: 10.3109/10799898409042538. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tsou A. P., Chan H., Thomas J., Petrie K., Eugui E. M., Allison A. C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew W., Oppenheim J. J., Matsushima K. Analysis of the suppression of IL-1 alpha and IL-1 beta production in human peripheral blood mononuclear adherent cells by a glucocorticoid hormone. J Immunol. 1988 Mar 15;140(6):1895–1902. [PubMed] [Google Scholar]
- Livnat S., Felten S. Y., Carlson S. L., Bellinger D. L., Felten D. L. Involvement of peripheral and central catecholamine systems in neural-immune interactions. J Neuroimmunol. 1985 Nov;10(1):5–30. doi: 10.1016/0165-5728(85)90031-1. [DOI] [PubMed] [Google Scholar]
- Livnat S., Madden K. S., Felten D. L., Felten S. Y. Regulation of the immune system by sympathetic neural mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11(2-3):145–152. doi: 10.1016/0278-5846(87)90052-2. [DOI] [PubMed] [Google Scholar]
- Loveland B. E., Jarrott B., McKenzie I. F. The detection of beta-adrenoceptors on murine lymphocytes. Int J Immunopharmacol. 1981;3(1):45–55. doi: 10.1016/0192-0561(81)90044-8. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Synthesis and secretion of ACTH, beta-endorphin, and related peptides. Adv Biochem Psychopharmacol. 1981;28:35–47. [PubMed] [Google Scholar]
- Mandler R. N., Biddison W. E., Mandler R., Serrate S. A. beta-Endorphin augments the cytolytic activity and interferon production of natural killer cells. J Immunol. 1986 Feb 1;136(3):934–939. [PubMed] [Google Scholar]
- Mathews P. M., Froelich C. J., Sibbitt W. L., Jr, Bankhurst A. D. Enhancement of natural cytotoxicity by beta-endorphin. J Immunol. 1983 Apr;130(4):1658–1662. [PubMed] [Google Scholar]
- McCain H. W., Lamster I. B., Bilotta J. Modulation of human T-cell suppressor activity by beta endorphin and glycyl-L-glutamine. Int J Immunopharmacol. 1986;8(4):443–446. doi: 10.1016/0192-0561(86)90130-x. [DOI] [PubMed] [Google Scholar]
- McCain H. W., Lamster I. B., Bozzone J. M., Grbic J. T. Beta-endorphin modulates human immune activity via non-opiate receptor mechanisms. Life Sci. 1982 Oct 11;31(15):1619–1624. doi: 10.1016/0024-3205(82)90054-6. [DOI] [PubMed] [Google Scholar]
- McKinnon W., Weisse C. S., Reynolds C. P., Bowles C. A., Baum A. Chronic stress, leukocyte subpopulations, and humoral response to latent viruses. Health Psychol. 1989;8(4):389–402. doi: 10.1037//0278-6133.8.4.389. [DOI] [PubMed] [Google Scholar]
- McLean R. G. Potentiation of keystone virus infection in cotton rats by glucocorticoid-induced stress. J Wildl Dis. 1982 Apr;18(2):141–148. doi: 10.7589/0090-3558-18.2.141. [DOI] [PubMed] [Google Scholar]
- Mehrishi J. N., Mills I. H. Opiate receptors on lymphocytes and platelets in man. Clin Immunol Immunopathol. 1983 May;27(2):240–249. doi: 10.1016/0090-1229(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Melmon K. L., Bourne H. R., Weinstein Y., Shearer G. M., Kram J., Bauminger S. Hemolytic plaque formation by leukocytes in vitro. Control by vasoactive hormones. J Clin Invest. 1974 Jan;53(1):13–21. doi: 10.1172/JCI107530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. A., Hanson R. P. Effect of social stress on Newcastle disease virus (LaSota) infection. Avian Dis. 1980 Oct-Dec;24(4):908–915. [PubMed] [Google Scholar]
- Motulsky H. J., Insel P. A. Adrenergic receptors in man: direct identification, physiologic regulation, and clinical alterations. N Engl J Med. 1982 Jul 1;307(1):18–29. doi: 10.1056/NEJM198207013070104. [DOI] [PubMed] [Google Scholar]
- Northrop J. P., Crabtree G. R., Mattila P. S. Negative regulation of interleukin 2 transcription by the glucocorticoid receptor. J Exp Med. 1992 May 1;175(5):1235–1245. doi: 10.1084/jem.175.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozherelkov S. V., Khozinsky V. V., Semenov B. F. Replication of Langat virus in immunocompetent cells of mice subjected to immobilization stress. Acta Virol. 1990 May;34(3):291–294. [PubMed] [Google Scholar]
- PAYNE F. E., LARSON A., FOSTER W. L., MEYER K. F. Studies on immunization against plague. IX. The effect of cortisone on mouse resistance to attenuated strains of Pasteurella pestis. J Infect Dis. 1955 Mar-Apr;96(2):168–173. doi: 10.1093/infdis/96.2.168. [DOI] [PubMed] [Google Scholar]
- Perry S., Fishman B., Jacobsberg L., Frances A. Relationships over 1 year between lymphocyte subsets and psychosocial variables among adults with infection by human immunodeficiency virus. Arch Gen Psychiatry. 1992 May;49(5):396–401. doi: 10.1001/archpsyc.1992.01820050060010. [DOI] [PubMed] [Google Scholar]
- Petry L. J., Weems L. B., 2nd, Livingstone J. N., 2nd Relationship of stress, distress, and the immunologic response to a recombinant hepatitis B vaccine. J Fam Pract. 1991 May;32(5):481–486. [PubMed] [Google Scholar]
- Pitchenik A. E., Fertel D. Medical management of AIDS patients. Tuberculosis and nontuberculous mycobacterial disease. Med Clin North Am. 1992 Jan;76(1):121–171. doi: 10.1016/s0025-7125(16)30375-3. [DOI] [PubMed] [Google Scholar]
- Potter M., O'Brien A. D., Skamene E., Gros P., Forget A., Kongshavn P. A., Wax J. S. A BALB/c congenic strain of mice that carries a genetic locus (Ityr) controlling resistance to intracellular parasites. Infect Immun. 1983 Jun;40(3):1234–1235. doi: 10.1128/iai.40.3.1234-1235.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASMUSSEN A. F., Jr, MARSH J. T., BRILL N. Q. Increased susceptibility to herpes simplex in mice subjected to avoidance-learning stress or restraint. Proc Soc Exp Biol Med. 1957 Oct;96(1):183–189. doi: 10.3181/00379727-96-23426. [DOI] [PubMed] [Google Scholar]
- ROBSON J. M., DIDCOCK K. A. The action of cortisone on corneal tuberculosis studied with the phase contrast microscope. Am Rev Tuberc. 1956 Jul;74(1):1–6. doi: 10.1164/artpd.1956.74.1.1. [DOI] [PubMed] [Google Scholar]
- ROSENBAUM H. E., HARFORD C. G. Effect of fatigue on susceptibility of mice to poliomyelitis. Proc Soc Exp Biol Med. 1953 Aug-Sep;83(4):678–681. doi: 10.3181/00379727-83-20456. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Hoon E. F., Massey J. K., Johnson J. H. Daily stress and recurrence of genital herpes simplex. Arch Intern Med. 1990 Sep;150(9):1889–1893. [PubMed] [Google Scholar]
- Reyes M. P., Lerner A. M. Interferon and neutralizing antibody in sera of exercised mice with coxsackievirus B-3 myocarditis. Proc Soc Exp Biol Med. 1976 Feb;151(2):333–338. doi: 10.3181/00379727-151-39204. [DOI] [PubMed] [Google Scholar]
- Rivier C., Brownstein M., Spiess J., Rivier J., Vale W. In vivo corticotropin-releasing factor-induced secretion of adrenocorticotropin, beta-endorphin, and corticosterone. Endocrinology. 1982 Jan;110(1):272–278. doi: 10.1210/endo-110-1-272. [DOI] [PubMed] [Google Scholar]
- SINGER I. The effect of cortisone on infections with Plasmodium berghei in the white mouse. J Infect Dis. 1954 Mar-Apr;94(2):164–172. doi: 10.1093/infdis/94.2.164. [DOI] [PubMed] [Google Scholar]
- Sadeghi R., Feldmann M., Hawrylowicz C. Upregulation of HLA class II, but not intercellular adhesion molecule 1 (ICAM-1) by granulocyte-macrophage colony stimulating factor (GM-CSF) or interleukin-3 (IL-3) in synergy with dexamethasone. Eur Cytokine Netw. 1992 Jul-Aug;3(4):373–380. [PubMed] [Google Scholar]
- Schmidt D. D., Zyzanski S., Ellner J., Kumar M. L., Arno J. Stress as a precipitating factor in subjects with recurrent herpes labialis. J Fam Pract. 1985 Apr;20(4):359–366. [PubMed] [Google Scholar]
- Schurr E., Skamene E., Nesbitt M., Hynes R., Gros P. Identification of a linkage group including the Bcg gene by restriction fragment length polymorphism analysis. Curr Top Microbiol Immunol. 1988;137:310–315. doi: 10.1007/978-3-642-50059-6_46. [DOI] [PubMed] [Google Scholar]
- Shavit Y., Lewis J. W., Terman G. W., Gale R. P., Liebeskind J. C. Opioid peptides mediate the suppressive effect of stress on natural killer cell cytotoxicity. Science. 1984 Jan 13;223(4632):188–190. doi: 10.1126/science.6691146. [DOI] [PubMed] [Google Scholar]
- Sheridan J. F., Feng N. G., Bonneau R. H., Allen C. M., Huneycutt B. S., Glaser R. Restraint stress differentially affects anti-viral cellular and humoral immune responses in mice. J Neuroimmunol. 1991 Mar;31(3):245–255. doi: 10.1016/0165-5728(91)90046-a. [DOI] [PubMed] [Google Scholar]
- Shimizu M., Shimizu Y., Kodama Y. Effects of ambient temperatures on induction of transmissible gastroenteritis in feeder pigs. Infect Immun. 1978 Sep;21(3):747–752. doi: 10.1128/iai.21.3.747-752.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P. S., Auerbach S. M., Vishniavsky N., Kaplowitz L. G. Psychological factors in recurrent genital herpes infection: stress, coping style, social support, emotional dysfunction, and symptom recurrence. J Psychosom Res. 1986;30(2):163–171. doi: 10.1016/0022-3999(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Brosnan P., Meyer W. J., Blalock J. E. An ACTH receptor on human mononuclear leukocytes. Relation to adrenal ACTH-receptor activity. N Engl J Med. 1987 Nov 12;317(20):1266–1269. doi: 10.1056/NEJM198711123172006. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Solano L., Costa M., Salvati S., Coda R., Aiuti F., Mezzaroma I., Bertini M. Psychosocial factors and clinical evolution in HIV-1 infection: a longitudinal study. J Psychosom Res. 1993 Jan;37(1):39–51. doi: 10.1016/0022-3999(93)90122-v. [DOI] [PubMed] [Google Scholar]
- Stead W. W., Senner J. W., Reddick W. T., Lofgren J. P. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990 Feb 15;322(7):422–427. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Carpenter C. B., Garovoy M. R., Austen K. F., Merrill J. P., Kaliner M. The modulating influence of cyclic nucleotides upon lymphocyte-mediated cytotoxicity. J Exp Med. 1973 Aug 1;138(2):381–393. doi: 10.1084/jem.138.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz M. N. Stress and the common cold. N Engl J Med. 1991 Aug 29;325(9):654–656. doi: 10.1056/NEJM199108293250910. [DOI] [PubMed] [Google Scholar]
- Thiry E., Saliki J., Bublot M., Pastoret P. P. Reactivation of infectious bovine rhinotracheitis virus by transport. Comp Immunol Microbiol Infect Dis. 1987;10(1):59–63. doi: 10.1016/0147-9571(87)90041-5. [DOI] [PubMed] [Google Scholar]
- VOLLMER E. P. The course of pneumococcal infection in mice during treatment with antibacterial substances and adrenocortical extract. J Infect Dis. 1951 Jan-Feb;88(1):27–31. doi: 10.1093/infdis/88.1.27. [DOI] [PubMed] [Google Scholar]
- Vacca A., Martinotti S., Screpanti I., Maroder M., Felli M. P., Farina A. R., Gismondi A., Santoni A., Frati L., Gulino A. Transcriptional regulation of the interleukin 2 gene by glucocorticoid hormones. Role of steroid receptor and antigen-responsive 5'-flanking sequences. J Biol Chem. 1990 May 15;265(14):8075–8080. [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Webster E. L., Battaglia G., De Souza E. B. Functional corticotropin-releasing factor (CRF) receptors in mouse spleen: evidence from adenylate cyclase studies. Peptides. 1989 Mar-Apr;10(2):395–401. doi: 10.1016/0196-9781(89)90049-1. [DOI] [PubMed] [Google Scholar]
- Webster E. L., Tracey D. E., Jutila M. A., Wolfe S. A., Jr, De Souza E. B. Corticotropin-releasing factor receptors in mouse spleen: identification of receptor-bearing cells as resident macrophages. Endocrinology. 1990 Jul;127(1):440–452. doi: 10.1210/endo-127-1-440. [DOI] [PubMed] [Google Scholar]
- Werb Z., Foley R., Munck A. Interaction of glucocorticoids with macrophages. Identification of glucocorticoid receptors in monocytes and macrophages. J Exp Med. 1978 Jun 1;147(6):1684–1694. doi: 10.1084/jem.147.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegeshaus E., Balasubramanian V., Smith D. W. Immunity to tuberculosis from the perspective of pathogenesis. Infect Immun. 1989 Dec;57(12):3671–3676. doi: 10.1128/iai.57.12.3671-3676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA A., JENSEN M. M., RASMUSSEN A. F., Jr STRESS AND SUSCEPTIBILITY TO VIRAL INFECTIONS. 3. ANTIBODY RESPONSE AND VIRAL RETENTION DURING AVOIDANCE LEARNING STRESS. Proc Soc Exp Biol Med. 1964 Jul;116:677–680. doi: 10.3181/00379727-116-29342. [DOI] [PubMed] [Google Scholar]
- Zamri-Saad M., Jasni S., Nurida A. B., Sheikh-Omar A. R. Experimental infection of dexamethasone-treated goats with Pasteurella haemolytica A2. Br Vet J. 1991 Nov-Dec;147(6):565–568. doi: 10.1016/0007-1935(91)90027-K. [DOI] [PubMed] [Google Scholar]
- Zwilling B. S., Brown D., Pearl D. Induction of major histocompatibility complex class II glycoproteins by interferon-gamma: attenuation of the effects of restraint stress. J Neuroimmunol. 1992 Mar;37(1-2):115–122. doi: 10.1016/0165-5728(92)90162-e. [DOI] [PubMed] [Google Scholar]
- Zwilling B. S., Dinkins M., Christner R., Faris M., Griffin A., Hilburger M., McPeek M., Pearl D. Restraint stress-induced suppression of major histocompatibility complex class II expression by murine peritoneal macrophages. J Neuroimmunol. 1990 Sep-Oct;29(1-3):125–130. doi: 10.1016/0165-5728(90)90154-f. [DOI] [PubMed] [Google Scholar]


